One-Pot Syntheses of [c2]Daisy-Chain Rotaxane Networks via Thiol-Ene Reaction and Its Application to Gel Electrolyte for Secondary Battery
Abstract
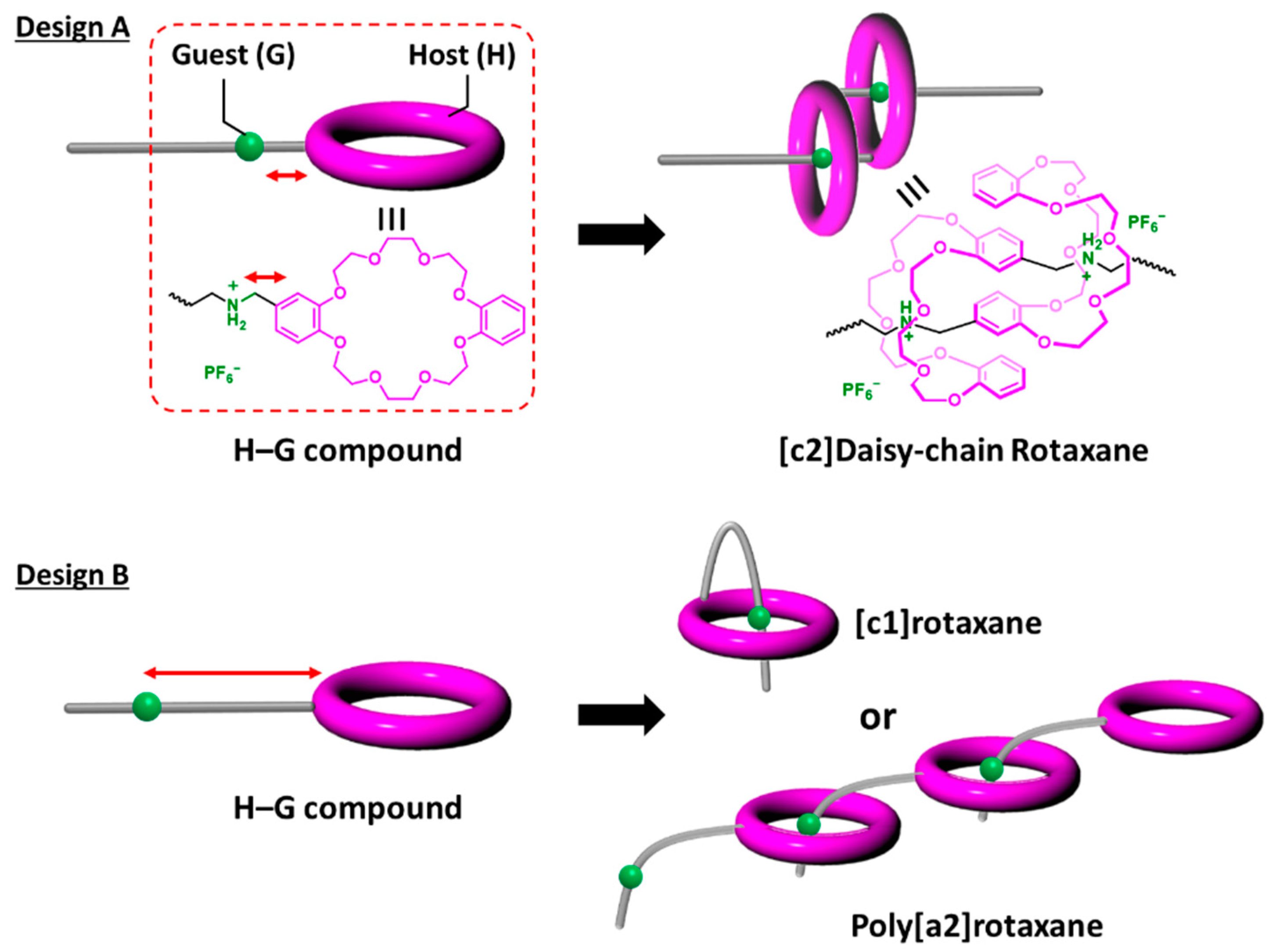
:1. Introduction
2. Experimental Section
2.1. Materials and Instruments
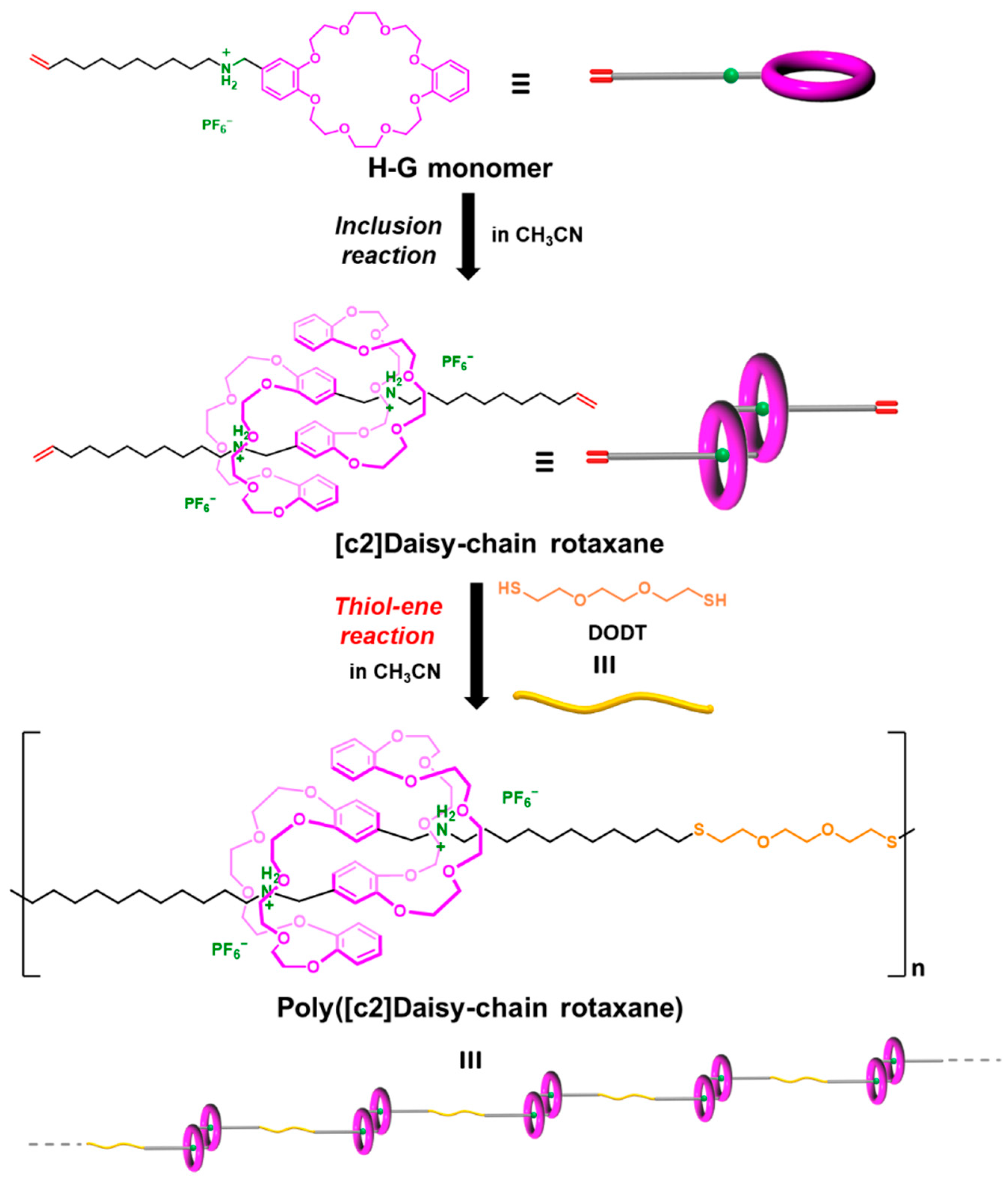
2.2. Synthesis of H–G Compound
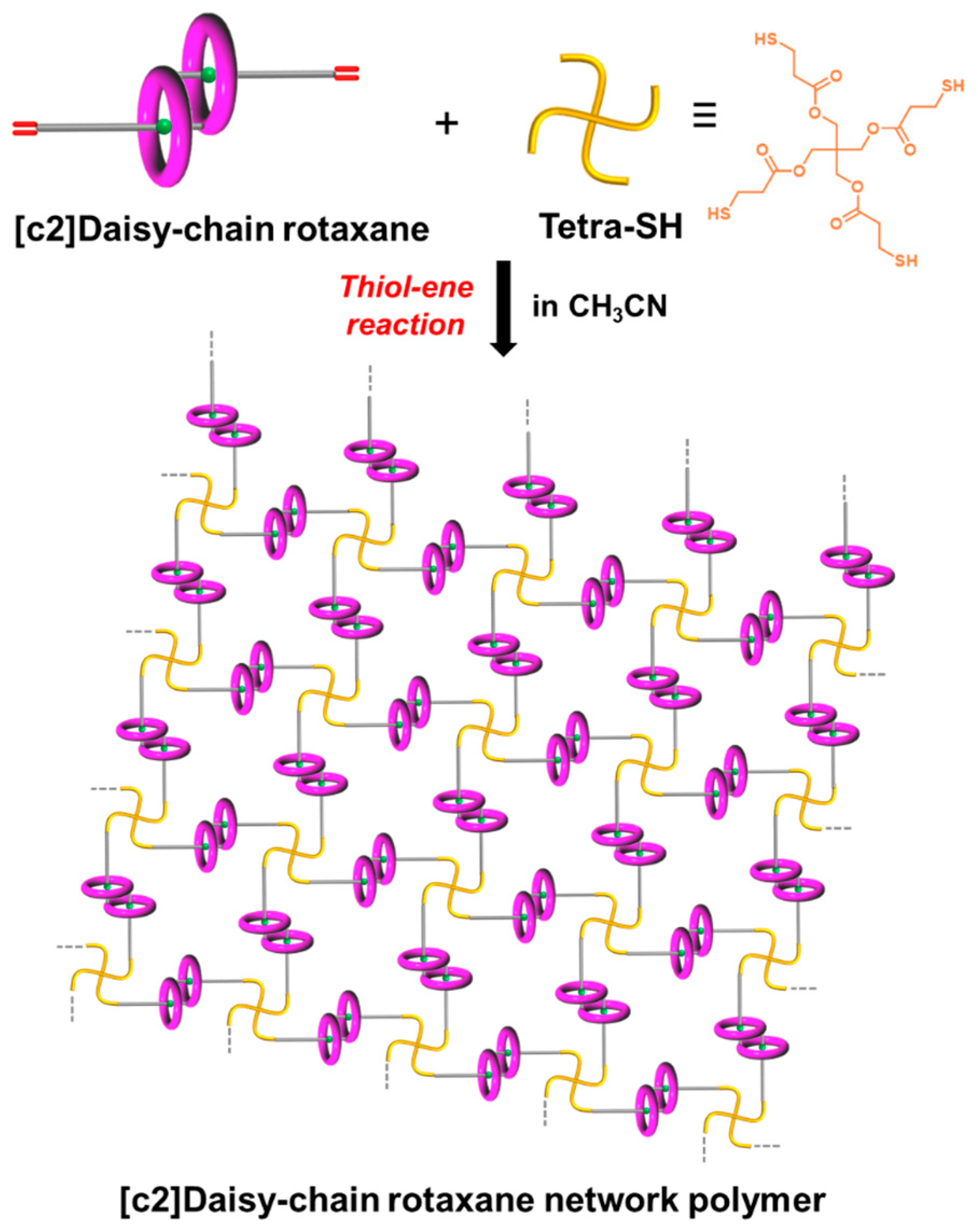
2.3. Synthesis of [c2]Daisy-Chain Rotaxane Network Polymer
2.4. Synthesis of Neutralized [c2]Daisy-Chain Rotaxane Network Polymer
2.5. Synthesis of DB24C8 Network Network
2.6. Compression Test
2.7. Ion Conductivity Measurement
3. Results and Discussion
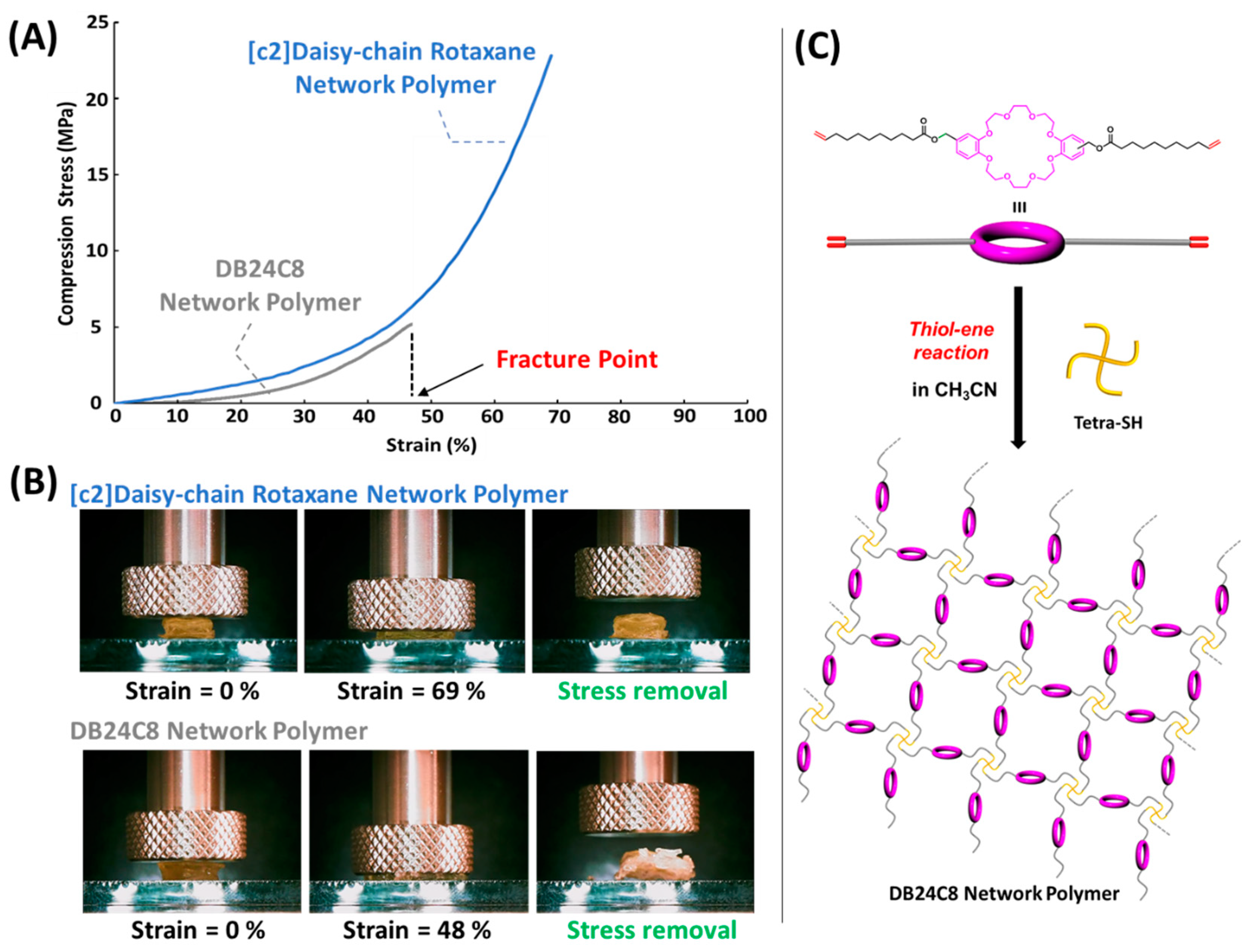
3.1. Preparation of [c2]Daisy-Chain Rotaxane Network Polymer
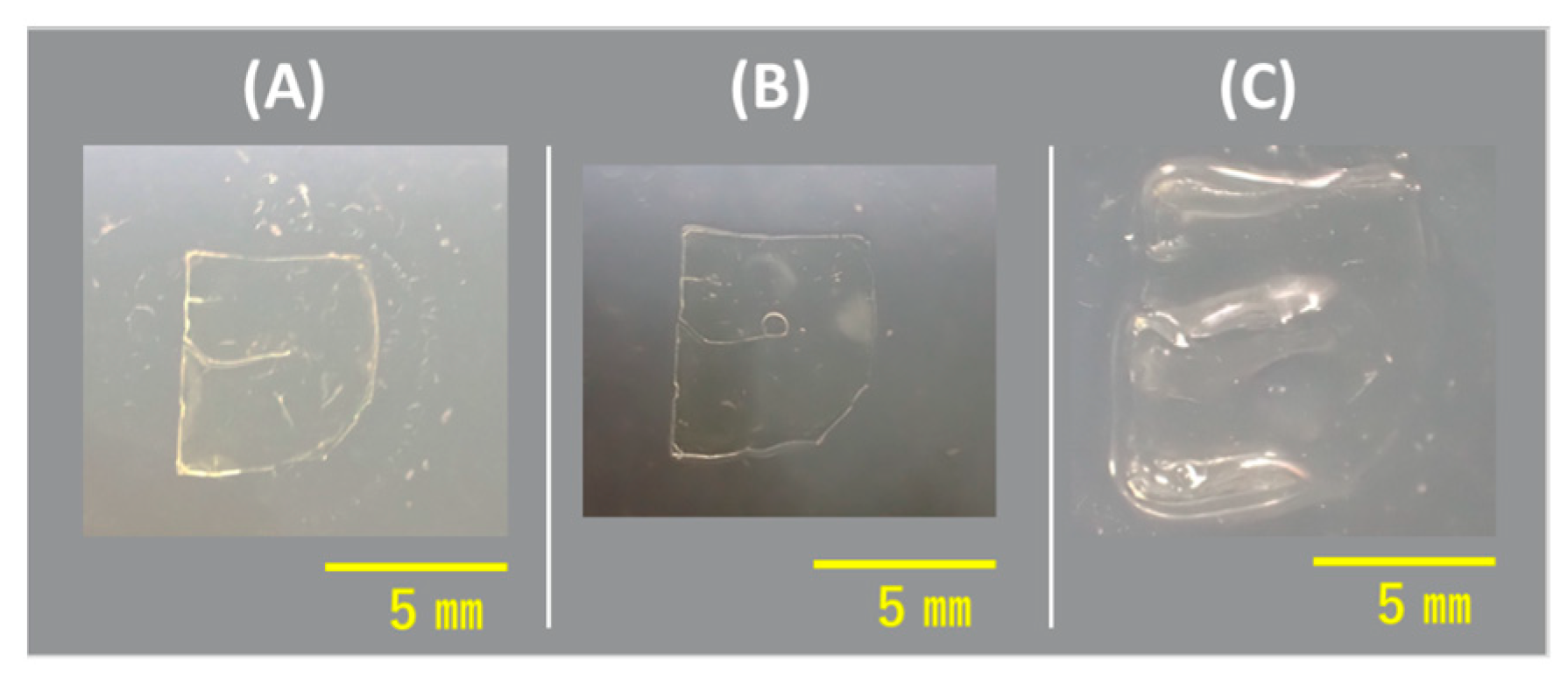
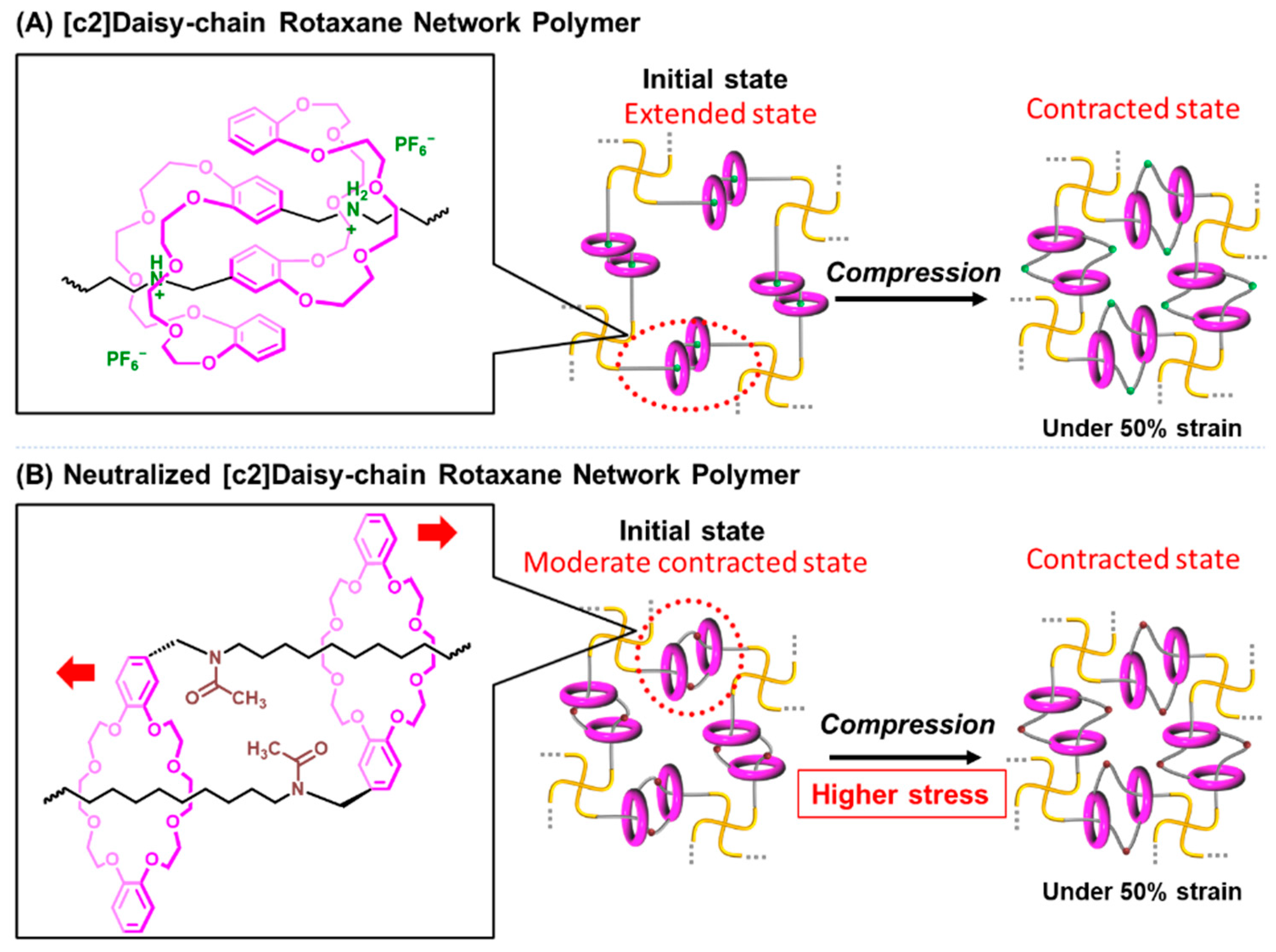
3.2. Compression Evaluation of [c2]Daisy-Chain Rotaxane Network Polymer
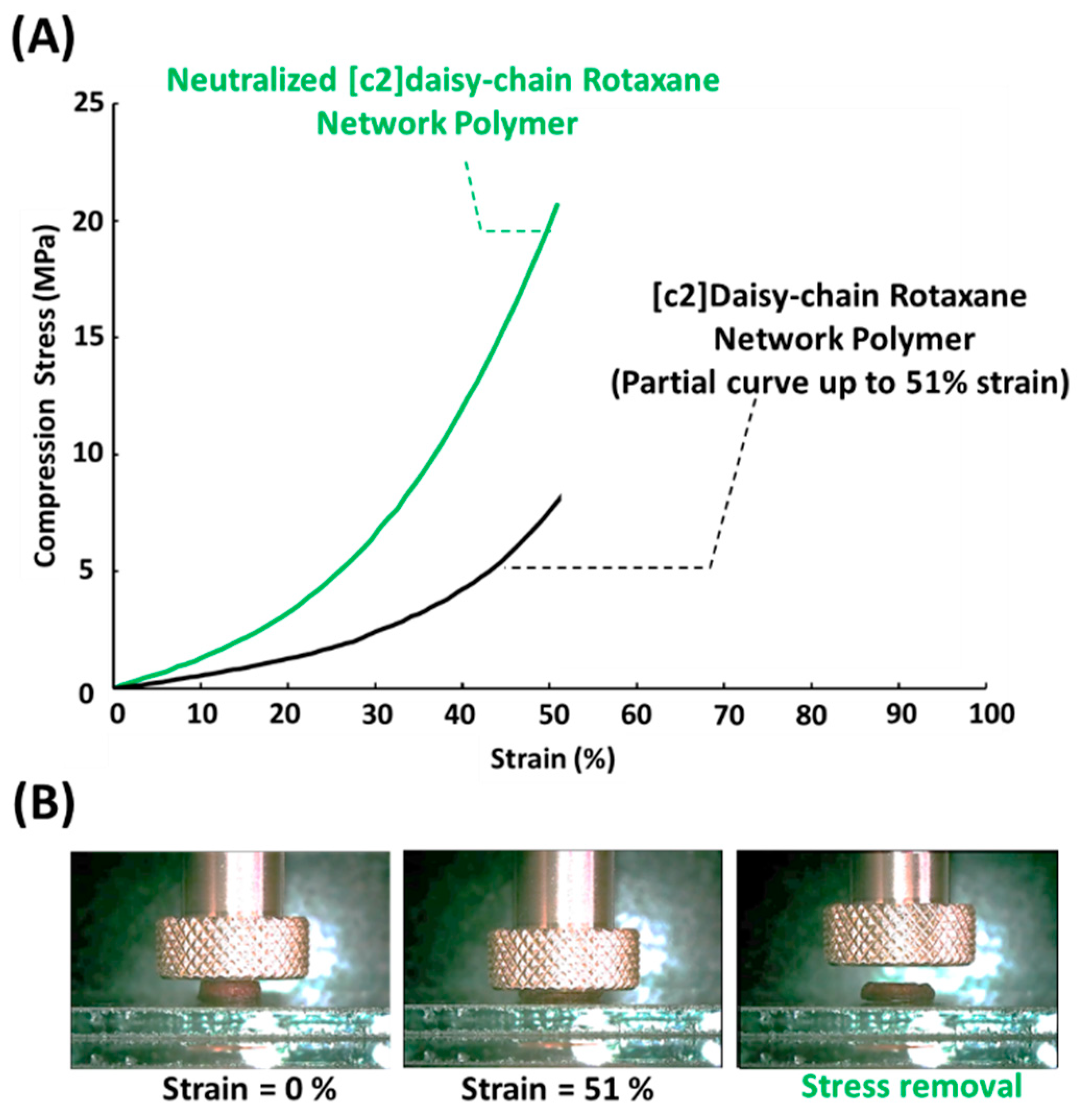
3.3. Compression Evaluation of Neutralized [c2]Daisy-Chain Rotaxane Network Polymer
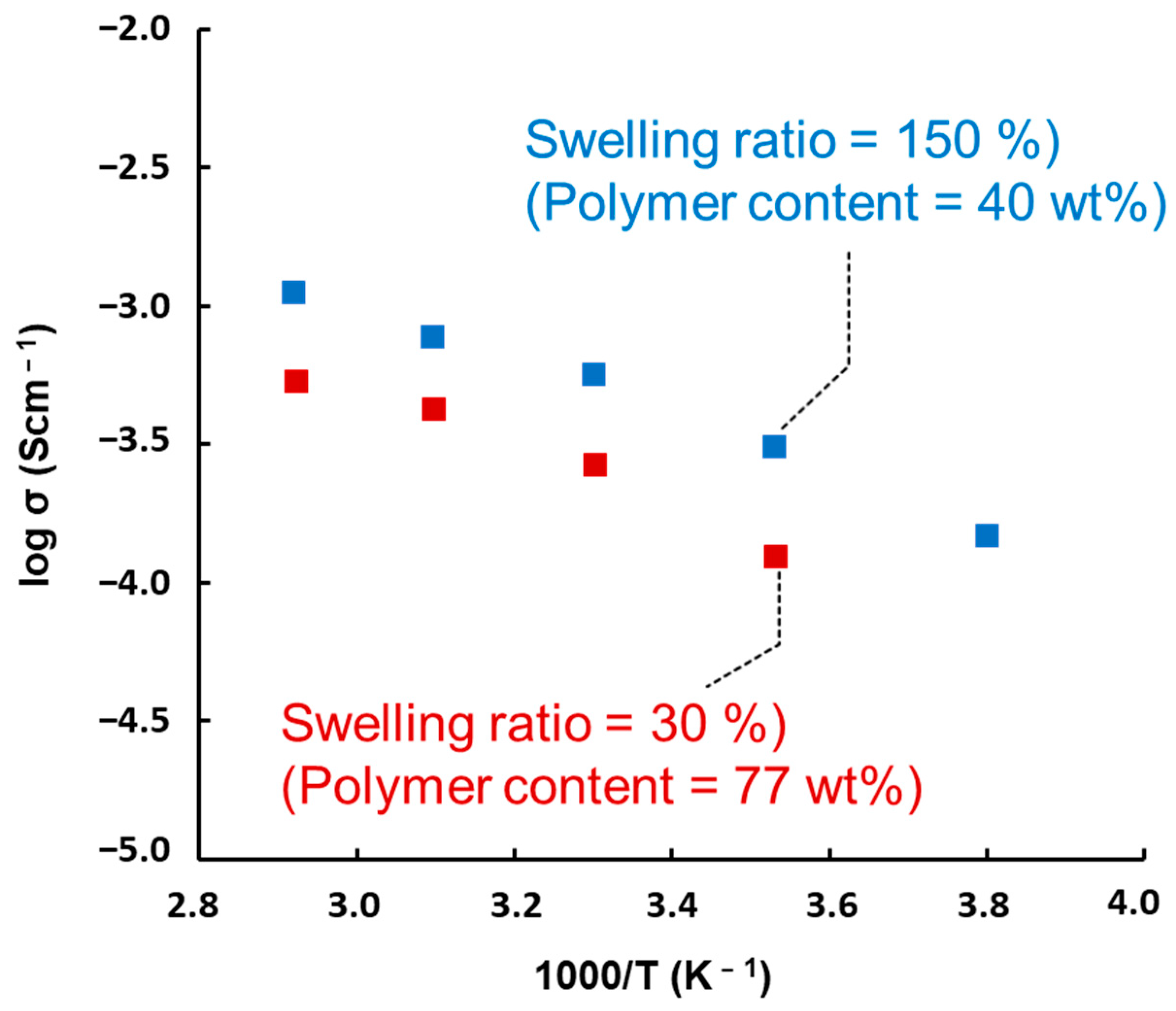
3.4. Ionic Conductivity of [c2]Daisy-Chain Rotaxane Network Polymer Gel Electrolyte
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cordova, E.; Bissell, R.A.; Spencer, N.; Ashton, P.R.; Stoddart, J.F.; Kaifer, A.E. Novel rotaxanes based on the inclusion complexation of biphenyl guests by cyclobis(paraquat-p-phenylene). J. Org. Chem. 1993, 58, 6550–6552. [Google Scholar] [CrossRef]
- Amabilino, D.B.; Stoddart, J.F. Interlocked and Intertwined Structures and Superstructures. Chem. Rev. 1995, 95, 2725–2828. [Google Scholar] [CrossRef]
- Voegtle, F.; Haendel, M.; Meier, S.; Ottens-H, S.; Ott, F.; Schmidt, T. Template synthesis of the first amide-based rotaxanes. Liebigs Ann. 1995, 1995, 739–743. [Google Scholar] [CrossRef]
- Born, M.; Koch, T.; Ritter, H. Side-chain polyrotaxanes. 3. Synthesis, characterization and enzymically catalyzed degradation of non-covalently anchored cyclodextrins in the side chains of poly(ether-ether-ketone)s. Macromol Chem. Phys. 1995, 196, 1761–1767. [Google Scholar] [CrossRef]
- Benniston, A.C.; Harriman, A.; Lynch, V.M. Photoactive [2]Rotaxanes: Structure and Photophysical Properties of Anthracene- and Ferrocene-Stoppered [2]Rotaxanes. J. Am. Chem. Soc. 1995, 117, 5275–5291. [Google Scholar] [CrossRef]
- Diederich, F.; Dietrich-Buchecker, C.; Nierengarten, J.-F.; Sauvage, J.-P. A copper(I)-complexed rotaxane with two fullerene stoppers. J. Chem. Soc. Chem. Commun. 1995, 1995, 781–782. [Google Scholar] [CrossRef]
- Takata, T.; Kawasaki, H.; Kihara, N.; Furusho, Y. Synthesis of Side-Chain Polyrotaxane by Radical Polymerizations of Pseudorotaxane Monomers Consisting of Crown Ether Wheel and Acrylate Axle Bearing Bulky End-Cap and Ammonium Group. Macromolecules 2001, 34, 5449–5456. [Google Scholar] [CrossRef]
- Yamabuki, K.; Isobe, Y.; Onimura, K.; Oishi, T. Dual polyrotaxane: One-pot synthesis of topological polymer by using metathesis reaction. Chem. Lett. 2007, 36, 1196–1197. [Google Scholar] [CrossRef]
- Yamabuki, K.; Isobe, Y.; Onimura, K.; Oishi, T. Pseudorotaxane-coupled gel: A new concept of interlocked gel synthesis by using metathesis reaction. Polym. J. 2008, 40, 205–211. [Google Scholar] [CrossRef]
- Koyanagi, K.; Takashima, Y.; Yamaguchi, H.; Harada, A. Movable Cross-Linked Polymeric Materials from Bulk Polymerization of Reactive Polyrotaxane Cross-Linker with Acrylate Monomers. Macromolecules 2017, 50, 5695–5700. [Google Scholar] [CrossRef]
- Nomura, T.; Onimura, K.; Yamabuki, K. Synthesis and Polymerization of Acid-degradable Rotaxane Using Boc Protecting Group. Chem. Lett. 2022, 51, 16–19. [Google Scholar] [CrossRef]
- Duan, Z.; Xu, F.; Huang, X.; Qian, Y.; Li, H.; Tian, W. Crown Ether-Based Supramolecular Polymers: From Synthesis to Self-Assembly. Macromol Rapid Commun. 2022, 43, 2100775. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Tang, M.; Ke, C. 3D-printed ketoenamine crosslinked polyrotaxane hydrogels and their mechanochromic responsiveness. Polym. Chem. 2023, 14, 2159–2163. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Y.; Liang, M.; Li, J.; Jiang, X.; Wu, W. Cyclodextrin-Catalyzed Diels-Alder Reaction for the Syntheses of Cyclodextrin Polyrotaxanes, Molecular Tubes, and Molecular Shuttles. Macromolecules 2024, 57, 5208–5217. [Google Scholar] [CrossRef]
- Ohtsuka, K.; Nakao, S.; Hatanaka, Y. Effect of catalysts on the mechanical, thermal, and adhesive properties of polyrotaxane-modified epoxy resin. Polymer 2024, 301, 126941. [Google Scholar] [CrossRef]
- Yi, J.; Ren, X.; Li, Y.; Yuan, Y.; Tang, W.; Wang, X.; Yu, J.; Yu, S.; Li, W.; Wang, J.; et al. Rapid-Response Water-Shrink Films with High Output Work Density Based on Polyethylene Oxide and α-Cyclodextrin for Autonomous Wound Closure. Adv. Mater. 2024, 36, 2403551. [Google Scholar] [CrossRef]
- Ashton, P.R.; Baxter, I.; Cantrill, S.J.; Fyfe, M.C.T.; Glink, P.T.; Stoddart, J.F.; White, A.J.P.; Williams, D.J. Supramolecular daisy chains. Angew. Chem. Int. Ed. 1998, 37, 1294–1297. [Google Scholar] [CrossRef]
- Jiménez, M.C.; Dietrich-Buchecker, C.; Sauvage, J.P. Towards Synthetic Molecular Muscles: Contraction and Stretching of a Linear Rotaxane Dimer. Angew. Chem. Int. Ed. 2000, 39, 3284–3287. [Google Scholar] [CrossRef]
- Amirsakis, D.G.; Elizarov, A.M.; Garcia-Garibay, M.A.; Glink, P.T.; Stoddart, J.F.; White, A.J.P.; Williams, D.J. Diastereospecific Photochemical Dimerization of a Stilbene-Containing Daisy Chain Monomer in Solution as well as in the Solid State. Angew. Chem. Int. Ed. 2003, 42, 1126–1132. [Google Scholar] [CrossRef]
- Wu, J.; Leung, K.C.F.; Bnitez, D.; Han, J.Y.; Cantrill, S.J.; Fang, L.; Stoddart, J.F. An Acid–Base-Controllable [c2]Daisy Chain. Angew. Chem. Int. Ed. 2008, 47, 7470–7474. [Google Scholar] [CrossRef]
- Zheng, B.; Klautzsch, F.; Xue, M.; Huang, F.; Schalley, C.A. Self-sorting of crown ether/secondary ammonium ion hetero-[c2]daisy chain pseudorotaxanes. Org. Chem. Front. 2014, 1, 532–540. [Google Scholar] [CrossRef]
- Bruns, C.J.; Stoddart, J.F. Rotaxane-Based Molecular Muscles. Acc. Chem. Res. 2014, 47, 2186–2199. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Gao, Z.; Yuan, M.; Zhu, J.; Wang, F. Mechanically linked poly[2]rotaxanes constructed from the benzo-21-crown-7/secondary ammonium salt recognition motif. Polym. Chem. 2016, 7, 3664–3668. [Google Scholar] [CrossRef]
- Zhang, Q.; Rao, S.; Xie, T.; Li, X.; Xu, T.; Li, D.; Qu, D.; Long, Y.; Tian, H. Muscle-like Artificial Molecular Actuators for Nanoparticles. Chem 2018, 4, 2670–2684. [Google Scholar] [CrossRef]
- Cai, K.; Shi, Y.; Zhuang, G.; Zhang, L.; Qiu, Y.; Shen, D.; Chen, H.; Jiao, Y.; Wu, H.; Cheng, C.; et al. Molecular-Pump-Enabled Synthesis of a Daisy Chain Polymer. J. Am. Chem. Soc. 2020, 142, 10308–10313. [Google Scholar] [CrossRef] [PubMed]
- Saura-Sanmartin, A.; Schalley, C.A. The Mobility of Homomeric Lasso- and Daisy Chain-Like Rotaxanes in Solution and in the Gas Phase as a means to Study Structure and Switching Behaviour. Isr. J. Chem. 2023, 63, e202300022. [Google Scholar] [CrossRef]
- Trung, N.T.; Nhien, P.Q.; Kim, C.T.T.; Wu, C.-H.; Buu Hue, B.T.; Wu, J.I.; Li, Y.-K.; Lin, H.-C. Controllable Aggregation-Induced Emission and Forster Resonance Energy Transfer Behaviors of Bistable [c2]Daisy Chain Rotaxanes for White-Light Emission and Temperature-Sensing Applications. ACS Appl. Mater. Interfaces 2023, 15, 15353–15366. [Google Scholar] [CrossRef]
- Clark, P.G.; Day, M.W.; Grubbs, R.H. Switching and Extension of a [c2]Daisy-Chain Dimer Polymer. J. Am. Chem. Soc. 2009, 131, 13631–13633. [Google Scholar] [CrossRef]
- Hmadeh, M.; Fang, L.; Trabolsi, A.; Elhabiri, M.; Albrecht-Gary, A.M.; Stoddart, J.F. On the thermodynamic and kinetic investigations of a [c2]daisy chain polymer. J. Mater. Chem. 2010, 20, 3422–3430. [Google Scholar] [CrossRef]
- Du, G.; Moulin, E.; Jouault, N.; Buhler, E.; Giuseppone, N. Muscle-like Supramolecular Polymers: Integrated Motion from Thousands of Molecular Machines. Angew. Chem. 2012, 51, 12504–12508. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, Z.; Zheng, B.; Huang, F. Construction of muscle-like metallo-supramolecular polymers from a pillar[5]arene-based [c2]daisy chain. Polym. Chem. 2014, 5, 5734–5739. [Google Scholar] [CrossRef]
- Fu, X.; Gu, R.R.; Zhang, Q.; Rao, S.J.; Zheng, X.L.; Qu, D.H.; Tian, H. Phototriggered supramolecular polymerization of a [c2]daisy chain rotaxane. Polym. Chem. 2016, 7, 2166–2170. [Google Scholar] [CrossRef]
- Goujon, A.; Mariani, G.; Lang, T.; Moulin, E.; Rawiso, M.; Buhler, E.; Giuseppone, E. Controlled Sol–Gel Transitions by Actuating Molecular Machine Based Supramolecular Polymers. J. Am. Chem. Soc. 2017, 139, 4923–4928. [Google Scholar] [CrossRef] [PubMed]
- Goujon, A.; Lang, T.; Mariani, G.; Moulin, E.; Fuks, G.; Raya, J.; Buhler, E.; Giuseppone, N. Bistable [c2]Daisy Chain Rotaxanes as Reversible Muscle-like Actuators in Mechanically Active Gel. J. Am. Chem. Soc. 2017, 139, 14825–14828. [Google Scholar] [CrossRef]
- Goujon, A.; Moulin, E.; Fuks, G.; Giuseppone, N. [c2]Daisy chain rotaxanes as molecular muscles. CCS Chem. 2019, 1, 83–96. [Google Scholar] [CrossRef]
- Zhang, Z.; You, W.; Li, P.; Zhao, J.; Guo, Z.; Xu, T.; Chen, J.; Yu, W.; Yan, X. Insights into the Correlation of Microscopic Motions of [c2]Daisy Chains with Macroscopic Mechanical Performance for Mechanically Interlocked Networks. J. Am. Chem. Soc. 2023, 145, 567–578. [Google Scholar] [CrossRef]
- Liao, S.; Tang, J.; Ma, C.; Yu, L.; Tan, Y.; Li, X.; Gan, Q. Foldaxane-Based Switchable [c2]Daisy Chains. Angew. Chem. Int. Ed. 2024, 63, e202315668. [Google Scholar] [CrossRef]
- Kamoto, R.; Onimura, K.; Yamabuki, K. One-Pot Synthesis of Stable Poly([c2]Daisy-chain Rotaxane) with Pseudo-Stopper via Metathesis Reaction and Thiol-Ene Reaction. Reactions 2023, 4, 448–464. [Google Scholar] [CrossRef]
- Zhou, S.-W.; Zhou, D.; Gu, R.; Ma, C.-S.; Yu, C.; Qu, D.-H. Mechanically interlocked [c2]daisy chain backbone enabling advanced shape-memory polymeric materials. Nat. Commun. 2024, 15, 1690. [Google Scholar] [CrossRef]
- Imholt, L.; Dong, D.; Bedrov, D.; Cekic-Laskovic, I.; Winter, M.; Brunklaus, G. Supramolecular self-assembly of methylated rotaxanes for solid polymer electrolyte application. ACS Macro Lett. 2018, 7, 881–885. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Xu, H.; Wang, Q.; Peng, W.; Chi, S.; Wang, C.; Wang, J.; Deng, Y. Polymer-in-Salt Electrolyte from Poly(caprolactone)-graft-polyrotaxane for Ni-Rich Lithium Metal Batteries at Room Temperature. ACS Appl. Mater. Interfaces 2023, 15, 31552–31560. [Google Scholar] [CrossRef] [PubMed]
- Ding, P.; Wu, L.; Lin, Z.; Lou, C.; Tang, M.; Guo, X.; Guo, H.; Wang, Y.; Yu, H. Molecular Self-Assembled Ether-Based Polyrotaxane Solid Electrolyte for Lithium Metal Batteries. J. Am. Chem. Soc. 2023, 145, 1548–1556. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Zhan, S.; Liu, W.; Liu, X.; Ning, F.; Liu, Y.; Zhang, J.; Yi, J. Targeted Delivery of Zinc Ion Derived by Pseudopolyrotaxane Gel Polymer Electrolyte for Long-Life Zn Anode. ACS Appl. Mater. Interfaces 2023, 15, 6839–6847. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamoto, R.; Onimura, K.; Yamabuki, K. One-Pot Syntheses of [c2]Daisy-Chain Rotaxane Networks via Thiol-Ene Reaction and Its Application to Gel Electrolyte for Secondary Battery. Reactions 2024, 5, 800-811. https://doi.org/10.3390/reactions5040041
Kamoto R, Onimura K, Yamabuki K. One-Pot Syntheses of [c2]Daisy-Chain Rotaxane Networks via Thiol-Ene Reaction and Its Application to Gel Electrolyte for Secondary Battery. Reactions. 2024; 5(4):800-811. https://doi.org/10.3390/reactions5040041
Chicago/Turabian StyleKamoto, Risako, Kenjiro Onimura, and Kazuhiro Yamabuki. 2024. "One-Pot Syntheses of [c2]Daisy-Chain Rotaxane Networks via Thiol-Ene Reaction and Its Application to Gel Electrolyte for Secondary Battery" Reactions 5, no. 4: 800-811. https://doi.org/10.3390/reactions5040041
APA StyleKamoto, R., Onimura, K., & Yamabuki, K. (2024). One-Pot Syntheses of [c2]Daisy-Chain Rotaxane Networks via Thiol-Ene Reaction and Its Application to Gel Electrolyte for Secondary Battery. Reactions, 5(4), 800-811. https://doi.org/10.3390/reactions5040041





