Interplay Between Diabetes, Obesity and Glioblastoma Multiforme, and the Role of Nanotechnology in Its Treatment
Abstract
:1. Introduction
2. Impact of Metabolic Disorders on Glioblastoma Multiforme
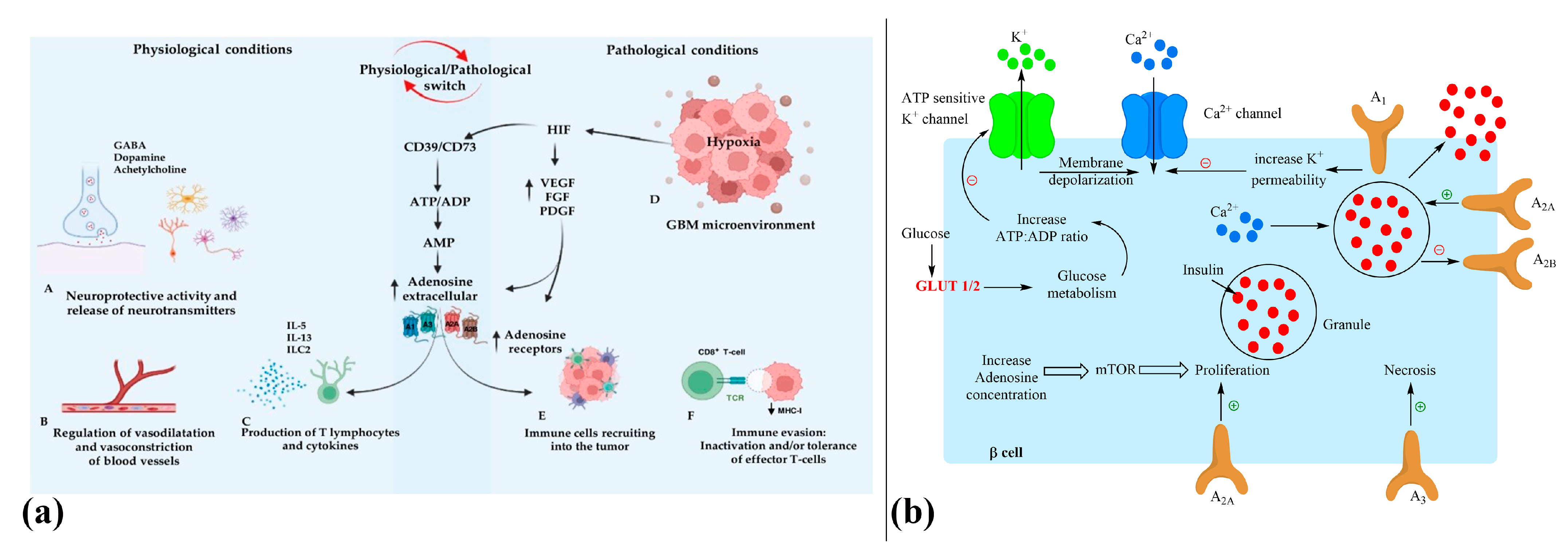
2.1. Role of Diabetes, Glioma Metabolism, and Adenosine Signaling in GBM
2.2. T2DM Clinical Risk to GBM Development
2.3. Influence of Diabetes and Obesity on GBM Prognosis and Survival Outcomes
2.4. Role of Hyperglycemia in Shaping GBM Patient Outcomes
2.5. Effects of Metformin and Oral Anti-Diabetic Medications on Patients with Glioblastoma Multiforme
2.6. Influence of Steroid Therapy on Treatment Outcomes in the Context of Diabetes and Obesity in GBM Patients
3. Ketogenic Diet
4. Biomaterials for Managing GBM in the Context of Diabetes and Obesity
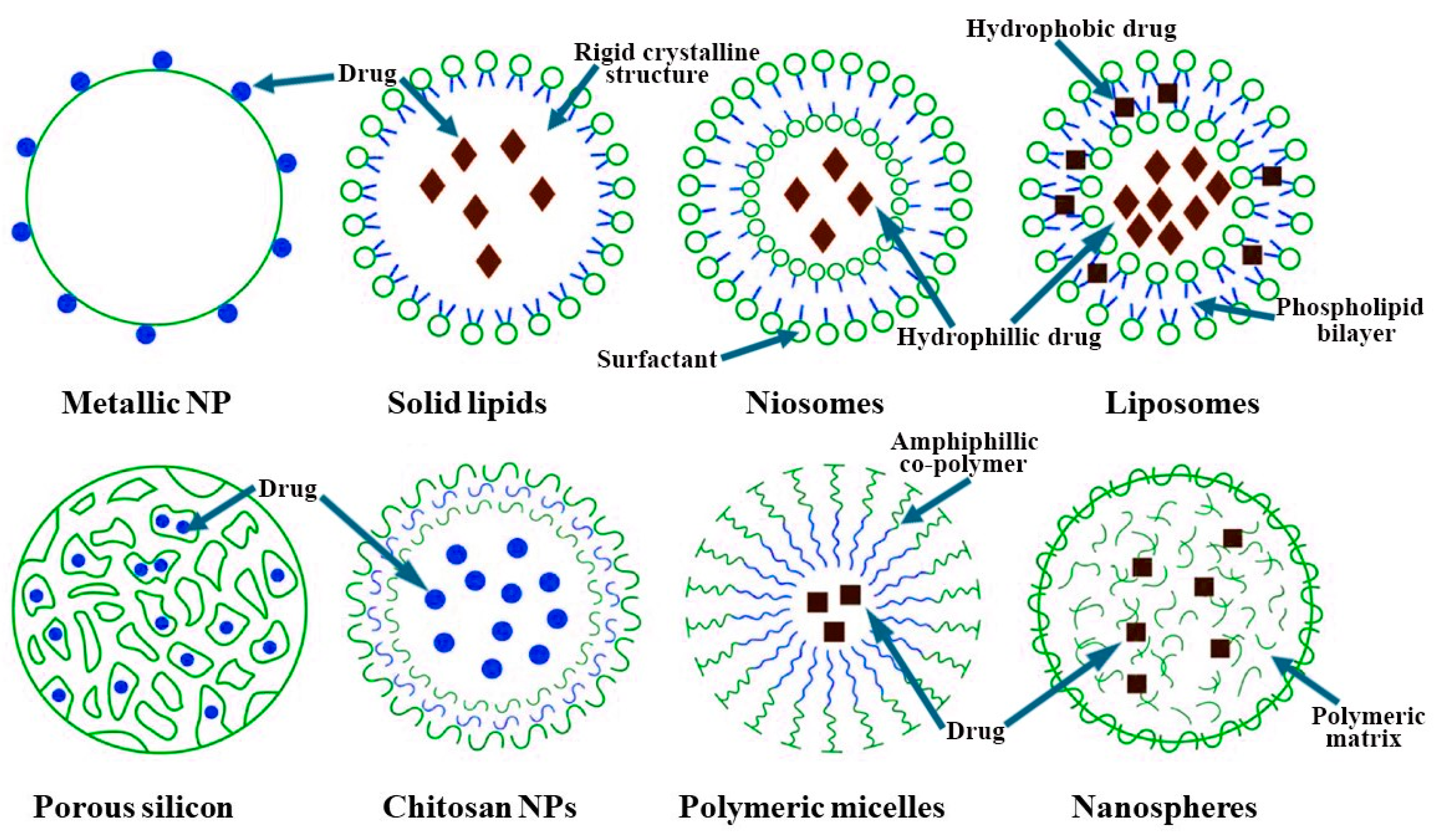
5. Nanocarriers Designed for the Delivery of Anticancer Agents
5.1. Nanocarrier Characteristics
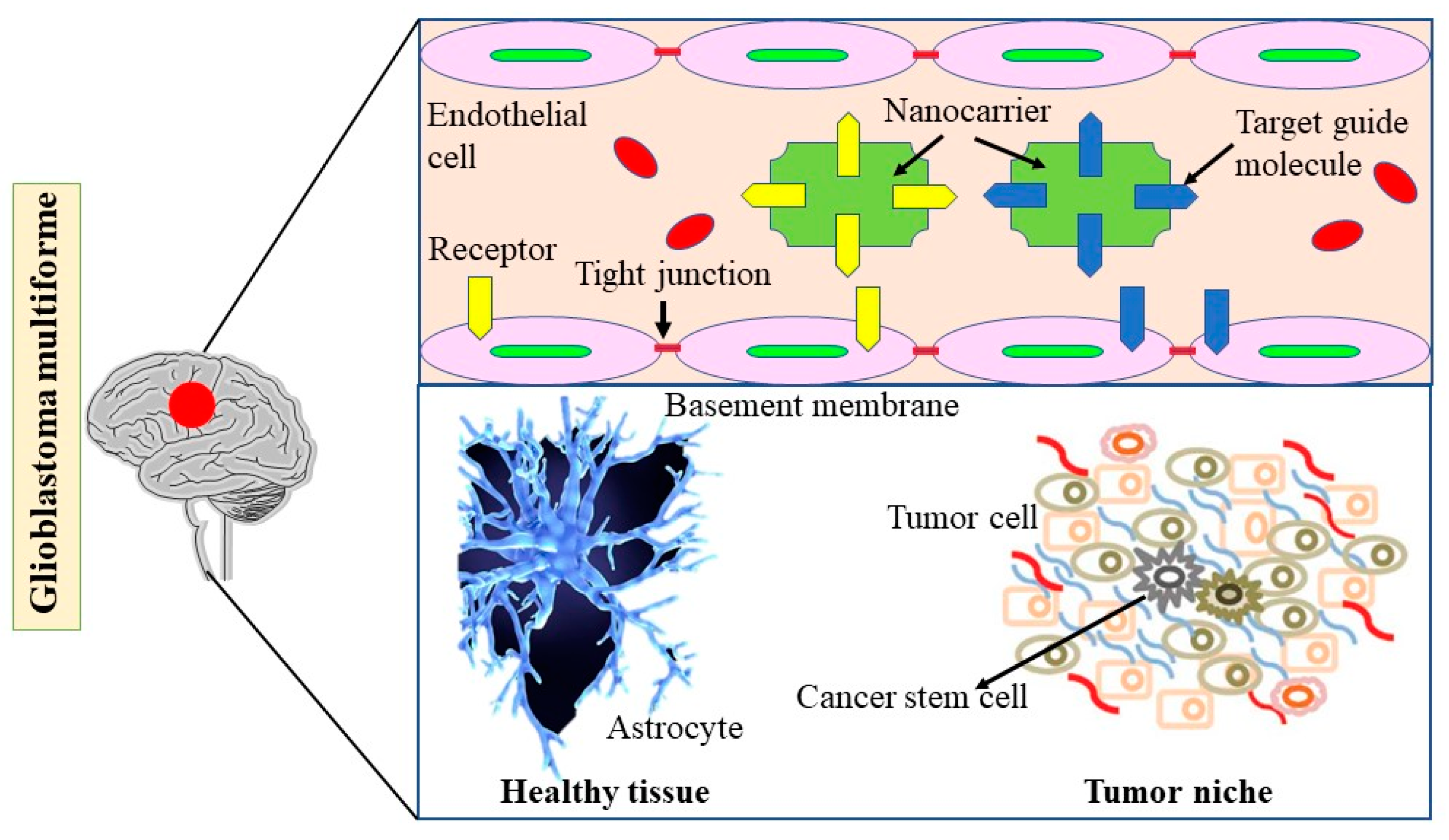
5.2. Nanotechnology-Based Strategies for Enhancing Drug Delivery in GBM: Exploring the Impact of Diabetes and Obesity
5.3. Impact of Diabetes, Obesity, and Nanotechnology on Glioblastoma Multiforme Cells and Glioblastoma Stem Cells
5.4. Current NCs and GBM Treatment Strategies
5.4.1. Liposomes
5.4.2. Polymeric Micelles
5.4.3. Dendrimers
5.4.4. Metal Nanoparticles
5.4.5. Silica Nanoparticles
5.5. Regulatory Hurdles and Ethical Considerations
6. Role of NCs Beta-Cell Function and Their Implications for Diabetes, Obesity, and GBM Treatment
6.1. Biomolecule-Based Nanomaterials for DM Therapy
6.2. Polymeric NPs for Drug Delivery
7. Clinical Trials Exploring the Impact of Diabetes, Obesity, and Nanotechnology on GBM Prognosis and Treatment
8. Limitations
9. Conclusions and Future Perspective
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| A2AAR | anti-adenosine A2A receptor |
| Akt | protein kinase B/Akt |
| ALDH1A3 | aldehyde dehydrogenase 1A3 |
| AMP | adenosine monophosphate |
| ATP | adenosine triphosphate |
| AuNP | gold nanoparticles |
| BBB | blood–brain barrier |
| CD73 | cluster of differentiation 73 |
| CED | convection-enhanced delivery |
| cIAP1 | cellular IAP 1 |
| CLL | chronic lymphocytic leukemia |
| CNS | central nervous system |
| DM | diabetes mellitus |
| EDVDox | EnGeneral delivery vehicle-doxorubicin |
| EGFR | epithelial growth factor receptor |
| FPR2 | formylpeptide receptor 2 |
| GBM | glioblastoma multiforme |
| GSC | glioblastoma stem cell |
| GTR | gross total resection |
| HbA1c | hemoglobin A1c |
| Hsp90 | heat shock protein 90 |
| IAP | inhibitors of apoptosis protein |
| IDH | isocitrate dehydrogenase |
| IGF | insulin-like growth factor |
| IL | interleukin |
| JNK | c-Jun N-terminal kinase |
| MDSC | myeloid-derived suppressor cells |
| Mes GSC | mesenchymal GSC |
| MGMT | O6-methylguanine methyltransferase |
| MNP | magnetic nanoparticle |
| NC | nanocarrier |
| NP | nanoparticle |
| OS | overall survival |
| PEG | polyethylene glycol |
| PEGPE | PEG-phosphatidylethanolamine |
| PEG-Dox | Pegylated liposomal doxorubicin |
| PN GSC | proneural GSC |
| SiNP | silica nanoparticles |
| siRNA | short interfering RNA |
| Smac | second mitochondrial-derived activator of caspases |
| TMZ | temozolomide |
| T2DM | type 2 diabetes mellitus |
| VEGF | vascular endothelial growth factor |
| XIAP | X-linked IAP |
References
- Kawamura, Y.; Takouda, J.; Yoshimoto, K.; Nakashima, K. New aspects of glioblastoma multiforme revealed by similarities between neural and glioblastoma stem cells. Cell Biol. Toxicol. 2018, 34, 425–440. [Google Scholar] [CrossRef] [PubMed]
- Disney-Hogg, L.; Sud, A.; Law, P.J.; Cornish, A.J.; Kinnersley, B.; Ostrom, Q.T.; Labreche, K.; Eckel-Passow, J.E.; Armstrong, G.N.; Claus, E.B. Influence of obesity-related risk factors in the aetiology of glioma. Br. J. Cancer 2018, 118, 1020–1027. [Google Scholar] [CrossRef]
- Behl, T.; Sharma, A.; Sharma, L.; Sehgal, A.; Singh, S.; Sharma, N.; Zengin, G.; Bungau, S.; Toma, M.M.; Gitea, D. Current perspective on the natural compounds and drug delivery techniques in glioblastoma multiforme. Cancers 2021, 13, 2765. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Zhai, X.; Liang, P.; Cui, H. Overcoming TRAIL resistance for glioblastoma treatment. Biomolecules 2021, 11, 572. [Google Scholar] [CrossRef] [PubMed]
- Frazzi, R. BIRC3 and BIRC5: Multi-faceted inhibitors in cancer. Cell Biosci. 2021, 11, 1–14. [Google Scholar] [CrossRef]
- Opo, F.A.D.M.; Rahman, M.M.; Ahammad, F.; Ahmed, I.; Bhuiyan, M.A.; Asiri, A.M. Structure based pharmacophore modeling, virtual screening, molecular docking and ADMET approaches for identification of natural anti-cancer agents targeting XIAP protein. Sci. Rep. 2021, 11, 4049. [Google Scholar] [CrossRef]
- Cetraro, P.; Plaza-Diaz, J.; MacKenzie, A.; Abadía-Molina, F. A review of the current impact of inhibitors of apoptosis proteins and their repression in cancer. Cancers 2022, 14, 1671. [Google Scholar] [CrossRef] [PubMed]
- Fukumura, D.; Incio, J.; Shankaraiah, R.C.; Jain, R.K. Obesity and cancer: An angiogenic and inflammatory link. Microcirculation 2016, 23, 191–206. [Google Scholar] [CrossRef]
- Edlinger, M.; Strohmaier, S.; Jonsson, H.; Bjørge, T.; Manjer, J.; Borena, W.T.; Häggström, C.; Engeland, A.; Tretli, S.; Concin, H. Blood pressure and other metabolic syndrome factors and risk of brain tumour in the large population-based Me-Can cohort study. J. Hypertens. 2012, 30, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E.; Harlan, D.M.; Archer, M.C.; Bergenstal, R.M.; Gapstur, S.M.; Habel, L.A.; Pollak, M.; Regensteiner, J.G.; Yee, D. Diabetes and cancer: A consensus report. CA A Cancer J. Clin. 2010, 60, 207–221. [Google Scholar] [CrossRef]
- Kyrgiou, M.; Kalliala, I.; Markozannes, G.; Gunter, M.J.; Paraskevaidis, E.; Gabra, H.; Martin-Hirsch, P.; Tsilidis, K.K. Adiposity and cancer at major anatomical sites: Umbrella review of the literature. Bmj 2017, 356, j477. [Google Scholar] [CrossRef] [PubMed]
- Niedermaier, T.; Behrens, G.; Schmid, D.; Schlecht, I.; Fischer, B.; Leitzmann, M.F. Body mass index, physical activity, and risk of adult meningioma and glioma: A meta-analysis. Neurology 2015, 85, 1342–1350. [Google Scholar] [CrossRef] [PubMed]
- Sergentanis, T.N.; Tsivgoulis, G.; Perlepe, C.; Ntanasis-Stathopoulos, I.; Tzanninis, I.-G.; Sergentanis, I.N.; Psaltopoulou, T. Obesity and risk for brain/CNS tumors, gliomas and meningiomas: A meta-analysis. PLoS ONE 2015, 10, e0136974. [Google Scholar] [CrossRef] [PubMed]
- Wiedmann, M.; Brunborg, C.; Lindemann, K.; Johannesen, T.; Vatten, L.; Helseth, E.; Zwart, J. Body mass index and the risk of meningioma, glioma and schwannoma in a large prospective cohort study (The HUNT Study). Br. J. Cancer 2013, 109, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Danaei, G.; Finucane, M.M.; Lu, Y.; Singh, G.M.; Cowan, M.J.; Paciorek, C.J.; Lin, J.K.; Farzadfar, F.; Khang, Y.-H.; Stevens, G.A. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: Systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2· 7 million participants. Lancet 2011, 378, 31–40. [Google Scholar] [CrossRef]
- Seliger, C.; Ricci, C.; Meier, C.R.; Bodmer, M.; Jick, S.S.; Bogdahn, U.; Hau, P.; Leitzmann, M.F. Diabetes, use of antidiabetic drugs, and the risk of glioma. Neuro-Oncology 2015, 18, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Kaul, K.; Tarr, J.M.; Ahmad, S.I.; Kohner, E.M.; Chibber, R. Introduction to diabetes mellitus. In Diabetes: An Old Disease, a New Insight; Springer: New York, NY, USA, 2013; pp. 1–11. [Google Scholar] [CrossRef]
- Heidemann, C.; Boeing, H.; Pischon, T.; Nöthlings, U.; Joost, H.-G.; Schulze, M.B. Association of a diabetes risk score with risk of myocardial infarction, stroke, specific types of cancer, and mortality: A prospective study in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam cohort. Eur. J. Epidemiol. 2009, 24, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Saltzman, B.S.; Doherty, J.A.; Hill, D.A.; Beresford, S.A.; Voigt, L.F.; Chen, C.; Weiss, N.S. Diabetes and endometrial cancer: An evaluation of the modifying effects of other known risk factors. Am. J. Epidemiol. 2008, 167, 607–614. [Google Scholar] [CrossRef]
- Ben, Q.; Cai, Q.; Li, Z.; Yuan, Y.; Ning, X.; Deng, S.; Wang, K. The relationship between new-onset diabetes mellitus and pancreatic cancer risk: A case–control study. Eur. J. Cancer 2011, 47, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-S.; Yao, W.-J.; Chang, T.-T.; Wang, S.-T.; Chou, P. The impact of type 2 diabetes on the development of hepatocellular carcinoma in different viral hepatitis statuses. Cancer Epidemiol. Biomark. Prev. 2009, 18, 2054–2060. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.T.; Newton, C.C.; Patel, A.V.; Jacobs, E.J.; Gapstur, S.M. Diabetes and cause-specific mortality in a prospective cohort of one million US adults. Diabetes Care 2012, 35, 1835–1844. [Google Scholar] [CrossRef] [PubMed]
- Rapone, B.; Corsalini, M.; Converti, I.; Loverro, M.T.; Gnoni, A.; Trerotoli, P.; Ferrara, E. Does periodontal inflammation affect type 1 diabetes in childhood and adolescence? A meta-analysis. Front. Endocrinol. 2020, 11, 278. [Google Scholar] [CrossRef]
- Van Den Berghe, G.; Wouters, P.; Weekers, F.; Verwaest, C.; Bruyninckx, F.; Schetz, M.; Vlasselaers, D.; Ferdinande, P.; Lauwers, P.; Bouillon, R. Intensive insulin therapy in critically ill patients. N. Engl. J. Med. 2001, 345, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.J.; Tao, H.; Hui, O.T.; Jian, C. Diabetes mellitus and risk of brain tumors: A meta-analysis. Exp. Ther. Med. 2012, 4, 877–882. [Google Scholar] [CrossRef]
- Polednak, A.P. Comorbid diabetes mellitus and risk of death after diagnosis of colorectal cancer: A population-based study. Cancer Detect. Prev. 2006, 30, 466–472. [Google Scholar] [CrossRef]
- Yancik, R.; Wesley, M.N.; Ries, L.A.; Havlik, R.J.; Edwards, B.K.; Yates, J.W. Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA 2001, 285, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Schwartzbaum, J.; Edlinger, M.; Zigmont, V.; Stattin, P.; Rempala, G.A.; Nagel, G.; Hammar, N.; Ulmer, H.; Föger, B.; Walldius, G. Associations between prediagnostic blood glucose levels, diabetes, and glioma. Sci. Rep. 2017, 7, 1436. [Google Scholar] [CrossRef]
- Van Hemelrijck, M.; Garmo, H.; Holmberg, L.; Walldius, G.; Jungner, I.; Hammar, N.; Lambe, M. Prostate cancer risk in the Swedish AMORIS study: The interplay among triglycerides, total cholesterol, and glucose. Cancer 2011, 117, 2086–2095. [Google Scholar] [CrossRef]
- Gritti, M.; Würth, R.; Angelini, M.; Barbieri, F.; Peretti, M.; Pizzi, E.; Pattarozzi, A.; Carra, E.; Sirito, R.; Daga, A. Metformin repositioning as antitumoral agent: Selective antiproliferative effects in human glioblastoma stem cells, via inhibition of CLIC1-mediated ion current. Oncotarget 2014, 5, 11252. [Google Scholar] [CrossRef]
- Sato, A.; Sunayama, J.; Okada, M.; Watanabe, E.; Seino, S.; Shibuya, K.; Suzuki, K.; Narita, Y.; Shibui, S.; Kayama, T. Glioma-initiating cell elimination by metformin activation of FOXO3 via AMPK. Stem Cells Transl. Med. 2012, 1, 811–824. [Google Scholar] [CrossRef] [PubMed]
- Würth, R.; Pattarozzi, A.; Gatti, M.; Bajetto, A.; Corsaro, A.; Parodi, A.; Sirito, R.; Massollo, M.; Marini, C.; Zona, G. Metformin selectively affects human glioblastoma tumor-initiating cell viability: A role for metformin-induced inhibition of Akt. Cell Cycle 2013, 12, 145–156. [Google Scholar] [CrossRef]
- Yu, Z.; Zhao, G.; Xie, G.; Zhao, L.; Chen, Y.; Yu, H.; Zhang, Z.; Li, C.; Li, Y. Metformin and temozolomide act synergistically to inhibit growth of glioma cells and glioma stem cells in vitro and in vivo. Oncotarget 2015, 6, 32930. [Google Scholar] [CrossRef]
- Müller, D.M.; Robe, P.A.; Eijgelaar, R.S.; Witte, M.G.; Visser, M.; de Munck, J.C.; Broekman, M.L.; Seute, T.; Hendrikse, J.; Noske, D.P. Comparing glioblastoma surgery decisions between teams using brain maps of tumor locations, biopsies, and resections. JCO Clin. Cancer Inform. 2019, 2, 1–12. [Google Scholar] [CrossRef]
- Bruhn, H.; Strandéus, M.; Milos, P.; Hallbeck, M.; Vrethem, M.; Lind, J. Improved survival of Swedish glioblastoma patients treated according to Stupp. Acta Neurol. Scand. 2018, 138, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Montemurro, N.; Perrini, P.; Blanco, M.O.; Vannozzi, R. Second surgery for recurrent glioblastoma: A concise overview of the current literature. Clin. Neurol. Neurosurg. 2016, 142, 60–64. [Google Scholar] [CrossRef]
- Schwartzbaum, J.; Jonsson, F.; Ahlbom, A.; Preston-Martin, S.; Malmer, B.; Lönn, S.; Söderberg, K.; Feychting, M. Prior hospitalization for epilepsy, diabetes, and stroke and subsequent glioma and meningioma risk. Cancer Epidemiol. Biomark. Prev. 2005, 14, 643–650. [Google Scholar] [CrossRef]
- Mauer, J.; Chaurasia, B.; Goldau, J.; Vogt, M.C.; Ruud, J.; Nguyen, K.D.; Theurich, S.; Hausen, A.C.; Schmitz, J.; Brönneke, H.S. Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nat. Immunol. 2014, 15, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.; Dziura, J.; Burgert, T.S.; Tamborlane, W.V.; Taksali, S.E.; Yeckel, C.W.; Allen, K.; Lopes, M.; Savoye, M.; Morrison, J. Obesity and the metabolic syndrome in children and adolescents. N. Engl. J. Med. 2004, 350, 2362–2374. [Google Scholar] [CrossRef]
- Chen, W.; Xia, T.; Wang, D.; Huang, B.; Zhao, P.; Wang, J.; Qu, X.; Li, X. Human astrocytes secrete IL-6 to promote glioma migration and invasion through upregulation of cytomembrane MMP14. Oncotarget 2016, 7, 62425. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, G.; Deng, L.; Liu, Q.; Dai, J.; Shen, J.; Zhang, J. IL-6 augments the invasiveness of U87MG human glioblastoma multiforme cells via up-regulation of MMP-2 and fascin-1. Oncol. Rep. 2010, 23, 1553–1559. [Google Scholar] [CrossRef]
- Liu, Q.; Li, G.; Li, R.; Shen, J.; He, Q.; Deng, L.; Zhang, C.; Zhang, J. IL-6 promotion of glioblastoma cell invasion and angiogenesis in U251 and T98G cell lines. J. Neuro-Oncol. 2010, 100, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Tchirkov, A.; Khalil, T.; Chautard, E.; Mokhtari, K.; Veronese, L.; Irthum, B.; Vago, P.; Kémény, J.; Verrelle, P. Interleukin-6 gene amplification and shortened survival in glioblastoma patients. Br. J. Cancer 2007, 96, 474–476. [Google Scholar] [CrossRef]
- Solinas, G.; Becattini, B. JNK at the crossroad of obesity, insulin resistance, and cell stress response. Mol. Metab. 2017, 6, 174–184. [Google Scholar] [CrossRef]
- Yeung, Y.; McDonald, K.; Grewal, T.; Munoz, L. Interleukins in glioblastoma pathophysiology: Implications for therapy. Br. J. Pharmacol. 2013, 168, 591–606. [Google Scholar] [CrossRef]
- Zeke, A.; Misheva, M.; Reményi, A.; Bogoyevitch, M.A. JNK signaling: Regulation and functions based on complex protein-protein partnerships. Microbiol. Mol. Biol. Rev. 2016, 80, 793–835. [Google Scholar] [CrossRef]
- Bielecka-Wajdman, A.M.; Ludyga, T.; Smyk, D.; Smyk, W.; Mularska, M.; Świderek, P.; Majewski, W.; Mullins, C.S.; Linnebacher, M.; Obuchowicz, E. Glucose influences the response of Glioblastoma cells to temozolomide and dexamethasone. Cancer Control 2022, 29, 10732748221075468. [Google Scholar] [CrossRef]
- Association, A.D. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010, 33, S62–S69. [Google Scholar] [CrossRef]
- Ding, C.-Z.; Guo, X.-F.; Wang, G.-L.; Wang, H.-T.; Xu, G.-H.; Liu, Y.-Y.; Wu, Z.-J.; Chen, Y.-H.; Wang, J.; Wang, W.-G. High glucose contributes to the proliferation and migration of non-small-cell lung cancer cells via GAS5-TRIB3 axis. Biosci. Rep. 2018, 38, BSR20171014. [Google Scholar] [CrossRef]
- Klil-Drori, A.J.; Azoulay, L.; Pollak, M.N. Cancer, obesity, diabetes, and antidiabetic drugs: Is the fog clearing? Nat. Rev. Clin. Oncol. 2017, 14, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Strickland, M.; Stoll, E.A. Metabolic reprogramming in glioma. Front. Cell Dev. Biol. 2017, 5, 43. [Google Scholar] [CrossRef]
- Oppermann, H.; Ding, Y.; Sharma, J.; Berndt Paetz, M.; Meixensberger, J.; Gaunitz, F.; Birkemeyer, C. Metabolic response of glioblastoma cells associated with glucose withdrawal and pyruvate substitution as revealed by GC-MS. Nutr. Metab. 2016, 13, 1–11. [Google Scholar] [CrossRef]
- Warburg, O. On respiratory impairment in cancer cells. Science 1956, 124, 269–270. [Google Scholar] [CrossRef]
- Xing, F.; Luan, Y.; Cai, J.; Wu, S.; Mai, J.; Gu, J.; Zhang, H.; Li, K.; Lin, Y.; Xiao, X. The anti-Warburg effect elicited by the cAMP-PGC1α pathway drives differentiation of glioblastoma cells into astrocytes. Cell Rep. 2017, 18, 468–481. [Google Scholar] [CrossRef]
- Singh, S.K.; Hawkins, C.; Clarke, I.D.; Squire, J.A.; Bayani, J.; Hide, T.; Henkelman, R.M.; Cusimano, M.D.; Dirks, P.B. Identification of human brain tumour initiating cells. Nature 2004, 432, 396–401. [Google Scholar] [CrossRef]
- Uribe, D.; Torres, Á.; Rocha, J.D.; Niechi, I.; Oyarzún, C.; Sobrevia, L.; San Martín, R.; Quezada, C. Multidrug resistance in glioblastoma stem-like cells: Role of the hypoxic microenvironment and adenosine signaling. Mol. Asp. Med. 2017, 55, 140–151. [Google Scholar] [CrossRef]
- Davidson, J.A.; Sloan, L. Fixed-dose combination of canagliflozin and metformin for the treatment of type 2 diabetes: An overview. Adv. Ther. 2017, 34, 41–59. [Google Scholar] [CrossRef]
- Torres, A.; Vargas, Y.; Uribe, D.; Jaramillo, C.; Gleisner, A.; Salazar-Onfray, F.; López, M.N.; Melo, R.; Oyarzún, C.; San Martín, R. Adenosine A3 receptor elicits chemoresistance mediated by multiple resistance-associated protein-1 in human glioblastoma stem-like cells. Oncotarget 2016, 7, 67373. [Google Scholar] [CrossRef] [PubMed]
- Oyarzún, C.; Garrido, W.; Alarcón, S.; Yáñez, A.; Sobrevia, L.; Quezada, C.; San Martín, R. Adenosine contribution to normal renal physiology and chronic kidney disease. Mol. Asp. Med. 2017, 55, 75–89. [Google Scholar] [CrossRef]
- Yang, L.; Lin, C.; Wang, L.; Guo, H.; Wang, X. Hypoxia and hypoxia-inducible factors in glioblastoma multiforme progression and therapeutic implications. Exp. Cell Res. 2012, 318, 2417–2426. [Google Scholar] [CrossRef] [PubMed]
- Perrini, P.; Gambacciani, C.; Weiss, A.; Pasqualetti, F.; Delishaj, D.; Paiar, F.; Morganti, R.; Vannozzi, R.; Lutzemberger, L. Survival outcomes following repeat surgery for recurrent glioblastoma: A single-center retrospective analysis. J. Neuro-Oncol. 2017, 131, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Montemurro, N. Glioblastoma multiforme and genetic mutations: The issue is not over yet. An overview of the current literature. J. Neurol. Surg. Part A Cent. Eur. Neurosurg. 2020, 81, 064–070. [Google Scholar] [CrossRef]
- Bobola, M.S.; Alnoor, M.; Chen, J.Y.-S.; Kolstoe, D.D.; Silbergeld, D.L.; Rostomily, R.C.; Blank, A.; Chamberlain, M.C.; Silber, J.R. O6-methylguanine-DNA methyltransferase activity is associated with response to alkylating agent therapy and with MGMT promoter methylation in glioblastoma and anaplastic glioma. BBA Clin. 2015, 3, 1–10. [Google Scholar] [CrossRef]
- Carr, M.T.; Hochheimer, C.J.; Rock, A.K.; Dincer, A.; Ravindra, L.; Zhang, F.L.; Opalak, C.F.; Poulos, N.; Sima, A.P.; Broaddus, W.C. Comorbid medical conditions as predictors of overall survival in glioblastoma patients. Sci. Rep. 2019, 9, 20018. [Google Scholar] [CrossRef]
- Evans, J.M.; Donnelly, L.A.; Emslie-Smith, A.M.; Alessi, D.R.; Morris, A.D. Metformin and reduced risk of cancer in diabetic patients. Bmj 2005, 330, 1304–1305. [Google Scholar] [CrossRef]
- Donihi, A.C.; Raval, D.; Saul, M.; Korytkowski, M.T.; DeVita, M.A. Prevalence and predictors of corticosteroid-related hyperglycemia in hospitalized patients. Endocr. Pract. 2006, 12, 358–362. [Google Scholar] [CrossRef]
- Derr, R.L.; Ye, X.; Islas, M.U.; Desideri, S.; Saudek, C.D.; Grossman, S.A. Association between hyperglycemia and survival in patients with newly diagnosed glioblastoma. J. Clin. Oncol. 2009, 27, 1082–1086. [Google Scholar] [CrossRef]
- Al-Goblan, A.S.; Al-Alfi, M.A.; Khan, M.Z. Mechanism linking diabetes mellitus and obesity. Diabetes Metab. Syndr. Obes. Targets Ther. 2014, 7, 587–591. [Google Scholar] [CrossRef]
- Kleinert, M.; Clemmensen, C.; Hofmann, S.M.; Moore, M.C.; Renner, S.; Woods, S.C.; Huypens, P.; Beckers, J.; De Angelis, M.H.; Schürmann, A. Animal models of obesity and diabetes mellitus. Nat. Rev. Endocrinol. 2018, 14, 140–162. [Google Scholar] [CrossRef]
- Antonioli, L.; Blandizzi, C.; Csóka, B.; Pacher, P.; Haskó, G. Adenosine signalling in diabetes mellitus—Pathophysiology and therapeutic considerations. Nat. Rev. Endocrinol. 2015, 11, 228–241. [Google Scholar] [CrossRef]
- Silva, L.; Subiabre, M.; Araos, J.; Sáez, T.; Salsoso, R.; Pardo, F.; Leiva, A.; San Martín, R.; Toledo, F.; Sobrevia, L. Insulin/adenosine axis linked signalling. Mol. Asp. Med. 2017, 55, 45–61. [Google Scholar] [CrossRef]
- Pardo, F.; Villalobos-Labra, R.; Chiarello, D.I.; Salsoso, R.; Toledo, F.; Gutierrez, J.; Leiva, A.; Sobrevia, L. Molecular implications of adenosine in obesity. Mol. Asp. Med. 2017, 55, 90–101. [Google Scholar] [CrossRef]
- Saez, T.; De Vos, P.; Sobrevia, L.; Faas, M.M. Is there a role for exosomes in foetoplacental endothelial dysfunction in gestational diabetes mellitus? Placenta 2018, 61, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Salsoso, R.; Farias, M.; Gutierrez, J.; Pardo, F.; Chiarello, D.I.; Toledo, F.; Leiva, A.; Mate, A.; Vazquez, C.M.; Sobrevia, L. Adenosine and preeclampsia. Mol. Asp. Med. 2017, 55, 126–139. [Google Scholar] [CrossRef]
- Sobrevia, L.; Fredholm, B.B. Adenosine-from molecular mechanisms to pathophysiology. Mol. Asp. Med. 2017, 55, 1–3. [Google Scholar] [CrossRef]
- Sebastiao, A.M.; Ribeiro, J.A. Neuromodulation and metamodulation by adenosine: Impact and subtleties upon synaptic plasticity regulation. Brain Res. 2015, 1621, 102–113. [Google Scholar] [CrossRef]
- Sperlágh, B.; Sylvester Vizi, E. The role of extracellular adenosine in chemical neurotransmission in the hippocampus and Basal Ganglia: Pharmacological and clinical aspects. Curr. Top. Med. Chem. 2011, 11, 1034–1046. [Google Scholar] [CrossRef] [PubMed]
- Dorotea, D.; Cho, A.; Lee, G.; Kwon, G.; Lee, J.; Sahu, P.K.; Jeong, L.S.; Cha, D.R.; Ha, H. Orally active, species-independent novel A3 adenosine receptor antagonist protects against kidney injury in db/db mice. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Fredholm, B.B.; IJzerman, A.P.; Jacobson, K.A.; Linden, J.; Müller, C.E. International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors—An update. Pharmacol. Rev. 2011, 63, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda, C.; Palomo, I.; Fuentes, E. Role of adenosine A2b receptor overexpression in tumor progression. Life Sci. 2016, 166, 92–99. [Google Scholar] [CrossRef]
- Mandapathil, M.; Szczepanski, M.J.; Szajnik, M.; Ren, J.; Lenzner, D.E.; Jackson, E.K.; Gorelik, E.; Lang, S.; Johnson, J.T.; Whiteside, T.L. Increased ectonucleotidase expression and activity in regulatory T cells of patients with head and neck cancer. Clin. Cancer Res. 2009, 15, 6348–6357. [Google Scholar] [CrossRef]
- Sitkovsky, M.V.; Kjaergaard, J.; Lukashev, D.; Ohta, A. Hypoxia-adenosinergic immunosuppression: Tumor protection by T regulatory cells and cancerous tissue hypoxia. Clin. Cancer Res. 2008, 14, 5947–5952. [Google Scholar] [CrossRef]
- Stagg, J.; Smyth, M. Extracellular adenosine triphosphate and adenosine in cancer. Oncogene 2010, 29, 5346–5358. [Google Scholar] [CrossRef]
- Vaupel, P.; Mayer, A. Hypoxia-driven adenosine accumulation: A crucial microenvironmental factor promoting tumor progression. In Proceedings of the Oxygen Transport to Tissue XXXVII; Springer: New York, NY, USA, 2016; pp. 177–183. [Google Scholar]
- Young, A.; Mittal, D.; Stagg, J.; Smyth, M.J. Targeting cancer-derived adenosine: New therapeutic approaches. Cancer Discov. 2014, 4, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Feoktistov, I.; Biaggioni, I.; Cronstein, B.N. Adenosine receptors in wound healing, fibrosis and angiogenesis. In Adenosine Receptors in Health and Disease; Springer: Berlin/Heidelberg, Germany, 2009; pp. 383–397. [Google Scholar] [CrossRef]
- Linden, J. Adenosine metabolism and cancer. Focus on “Adenosine downregulates DPPIV on HT-29 colon cancer cells by stimulating protein tyrosine phosphatases and reducing ERK1/2 activity via a novel pathway”. Am. J. Physiol.-Cell Physiol. 2006, 291, C405–C406. [Google Scholar] [CrossRef] [PubMed]
- Novitskiy, S.V.; Ryzhov, S.; Zaynagetdinov, R.; Goldstein, A.E.; Huang, Y.; Tikhomirov, O.Y.; Blackburn, M.R.; Biaggioni, I.; Carbone, D.P.; Feoktistov, I. Adenosine receptors in regulation of dendritic cell differentiation and function. Blood J. Am. Soc. Hematol. 2008, 112, 1822–1831. [Google Scholar] [CrossRef] [PubMed]
- Antonioli, L.; Pacher, P.; Vizi, E.S.; Haskó, G. CD39 and CD73 in immunity and inflammation. Trends Mol. Med. 2013, 19, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Shao, Q.-Q.; Sun, J.-T.; Yang, N.; Xie, Q.; Wang, D.-H.; Huang, Q.-B.; Huang, B.; Wang, X.-Y.; Li, X.-G. Synergy between the ectoenzymes CD39 and CD73 contributes to adenosinergic immunosuppression in human malignant gliomas. Neuro-oncology 2013, 15, 1160–1172. [Google Scholar] [CrossRef] [PubMed]
- Vaisitti, T.; Arruga, F.; Deaglio, S. Targeting the adenosinergic axis in chronic lymphocytic leukemia: A way to disrupt the tumor niche? Int. J. Mol. Sci. 2018, 19, 1167. [Google Scholar] [CrossRef]
- Ledur, P.F.; Villodre, E.S.; Paulus, R.; Cruz, L.A.; Flores, D.G.; Lenz, G. Extracellular ATP reduces tumor sphere growth and cancer stem cell population in glioblastoma cells. Purinergic Signal. 2012, 8, 39–48. [Google Scholar] [CrossRef]
- Morrone, F.B.; Horn, A.P.; Stella, J.; Spiller, F.; Sarkis, J.J.; Salbego, C.G.; Lenz, G.; Battastini, A.M.O. Increased resistance of glioma cell lines to extracellular ATP cytotoxicity. J. Neuro-Oncol. 2005, 71, 135–140. [Google Scholar] [CrossRef]
- Wink, M.R.; Lenz, G.; Braganhol, E.; Tamajusuku, A.S.; Schwartsmann, G.; Sarkis, J.J.; Battastini, A.M. Altered extracellular ATP, ADP and AMP catabolism in glioma cell lines. Cancer Lett. 2003, 198, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, T.L. Targeting adenosine in cancer immunotherapy: A review of recent progress. Expert Rev. Anticancer. Ther. 2017, 17, 527–535. [Google Scholar] [CrossRef]
- Pastor-Anglada, M.; Pérez-Torras, S. Emerging roles of nucleoside transporters. Front. Pharmacol. 2018, 9, 606. [Google Scholar] [CrossRef]
- Parkinson, F.E.; Damaraju, V.L.; Graham, K.; Yao, S.Y.; Baldwin, S.A.; Cass, C.E.; Young, J.D. Molecular biology of nucleoside transporters and their distributions and functions in the brain. Curr. Top. Med. Chem. 2011, 11, 948–972. [Google Scholar] [CrossRef] [PubMed]
- Pardo, F.; Arroyo, P.; Salomón, C.; Westermeier, F.; Guzmán-Gutiérrez, E. Gestational Diabetes Mellitus and the Role of Adenosine in the Human Placental En-dothelium and Central Nervous System. J. Diabetes Metab. S 2012, 2. [Google Scholar] [CrossRef]
- Pawelczyk, T.; Podgorska, M.; Sakowicz, M. The effect of insulin on expression level of nucleoside transporters in diabetic rats. Mol. Pharmacol. 2003, 63, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Podgorska, M.; Kocbuch, K.; Grden, M.; Szulc, A.; Szutowicz, A.; Pawelczyk, T. Different signaling pathways utilized by insulin to regulate the expression of ENT2, CNT1, CNT2 nucleoside transporters in rat cardiac fibroblasts. Arch. Biochem. Biophys. 2007, 464, 344–349. [Google Scholar] [CrossRef]
- Esser, N.; Legrand-Poels, S.; Piette, J.; Scheen, A.J.; Paquot, N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract. 2014, 105, 141–150. [Google Scholar] [CrossRef]
- Tan, B.K.; Adya, R.; Randeva, H.S. Omentin: A novel link between inflammation, diabesity, and cardiovascular disease. Trends Cardiovasc. Med. 2010, 20, 143–148. [Google Scholar] [CrossRef]
- Allard, D.; Turcotte, M.; Stagg, J. Targeting A2 adenosine receptors in cancer. Immunol. Cell Biol. 2017, 95, 333–339. [Google Scholar] [CrossRef]
- Liu, C.; Mukienko, Y.; Wu, C.; Zavialov, A. Human adenosine deaminases control the immune cell responses to activation signals by reducing extracellular adenosine concentration. J. Immunol. 2016, 196, 124.163. [Google Scholar] [CrossRef]
- Cronstein, B.N. Adenosine, an endogenous anti-inflammatory agent. J. Appl. Physiol. 1994, 76, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.F.; Takedachi, M.; Ebisuno, Y.; Tanaka, T.; Miyasaka, M.; Mills, J.H.; Bynoe, M.S. Regulation of leukocyte migration across endothelial barriers by ECTO-5′-nucleotidase-generated adenosine. Nucleosides Nucleotides Nucleic Acids 2008, 27, 755–760. [Google Scholar] [CrossRef]
- Merighi, S.; Mirandola, P.; Varani, K.; Gessi, S.; Leung, E.; Baraldi, P.G.; Tabrizi, M.A.; Borea, P.A. A glance at adenosine receptors: Novel target for antitumor therapy. Pharmacol. Ther. 2003, 100, 31–48. [Google Scholar] [CrossRef]
- Bova, V.; Filippone, A.; Casili, G.; Lanza, M.; Campolo, M.; Capra, A.P.; Repici, A.; Crupi, L.; Motta, G.; Colarossi, C. Adenosine targeting as a new strategy to decrease glioblastoma aggressiveness. Cancers 2022, 14, 4032. [Google Scholar] [CrossRef]
- Barami, K.; Lyon, L.; Conell, C. Type 2 diabetes mellitus and glioblastoma multiforme–assessing risk and survival: Results of a large retrospective study and systematic review of the literature. World Neurosurg. 2017, 106, 300–307. [Google Scholar] [CrossRef]
- Rapone, B.; Ferrara, E.; Corsalini, M.; Converti, I.; Grassi, F.R.; Santacroce, L.; Topi, S.; Gnoni, A.; Scacco, S.; Scarano, A. The effect of gaseous ozone therapy in conjunction with periodontal treatment on glycated hemoglobin level in subjects with type 2 diabetes mellitus: An unmasked randomized controlled trial. Int. J. Environ. Res. Public Health 2020, 17, 5467. [Google Scholar] [CrossRef]
- Dankner, R.; Boffetta, P.; Balicer, R.D.; Boker, L.K.; Sadeh, M.; Berlin, A.; Olmer, L.; Goldfracht, M.; Freedman, L.S. Time-dependent risk of cancer after a diabetes diagnosis in a cohort of 2.3 million adults. Am. J. Epidemiol. 2016, 183, 1098–1106. [Google Scholar] [CrossRef]
- Zhao, L.; Zheng, Z.; Huang, P. Diabetes mellitus and the risk of glioma: A meta-analysis. Oncotarget 2016, 7, 4483. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, T.; Zhang, H. Causal relationship between type 2 diabetes and glioblastoma: Bidirectional Mendelian randomization analysis. Sci. Rep. 2024, 14, 16544. [Google Scholar] [CrossRef]
- Fogel, D.B. Factors associated with clinical trials that fail and opportunities for improving the likelihood of success: A review. Contemp. Clin. Trials Commun. 2018, 11, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Montemurro, N.; Murrone, D.; Romanelli, B.; Ierardi, A. Postoperative textiloma mimicking intracranial rebleeding in a patient with spontaneous hemorrhage: Case report and review of the literature. Case Rep. Neurol. 2020, 12, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Akiboye, F.; Rayman, G. Management of hyperglycemia and diabetes in orthopedic surgery. Curr. Diabetes Rep. 2017, 17, 13. [Google Scholar] [CrossRef]
- Montemurro, N.; Perrini, P.; Mangini, V.; Galli, M.; Papini, A. The Y-shaped trabecular bone structure in the odontoid process of the axis: A CT scan study in 54 healthy subjects and biomechanical considerations. J. Neurosurg. Spine 2019, 30, 585–592. [Google Scholar] [CrossRef]
- Corsalini, M.; Di Venere, D.; Sportelli, P.; Magazzino, D.; Ripa, M.; Cantatore, F.; Cagnetta, C.; De Rinaldis, C.; Montemurro, N.; De Giacomo, A. Evaluation of prosthetic quality and masticatory efficiency in patients with total removable prosthesis: Study of 12 cases. Oral Implantol. 2018, 11, 230–240. [Google Scholar]
- Perrini, P.; Gambacciani, C.; Martini, C.; Montemurro, N.; Lepori, P. Anterior cervical corpectomy for cervical spondylotic myelopathy: Reconstruction with expandable cylindrical cage versus iliac crest autograft. A retrospective study. Clin. Neurol. Neurosurg. 2015, 139, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, M.; Kahari, J.; Vestman, A.; Hallmans, M.; Johansson, M.; Bergenheim, A.T.; Sandström, M. Improved treatment of glioblastoma–changes in survival over two decades at a single regional Centre. Acta Oncol. 2019, 58, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Adeberg, S.; Bernhardt, D.; Harrabi, S.B.; Bostel, T.; Mohr, A.; Koelsche, C.; Diehl, C.; Rieken, S.; Debus, J. Metforminbeeinflusst die Progression bei diabetischen Glioblastompatienten. Strahlenther. Und. Onkol. 2015, 191, 928–935. [Google Scholar] [CrossRef]
- Yang, T.O.; Cairns, B.J.; Kroll, M.E.; Reeves, G.K.; Green, J.; Beral, V.; Collaborators, M.W.S. Body size in early life and risk of lymphoid malignancies and histological subtypes in adulthood. Int. J. Cancer 2016, 139, 42–49. [Google Scholar] [CrossRef]
- Chambless, L.B.; Parker, S.L.; Hassam-Malani, L.; McGirt, M.J.; Thompson, R.C. Type 2 diabetes mellitus and obesity are independent risk factors for poor outcome in patients with high-grade glioma. J. Neuro-Oncol. 2012, 106, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Welch, M.R.; Grommes, C. Retrospective analysis of the effects of steroid therapy and antidiabetic medication on survival in diabetic glioblastoma patients. CNS Oncol. 2013, 2, 237–246. [Google Scholar] [CrossRef]
- Siegel, E.M.; Nabors, L.B.; Thompson, R.C.; Olson, J.J.; Browning, J.E.; Madden, M.H.; Han, G.; Egan, K.M. Prediagnostic body weight and survival in high grade glioma. J. Neuro-Oncol. 2013, 114, 79–84. [Google Scholar] [CrossRef]
- Petrelli, F.; Cortellini, A.; Indini, A.; Tomasello, G.; Ghidini, M.; Nigro, O.; Salati, M.; Dottorini, L.; Iaculli, A.; Varricchio, A. Obesity paradox in patients with cancer: A systematic review and meta-analysis of 6,320,365 patients. MedRxiv 2020. [Google Scholar] [CrossRef]
- Vucenik, I.; Jones, L.P.; McLenithan, J.C. Linking obesity, metabolism, and cancer. In Metabolic Syndrome: A Comprehensive Textbook; Springer: Berlin/Heidelberg, Germany, 2024; pp. 603–620. [Google Scholar]
- Behrooz, A.B.; Cordani, M.; Fiore, A.; Donadelli, M.; Gordon, J.W.; Klionsky, D.J.; Ghavami, S. The obesity-autophagy-cancer axis: Mechanistic insights and therapeutic perspectives. Semin. Cancer Biol. 2024, 99, 24–44. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Fu, X.-L.; Wang, J.-J.; Guan, R.; Tang, X.-J. Novel strategies to discover effective drug targets in metabolic and immune therapy for glioblastoma. Curr. Cancer Drug Targets 2017, 17, 17–39. [Google Scholar] [CrossRef]
- Lin, H.; Liu, C.; Hu, A.; Zhang, D.; Yang, H.; Mao, Y. Understanding the immunosuppressive microenvironment of glioma: Mechanistic insights and clinical perspectives. J. Hematol. Oncol. 2024, 17, 31. [Google Scholar]
- Mayer, A.; Vaupel, P.; Struss, H.-G.; Giese, A.; Stockinger, M.; Schmidberger, H. Strong adverse prognostic impact of hyperglycemic episodes during adjuvant chemoradiotherapy of glioblastoma multiforme. Strahlenther Onkol. 2014, 190, 933–938. [Google Scholar] [CrossRef]
- McGirt, M.J.; Chaichana, K.L.; Gathinji, M.; Attenello, F.; Than, K.; Ruiz, A.J.; Olivi, A.; Quiñones-Hinojosa, A. Persistent outpatient hyperglycemia is independently associated with decreased survival after primary resection of malignant brain astrocytomas. Neurosurgery 2008, 63, 286–291. [Google Scholar] [CrossRef]
- Stevens, G.; Ahluwalia, M. Elevated preoperative glucose levels and survival in elderly newly diagnosed glioblastoma patients. Neurology 2012, 78, P07.111. [Google Scholar] [CrossRef]
- Tieu, M.T.; Lovblom, L.E.; McNamara, M.G.; Mason, W.; Laperriere, N.; Millar, B.-A.; Ménard, C.; Kiehl, T.-R.; Perkins, B.A.; Chung, C. Impact of glycemia on survival of glioblastoma patients treated with radiation and temozolomide. J. Neuro-Oncol. 2015, 124, 119–126. [Google Scholar] [CrossRef]
- Hagan, K.; Bhavsar, S.; Arunkumar, R.; Grasu, R.; Dang, A.; Carlson, R.; Cowles, C.; Arnold, B.; Potylchansky, Y.; Rahlfs, T.F. Association between perioperative hyperglycemia and survival in patients with glioblastoma. J. Neurosurg. Anesthesiol. 2017, 29, 21–29. [Google Scholar] [CrossRef]
- Decker, M.; Sacks, P.; Abbatematteo, J.; De Leo, E.; Brennan, M.; Rahman, M. The effects of hyperglycemia on outcomes in surgical high-grade glioma patients. Clin. Neurol. Neurosurg. 2019, 179, 9–13. [Google Scholar] [CrossRef]
- Bao, Z.; Chen, K.; Krepel, S.; Tang, P.; Gong, W.; Zhang, M.; Liang, W.; Trivett, A.; Zhou, M.; Wang, J.M. High glucose promotes human glioblastoma cell growth by increasing the expression and function of chemoattractant and growth factor receptors. Transl. Oncol. 2019, 12, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos-Dos-Santos, A.; Loponte, H.; Mantuano, N.; Oliveira, I.; De Paula, I.; Teixeira, L.; De-Freitas-Junior, J.; Gondim, K.; Heise, N.; Mohana-Borges, R. Hyperglycemia exacerbates colon cancer malignancy through hexosamine biosynthetic pathway. Oncogenesis 2017, 6, e306. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Bao, Z.; Wang, X.; Gong, W.; Chen, H.; Guan, H.; Le, Y.; Su, S.; Chen, K.; Wang, J.M. The G-protein-coupled chemoattractant receptor Fpr2 exacerbates high glucose-mediated proinflammatory responses of müller glial cells. Front. Immunol. 2017, 8, 1852. [Google Scholar] [CrossRef]
- Grommes, C.; Conway, D.S.; Alshekhlee, A.; Barnholtz-Sloan, J.S. Inverse association of PPARγ agonists use and high grade glioma development. J. Neuro-Oncol. 2010, 100, 233–239. [Google Scholar] [CrossRef]
- Puzio-Kuter, A.M. The role of p53 in metabolic regulation. Genes Cancer 2011, 2, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Sudderth, J.; Dang, T.; Bachoo, R.G.; McDonald, J.G.; DeBerardinis, R.J. Glioblastoma cells require glutamate dehydrogenase to survive impairments of glucose metabolism or Akt signaling. Cancer Res. 2009, 69, 7986–7993. [Google Scholar] [CrossRef]
- Zhou, Y.; Bian, X.; Le, Y.; Gong, W.; Hu, J.; Zhang, X.; Wang, L.; Iribarren, P.; Salcedo, R.; Howard, O.Z. Formylpeptide receptor FPR and the rapid growth of malignant human gliomas. J. Natl. Cancer Inst. 2005, 97, 823–835. [Google Scholar] [CrossRef]
- Woolf, E.C.; Scheck, A.C. The ketogenic diet for the treatment of malignant glioma. J. Lipid Res. 2015, 56, 5–10. [Google Scholar] [CrossRef]
- Nathan, D.M. Finding new treatments for diabetes—How many, how fast... how good? N. Engl. J. Med. 2007, 356, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Zander, T.; Kraus, J.A.; Grommes, C.; Schlegel, U.; Feinstein, D.; Klockgether, T.; Landreth, G.; Koenigsknecht, J.; Heneka, M.T. Induction of apoptosis in human and rat glioma by agonists of the nuclear receptor PPARγ. J. Neurochem. 2002, 81, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Esteva, F.; Ensor, J.; Hortobagyi, G.; Lee, M.-H.; Yeung, S.-C. Metformin and thiazolidinediones are associated with improved breast cancer-specific survival of diabetic women with HER2+ breast cancer. Ann. Oncol. 2012, 23, 1771–1780. [Google Scholar] [CrossRef]
- Bowker, S.L.; Majumdar, S.R.; Veugelers, P.; Johnson, J.A. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care 2006, 29, 254–258. [Google Scholar] [CrossRef]
- Soritau, O.; Tomuleasa, C.; Aldea, M.; Petrushev, B.; Susman, S.; Gheban, D.; Ioani, H.; Cosis, A.; Brie, I.; Irimie, A. Metformin plus temozolomide-based chemotherapy as adjuvant treatment for WHO grade III and IV malignant gliomas. J. Buon 2011, 16, 282–289. [Google Scholar] [PubMed]
- Sesen, J.; Dahan, P.; Scotland, S.J.; Saland, E.; Dang, V.-T.; Lemarié, A.; Tyler, B.M.; Brem, H.; Toulas, C.; Cohen-Jonathan Moyal, E. Metformin inhibits growth of human glioblastoma cells and enhances therapeutic response. PLoS ONE 2015, 10, e0123721. [Google Scholar] [CrossRef]
- Xiao, Z.X.; Chen, R.Q.; Hu, D.X.; Xie, X.Q.; Yu, S.B.; Chen, X.Q. Identification of repaglinide as a therapeutic drug for glioblastoma multiforme. Biochem. Biophys. Res. Commun. 2017, 488, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Perrini, P.; Montemurro, N. Congenital absence of a cervical spine pedicle. Neurol. India 2016, 64, 189–190. [Google Scholar] [CrossRef]
- Montemurro, N.; Ortenzi, V.; Naccarato, G.A.; Perrini, P. Angioleiomyoma of the knee: An uncommon cause of leg pain. A systematic review of the literature. Interdiscip. Neurosurg. 2020, 22, 100877. [Google Scholar] [CrossRef]
- Perrini, P.; Montemurro, N.; Iannelli, A. The contribution of Carlo Giacomini (1840–1898): The limbus Giacomini and beyond. Neurosurgery 2013, 72, 475–482. [Google Scholar] [CrossRef]
- Klement, R.J.; Champ, C.E. Calories, carbohydrates, and cancer therapy with radiation: Exploiting the five R’s through dietary manipulation. Cancer Metastasis Rev. 2014, 33, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Pitter, K.L.; Tamagno, I.; Alikhanyan, K.; Hosni-Ahmed, A.; Pattwell, S.S.; Donnola, S.; Dai, C.; Ozawa, T.; Chang, M.; Chan, T.A. Corticosteroids compromise survival in glioblastoma. Brain 2016, 139, 1458–1471. [Google Scholar] [CrossRef] [PubMed]
- Panhans, C.M.; Gresham, G.; Amaral, L.; Hu, J. Exploring the feasibility and effects of a ketogenic diet in patients with CNS malignancies: A retrospective case series. Front. Neurosci. 2020, 14, 390. [Google Scholar] [CrossRef]
- Poff, A.; Koutnik, A.P.; Egan, K.M.; Sahebjam, S.; D’Agostino, D.; Kumar, N.B. Targeting the Warburg effect for cancer treatment: Ketogenic diets for management of glioma. Semin. Cancer Biol. 2019, 56, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Rapone, B.; Ferrara, E.; Montemurro, N.; Converti, I.; Loverro, M.; Loverro, M.T.; Gnoni, A.; Scacco, S.; Siculella, L.; Corsalini, M. Oral microbiome and preterm birth: Correlation or coincidence? A narrative review. Open Access Maced. J. Med. Sci. 2020, 8, 123–132. [Google Scholar] [CrossRef]
- Valerio, J.; Borro, M.; Proietti, E.; Pisciotta, L.; Olarinde, I.O.; Fernandez Gomez, M.; Alvarez Pinzon, A.M. Systematic Review and Clinical Insights: The Role of the Ketogenic Diet in Managing Glioblastoma in Cancer Neuroscience. J. Pers. Med. 2024, 14, 929. [Google Scholar] [CrossRef] [PubMed]
- Puig-Saenz, C.; Pearson, J.R.; Thomas, J.E.; McArdle, S.E. A Holistic Approach to Hard-to-Treat Cancers: The Future of Immunotherapy for Glioblastoma, Triple Negative Breast Cancer, and Advanced Prostate Cancer. Biomedicines 2023, 11, 2100. [Google Scholar] [CrossRef]
- Dal Bello, S.; Valdemarin, F.; Martinuzzi, D.; Filippi, F.; Gigli, G.L.; Valente, M. Ketogenic diet in the treatment of gliomas and glioblastomas. Nutrients 2022, 14, 3851. [Google Scholar] [CrossRef]
- Zhao, M.; van Straten, D.; Broekman, M.L.; Préat, V.; Schiffelers, R.M. Nanocarrier-based drug combination therapy for glioblastoma. Theranostics 2020, 10, 1355. [Google Scholar] [CrossRef]
- Liu, D.; Dai, X.; Ye, L.; Wang, H.; Qian, H.; Cheng, H.; Wang, X. Nanotechnology meets glioblastoma multiforme: Emerging therapeutic strategies. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2023, 15, e1838. [Google Scholar] [CrossRef] [PubMed]
- Frellsen, A.F.; Hansen, A.E.; Jølck, R.I.; Kempen, P.J.; Severin, G.W.; Rasmussen, P.H.; Kjær, A.; Jensen, A.T.; Andresen, T.L. Mouse positron emission tomography study of the biodistribution of gold nanoparticles with different surface coatings using embedded copper-64. ACS Nano 2016, 10, 9887–9898. [Google Scholar] [CrossRef]
- Guo, Q.-L.; Dai, X.-L.; Yin, M.-Y.; Cheng, H.-W.; Qian, H.-S.; Wang, H.; Zhu, D.-M.; Wang, X.-W. Nanosensitizers for sonodynamic therapy for glioblastoma multiforme: Current progress and future perspectives. Mil. Med. Res. 2022, 9, 26. [Google Scholar] [CrossRef]
- Sharma, G.; Sharma, A.R.; Lee, S.-S.; Bhattacharya, M.; Nam, J.-S.; Chakraborty, C. Advances in nanocarriers enabled brain targeted drug delivery across blood brain barrier. Int. J. Pharm. 2019, 559, 360–372. [Google Scholar] [CrossRef]
- Zhou, Y.; Peng, Z.; Seven, E.S.; Leblanc, R.M. Crossing the blood-brain barrier with nanoparticles. J. Control. Release 2018, 270, 290–303. [Google Scholar] [CrossRef]
- Khongkow, M.; Yata, T.; Boonrungsiman, S.; Ruktanonchai, U.R.; Graham, D.; Namdee, K. Surface modification of gold nanoparticles with neuron-targeted exosome for enhanced blood–brain barrier penetration. Sci. Rep. 2019, 9, 8278. [Google Scholar] [CrossRef]
- Abdul Razzak, R.; Florence, G.J.; Gunn-Moore, F.J. Approaches to CNS drug delivery with a focus on transporter-mediated transcytosis. Int. J. Mol. Sci. 2019, 20, 3108. [Google Scholar] [CrossRef]
- Jain, A.; Jain, A.; Garg, N.K.; Tyagi, R.K.; Singh, B.; Katare, O.P.; Webster, T.J.; Soni, V. Surface engineered polymeric nanocarriers mediate the delivery of transferrin–methotrexate conjugates for an improved understanding of brain cancer. Acta Biomater. 2015, 24, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, M.; Borri, C. Polymer nanoparticles as smart carriers for the enhanced release of therapeutic agents to the CNS. Curr. Pharm. Des. 2017, 23, 393–410. [Google Scholar] [CrossRef]
- Song, Q.; Song, H.; Xu, J.; Huang, J.; Hu, M.; Gu, X.; Chen, J.; Zheng, G.; Chen, H.; Gao, X. Biomimetic ApoE-reconstituted high density lipoprotein nanocarrier for blood–brain barrier penetration and amyloid beta-targeting drug delivery. Mol. Pharm. 2016, 13, 3976–3987. [Google Scholar] [CrossRef]
- Shi, C.; Guo, D.; Xiao, K.; Wang, X.; Wang, L.; Luo, J. A drug-specific nanocarrier design for efficient anticancer therapy. Nat. Commun. 2015, 6, 7449. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Moosavi, M.A.; Tavakol, S.; Vardar, D.Ö.; Hosseini, A.; Rahmati, M.; Dini, L.; Hussain, S.; Mandegary, A.; Klionsky, D.J. Necrotic, apoptotic and autophagic cell fates triggered by nanoparticles. Autophagy 2019, 15, 4–33. [Google Scholar] [CrossRef] [PubMed]
- Jo, D.H.; Kim, J.H.; Lee, T.G.; Kim, J.H. Size, surface charge, and shape determine therapeutic effects of nanoparticles on brain and retinal diseases. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1603–1611. [Google Scholar] [CrossRef]
- Shin, S.W.; Song, I.H.; Um, S.H. Role of physicochemical properties in nanoparticle toxicity. Nanomaterials 2015, 5, 1351–1365. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Ahn, S.I.; Kim, Y. Nanotherapeutics engineered to cross the blood-brain barrier for advanced drug delivery to the central nervous system. J. Ind. Eng. Chem. 2019, 73, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Monsalve, Y.; Tosi, G.; Ruozi, B.; Belletti, D.; Vilella, A.; Zoli, M.; Vandelli, M.A.; Forni, F.; Lopez, B.L.; Sierra, L. PEG-g-chitosan nanoparticles functionalized with the monoclonal antibody OX26 for brain drug targeting. Nanomedicine 2015, 10, 1735–1750. [Google Scholar] [CrossRef]
- Cai, Q.; Wang, L.; Deng, G.; Liu, J.; Chen, Q.; Chen, Z. Systemic delivery to central nervous system by engineered PLGA nanoparticles. Am. J. Transl. Res. 2016, 8, 749. [Google Scholar]
- Alli, S.; Figueiredo, C.A.; Golbourn, B.; Sabha, N.; Wu, M.Y.; Bondoc, A.; Luck, A.; Coluccia, D.; Maslink, C.; Smith, C. Brainstem blood brain barrier disruption using focused ultrasound: A demonstration of feasibility and enhanced doxorubicin delivery. J. Control. Release 2018, 281, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Timbie, K.F.; Afzal, U.; Date, A.; Zhang, C.; Song, J.; Miller, G.W.; Suk, J.S.; Hanes, J.; Price, R.J. MR image-guided delivery of cisplatin-loaded brain-penetrating nanoparticles to invasive glioma with focused ultrasound. J. Control. Release 2017, 263, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Lundy, D.J.; Lee, K.-J.; Peng, I.-C.; Hsu, C.-H.; Lin, J.-H.; Chen, K.-H.; Tien, Y.-W.; Hsieh, P.C. Inducing a transient increase in blood–brain barrier permeability for improved liposomal drug therapy of glioblastoma multiforme. Acs Nano 2018, 13, 97–113. [Google Scholar] [CrossRef]
- Wen, L.; Tan, Y.; Dai, S.; Zhu, Y.; Meng, T.; Yang, X.; Liu, Y.; Liu, X.; Yuan, H.; Hu, F. VEGF-mediated tight junctions pathological fenestration enhances doxorubicin-loaded glycolipid-like nanoparticles traversing BBB for glioblastoma-targeting therapy. Drug Deliv. 2017, 24, 1843–1855. [Google Scholar] [CrossRef]
- Van Tellingen, O.; Yetkin-Arik, B.; De Gooijer, M.; Wesseling, P.; Wurdinger, T.; De Vries, H. Overcoming the blood–brain tumor barrier for effective glioblastoma treatment. Drug Resist. Updates 2015, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Salaroglio, I.C.; Abate, C.; Rolando, B.; Battaglia, L.; Gazzano, E.; Colombino, E.; Costamagna, C.; Annovazzi, L.; Mellai, M.; Berardi, F. Validation of thiosemicarbazone compounds as P-Glycoprotein inhibitors in human primary brain–blood barrier and glioblastoma stem cells. Mol. Pharm. 2019, 16, 3361–3373. [Google Scholar] [CrossRef] [PubMed]
- Mittapalli, R.K.; Chung, A.H.; Parrish, K.E.; Crabtree, D.; Halvorson, K.G.; Hu, G.; Elmquist, W.F.; Becher, O.J. ABCG2 and ABCB1 limit the efficacy of dasatinib in a PDGF-B–Driven brainstem glioma model. Mol. Cancer Ther. 2016, 15, 819–829. [Google Scholar] [CrossRef]
- Brown, C.B.; Jacobs, S.; Johnson, M.P.; Southerland, C.; Threatt, S. Convection-enhanced delivery in the treatment of glioblastoma. Semin. Oncol. Nurs. 2018, 34, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Ung, T.H.; Malone, H.; Canoll, P.; Bruce, J.N. Convection-enhanced delivery for glioblastoma: Targeted delivery of antitumor therapeutics. CNS Oncol. 2015, 4, 225–234. [Google Scholar] [CrossRef]
- Mehta, A.; Sonabend, A.; Bruce, J. Convection-enhanced delivery. Neurotherapeutics 2017, 14, 358–371. [Google Scholar] [CrossRef]
- Chen, E.M.; Quijano, A.R.; Seo, Y.-E.; Jackson, C.; Josowitz, A.D.; Noorbakhsh, S.; Merlettini, A.; Sundaram, R.K.; Focarete, M.L.; Jiang, Z. Biodegradable PEG-poly (ω-pentadecalactone-co-p-dioxanone) nanoparticles for enhanced and sustained drug delivery to treat brain tumors. Biomaterials 2018, 178, 193–203. [Google Scholar] [CrossRef]
- Zhang, C.; Nance, E.A.; Mastorakos, P.; Chisholm, J.; Berry, S.; Eberhart, C.; Tyler, B.; Brem, H.; Suk, J.S.; Hanes, J. Convection enhanced delivery of cisplatin-loaded brain penetrating nanoparticles cures malignant glioma in rats. J. Control. Release 2017, 263, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Ganipineni, L.P.; Danhier, F.; Préat, V. Drug delivery challenges and future of chemotherapeutic nanomedicine for glioblastoma treatment. J. Control. Release 2018, 281, 42–57. [Google Scholar] [CrossRef]
- Alphandéry, E.; Idbaih, A.; Adam, C.; Delattre, J.-Y.; Schmitt, C.; Guyot, F.; Chebbi, I. Development of non-pyrogenic magnetosome minerals coated with poly-l-lysine leading to full disappearance of intracranial U87-Luc glioblastoma in 100% of treated mice using magnetic hyperthermia. Biomaterials 2017, 141, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Alphandéry, E.; Idbaih, A.; Adam, C.; Delattre, J.-Y.; Schmitt, C.; Guyot, F.; Chebbi, I. Chains of magnetosomes with controlled endotoxin release and partial tumor occupation induce full destruction of intracranial U87-Luc glioma in mice under the application of an alternating magnetic field. J. Control. Release 2017, 262, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Bayoumi, M.; Youshia, J.; Arafa, M.G.; Nasr, M.; Sammour, O.A. Nanocarriers for the treatment of glioblastoma multiforme: A succinct review of conventional and repositioned drugs in the last decade. Arch. Der Pharm. 2024, 357, e2400343. [Google Scholar] [CrossRef]
- Sukumar, U.K.; Bose, R.J.; Malhotra, M.; Babikir, H.A.; Afjei, R.; Robinson, E.; Zeng, Y.; Chang, E.; Habte, F.; Sinclair, R. Intranasal delivery of targeted polyfunctional gold–iron oxide nanoparticles loaded with therapeutic microRNAs for combined theranostic multimodality imaging and presensitization of glioblastoma to temozolomide. Biomaterials 2019, 218, 119342. [Google Scholar] [CrossRef]
- Bruinsmann, F.A.; Richter Vaz, G.; de Cristo Soares Alves, A.; Aguirre, T.; Raffin Pohlmann, A.; Stanisçuaski Guterres, S.; Sonvico, F. Nasal drug delivery of anticancer drugs for the treatment of glioblastoma: Preclinical and clinical trials. Molecules 2019, 24, 4312. [Google Scholar] [CrossRef] [PubMed]
- Parodi, A.; Rudzińska, M.; Deviatkin, A.A.; Soond, S.M.; Baldin, A.V.; Zamyatnin, A.A., Jr. Established and emerging strategies for drug delivery across the blood-brain barrier in brain cancer. Pharmaceutics 2019, 11, 245. [Google Scholar] [CrossRef]
- Coluccia, D.; Figueiredo, C.A.; Wu, M.Y.; Riemenschneider, A.N.; Diaz, R.; Luck, A.; Smith, C.; Das, S.; Ackerley, C.; O’Reilly, M. Enhancing glioblastoma treatment using cisplatin-gold-nanoparticle conjugates and targeted delivery with magnetic resonance-guided focused ultrasound. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, C.; Wang, L.; Chen, Y. A comprehensive review in improving delivery of small-molecule chemotherapeutic agents overcoming the blood-brain/brain tumor barriers for glioblastoma treatment. Drug Deliv. 2019, 26, 551–565. [Google Scholar] [CrossRef]
- Singh, M.S.; Lamprecht, A. Cargoing P-gp inhibitors via nanoparticle sensitizes tumor cells against doxorubicin. Int. J. Pharm. 2015, 478, 745–752. [Google Scholar] [CrossRef]
- Gupta, R.; Sharma, D. Evolution of magnetic hyperthermia for glioblastoma multiforme therapy. ACS Chem. Neurosci. 2019, 10, 1157–1172. [Google Scholar] [CrossRef] [PubMed]
- Rego, G.N.; Nucci, M.P.; Mamani, J.B.; Oliveira, F.A.; Marti, L.C.; Filgueiras, I.S.; Ferreira, J.M.; Real, C.C.; Faria, D.d.P.; Espinha, P.L. Therapeutic efficiency of multiple applications of magnetic hyperthermia technique in glioblastoma using aminosilane coated iron oxide nanoparticles: In vitro and in vivo study. Int. J. Mol. Sci. 2020, 21, 958. [Google Scholar] [CrossRef] [PubMed]
- Grillone, A.; Battaglini, M.; Moscato, S.; Mattii, L.; de Julián Fernández, C.; Scarpellini, A.; Giorgi, M.; Sinibaldi, E.; Ciofani, G. Nutlin-loaded magnetic solid lipid nanoparticles for targeted glioblastoma treatment. Nanomedicine 2019, 14, 727–752. [Google Scholar] [CrossRef] [PubMed]
- Pucci, C.; De Pasquale, D.; Marino, A.; Martinelli, C.; Lauciello, S.; Ciofani, G. Hybrid magnetic nanovectors promote selective glioblastoma cell death through a combined effect of lysosomal membrane permeabilization and chemotherapy. ACS Appl. Mater. Interfaces 2020, 12, 29037–29055. [Google Scholar] [CrossRef]
- Agarwal, S.; Muniyandi, P.; Maekawa, T.; Kumar, D.S. Vesicular systems employing natural substances as promising drug candidates for MMP inhibition in glioblastoma: A nanotechnological approach. Int. J. Pharm. 2018, 551, 339–361. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, X.; Liu, X.; Lv, W.; Zhang, H.; Zhang, M.; Li, X.; Xin, H.; Xu, Q. Enhanced antiglioma efficacy of ultrahigh loading capacity paclitaxel prodrug conjugate self-assembled targeted nanoparticles. ACS Appl. Mater. Interfaces 2017, 9, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.C.; Roth, I.M.; Wickremesekera, A.C.; Davis, P.F.; Kaye, A.H.; Mantamadiotis, T.; Stylli, S.S.; Tan, S.T. Therapeutic targeting of cancer stem cells in human glioblastoma by manipulating the renin-angiotensin system. Cells 2019, 8, 1364. [Google Scholar] [CrossRef] [PubMed]
- Kalkan, R. Glioblastoma stem cells as a new therapeutic target for glioblastoma. Clin. Med. Insights Oncol. 2015, 9, CMO-S30271. [Google Scholar] [CrossRef]
- Tarasov, V.V.; Svistunov, A.A.; Chubarev, V.N.; Zatsepilova, T.A.; Preferanskaya, N.G.; Stepanova, O.I.; Sokolov, A.V.; Dostdar, S.A.; Minyaeva, N.N.; Neganova, M.E. Feasibility of targeting glioblastoma stem cells: From concept to clinical trials. Curr. Top. Med. Chem. 2019, 19, 2974–2984. [Google Scholar] [CrossRef] [PubMed]
- Kunoh, T.; Shimura, T.; Kasai, T.; Matsumoto, S.; Mahmud, H.; Khayrani, A.C.; Seno, M.; Kunoh, H.; Takada, J. Use of DNA-generated gold nanoparticles to radiosensitize and eradicate radioresistant glioma stem cells. Nanotechnology 2018, 30, 055101. [Google Scholar] [CrossRef] [PubMed]
- Lépinoux-Chambaud, C.; Eyer, J. The NFL-TBS. 40–63 peptide targets and kills glioblastoma stem cells derived from human patients and also targets nanocapsules into these cells. Int. J. Pharm. 2019, 566, 218–228. [Google Scholar] [CrossRef]
- Säälik, P.; Lingasamy, P.; Toome, K.; Mastandrea, I.; Rousso-Noori, L.; Tobi, A.; Simón-Gracia, L.; Hunt, H.; Paiste, P.; Kotamraju, V.R. Peptide-guided nanoparticles for glioblastoma targeting. J. Control. Release 2019, 308, 109–118. [Google Scholar] [CrossRef]
- Gonçalves, D.P.; Rodriguez, R.D.; Kurth, T.; Bray, L.J.; Binner, M.; Jungnickel, C.; Gür, F.N.; Poser, S.W.; Schmidt, T.L.; Zahn, D.R. Enhanced targeting of invasive glioblastoma cells by peptide-functionalized gold nanorods in hydrogel-based 3D cultures. Acta Biomater. 2017, 58, 12–25. [Google Scholar] [CrossRef]
- Cho, J.-H.; Kim, A.-R.; Kim, S.-H.; Lee, S.-J.; Chung, H.; Yoon, M.-Y. Development of a novel imaging agent using peptide-coated gold nanoparticles toward brain glioma stem cell marker CD133. Acta Biomater. 2017, 47, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Glaser, T.; Han, I.; Wu, L.; Zeng, X. Targeted nanotechnology in glioblastoma multiforme. Front. Pharmacol. 2017, 8, 166. [Google Scholar] [CrossRef]
- Hosseini, M.; Haji-Fatahaliha, M.; Jadidi-Niaragh, F.; Majidi, J.; Yousefi, M. The use of nanoparticles as a promising therapeutic approach in cancer immunotherapy. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1051–1061. [Google Scholar] [CrossRef]
- Madhankumar, A.B.; Slagle-Webb, B.; Wang, X.; Yang, Q.X.; Antonetti, D.A.; Miller, P.A.; Sheehan, J.M.; Connor, J.R. Efficacy of interleukin-13 receptor–targeted liposomal doxorubicin in the intracranial brain tumor model. Mol. Cancer Ther. 2009, 8, 648–654. [Google Scholar] [CrossRef]
- Yang, F.-Y.; Wong, T.-T.; Teng, M.-C.; Liu, R.-S.; Lu, M.; Liang, H.-F.; Wei, M.-C. Focused ultrasound and interleukin-4 receptor-targeted liposomal doxorubicin for enhanced targeted drug delivery and antitumor effect in glioblastoma multiforme. J. Control. Release 2012, 160, 652–658. [Google Scholar] [CrossRef]
- Limasale, Y.D.P.; Tezcaner, A.; Özen, C.; Keskin, D.; Banerjee, S. Epidermal growth factor receptor-targeted immunoliposomes for delivery of celecoxib to cancer cells. Int. J. Pharm. 2015, 479, 364–373. [Google Scholar] [CrossRef]
- Nishiyama, N.; Matsumura, Y.; Kataoka, K. Development of polymeric micelles for targeting intractable cancers. Cancer Sci. 2016, 107, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Karim, R.; Palazzo, C.; Evrard, B.; Piel, G. Nanocarriers for the treatment of glioblastoma multiforme: Current state-of-the-art. J. Control. Release 2016, 227, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Saxena, V.; Hussain, M.D. Formulation and in vitro evaluation of 17-allyamino-17-demethoxygeldanamycin (17-AAG) loaded polymeric mixed micelles for glioblastoma multiforme. Colloids Surf. B Biointerfaces 2013, 112, 350–355. [Google Scholar] [CrossRef]
- Talaei, S.; Mellatyar, H.; Asadi, A.; Akbarzadeh, A.; Sheervalilou, R.; Zarghami, N. Spotlight on 17-AAG as an Hsp90 inhibitor for molecular targeted cancer treatment. Chem. Biol. Drug Des. 2019, 93, 760–786. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Xiao, Y.; Di, Q.; Ma, W.; Ma, X.; Wang, Q.; Chen, W. Transferrin receptor-targeted PEG-PLA polymeric micelles for chemotherapy against glioblastoma multiforme. Int. J. Nanomed. 2020, 15, 6673–6688. [Google Scholar] [CrossRef] [PubMed]
- Van Woensel, M.; Wauthoz, N.; Rosière, R.; Mathieu, V.; Kiss, R.; Lefranc, F.; Steelant, B.; Dilissen, E.; Van Gool, S.W.; Mathivet, T. Development of siRNA-loaded chitosan nanoparticles targeting Galectin-1 for the treatment of glioblastoma multiforme via intranasal administration. J. Control. Release 2016, 227, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Alswailem, R.; Alqahtani, F.Y.; Aleanizy, F.S.; Alrfaei, B.M.; Badran, M.; Alqahtani, Q.H.; Abdelhady, H.G.; Alsarra, I. MicroRNA-219 loaded chitosan nanoparticles for treatment of glioblastoma. Artif. Cells Nanomed. Biotechnol. 2022, 50, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Stenström, P.; Manzanares, D.; Zhang, Y.; Ceña, V.; Malkoch, M. Evaluation of amino-functional polyester dendrimers based on Bis-MPA as nonviral vectors for siRNA delivery. Molecules 2018, 23, 2028. [Google Scholar] [CrossRef]
- Tambe, V.; Thakkar, S.; Raval, N.; Sharma, D.; Kalia, K.; Tekade, R.K. Surface engineered dendrimers in siRNA delivery and gene silencing. Curr. Pharm. Des. 2017, 23, 2952–2975. [Google Scholar] [CrossRef]
- Dhanikula, R.S.; Argaw, A.; Bouchard, J.-F.; Hildgen, P. Methotrexate loaded polyether-copolyester dendrimers for the treatment of gliomas: Enhanced efficacy and intratumoral transport capability. Mol. Pharm. 2008, 5, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Kong, L.; Cao, X.; Li, A.; Wei, P.; Wang, L.; Mignani, S.; Caminade, A.-M.; Majoral, J.-P.; Shi, X. Enhanced delivery of therapeutic siRNA into glioblastoma cells using dendrimer-entrapped gold nanoparticles conjugated with β-cyclodextrin. Nanomaterials 2018, 8, 131. [Google Scholar] [CrossRef]
- Ghaffari, M.; Dehghan, G.; Abedi-Gaballu, F.; Kashanian, S.; Baradaran, B.; Dolatabadi, J.E.N.; Losic, D. Surface functionalized dendrimers as controlled-release delivery nanosystems for tumor targeting. Eur. J. Pharm. Sci. 2018, 122, 311–330. [Google Scholar] [CrossRef]
- Li, J.; Liang, H.; Liu, J.; Wang, Z. Poly (amidoamine)(PAMAM) dendrimer mediated delivery of drug and pDNA/siRNA for cancer therapy. Int. J. Pharm. 2018, 546, 215–225. [Google Scholar] [CrossRef]
- Kong, L.; Wu, Y.; Alves, C.S.; Shi, X. Efficient delivery of therapeutic siRNA into glioblastoma cells using multifunctional dendrimer-entrapped gold nanoparticles. Nanomedicine 2016, 11, 3103–3115. [Google Scholar] [CrossRef] [PubMed]
- Bobyk, L.; Edouard, M.; Deman, P.; Vautrin, M.; Pernet-Gallay, K.; Delaroche, J.; Adam, J.-F.; Estève, F.; Ravanat, J.-L.; Elleaume, H. Photoactivation of gold nanoparticles for glioma treatment. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Her, S.; Jaffray, D.A.; Allen, C. Gold nanoparticles for applications in cancer radiotherapy: Mechanisms and recent advancements. Adv. Drug Deliv. Rev. 2017, 109, 84–101. [Google Scholar] [CrossRef]
- Liu, J.; Peng, Q. Protein-gold nanoparticle interactions and their possible impact on biomedical applications. Acta Biomater. 2017, 55, 13–27. [Google Scholar] [CrossRef]
- Peng, L.; Liang, Y.; Zhong, X.; Liang, Z.; Tian, Y.; Li, S.; Liang, J.; Wang, R.; Zhong, Y.; Shi, Y. Aptamer-conjugated gold nanoparticles targeting epidermal growth factor receptor variant III for the treatment of glioblastoma. Int. J. Nanomed. 2020, 23, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- Bourquin, J.; Milosevic, A.; Hauser, D.; Lehner, R.; Blank, F.; Petri-Fink, A.; Rothen-Rutishauser, B. Biodistribution, clearance, and long-term fate of clinically relevant nanomaterials. Adv. Mater. 2018, 30, 1704307. [Google Scholar] [CrossRef]
- Krętowski, R.; Kusaczuk, M.; Naumowicz, M.; Kotyńska, J.; Szynaka, B.; Cechowska-Pasko, M. The effects of silica nanoparticles on apoptosis and autophagy of glioblastoma cell lines. Nanomaterials 2017, 7, 230. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.-Y.; Joachim, E.; Choi, H.; Kim, K. Toxicity of silica nanoparticles depends on size, dose, and cell type. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1407–1416. [Google Scholar] [CrossRef]
- Yazdimamaghani, M.; Moos, P.J.; Dobrovolskaia, M.A.; Ghandehari, H. Genotoxicity of amorphous silica nanoparticles: Status and prospects. Nanomed. Nanotechnol. Biol. Med. 2019, 16, 106–125. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Wu, Z.; Li, X.; Xiao, L.; Yang, M.; Li, Y.; Duan, J.; Sun, Z. The size-dependent cytotoxicity of amorphous silica nanoparticles: A systematic review of in vitro studies. Int. J. Nanomed. 2020, 15, 9089–9113. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Lewik, G.; Ratcliffe, J.C.; Choi, C.H.J.; Mäkilä, E.; Tong, W.Y.; Voelcker, N.H. Systematic evaluation of transferrin-modified porous silicon nanoparticles for targeted delivery of doxorubicin to glioblastoma. ACS Appl. Mater. Interfaces 2019, 11, 33637–33649. [Google Scholar] [CrossRef]
- Sheykhzadeh, S.; Luo, M.; Peng, B.; White, J.; Abdalla, Y.; Tang, T.; Mäkilä, E.; Voelcker, N.H.; Tong, W.Y. Transferrin-targeted porous silicon nanoparticles reduce glioblastoma cell migration across tight extracellular space. Sci. Rep. 2020, 10, 2320. [Google Scholar] [CrossRef]
- Turan, O.; Bielecki, P.A.; Perera, V.; Lorkowski, M.; Covarrubias, G.; Tong, K.; Yun, A.; Loutrianakis, G.; Raghunathan, S.; Park, Y. Treatment of glioblastoma using multicomponent silica nanoparticles. Adv. Ther. 2019, 2, 1900118. [Google Scholar] [CrossRef] [PubMed]
- Ghaznavi, H.; Afzalipour, R.; Khoei, S.; Sargazi, S.; Shirvalilou, S.; Sheervalilou, R. New insights into targeted therapy of glioblastoma using smart nanoparticles. Cancer Cell Int. 2024, 24, 160. [Google Scholar] [CrossRef]
- Đorđević, S.; Gonzalez, M.M.; Conejos-Sánchez, I.; Carreira, B.; Pozzi, S.; Acúrcio, R.C.; Satchi-Fainaro, R.; Florindo, H.F.; Vicent, M.J. Current hurdles to the translation of nanomedicines from bench to the clinic. Drug Deliv. Transl. Res. 2022, 12, 500–525. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Rosenblum, D.; Joshi, N.; Tao, W.; Karp, J.M.; Peer, D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 2018, 9, 1410. [Google Scholar] [CrossRef] [PubMed]
- Bawa, R.; Johnson, S. Emerging issues in nanomedicine and ethics. In Nanotechnology & Society: Current and Emerging Ethical Issues; Springer: Berlin/Heidelberg, Germany, 2009; pp. 207–223. [Google Scholar]
- Ma, X.; Tian, Y.; Yang, R.; Wang, H.; Allahou, L.W.; Chang, J.; Williams, G.; Knowles, J.C.; Poma, A. Nanotechnology in healthcare, and its safety and environmental risks. J. Nanobiotechnol. 2024, 22, 715. [Google Scholar] [CrossRef]
- Wasti, S.; Lee, I.H.; Kim, S.; Lee, J.-H.; Kim, H. Ethical and legal challenges in nanomedical innovations: A scoping review. Front. Genet. 2023, 14, 1163392. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, X.; Fu, X.; Huang, X.; Zhang, S.; Zhao, N.; Ma, X.; Saiding, Q.; Yang, M.; Tao, W. Innovative Nanotechnology in Drug Delivery Systems for Advanced Treatment of Posterior Segment Ocular Diseases. Adv. Sci. 2024, 11, 2403399. [Google Scholar] [CrossRef]
- Nikalje, A.P. Nanotechnology and its applications in medicine. Med. Chem. 2015, 5, 81–89. [Google Scholar] [CrossRef]
- DiSanto, R.M.; Subramanian, V.; Gu, Z. Recent advances in nanotechnology for diabetes treatment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 548–564. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R. Diabetes treatment by nanotechnology. J. Biotechnol. Biomater. 2017, 7, 268. [Google Scholar] [CrossRef]
- Miñon-Hernández, D.; Villalobos-Espinosa, J.; Santiago-Roque, I.; González-Herrera, S.L.; Herrera-Meza, S.; Meza-Alvarado, E.; Bello-Pérez, A.; Osorio-Díaz, P.; Chanona-Pérez, J.; Méndez-Méndez, J.V. Biofunctionality of native and nano-structured blue corn starch in prediabetic Wistar rats. CyTA-J. Food 2018, 16, 477–483. [Google Scholar] [CrossRef]
- Antwi-Baah, R.; Wang, Y.; Chen, X.; Yu, K. Metal-based nanoparticle magnetic resonance imaging contrast agents: Classifications, issues, and countermeasures toward their clinical translation. Adv. Mater. Interfaces 2022, 9, 2101710. [Google Scholar] [CrossRef]
- Su, C.; Liu, Y.; Li, R.; Wu, W.; Fawcett, J.P.; Gu, J. Absorption, distribution, metabolism and excretion of the biomaterials used in Nanocarrier drug delivery systems. Adv. Drug Deliv. Rev. 2019, 143, 97–114. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Gorain, B.; Low, S.Y.; Tan, S.A.; Ling, E.C.S.; Lim, Y.K.; Chin, C.M.; Lee, P.Y.; Lee, C.M.; Ooi, C.H. Nanotechnology based approaches for anti-diabetic drugs delivery. Diabetes Res. Clin. Pract. 2018, 136, 52–77. [Google Scholar] [CrossRef]
- Moros, M.; Mitchell, S.; Grazu, V.; Fuente, J.d.l. The fate of nanocarriers as nanomedicines in vivo: Important considerations and biological barriers to overcome. Curr. Med. Chem. 2013, 20, 2759–2778. [Google Scholar] [CrossRef]
- Ding, C.; Li, Z. A review of drug release mechanisms from nanocarrier systems. Mater. Sci. Eng. C 2017, 76, 1440–1453. [Google Scholar] [CrossRef]
- Farokhzad, O.C.; Langer, R. Impact of nanotechnology on drug delivery. ACS Nano 2009, 3, 16–20. [Google Scholar] [CrossRef]
- Xing, H.; Hwang, K.; Lu, Y. Recent developments of liposomes as nanocarriers for theranostic applications. Theranostics 2016, 6, 1336. [Google Scholar] [CrossRef]
- Veiseh, O.; Tang, B.C.; Whitehead, K.A.; Anderson, D.G.; Langer, R. Managing diabetes with nanomedicine: Challenges and opportunities. Nat. Rev. Drug Discov. 2015, 14, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Yang, T.; Wang, L.; Yu, J.; Wei, X.; Zhou, Y.; Wang, C.; Liang, W. Nano-cage-mediated refolding of insulin by PEG-PE micelle. Biomaterials 2016, 77, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Yousaf, M.; Huang, Q.; Yang, Y.; Wang, C. Dual effect of PEG-PE micelle over the oligomerization and fibrillation of human islet amyloid polypeptide. Sci. Rep. 2018, 8, 4463. [Google Scholar] [CrossRef]
- Consultation, W. Definition, diagnosis and classification of diabetes mellitus and its complications. Diabet. Med. 1999, 15, 539–553. [Google Scholar]
- Group, N.D.D. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979, 28, 1039–1057. [Google Scholar] [CrossRef]
- Maier-Hauff, K.; Ulrich, F.; Nestler, D.; Niehoff, H.; Wust, P.; Thiesen, B.; Orawa, H.; Budach, V.; Jordan, A. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J. Neuro-Oncol. 2011, 103, 317–324. [Google Scholar] [CrossRef]
- Whittle, J.R.; Lickliter, J.D.; Gan, H.K.; Scott, A.M.; Simes, J.; Solomon, B.J.; MacDiarmid, J.A.; Brahmbhatt, H.; Rosenthal, M.A. First in human nanotechnology doxorubicin delivery system to target epidermal growth factor receptors in recurrent glioblastoma. J. Clin. Neurosci. 2015, 22, 1889–1894. [Google Scholar] [CrossRef]
- Ren, H.; Boulikas, T.; Söling, A.; Warnke, P.; Rainov, N. Immunogene therapy of recurrent glioblastoma multiforme with a liposomally encapsulated replication-incompetent Semliki forest virus vector carrying the human interleukin-12 gene–a phase I/II clinical protocol. J. Neuro-Oncol. 2003, 64, 147–154. [Google Scholar] [CrossRef]
- Menei, P.; Capelle, L.; Guyotat, J.; Fuentes, S.; Assaker, R.; Bataille, B.; François, P.; Dorwling-Carter, D.; Paquis, P.; Bauchet, L. Local and sustained delivery of 5-fluorouracil from biodegradable microspheres for the radiosensitization of malignant glioma: A randomized phase II trial. Neurosurgery 2005, 56, 242–248. [Google Scholar] [CrossRef]
- Beier, C.P.; Schmid, C.; Gorlia, T.; Kleinletzenberger, C.; Beier, D.; Grauer, O.; Steinbrecher, A.; Hirschmann, B.; Brawanski, A.; Dietmaier, C. RNOP-09: Pegylated liposomal doxorubicine and prolonged temozolomide in addition to radiotherapy in newly diagnosed glioblastoma-a phase II study. BMC Cancer 2009, 9, 1–10. [Google Scholar] [CrossRef]
- Ananda, S.; Nowak, A.K.; Cher, L.; Dowling, A.; Brown, C.; Simes, J.; Rosenthal, M.A.; Neuro-Oncology, C.T.G.f. Phase 2 trial of temozolomide and pegylated liposomal doxorubicin in the treatment of patients with glioblastoma multiforme following concurrent radiotherapy and chemotherapy. J. Clin. Neurosci. 2011, 18, 1444–1448. [Google Scholar] [CrossRef] [PubMed]
| Brand Name | Formulation | Results | Patients No | Reference |
|---|---|---|---|---|
| Nano-thermotherapy Phase II | Thermotherapy and magnetic iron oxide nanoparticles + reduced dose radiotherapy. | The amalgamation of these elements has been deemed secure and efficacious, resulting in an extended duration of survival on the whole. | 59 | [272] |
| EDV-doxorubicin Phase I | EnGenelC delivery vehicle (EDV)-doxorubicin + radiation and oral TMZ. | The EnGenelC delivery vehicle (EDV) has been utilized in combination with doxorubicin and radiation, as well as oral TMZ. | 14 | [273] |
| Interleukin-12 Phase I, II | The utilization of a Semliki Forest virus vector that carries the IL-12 gene, which has been encapsulated in cationic liposomes. | The efficient delivery of liposomally encapsulated virus to GBM can be achieved through the utilization of convection-enhanced delivery. | Adult patients | [274] |
| 5-fluorouracil Phase II | 5-fluorouracil-releasing microspheres followed by early radiotherapy. | The study group exhibited a marginal improvement in overall survival as compared to those who received radiotherapy alone. | 95 | [275] |
| Caelyx, PEG-Dox Phase I, II | Pegylated liposomal doxorubicin + prolonged TMZ and radiotherapy. | The rate of progression-free survival at the end of 12 months was observed to be 30.2%, while the median overall survival was found to be 17.6 months. The incorporation of PEG-Dox or extended TMZ administration did not yield a significant enhancement. | 63 | [276] |
| PEG-Dox Phase II | The utilization of TMZ and Pegylated liposomal doxorubicin following radiotherapy and surgery. | The rate of progression-free survival at the six-month mark was determined to be 58%, while the median OS was found to be 13.6 months. The co-administration of TMZ and PEG-Dox has not been observed to confer any discernible clinical advantage. | 40 | [277] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De, S.; Banerjee, S.; Dey, G.; Banerjee, S.; Kumar, S.K.A. Interplay Between Diabetes, Obesity and Glioblastoma Multiforme, and the Role of Nanotechnology in Its Treatment. J. Nanotheranostics 2025, 6, 7. https://doi.org/10.3390/jnt6010007
De S, Banerjee S, Dey G, Banerjee S, Kumar SKA. Interplay Between Diabetes, Obesity and Glioblastoma Multiforme, and the Role of Nanotechnology in Its Treatment. Journal of Nanotheranostics. 2025; 6(1):7. https://doi.org/10.3390/jnt6010007
Chicago/Turabian StyleDe, Sourav, Sabyasachi Banerjee, Gourab Dey, Subhasis Banerjee, and S.K. Ashok Kumar. 2025. "Interplay Between Diabetes, Obesity and Glioblastoma Multiforme, and the Role of Nanotechnology in Its Treatment" Journal of Nanotheranostics 6, no. 1: 7. https://doi.org/10.3390/jnt6010007
APA StyleDe, S., Banerjee, S., Dey, G., Banerjee, S., & Kumar, S. K. A. (2025). Interplay Between Diabetes, Obesity and Glioblastoma Multiforme, and the Role of Nanotechnology in Its Treatment. Journal of Nanotheranostics, 6(1), 7. https://doi.org/10.3390/jnt6010007







