Abstract
Ultrasound technique was used to produce native and acetylated cassava starch particles containing potassium sorbate (KS). In order to obtain an active packaging, films with addition of native starch particles containing KS (NKSPF) or added with acetylated starch particles containing KS (AKSPF) were formulated. As control systems, films without KS (CF) or added with KS that was not retained in particles (KSF), were produced. The NKSPF and AKSPF microstructure was consistent with composite materials. Tensile test revealed that CF and KSF were ductile and extensible (stress at break (σb) 2.8–2.5 MPa and strain at break (εb) 284–206%), while NKSPF and AKSPF were more resistant films with higher Young’s Modulus (148–477 MPa) and σb (3.6–17 MPa) but lower εb (40–11%). Moreover, NKSPF and AKSPF developed lower Yellowness Index (6.6–6.5) but higher opacity (19–23%) and solubility in water (31–35%) than KSF (9, 10.8% and 9%, respectively). It was observed that KSF and NKSPF moderately reduced the Zygosaccharomyces bailii growth while AKSPF showed the highest yeast inhibition, three Log-cycles, compared to CF. Additionally, FTIR spectroscopy revealed intensified interactions between KS and modified starch. It was concluded that starch sonication and acetylation were useful modifications to produce particles carrier of KS that improved the physical and antimicrobial performance of active films.
1. Introduction
Starch is a natural biopolymer (carbohydrates) extensively used in a wide range of food and non-food products, as a raw material or as an additive. Therefore, it performs many functions mainly in food and pharmaceutical industry, but also in adhesives, paints, textile, and paper production. Some advantages of using this biopolymer are low cost, absence of toxicity, being renewable, biodegradable, and compatible with other materials [1].
The granule is semi-crystalline in structure with a variable amount of two main components, amylose (amorphous) and amylopectin (crystalline), depending on botanical origin. Their technological properties are deeply modulated by the amylose:amylopectin ratio, distribution of branched chains in amylopectin, granule architecture, phospholipid, phosphate-monoester, and lipids content [2].
In food manufacturing, this carbohydrate is commonly used as a thickener, gelling agent, water absorber, as a source of energy in fermentation, and as an agent to increase volume, among others [3]. In this context, the food industry continuously demands for new strategies that allow both to obtain safer and healthier food as well as an optimal stabilization of its products, through processes and ingredients that reduce physical, organoleptic, nutritional, and microbiological deterioration [4].
However, gelatinized native starch presents technological limitations related to its high hydrophilicity, retrogradation, low resistance to mechanical stress, and limited stability in an acidic medium [5]. Therefore, chemical, physical or enzymatic modification has been carried out to overcome these deficiencies and increase industrial applications [6]. It has been reported that the esterification with long or short acyl chain or organic acid groups, as well as crosslinking, gives desirable modifications in biopolymer properties, such as delay or elimination of the retrogradation process, less syneresis of gels, lower crystallinity of the granule, lower temperatures, and enthalpies of gelatinization [7].
Biodegradable and edible films constituted with polymers from natural sources such as cellulose and derivatives, pectins, chitosan, gums, etc., are a promising alternative that can reduce the negative impact of petroleum derived materials on the environment. In this context, starch is commonly used for the formulation of edible films, due to its availability and biodegradability [8]. The most widely used technique for the laboratory production of films is casting, which consists of pouring a suspension on small containers or plates, controlling the average thickness of the resulting films from the mass of suspension poured on the plate [9].
At a global level, one of the innovations with high potential in food science and technology is nanotechnology. Using different techniques, nanosystems can be produced with dimensions in the order of 1 to 100 nm with unique properties [10]. Applications of these nanomaterials are growing widely due to their ability to increase solubility and bioavailability and to protect bioactive components during processing and storage. In recent decades, micro and nanostructured systems based on biopolymers, such as starch, with the ability to control, stabilize and/or release preservatives, nutraceuticals, aromas or flavorings, present in food formulations, have been proposed [11,12]. Starch nanoparticles have been used successfully as support for nutraceuticals, as a food additive, as an emulsion stabilizer, as a fat substitute, as a thickener or as a rheology modifier, etc. [6,13,14].
According to this trend, our previous research was focused on the development of micro-nanoparticles of native and acetylated cassava starch as carriers of the antifungal potassium sorbate (KS) using the dialysis technique [15]. More recently, sonication was applied [16] to obtain nanoparticles from native (NKSP) or acetylated starch (AKSP), observing a noticeable reduction of the molecular weight, an increase of amylose content and high KS retention capacity and mass yield.
Sorbic acid (2,4-hexadienoic acid) and its sodium and potassium salts are recognized antimicrobial agents in food industry. These preservatives prevent the growth of fungi, yeasts and some bacteria. The Codex Alimentarius regulate the maximum dose, being 200 to 2000 mg/kg of food depending on food category. Sorbates are classified as generally recognized as safe (GRAS) [15]. It has been demonstrated the advantages of retaining KS in biopolymer films or particles to modulate its release in a gradual and controlled manner into the target matrix [16].
Regarding environmental aspects, there is a growing interest in biodegradable and edible packaging. In order to control food contamination and quality loss, active packages added with antimicrobial agents have been introduced [17]. Among the most used materials to produce biodegradable and edible films, starch offers advantages as sustainability. The preparation of films incorporated with nanoparticles has been studied in recent years. An improvement in the mechanical, permeability, thermal and optical properties of these films might be achieved by adjusting the quantity, size, preparation method and the degree of dispersion of the nanoparticles used [18]. Adding nanoparticles, packages can be stronger and lighter, with less gas sorption [19].
Many additives and ingredients can lose their functionality when exposed to degradative interactions within the food. The incorporation of these compounds in starchy matrices that can retain and protect them to optimize their performance is a strategy that has shown potential. However, it needs to be further explored in order to expand its technological applications and to better understand the features that modulate active compound interactions. It is important to highlight that the presence of antimicrobials in the film formulation can alter their mechanical properties, barrier to water vapor and oxygen, solubility and organoleptic properties [20].
The general purpose of this research was to use nanostructured particles based on native or acetylated cassava starch containing KS (NKSP and AKSP, respectively) and obtained by sonication, to contribute with the optimization of physical and antimicrobial functionality of biodegradable films. Specifically, the objectives of this work were: (a) to analyze the impact of NKSP and AKSP addition to biodegradable film formulation on the microstructure, mechanical properties, solubility in water and color of films; and (b) to study the antimicrobial capacity of biodegradable films incorporated with NKSP and AKSP against the yeast Zygosaccharomyces bailii.
2. Materials and Methods
2.1. Materials
Food grade native cassava starch (NCS), 92–98% w/w purity grade, 24 ± 4% amylose content w/w d.b. [21] molecular weights of 68 × 106 g/mol (amylopectin) and 0.8 × 106 g/mol (amylose) [15], was obtained from Bernesa S.A. (Lomas de Zamora, Buenos Aires, Argentina). Potassium sorbate (KS) (Sigma Aldrich, St. Louis, MO, USA), iodine (Biopack, CABA, Argentina), acetic anhydride (Sintorgan, Villa Martelli—Buenos Aires, Argentina), sodium tiosulphate (Biopack, CABA, Argentina) and other used chemicals were of analytical grade. Ethanol (Purocol, Claypole, Buenos Aires, Argentina) was USP grade.
2.2. Methods
2.2.1. Microwave Assisted Starch Acetylation
Acetylation of NCS was performed according to previous research [15]. Briefly, 10.25 g of NCS were weighed in a Teflon® reactor (50 mL). Acetic anhydride (19.1 mL) and iodine (I2, 0.05 g) were added to the reactor and mixed vigorously. Then, the reactor was introduced in a microwave device (Ethos Plus, Milestone, Sorisole, BG, Italy). The process conditions for microwave heating were as follows: the maximum power applied was 450 Watts; temperature: a period of 1 min to reach a maximum temperature of ~100 °C, 3.5 min of sample maintenance at 100 °C and, finally, 1 min of ventilation without power application before opening the microwave chamber. Once the reacting mixture for acetylated cassava starch (ACS) synthesis was cooled to ≈25 °C, 4 mL of Na2S2O3 were added to reduce the I2 to iodide. The ACS was subjected to alternating washings, at room temperature, with water and ethanol. After each washing, the slurry was filtered with glass fiber. The obtained product was dried in a vacuum oven at 60 °C overnight and subsequently milled and sieved through a 400 μm mesh.
- Degree of substitution
A volumetric technique was used to determine the degree of substitution (DS) [15]. Samples of around 1.0000 g of ACS were placed in Erlenmeyer flasks and 50 mL of a 75% v/v aqueous ethanol solution was added. The above mixture was stirred for 30 min in a magnetic stirrer maintaining a temperature of 50 °C. After cooling to room temperature, 20 mL of 0.5 N NaOH solution were added. The Erlenmeyer flasks were shaken for 24 h at room temperature in order to proceed to the saponification. Excess alkali was titrated with 0.5 N HCl using phenolphthalein as an indicator. The procedure was also accomplished with a blank sample prepared with NCS. The percentage of acetyl and the corresponding DS were calculated according to the Equations (1) and (2):
where mLblank is the volume of 0.5 N HCl consumed for the NCS sample at titration and mLsample is the volume of 0.5 N HCl consumed for ACS at titration.
% Acetyl = [(mLblank − mLsample) × NHCl × 0.043/g sample] × 100,
DS acetyl = (162 × % acetyl)/[4300 − (42 × % acetyl)],
2.2.2. Ultrasound Application to Obtain Starch Particles as Carriers of KS
Starch particles containing KS were produced considering previous assays [16]. Briefly, NCS and ACS aqueous suspensions (3.0% w/w) were prepared and subjected to heating until starch gelatinization (85 °C). They were subsequently cooled to room temperature and were later treated with a 20 kHz frequency 750 W ultrasound generator (Vibra Cell VCX-750, Sonics, Newtown, CT, USA) equipped with a 13 mm diameter titanium tip. Wave amplitude of 80% was applied during 10 min. The temperature of the samples was maintained lower than 25 °C, introducing the solutions in an ice bath. Later, the antimicrobial KS was added to each solution in a KS:NCS or KS:ACS ratio of 0.1:1 and stirred for 30 min to equilibration. After that, NCS and ACS suspensions containing KS were freeze-dried (Martin Christ Alpha 1–4, Osterode am Harz, Germany) for 48 h (4.5 Pa, 25 °C), subsequently milled in order to obtain a powder and finally sieved through a 104 μm mesh. The described procedure lets us obtain nanostructured particles as carriers of KS based on NCS or ACS that were labeled NKSP and AKSP, respectively.
2.2.3. Films Production
Four film formulations were manufactured based on NCS and glycerol by casting technique, according to previous assays [20,22]. Briefly, NCS aqueous suspensions (4% w/w) added with glycerol (1% w/w) as a plasticizer, were prepared and subjected to heating until the starch gelatinization (85 °C). This plain film formulation was used to prepare the following four systems: (a) without KS neither particles addition, as a control system, labeled “CF”, (b) with addition of KS that was not retained in particles, called “KSF”, (c) with addition of NKSP particles containing KS, named “NKSPF” and (d) with addition of AKSP particles containing KS, named “AKSPF”. The KS, NKSP and AKSP amounts in (b), (c) and (d) were selected in order to produce films with similar KS final content (≈7.2 ± 0.9 mg/g film on average) since starch particles, NKSP and AKSP, developed distinctive capacity for antimicrobial retention [16]. All films containing KS were gently stirred after antimicrobial addition. An aliquot of each film-forming solution (20 g) was dispensed into 9-cm diameter silicon Petri dishes (Silikomart, Pianiga, Venice, Italy). Subsequently, the film-forming solutions were dehydrated in a forced air convection oven at 38–40 °C for 24 h. Once constituted, all film systems were stabilized in a 57% H.R. atmosphere and 25 °C for 15 d, prior to characterization.
2.2.4. KS Content
The preservative quantification in films was performed using the AOAC colorimetric technique (1990) [23]; performing first a steam distillation of the sample in acidic media and subsequently KS oxidation to malonaldehyde to finally quantify the red pigment formed using a spectrophotometer at a wavelength of 532 nm. The average and standard deviation of at least two determinations is reported.
2.2.5. Physicochemical Characterization of Films Based on NCS and Containing NKSP
- Microstructure of films
Scanning Electron Microscopy (SEM) was performed to analyze the microstructure of films. Samples were maintained in a desiccator with CaCl2 to remove the moisture content. All samples were mounted on a bronze stub and sputter-coated (Cressington Scientific Instruments Sputter Coater, Watford, UK) with a layer of gold prior to imaging. Micrographs of the film surface were obtained using a scanning electron microscope (Zeiss Supra 40, Oberkochen, Germany) operated at an accelerated voltage of 10 kV.
- Mechanical properties
The tensile force (N) and extension (mm) profile of the CF, KSF and NKSPF films until the moment of rupture was obtained by using a universal testing machine (Instron, USA). Tested filmstrips (60 mm length and 6 mm wide) were mounted between pneumatic grips. The initial grip separation and crosshead speed were 20 mm and 0.8 mm/s, respectively. Force and extension data were used to generate stress (σ, MPa) versus strain (ε) curves. The stress is defined as σ = F/A, with F as the force (N) and A as the cross-sectional area (mm2) of the specimen; and the strain as ε = H/L0, with H as the crosshead displacement (extension of the sample) and L0 as the initial effective length of the sample. In addition, the stress (σb) and the strain at break (εb) were registered. The film thickness was measured in triplicate using a thickness gauge (Mitutoyo, Kawasaki, Japan) with a precision of 0.001 mm. Nine specimens were tested for each sample and the main value ± standard deviation is reported.
- Moisture content
Moisture content of the films was determined by drying in a vacuum oven at 100 °C for 24 h. The result was expressed as percentage moisture on a dry basis (% b.s.).
- Solubility in water (SW)
Initial percentage of dry matter was determined by drying 2 cm diameter disks in a vacuum oven at 100 °C for 24 h. Other disks were cut, weighed, and immersed in 50 mL of distilled water, with periodic stirring, at 25 °C for 24 h. The films were taken out by filtration and dried at 100 °C for 24 h to determine the final weight of the dry matter. Solubility is reported as the difference between initial and final (after solubilization) dry matter with respect to the initial dry matter. Three specimens were tested for each sample and the main value ± standard deviation is reported.
- Color evaluation
Color measurements were performed with a photocolorimeter (Minolta, Tokyo, Japan) using a 1.5 cm aperture diameter. The films were rested on a standard white background and the color parameters L*, a*, b*, according to the International Commission of Illumination (CIELab), and the Yellowness Index (YI) [24] were determined in at least five different positions for each specimen. Color parameters range from L* = 0 (black) to L* = 100 (white), −a* (greenness) to +a* (redness), and −b* (blueness) to +b* (yellowness). Calculations were made for the illuminant D65 and 2-degree observer. Three specimens were tested for each sample.
Once the CIELab parameters were obtained, the color difference, ΔE (Delta E), was calculated using the following equations:
where L0*, a0* and b0* correspond to the CF film as the reference values.
ΔE = (ΔL* + Δa* + Δb*)1/2,
ΔL* = L* − L0*; Δa* = a* − a0*; Δb* = b* − b0*,
Additionally, the opacity of films was calculated following the technique used by Farroni [25], where the ratio between the L* value obtained with a black background and L* obtained with a white background is determined.
2.2.6. Antimicrobial Effectiveness of Films Based on NCS and Containing NKSP or AKSP
An inoculum of Zygosaccharomyces bailii (NRRL 7256), a common yeast spoilage found in foods, was prepared using Sabouraud broth (Biokar Diagnostics, CEDEX, France) until an early stationary phase was achieved (≈24 h) at 25 °C.
In order to study the effectiveness of the films as an antimicrobial barrier [26] to protect a product with high water activity (aw), a model food was formulated from Saboraud agar (Biokar Diagnostics, CEDEX, France), with an aw value around 0.98 by dextrose addition (Biopack, CABA, Argentina) and pH adjusted to 4.5 with the help of a sterile citric acid solution (Biopack, CABA, Argentina) at 50% (w/w). Disks of 1 cm in diameter of the studied films were cut aseptically, weighed, and applied on the surface of the plates containing the model food. Next, 10 µL of an inoculum of Z. bailii (105 CFU/mL) were seeded on the film disks. The systems were incubated at 25 °C for 2 days. At selected times (0, 24 and 48 h), two disks were sampled and suspended, each one in 1 mL of sterile peptone water (Biokar Diagnostics, CEDEX, France), contained in a glass tube. The microorganism was resuspended by shaking for 2 min at 2500 RPM with a vortex (Ika Works Inc., Wilmington, NC, USA). Afterward, serial dilutions in peptone water were prepared for the cell count, which was expressed as colony forming units per gram of film (CFU/g). The yeast was enumerated by surface seeding on Sabouraud agar and was incubated at 25 °C for 5 days prior to counting. The determinations were made in duplicate.
2.2.7. Fourier Transform Infrared Spectroscopy (FTIR)
FTIR spectra were obtained between 500 and 4000 cm−1 (2 cm−1 resolution) to identify the signals with minimum transmittance for KS, NKSP, and AKSP. Diffuse Reflectance Infrared Fourier Transform Spectroscopy (DRIFTS) analysis was performed on the studied samples using a spectrometer Spectrum V5.3.1 (Perkin Elmer, Inc., Waltham, MA, USA).
2.3. Statistical Analysis
The significant differences between the systems were evaluated by means of one-way analysis of variance (ANOVA) with a significance level of 95% (p < 0.05) and applying a posteriori test (LSD). Results are reported based on their average and standard deviation. The Statgraphics Centurion XV program for Windows, version 15.2.06, was used for the treatment and analysis of the data.
3. Results and Discussion
3.1. Physicochemical Characterization of Films Based on NCS and Containing NKSP or AKSP
In order to analyze the impact of NKSP and AKSP addition to film formulation on the microstructure, mechanical properties, moisture content, solubility in water, and color parameters, NKSPF and AKSPF films were tested and compared with the corresponding results for KSF (films added with KS which was not retained in particles) and CF (films without KS).
The acetylated starch used for AKSP production had a DS of 0.24 ± 0.02. The KS content determined in KSF, NKSPF and AKSPF films was 7.1 ± 0.7, 6.5 ± 0.8 and 8.0 ± 0.8 mg KS/g film, respectively, not observing significant differences (p > 0.05) between values.
3.1.1. Microstructure of Films
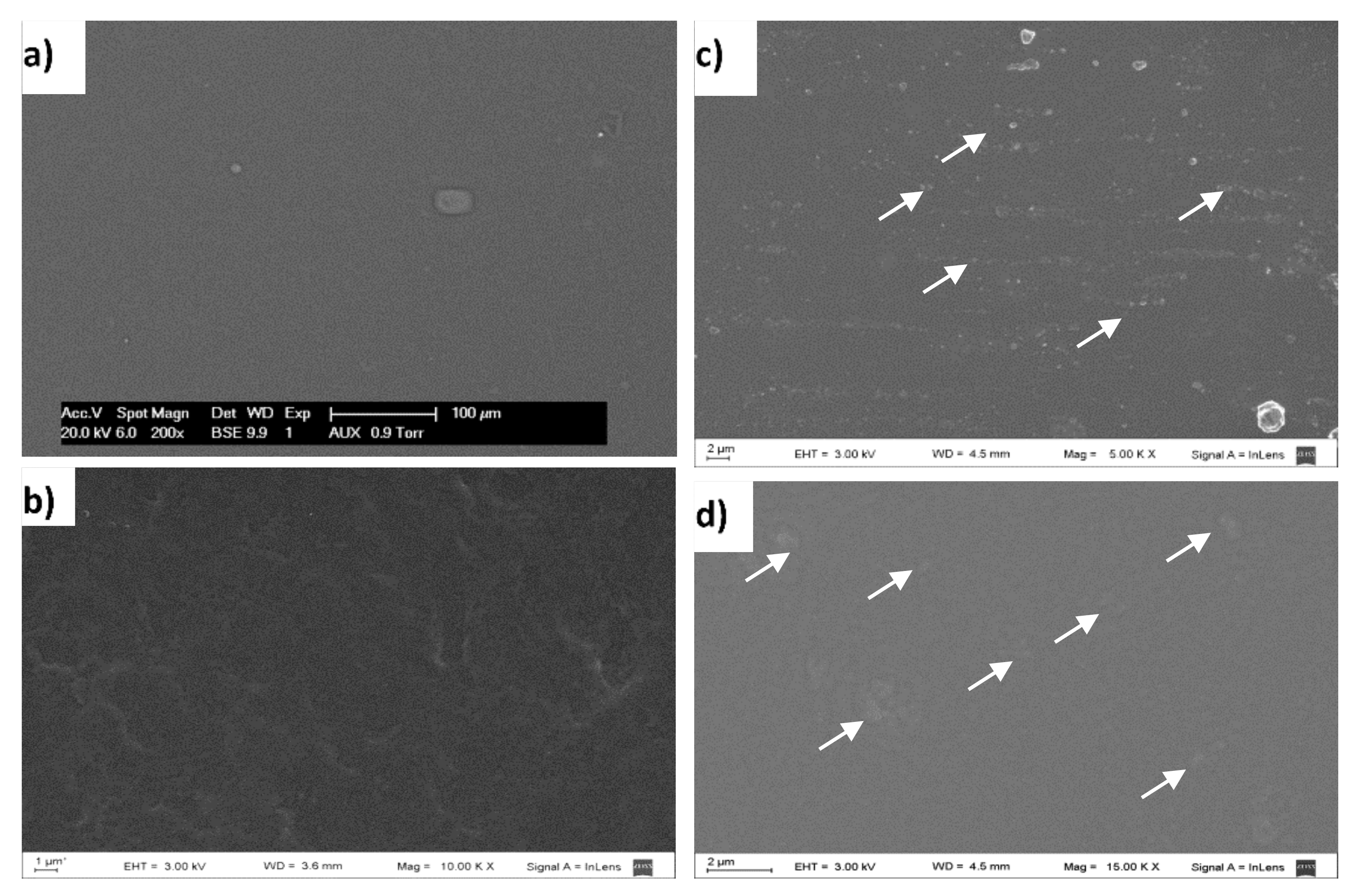
The methodology applied to produce films (casting technique), allowed us to obtain self-supported, transparent, continuous, and handleable films as seen to the naked eye. Surface microstructure of CF, KSF, NKSPF, and AKSPF films surface can be seen in SEM micrographs (Figure 1).
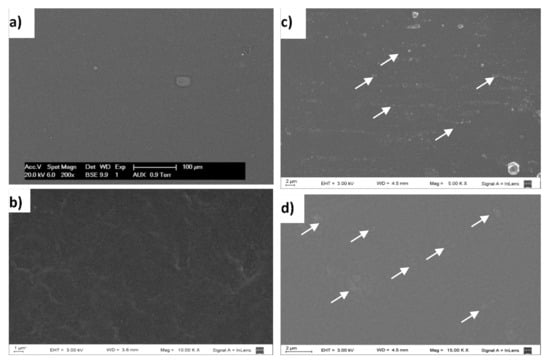
Figure 1.
SEM micrographs of the film surface: (a) CF (films without KS), magnification: 200× (b) KSF (films added with KS that was not retained in particles), magnification: 10 kX; (c) NKSPF (films added with NKSP), magnification: 5 kX; (d) AKSPF (films added with AKSP), magnification: 15 kX. Arrows point out NKSP and AKSP particles.
In general, the presence of holes or cracks in all the studied films was not observed, indicating good integrity and continuity of film matrices. Particularly, a smooth, compact and homogenous surface in CF sample was appreciated (Figure 1a). The KSF film also showed a uniform surface but with a higher roughness than CF (Figure 1b). In contrast, NKSPF and AKSPF films exhibited regularly distributed particles (Figure 1c,d). According to previous results [16], produced starch nanoparticles containing KS, rendering nanostructured particles in a powder form after freeze-drying. It could be observed in SEM images that particles were lower than 2 μm (mainly 0.5 and 1 μm for NKSPF and AKSPF, respectively) in size, showing compact round shape for NKSPF while AKSPF were irregular in shape and diffuse. In both films, particles were immersed in a continuous starch matrix (Figure 1c,d) revealing, as a consequence, a heterogeneous microstructure. Therefore, it can be inferred that NKSP and AKSP were not totally solubilized during the filmmaking process, suggesting that these nanostructured particles could act as fillers, producing a composite film. In a previous work [16], relatively low solubility in water (SW) of around 8.5% (85 °C) was reported for NKSP, which was attributed to increased interaction among KS and sonicated starch chains into NKSP that might help to prevent their susceptibility to liquid water. Such SW value could be low enough to infer a significant insolubilization of the NKSP particles in the matrix at the addition and film-forming temperatures (~25 °C and 40 °C, respectively). On the other hand, the 53.3% of AKSP mass was soluble in water [16], suggesting that the presence of acetate groups could hinder the associations among starch chains producing particles more susceptible to the aqueous film slurry. Therefore, AKSP were well integrated with the film matrix, as can be seen in the SEM micrograph (Figure 1d). Bodirlau et al. [27] prepared composite films adding modified corn starch microparticles (CSM) to glycerol plasticized-corn starch matrix and used the SEM technique to establish the filler dispersion within the films. These authors established that CSM could be dispersed without aggregation within the plasticized starch matrix.
3.1.2. Mechanical Properties
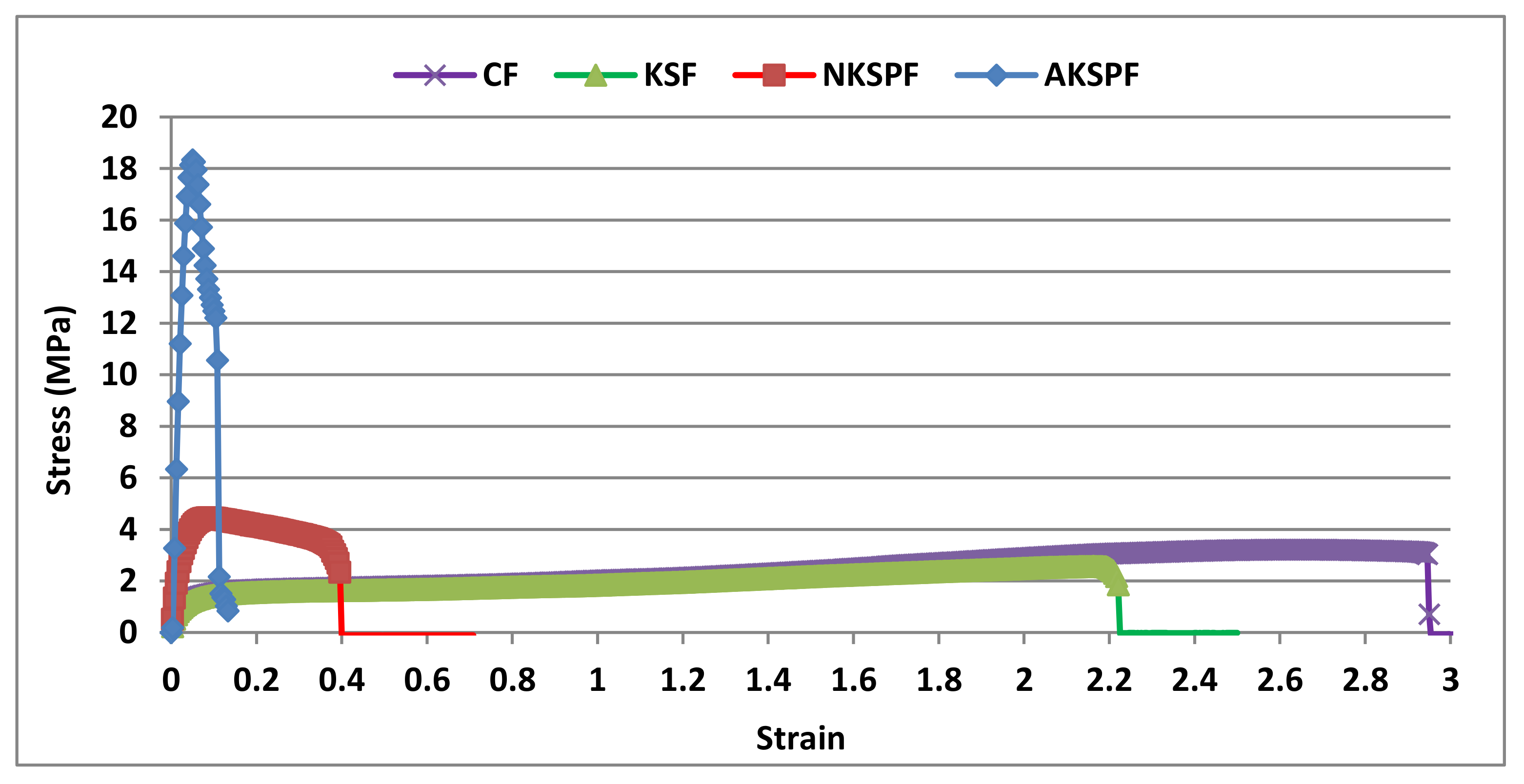
The film resistance to tensile deformation is one of the main properties to be analyzed for the evaluation of material suitability as a packaging. Figure 2 shows the stress versus strain curves of CF, KSF, NKSPF, and AKSPF films after stabilization at 25 °C and 57% R.H. A ductile profile in CF and KSF was observed, common in plasticized biopolymer matrices [20,22]. Both CF and KSF systems developed an analogous plastic deformation showing very high extensibility (≈245%) and stress at break (σb) around 2.6 MPa.
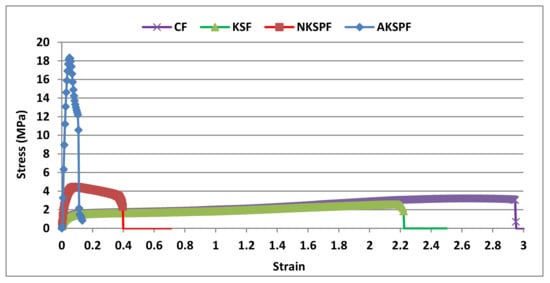
Figure 2.
Representative tensile curves for the CF (films without KS), KSF (films with KS that was not retained in particles), NKSPF (films with KS retained in NKSP) and AKSPF (films with KS retained in AKSP).
The mechanical parameters of the studied systems are summarized in Table 1. In addition, it could be seen that CF and KSF thicknesses were similar (p > 0.05) but lower (54 and 38%) than the corresponding value of NKSPF and AKSPF, respectively (p < 0.05). Thickness of films containing particles did not differ significantly (Table 1).

Table 1.
Thickness, mechanical parameters, moisture content, solubility in water and color of films: CF (without KS), KSF (added with KS that was not retained in particles), NKSPF (added with KS retained in starch nanostructured particles) and AKSPF (added with KS retained in acetylated starch nanostructured particles).
Similar Young’s Modulus (YM) and σb (p > 0.05) for CF and KSF was observed. It is well known that film formulation modulates the mechanical response, being the addition of hydrophilic small molecules responsible for a plasticizer effect together with an inferior elastic response and increased extensibility. Molecules of KS also plasticized tapioca starch films when added to the filmmaking solution at a level of 0.3% w/w [20]. Likewise, Basch et al. [22] reported a significant decrease (89%) of the YM in NCS based films added with 0.06 g KS/g starch in comparison with films without KS. In the present research, the KS added to KSF was 0.012 g KS/g starch, and was most likely not enough to modify significantly the YM. Moreover, KSF films tended to a lower moisture content value (Table 1), which may have contributed to less deformation compared to CF, due to a diminished amount of water molecules to plasticize the starch matrix.
On the contrary, NKSPF and AKSPF presented a more resistant material behavior. Indeed, curves showed a maximum stress corresponding to a first internal structural rupture (yield strength) with a posterior stress decrease until rupture [28]. A significant increase was determined (p < 0.05) in the YM parameter compared with CF and KSF (around 225%) evidencing a very higher solid character in NKSPF films. Likewise, the AKSPF developed the highest solid component with a 222% of YM increase in relation with NKSPF. Accordingly, the σb was also higher (p < 0.05) for NKSPF and AKSPF (33% and 507–580%, respectively) in relation to CF or KSF. However, strain at break (εb) was lower (86–81%) for NKSPF than CF and KSF films (p < 0.05). The same trend was observed for AKSPF systems, where εb diminished 95–96% compared with CF and KSF. Such stiffer character observed in NKSPF and AKSPF, could be attributed to the higher solids content after NKSP or AKSP addition as well as to the presence of particles (filler) as was observed in SEM micrographs (Figure 1c,d). The higher solubility of AKSP could have promoted the constitution of a stronger starch matrix for AKSPF. In addition, the good compatibility between AKSP and starch matrix, might have significantly increased the YM and σb and, at the same time, decreased the deformation in AKSPF formulation in comparison with NKSPF. Therefore, AKSPF turned out a more reinforced films than NKSPF, as tensile test revealed. Some researchers have postulated that the mechanical properties of the reinforced films are affected by both the distribution and the interactions between the particles of the filler material and the film-forming network [29]. Likewise, García et al. [30] concluded that starch nanoparticles produced by acidic hydrolysis added to cassava starch based edible film, acted as reinforcement and rendering a more rigid material. The results in the present research are similar to those reported by Jiang et al. [31] for films made with pea starch and containing nanoparticles based on potato starch in their formulation. In such work, a low value of tensile strength (TS) (8.8 MPa) and high deformation (53%) were obtained for control films (without nanoparticles) while the highest increase in TS (15 MPa) and a lower deformation (47%) were observed for films made with nanoparticles (0.06 g/g pea starch). Similarly, Bodirlau et al. [27] observed an effective reinforcing effect in corn starch-based matrices added with corn starch microparticles.
3.1.3. Solubility in Water (SW)
Solubility is a crucial parameter when defining the application of a film composed of biopolymers. Some applications may require low SW to maintain material integrity while other applications, such as encapsulation for control release, may require higher solubility [32]. In this work it was observed that the CF and KSF systems did not show significant differences (p > 0.05) in terms of SW (10.5% on average) (Table 1). However, the NKSPF and AKSPF systems increased (p < 0.05) this parameter compared to the CF and KSF. These results would be linked to the generation of a more cohesive film matrix in CF and KSF while the composite matrix of NKSPF and AKSPF might produce a discontinuous structure. According to García et al. [33], more compact films with a homogenous structure present a restrictive interaction with water molecules. Thus, SW results agree with SEM observations (Figure 1) and with the higher value of NKSPF and AKSPF thickness (Table 1), which suggest a more heterogeneous and less cohesive structure of films added with NKSP or AKSP than CF and KSF. The NKSPF and AKSPF had a SW value of the same order as those reported by Mukurumbira et al. [32] for amadumbe or potato starch films incorporated with different levels of amadumbe starch nanocrystals (SW ranged 30–35%). Contrarily, other authors [29,34] reported a decrease in SW properties when natural nanoparticles were added to biopolymeric matrices because of an enhanced interaction of filler and film network.
3.1.4. Color Evaluation
It can be observed in Table 1 that, in general, all films had similar values of L* and a* (p > 0.05), with the exception of AKSPF, which developed a slightly higher but significantly more negative value of a*, indicating a color with more a green component for this film. Regarding b* and YI parameters, CF, NKSPF, and AKSPF did not show significant differences (p > 0.05). Contrarily, the highest values were observed for the KSF film, reaching increases of 27% and 36% in relation to b* and YI mean values for CF, NKSPF, and AKSPF, respectively. These trends can also be observed through the ΔE value, which is significantly higher (p < 0.05) for KSF than NSKPF or AKSPF, mainly due to the b* increase in KSF film. The greater tendency to browning in KSF films could indicate that KS would be more exposed to oxidative degradation [35]. It could be concluded that the KS supported in native or acetylated nanostructured particles was effectively protected from the oxidation, preventing the darkness of the material. This result might also represent an advantage from the antimicrobial action point of view since the original level of preservative could be better maintained.
Even though all films were translucent, there were significant differences in the opacity values for all analyzed systems (p < 0.05). Both NKSPF and AKSPF films greatly increased this parameter around 76 to 113% and 137 to 187% compared to KSF and CF respectively, most likely because of the nanostructured particles presence. Opacity is an important parameter in food packaging since many products are affected by light. Mukurumbira et al. [32] reported that the inclusion of amadumbe starch nanoparticles increased the opacity of the films obtained from potato starch. These authors proposed that clusters of particles prevent the transmittance of light due to the promotion of the light scattering.
3.2. Antimicrobial Capacity of Films Based on NCS and Containing NKSP or AKSP
In order to study the protective action of films added with nanostructured particles containing KS (NKSPF and AKSPF), their capacity to act as a hurdle to prevent a yeast external contamination of a model food was evaluated at 25 °C. As control systems, the KSF (added with KS that was not retained in particles) and CF (without KS) films were incorporated to the assay.
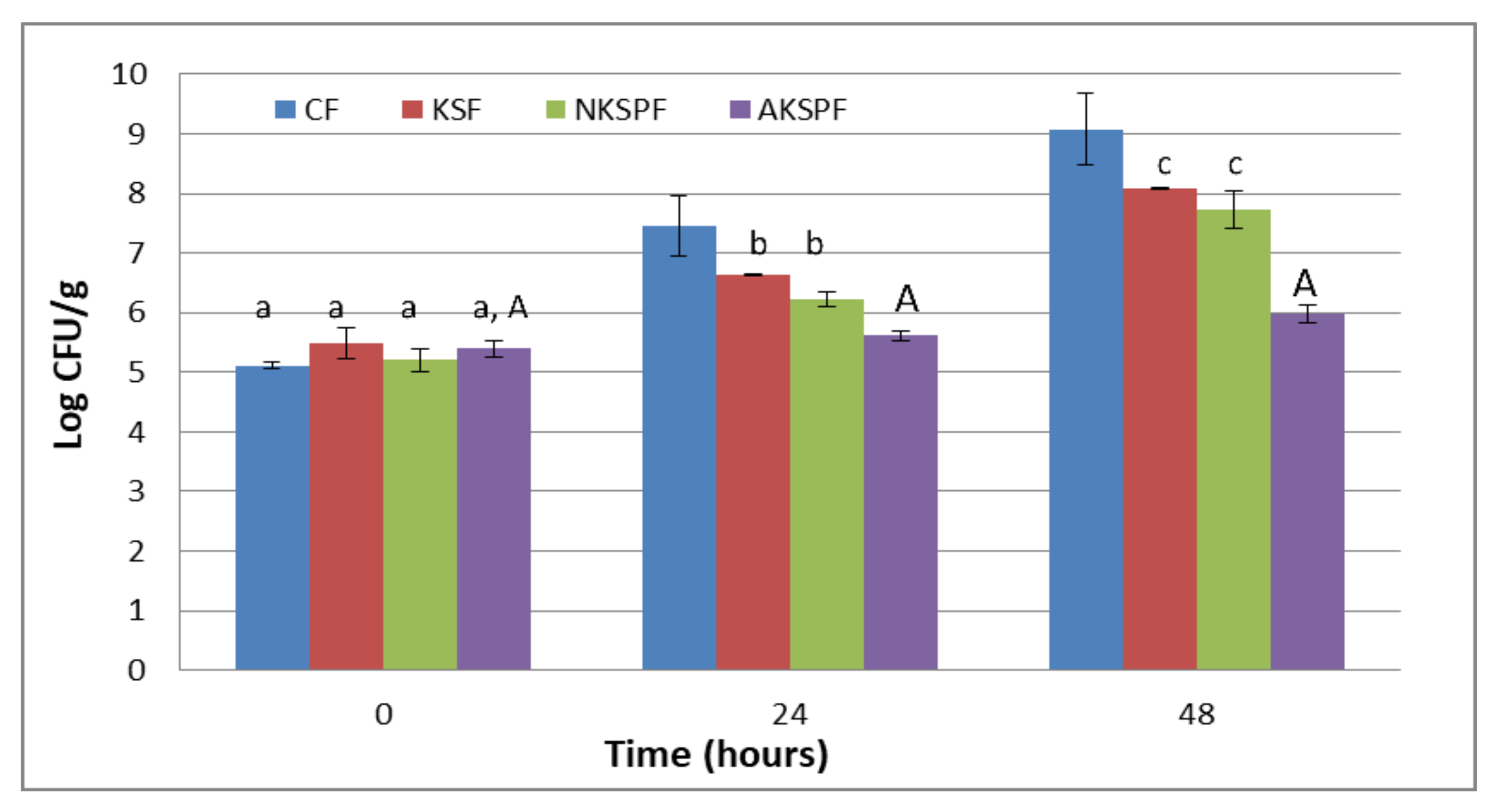
The results of the barrier test against the yeast Z. bailii for CF, KSF, NKSPF and AKSPF films are shown in Figure 3.
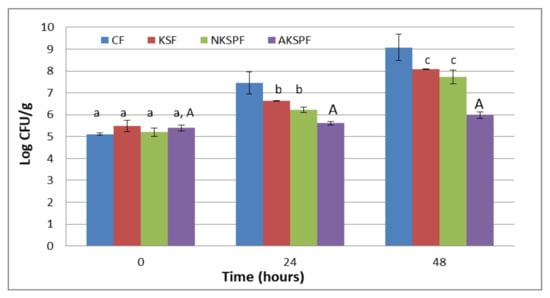
Figure 3.
Antimicrobial capacity of films (Barrier test) against Z. bailii: CF (without KS neither particles), KSF (with KS not retained in particles), NKSPF (with KS retained in NKSP particles) and AKSPF (with KS retained in AKSP particles). Same lower-case letters indicate non-significant differences among films at the same time (p > 0.05). Same capital letters indicate non-significant differences for the same film at different times (p > 0.05).
It was observed that CF allowed a continuous yeast growth reaching an increase of 4 Log cycles at the end of the storage (9.1 ± 0.6 CFU/g). On the other hand, KSF and NKSPF films reduced the growth of the Z. bailii by approximately 1 to 1.3 Log cycles compared to the CF system at 48 h (8.08 ± 0.01 CFU/g and 7.7 ± 0.3 CFU/g, respectively). However, the film added with the NKSP had a non-significant (p > 0.05) tendency to increase the inhibition of the yeast growth with respect to the KSF system throughout the test. On the contrary, AKSPF showed the best antimicrobial performance since it maintained the yeast count approximately constant along 48 h (≈5.6 CFU/g). The Z. bailii population in AKSPF was 3.1 Log cycles inferior to control film (CF) and around 1.9 Log cycles lower than KSF and NKSPF at the end of the storage.
These results indicate that the KS contained in films was available to act as an antimicrobial agent, regardless of the type of starch used for its preparation. However, in the case of AKSPF, a higher antimicrobial efficiency was observed compared to NKSPF, demonstrating better protection and availability of KS in the acetylated starch systems.
According to previous research [16], the nanostructured particles based on native or acetylated cassava starch obtained with ultrasound application, presented a reduced molecular weight (≈5–7 × 105 Da), because of the de-polymerization produced by cavitation. The resulting particles were amorphous nanostructured starches with higher amylose content (66–68% w/w) compared with original starch granules (24% w/w) before gelatinization and sonication. Such characteristics promoted an important capacity to retain KS for NKSP and AKSP, 41.5 and 90 mg KS/g, respectively, being the starch acetate the system with an increased KS retention ability. It was postulated that after ultrasound application, small fragments of amylose and amylopectin were generated with improved capacity to retain KS through hydrogen bonds and/or lipid-amylose complexes conformation, along with high surface area/volume ratio of the particles [16]. Most likely, the higher amount of single helices able to interact with sorbates (a short chain fatty acid) was developed in AKSP promoting the KS protection against oxidative degradation and negative interaction with other food components. Because of these AKSP characteristics, the AKSPF could better retain, protect, and control the release of the preservative, optimizing the KS performance in the antimicrobial assay.
It is important to mention that the preservative concentrations in films were sufficient to inhibit yeast growth in ≈1 (NKSPF) and ≈3 (AKSPF) Log cycles at 48 h with respect to the CF film, under the conditions of the assay. However, no reduction of the yeast population was observed during storage, most likely due to not having reached the minimum inhibitory concentration required on the surface, according to the conditions used in the present work. In a previous research reported by Alzate et al. [26], films based on native cassava starch with a KS level of 32.2 mg KS/g film, were able to inhibit the Z. bailii growth in 2 Log cycles after 24 h of storage at 25 °C, compared to a film without antimicrobial. It should be noted that in the mentioned research [26], the release of KS to a simulated food was reported for a similar assay, indicating that the amount of preservative in that film decreased by diffusion to the receptor medium. According to results of the present work, despite AKSPF being able to develop a higher control of the KS release than KSF and NKSPF, as was explained, it did not prevent the KS from being available to act as antimicrobial and possibly maintained a higher concentration of the preservative for longer on the surface. Therefore, the studied AKSPF is an auspicious alternative for reducing the preservative level in films matrix maintaining, at the same time, an effective antimicrobial action at the surface of food.
3.3. Analysis of Interactions by Fourier Transform Infrared Spectroscopy (FTIR)
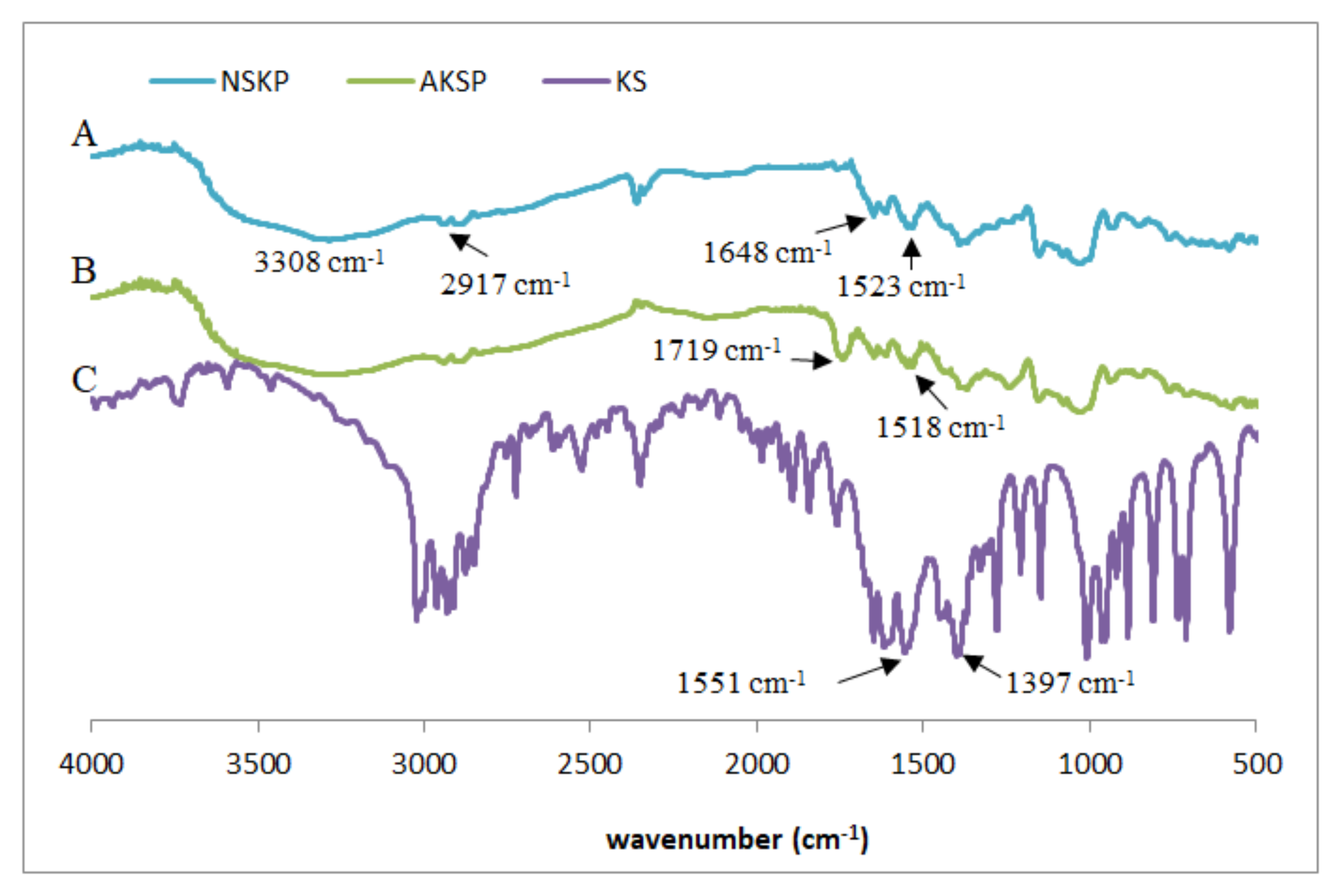
In order to analyze the existence of possible interactions between native or acetylated nanostructured starch and KS, the corresponding FTIR spectra of NKSP and AKSP were obtained (Figure 4). For comparative purposes, the KS spectrum was included.
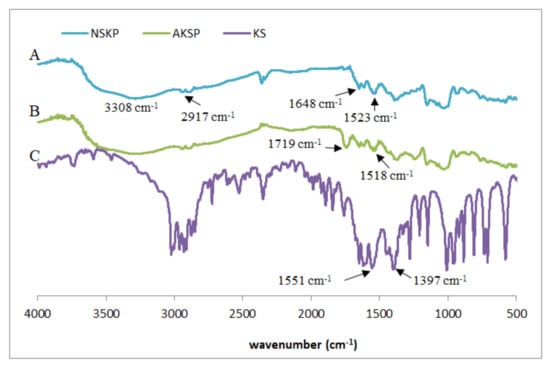
Figure 4.
FTIR spectra (A) NKSP, (B) AKSP and (C) potassium sorbate.
The FTIR spectra of NKSP and AKSP showed the expected transmission bands of starch matrixes (Figure 4, traces A and B): a broad band around 3308 cm−1 related to the stretching of the O–H groups (intra and intermolecular hydrogen bonds), a band around 2917 cm−1 assigned to the C-H bond stretching, a signal at 1648 cm−1 linked to the O-H flexing of the water in the starch (water bound to the structure) and the bands in the range from 1300 to 900 cm−1 associated to C-O and C-C stretching bonds of the anhydroglucose ring [27]. In AKSP, spectra also appeared a band at 1719 cm−1 related to the C=O stretching of the acetate ester group (Figure 4, trace B). The characteristic absorption bands of KS are linked to the carboxylate anion: a strong asymmetrical stretching of C(=O)2− near 1550 cm−1 and a weaker symmetrical stretching around 1440 cm−1 [36] (Figure 4, trace C). It is possible to observe, in both types of particles, that the signal corresponding to the asymmetric stretching of the C(=O)2− group of the KS (1551 cm−1) was shifted towards lower wavenumbers, such a shift being greater in the case of acetylated particles (1518 cm−1) compared to the native one (1523 cm−1). This displacement is an evidence of the increase in hydrogen bonds interactions between the -OH of starch and the C(=O)2− group of sorbate [37]. Flores et al. [38] also confirmed interactions between starch film matrix and KS directly added (not retained in particles) to film formulation analyzing the C(=O)2− signal in FTIR spectra but with small shift (displacement from 1559 to 1537 cm−1).
It is interesting to remark that for NKSP and AKSP spectra, most of the antimicrobial signals are not visible. Overlapping of the bands might indicate that most of the KS remained associated with the starchy matrix instead of remaining as free molecules [39]. In a similar study of FTIR spectra, El Feky et al. [40] established that an anti-inflammatory drug (indomethacin) was incorporated through H-bridge-type interactions, without evidence of new chemical bonds, to cross-linked starch nanoparticles.
4. Conclusions
Sonication and microwave-assisted acetylation of native cassava starch (NCS) has been proposed as novel and ecofriendly techniques to produce nanostructured particles useful for the KS retention. Such particles containing KS from native or acetylated starch (NKSP and AKSP, respectively) were added to biodegradable film formulations based on NCS, seeking to generate an active packaging material.
According to results, films added with NKSP and AKSP showed an improved mechanical response, reduced color changes and an increased SW, in comparison with films containing KS that was not retained in particles or films without KS. On the other hand, films added with AKSP demonstrated the highest antimicrobial action against the yeast Z. bailii, suggesting the expression of mechanisms that enhanced the interactions, protection, and performance of the preservative confined in films. Therefore, the studied AKSPF presents potentiality to be used as active packaging material for food preservation. Some possible applications of films in order to prevent external yeast contamination and increase food stability are packaging of hard and semi-hard cheeses, salty or sweet snacks made from vegetables, dividers of meat or chicken burgers and sliced ham or cheeses.
It could be concluded that both structural and chemical modification of starch matrices were relevant processes to introduce significant improvements on the physical and antimicrobial functionality of active films that can be used as an additional stress factor for the inhibition of microbial growth in foods since they constitute a suitable medium for the provision of preservative agents.
Author Contributions
Conceptualization, P.A., L.G. and S.F.; methodology, P.A. and S.F.; validation, L.G. and S.F.; formal analysis and investigation, P.A. and S.F.; resources, L.G. and S.F.; data curation, P.A.; writing—original draft preparation, P.A. and S.F.; writing—review and editing, P.A., L.G. and S.F.; visualization, P.A. and S.F.; supervision, S.F.; project administration and funding acquisition, L.G. and S.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Universidad de Buenos Aires (UBACYT 2011-2014/726, 2014–2017/550BA), Consejo Nacional de Investigaciones Científicas y Técnicas de la República Argentina (CONICET) (PIP 2010-2012/531, 2013-2015/507), Agencia Nacional de Promoción Científica y Tecnológica de la República Argentina (PICT-2012-0183, PICT 2015-2109).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article. Data analysis and replicates are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rodrigues, A.; Emeje, M. Recent applications of starch derivatives in nanodrug delivery. Carbohydr. Polym. 2012, 87, 987–994. [Google Scholar] [CrossRef]
- Bashir, K.; Aggarwal, M. Physicochemical, structural and functional properties of native and irradiated starch: A review. J. Food Sci. Technol. 2019, 56, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Eliasson, A.-C.; Gudmundsson, M. Starch: Physicochemical and Functional Aspects. In Carbohydrates in Food, 3rd ed.; Eliasson, A.-C., Ed.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar] [CrossRef]
- Yuan, C.; Thomas, D.S.; Hook, J.M.; Qin, G.; Qi, K.; Zhao, J. Molecular Encapsulation of Eucalyptus staigeriana Essential Oil by Forming Inclusion Complexes with Hydroxypropyl-β-Cyclodextrin. Food Bioprocess Technol. 2019, 12, 1264–1272. [Google Scholar] [CrossRef]
- Kong, X. Starches Modified by Nonconventional Techniques and Food Applications. In Starches for Food Application, 1st ed.; Pedrosa Silva Clerici, M.T., Schmiele, M., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 271–295. [Google Scholar] [CrossRef]
- Kaur, B.; Ariffin, F.; Bhat, R.; Karim, A. Progress in starch modification in the last decade. Food Hydrocoll. 2012, 26, 398–404. [Google Scholar] [CrossRef]
- Guarás, M.; Ludueña, L.; Álvarez, V. Development of Biodegradable Products from Modified Starches. In Starch-Based Materials in Food Packaging. Processing, Characterization and Applications, 1st ed.; Barbosa, S.E., García, M.A., Castillo, L., Lopez, O.V., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 77–124. [Google Scholar] [CrossRef]
- Otalora Gonzalez, C.; Flores, S.K.; Basanta, M.F.; Gerschenson, L. Effect of beetroot (Beta vulgaris L. var conditiva) fiber filler and corona treatment on cassava starch films properties. Food Packag. Shelf-Life 2020, 26, 100605. [Google Scholar] [CrossRef]
- Oliveira de Moraes, J.; Scheibe, A.S.; Sereno, A.; Borges, L. Scale-up of the production of cassava starch based films using tape casting. J. Food Eng. 2013, 1–36. [Google Scholar] [CrossRef]
- He, X.; Hwang, H. Nanotechnology in food science: Functionality, applicability, and safety assessment. J. Food Drug Anal. 2016, 24, 671–681. [Google Scholar] [CrossRef]
- Odeniyi, M.; Omoteso, O.; Adepoju, A.; Jaiyeoba, K. Starch nanoparticles in drug delivery: A review. Polim. Med. 2018, 48, 41–45. [Google Scholar] [CrossRef]
- Matalanis, A.; Griffith Jones, O.; McClements, D. Structured biopolymer-based delivery systems for encapsulation, protection, and release of lipophilic compounds. Food Hydrocoll. 2011, 25, 1865–1880. [Google Scholar] [CrossRef]
- Hasanvand, E.; Fathi, M.; Bassiri, A. Production and characterization of vitamin D3 loaded starch nanoparticles: Effect of amylose to amylopectin ratio and sonication parameters. J. Food Sci. Technol. 2018, 55, 1314–1324. [Google Scholar] [CrossRef]
- Chaves Da Silva, N.; Freitas de Lima, F.; Lopes Lima Fialho, R.; de Magalhães Cabral Albuquerque, E.C.; Velasco, J.I.; Matta Fakhouri, F. Production and Characterization of Starch Nanoparticles. In Applications of Modified Starches, 1st ed.; Flores Huicochea, E., Rendon, R., Eds.; IntechOpen: London, UK, 2018; pp. 41–48. [Google Scholar] [CrossRef]
- Alzate, P.; Gerschenson, L.; Flores, S. Micro/nanoparticles containing potassium sorbate obtained by the dialysis technique: Effect of starch concentration and starch ester type on the particle properties. Food Hydrocoll. 2019, 95, 540–550. [Google Scholar] [CrossRef]
- Alzate, P.; Gerschenson, L.; Flores, S. Ultrasound application for production of nano-structured particles from esterified starches to retain potassium sorbate. Carbohydr. Polym. 2020, 247, 116759. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pérez, C.; Regalado-González, C.; Rodríguez-Rodríguez, C.A.; Barbosa-Rodríguez, J.R.; Villaseñor-Ortega, F. Incorporation of antimicrobial agents in food packaging films and coatings. In Advances in Agricultural and Food Biotechnology; Guevara-González, R.G., Torres-Pacheco, I., Eds.; Research Signpost: Trivandrum, India, 2006; pp. 193–216. [Google Scholar]
- Teodoro, A.P.; Mali, S.; Romero, N.; De Carvalho, G.M. Cassava starch films containing acetylated starch nanoparticles as reinforcement: Physical and mechanical characterization. Carbohyd. Polym. 2015, 126, 9–16. [Google Scholar] [CrossRef]
- Sorrentino, A. Nanocoatings and ultra-thin films for packaging applications. In Woodhead Publishing Series in Metals and Surface Engineering, Nanocoatings and Ultra-Thin Films, 1st ed.; Hamdy Makhlouf, A.S., Tiginyanu, I., Eds.; Woodhead Publishing: Salerno, Italy, 2011; pp. 203–234. [Google Scholar] [CrossRef]
- Flores, S.; Famá, L.; Rojas, A.M.; Goyanes, S.; Gerschenson, L. Physical properties of tapioca-starch edible films: Influence of filmmaking and potassium sorbate. Food Res. Int. 2007, 40, 257–265. [Google Scholar] [CrossRef]
- ISO6647-1. Rice—Determination of Amylose Content—Part 1: Reference Method; International Organization for Standardization: Geneva, Switzerland, 2007. [Google Scholar]
- Basch, C.Y.; Jagus, R.J.; Flores, S.K. Physical and antimicrobial properties of tapioca starch-HPMC edible films incorporated with nisin and/or potassium sorbate. Food Bioproc. Technol. 2013, 6, 2419–2428. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 13th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1990. [Google Scholar]
- ASTM D1925. Standard Test Method for Yellowness Index of Plastics; American Society for Testing and Materials: Philadelphia, PA, USA, 1988. [Google Scholar]
- Farroni, A.E. Transformaciones Estructurales y Físico-Químicas de Maíces Argentinos en la Producción de Alimentos Obtenidos por Procesos de Gelatinización-Laminación. Ph.D. Thesis, University of Buenos Aires, Buenos Aires, Argentina, 2011. [Google Scholar]
- Alzate, P.; Miramont, S.; Flores, S.; Gerschenson, L. Effect of the potassium sorbate and carvacrol addition on the properties and antimicrobial activity of tapioca starch—Hydroxypropyl methylcellulose edible films. Starch/Stärke 2017, 69, 1–9. [Google Scholar] [CrossRef]
- Bodirlau, R.; Teaca, C.-A.; Spiridon, I. Influence of natural fillers on the properties of starch-based biocomposite films. Compos. Part B Eng. 2013, 44, 575–583. [Google Scholar] [CrossRef]
- Bower, D.I. An Introduction to Polymer Physics; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Sahraee, S.; Milani, J.M.; Ghanbarzadeh, B.; Hamishehkar, H.; Kafil, H.S. Physicochemical and antifungal properties of bio-nanocomposite film based on gelatin-chitin nanoparticles. Int. J. Biol. Macromol. 2017, 97, 373–381. [Google Scholar] [CrossRef] [PubMed]
- García, N.L.; Ribba, L.; Dufresne, A.; Aranguren, M.I.; Goyanes, S. Physico-mechanical properties of biodegradable starch nanocomposites. Macromol. Mater. Eng. 2009, 294, 169–177. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, C.; Wang, X.; Xiong, L.; Sun, Q. Physicochemical properties of starch nanocomposite films enhanced by self-assembled potato starch nanoparticles. LWT 2016, 69, 251–257. [Google Scholar] [CrossRef]
- Mukurumbira, A.R.; Mellem, J.J.; Amonsou, E.O. Effects of amadumbe starch nanocrystals on the physicochemical properties of starch biocomposite films. Carbohyd. Polym. 2017, 165, 142–148. [Google Scholar] [CrossRef]
- Garcia, P.S.; Grossmann, M.V.; Shirai, M.A.; Lazaretti, M.M.; Yamashita, F.; Muller, C.M.O.; Mali, S. Improving action of citric acid as compatibiliser in starch/polyester blown films. Ind. Crops Prod. 2014, 52, 305–312. [Google Scholar] [CrossRef]
- Edhirej, A.; Sapuan, S.; Jawaid, M.; Zahari, N. Cassava/sugar palm fiber reinforced cassava starch hybrid composites: Physical, thermal and structural properties. Int. J. Biol. Macromol. 2017, 101, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Gliemmo, M.F.; Campos, C.A.; Gerschenson, L.N. Effect of Sweet Humectants on Stability and Antimicrobial Action of Sorbates. JFS Food Microbiol. Saf. 2004, 69, 39–44. [Google Scholar] [CrossRef]
- Silverstein, M.R.; Bassler, G.C.; Morrill, T.C. Spectrometric Identification of Organic Compounds, 5th ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1991. [Google Scholar]
- Ma, X.; Jian, R.; Chang, P.R.; Yu, J. Fabrication and Characterization of Citric Acid-Modified Starch Nanoparticles/Plasticized-Starch Composites. Biomacromolecules 2008, 9, 3314–3320. [Google Scholar] [CrossRef] [PubMed]
- Flores, S.; Haedo, A.S.; Campos, C.; Gerschenson, L. Antimicrobial performance of sorbates supported in a tapioca starch edible film. Eur. Food Res. Technol. 2007, 225, 375–384. [Google Scholar] [CrossRef]
- Acevedo-Guevara, L.; Nieto-Suaza, L.; Sanchez, L.; Pinzon, M.; Villa, C. Development of native and modified banana starch nanoparticles as vehicles for curcumin. Int. J. Biol. Macromol. 2018, 111, 498–504. [Google Scholar] [CrossRef]
- El-Feky, G.; El-Rafle, M.; El-Sheikh, M.; El-Naggar, M.; Hebeish, A. Utilization of crosslinked starch nanoparticles as a carrier for indomethacin and acyclovir drugs. J. Nanomed. Nanotechnol. 2015, 6, 254. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).