The Degradation of Furfural from Petroleum Refinery Wastewater Employing a Packed Bubble Column Reactor Using O3 and a CuO Nanocatalyst
Abstract
:1. Introduction
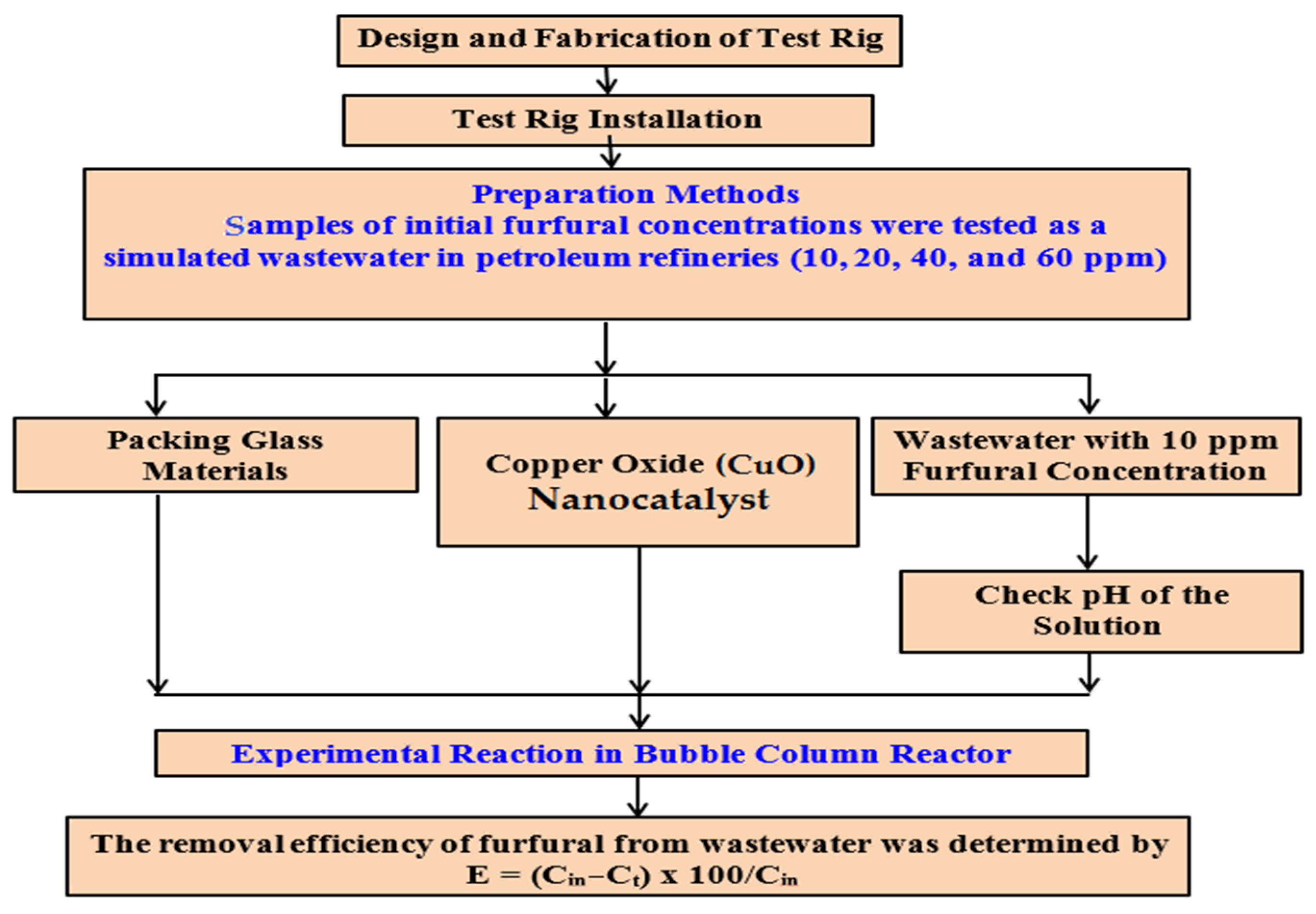
2. Materials and Methods
2.1. Chemicals
2.2. Experimental Unit
2.3. Furfural Removal Procedure
3. Results and Discussion
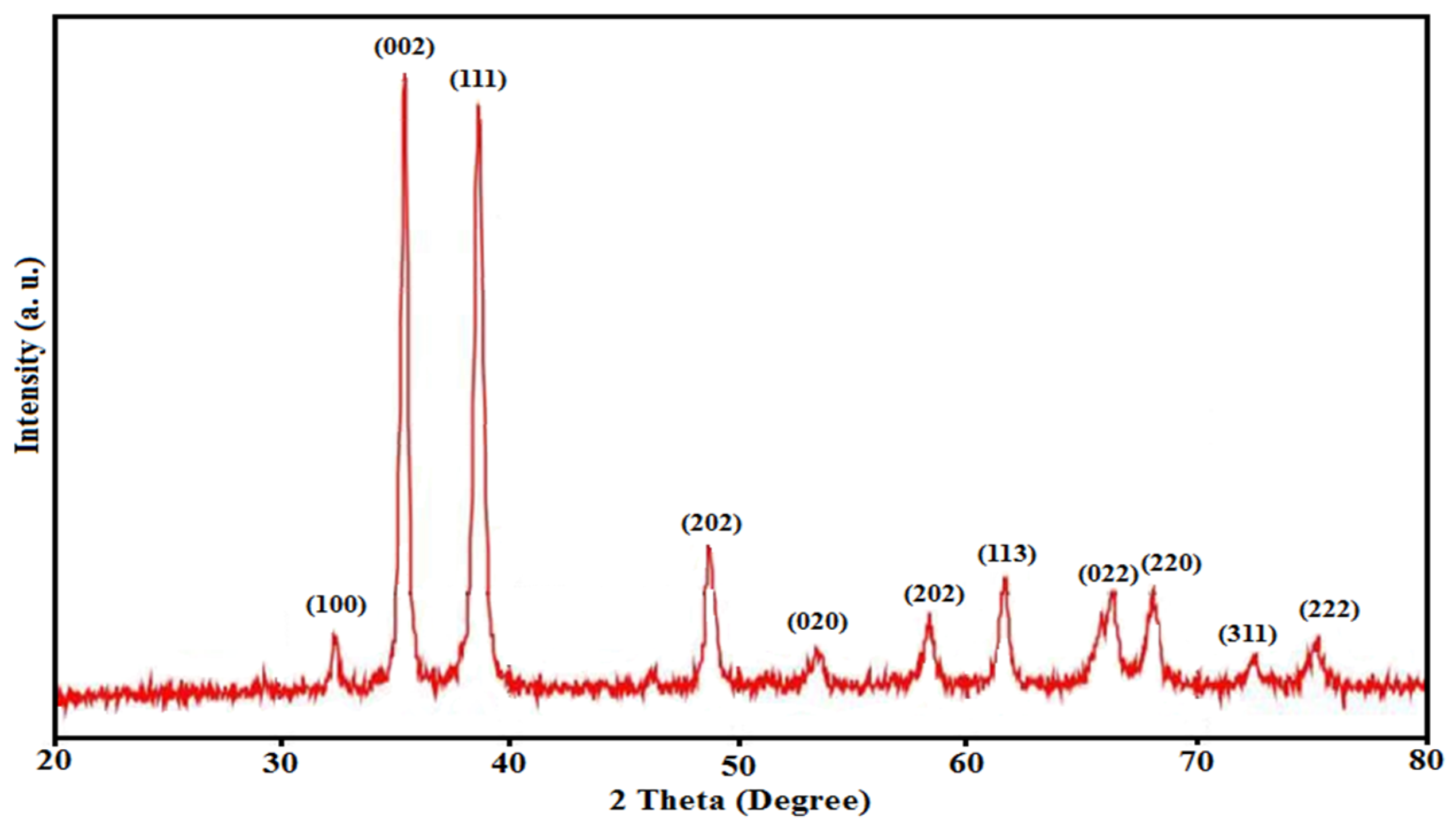
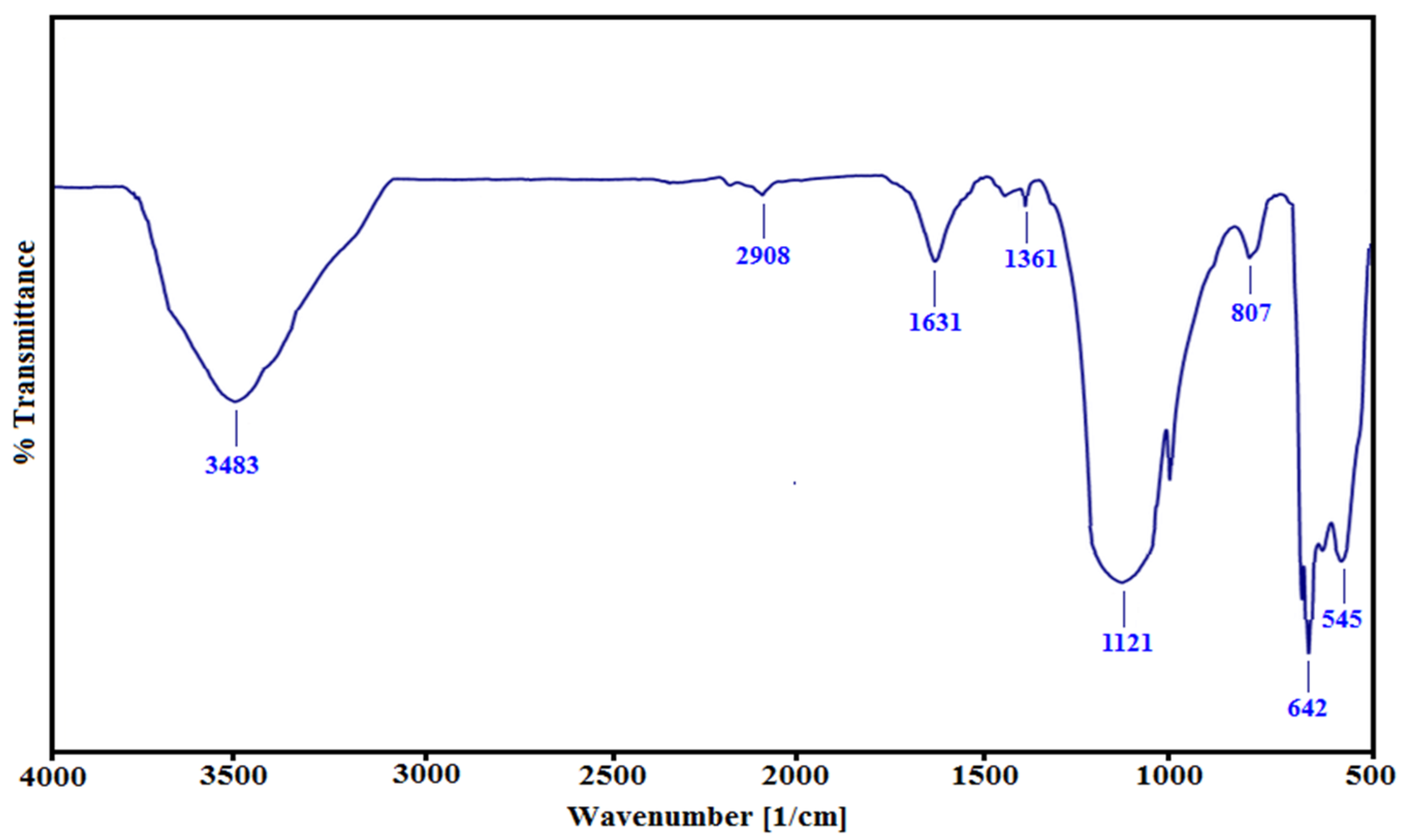
3.1. Characterization of CuO Nanocatalyst
3.2. Furfural Removal in the Presence of Ozone Gas Alone
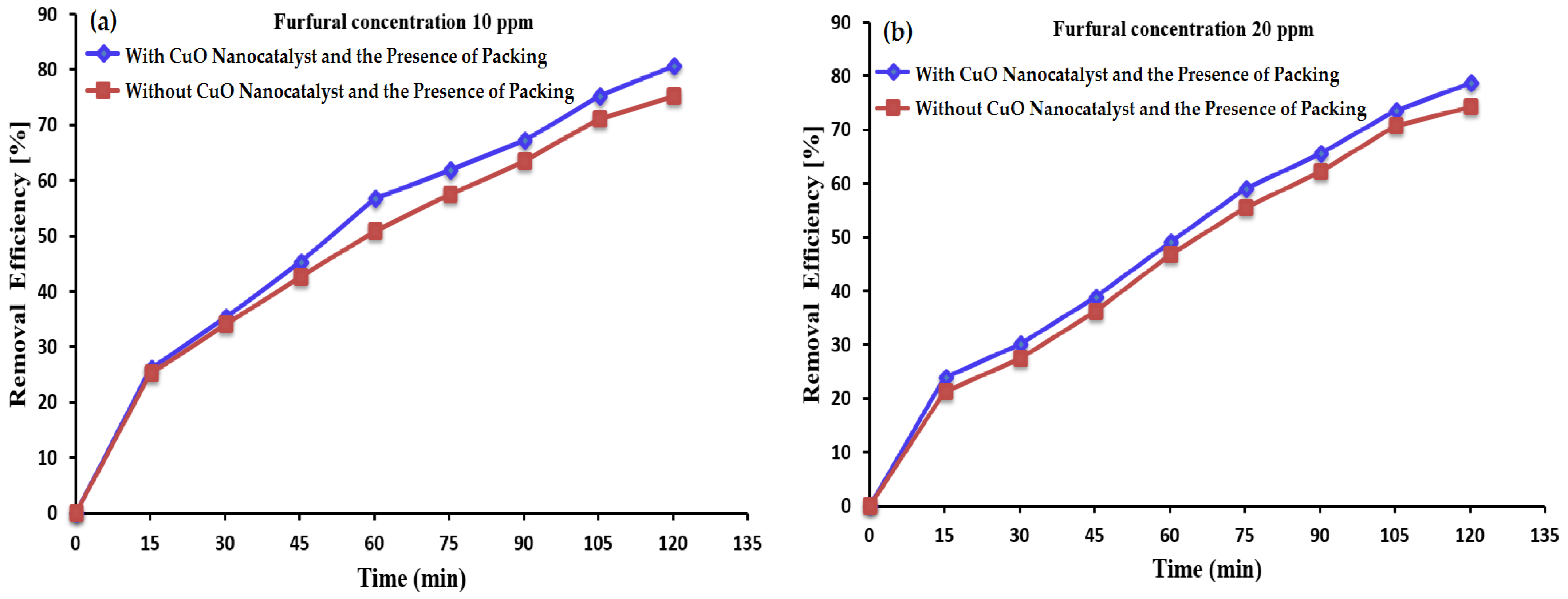
3.3. Furfural Removal in the Presence of Ozone and CuO Nanocatalyst
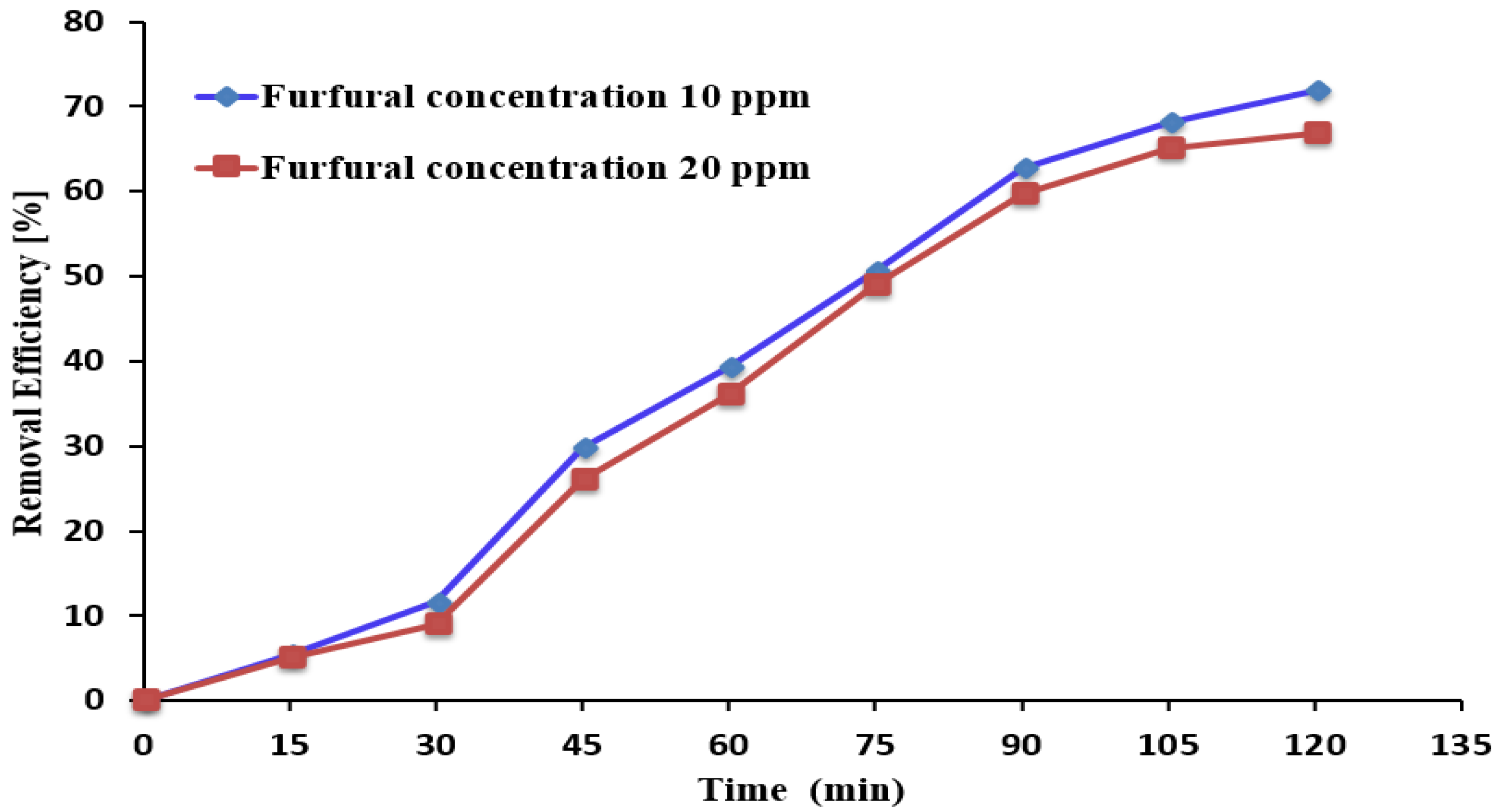
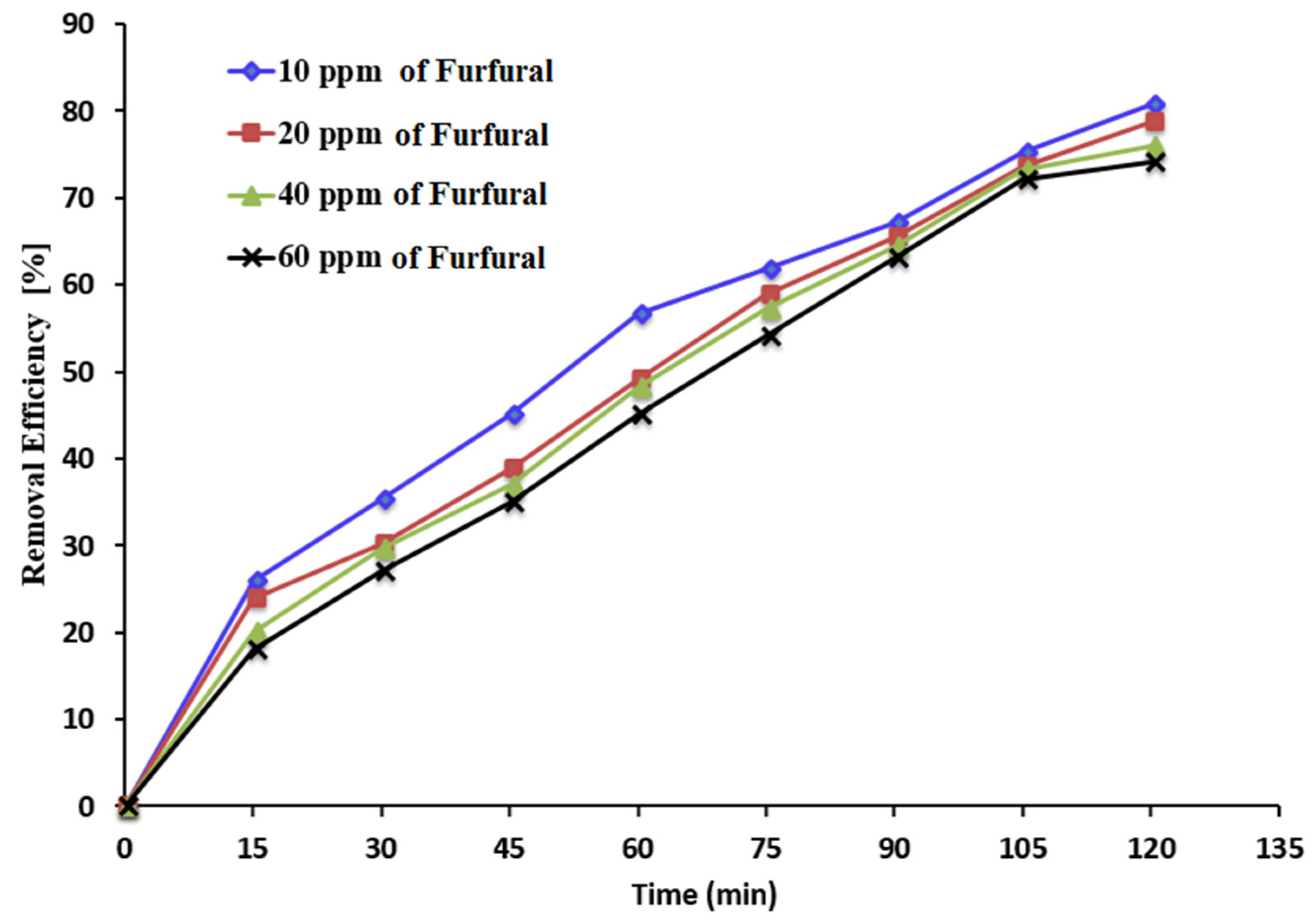
3.4. Impact of Initial Furfural Concentrations
3.5. Influence of the CuO Nanocatalyst Dose on the Removal Efficiency
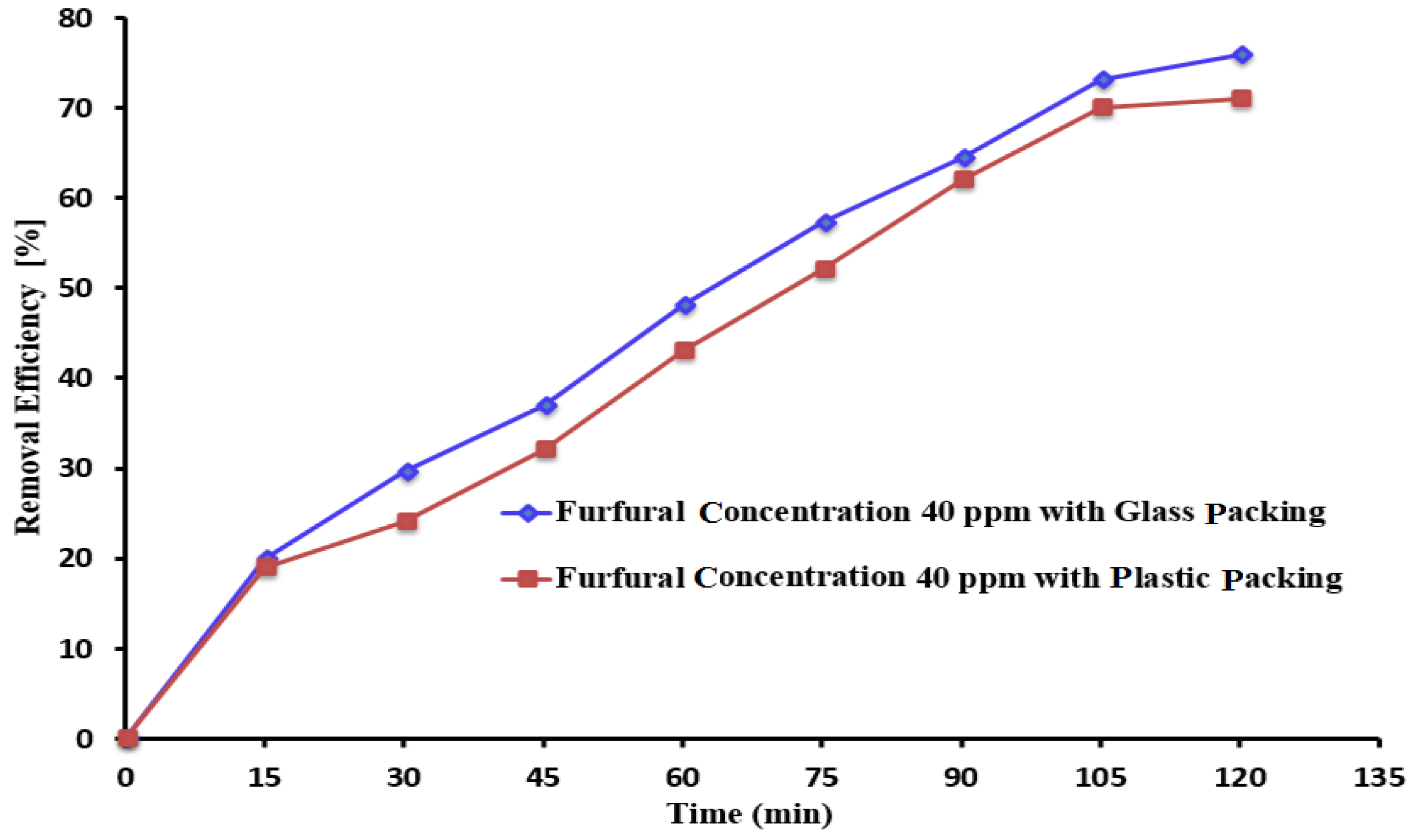
3.6. The Impact of the Packing Material
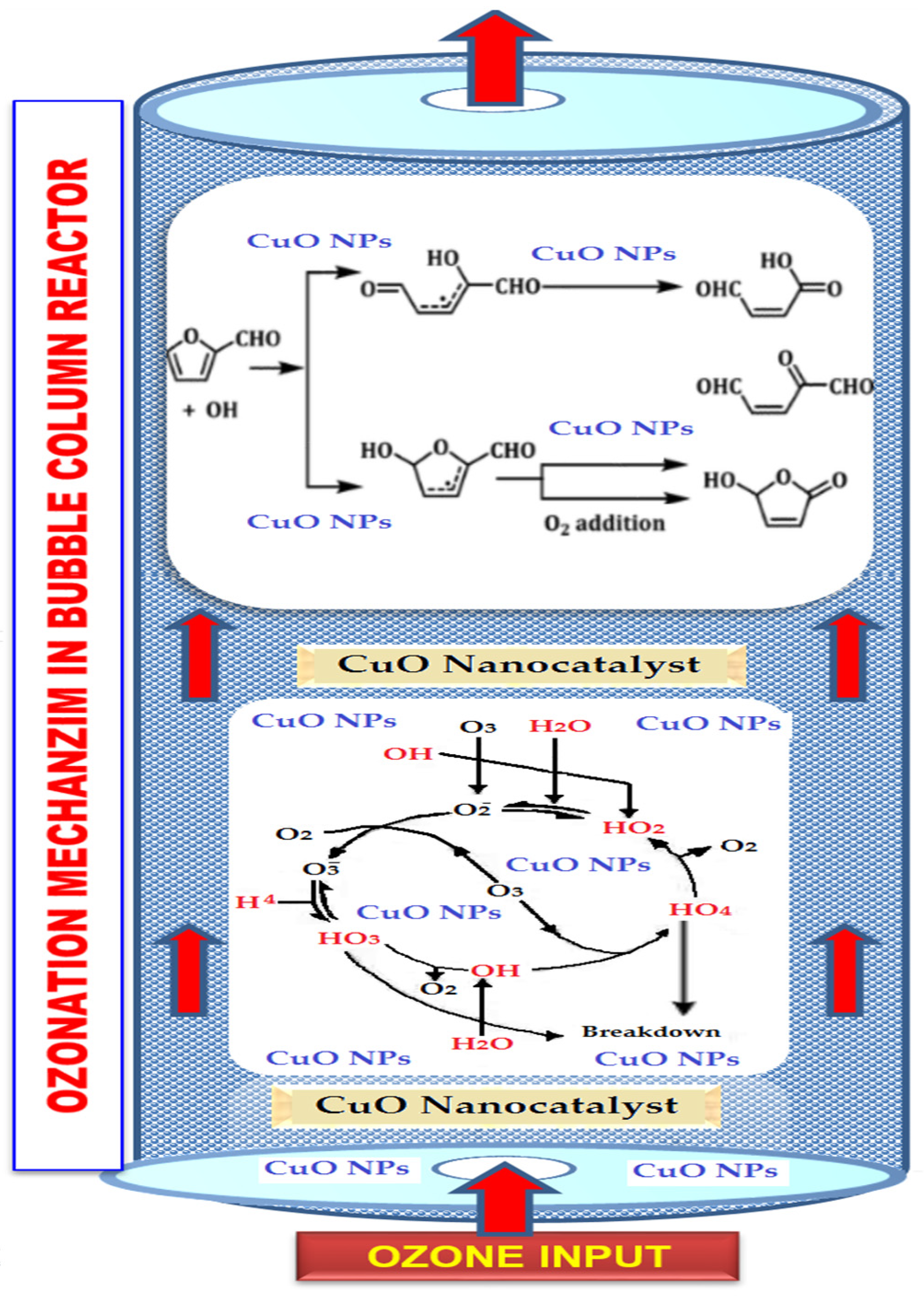
3.7. Furfural Degradation Mechanism
3.8. Kinetics Study of Furfural Degradation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Shao, S.; Ding, X.; Li, Z.; Jing, J.; Jiao, W.; Liu, Y. Removal of phenol from wastewater by high-gravity intensified heterogeneous catalytic ozonation with activated carbon. Environ. Pollut. 2022, 29, 34830–34840. [Google Scholar] [CrossRef] [PubMed]
- Dalali, N.; Kazeraninejad, M.; Akhavan, A. Treatment of petroleum refinery wastewater containing furfural by electron beam irradiation. Desalination Water Treat. 2016, 57, 24124–24131. [Google Scholar] [CrossRef]
- Guo, H.; Qin, Q.; Hu, M.; Chang, J.S.; Lee, D.J. Treatment of refinery wastewater: Current status and prospects. J. Environ. Chem. Eng. 2024, 12, 112508. [Google Scholar] [CrossRef]
- Pourali, P.; Fazlzadeh, M.; Aaligadri, M.; Dargahi, A.; Poureshgh, Y.; Kakavandi, B. Enhanced three-dimensional electrochemical process using magnetic recoverable of Fe3O4@GAC towards furfural degradation and mineralization. Arab. J. Chem. 2022, 15, 103980. [Google Scholar] [CrossRef]
- Ghosh, S.; Falyouna, O.; Malloum, A.; Othmani, A.; Bornman, C.; Bedair, H.; Onyeaka, H.; Al-Sharify, Z.T.; Jacob, A.O.; Miri, T.; et al. A general review on the use of advance oxidation and adsorption processes for the removal of furfural from industrial effluents. Microporous Mesoporous Mater. 2022, 331, 111638. [Google Scholar] [CrossRef]
- Majhool, A.; Sukkar, K.; Alsaffar, M. Combining α-Al2O3 packing material and a ZnO nanocatalyst in an ozonized bubble column reactor to increase the phenol degradation from wastewater. Processes 2023, 11, 2416. [Google Scholar] [CrossRef]
- Contreras-Zarazúa, G.; Jasso-Villegas, M.E.; Ramírez-Márquez, C.; Sánchez-Ramírez, E.; Vázquez-Castillo, J.A.; Segovia-Hernandez, J.G. Design and intensification of distillation processes for furfural and co-products purification considering economic, environmental, safety and control issues. Chem. Eng. Process.-Process Intensif. 2021, 159, 108218. [Google Scholar] [CrossRef]
- Barlak, M.S.; Değermenci, N.; Cengiz, I.; Özel, H.U.; Yildiz, E. Comparison of phenol removal with ozonation in jet loop reactor and bubble column. J. Environ. Chem. Eng. 2020, 8, 104402. [Google Scholar] [CrossRef]
- Jin, X.; Wu, C.; Fu, L.; Tian, X.; Wang, P.; Zhou, Y.; Zuo, J. Development, dilemma and potential strategies for the application of nanocatalysts in wastewater catalytic ozonation: A review. J. Environ. Sci. 2023, 124, 330–349. [Google Scholar] [CrossRef]
- Grmasha, R.A.; Stenger-Kovács, C.; Bedewy, B.A.H.; Al-Sareji, O.J.; Al-Juboori, R.A.; Meiczinger, M.; Hashim, K.S. Ecological and human health risk assessment of polycyclic aromatic hydrocarbons (PAH) in Tigris river near the oil refineries in Iraq. Environ. Res. 2023, 227, 115791. [Google Scholar] [CrossRef]
- Kermani, M.; Farzadkia, M.; Morovati, M.; Taghavi, M.; Fallahizadeh, S.; Khaksefidi, R.; Norzaee, S. Degradation of furfural in aqueous solution using activated persulfate and peroxymonosulfate by ultrasound irradiation. J. Environ. Manag. 2020, 266, 110616. [Google Scholar] [CrossRef] [PubMed]
- Alattar, S.; Sukkar, K.; Alsaffar, M. The role of TiO2 NPs catalyst and packing material in removal of phenol from wastewater using an ozonized bubble column reactor. Acta Innov. 2023, 46, 90–101. [Google Scholar] [CrossRef]
- Shabanloo, A.; Salari, M.; Shabanloo, N.; Dehghani, M.H.; Pittman, C.U., Jr.; Mohan, D. Heterogeneous persulfate activation by nano-sized Mn3O4 to degrade furfural from wastewater. J. Mol. Liq. 2020, 298, 112088. [Google Scholar] [CrossRef]
- Nsaif-Al Ezzi, A.A.R.; Balasim, N. Removal of chloroform and phenol from water by expanded bed air lift loop reactor. Solid State Technol. 2020, 63, 5213–5223. [Google Scholar]
- Khudhair, H.A.; Ismail, Z.Z. New application of single and mixed immobilized cells for furfural biodegradation. Bioremediat. J. 2019, 23, 32–41. [Google Scholar] [CrossRef]
- Cheng, P.; Huang, J.; Song, X.; Yao, T.; Jiang, J.; Zhou, C.; Yan, X.; Ruan, R. Heterotrophic and mixotrophic cultivation of microalgae to simultaneously achieve furfural wastewater treatment and lipid production. Bioresour. Technol. 2022, 349, 126888. [Google Scholar] [CrossRef]
- Alomar, T.; Hameed, B.H.; Al-Ghouti, M.A.; Almomani, F.A.; Han, D.S. A review on recent developments and future prospects in the treatment of oily petroleum refinery wastewater by adsorption. J. Water Process Eng. 2024, 64, 105616. [Google Scholar] [CrossRef]
- Shokoohi, R.; Bajalan, S.; Salari, M.; Shabanloo, A. Thermochemical degradation of furfural by sulfate radicals in aqueous solution: Optimization and synergistic effect studies. Environ. Sci. Pollut. Res. 2019, 26, 8914–8927. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, S.; Li, Z.; Liang, X.; Zhang, Z.; Liu, R.; Yun, J. Efficient degradation of phenol in aqueous solution by catalytic ozonation over MgO/AC. J. Water Process Eng. 2020, 36, 101168. [Google Scholar] [CrossRef]
- Derco, J.; Gotvajn, A.Ž.; Čižmárová, O.; Dudáš, J.; Sumegová, L.; Šimovičová, K. Removal of micropollutants by ozone-based processes. Processes 2021, 9, 1013. [Google Scholar] [CrossRef]
- Mohamadi, L.; Bazrafshan, E.; Rahdar, A.; Labuto, G.; Kamali, A.R. Nanostructured MgO-enhanced catalytic ozonation of petrochemical wastewater. Boletín Soc. Española Cerámica Vidr. 2021, 60, 391–400. [Google Scholar] [CrossRef]
- Zhang, F.; Wei, C.; Hu, Y.; Wu, H. Zinc ferrite catalysts for ozonation of aqueous organic contaminants: Phenol and bio-treated coking wastewater. Sep. Purif. Technol. 2015, 156, 625–635. [Google Scholar] [CrossRef]
- Li, X.; Fu, L.; Chen, F.; Zhao, S.; Zhu, J.; Yin, C. Application of heterogeneous catalytic ozonation in wastewater treatment: An overview. Catalysts 2023, 13, 342. [Google Scholar] [CrossRef]
- Cao, Q.; Sang, L.; Tu, J.; Xiao, Y.; Liu, N.; Wu, L.; Zhang, J. Rapid degradation of refractory organic pollutants by continuous ozonation in a micro-packed bed reactor. Chemosphere 2021, 270, 128621. [Google Scholar] [CrossRef]
- Dehdar, A.; Rahmani, A.R.; Azarian, G.; Jamshidi, R.; Moradi, S. Removal of furfural using zero gap electrocoagulation by a scrap iron anode from aqueous solution. J. Mol. Liq. 2022, 367, 120368. [Google Scholar] [CrossRef]
- Zabermawi, N.M.; Bestawy, E.E. Effective treatment of petroleum oil–contaminated wastewater using activated sludge modified with magnetite/silicon nanocomposite. Environ. Sci. Pollut. Res. 2024, 31, 17634–17650. [Google Scholar] [CrossRef]
- Ezzi, R.A.; Abdul, A.; Alhamdiny, S.H. A new treatment design for water contaminated with phenol. Environ. Health Eng. Manag. J. 2019, 6, 185–190. [Google Scholar] [CrossRef]
- Mousa, N.E.; Mohammed, S.S.; Shnain, Z.Y.; Abid, M.F.; Alwasiti, A.A.; Sukkar, K.A. Catalytic photodegradation of cyclic sulfur compounds in a model fuel using a bench-scale falling-film reactor irradiated by a visible light. Bull. Chem. React. Eng. Catal. 2022, 17, 755–767. [Google Scholar] [CrossRef]
- Can-Güven, E.; Daniser, Y.; Guvenc, S.Y.; Ghanbari, F.; Varank, G. Effective removal of furfural by ultraviolet activated persulfate, peroxide, and percarbonate oxidation: Focus on influencing factors, kinetics, and water matrix effect. J. Photochem. Photobiol. A Chem. 2022, 433, 114139. [Google Scholar] [CrossRef]
- Alattar, S.A.; Sukkar, K.A.; Alsaffar, M.A. Phenol removal from wastewater in petroleum refineries by managing flow characteristics and nanocatalyst in ozonized bubble column. Pet. Chem. 2024, 64, 159–169. [Google Scholar] [CrossRef]
- Chen, C.; Yoza, B.A.; Wang, Y.; Wang, P.; Li, Q.X.; Guo, S.; Yan, G. Catalytic ozonation of petroleum refinery wastewater utilizing Mn-Fe-Cu/Al2O3 catalyst. Environ. Sci. Pollut. Res. 2015, 22, 5552–5562. [Google Scholar] [CrossRef]
- Hamied, R.S.; Sukkar, K.A.; Majdi, H.S.; Shnain, Z.Y.; Graish, M.S.; Mahmood, L.H. Catalytic-level identification of prepared Pt/HY, Pt-Zn/HY, and Pt-Rh/HY nanocatalysts on the reforming reactions of n-Heptane. Processes 2023, 11, 270. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, H.; Wang, F.; Xiong, X.; Tian, K.; Sun, Y.; Yu, T. Application of heterogeneous catalytic ozonation for refractory organics in wastewater. Catalysts 2019, 9, 241. [Google Scholar] [CrossRef]
- Zoumpouli, G.A.; Zhang, Z.; Wenk, J.; Prasse, C. Aqueous ozonation of furans: Kinetics and transformation mechanisms leading to the formation of α, β-unsaturated dicarbonyl compounds. Water Res. 2021, 203, 117487. [Google Scholar] [CrossRef]
- Majhool, A.K.; Sukkar, K.A.; Alsaffar, M.A.; Majdi, H.S. Integrated process for high phenol removal from wastewater employing a ZnO nanocatalyst in an ozonation reaction in a packed bubble column reactor. ChemEngineering 2023, 7, 112. [Google Scholar] [CrossRef]
- Wang, S.; Ma, J.; Li, H.; Li, G.; Zhou, L.; Cao, X.; Yun, J. Mineralization of high concentration of aniline and other organics in wastewater by catalytic ozonation on CaMn2O4. J. Water Process Eng. 2024, 60, 105160. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, G.; Xu, Y.; Yu, P. The Effect of Magnetic Composites (γ-Al2O3/TiO2/γ-Fe2O3) as Ozone Catalysts in Wastewater Treatment. Materials 2022, 15, 8459. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, D.; Fan, P.P.; Zhou, L. Zeolite-induced catalytic ozonation of p-aminobenzenesulfonic acid in a bubbling reactor. Desalination Water Treat. 2016, 57, 1949–1958. [Google Scholar] [CrossRef]
- Al Ezzi, A.A.R. Removal of phenol by expanded bed airlift loop reactor. Iran. J. Chem. Chem. Eng. 2022, 41, 154–162. [Google Scholar]
- Faramarzpour, M.; Vossoughi, M.; Borghei, M. Photocatalytic degradation of furfural by titania nanoparticles in a floating-bed photoreactor. Chem. Eng. J. 2009, 146, 79–85. [Google Scholar] [CrossRef]
- Hosseini, L.; Ghafurian, N.; Hosseini, S.N.; Hassanzadeh, S.M. Immobilization of TiO2 on leca granules for photocatalytic degradation of furfural. Casp. J. Appl. Sci. Res. 2014, 3, 23. [Google Scholar]
- Jothinathan, L.; Cai, Q.Q.; Ong, S.L.; Hu, J.Y. Organics removal in high strength petrochemical wastewater with combined microbubble-catalytic ozonation process. Chemosphere 2021, 263, 127980. [Google Scholar] [CrossRef]
- Mousavi-Mortazavi, S.; Nezamzadeh-Ejhieh, A. Supported iron oxide onto an Iranian clinoptilolite as a heterogeneous catalyst for photodegradation of furfural in a wastewater sample. Desalination Water Treat. 2016, 57, 10802–10814. [Google Scholar] [CrossRef]
- Wei, X.; Shao, S.; Ding, X.; Jiao, W.; Liu, Y. Degradation of phenol with heterogeneous catalytic ozonation enhanced by high gravity technology. J. Clean. Prod. 2020, 248, 119179. [Google Scholar] [CrossRef]
- Rahman, A.A. Phenol removal using pulsation bubble column with inverse fluidization airlift loop reactor. Chem. Ind. Chem. Eng. Q. 2021, 27, 99–106. [Google Scholar]
- Alattar, S.A.; Sukkar, K.A.; Alsaffar, M.A. Enhancement of ozonation reaction for efficient removal of phenol from wastewater using a packed bubble column reactor. Indones. J. Chem. 2023, 23, 383–394. [Google Scholar] [CrossRef]
- Bello, M.M.; Y’ng, T.S.; Abdul Raman, A.A. Response surface methodology optimization of integrated fluidized bed adsorption–Fenton oxidation for removal of Reactive Black 5. Chem. Eng. Commun. 2020, 207, 1567–1578. [Google Scholar] [CrossRef]
- Abid, M.F.; Alwan, G.M.; Abdul-Ridha, L.A. Study on catalytic wet air oxidation process for phenol degradation in synthetic wastewater using trickle bed reactor. Arab. J. Sci. Eng. 2016, 41, 2659–2670. [Google Scholar] [CrossRef]
- Das, A.; Adak, M.K.; Mahata, N.; Biswas, B. Wastewater treatment with the advent of TiO2 endowed photocatalysts and their reaction kinetics with scavenger effect. J. Mol. Liq. 2021, 338, 116479. [Google Scholar] [CrossRef]
- Kamarehie, B.; Jafari, A.; Ghaderpoori, M.; Amin Karami, M.; Mousavi, K.; Ghaderpoury, A. Catalytic ozonation process using PAC/γ-Fe2O3 to Alizarin Red S degradation from aqueous solutions: A batch study. Chem. Eng. Commun. 2019, 206, 898–908. [Google Scholar] [CrossRef]
- Chen, J.; Tian, S.; Kong, L.; Tu, Y.; Lu, J.; Xiong, Y. Efficient degradation of nitrobenzene by an integrated heterogeneous catalytic ozonation and membrane separation system with active MgO (111) catalyst. Desalination Water Treat. 2015, 56, 2168–2180. [Google Scholar] [CrossRef]
- Duan, L.; Ma, C.; Lou, F.; Yin, J.; Sang, L.; Zhang, J. Investigation of external mass transfer in a micropacked bed reactor with a Pd/Al2O3/nickel foam. Ind. Eng. Chem. Res. 2022, 61, 13710–13719. [Google Scholar] [CrossRef]
- Gholami, N.; Ghasemi, B.; Anvaripour, B.; Jorfi, S. Enhanced photocatalytic degradation of furfural and a real wastewater using UVC/TiO2 nanoparticles immobilized on white concrete in a fixed-bed reactor. J. Ind. Eng. Chem. 2018, 62, 291–301. [Google Scholar] [CrossRef]
- Veisi, F.; Zazouli, M.A.; Ebrahimzadeh, M.A.; Charati, J.Y.; Dezfoli, A.S. Photocatalytic degradation of furfural in aqueous solution by N-doped titanium dioxide nanoparticles. Environ. Sci. Pollut. Res. 2016, 23, 21846–21860. [Google Scholar] [CrossRef]
- Mazumder, A.; Sarkar, S.; Sen, D.; Bhattacharjee, C. Environmental effects and human health challenges originated from oily wastewater. In Advanced Technologies in Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2023; pp. 29–47. [Google Scholar]
- Gu, Y.; Dai, P.; Wu, T.; Yuan, F.; Yang, Q. A novel physical-biochemical treatment of refinery wastewater. J. Environ. Manag. 2024, 354, 120356. [Google Scholar] [CrossRef]
- Ismail, Z.Z.; Jasim, A.S. Ultrasonic treatment of wastewater contaminated with furfural. IDA J. Desalination Water Reuse 2014, 6, 103–111. [Google Scholar] [CrossRef]
- Ayhan, N.N.; Aldemir, A.; Özgüven, A. Treatment of petroleum refinery wastewater by chemical coagulation method: Determination of optimum removal conditions using experimental design. Braz. J. Chem. Eng. 2024, 41, 121–137. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Sukkar, K.A.; Shnain, Z.Y. Effect of graphene and multiwalled carbon nanotube additives on the properties of nano-reinforced rubber. Chem. Pap. 2021, 75, 3265–3272. [Google Scholar] [CrossRef]
- Baqur, M.S.; Hamied, R.S.; Sukkar, K.A. An eco-friendly process to produce high-purity nano-γ-Al2O3 from aluminum scrap using a novel electrolysis technique for petroleum industry applications. Arab. J. Sci. Eng. 2023, 48, 15915–15925. [Google Scholar] [CrossRef]
- Wang, J.; Smit, M.G.; Verhaegen, Y.; Nolte, T.M.; Redman, A.D.; Hendriks, A.J.; Hjort, M. Petroleum refinery effluent contribution to chemical mixture toxic pressure in the environment. Chemosphere 2023, 311, 137127. [Google Scholar] [CrossRef]
- Al-Tameeemi, H.M.; Sukkar, K.A.; Abbar, A.H. Treatment of petroleum refinery wastewater by a combination of anodic oxidation with photocatalyst process: Recent advances, affecting factors and future perspectives. Chem. Eng. Res. Des. 2024, 204, 487–508. [Google Scholar] [CrossRef]
- ASTM D7573; Standard Test Method for Total Carbon and Organic Carbon in Water by High Temperature Catalytic Combustion and Infrared Detection. ASTM: West Conshohocken, PA, USA, 2017.
- Luo, X.; Su, T.; Xie, X.; Qin, Z.; Ji, H. The adsorption of ozone on the solid catalyst surface and the catalytic reaction mechanism for organic components. ChemistrySelect 2020, 5, 15092–15116. [Google Scholar] [CrossRef]





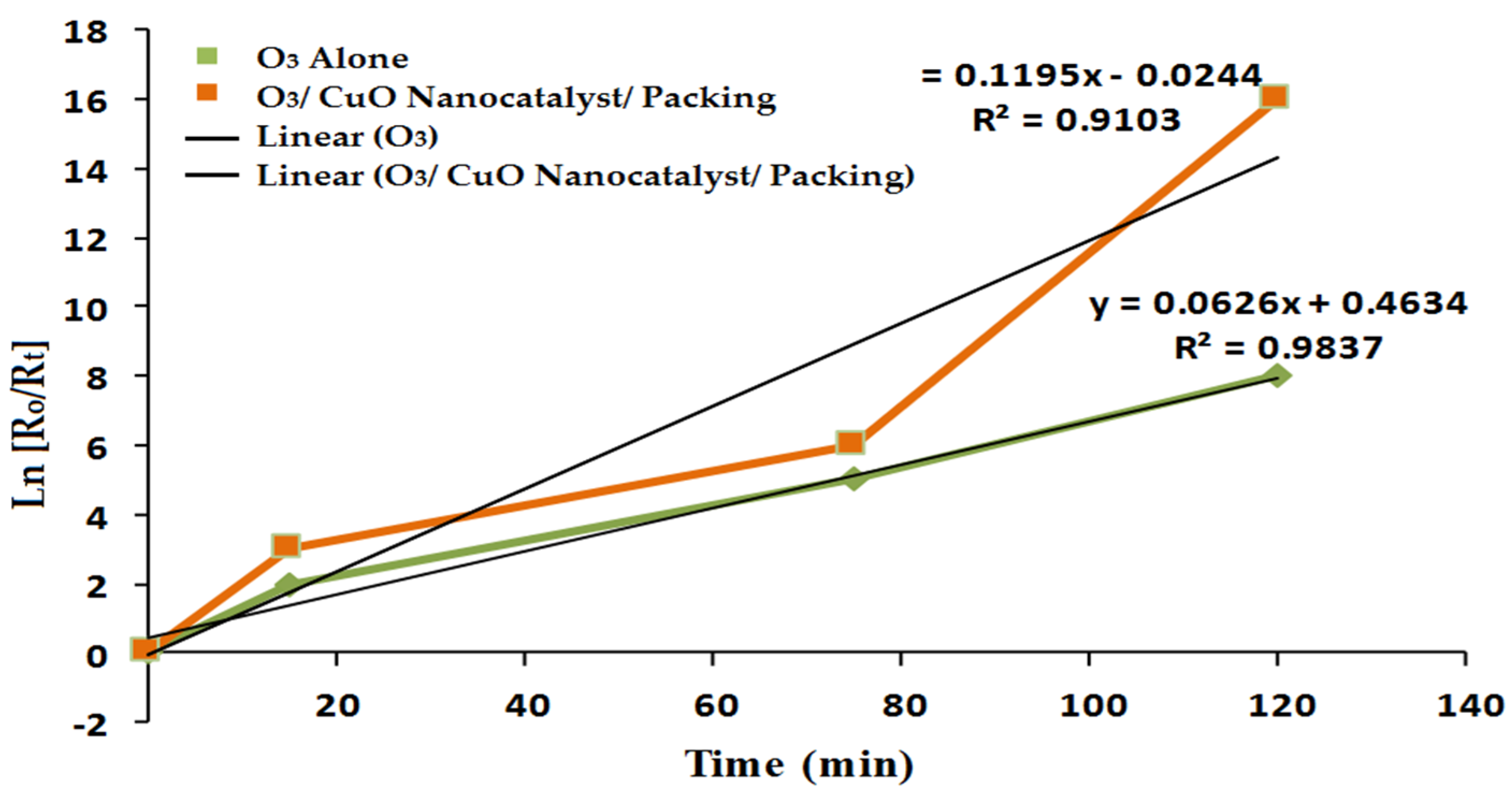
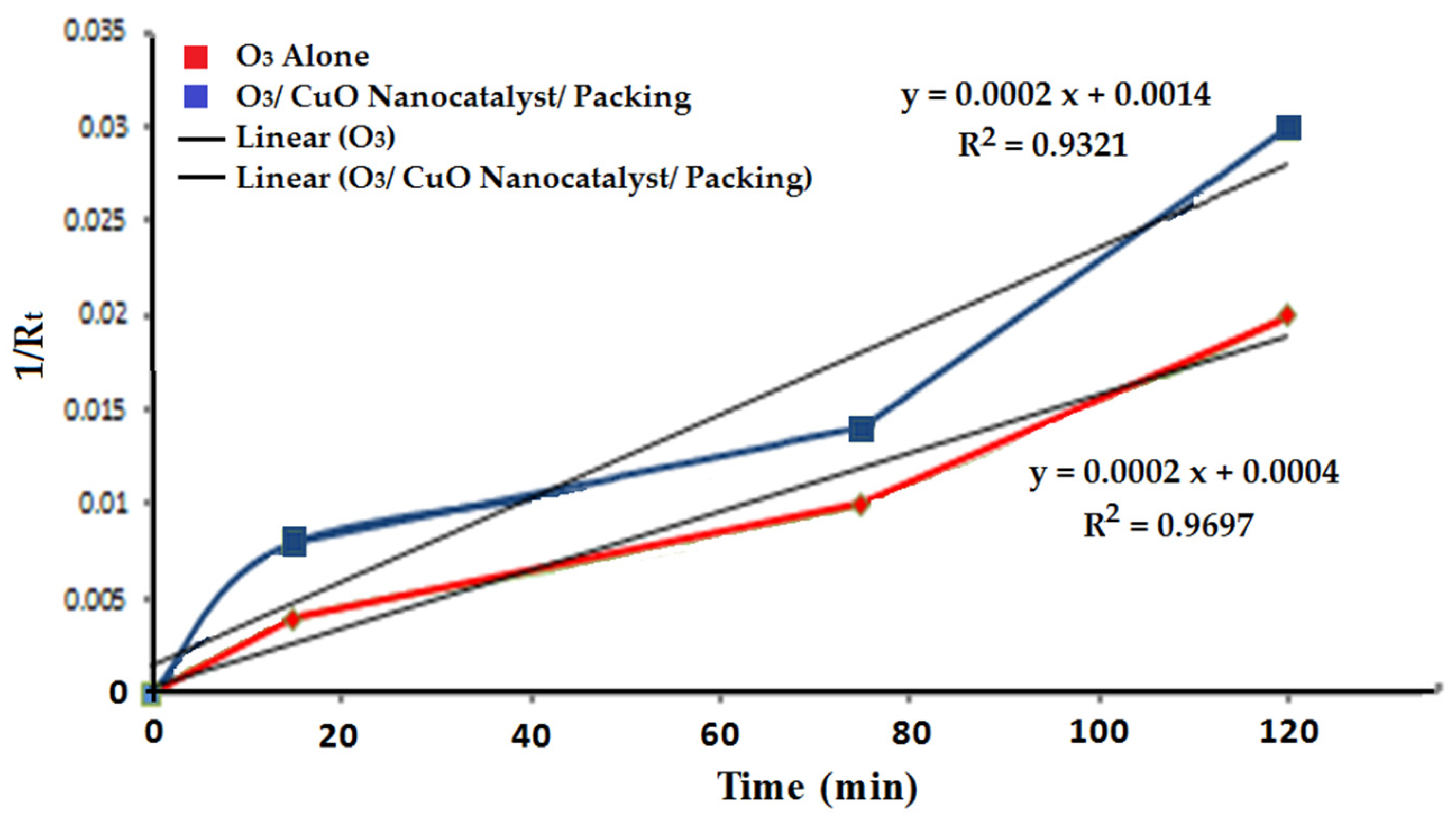
| The First-Order Equation | |||
|---|---|---|---|
| Type of treatment | Equation, Y = Ln(Ro/Rt), and x = t | k1 (1/min) | R² (L/mg·min) |
| O3 alone | y = 0.0626x + 0.4634 | 0.06712 | 0.9837 |
| O3/packing/CuO nanocatalyst | y = 0.1195x − 0.0521 | 0.131 | 0.9103 |
| The Second-Order Equation | |||
| Type of treatment | Equation, Y = (1/Rt), and x = t | k2 (L/mg·min) | R² (L/mg·min) |
| O3 alone | y = 0.0298x + 0.1405 | 0.00019 | 0.9697 |
| O3/packing/CuO nanocatalyst | y = 0.0002x − 0.0014 | 0.00017 | 0.9321 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed, S.M.; Al Ezzi, A.A.R.; Majdi, H.S.; Sukkar, K.A. The Degradation of Furfural from Petroleum Refinery Wastewater Employing a Packed Bubble Column Reactor Using O3 and a CuO Nanocatalyst. Reactions 2024, 5, 883-899. https://doi.org/10.3390/reactions5040047
Mohammed SM, Al Ezzi AAR, Majdi HS, Sukkar KA. The Degradation of Furfural from Petroleum Refinery Wastewater Employing a Packed Bubble Column Reactor Using O3 and a CuO Nanocatalyst. Reactions. 2024; 5(4):883-899. https://doi.org/10.3390/reactions5040047
Chicago/Turabian StyleMohammed, Safiaa M., Ali Abdul Rahman Al Ezzi, Hasan Shakir Majdi, and Khalid A. Sukkar. 2024. "The Degradation of Furfural from Petroleum Refinery Wastewater Employing a Packed Bubble Column Reactor Using O3 and a CuO Nanocatalyst" Reactions 5, no. 4: 883-899. https://doi.org/10.3390/reactions5040047
APA StyleMohammed, S. M., Al Ezzi, A. A. R., Majdi, H. S., & Sukkar, K. A. (2024). The Degradation of Furfural from Petroleum Refinery Wastewater Employing a Packed Bubble Column Reactor Using O3 and a CuO Nanocatalyst. Reactions, 5(4), 883-899. https://doi.org/10.3390/reactions5040047







