Magnetic Nanoparticles: Current Advances in Nanomedicine, Drug Delivery and MRI
Abstract
:1. Introduction
2. Justification and Design Strategies for Magnetic Nanoplatforms
2.1. Synthetic Strategies and Feedback-Driven Design
2.2. Physical Characterization
2.3. MNPs with Improved Magnetic Properties: Substitution/Doping Effect
2.4. Flow Characteristics and Simulated Models for MNPs in Biologic Fluids and High-Performance Ferrofluids
2.5. Morphology—Role of Size and Shape
2.6. Intra- and Interparticle Interactions: Colloidal Stability and Size Differentiation
3. Coating and Surface Functionalization of MNPs: Polymers, Acids, Amines, Siloxanes, Other Coatings
4. MR Imaging
4.1. Radiolabeling
4.2. SARS-CoV-2 and MRI with SPIONs
4.3. Functionalization Agents and Strategies
5. Therapeutic Features
5.1. Drug Delivery
5.2. Cell Drug Uptake
5.3. Hyperthermia
5.4. Hypoxia
5.5. MDT: Magnetic Drug Targeting
5.6. On-Demand Drug Release
5.7. Apoptosis
6. Biocompatibility and Toxicity
6.1. Cytotoxicity
6.2. NPs Accumulation in Tissue: The Fate of MNPs in Biological Systems
7. Conclusions and Outlook
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fadli, A.; Amri, A.; Sari, E.O.; Sukoco, S.; Saprudin, D. Superparamagnetic nanoparticles with mesoporous structure prepared through hydrothermal technique. Mater. Sci. Forum 2020, 1000, 203–209. [Google Scholar] [CrossRef]
- Ghaemi, A.; Mohave, F.; Farhadi, A.; Takassi, M.A.; Tavakkoli, H. Hydrothermal synthesis of mesoporous cobalt ferrite by ionic liquid-assisted process; catalytic performance, morphology, and magnetic studies. J. Aust. Ceram. Soc. 2021, 57, 1321–1330. [Google Scholar] [CrossRef]
- Tombuloglu, H.; Khan, F.A.; Almessiere, M.A.; Aldakheel, S.; Baykal, A. Synthesis of niobium substituted cobalt-nickel nano-ferrite (Co0.5Ni0.5NbxFe2−xO4 (x ≤ 0.1) by hydrothermal approach show strong anti-colon cancer activities. J. Biomol. Struct. Dyn. 2021, 39, 2257–2265. [Google Scholar] [CrossRef] [PubMed]
- Panda, J.; Das, S.; Kumar, S.; Tudu, B.; Sarkar, R. Investigation of antibacterial, antioxidant, and anticancer properties of hydrothermally synthesized cobalt ferrite nanoparticles. Appl. Phys. A Mater. Sci. Process. 2022, 128, 562. [Google Scholar] [CrossRef]
- Albornoz, C.A.; Paulin, M.A.; Cristóbal, A.A.; Vega, D.R.; Leyva, A.G.; Ramos, C.P. Microwave-assisted hydrothermal nanoarchitectonics of polyethyleneimine-coated iron oxide nanoparticles. Appl. Phys. A Mater. Sci. Process. 2022, 128, 68. [Google Scholar] [CrossRef]
- Fayazzadeh, S.; Khodaei, M.; Arani, M.; Mahdavi, S.R.; Nizamov, T.; Majouga, A. Magnetic Properties and Magnetic Hyperthermia of Cobalt Ferrite Nanoparticles Synthesized by Hydrothermal Method. J. Supercond. Nov. Magn. 2020, 33, 2227–2233. [Google Scholar] [CrossRef]
- Park, Y.; Yoon, H.J.; Lee, S.E.; Lee, L.P. Multifunctional Cellular Targeting, Molecular Delivery, and Imaging by Integrated Mesoporous-Silica with Optical Nanocrescent Antenna: MONA. ACS Nano 2022, 16, 2013–2023. [Google Scholar] [CrossRef]
- Wang, F.; Qi, X.; Geng, J.; Liu, X.; Li, D.; Zhang, H.; Zhang, P.; He, X.; Li, B.; Li, Z.; et al. Template-free construction of hollow mesoporous Fe3O4 nanospheres as controlled drug delivery with enhanced drug loading capacity. J. Mol. Liq. 2022, 347, 118000. [Google Scholar] [CrossRef]
- Gao, Y.; Shi, X.; Shen, M. Intelligent Design of Ultrasmall Iron Oxide Nanoparticle-Based Theranostics. ACS Appl. Mater. Interfaces 2021, 13, 45119–45129. [Google Scholar] [CrossRef]
- Saputra, O.A.; Wibowo, F.R.; Lestari, W.W.; Handayani, M. Highly monodisperse and colloidal stable of L-serine capped magnetite nanoparticles synthesized via sonochemistry assisted co-precipitation method. Adv. Nat. Sci. Nanosci. Nanotechnol. 2020, 11, 025012. [Google Scholar] [CrossRef]
- Kafi-Ahmadi, L.; Khademinia, S.; Nansa, M.N.; Alemi, A.A.L.I.; Mahdavi, M.; Marjani, A.P. Co-precipitation synthesis, characterization of CoFe2O4 nanomaterial and evaluation of its toxicity behavior on human leukemia cancer K562 cell line. J. Chil. Chem. Soc. 2020, 65, 4845–4848. [Google Scholar] [CrossRef]
- Tadic, M.; Lazovic, J.; Panjan, M.; Kralj, S. Hierarchical iron oxide nanocomposite: Bundle-like morphology, magnetic properties and potential biomedical application. Ceram. Int. 2022, 48, 16015–16022. [Google Scholar] [CrossRef]
- Porrawatkul, P.; Nuengmatcha, P.; Tangwatanakul, W.; Chanthai, S. Effect of Zn, Ni, and Mn doping ions on magnetic properties of MFe2O4 (M = Mn, Zn, and Ni) nanoparticles synthesized via sol–gel autocombustion using PVA/sago starch blend as a chelating agent. J. Korean Ceram. Soc. 2020, 57, 676–683. [Google Scholar] [CrossRef]
- Qayoom, M.; Bhat, R.; Shah, K.A.; Pandit, A.H.; Firdous, A.; Dar, G.N. Modified Solution Combustion Synthesis of Nickel-Doped Magnetite Nanoparticles and the Influence of Annealing on Their Optical, Electrical, and Magnetic Properties. J. Electron. Mater. 2020, 49, 1215–1229. [Google Scholar] [CrossRef]
- Haydar, M.S.; Das, D.; Ghosh, S.; Mandal, P. Implementation of mature tea leaves extract in bioinspired synthesis of iron oxide nanoparticles: Preparation, process optimization, characterization, and assessment of therapeutic potential. Chem. Pap. 2022, 76, 491–514. [Google Scholar] [CrossRef]
- Mangamma, J.L.; Devi, D.R.; Sagar, P.S.R.V.; Babu, M.R.; Basavaiah, K. Review on Plant Mediated Green Synthesis of Magnetite Nanoparticles for Pollution Abatement, Biomedical and Electronic Applications. Asian J. Chem. 2022, 34, 1047–1054. [Google Scholar] [CrossRef]
- Loiola, A.R.; Bessa, R.A.; Oliveira, C.P.; Freitas, A.D.L.; Soares, S.A.; Bohn, F.; Pergher, S.B.C. Magnetic zeolite composites: Classification, synthesis routes, and technological applications. J. Magn. Magn. Mater. 2022, 560, 169651. [Google Scholar] [CrossRef]
- Bertran, A.; Sandoval, S.; Oro-Sole, J.; Sanchez, A.; Tobias, G. Particle size determination from magnetization curves in reduced graphene oxide decorated with monodispersed superparamagnetic iron oxide nanoparticles. J. Colloid Interface Sci. 2020, 566, 107–119. [Google Scholar] [CrossRef]
- Harada, M.; Kuwa, M.; Sato, R.; Teranishi, T.; Takahashi, M.; Maenosono, S. Cation Distribution in Monodispersed MFe2O4 (M = Mn, Fe, Co, Ni, and Zn) Nanoparticles Investigated by X-ray Absorption Fine Structure Spectroscopy: Implications for Magnetic Data Storage, Catalysts, Sensors, and Ferrofluids. ACS Appl. Nano Mater. 2020, 3, 8389–8402. [Google Scholar] [CrossRef]
- Wahba, A.M.; Mohamed, M.B. Correlating cation distribution with the structural and magnetic properties of Co0.5Zn0.5AlxFe2–xO4 nanoferrites. Appl. Phys. A Mater. Sci. Process. 2020, 126, 488. [Google Scholar] [CrossRef]
- Palade, P.; Comanescu, C.; Kuncser, A.; Berger, D.; Matei, C.; Iacob, N.; Kuncser, V. Mesoporous Cobalt Ferrite Nanosystems Obtained by Surfactant-Assisted Hydrothermal Method: Tuning Morpho-structural and Magnetic Properties via pH-Variation. Nanomaterials 2020, 10, 476. [Google Scholar] [CrossRef] [PubMed]
- Orozco-Henao, J.M.; Muraca, D.; Sánchez, F.H.; Mendoza Zélis, P. Determination of the effective anisotropy of magnetite/maghemite nanoparticles from Mössbauer effect spectra. J. Phys. D Appl. Phys. 2022, 55, 335302. [Google Scholar] [CrossRef]
- Kahmann, T.; Ludwig, F. Magnetic field dependence of the effective magnetic moment of multi-core nanoparticles. J. Appl. Phys. 2020, 127, 233901. [Google Scholar] [CrossRef]
- Wang, S.; Xu, J.; Li, W.; Sun, S.; Gao, S.; Hou, Y. Magnetic Nanostructures: Rational Design and Fabrication Strategies toward Diverse Applications. Chem. Rev. 2022, 122, 5411–5475. [Google Scholar] [CrossRef] [PubMed]
- Pyatakov, A.; Pyatakova, Z.; Tishin, A.M. Short history overview of magnetism and magnetic technologies for medical applications. In Materials and Technologies for Medical Applications; Woodhead Publishing: Cambridge, UK, 2022; pp. 3–21. [Google Scholar] [CrossRef]
- Krasia-Christoforou, T.; Socoliuc, V.; Knudsen, K.D.; Tombácz, E.; Turcu, R.; Vekas, L. From single-core nanoparticles in ferrofluids to multi-core magnetic nanocomposites: Assembly strategies, structure and magnetic behavior. Nanomaterials 2020, 10, 2178. [Google Scholar] [CrossRef]
- Scanone, A.C.; Gsponer, N.S.; Alvarez, M.G.; Heredia, D.A.; Durantini, A.M.; Durantini, E.N. Magnetic Nanoplatforms for in Situ Modification of Macromolecules: Synthesis, Characterization, and Photoinactivating Power of Cationic Nanoiman–Porphyrin Conjugates. ACS Appl. Bio Mater. 2020, 3, 5930–5940. [Google Scholar] [CrossRef]
- Shi, Y.; Jyoti, D.; Gordon-Wylie, S.W.; Weaver, J.B. Quantification of magnetic nanoparticles by compensating for multiple environment changes simultaneously. Nanoscale 2020, 12, 195–200. [Google Scholar] [CrossRef]
- Papadopoulos, C.; Efthimiadou, E.K.; Pissas, M.; Fuentes, D.; Boukos, N.; Psycharis, V.; Kordas, G.; Loukopoulos, V.C.; Kagadis, G.C. Magnetic fluid hyperthermia simulations in evaluation of SAR calculation methods. Phys. Med. 2020, 71, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Honecker, D.; Bersweiler, M.; Erokhin, S.; Berkov, D.; Chesnel, K.; Venero, D.A.; Qdemat, A.; Disch, S.; Jochum, J.K.; Michels, A.; et al. Using small-angle scattering to guide functional magnetic nanoparticle design. Nanoscale Adv. 2022, 4, 1026–1059. [Google Scholar] [CrossRef]
- Priyadarshi, H.; Gaur, U. Paramagnetism-enhanced doped maghemite nanoparticles for targeted drug delivery. Mater. Today Proc. 2021, 43, 3030–3033. [Google Scholar] [CrossRef]
- Pardo, A.; Pelaz, B.; Gallo, J.; Bañobre-López, M.; Parak, W.J.; Barbosa, S.; Del Pino, P.; Taboada, P. Synthesis, Characterization, and Evaluation of Superparamagnetic Doped Ferrites as Potential Therapeutic Nanotools. Chem. Mater. 2020, 32, 2220–2231. [Google Scholar] [CrossRef]
- Dhillon, G.; Kumar, N.; Chitkara, M.; Sandhu, I.S. Effect of Gd3+ substitution on physicochemical properties of superparamagnetic Fe3O4 nanoparticles. J. Mater. Sci. Mater. Electron. 2021, 32, 22387–22397. [Google Scholar] [CrossRef]
- Saini, A.; Borchers, J.A.; George, S.; Maranville, B.B.; Krycka, K.L.; Dura, J.A.; Theis-Bröhl, K.; Wolff, M. Layering of Magnetic Nanoparticles at Amorphous Magnetic Templates with Perpendicular Anisotropy. Soft Matter 2020, 16, 7676. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Zhao, J.; Deng, Y.; Zeng, G.; Jiang, Y.; Liao, L.; Zhang, S.; Tao, Q.; Liu, Z.; Tang, X.; et al. Application of iron/barium ferrite/carbon-coated iron nanocrystal composites in transcatheter arterial chemoembolization of hepatocellular carcinoma. J. Colloid Interface Sci. 2021, 601, 30–41. [Google Scholar] [CrossRef]
- Hölscher, J.; Petrecca, M.; Albino, M.; Garbus, P.G.; Saura-Múzquiz, M.; Sangregorio, C.; Christensen, M. Magnetic Property Enhancement of Spinel Mn-Zn Ferrite through Atomic Structure Control. Inorg. Chem. 2020, 59, 11184–11192. [Google Scholar] [CrossRef]
- Riaz, H.; Hashmi, R.; Abid, S.; Shareef, N.; Faqir, A.; Amir, A.; Shahzad, M.S.; Shakeel, M.; Akhtar, S.; Ashiq, M.N.; et al. Intraperitoneal injections of copper ferrite nanoparticles disturb blood, plasma, and antioxidant parameters of Wistar rats in a sex-specific manner. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 2019–2028. [Google Scholar] [CrossRef]
- Sarkar, B.J.; Bandyopadhyay, A. Studies of magnetic behavior of chemically synthesized interacting superparamagnetic copper ferrite nanoparticles. J. Mater. Sci. Mater. Electron. 2021, 32, 1491–1505. [Google Scholar] [CrossRef]
- Manju, B.G.; Raji, P. Green synthesis, characterization, and antibacterial activity of lime-juice-mediated copper–nickel mixed ferrite nanoparticles. Appl. Phys. A Mater. Sci. Process. 2020, 126, 156. [Google Scholar] [CrossRef]
- Althubayti, M.M.; Hjiri, M.; Alonizan, N.H.; Lemine, O.M.; Aida, M.S. Influence of divalent metals (Zn, Cu and Co) on the synthesis and magnetic properties of spinel ferrite nanopowders. J. Mater. Sci. Mater. Electron. 2020, 31, 8194–8205. [Google Scholar] [CrossRef]
- Silvestri, N.; Gavilán, H.; Guardia, P.; Brescia, R.; Fernandes, S.; Samia, A.C.S.; Teran, F.J.; Pellegrino, T. Di- and tri-component spinel ferrite nanocubes: Synthesis and their comparative characterization for theranostic applications. Nanoscale 2021, 13, 13665–13680. [Google Scholar] [CrossRef]
- Hossain, M.D.; Khan, M.N.I.; Nahar, A.; Ali, M.A.; Matin, M.A.; Hoque, S.M.; Hakim, M.A.; Jamil, A.T.M.K. Tailoring the properties of Ni-Zn-Co ferrites by Gd3+ substitution. J. Magn. Magn. Mater. 2020, 497, 165978. [Google Scholar] [CrossRef]
- Vangijzegem, T.; Stanicki, D.; Panepinto, A.; Socoliuc, V.; Vekas, L.; Muller, R.N.; Laurent, S. Influence of Experimental Parameters of a Continuous Flow Process on the Properties of Very Small Iron Oxide Nanoparticles (VSION) Designed for T1-Weighted Magnetic Resonance Imaging (MRI). Nanomaterials 2020, 10, 757. [Google Scholar] [CrossRef] [PubMed]
- Morillas, J.R.; de Vicente, J. Magnetorheology: A review. Soft Matter 2020, 16, 9614–9642. [Google Scholar] [CrossRef] [PubMed]
- Zablotsky, D.; Kralj, S.; Maiorov, M.M. Features of magnetorheology of biocompatible chain-forming ferrofluids with multi-core magnetic nanoparticles: Experiment and simulation. Colloids Surf. A 2020, 603, 125079. [Google Scholar] [CrossRef]
- Craciunescu, I.; Chiţanu, E.; Codescu, M.M.; Iacob, N.; Kuncser, A.; Kuncser, V.; Socoliuc, V.; Susan-Resiga, D.; Bălănean, F.; Ispas, G.; et al. High performance magnetorheological fluids: Very high magnetization FeCo-Fe3O4 nanoclusters in ferrofluid carrier. Soft Matter 2022, 18, 626–639. [Google Scholar] [CrossRef]
- Lu, Q.; Choi, K.; Nam, J.-D.; Choi, H.J. Magnetic Polymer Composite Particles: Design and Magnetorheology. Polymers 2021, 13, 512. [Google Scholar] [CrossRef]
- Willis, A.J.; Pernal, S.P.; Gaertner, Z.A.; Lakka, S.S.; Sabo, M.E.; Creighton, F.M.; Engelhard, H.H. Rotating magnetic nanoparticle clusters as microdevices for drug delivery. Int. J. Nanomed. 2020, 15, 4105–4123. [Google Scholar] [CrossRef]
- Esfe, M.H.; Afrand, M.; Esfandeh, S. Investigation of the effects of various parameters on the natural convection of nanofluids in various cavities exposed to magnetic fields: A comprehensive review. J. Therm. Anal. Calorim. 2020, 140, 2055–2075. [Google Scholar] [CrossRef]
- Ma, W.; Xu, M.; Zhong, Z.; Li, X.; Huan, Z. Closed-Loop Control for Trajectory Tracking of a Microparticle Based on Input-to-State Stability Through an Electromagnetic Manipulation System. IEEE Access 2020, 8, 46537–46545. [Google Scholar] [CrossRef]
- van Silfhout, A.M.; Engelkamp, H.; Erné, B.H. Magnetic Sedimentation Velocities and Equilibria in Dilute Aqueous Ferrofluids. J. Phys. Chem. B 2020, 124, 7989–7998. [Google Scholar] [CrossRef]
- Socoliuc, V.; Turcu, R. Large scale aggregation in magnetic colloids induced by high frequency magnetic fields. J. Magn. Magn. Mater. 2020, 500, 166348. [Google Scholar] [CrossRef]
- Ganguly, S.; Margel, S. Review: Remotely controlled magneto-regulation of therapeutics from magnetoelastic gel matrices. Biotechnol. Adv. 2020, 44, 107611. [Google Scholar] [CrossRef] [PubMed]
- Podoliak, N.; Richardson, G. Correctly computing targeting efficiency in magnetically targeted delivery from particle tracking models. J. Magn. Magn. Mater. 2022, 549, 168960. [Google Scholar] [CrossRef]
- Russo, T.; Peluso, V.; Gloria, A.; Oliviero, O.; Rinaldi, L.; Improta, G.; de Santis, R.; D’Antò, V. Combination Design of Time-Dependent Magnetic Field and Magnetic Nanocomposites to Guide Cell Behavior. Nanomaterials 2020, 10, 577. [Google Scholar] [CrossRef]
- Bhatt, A.; Sakai, K.; Madhyastha, R.; Murayama, M.; Madhyastha, H.; Rath, S.N. Biosynthesis and characterization of nano magnetic hydroxyapatite (nMHAp): An accelerated approach using simulated body fluid for biomedical applications. Ceram. Int. 2020, 46, 27866–27876. [Google Scholar] [CrossRef]
- Vijayakanth, V.; Chintagumpala, K. A review on an effect of dispersant type and medium viscosity on magnetic hyperthermia of nanoparticles. Polym. Bull. 2022. [Google Scholar] [CrossRef]
- Zuluaga-Parra, J.D.; Sánchez-Valdés, S.; Ramos-deValle, L.F.; Beltrán-Ramírez, F.I.; da-Silva, L.; Ramírez-Vargas, E.; Vázquez-Rodríguez, S.; Flores-Gallardo, S.; Méndez-Nonell, J.; Valera-Zaragoza, M.; et al. A novel method for the modification of magnetite nanoparticles for the enhancement of its dispersibility in hydrophobic media. J. Magn. Magn. Mater. 2020, 514, 167169. [Google Scholar] [CrossRef]
- Park, Y.; Cho, H. Improvement in the dispersion stability of iron oxide nanoparticles in highly concentrated brine solution using encapsulation with polymer-polymer crosslinked shells. Adv. Powder Technol. 2020, 31, 4743–4750. [Google Scholar] [CrossRef]
- Roa-Barrantes, L.M.; Patarroyo, D.J.R. Magnetic Field Effect on the Magnetic Nanoparticles Trajectories in Pulsating Blood Flow: A Computational Model. BioNanoScience 2022, 12, 571–581. [Google Scholar] [CrossRef]
- Salem, S.F.; Tuchin, V.V. Numerical simulation of magnetic nanoparticles in the blood stream. In Proceedings of the Saratov Fall Meeting 2019: Optical and Nano-Technologies for Biology and Medicine, Saratov, Russia, 23–27 September 2019; Volume 11457, p. 114571N. [Google Scholar] [CrossRef]
- Badfar, H.; Motlagh, S.Y.; Sharifi, A. Numerical Simulation of Magnetic Drug Targeting to the Stenosis Vessel Using Fe3O4 Magnetic Nanoparticles Under the Effect of Magnetic Field of Wire. Cardiovasc. Eng. Technol. 2020, 11, 162–175. [Google Scholar] [CrossRef]
- Kreissl, P.; Holm, C.; Weeber, R. Frequency-dependent magnetic susceptibility of magnetic nanoparticles in a polymer solution: A simulation study. Soft Matter 2021, 17, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-H.; Hsu, M.-H.; Lee, K.-H.; Chen, W.-Y.; Yang, S.-Y. Real-time changes in the AC magnetic susceptibility of reagents during immunomagnetic reduction assays. AIP Adv. 2022, 12, 065220. [Google Scholar] [CrossRef]
- Labbafzadeh, M.R.; Vakili, M.H. Application of magnetic electrospun polyvinyl alcohol/collagen nanofibres for drug delivery systems. Mol. Simul. 2022, 48, 1–7. [Google Scholar] [CrossRef]
- Harris, R.A. Simulation study on the physicochemical properties of Fe3O4 nanoparticles as drug delivery vehicles for dopamine replacement therapy of Parkinson’s disease. Mater. Today Commun. 2022, 31, 103829. [Google Scholar] [CrossRef]
- Cai, J.; Dao, P.; Chen, H.; Yan, L.; Li, Y.L.; Zhang, W.; Li, L.; Du, Z.; Dong, C.-Z.; Meunier, B. Ultrasmall superparamagnetic iron oxide nanoparticles-bound NIR dyes: Novel theranostic agents for Alzheimer’s disease. Dye Pigment. 2020, 173, 107968. [Google Scholar] [CrossRef]
- Sadeghi-Goughari, M.; Jeon, S.; Kwon, H.-J. Magnetic nanoparticles-enhanced focused ultrasound heating: Size effect, mechanism, and performance analysis. Nanotechnology 2020, 31, 245101. [Google Scholar] [CrossRef]
- Contreras-Montoya, R.; Jabalera, Y.; Blanco, V.; Cuerva, J.M.; Jimenez-Lopez, C.; Alvarez De Cienfuegos, L. Lysine as Size-Control Additive in a Bioinspired Synthesis of Pure Superparamagnetic Magnetite Nanoparticles. Cryst. Growth Des. 2020, 20, 533–542. [Google Scholar] [CrossRef]
- Craciunescu, I.; Palade, P.; Iacob, N.; Ispas, G.M.; Stanciu, A.E.; Kuncser, V.; Turcu, R.P. High Performance Functionalized Magnetic Nanoparticles with Tailored Sizes and Shapes for Localized Hyperthermia Applications. J. Phys. Chem. C 2021, 125, 11132–11146. [Google Scholar] [CrossRef]
- Islam, K.; Haque, M.; Kumar, A.; Hoq, A.; Hyder, F.; Hoque, S.M. Manganese ferrite nanoparticles (MnFe2O4): Size dependence for hyperthermia and negative/positive contrast enhancement in MRI. Nanomaterials 2020, 10, 2297. [Google Scholar] [CrossRef]
- Malekie, S.; Rajabi, A. Study on Fe3O4 Magnetic Nanoparticles Size Effect on Temperature Distribution of Tumor in Hyperthermia: A Finite Element Method. Int. J. Nanosci. Nanotechnol. 2020, 16, 181–188. [Google Scholar]
- Bilmez, B.; Toker, M.Ö.; Toker, O.; İçelli, O. Monte Carlo study on size-dependent radiation enhancement effects of spinel ferrite nanoparticles. Radiat. Phys. Chem. 2022, 199, 110364. [Google Scholar] [CrossRef]
- Das, R.; Masa, J.; Kalappattil, V.; Nemati, Z.; Rodrigo, I.; Garaio, E.; García, J.; Phan, M.-H.; Srikanth, H. Iron Oxide Nanorings and Nanotubes for Magnetic Hyperthermia: The Problem of Intraparticle Interactions. Nanomaterials 2021, 11, 1380. [Google Scholar] [CrossRef] [PubMed]
- van Silfhout, A.M.; Engelkamp, H.; Erné, B.H. Colloidal Stability of Aqueous Ferrofluids at 10 T. J. Phys. Chem. Lett. 2020, 11, 5908–5912. [Google Scholar] [CrossRef] [PubMed]
- Pilati, V.; Gomide, G.; Gomes, R.C.; Goya, G.F.; Depeyrot, J. Colloidal Stability and Concentration Effects on Nanoparticle Heat Delivery for Magnetic Fluid Hyperthermia. Langmuir 2021, 37, 1129–1140. [Google Scholar] [CrossRef] [PubMed]
- Castro, L.L.; Amorim, C.C.C.; Miranda, J.P.V.; Cassiano, T.S.A.; Paula, F.L.O. The role of small separation interactions in ferrofluid structure. Colloids Surf. A 2022, 635, 128082. [Google Scholar] [CrossRef]
- Riedl, J.C.; Sarkar, M.; Fiuza, T.; Cousin, F.; Depeyrot, J.; Dubois, E.; Mériguet, G.; Perzynski, R.; Peyre, V. Design of concentrated colloidal dispersions of iron oxide nanoparticles in ionic liquids: Structure and thermal stability from 25 to 200 °C. J. Colloid Interface Sci. 2022, 607, 584–594. [Google Scholar] [CrossRef]
- Boskovic, M.; Fabián, M.; Vranjes-Djuric, S.; Antic, B. Magnetic nano- and micro-particles based on Gd-substituted magnetite with improved colloidal stability. Appl. Phys. A Mater. Sci. Process. 2021, 127, 372. [Google Scholar] [CrossRef]
- Aguilar, N.M.; Perez-Aguilar, J.M.; González-Coronel, V.J.; Moro, J.G.S.; Sanchez-Gaytan, B.L. Polymers as Versatile Players in the Stabilization, Capping, and Design of Inorganic Nanostructures. ACS Omega 2021, 6, 35196–35203. [Google Scholar] [CrossRef]
- Mahin, J.; Franck, C.O.; Fanslau, L.; Patra, H.K.; Mantle, M.D.; Fruk, L.; Torrente-Murciano, L. Green, scalable, low cost and reproducible flow synthesis of biocompatible PEG-functionalized iron oxide nanoparticles. React. Chem. Eng. 2021, 6, 1961–1973. [Google Scholar] [CrossRef]
- Parham, N.; Panahi, H.A.; Feizbakhsh, A.; Moniri, E. Synthesis of PEGylated superparamagnetic dendrimers and their applications as a drug delivery system. Polym. Adv. Technol. 2021, 32, 1568–1578. [Google Scholar] [CrossRef]
- Taghizadeh, A.; Pesyan, N.N.; Alamgholiloo, H.; Sheykhaghaei, G. Immobilization of Nickel on Kryptofix 222 Modified Fe3O4 @PEG Core-Shell Nanosphere for the Clean Synthesis of 2-Aryl-2,3-dihydroquinazolin-4(1 H )-ones. Appl. Organomet. Chem. 2022, e6787. [Google Scholar] [CrossRef]
- Da, X.; Li, R.; Li, X.; Lu, Y.; Gu, F.; Liu, Y. Synthesis and characterization of PEG coated hollow Fe3O4 magnetic nanoparticles as a drug carrier. Mater. Lett. 2022, 309, 131357. [Google Scholar] [CrossRef]
- Shi, D.; Beasock, D.; Fessler, A.; Szebeni, J.; Ljubimova, J.Y.; Afonin, K.A.; Dobrovolskaia, M.A. To PEGylate or not to PEGylate: Immunological properties of nanomedicine’s most popular component, polyethylene glycol and its alternatives. Adv. Drug Deliv. Rev. 2022, 180, 114079. [Google Scholar] [CrossRef] [PubMed]
- Karaagac, O.; Köçkar, H. Improvement of the saturation magnetization of PEG coated superparamagnetic iron oxide nanoparticles. J. Magn. Magn. Mater. 2022, 551, 169140. [Google Scholar] [CrossRef]
- Mohammadi, M.A.; Asghari, S.; Aslibeiki, B. Surface modified Fe3O4 nanoparticles: A cross-linked polyethylene glycol coating using plasma treatment. Surf. Interfaces 2021, 25, 101271. [Google Scholar] [CrossRef]
- Suciu, M.; Mirescu, C.; Crăciunescu, I.; Macavei, S.G.; Leoștean, C.; Ştefan, R.; Olar, L.E.; Tripon, S.-C.; Ciorîță, A.; Barbu-Tudoran, L. In vivo distribution of poly(Ethylene glycol) functionalized iron oxide nanoclusters: An ultrastructural study. Nanomaterials 2021, 11, 2184. [Google Scholar] [CrossRef]
- Chroni, A.; Forys, A.; Trzebicka, B.; Alemayehu, A.; Tyrpekl, V.; Pispas, S. Poly[oligo(ethylene glycol) methacrylate]-bpoly[(vinyl benzyl trimethylammonium chloride)] based multifunctional hybrid nanostructures encapsulating magnetic nanoparticles and DNA. Polymers 2020, 12, 1283. [Google Scholar] [CrossRef] [PubMed]
- Akkurt, N.; Altan, C.L.; Sarac, M.F. Continuous Flow–Assisted Polyol Synthesis of Citric Acid Functionalized Iron Oxide Nanoparticles. J. Supercond. Nov. Magn. 2022, 35, 615–623. [Google Scholar] [CrossRef]
- Răcuciu, M.; Tecucianu, A.; Oancea, S. Impact of Magnetite Nanoparticles Coated with Aspartic Acid on the Growth, Antioxidant Enzymes Activity and Chlorophyll Content of Maize. Antioxidants 2022, 11, 1193. [Google Scholar] [CrossRef]
- Li, K.; Dugas, P.-Y.; Lansalot, M.; Bourgeat-Lami, E. Synthesis of Iron Oxide-Armored Latex Particles by Pickering Emulsion Polymerization Using 2-Acrylamido-2-methyl-1-propane Sulfonic Acid as an Auxiliary Comonomer. Macromolecules 2022, 55, 4284–4296. [Google Scholar] [CrossRef]
- Sood, A.; Arora, V.; Kumari, S.; Sarkar, A.; Kumaran, S.S.; Chaturvedi, S.; Jain, T.K.; Agrawal, G. Imaging application and radiosensitivity enhancement of pectin decorated multifunctional magnetic nanoparticles in cancer therapy. Int. J. Biol. Macromol. 2021, 189, 443. [Google Scholar] [CrossRef] [PubMed]
- Viratchaiboott, N.; Sakunpongpitiporn, P.; Niamlang, S.; Sirivat, A. Release of 5-FU loaded pectin/Fe3O4 from porous PBSA matrix under magnetic and electric fields. J. Alloys Compd. 2022, 906, 164239. [Google Scholar] [CrossRef]
- Rozhina, E.; Danilushkina, A.; Akhatova, F.; Fakhrullin, R.; Rozhin, A.; Batasheva, S. Biocompatibility of magnetic nanoparticles coating with polycations using A549 cells. J. Biotechnol. 2021, 325, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Jary, J.; Defort, A.; Kozioƚ, J.J. Modified Physicochemical Properties of Acidic Model Drugs Immobilized on Fe3O4Magnetic Iron Oxide Nanoparticles. Pharm. Chem. J. 2020, 53, 1025–1035. [Google Scholar] [CrossRef]
- Bealer, E.J.; Kavetsky, K.; Dutko, S.; Lofland, S.; Hu, X. Protein and polysaccharide-based magnetic composite materials for medical applications. Int. J. Mol. Sci. 2020, 21, 186. [Google Scholar] [CrossRef] [PubMed]
- Sharifianjazi, F.; Irani, M.; Esmaeilkhanian, A.; Bazli, L.; Asl, M.S.; Jang, H.W.; Kim, S.Y.; Ramakrishna, S.; Shokouhimehr, M.; Varma, R.S. Polymer incorporated magnetic nanoparticles: Applications for magnetoresponsive targeted drug delivery. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2021, 272, 115358. [Google Scholar] [CrossRef]
- Sang-Mook, Y.; Jin-Sung, P.; Ke, L.; Ki-Baek, J.; Joy, A.H.; Young-Rok, K. Modulation of the peroxidase-like activity of iron oxide nanoparticles by surface functionalization with polysaccharides and its application for the detection of glutathione. Carbohydr. Polym. 2021, 267, 118164. [Google Scholar]
- Guzmán-Rocha, D.A.; Córdova-Fraga, T.; Bernal-Alvarado, J.J.; López, Z.; Cholico, F.A.; Quintero, L.H.; Paz, J.A.; Cano, M.E. A Ferrofluid with High Specific Absorption Rate Prepared in a Single Step Using a Biopolymer. Materials 2022, 15, 788. [Google Scholar] [CrossRef]
- Laborie, E.; Le-Minh, V.; Mai, T.D.; Ammar, M.; Taverna, M.; Smadja, C. Analytical methods of antibody surface coverage and orientation on bio-functionalized magnetic beads: Application to immunocapture of TNF-α. Anal. Bioanal. Chem. 2021, 413, 6425. [Google Scholar] [CrossRef]
- Zhou, Y.; Que, K.-T.; Tang, H.-M.; Zhang, P.; Fu, Q.-M.; Liu, Z.-J. Anti CD206 antibody conjugated Fe3O4 based PLGA nanoparticles selectively promote tumor associated macrophages to polarize to the pro inflammatory subtype. Oncol. Lett. 2020, 20, 12161. [Google Scholar] [CrossRef]
- He, C.; Zeng, W.; Su, Y.; Sun, R.; Xiao, Y.; Zhang, B.; Liu, W.; Wang, R.; Zhang, X.; Chen, C. Microfluidic-based fabrication and characterization of drug-loaded PLGA magnetic microspheres with tunable shell thickness. Drug Deliv. 2021, 28, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Zumaya, A.L.V.; Martynek, D.; Bautkinová, T.; Šoóš, M.; Ulbrich, P.; Raquez, J.-M.; Dendisová, M.; Merna, J.; Vilčáková, J.; Kopecký, D.; et al. Self-assembly of poly(L-lactide-co-glycolide) and magnetic nanoparticles into nanoclusters for controlled drug delivery. Eur. Polym. J. 2020, 133, 109795. [Google Scholar] [CrossRef]
- Kuger, L.; Arlt, C.-R.; Franzreb, M. Magnetic/flow controlled continuous size fractionation of magnetic nanoparticles using simulated moving bed chromatography. Talanta 2022, 240, 123160. [Google Scholar] [CrossRef] [PubMed]
- Aghazadeh, M.; Ganjali, M.R.; Morad, M.M.; Gharailou, D. Saccharide-capped superparamagnetic copper cations-doped magnetite nanoparticles for biomedical applications: A novel and simple synthesis procedure, in-situ surface engineering and characterization. Curr. Nanosci. 2020, 16, 770–778. [Google Scholar] [CrossRef]
- Metaxa, A.-F.; Vrontaki, E.; Efthimiadou, E.K.; Mavromoustakos, T. Drug Delivery Systems Based on Modified Polysaccharides: Synthesis and Characterization. Methods Mol. Biol. 2021, 2207, 151–161. [Google Scholar] [CrossRef]
- Rajabzadeh, M.; Najdi, N.; Zarei, Z.; Khalifeh, R. CuI Immobilized on Tricationic Ionic Liquid Anchored on Functionalized Magnetic Hydrotalcite (Fe3O4/HT-TIL-CuI) as a Novel, Magnetic and Efficient Nanocatalyst for Ullmann-Type C–N Coupling Reaction. J. Inorg. Organomet. Polym. Mater. 2022, 32, 2696–2711. [Google Scholar] [CrossRef]
- Ghasemi-Ghahsareh, A.; Safaei-Ghomi, J.; Oboudatian, H.S. Supported l-tryptophan on Fe3O4@SiO2 as an efficient and magnetically separable catalyst for one-pot construction of spiro[indene-2,2′-naphthalene]-4′-carbonitrile derivatives. RSC Adv. 2022, 12, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Nikoofar, K.; Arian, Z.; Djahaniani, H. Novel AlCl3@nano Fe3O4-SiO2: A benign multi-layer magnetite nanocatalyst for the three-component one-pot synthesis of spiro[benzochromeno [2,3-d]pyrimidin-indolines]. Inorg. Nano-Met. Chem. 2022, 52, 365–374. [Google Scholar] [CrossRef]
- Berret, J.-F.; Graillot, A. Versatile Coating Platform for Metal Oxide Nanoparticles: Applications to Materials and Biological Science. Langmuir 2022, 18, 5323–5338. [Google Scholar] [CrossRef]
- Pinelli, F.; Perale, G.; Rossi, F. Coating and functionalization strategies for nanogels and nanoparticles for selective drug delivery. Gels 2020, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Aurélio, D.; Mikšátko, J.; Veverka, M.; Michlová, M.; Kalbáč, M.; Vejpravová, J. Thermal traits of MNPs under high-frequency magnetic fields: Disentangling the effect of size and coating. Nanomaterials 2021, 11, 797. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Tomar, R.; Sahni, M.; Chauhan, S. Cobalt, nickel and copper doped non-stoichiometric cadmium gallate as a prominent magnetic and photocatalytic material. Chem. Phys. 2022, 554, 111419. [Google Scholar] [CrossRef]
- Hezam, F.A.; Rajeh, A.; Nur, O.; Mustafa, M.A. Synthesis and physical properties of spinel ferrites/MWCNTs hybrids nanocomposites for energy storage and photocatalytic applications. Phys. B Condens. Matter. 2020, 596, 412389. [Google Scholar] [CrossRef]
- Lei, Y.; Zhang, X.; Meng, X.; Wang, Z. The preparation of core–shell Fe3O4@SiO2 magnetic nanoparticles with different surface carboxyl densities and their application in the removal of methylene blue. Inorg. Chem. Commun. 2022, 139, 109381. [Google Scholar] [CrossRef]
- Mubarak, M.F.; Selim, H.; Elshypany, R. Hybrid magnetic core–shell TiO2@CoFe3O4 composite towards visible light-driven photodegradation of Methylene blue dye and the heavy metal adsorption: Isotherm and kinetic study. J. Environ. Heal. Sci. Eng. 2022, 20, 265–280. [Google Scholar] [CrossRef]
- Li, M.; Zhang, H.; Hou, Y.; Wang, X.; Xue, C.; Li, W.; Cai, K.; Zhao, Y.; Luo, Z. State-of-the-art iron-based nanozymes for biocatalytic tumor therapy. Nanoscale Horiz. 2020, 5, 202–217. [Google Scholar] [CrossRef]
- da Silva, N.M.; Bajgiran, K.R.; Melvin, A.T.; Dooley, K.M.; Dorman, J.A. Direct Probing of Fe3O4 Nanoparticle Surface Temperatures during Magnetic Heating: Implications for Induction Catalysis. ACS Appl. Nano Mater. 2021, 4, 13778–13787. [Google Scholar] [CrossRef]
- da Silva, R.T.P.; de Barros, H.R.; Sandrini, D.M.F.; de Torresi, S.I.C. Stimuli-Responsive Regulation of Biocatalysis through Metallic Nanoparticle Interaction. Bioconjugate Chem. 2021, 33, 53–66. [Google Scholar] [CrossRef]
- Kumar, R.; Mondal, K.; Panda, P.K.; Kaushik, A.; Abolhassani, R.; Ahuja, R.; Rubahn, H.-G.; Mishra, Y.K. Core-shell nanostructures: Perspectives towards drug delivery applications. J. Mater. Chem. B 2020, 8, 8992–9027. [Google Scholar] [CrossRef]
- Wankar, J.; Kotla, N.G.; Gera, S.; Rasala, S.; Pandit, A.; Rochev, Y.A. Recent Advances in Host–Guest Self-Assembled Cyclodextrin Carriers: Implications for Responsive Drug Delivery and Biomedical Engineering. Adv. Funct. Mater. 2020, 30, 1909049. [Google Scholar] [CrossRef]
- Fatimah, I.; Fadillah, G.; Purwiandono, G.; Sahroni, I.; Purwaningsih, D.; Riantana, H.; Avif, A.N.; Sagadevan, S. Magnetic-silica nanocomposites and the functionalized forms for environment and medical applications: A review. Inorg. Chem. Commun. 2022, 137, 109213. [Google Scholar] [CrossRef]
- Mehta, S.; Suresh, A.; Nayak, Y.; Narayan, R.; Nayak, U.Y. Hybrid nanostructures: Versatile systems for biomedical applications. Coord. Chem. Rev. 2022, 460, 214482. [Google Scholar] [CrossRef]
- Taheri-Ledari, R.; Rahimi, J.; Maleki, A. Method screening for conjugation of the small molecules onto the vinyl-coated Fe3O4/silica nanoparticles: Highlighting the efficiency of ultrasonication. Mater. Res. Express 2020, 7, 015067. [Google Scholar] [CrossRef]
- Stergar, J.; Maver, U.; Bele, M.; Gradišnik, L.; Kristl, M.; Ban, I. NiCu-silica nanoparticles as a potential drug delivery system. J. Sol-Gel Sci. Technol. 2022, 101, 493–504. [Google Scholar] [CrossRef]
- Ali, T.H.; Mandal, A.M.; Heidelberg, T.; Hussen, R.S.D. Sugar based cationic magnetic core-shell silica nanoparticles for nucleic acid extraction. RSC Adv. 2022, 12, 13566–13579. [Google Scholar] [CrossRef]
- Demirdogen, R.E.; Emen, F.M.; Karaçolak, A.I.; Kılıç, D.; Kutlu, E.; Meral, O. Preparation of novel CaMoO4:Eu3+-MCM-41 nanocomposites and their applications and monitoring as drug release systems. J. Drug Deliv. Sci. Technol. 2021, 66, 102792. [Google Scholar] [CrossRef]
- Goderski, S.; Kanno, S.; Yoshihara, K.; Komiya, H.; Goto, K.; Tanaka, T.; Kawaguchi, S.; Ishii, A.; Shimoyama, J.-I.; Hasegawa, M.; et al. Lanthanide Luminescence Enhancement of Core-Shell Magnetite-SiO2Nanoparticles Covered with Chain-Structured Helical Eu/Tb Complexes. ACS Omega 2020, 5, 32930–32938. [Google Scholar] [CrossRef]
- Sanoh, N.C.; Salazar, G.M.; Penaloza, D.P. Magnetic biopolymeric hydrogel composite material with self-healing attribute. Biointerface Res. Appl. Chem. 2021, 11, 14881–14888. [Google Scholar] [CrossRef]
- Asghari, S.; Mohammadi, M.A.; Julaei, R.; Taheri, R.A. Surface Modification of Superparamagnetic Iron Oxide Nanoparticles by Argon Plasma for Medical Applications. J. Appl. Biotechnol. Rep. 2022, 9, 563–568. [Google Scholar] [CrossRef]
- Ahmad, F.; Salem-Bekhit, M.M.; Khan, F.; Alshehri, S.; Khan, A.; Ghoneim, M.M.; Wu, H.-F.; Taha, E.I.; Elbagory, I. Unique Properties of Surface-Functionalized Nanoparticles for Bio-Application: Functionalization Mechanisms and Importance in Application. Nanomaterials 2022, 12, 1333. [Google Scholar] [CrossRef]
- Hupfeld, T.; Salamon, S.; Landers, J.; Sommereyns, A.; Doñate-Buendía, C.; Schmidt, J.; Wende, H.; Schmidt, M.; Barcikowski, S.; Gökce, B. 3D printing of magnetic parts by laser powder bed fusion of iron oxide nanoparticle functionalized polyamide powders. J. Mater. Chem. C 2020, 8, 12204–12217. [Google Scholar] [CrossRef]
- Pavón-Hernández, A.I.; Rodríguez-Velázquez, E.; Alatorre-Meda, M.; Elizalde Galindo, J.T.; Paraguay-Delgado, F.; Tirado-Guízar, A.; Pina-Luis, G. Magnetic nanocomposite with fluorescence enhancement effect based on amino acid coated-Fe3O4 functionalized with quantum dots. Mater. Chem. Phys. 2020, 251, 123082. [Google Scholar] [CrossRef]
- Jambhulkar, S.; Xu, W.; Ravichandran, D.; Prakash, J.; Kannan, A.N.M.; Song, K. Scalable Alignment and Selective Deposition of Nanoparticles for Multifunctional Sensor Applications. Nano Lett. 2020, 20, 3199–3206. [Google Scholar] [CrossRef]
- Russo, T.; De Santis, R.; Peluso, V.; Gloria, A. Multifunctional Bioactive Magnetic Scaffolds with Tailored Features for Bone Tissue Engineering. In Magnetic Nanoparticles in Human Health and Medicine; Wiley: Hoboken, NJ, USA, 2021; pp. 87–112. [Google Scholar] [CrossRef]
- Aslam, H.; Shukrullah, S.; Naz, M.Y.; Fatima, H.; Ullah, S.; Al-Sehemi, A.G. Multifunctional Magnetic Nanomedicine Drug Delivery and Imaging-Based Diagnostic Systems. Part. Part. Syst. Charact. 2021, 38, 2100179. [Google Scholar] [CrossRef]
- Al-Terke, H.H.; Latikka, M.; Timonen, J.V.I.; Vékás, L.; Paananen, A.; Joensuu, J.; Ras, R.H.A. Functional Magnetic Microdroplets for Antibody Extraction. Adv. Mater. Interfaces 2022, 9, 2101317. [Google Scholar]
- Besenhard, M.O.; Panariello, L.; Kiefer, C.; LaGrow, A.P.; Storozhuk, L.; Perton, F.; Begin, S.; Mertz, D.; Thanh, N.T.K.; Gavriilidis, A. Small iron oxide nanoparticles as MRI T1 contrast agent: Scalable inexpensive water-based synthesis using a flow reactor. Nanoscale 2021, 13, 8795–8805. [Google Scholar]
- Gradinaru, L.M.; Mandru, M.B.; Drobota, M.; Aflori, M.; Butnaru, M.; Spiridon, M.; Doroftei, F.; Aradoaei, M.; Ciobanu, R.C.; Vlad, S. Composite materials based on iron oxide nanoparticles and polyurethane for improving the quality of mri. Polymers 2021, 13, 4316. [Google Scholar] [CrossRef]
- Harvell-Smith, S.; Tung, L.D.; Thanh, N.T.K. Magnetic particle imaging: Tracer development and the biomedical applications of a radiation-free, sensitive, and quantitative imaging modality. Nanoscale 2021, 14, 3658–3697. [Google Scholar] [CrossRef]
- Svenskaya, Y.; Garello, F.; Lengert, E.; Kozlova, A.; Verkhovskii, R.; Bitonto, V.; Ruggiero, M.R.; German, S.; Gorin, D.; Terreno, E. Biodegradable polyelectrolyte/magnetite capsules for MR imaging and magnetic targeting of tumors. Nanotheranostics 2021, 5, 362–377. [Google Scholar] [CrossRef] [PubMed]
- Rosch, E.L.; Zhong, J.; Lak, A.; Liu, Z.; Etzkorn, M.; Schilling, M.; Ludwig, F.; Viereck, T.; Lalkens, B. Point-of-need detection of pathogen-specific nucleic acid targets using magnetic particle spectroscopy. Biosens. Bioelectron. 2021, 192, 113536. [Google Scholar]
- Bruno, F.; Granata, V.; Bellisari, F.C.; Sgalambro, F.; Tommasino, E.; Palumbo, P.; Arrigoni, F.; Cozzi, D.; Grassi, F.; Brunese, M.C.; et al. Advanced Magnetic Resonance Imaging (MRI) Techniques: Technical Principles and Applications in Nanomedicine. Cancers 2022, 14, 1626. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, M.; Zeng, J.; Huo, L.; Liu, K.; Wei, R.; Ni, K.; Gao, J. Recent advances in engineering iron oxide nanoparticles for effective magnetic resonance imaging. Bioact. Mater. 2022, 12, 214–245. [Google Scholar] [CrossRef]
- Wu, K.; Liu, J.; Saha, R.; Ma, B.; Su, D.; Peng, C.; Sun, J.; Wang, J.-P. Irregularly Shaped Iron Nitride Nanoparticles as a Potential Candidate for Biomedical Applications: From Synthesis to Characterization. ACS Omega 2020, 5, 11756–11767. [Google Scholar] [CrossRef]
- Balk, M.; Haus, T.; Band, J.; Unterweger, H.; Schreiber, E.; Friedrich, R.P.; Alexiou, C.; Gostian, A.-O. Cellular spion uptake and toxicity in various head and neck cancer cell lines. Nanomaterials 2021, 11, 726. [Google Scholar] [CrossRef]
- Sokolov, A.E.; Ivanova, O.S.; Fedorov, A.S.; Kovaleva, E.A.; Vysotin, M.A.; Lin, C.-R.; Ovchinnikov, S.G. Why the Magnetite–Gold Core–Shell Nanoparticles Are Not Quite Good and How to Improve Them. Phys. Solid State 2021, 63, 1536–1540. [Google Scholar] [CrossRef]
- Bhatia, P.; Verma, S.S.; Sinha, M.M. Magneto-plasmonic Co@M (M = Au/Ag/Au-Ag) core-shell nanoparticles for biological imaging and therapeutics. J. Quant. Spectrosc. Radiat. Transf. 2020, 251, 107095. [Google Scholar] [CrossRef]
- Wei, D.-H.; Lin, T.-K.; Liang, Y.-C.; Chang, H.-W. Formation and Application of Core–Shell of FePt-Au Magnetic–Plasmonic Nanoparticles. Front. Chem. 2021, 9, 653718. [Google Scholar] [CrossRef]
- Mohajer, F.; Ziarani, G.M.; Badiei, A. New advances on Au-magnetic organic hybrid core-shells in MRI, CT imaging, and drug delivery. RSC Adv. 2021, 11, 6517–6525. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, J.; Papanastasiou, G.; Tai, C.-W.; Moran, C.M.; Jansen, M.A.; Tavares, A.A.S.; Lennen, R.J.; Corral, C.A.; Wang, C.; Thomson, A.J.W.; et al. Tri-modal imaging of gold-dotted magnetic nanoparticles for magnetic resonance imaging, computed tomography and intravascular ultrasound: An in vitro study. Nanomedicine 2020, 15, 2433–2445. [Google Scholar] [CrossRef]
- Iancu, S.D.; Albu, C.; Chiriac, L.; Moldovan, R.; Stefancu, A.; Moisoiu, V.; Coman, V.; Szabo, L.; Leopold, N.; Bálint, Z. Assessment of gold-coated iron oxide nanoparticles as negative T2 contrast agent in small animal MRI studies. Int. J. Nanomed. 2020, 15, 4811–4824. [Google Scholar] [CrossRef]
- Dheyab, M.A.; Aziz, A.A.; Jameel, M.S.; Noqta, O.A.; Mehrdel, B. Synthesis and coating methods of biocompatible iron oxide/gold nanoparticle and nanocomposite for biomedical applications. Chin. J. Phys. 2020, 64, 305–325. [Google Scholar] [CrossRef]
- Schwaminger, S.P.; Bauer, D.; Fraga-García, P. Gold-iron oxide nanohybrids: Insights into colloidal stability and surface-enhanced Raman detection. Nanoscale Adv. 2021, 3, 6438. [Google Scholar] [CrossRef]
- Chaturvedi, A.; Pranjali, P.; Meher, M.K.; Raj, R.; Basak, M.; Singh, R.K.; Poluri, K.M.; Kumar, D.; Guleria, A. In vitro and ex vivo relaxometric properties of ethylene glycol coated gadolinium oxide nanoparticles for potential use as contrast agents in magnetic resonance imaging. J. Appl. Phys. 2020, 128, 034903. [Google Scholar] [CrossRef]
- Forte, E.; Fiorenza, D.; Torino, E.; Di Polidoro, A.C.; Cavaliere, C.; Netti, P.A.; Salvatore, M.; Aiello, M. Radiolabeled PET/MRI nanoparticles for tumor imaging. J. Clin. Med. 2020, 9, 89. [Google Scholar] [CrossRef]
- Belderbos, S.; González-Gómez, M.A.; Cleeren, F.; Wouters, J.; Piñeiro, Y.; Deroose, C.M.; Coosemans, A.; Gsell, W.; Bormans, G.; Rivas, J.; et al. Simultaneous in vivo PET/MRI using fluorine-18 labeled Fe3O4@Al(OH)3 nanoparticles: Comparison of nanoparticle and nanoparticle-labeled stem cell distribution. EJNMMI Res. 2020, 10, 73. [Google Scholar] [CrossRef]
- Yan, Q.; Dong, X.; Xie, R.; Xu, X.; Wang, X.; Zhang, K.; Xia, J.; Ling, J.; Zhou, F.; Sun, J. Preparation of Mn2+@PolyDOPA-b-polysarcosine micelle as MRI contrast agent with high longitudinal relaxivity. J. Macromol. Sci. Part A Pure Appl. Chem. 2021, 58, 175–181. [Google Scholar] [CrossRef]
- Ramazanov, M.; Karimova, A.; Shirinova, H. Magnetism for drug delivery, mri and hyperthermia applications: A review. Biointerface Res. Appl. Chem. 2021, 11, 8654–8668. [Google Scholar] [CrossRef]
- Varghese, R.; Vijay, N.; Dalvi, Y.B. Magnetic Nanoparticles for Image-Guided Drug Delivery. In Magnetic Nanoparticles; Springer: Singapore, 2021; pp. 45–71. [Google Scholar] [CrossRef]
- Basina, G.; Diamantopoulos, G.; Devlin, E.; Psycharis, V.; Alhassan, S.M.; Pissas, M.; Hadjipanayis, G.; Tomou, A.; Bouras, A.; Hadjipanayis, C.; et al. LAPONITE® nanodisk-“decorated” Fe3O4 nanoparticles: A biocompatible nano-hybrid with ultrafast magnetic hyperthermia and MRI contrast agent ability. J. Mater. Chem. B 2022, 10, 4935. [Google Scholar] [CrossRef]
- Salunkhe, A.B.; Khot, V.M.; Patil, S.I.; Tofail, S.A.M.; Bauer, J.; Thorat, N.D. MRI Guided Magneto-chemotherapy with High-Magnetic-Moment Iron Oxide Nanoparticles for Cancer Theranostics. ACS Appl. Bio Mater. 2020, 3, 2305–2313. [Google Scholar] [CrossRef]
- Antal, I.; Strbak, O.; Khmara, I.; Koneracka, M.; Kubovcikova, M.; Zavisova, V.; Kmetova, M.; Baranovicova, E.; Dobrota, D. MRI relaxivity changes of the magnetic nanoparticles induced by different amino acid coatings. Nanomaterials 2020, 10, 394. [Google Scholar] [CrossRef]
- Nagornyi, A.V.; Shlapa, Y.; Avdeev, M.V.; Solopan, S.O.; Belous, A.G.; Shulenina, A.V.; Ivankov, O.I.; Bulavin, L.A. Structural characterization of aqueous magnetic fluids with nanomagnetite of different origin stabilized by sodium oleate. J. Mol. Liq. 2020, 312, 113430. [Google Scholar] [CrossRef]
- Shaumbwa, V.R.; Liu, D.; Archer, B.; Li, J.; Su, F. Preparation and application of magnetic chitosan in environmental remediation and other fields: A review. J. Appl. Polym. Sci. 2021, 138, 51241. [Google Scholar] [CrossRef]
- Arias, L.S.; Pessan, J.P.; de Souza, F.N.N.; Lima, B.H.R.; de Camargo, E.R.; Ramage, G.; Delbem, A.C.B.; Monteiro, D.R. Novel nanocarrier of miconazole based on chitosan-coated iron oxide nanoparticles as a nanotherapy to fight Candida biofilms. Colloids Surf. B Biointerfaces 2020, 192, 111080. [Google Scholar] [CrossRef] [PubMed]
- Bindu, V.U.; Mohanan, P.V. Thermal deactivation of α-amylase immobilized magnetic chitosan and its modified forms: A kinetic and thermodynamic study. Carbohydr. Res. 2020, 498, 108185. [Google Scholar] [CrossRef]
- Balan, V.; Malihin, S.; Verestiuc, L. Chitosan-based systems for theranostic applications. In Functional Chitosan: Drug Delivery and Biomedical Applications; Springer: Singapore, 2020; pp. 343–384. [Google Scholar] [CrossRef]
- Lawai, V.; Ngaini, Z. Chitosan magnetic nanocomposites for gene delivery. In Polysaccharide-Based Nanocomposites for Gene Delivery and Tissue Engineering; Woodhead Publishing: Cambridge, UK, 2021; pp. 279–294. [Google Scholar] [CrossRef]
- Pop, N.L.; Nan, A.; Urda-Cimpean, A.E.; Florea, A.; Toma, V.A.; Moldovan, R.; Decea, N.; Mitrea, D.R.; Orasan, R. Chitosan functionalized magnetic nanoparticles to provide neural regeneration and recovery after experimental model induced peripheral nerve injury. Biomolecules 2021, 11, 676. [Google Scholar] [CrossRef]
- Mohammad Gholiha, H.; Ehsani, M.; Saeidi, A.; Ghadami, A.; Alizadeh, N. Magnetic dual-responsive semi-IPN nanogels based on chitosan/PNVCL and study on BSA release behavior. Prog. Biomater. 2021, 10, 173–183. [Google Scholar] [CrossRef]
- Borges, M.M.C.; Pires, B.C.; Vieira, S.S.; Borges, K.B.; Guimarães, L.G.D.L. Magnetic and pH responsive composite hydrogel-based on poly(2-(diethylamino)ethyl methacrylate)/chitosan for fipronil removal from aqueous medium. React. Funct. Polym. 2021, 168, 105050. [Google Scholar] [CrossRef]
- González-Martínez, E.; Pérez, A.G.; González-Martínez, D.A.; Águila, C.R.D.; Urbina, E.C.; Ramírez, D.U.; Yee-Madeira, H. Chitosan-coated magnetic nanoparticles; exploring their potentialities for DNA and Cu(II) recovery. Inorg. Nano-Met. Chem. 2021, 51, 1098–1107. [Google Scholar] [CrossRef]
- Vaewbundit, S.; Siriphannon, P. Soft solution growth of magnetite-maghemite nanocrystals in crosslinked chitosan templates and their superparamagnetic properties. Nanocomposites 2022, 8, 142–154. [Google Scholar] [CrossRef]
- Bianchetti, E.; Di Valentin, C. Mechanism of spin ordering in Fe3O4 nanoparticles by surface coating with organic acids. Mater. Today Nano 2022, 17, 100169. [Google Scholar] [CrossRef]
- Yıldırım, E.; Arıkan, B.; Yücel, O.; Çakır, O.; Kara, N.T.; İyim, T.B.; Gürdağ, G.; Emik, S. Synthesis and characterization of amino functional poly(acrylamide) coated Fe3O4 nanoparticles and investigation of their potential usage in DNA isolation. Chem. Pap. 2022, 76, 5747–5759. [Google Scholar] [CrossRef]
- Yan, Y.-Q.; Wang, H.; Zhao, Y. Radiolabeled peptide probe for tumor imaging. Chin. Chem. Lett. 2022, 33, 3361–3370. [Google Scholar] [CrossRef]
- Yilmaz, S.; Ichedef, C.; Karatay, K.B.; Teksöz, S. Polymer coated iron nanoparticles: Radiolabeling & in vitro studies. Curr. Radiopharm. 2021, 14, 37–45. [Google Scholar] [CrossRef]
- Shi, S.; Chen, Y.; Yao, X. In Vivo Computing Strategies for Tumor Sensitization and Targeting. IEEE Trans. Cybern. 2022, 52, 4970–4980. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, J.; Yuan, Z. Strategies and challenges to improve the performance of tumor-associated active targeting. J. Mater. Chem. B 2020, 8, 3959–3971. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Wang, X.; Chang, K.; Shen, W.; Yu, G.; Du, J. The bright future of nanotechnology in lymphatic system imaging and imaging-guided surgery. J. Nanobiotechnol. 2022, 20, 24. [Google Scholar] [CrossRef]
- Kubelick, K.P.; Mehrmohammadi, M. Magnetic particles in motion: Magneto-motive imaging and sensing. Theranostics 2022, 12, 1783–1799. [Google Scholar] [CrossRef] [PubMed]
- Grudzinski, I.P.; Bystrzejewski, M.; Bogorodzki, P.; Cieszanowski, A.; Szeszkowski, W.; Poplawska, M.; Bamburowicz-Klimkowska, M. Comprehensive magnetic resonance characteristics of carbon-encapsulated iron nanoparticles: A new frontier for the core-shell–type contrast agents. J. Nanoparticle Res. 2020, 22, 82. [Google Scholar] [CrossRef]
- Zhao, W.; Yu, X.; Peng, S.; Luo, Y.; Li, J.; Lu, L. Construction of nanomaterials as contrast agents or probes for glioma imaging. J. Nanobiotechnol. 2021, 19, 125. [Google Scholar] [CrossRef]
- Marasini, S.; Yue, H.; Ho, S.L.; Cha, H.; Park, J.A.; Jung, K.-H.; Ghazanfari, A.; Ahmad, M.Y.; Liu, S.; Chae, K.-S.; et al. A Novel Paramagnetic Nanoparticle T2 Magnetic Resonance Imaging Contrast Agent with High Colloidal Stability: Polyacrylic Acid-Coated Ultrafine Dysprosium Oxide Nanoparticles. Bull. Korean Chem. Soc. 2020, 41, 829–836. [Google Scholar] [CrossRef]
- Ahmad, M.Y.; Ahmad, M.W.; Yue, H.; Ho, S.L.; Park, J.A.; Jung, K.-H.; Cha, H.; Marasini, S.; Ghazanfari, A.; Liu, S.; et al. In vivo positive magnetic resonance imaging applications of poly(methyl vinyl ether-alt-maleic acid)-coated ultra-small paramagnetic gadolinium oxide nanoparticles. Molecules 2020, 25, 1159. [Google Scholar] [CrossRef] [PubMed]
- Marasini, S.; Yue, H.; Ghazanfari, A.; Ho, S.L.; Park, J.A.; Kim, S.; Cha, H.; Liu, S.; Tegafaw, T.; Ahmad, M.Y.; et al. Polyaspartic acid-coated paramagnetic gadolinium oxide nanoparticles as a dual-modal t1 and t2 magnetic resonance imaging contrast agent. Appl. Sci. 2021, 11, 8222. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, Y.; Wu, M.; Li, W.; Zuo, H.; Xiao, B.; Zhang, X.; Wu, J.; He, H.; Xia, Q. Sericin-based gadolinium nanoparticles as synergistically enhancing contrast agents for pH-responsive and tumor targeting magnetic resonance imaging. Mater. Des. 2021, 203, 109600. [Google Scholar] [CrossRef]
- Fei, M.-Y.; Song, M.-M.; Wang, P.; Pang, G.-Z.; Chen, J.; Lu, D.-P.; Liu, R.; Zhang, G.-Y.; Zhao, T.-T.; Shen, Y.-X.; et al. Folic acid modified Fe3O4 nanoclusters by a one-step ultrasonic technique for drug delivery and MR imaging. RSC Adv. 2020, 10, 5294–5303. [Google Scholar] [CrossRef]
- Rodriguez, G.G.; Erro, E.M. Fast iron oxide-induced low-field magnetic resonance imaging. J. Phys. D Appl. Phys. 2021, 54, 025003. [Google Scholar] [CrossRef]
- Creţu, B.E.-B.; Dodi, G.; Shavandi, A.; Gardikiotis, I.; Şerban, I.L.; Balan, V. Imaging constructs: The rise of iron oxide nanoparticles. Molecules 2021, 26, 3437. [Google Scholar] [CrossRef] [PubMed]
- Almijalli, M.; Saad, A.; Alhussaini, K.; Aleid, A.; Alwasel, A. Towards drug delivery control using iron oxide nanoparticles in three-dimensional magnetic resonance imaging. Nanomaterials 2021, 11, 1876. [Google Scholar] [CrossRef]
- Billings, C.; Langley, M.; Warrington, G.; Mashali, F.; Johnson, J.A. Magnetic particle imaging: Current and future applications, magnetic nanoparticle synthesis methods and safety measures. Int. J. Mol. Sci. 2021, 22, 7651. [Google Scholar] [CrossRef]
- Chow, J.C.L. Magnetic nanoparticles as contrast agents in magnetic resonance imaging and radiosensitizers in radiotherapy. In Fundamentals and Industrial Applications of Magnetic Nanoparticles; Woodhead Publishing: Cambridge, UK, 2022; pp. 291–316. [Google Scholar] [CrossRef]
- Dash, A.; Blasiak, B.; Tomanek, B.; Banerjee, A.; Trudel, S.; Latta, P.; Van Veggel, F.C.J.M. Colloidally Stable Monodisperse Fe Nanoparticles as T2Contrast Agents for High-Field Clinical and Preclinical Magnetic Resonance Imaging. ACS Appl. Nano Mater. 2021, 4, 1235–1242. [Google Scholar] [CrossRef]
- Liu, X.; Tian, Y.; Jiang, L. Manipulating Dispersions of Magnetic Nanoparticles. Nano Lett. 2021, 21, 2699–2708. [Google Scholar] [CrossRef]
- Mair, L.O.; Hale, O.; Jafari, S.; Chen, C.; Udalov, O.; Probst, R.; Baum, I.; Hevaganinge, A.; Wang, E.Y.; Rodriguez, O.C.; et al. Magnetic Microdevices for MRI-Based Detection of SARS-CoV-2 Viruses. IEEE Open J. Eng. Med. Biol. 2020, 1, 265–267. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Saha, R.; Su, D.; Krishna, V.D.; Liu, J.; Cheeran, M.C.-J.; Wang, J.-P. Magnetic-Nanosensor-Based Virus and Pathogen Detection Strategies before and during COVID-19. ACS Appl. Nano Mater. 2020, 3, 9560–9580. [Google Scholar] [CrossRef]
- Medhi, R.; Srinoi, P.; Ngo, N.; Tran, H.-V.; Lee, T.R. Nanoparticle-Based Strategies to Combat COVID-19. ACS Appl. Nano Mater. 2020, 3, 8557–8580. [Google Scholar] [CrossRef]
- Ebrahimi, S.; Shamloo, A.; Alishiri, M.; Mofrad, Y.M.; Akherati, F. Targeted pulmonary drug delivery in coronavirus disease (COVID-19) therapy: A patient-specific in silico study based on magnetic nanoparticles-coated microcarriers adhesion. Int. J. Pharm. 2021, 609, 121133. [Google Scholar] [CrossRef]
- Wang, W.; Huang, Z.; Huang, Y.; Zhang, X.; Huang, J.; Cui, Y.; Yue, X.; Ma, C.; Fu, F.; Wang, W.; et al. Pulmonary delivery nanomedicines towards circumventing physiological barriers: Strategies and characterization approaches. Adv. Drug Deliv. Rev. 2022, 185, 114309. [Google Scholar] [CrossRef]
- Chelluri, L.K.; Mohanram, Y.; Jain, R.; Mallarpu, C.S.; Ponnana, M.; Kumar, D.; Krishna Venuganti, V.V.; Kancherla, R.; Papineni, R.V.; Towner, R.; et al. Effect of engineered superparamagnetic iron oxide nanoparticles in targeted cardiac precursor cell delivery by MRI. Biochem. Biophys. Res. Commun. 2021, 541, 15–21. [Google Scholar] [CrossRef]
- Marasini, R.; Rayamajhi, S.; Moreno-Sanchez, A.; Aryal, S. Iron(iii) chelated paramagnetic polymeric nanoparticle formulation as a next-generation: T 1-weighted MRI contrast agent. RSC Adv. 2021, 11, 32216–32226. [Google Scholar] [CrossRef]
- Hou, Z.; Liu, Y.; Xu, J.; Zhu, J. Surface Engineering of Magnetic Iron Oxide Nanoparticles by Polymer Grafting: Synthesis Progress and Biomedical Applications. Nanoscale 2020, 12, 14957. [Google Scholar] [CrossRef]
- Qin, M.; Xu, M.; Niu, L.; Cheng, Y.; Niu, X.; Kong, J.; Zhang, X.; Wei, Y.; Huang, D. Multifunctional modification of Fe3O4 nanoparticles for diagnosis and treatment of diseases: A review. Front. Mater. Sci. 2021, 15, 36–53. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M. Surface Engineering of Nanomaterials with Polymers, Biomolecules, and Small Ligands for Nanomedicine. Materials 2022, 15, 3251. [Google Scholar] [CrossRef]
- Li, Y.; Wang, N.; Huang, X.; Li, F.; Davis, T.P.; Qiao, R.; Ling, D. Polymer-Assisted Magnetic Nanoparticle Assemblies for Biomedical Applications. ACS Appl. Biol. Mater. 2020, 3, 121–142. [Google Scholar] [CrossRef] [PubMed]
- Sanadgol, N.; Wackerlig, J. Developments of smart drug-delivery systems based on magnetic molecularly imprinted polymers for targeted cancer therapy: A short review. Pharmaceutics 2020, 12, 831. [Google Scholar] [CrossRef]
- Jarak, I.; Varela, C.L.; da Silva, E.T.; Roleira, F.F.M.; Veiga, F.; Figueiras, A. Pluronic-based nanovehicles: Recent advances in anticancer therapeutic applications. Eur. J. Med. Chem. 2020, 206, 112526. [Google Scholar] [CrossRef] [PubMed]
- Singla, P.; Garg, S.; McClements, J.; Jamieson, O.; Peeters, M.; Mahajan, R.K. Advances in the therapeutic delivery and applications of functionalized Pluronics: A critical review. Adv. Colloid Interface Sci. 2022, 299, 102563. [Google Scholar] [CrossRef] [PubMed]
- Mostarac, D.; Xiong, Y.; Gang, O.; Kantorovich, S.S. Nanopolymers for magnetic applications: How to choose the architecture? Nanoscale 2022, 14, 11139–11151. [Google Scholar] [CrossRef] [PubMed]
- Saengruengrit, C.; Rodponthukwaji, K.; Sucharitakul, J.; Tummamunkong, P.; Palaga, T.; Ritprajak, P.; Insin, N. Effective gene delivery into primary dendritic cells using synthesized PDMAEMA-iron oxide nanocubes. Mater. Today Chem. 2021, 20, 100481. [Google Scholar] [CrossRef]
- Samim, M. Aarzoo Hyaluronic acid-magnetic nanocomposites for gene delivery. In Polysaccharide-Based Nanocomposites for Gene Delivery and Tissue Engineering; Woodhead Publishing: Cambridge, UK, 2021; pp. 311–323. [Google Scholar] [CrossRef]
- Dalmartello, M.; La Vecchia, C.; Bertuccio, P.; Boffetta, P.; Levi, F.; Negri, E.; Malvezzi, M. European cancer mortality predictions for the year 2022 with focus on ovarian cancer. Ann. Oncol. 2022, 33, 330–339. [Google Scholar] [CrossRef]
- Low, L.E.; Wu, J.; Lee, J.; Tey, B.T.; Goh, B.; Gao, J.; Li, F.; Ling, D. Tumor-responsive dynamic nanoassemblies for targeted imaging, therapy and microenvironment manipulation. J. Control. Release 2020, 324, 69–103. [Google Scholar] [CrossRef]
- Gauger, A.; Hershberger, K.K.; Bronstein, L.M. Theranostics Based on Magnetic Nanoparticles and Polymers: Intelligent Design for Efficient Diagnostics and Therapy. Front. Chem. 2020, 8, 561. [Google Scholar] [CrossRef]
- Wang, W.; Huang, Z.; Huang, Y.; Pan, X.; Wu, C. Updates on the applications of iron-based nanoplatforms in tumor theranostics. Int. J. Pharm. 2020, 589, 119815. [Google Scholar] [CrossRef] [PubMed]
- Koksharov, Y.A.; Gubin, S.P.; Taranov, I.V.; Khomutov, G.B.; Gulyaev, Y.V. Magnetic Nanoparticles in Medicine: Progress, Problems, and Advances. J. Commun. Technol. Electron. 2022, 67, 101–116. [Google Scholar] [CrossRef]
- Manescu, V.; Paltanea, G.; Antoniac, I.; Vasilescu, M. Magnetic nanoparticles used in oncology. Materials 2021, 14, 5948. [Google Scholar] [CrossRef] [PubMed]
- Cotin, G.; Blanco-Andujar, C.; Perton, F.; Asin, L.; de la Fuente, J.M.; Reichardt, W.; Schaffner, D.; Nguyen, D.; Mertz, D.; Kiefer, C.; et al. Unveiling the role of surface, size, shape and defects for theranostic applications of iron oxide nanoparticles. Nanoscale 2021, 13, 14552–14571. [Google Scholar]
- Khizar, S.; Ahmad, N.M.; Zine, N.; Jaffrezic-Renault, N.; Errachid-el-salhi, A.; Elaissari, A. Magnetic Nanoparticles: From Synthesis to Theranostic Applications. ACS Appl. Nano Mater. 2021, 4, 4284–4306. [Google Scholar] [CrossRef]
- Stueber, D.D.; Villanova, J.; Aponte, I.; Xiao, Z.; Colvin, V.L. Magnetic nanoparticles in biology and medicine: Past, present, and future trends. Pharmaceutics 2021, 13, 943. [Google Scholar] [CrossRef]
- Singh, S.; Chawla, H.; Chandra, A.; Garg, S. Magnetic hybrid nanoparticles for drug delivery. In Magnetic Nanoparticle-Based Hybrid Materials: Fundamentals and Applications; Woodhead Publishing: Cambridge, UK, 2021; pp. 319–342. [Google Scholar] [CrossRef]
- Gao, S.; Guisán, J.M.; Rocha-Martin, J. Oriented immobilization of antibodies onto sensing platforms—A critical review. Anal. Chim. Acta 2022, 1189, 338907. [Google Scholar] [CrossRef]
- Shabatina, T.I.; Vernaya, O.I.; Shabatin, V.P.; Melnikov, M.Y. Magnetic nanoparticles for biomedical purposes: Modern trends and prospects. Magnetochemistry 2020, 6, 30. [Google Scholar] [CrossRef]
- Abdelgawad, M.A.; Elkanzi, N.A.A.; Nayl, A.A.; Musa, A.; Alotaibi, N.H.; Arafa, W.A.A.; Gomha, S.M.; Bakr, R.B. Targeting tumor cells with pyrazolo[3,4-d]pyrimidine scaffold: A literature review on synthetic approaches, structure activity relationship, structural and target-based mechanisms. Arab. J. Chem. 2022, 15, 103781. [Google Scholar] [CrossRef]
- Ignatovich, Z.; Novik, K.; Abakshonok, A.; Koroleva, E.; Beklemisheva, A.; Panina, L.; Kaniukov, E.; Anisovich, M.; Shumskaya, A. One-step synthesis of magnetic nanocomposite with embedded biologically active substance. Molecules 2021, 26, 937. [Google Scholar] [CrossRef]
- Teimouri, K.; Tavakoli, M.R.; Ghafari, A.; Kim, K.C. Effect of plaque geometry on targeted delivery of stem cells containing magnetic particles in a rigid and elastic curved artery with stenosis. J. Magn. Magn. Mater. 2022, 542, 168580. [Google Scholar] [CrossRef]
- Sanz-Ortega, L.; Rojas, J.M.; Barber, D.F. Improving tumor retention of effector cells in adoptive cell transfer therapies by magnetic targeting. Pharmaceutics 2020, 12, 812. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.T.-W.; Martino, U.; Khan, R.; Bazzar, M.; Southern, P.; Tuncel, D.; Al-Jamal, K.T. Engineering red-emitting multi-functional nanocapsules for magnetic tumour targeting and imaging. Biomater. Sci. 2020, 8, 2590–2599. [Google Scholar] [CrossRef]
- Shasha, C.; Krishnan, K.M. Nonequilibrium Dynamics of Magnetic Nanoparticles with Applications in Biomedicine. Adv. Mater. 2020, 33, 23. Available online: https://onlinelibrary.wiley.com/doi/10.1002/adma.201904131 (accessed on 30 June 2022). [CrossRef]
- Yang, X.; Shao, G.; Zhang, Y.; Wang, W.; Qi, Y.; Han, S.; Li, H. Applications of Magnetic Particle Imaging in Biomedicine: Advancements and Prospects. Front. Physiol. 2022, 13, 898426. [Google Scholar] [CrossRef] [PubMed]
- Nisticò, R.; Cesano, F.; Garello, F. Magnetic materials and systems: Domain structure visualization and other characterization techniques for the application in the materials science and biomedicine. Inorganics 2020, 8, 6. [Google Scholar] [CrossRef]
- Du, H.; Yao, C.-Y.; Peng, H.; Jiang, B.; Li, S.-X.; Yao, J.-L.; Zheng, F.; Yang, F.; Wu, A.-G. Applications of Transition Metal-doped Iron-based Nanoparticles in Biomedicine. Chin. J. Appl. Chem. 2022, 39, 391. [Google Scholar] [CrossRef]
- Kee, H.; Lee, H.; Park, S. Optimized Halbach array for focused magnetic drug targeting. J. Magn. Magn. Mater. 2020, 514, 167180. [Google Scholar] [CrossRef]
- Garg, R.; Kaur, S.; Ritika Khatoon, S.; Naina Verma, H. A complete and updated review on various types of drug delivery systems. Int. J. Appl. Pharm. 2020, 12, 4. [Google Scholar] [CrossRef]
- Das, S.S.; Bharadwaj, P.; Bilal, M.; Barani, M.; Rahdar, A.; Taboada, P.; Bungau, S.; Kyzas, G.Z. Stimuli-responsive polymeric nanocarriers for drug delivery, imaging, and theragnosis. Polymers 2020, 12, 1397. [Google Scholar] [CrossRef]
- Al-Rawi, N.N.; Anwer, B.A.; Al-Rawi, N.H.; Uthman, A.T.; Ahmed, I.S. Magnetism in drug delivery: The marvels of iron oxides and substituted ferrites nanoparticles. Saudi Pharm. J. 2020, 28, 876–887. [Google Scholar] [CrossRef]
- Mahmoodpour, M.; Goharkhah, M.; Ashjaee, M. Investigation on trajectories and capture of magnetic drug carrier nanoparticles after injection into a direct vessel. J. Magn. Magn. Mater. 2020, 497, 166065. [Google Scholar] [CrossRef]
- Kiaie, N.; Emami, S.H.; Rabbani, S.; Aghdam, R.M.; Tafti, H.A. Targeted and Controlled Drug Delivery to a Rat Model of Heart Failure Through a Magnetic Nanocomposite. Ann. Biomed. Eng. 2020, 48, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-I.; Lee, H.; Kwon, S.-H.; Sung, Y.J.; Song, W.K.; Park, S. Bilayer Hydrogel Sheet-Type Intraocular Microrobot for Drug Delivery and Magnetic Nanoparticles Retrieval. Adv. Healthc. Mater. 2020, 9, 2000118. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.T.; Kim, C.R.; Le, T.H.; Koo, K.-I.; Hwang, C.H. Magnetically guided targeted delivery of erythropoietin using magnetic nanoparticles: Proof of concept. Medicine 2020, 99, e19972. [Google Scholar] [CrossRef]
- Wang, C.H. Targeted delivery of erythropoietin hybridized with magnetic nanocarriers for the treatment of central nervous system injury: A literature review. Int. J. Nanomed. 2020, 15, 9683–9701. [Google Scholar] [CrossRef]
- Timerbaev, A.R. Analytical methodology for developing nanomaterials designed for magnetically-guided delivery of platinum anticancer drugs. Talanta 2022, 243, 123371. [Google Scholar] [CrossRef]
- Ferreira, B.J.M.L.; Martel, F.; Silva, C.; Santos, T.M.; Daniel-da-Silva, A.L. Nanostructured functionalized magnetic platforms for the sustained delivery of cisplatin: Synthesis, characterization and in vitro cytotoxicity evaluation. J. Inorg. Biochem. 2020, 213, 111258. [Google Scholar] [CrossRef]
- Schroffenegger, M.; Leitner, N.S.; Morgese, G.; Ramakrishna, S.N.; Willinger, M.; Benetti, E.M.; Reimhult, E. Polymer Topology Determines the Formation of Protein Corona on Core–Shell Nanoparticles. ACS Nano 2020, 14, 12708–12718. [Google Scholar] [CrossRef]
- Shadmani, P.; Mehrafrooz, B.; Montazeri, A.; Naghdabadi, R. Protein corona impact on nanoparticle-cell interactions: Toward an energy-based model of endocytosis. J. Phys. Condens. Matter 2020, 32, 115101. [Google Scholar] [CrossRef]
- Costa, L.S.D.; Khan, L.U.; Franqui, L.S.; Delite, F.D.S.; Muraca, D.; Martinez, D.S.T.; Knobel, M. Hybrid magneto-luminescent iron oxide nanocubes functionalized with europium complexes: Synthesis, hemolytic properties and protein corona formation. J. Mater. Chem. B 2021, 9, 428–439. [Google Scholar] [CrossRef]
- Nowak-Jary, J.; Machnicka, B. Pharmacokinetics of magnetic iron oxide nanoparticles for medical applications. J. Nanobiotechnol. 2022, 20, 305. [Google Scholar] [CrossRef]
- Rezaei-Aghdam, E.; Shamel, A.; Khodadadi-Moghaddam, M.; Rajaei, G.E.; Mohajeri, S. Synthesis of TiO2 and ZnO Nanoparticles and CTAB-Stabilized Fe3O4 nanocomposite: Kinetics and thermodynamics of adsorption. Res. Chem. Intermed. 2021, 47, 1759–1774. [Google Scholar] [CrossRef]
- Saifullah, S.; Ali, I.; Kawish, M.; El-Shabasy, R.M.; Chen, L.; El-Seedi, H.R. Surface functionalized magnetic nanoparticles for targeted cancer therapy and diagnosis. In Metal Nanoparticles for Drug Delivery and Diagnostic Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 215–236. [Google Scholar] [CrossRef]
- Mirza, S.; Ahmad, M.S.; Shah, M.I.A.; Ateeq, M. Magnetic nanoparticles: Drug delivery and bioimaging applications. In Metal Nanoparticles for Drug Delivery and Diagnostic Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 189–213. [Google Scholar] [CrossRef]
- Patra, P.; Chattopadhyay, D. Sustainable release of nanodrugs: A new biosafe approach. In Green Approaches in Medicinal Chemistry for Sustainable Drug Design; Elsevier: Amsterdam, The Netherlands, 2020; pp. 603–615. [Google Scholar] [CrossRef]
- Sun, T.; Dasgupta, A.; Zhao, Z.; Nurunnabi, M.; Mitragotri, S. Physical triggering strategies for drug delivery. Adv. Drug Deliv. Rev. 2020, 158, 36–62. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Ahmad, J.; Warsi, M.H.; Abdel-Wahab, B.A.; Akhter, S. Metallic nanoparticulate delivery systems. In Nanoengineered Biomaterials for Advanced Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2020; pp. 279–328. [Google Scholar] [CrossRef]
- Jafari, R.M.; Ala, M.; Goodarzi, N.; Dehpour, A.R. Does Pharmacodynamics of Drugs Change After Presenting them as Nanoparticles Like their Pharmacokinetics? Curr. Drug Targets 2020, 21, 807–818. [Google Scholar] [CrossRef]
- Kakaei, S.; Khameneh, E.S.; Ghasemi, E.; Aghazadeh, M. Targeted Drug Delivery of Teniposide by Magnetic Nanocarrier. Curr. Nanosci. 2020, 16, 608–616. [Google Scholar] [CrossRef]
- Aghajanzadeh, M.; Naderi, E.; Zamani, M.; Sharafi, A.; Naseri, M.; Danafar, H. In vivo and in vitro biocompatibility study of MnFe2O4 and Cr2Fe6O12 as photosensitizer for photodynamic therapy and drug delivery of anti-cancer drugs. Drug Dev. Ind. Pharm. 2020, 46, 846–851. [Google Scholar] [CrossRef]
- Al-Musawi, S.; Albukhaty, S.; Al-Karagoly, H.; Sulaiman, G.M.; Jabir, M.S.; Naderi-Manesh, H. Dextran-coated superparamagnetic nanoparticles modified with folate for targeted drug delivery of camptothecin. Adv. Nat. Sci. Nanosci. Nanotechnol. 2020, 11, 045009. [Google Scholar] [CrossRef]
- Gholami, A.; Mousavi, S.M.; Hashemi, S.A.; Ghasemi, Y.; Chiang, W.-H.; Parvin, N. Current trends in chemical modifications of magnetic nanoparticles for targeted drug delivery in cancer chemotherapy. Drug Metab. Rev. 2020, 52, 205–224. [Google Scholar] [CrossRef]
- Nabipour, H.; Hu, Y. Sustainable drug delivery systems through green nanotechnology. In Nanoengineered Biomaterials for Advanced Drug Delivery; Mozafari, M., Ed.; Elsevier: San Diego, CA, USA, 2020; pp. 61–89. [Google Scholar] [CrossRef]
- Sadhasivam, J.; Sugumaran, A. Magnetic nanocarriers: Emerging tool for the effective targeted treatment of lung cancer. J. Drug Deliv. Sci. Technol. 2020, 55, 101493. [Google Scholar] [CrossRef]
- Saadat, M.; Manshadi, M.K.D.; Mohammadi, M.; Zare, M.J.; Zarei, M.; Kamali, R.; Sanati-Nezhad, A. Magnetic particle targeting for diagnosis and therapy of lung cancers. Control. Release 2020, 328, 776–791. [Google Scholar] [CrossRef]
- Ngema, L.M.; Adeyemi, S.A.; Marimuthu, T.; Choonara, Y.E. A review on engineered magnetic nanoparticles in Non-Small-Cell lung carcinoma targeted therapy. Int. J. Pharm. 2021, 606, 120870. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, A.; Yadav, K.; Mishra, A.; Singh, M.S.; Chaudhary, S.; Manohar, R.; Parmar, A.S. Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems. Nanotechnol. Rev. 2022, 11, 544–574. [Google Scholar] [CrossRef]
- Hatami, E.; Nagesh, P.K.B.; Chauhan, N.; Jaggi, M.; Chauhan, S.C.; Yallapu, M.M. In Situ Nanoparticle Self-Assembly for Combination Delivery of Therapeutics to Non-Small Cell Lung Cancer. ACS Appl. Bio Mater. 2022, 5, 1104–1119. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Li, P.; Wang, Y.; Han, S. Manganese-Based Targeted Nanoparticles for Postoperative Gastric Cancer Monitoring via Magnetic Resonance Imaging. Front. Oncol. 2020, 10, 601538. [Google Scholar] [CrossRef]
- Zhan, W.; Cai, X.; Li, H.; Du, G.; Hu, H.; Wu, Y.; Wang, L. GMBP1-conjugated manganese oxide nanoplates for: In vivo monitoring of gastric cancer MDR using magnetic resonance imaging. RSC Adv. 2020, 10, 13687–13695. [Google Scholar] [CrossRef]
- Sharif, A.P.; Habibi, K.; Bijarpas, Z.K.; Tolami, H.F.; Alkinani, T.A.; Jameh, M.; Dehkaei, A.A.; Monhaser, S.K.; Daemi, H.B.; Mahmoudi, A.; et al. Cytotoxic Effect of a Novel GaFe2O4@Ag Nanocomposite Synthesized by Scenedesmus obliquus on Gastric Cancer Cell Line and Evaluation of BAX, Bcl-2 and CASP8 Genes Expression. J. Clust. Sci. 2022. [Google Scholar] [CrossRef]
- Hajializadeh, D.; Saber, A.A.; Jameh, M.; Ahang, B.; Moafy, A.; Bijarpas, Z.K.; Masouleh, R.S.; Kia, M.B.; Mojdehi, S.R.; Salehzadeh, A. Potential of Apoptosis-Inducing by a Novel Bio-synthesized CoFe2O4@Ag Nanocomposite in Gastric Cell Line at the Cellular and Molecular Level. J. Clust. Sci. 2022. [Google Scholar] [CrossRef]
- Zou, J.; Chen, S.; Li, Y.; Zeng, L.; Lian, G.; Li, J.; Chen, S.; Huang, K.; Chen, Y. Nanoparticles modified by triple single chain antibodies for MRI examination and targeted therapy in pancreatic cancer. Nanoscale 2020, 12, 4473–4490. [Google Scholar] [CrossRef]
- Karahaliloglu, Z.; Kilicay, E.; Hazer, B. PLinaS-g-PEG coated magnetic nanoparticles as a contrast agent for hepatocellular carcinoma diagnosis. J. Biomater. Sci. Polym. Ed. 2020, 31, 1580–1603. [Google Scholar] [CrossRef]
- Fu, Y.; He, G.; Liu, Z.; Wang, J.; Li, M.; Zhang, Z.; Bao, Q.; Wen, J.; Zhu, X.; Zhang, C.; et al. DNA Base Pairing-Inspired Supramolecular Nanodrug Camouflaged by Cancer-Cell Membrane for Osteosarcoma Treatment. Small 2022, 18, 2202337. [Google Scholar] [CrossRef]
- Zivarpour, P.; Hallajzadeh, J.; Asemi, Z.; Sadoughi, F.; Sharifi, M. Chitosan as possible inhibitory agents and delivery systems in leukemia. Cancer Cell Int. 2021, 21, 544. [Google Scholar] [CrossRef] [PubMed]
- Goorbandi, R.G.; Mohammadi, M.R.; Malekzadeh, K. Synthesizing efficacious genistein in conjugation with superparamagnetic Fe3O4 decorated with bio-compatible carboxymethylated chitosan against acute leukemia lymphoma. Biomater. Res. 2020, 24, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khutsishvili, S.S.; Aleksandrova, G.P.; Vakul’Skaya, T.I.; Sukhov, B.G. Structural and Magnetic Properties of Biocompatible-Coated Magnetite Nanoparticles for Treating Antianemia. IEEE Trans. Magn. 2021, 57, 5200309. [Google Scholar] [CrossRef]
- Hafez, A.A.; Salimi, A.; Jamali, Z.; Shabani, M.; Sheikhghaderi, H. Overview of the application of inorganic nanomaterials in breast cancer diagnosis. In Inorganic and Nano-Metal Chemistry; Taylor & Francis: Abingdon, UK, 2021. [Google Scholar] [CrossRef]
- Bai, X.; Su, G.; Zhai, S. Recent advances in nanomedicine for the diagnosis and therapy of liver fibrosis. Nanomaterials 2020, 10, 1945. [Google Scholar] [CrossRef]
- Farid, R.M.; Gaafar, P.M.E.; Hazzah, H.A.; Helmy, M.W.; Abdallah, O.Y. Chemotherapeutic potential of L-carnosine from stimuli-responsive magnetic nanoparticles against breast cancer model. Nanomedicine 2020, 15, 891–911. [Google Scholar] [CrossRef]
- Taheri-Ledari, R.; Zhang, W.; Radmanesh, M.; Mirmohammadi, S.S.; Maleki, A.; Cathcart, N.; Kitaev, V. Multi-Stimuli Nanocomposite Therapeutic: Docetaxel Targeted Delivery and Synergies in Treatment of Human Breast Cancer Tumor. Small 2020, 16, 2002733. [Google Scholar] [CrossRef]
- Panda, J.; Satapathy, B.S.; Mandal, B.; Sen, R.; Mukherjee, B.; Sarkar, R.; Tudu, B. Anticancer potential of docetaxel-loaded cobalt ferrite nanocarrier: An in vitro study on MCF-7 and MDA-MB-231 cell lines. J. Microencapsul. 2021, 38, 36–46. [Google Scholar] [CrossRef]
- Li, Z.; Wu, X.; Wang, W.; Gai, C.; Zhang, W.; Li, W.; Ding, D. Fe(II) and Tannic Acid-Cloaked MOF as Carrier of Artemisinin for Supply of Ferrous Ions to Enhance Treatment of Triple-Negative Breast Cancer. Nanoscale Res. Lett. 2021, 16, 37. [Google Scholar] [CrossRef]
- Helmy, L.A.; Abdel-Halim, M.; Hassan, R.; Sebak, A.; Farghali, H.A.M.; Mansour, S.; Tammam, S.N. The other side to the use of active targeting ligands; the case of folic acid in the targeting of breast cancer. Colloids Surf. B Biointerfaces 2022, 211, 112289. [Google Scholar] [CrossRef]
- Jani, K.; Kaushal, N.; Sadoqi, M.; Long, G.; Chen, Z.-S.; Squillante, E. Formulation and characterization of oleic acid magnetic PEG PLGA nanoparticles for targeting glioblastoma multiforme. J. Magn. Magn. Mater. 2021, 533, 167970. [Google Scholar] [CrossRef]
- Gandhi, H.; Sharma, A.K.; Mahant, S.; Kapoor, D.N. Recent advancements in brain tumor targeting using magnetic nanoparticles. Ther. Deliv. 2020, 11, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Guigou, C.; Lalande, A.; Millot, N.; Belharet, K.; Grayeli, A.B. Use of super paramagnetic iron oxide nanoparticles as drug carriers in brain and ear: State of the art and challenges. Brain Sci. 2021, 11, 358. [Google Scholar] [CrossRef] [PubMed]
- Estelrich, J.; Busquets, M.A. Magnetic Nanoparticles as Delivery Systems to Penetrate the Blood-Brain Barrier. Neuromethods 2021, 157, 173–208. [Google Scholar] [CrossRef]
- Joshi, B.; Joshi, A. Polymeric magnetic nanoparticles: A multitargeting approach for brain tumour therapy and imaging. Drug Deliv. Transl. Res. 2022, 12, 1588–1604. [Google Scholar] [CrossRef]
- Dhar, D.; Ghosh, S.; Das, S.; Chatterjee, J. A review of recent advances in magnetic nanoparticle-based theranostics of glioblastoma. Nanomedicine 2022, 17, 107–132. [Google Scholar] [CrossRef]
- Yasaswi, P.S.; Shetty, K.; Yadav, K.S. Temozolomide nano enabled medicine: Promises made by the nanocarriers in glioblastoma therapy. J. Control. Release 2021, 336, 549–571. [Google Scholar] [CrossRef]
- Chaudhary, R.; Morris, R.J.; Steinson, E. The multifactorial roles of microglia and macrophages in the maintenance and progression of glioblastoma. J. Neuroimmunol. 2021, 357, 577633. [Google Scholar] [CrossRef]
- Gupta, R.; Kaur, T.; Chauhan, A.; Kumar, R.; Kuanr, B.K.; Sharma, D. Tailoring nanoparticles design for enhanced heating efficiency and improved magneto-chemo therapy for glioblastoma. Biomater. Adv. 2022, 139, 213021. [Google Scholar] [CrossRef]
- Guo, L.; Yang, C.; Yang, R.; Zhao, W. Magnetically anchored antibody-coupled nanocomposite as α-Amylase inhibitor for long-time protection against glycemic variability. Chem. Eng. J. 2022, 430, 132984. [Google Scholar] [CrossRef]
- Zhou, H.; Alici, G. A Magnetically Actuated Novel Robotic Capsule for Site-Specific Drug Delivery Inside the Gastrointestinal Tract. IEEE Trans. Syst. Man Cybern. Syst. 2022, 52, 4010–4020. [Google Scholar] [CrossRef]
- Cicha, I.; Alexiou, C. Cardiovascular applications of magnetic particles. J. Magn. Magn. Mater. 2021, 518, 167428. [Google Scholar] [CrossRef]
- Zhao, Y.-Z.; Chen, R.; Xue, P.-P.; Luo, L.-Z.; Zhong, B.; Tong, M.-Q.; Chen, B.; Yao, Q.; Yuan, J.-D.; Xu, H.-L. Magnetic PLGA microspheres loaded with SPIONs promoted the reconstruction of bone defects through regulating the bone mesenchymal stem cells under an external magnetic field. Mater. Sci. Eng. C 2021, 122, 111877. [Google Scholar] [CrossRef] [PubMed]
- Modak, M.; Bobbala, S.; Lescott, C.; Liu, Y.-G.; Nandwana, V.; Dravid, V.P.; Scott, E.A. Magnetic Nanostructure-Loaded Bicontinuous Nanospheres Support Multicargo Intracellular Delivery and Oxidation-Responsive Morphological Transitions. ACS Appl. Mater. Interfaces 2020, 12, 55584–55595. [Google Scholar] [CrossRef] [PubMed]
- Janik-Olchawa, N.; Drozdz, A.; Ryszawy, D.; Pudełek, M.; Planeta, K.; Setkowicz, Z.; Śniegocki, M.; Żądło, A.; Ostachowicz, B.; Chwiej, J. Comparison of ultrasmall IONPs and Fe salts biocompatibility and activity in multi-cellular in vitro models. Sci. Rep. 2020, 10, 15447. [Google Scholar] [CrossRef]
- Nguyen, K.; Nuß, B.; Mühlberger, M.; Unterweger, H.; Friedrich, R.P.; Alexiou, C.; Janko, C. Superparamagnetic iron oxide nanoparticles carrying chemotherapeutics improve drug efficacy in monolayer and spheroid cell culture by enabling active accumulation. Nanomaterials 2020, 10, 1577. [Google Scholar] [CrossRef]
- Friedrich, R.P.; Schreiber, E.; Tietze, R.; Yang, H.; Pilarsky, C.; Alexiou, C. Intracellular quantification and localization of label-free iron oxide nanoparticles by holotomographic microscopy. Nanotechnol. Sci. Appl. 2020, 13, 119–130. [Google Scholar] [CrossRef]
- Raval, Y.S.; Samstag, A.; Taylor, C.; Huang, G.; Mefford, O.T.; Tzeng, T.-R.J. Assessing the biocompatibility of multi-anchored glycoconjugate functionalized iron oxide nanoparticles in a normal human colon cell line ccd-18co. Nanomaterials 2021, 11, 2465. [Google Scholar] [CrossRef]
- Pardo, A.; Gómez-Florit, M.; Barbosa, S.; Taboada, P.; Domingues, R.M.A.; Gomes, M.E. Magnetic Nanocomposite Hydrogels for Tissue Engineering: Design Concepts and Remote Actuation Strategies to Control Cell Fate. ACS Nano 2021, 15, 175–209. [Google Scholar] [CrossRef]
- Ivanova, A.V.; Nikitin, A.A.; Gabashvily, A.N.; Vishnevskiy, D.A.; Abakumov, M.A. Synthesis and intensive analysis of antibody labeled single core magnetic nanoparticles for targeted delivery to the cell membrane. J. Magn. Magn. Mater. 2021, 521, 167487. [Google Scholar] [CrossRef]
- Hillion, A.; Hallali, N.; Clerc, P.; Lopez, S.; Lalatonne, Y.; Noûs, C.; Motte, L.; Gigoux, V.; Carrey, J. Real-Time Observation and Analysis of Magnetomechanical Actuation of Magnetic Nanoparticles in Cells. Nano Lett. 2022, 22, 1986–1991. [Google Scholar] [CrossRef]
- Goren, K.; Neelam, N.; Yuval, J.B.; Weiss, D.J.; Kunicher, N.; Margel, S.; Mintz, Y. Targeting tumor cells using magnetic nanoparticles–A feasibility study in animal models. In Minimally Invasive Therapy and Allied Technologies; Taylor&Francis: Abingdon, UK, 2022. [Google Scholar] [CrossRef]
- Ishmukhametov, I.; Batasheva, S.; Rozhina, E.; Akhatova, F.; Mingaleeva, R.; Rozhin, A.; Fakhrullin, R. DNA/Magnetic Nanoparticles Composite to Attenuate Glass Surface Nanotopography for Enhanced Mesenchymal Stem Cell Differentiation. Polymers 2022, 14, 344. [Google Scholar] [CrossRef] [PubMed]
- Mohammadalizadeh, M.; Dabirian, S.; Akrami, M.; Hesari, Z. SPION based magnetic PLGA nanofibers for neural differentiation of mesenchymal stem cells. Nanotechnology 2022, 33, 375101. [Google Scholar] [CrossRef] [PubMed]
- Mazloum Tabaei, M.A.; Bamoniri, A.; Mirjalili, B.B.F. One-pot Biginelli synthesis of 3,4-dihydropyrimidin-2(1H)-ones using nano-cellulose/BF3/Fe3O4. J. Iran. Chem. Soc. 2022, 19, 2679–2691. [Google Scholar] [CrossRef]
- Panina, L.V.; Gurevich, A.; Beklemisheva, A.; Omelyanchik, A.; Levada, K.; Rodionova, V. Spatial Manipulation of Particles and Cells at Micro-and Nanoscale via Magnetic Forces. Cells 2022, 11, 950. [Google Scholar] [CrossRef] [PubMed]
- Dalal, V.; Biswas, S. Nanoscience: Convergence with Biomedical and Biological Applications. In Functional Bionanomaterials; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–25. [Google Scholar] [CrossRef]
- Puri, R.; Arora, V.; Kabra, A.; Dureja, H.; Hamaal, S. Magnetosomes: A Tool for Targeted Drug Delivery in the Management of Cancer. J. Nanomater. 2022, 2022, 6414585. [Google Scholar] [CrossRef]
- Kotakadi, S.M.; Borelli, D.P.R.; Nannepaga, J.S. Therapeutic Applications of Magnetotactic Bacteria and Magnetosomes: A Review Emphasizing on the Cancer Treatment. Front. Bioeng. Biotechnol. 2022, 10, 789016. [Google Scholar] [CrossRef]
- Ying, G.; Zhang, G.; Yang, J.; Hao, Z.; Xing, W.; Lu, D.; Zhang, S.; Yan, L. Biomineralization and biotechnological applications of bacterial magnetosomes. Colloids Surf. B Biointerfaces 2022, 216, 112556. [Google Scholar] [CrossRef]
- Zwiener, T.; Dziuba, M.; Mickoleit, F.; Rückert, C.; Busche, T.; Kalinowski, J.; Uebe, R.; Schüler, D. Towards a ‘chassis’ for bacterial magnetosome biosynthesis: Genome streamlining of Magnetospirillum gryphiswaldense by multiple deletions. Microb. Cell Factories 2021, 20, 35. [Google Scholar] [CrossRef]
- Jubran, A.S.; Al-Zamely, O.M.; Al-Ammar, M.H. A study of iron oxide nanoparticles synthesis by using bacteria. Int. J. Pharm. Qual. Assur. 2020, 11, 88–92. [Google Scholar] [CrossRef]
- Nemati, Z.; Um, J.; Zamani Kouhpanji, M.R.; Zhou, F.; Gage, T.; Shore, D.; Makielski, K.; Donnelly, A.; Alonso, J. Magnetic Isolation of Cancer-Derived Exosomes Using Fe/Au Magnetic Nanowires. ACS Appl. Nano Mater. 2020, 3, 2058–2069. [Google Scholar] [CrossRef]
- Talukdar, S.; Saha, P.; Mandal, K. Folate modified zinc ferrite nano-hollowspheres for drug delivery and intrinsic fluorescence. AIP Conf. Proc. 2020, 2265, 030131. [Google Scholar] [CrossRef]
- Rani, V.; Venkatesan, J.; Prabhu, A. Nanotherapeutics in glioma management: Advances and future perspectives. J. Drug Deliv. Sci. Technol. 2020, 57, 101626. [Google Scholar] [CrossRef]
- D’souza, U.P.; Rao, N.; Shriram, R.G.; Dubey, A. In vitro and in vivo exploration of super-magnetically modulated novel parenteral carrier: A targeted drug delivery of polymeric carboplatin nanosphere capped with the vitamin c to treat breast cancer in rats. Indian J. Pharm. Educ. Res. 2020, 54, S140–S153. [Google Scholar] [CrossRef]
- Lim, E.-B.; Vy, T.A.; Lee, S.-W. Comparative release kinetics of small drugs (ibuprofen and acetaminophen) from multifunctional mesoporous silica nanoparticles. J. Mater. Chem. B 2020, 8, 2096–2106. [Google Scholar] [CrossRef] [PubMed]
- Anirudhan, T.S.; Christa, J. Temperature and pH sensitive multi-functional magnetic nanocomposite for the controlled delivery of 5-fluorouracil, an anticancer drug. J. Drug Deliv. Sci. Technol. 2020, 55, 101476. [Google Scholar] [CrossRef]
- Ayyanaar, S.; Bhaskar, R.; Esthar, S.; Vadivel, M.; Rajesh, J.; Rajagopal, G. Design and development of 5-fluorouracil loaded biodegradable magnetic microspheres as site-specific drug delivery vehicle for cancer therapy. J. Magn. Magn. Mater. 2022, 546, 168853. [Google Scholar] [CrossRef]
- Truong, D.H.; Le, V.K.H.; Pham, T.T.; Dao, A.H.; Pham, T.P.D.; Tran, T.H. Delivery of erlotinib for enhanced cancer treatment: An update review on particulate systems. J. Drug Deliv. Sci. Technol. 2020, 55, 101348. [Google Scholar] [CrossRef]
- Jithendra, T.; Sreekanth Reddy, O.; Subha, M.C.S.; Chowdoji Rao, K. Fabrication of drug delivery system for controlled release of curcumin, intercalated with magnetite nanoparticles through sodium alginate/polyvinylpyrrolidone-co-vinyl acetate semi ipn microbeads. Int. J. Appl. Pharm. 2020, 12, 249–257. [Google Scholar] [CrossRef]
- de Santana, W.M.O.S.; Caetano, B.L.; de Annunzio, S.R.; Pulcinelli, S.H.; Ménager, C.; Fontana, C.R.; Santilli, C.V. Conjugation of superparamagnetic iron oxide nanoparticles and curcumin photosensitizer to assist in photodynamic therapy. Colloids Surf. B Biointerfaces 2020, 196, 111297. [Google Scholar] [CrossRef]
- Dutta, B.; Rawoot, Y.A.; Checker, S.; Shelar, S.B.; Barick, K.C.; Kumar, S.; Somani, R.R.; Hassan, P.A. Micellar assisted aqueous stabilization of iron oxide nanoparticles for curcumin encapsulation and hyperthermia application. Nano-Struct. Nano-Objects 2020, 22, 100466. [Google Scholar] [CrossRef]
- Santos, E.C.S.; Cunha, J.A.; Martins, M.G.; Galeano-Villar, B.M.; Caraballo-Vivas, R.J.; Leite, P.B.; Rossi, A.L.; Garcia, F.; Finotelli, P.V.; Ferraz, H.C. Curcuminoids-conjugated multicore magnetic nanoparticles: Design and characterization of a potential theranostic nanoplatform. J. Alloys Compd. 2021, 879, 160448. [Google Scholar] [CrossRef]
- Molaei, H.; Zaaeri, F.; Sharifi, S.; Ramazani, A.; Safaei, S.; Abdolmohammadi, J.; Khoobi, M. Polyethylenimine-graft-poly (maleic anhydride-alt-1-octadecene) coated Fe3O4 magnetic nanoparticles: Promising targeted pH-sensitive system for curcumin delivery and MR imaging. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 1344–1353. [Google Scholar] [CrossRef]
- Gharagozlou, M. NiFe2O4@SiO2@HKUST-1 as Novel Magnetic Metal-Organic Framework Nanocomposites for the Curcumin Adsorption. J. Nanostructures 2022, 12, 455–473. [Google Scholar] [CrossRef]
- Gharehaghaji, N.; Divband, B. PEGylated Magnetite/Hydroxyapatite: A Green Nanocomposite for T2-Weighted MRI and Curcumin Carrying. Evid. Based Complementary Altern. Med. 2022, 2022, 1337588. [Google Scholar] [CrossRef] [PubMed]
- Kowalik, P.; Mikulski, J.; Borodziuk, A.; Duda, M.; Kamińska, I.; Zajdel, K.; Rybusinski, J.; Szczytko, J.; Wojciechowski, T.; Sobczak, K.; et al. Yttrium-Doped Iron Oxide Nanoparticles for Magnetic Hyperthermia Applications. J. Phys. Chem. C 2020, 124, 6871–6883. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Sahu, N.K. Folate encapsulation in PEG-diamine grafted mesoporous Fe3O4 nanoparticles for hyperthermia and in vitro assessment. IET Nanobiotechnol. 2020, 14, 881–888. [Google Scholar] [CrossRef]
- Gahrouei, Z.E.; Imani, M.; Soltani, M.; Shafyei, A. Synthesis of iron oxide nanoparticles for hyperthermia application: Effect of ultrasonic irradiation assisted co-precipitation route. Adv. Nat. Sci. Nanosci. Nanotechnol. 2020, 11, 025001. [Google Scholar] [CrossRef]
- Barrera, G.; Coisson, M.; Celegato, F.; Martino, L.; Tiwari, P.; Verma, R.; Kane, S.N.; Mazaleyrat, F.; Tiberto, P. Specific loss power of Co/Li/Zn-mixed ferrite powders for magnetic hyperthermia. Sensors 2020, 20, 2151. [Google Scholar] [CrossRef]
- Salati, A.; Ramazani, A.; Kashi, M.A. Tuning hyperthermia properties of FeNiCo ternary alloy nanoparticles by morphological and magnetic characteristics. J. Magn. Magn. Mater. 2020, 498, 166172. [Google Scholar] [CrossRef]
- Leonel, A.G.; Mansur, A.A.P.; Carvalho, S.M.; Outon, L.E.F.; Ardisson, J.D.; Krambrock, K.; Mansur, H.S. Tunable magnetothermal properties of cobalt-doped magnetite–carboxymethylcellulose ferrofluids: Smart nanoplatforms for potential magnetic hyperthermia applications in cancer therapy. Nanoscale Adv. 2021, 3, 1029. [Google Scholar] [CrossRef]
- Derakhshankhah, H.; Jahanban-Esfahlan, R.; Vandghanooni, S.; Akbari-Nakhjavani, S.; Massoumi, B.; Haghshenas, B.; Rezaei, A.; Farnudiyan-Habibi, A.; Samadian, H.; Jaymand, M. A bio-inspired gelatin-based pH - and thermal-sensitive magnetic hydrogel for in vitro chemo/hyperthermia treatment of breast cancer cells. J. Appl. Polym. Sci. 2021, 138, 50578. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Asgharnasl, S.; Bani, M.S.; Radinekiyan, F.; Maleki, A.; Mahdavi, M.; Babaniamansour, P.; Bahreinizad, H.; Shalan, A.E.; Lanceros-Méndez, S. Magnetic Copper Ferrite Nanoparticles Functionalized by Aromatic Polyamide Chains for Hyperthermia Applications. Langmuir 2021, 37, 8847–8854. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Kim, N.P.; Attanayake, S.B.; Phan, M.-H.; Srikanth, H. Role of magnetic anisotropy on the hyperthermia efficiency in spherical Fe3−xCoxO4 (X = 0–1) nanoparticles. Appl. Sci. 2021, 11, 930. [Google Scholar] [CrossRef]
- Fiorito, S.; Soni, N.; Silvestri, N.; Brescia, R.; Gavilán, H.; Conteh, J.S.; Mai, B.T.; Pellegrino, T. Fe3O4@Au@Cu2−xS Heterostructures Designed for Tri-Modal Therapy: Photo- Magnetic Hyperthermia and 64Cu Radio-Insertion. Small 2022, 18, 2200174. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Hasan, M.R.; Haque, M.M.; Rashid, R.; Syed, I.M.; Hoque, S.M. Efficacy of surface-functionalized Mg1−xCoxFe2O4 (0 < x < 1; Δx = 0.1) for hyperthermia and in vivo MR imaging as a contrast agent. RSC Adv. 2022; 12, 7835–7849. [Google Scholar] [CrossRef]
- Benjamin, A.S.; Remya, V.; Arunai Nambi Raj, N.; Nayak, S. Evaluation of Doxorubicin Loaded Gelatin Coated Iron Oxide Nanoparticles for Drug Delivery and Magnetic Hyperthermia for Anti-Cancer Treatment. Trends Biomater. Artif. Organs 2022, 36, 3–10. [Google Scholar]
- Kovrigina, E.; Chubarov, A.; Dmitrienko, E. High Drug Capacity Doxorubicin-Loaded Iron Oxide Nanocomposites for Cancer Therapy. Magnetochemistry 2022, 8, 54. [Google Scholar] [CrossRef]
- Spiridonov, V.; Panova, I.; Kusaia, V.; Makarova, L.; Romodina, M.; Fedyanin, A.; Pozdnyakova, N.; Shibaeva, A.; Zezin, S.; Sybachin, A.; et al. Doxorubicin Loaded Magnetosensitive Water-Soluble Nanogel Based on NIPAM and Iron (3+) Containing Nanoparticles. Macromol. Symp. 2020, 389, 1900072. [Google Scholar] [CrossRef]
- Obireddy, S.R.; Chintha, M.; Kashayi, C.R.; Venkata, K.R.K.S.; Subbarao, S.M.C. Gelatin-Coated Dual Cross-Linked Sodium Alginate/Magnetite Nanoparticle Microbeads for Controlled Release of Doxorubicin. ChemistrySelect 2020, 5, 10276–10284. [Google Scholar] [CrossRef]
- Prospero, A.G.; Fidelis-De-Oliveira, P.; Soares, G.A.; Miranda, M.F.; Pinto, L.A.; Dos Santos, D.C.; Silva, V.D.S.; Zufelato, N.; Bakuzis, A.F.; Miranda, J.R.A. AC biosusceptometry and magnetic nanoparticles to assess doxorubicin-induced kidney injury in rats. Nanomedicine 2020, 15, 511–525. [Google Scholar] [CrossRef]
- Javadian, S.; Najafi, K.; Sadrpoor, S.M.; Ektefa, F.; Dalir, N.; Nikkhah, M. Graphene quantum dots based magnetic nanoparticles as a promising delivery system for controlled doxorubicin release. J. Mol. Liq. 2021, 331, 115746. [Google Scholar] [CrossRef]
- Fant, C.; Granzotto, A.; Mestas, J.-L.; Ngo, J.; Lafond, M.; Lafon, C.; Foray, N.; Padilla, F. DNA Double-Strand Breaks in Murine Mammary Tumor Cells Induced by Combined Treatment with Doxorubicin and Controlled Stable Cavitation. Ultrasound Med. Biol. 2021, 47, 2941–2957. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J.V.S.; Da Silva, M.R.; Da Silva, M.A.C. Magnetic gelatin microspheres for targeted release of doxorubicin. Mater. Res. 2021, 24, e20210176. [Google Scholar] [CrossRef]
- Ramnandan, D.; Mokhosi, S.; Daniels, A.; Singh, M. Chitosan, polyethylene glycol and polyvinyl alcohol modified MgFe2O4 ferrite magnetic nanoparticles in doxorubicin delivery: A comparative study in vitro. Molecules 2021, 26, 3893. [Google Scholar] [CrossRef] [PubMed]
- Ehsanimehr, S.; Moghadam, P.N.; Dehaen, W.; Irannejad, V.S. PEI grafted Fe3O4@SiO2@SBA-15 labeled FA as a pH-sensitive mesoporous magnetic and biocompatible nanocarrier for targeted delivery of doxorubicin to MCF-7 cell line. Colloids Surf. A Physicochem. Eng. Asp. 2021, 615, 126302. [Google Scholar] [CrossRef]
- Obireddy, S.R.; Lai, W.-F. ROS-Generating Amine-Functionalized Magnetic Nanoparticles Coupled with Carboxymethyl Chitosan for pH-Responsive Release of Doxorubicin. Int. J. Nanomed. 2022, 17, 589–601. [Google Scholar] [CrossRef]
- Korolkov, I.V.; Ludzik, K.; Kozlovskiy, A.L.; Fadeev, M.S.; Shumskaya, A.E.; Gorin, Y.G.; Jazdzewska, M.; Anisovich, M.; Rusakov, V.S.; Zdorovets, M.V. Immobilization of carboranes on Fe3O4-polymer nanocomposites for potential application in boron neutron cancer therapy. Colloids Surfaces A: Physicochem. Eng. Asp. 2020, 601, 125035. [Google Scholar] [CrossRef]
- Rashid, Z.; Shokri, F.; Abbasi, A.; Khoobi, M.; Zarnani, A. Surface modification and bioconjugation of anti-CD4 monoclonal antibody to magnetic nanoparticles as a highly efficient affinity adsorbent for positive selection of peripheral blood T CD4+ lymphocytes. Int. J. Biol. Macromol. 2020, 161, 729–737. [Google Scholar] [CrossRef]
- Sundar, S.; Ganesh, V. Bio-assisted preparation of efficiently architectured nanostructures of γ-Fe2O3 as a molecular recognition platform for simultaneous detection of biomarkers. Sci. Rep. 2020, 10, 15071. [Google Scholar] [CrossRef] [PubMed]
- Parsian, M.; Mutlu, P.; Yalcin, S.; Gunduz, U. Characterization of gemcitabine loaded polyhydroxybutyrate coated magnetic nanoparticles for targeted drug delivery. Anti-Cancer Agents Med. Chem. 2020, 20, 1233–1240. [Google Scholar] [CrossRef]
- Lee, X.J.; Lim, H.N.; Gowthaman, N.S.K.; Rahman, M.B.A.; Abdullah, C.A.C.; Muthoosamy, K. In-situ surface functionalization of superparamagnetic reduced graphene oxide—Fe3O4 nanocomposite via Ganoderma lucidum extract for targeted cancer therapy application. Appl. Surf. Sci. 2020, 512, 145738. [Google Scholar] [CrossRef]
- Ashjari, M.; Panahandeh, F.; Niazi, Z.; Abolhasani, M.M. Synthesis of PLGA–mPEG star-like block copolymer to form micelle loaded magnetite as a nanocarrier for hydrophobic anticancer drug. J. Drug Deliv. Sci. Technol. 2020, 56, 101563. [Google Scholar] [CrossRef]
- Khan, B.; Nawaz, M.; Price, G.J.; Hussain, R.; Baig, A.; Haq, S.; Rehman, W.; Waseem, M. In vitro sustained release of gallic acid from the size-controlled PEGylated magnetite nanoparticles. Chem. Pap. 2021, 75, 5339–5352. [Google Scholar] [CrossRef]
- Harris, R.A. The PEGylated and non-PEGylated interaction of the anticancer drug 5-fluorouracil with paramagnetic Fe3O4 nanoparticles as drug carrier. J. Mol. Liq. 2022, 360, 119515. [Google Scholar] [CrossRef]
- Fuentes-Baile, M.; Pérez-Valenciano, E.; García-Morales, P.; Romero, C.d.; Bello-Gil, D.; Barberá, V.M.; Rodríguez-Lescure, Á.; Sanz, J.M.; Alenda, C.; Saceda, M. CLytA-DAAO Chimeric Enzyme Bound to Magnetic Nanoparticles. A New Therapeutical Approach for Cancer Patients? Int. J. Mol. Sci. 2021, 22, 1477. [Google Scholar] [CrossRef]
- Rezaei, A.; Morsali, A.; Bozorgmehr, M.R.; Nasrabadi, M. Quantum chemical analysis of 5-aminolevulinic acid anticancer drug delivery systems: Carbon nanotube, –COOH functionalized carbon nanotube and iron oxide nanoparticle. J. Mol. Liq. 2021, 340, 117182. [Google Scholar] [CrossRef]
- Karade, V.C.; Sharma, A.; Dhavale, R.P.; Dhavale, R.P.; Shingte, S.R.; Patil, P.S.; Kim, J.H.; Zahn, D.R.T.; Chougale, A.D.; Salvan, G.; et al. APTES monolayer coverage on self-assembled magnetic nanospheres for controlled release of anticancer drug Nintedanib. Sci. Rep. 2021, 11, 5674. [Google Scholar] [CrossRef]
- Marycz, K.; Kornicka-Garbowska, K.; Patej, A.; Sobierajska, P.; Kotela, A.; Turlej, E.; Kepska, M.; Bienko, A.; Wiglusz, R.J. Aminopropyltriethoxysilane (APTES)-Modified Nanohydroxyapatite (nHAp) Incorporated with Iron Oxide (IO) Nanoparticles Promotes Early Osteogenesis, Reduces Inflammation and Inhibits Osteoclast Activity. Materials 2022, 15, 2095. [Google Scholar] [CrossRef]
- Dhavale, R.P.; Dhavale, R.P.; Sahoo, S.C.; Kollu, P.; Jadhav, S.U.; Patil, P.S.; Dongale, T.D.; Chougale, A.D.; Patil, P.B. Chitosan coated magnetic nanoparticles as carriers of anticancer drug Telmisartan: pH-responsive controlled drug release and cytotoxicity studies. J. Phys. Chem. Solids 2021, 148, 109749. [Google Scholar] [CrossRef]
- Taheri-Kafrani, A.; Shirzadfar, H.; Abbasi Kajani, A.; Kudhair, B.K.; Jasim Mohammed, L.; Mohammadi, S.; Lotfi, F. Functionalized graphene oxide/Fe3O4 nanocomposite: A biocompatible and robust nanocarrier for targeted delivery and release of anticancer agents. J. Biotechnol. 2021, 331, 26–36. [Google Scholar] [CrossRef]
- Al-Jameel, S.S.; Rehman, S.; Almessiere, M.A.; Khan, F.A.; Slimani, Y.; Al-Saleh, N.S.; Manikandan, A.; Al-Suhaimi, E.A.; Baykal, A. Anti-microbial and anti-cancer activities of Mn0.5Zn0.5DyxFe2−xO4 (x ≤ 0.1) nanoparticles. Artif. Cells Nanomed. Biotechnol. 2021, 49, 493–499. [Google Scholar] [CrossRef]
- Tomeh, M.A.; Hadianamrei, R.; Xu, D.; Brown, S.; Zhao, X. Peptide-functionalised magnetic silk nanoparticles produced by a swirl mixer for enhanced anticancer activity of ASC-J9. Colloids Surf. B Biointerfaces 2022, 216, 112549. [Google Scholar] [CrossRef] [PubMed]
- Sugito, S.F.A.; Firdaus, F.; Aung, Y.; Sakti, S.C.W.; Chiu, H.-T.; Fahmi, M.Z. In situ tailoring of carbon dots-metal ferrite nanohybrid as multipurpose marker agent of HeLa cancer cells. J. Mater. Res. 2022, 37, 1941–1951. [Google Scholar] [CrossRef]
- Ramezanpour, A.; Karami, K.; Kharaziha, M.; Bayat, P.; Jamshidian, N. Smart poly(amidoamine) dendron-functionalized magnetic graphene oxide for cancer therapy. New J. Chem. 2022, 46, 5052–5064. [Google Scholar] [CrossRef]
- Sagir, T.; Huysal, M.; Senel, M.; Isık, S.; Burgucu, N.; Tabakoglu, O.; Zaim, M. Folic acid conjugated PAMAM-modified mesoporous silica-coated superparamagnetic iron oxide nanoparticles for potential cancer therapy. J. Colloid Interface Sci. 2022, 625, 711–721. [Google Scholar] [CrossRef]
- Yeganeh, F.E.; Yeganeh, A.E.; Yousefi, M.; Far, B.F.; Akbarzadeh, I.; Bokov, D.O.; Raahemifar, K.; Soltani, M. Formulation and Characterization of Poly (Ethylene Glycol)-Coated Core-Shell Methionine Magnetic Nanoparticles as a Carrier for Naproxen Delivery: Growth Inhibition of Cancer Cells. Cancers 2022, 14, 1797. [Google Scholar] [CrossRef]
- Serio, F.; Silvestri, N.; Avugadda, S.K.; Nucci, G.E.P.; Nitti, S.; Onesto, V.; Catalano, F.; D’Amone, E.; Gigli, G.; del Mercato, L.L.; et al. Co-loading of doxorubicin and iron oxide nanocubes in polycaprolactone fibers for combining Magneto-Thermal and chemotherapeutic effects on cancer cells. J. Colloid Interface Sci. 2022, 607, 34–44. [Google Scholar] [CrossRef]
- Suhasini, A.; Kumar, K.P.V.; Sheela, A.M.; Sheenkumar, N. Electrical and Electrochemical studies of Polyurethane diol/Polycaprolactone-Iron Oxide nanocomposites. IOP Conf. Ser. Mater. Sci. Eng. 2020, 983, 012009. [Google Scholar] [CrossRef]
- Bahjat, H.H.; Ismail, R.A.; Sulaiman, G.M.; Mohammed, H.A.; Al-Omar, M.; Mohammed, S.A.A.; Khan, R.A. Preparation of Iron Oxide and Titania-Based Composite, Core-Shell Populated, Nanoparticulates Material by Two-Step LASER Ablation in Aqueous Media as Antimicrobial and Anticancer Agents. Bioinorg. Chem. Appl. 2022, 2022, 1854473. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.F.; Eshaghi, Z. Synthesis of Bio-Nanomagnetite and Its Optimized Conditions for Phthalate Absorption. Int. J. Eng. Sci. Technol. 2020, 4, 3. [Google Scholar] [CrossRef]
- Kurniawan, D.W.; Booijink, R.; Pater, L.; Wols, I.; Vrynas, A.; Storm, G.; Prakash, J.; Bansal, R. Fibroblast growth factor 2 conjugated superparamagnetic iron oxide nanoparticles (FGF2-SPIONs) ameliorate hepatic stellate cells activation in vitro and acute liver injury in vivo. J. Control. Release 2020, 328, 640–652. [Google Scholar] [CrossRef]
- Zachanowicz, E.; Kulpa-Greszta, M.; Tomaszewska, A.; Gazińska, M.; Marędziak, M.; Marycz, K.; Pazik, R. Multifunctional properties of binary polyrhodanine manganese ferrite nanohybrids—from the energy converters to biological activity. Polymers 2020, 12, 2934. [Google Scholar] [CrossRef] [PubMed]
- Rong, R.; Zhang, Y.; Tan, W.; Hu, T.; Wang, X.; Gui, Z.; Gong, J.; Xu, X. Evidence of Translocation of Oral Zn2+ Doped Magnetite Nanoparticles across the Small Intestinal Wall of Mice and Deposition in Spleen: Unique Advantage in Biomedical Applications. ACS Appl. Bio Mater. 2020, 3, 7919–7929. [Google Scholar] [CrossRef]
- Yang, L.; Fu, L.; Li, B.; Ma, J.; Li, C.; Jin, T.; Gu, W. Fluorescence enhancement method for enrofloxacin extraction by core-shell magnetic microspheres. Aust. J. Chem. 2020, 73, 1105–1111. [Google Scholar] [CrossRef]
- Yeganeh, F.E.; Yousefi, M.; Hekmati, M.; Bikhof, M. An experimental research on pH-responsive amino acid-coated Ni(1−x)CoxFe2O4 nanoparticles as a nano-carrier for drug delivery and biological applications. Chem. Pap. 2021, 75, 6047–6057. [Google Scholar] [CrossRef]
- Amiri, M.A.; Aghamaali, M.R.; Parsian, H.; Tashakkorian, H. Coenzyme Q0 immobilized on magnetic nanoparticle: Synthesis and antitumoral effect on saos, MCF7 and hela cell lines. Iran. J. Pharm. Res. 2020, 19, 394–409. [Google Scholar] [CrossRef]
- Micheletti, G.; Boga, C.; Telese, D.; Cassani, M.C.; Boanini, E.; Nitti, P.; Ballarin, B.; Ghirri, A.; Barucca, G.; Rinaldi, D. Magnetic Nanoparticles Coated with (R)-9-Acetoxystearic Acid for Biomedical Applications. ACS Omega 2020, 5, 12707–12715. [Google Scholar] [CrossRef]
- Kawish, M.; Elhissi, A.; Jabri, T.; Iqbal, K.M.; Zahid, H.; Shah, M.R. Enhancement in oral absorption of ceftriaxone by highly functionalized magnetic iron oxide nanoparticles. Pharmaceutics 2020, 12, 492. [Google Scholar] [CrossRef]
- Huang, J.; Guo, J.; Zou, X.; Zhu, J.; Wu, S.; Zhang, T. Bioinspired Heteromultivalent Chitosan- α -Fe2O3 /Gadofullerene Hybrid Composite for Enhanced Antibiotic-Resistant Bacterial Pneumonia. J. Biomed. Nanotechnol. 2021, 17, 1217–1228. [Google Scholar] [CrossRef]
- Senthilkumar, M.; Pandimurugan, R.; Muthuvinayagam, M. Investigations on chitosan/α-Fe2O3 nanocomposite for efficient antibacterial activity. Indian J. Appl. Res. 2021, 67–69. [Google Scholar] [CrossRef]
- Fiejdasz, S.; Gilarska, A.; Strączek, T.; Nowakowska, M.; Kapusta, C. Magnetic Properties of Collagen–Chitosan Hybrid Materials with Immobilized Superparamagnetic Iron Oxide Nanoparticles (SPIONs). Materials 2021, 14, 7652. [Google Scholar] [CrossRef]
- Ahmadi, M.; Pourmadadi, M.; Ghorbanian, S.A.; Yazdian, F.; Rashedi, H. Ultra pH-sensitive nanocarrier based on Fe2O3/chitosan/montmorillonite for quercetin delivery. Int. J. Biol. Macromol. 2021, 191, 738–745. [Google Scholar] [CrossRef]
- Forouzan, M.M.; Ghasemzadeh, M.A.; Razavian, S.M.H. Preparation and characterization of a novel Fe3O4@PAA@MIL-100(Cr) metal-organic framework for the drug delivery of ciprofloxacin and investigation of its antibacterial activities. Inorg. Nano-Metal Chem. 2022, 52, 1318–1324. [Google Scholar] [CrossRef]
- Mehrabi, F.; Shamspur, T.; Sheibani, H.; Mostafavi, A.; Mohamadi, M.; Hakimi, H.; Bahramabadi, R.; Salari, E. Silver-coated magnetic nanoparticles as an efficient delivery system for the antibiotics trimethoprim and sulfamethoxazole against E. Coli and S. aureus: Release kinetics and antimicrobial activity. BioMetals 2021, 34, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Casillas-Popova, S.N.; Bernad-Bernad, M.J.; Gracia-Mora, J. Modeling of adsorption and release kinetics of methotrexate from thermo/magnetic responsive CoFe2O4–BaTiO3, CoFe2O4–Bi4Ti3O12 and Fe3O4–BaTiO3 core-shell magnetoelectric nanoparticles functionalized with PNIPAm. J. Drug Deliv. Sci. Technol. 2022, 68, 103121. [Google Scholar] [CrossRef]
- Mathur, R.; Chauhan, R.P.; Singh, G.; Singh, S.; Varshney, R.; Kaul, A.; Jain, S.; Mishra, A.K. Tryptophan conjugated magnetic nanoparticles for targeting tumors overexpressing indoleamine 2,3 dioxygenase (IDO) and L-type amino acid transporter. J. Mater. Sci. Mater. Med. 2020, 31, 87. [Google Scholar] [CrossRef]
- Li, S.; Liu, X.; Xu, J.; Wei, D.; Li, C.; Zhao, R.; Yang, L. Magnetic solid-phase extraction of norfloxacin by core-shell magnetic nanoparticles. J. Coord. Chem. 2022. [Google Scholar] [CrossRef]
- Zhong, B.; Mateu-Roldán, A.; Fanarraga, M.L.; Han, W.; Muñoz-Guerra, D.; González, J.; Tao Weng, L.; Ibarra, M.R.; Marquina, C.; Yeung, K.L. Graphene-encapsulated magnetic nanoparticles for safe and steady delivery of ferulic acid in diabetic mice. Chem. Eng. J. 2022, 435, 134466. [Google Scholar] [CrossRef]
- Ali, T.H.; Mandal, A.M.; Heidelberg, T.; Hussen, R.S.D.; Goh, E.W. Ionic magnetic core-shell nanoparticles for DNA extraction. RSC Adv. 2020, 10, 38818–38830. [Google Scholar] [CrossRef]
- Bilici, K.; Atac, N.; Muti, A.; Baylam, I.; Dogan, O.; Sennaroglu, A.; Can, F.; Acar, H.Y. Broad spectrum antibacterial photodynamic and photothermal therapy achieved with indocyanine green loaded SPIONs under near infrared irradiation. Biomater. Sci. 2020, 8, 4616–4625. [Google Scholar] [CrossRef]
- Binandeh, M.; Rostamnia, S.; Karimi, F. MNPs-IHSPN nanoparticles in multi-application with absorption of bio drugs in vitro. Biochem. Biophys. Rep. 2021, 28, 101159. [Google Scholar] [CrossRef] [PubMed]
- Gera, T.; Smausz, T.; Ajtai, T.; Kurilla, B.; Homik, Z.; Kopniczky, J.; Bozóki, Z.; Szabó-Révész, P.; Ambrus, R.; Hopp, B. Production of ibuprofen-magnetite nanocomposites by pulsed laser ablation. J. Phys. D Appl. Phys. 2021, 54, 395401. [Google Scholar] [CrossRef]
- Avarand, S.; Morsali, A.; Heravi, M.M.; Beyramabadi, S.A. A quantum chemical study on the magnetic nanocarrier-tirapazamine drug delivery system. Nanosyst. Phys. Chem. Math. 2021, 12, 167–174. [Google Scholar] [CrossRef]
- Parvaneh, S.; Khademi, F.; Abdi, G.; Alizadeh, A.; Mostafaie, A. Efficient conjugation of anti-HBsAg antibody to modified core-shell magnetic nanoparticles (Fe3O4@SiO2/NH2). BioImpacts 2021, 11, 237–244. [Google Scholar] [CrossRef]
- Kawish, M.; Jabri, T.; Elhissi, A.; Zahid, H.; Iqbal, K.M.; Rao, K.; Gul, J.; Abdullah, M.; Shah, M.R. Galactosylated iron oxide nanoparticles for enhancing oral bioavailability of ceftriaxone. Pharm. Dev. Technol. 2021, 26, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Turrina, C.; Berensmeier, S.; Schwaminger, S.P. Bare iron oxide nanoparticles as drug delivery carrier for the short cationic peptide lasioglossin. Pharmaceuticals 2021, 14, 405. [Google Scholar] [CrossRef]
- Shima, P. Damodaran, Mesoporous Magnetite Nanoclusters as Efficient Nanocarriers for Paclitaxel Delivery. ChemistrySelect 2020, 5, 9261–9268. [Google Scholar] [CrossRef]
- Dabaghi, M.; Hilger, I. Magnetic nanoparticles behavior in biological solutions; The impact of clustering tendency on sedimentation velocity and cell uptake. Materials 2020, 13, 1644. [Google Scholar] [CrossRef]
- Rauwel, E.; Al-Arag, S.; Salehi, H.; Amorim, C.O.; Cuisinier, F.; Guha, M.; Rosario, M.S.; Rauwel, P. Assessing cobalt metal nanoparticles uptake by cancer cells using live Raman spectroscopy. Int. J. Nanomed. 2020, 15, 7051–7062. [Google Scholar] [CrossRef]
- Ilkhani, H.; Zhong, C.-J.; Hepel, M. Magneto-plasmonic nanoparticle grid biosensor with enhanced raman scattering and electrochemical transduction for the development of nanocarriers for targeted delivery of protected anticancer drugs. Nanomaterials 2021, 11, 1326. [Google Scholar] [CrossRef]
- Vurro, F.; Jabalera, Y.; Mannucci, S.; Glorani, G.; Sola-Leyva, A.; Gerosa, M.; Romeo, A.; Romanelli, M.G.; Malatesta, M.; Calderan, L.; et al. Improving the cellular uptake of biomimetic magnetic nanoparticles. Nanomaterials 2021, 11, 766. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhang, Q.; Guo, X.; Villanova, J.; Hu, Y.; Külaots, I.; Garcia-Rojas, D.; Guo, W.; Colvin, V.L. Libraries of Uniform Magnetic Multicore Nanoparticles with Tunable Dimensions for Biomedical and Photonic Applications. ACS Appl. Mater. Interfaces 2020, 12, 41932–41941. [Google Scholar] [CrossRef] [PubMed]
- Socoliuc, V.; Peddis, D.; Petrenko, V.I.; Avdeev, M.V.; Susan-Resiga, D.; Szabó, T.; Turcu, R.; Tombácz, E.; Vekas, L. Magnetic nanoparticle systems for nanomedicine–A materials science perspective. Magnetochemistry 2020, 6, 2. [Google Scholar] [CrossRef]
- Schneider, M.G.M.; Martín, M.J.; Otarola, J.; Vakarelska, E.; Simeonov, V.; Lassalle, V.; Nedyalkova, M. Biomedical Applications of Iron Oxide Nanoparticles: Current Insights Progress and Perspectives. Pharmaceutics 2022, 14, 204. [Google Scholar] [CrossRef] [PubMed]
- Aslam, H.; Shukrullah, S.; Naz, M.Y.; Fatima, H.; Hussain, H.; Ullah, S.; Assiri, M.A. Current and future perspectives of multifunctional magnetic nanoparticles based controlled drug delivery systems. J. Drug Deliv. Sci. Technol. 2022, 67, 102946. [Google Scholar] [CrossRef]
- Lavorato, G.C.; Das, R.; Masa, J.A.; Phan, M.-H.; Srikanth, H. Hybrid magnetic nanoparticles as efficient nanoheaters in biomedical applications. Nanoscale Adv. 2021, 3, 867–888. [Google Scholar] [CrossRef]
- Ovejero, J.G.; Gallo-Cordova, A.; Roca, A.G.; Morales, M.P.; Veintemillas-Verdaguer, S. Reproducibility and Scalability of Magnetic Nanoheater Synthesis. Nanomaterials 2021, 11, 2059. [Google Scholar] [CrossRef]
- Das, R.; Witanachchi, C.; Nemati, Z.; Kalappattil, V.; Rodrigo, I.; García, J.Á.; Garaio, E.; Alonso, J.; Lam, V.D.; Le, A.; et al. Magnetic Vortex and Hyperthermia Suppression in Multigrain Iron Oxide Nanorings. Appl. Sci. 2020, 10, 787. [Google Scholar] [CrossRef]
- Keshavarz, H.; Khavandi, A.; Alamolhoda, S.; Naimi-Jamal, M.R. PH-Sensitive magnetite mesoporous silica nanocomposites for controlled drug delivery and hyperthermia. RSC Adv. 2020, 10, 39008–39016. [Google Scholar] [CrossRef]
- Jabalera, Y.; Oltolina, F.; Peigneux, A.; Sola-Leyva, A.; Carrasco-Jiménez, M.P.; Prat, M.; Jimenez-Lopez, C.; Iglesias, G.R. Nanoformulation design including MamC-mediated biomimetic nanoparticles allows the simultaneous application of targeted drug delivery and magnetic hyperthermia. Polymers 2020, 12, 1832. [Google Scholar] [CrossRef]
- Gahrouei, Z.E.; Labbaf, S.; Kermanpur, A. Cobalt doped magnetite nanoparticles: Synthesis, characterization, optimization and suitability evaluations for magnetic hyperthermia applications. Phys. E Low-Dimens. Syst. Nanostruct. 2020, 116, 113759. [Google Scholar] [CrossRef]
- Rajan, A.; Sahu, N.K. Review on magnetic nanoparticle-mediated hyperthermia for cancer therapy. J. Nanoparticle Res. 2020, 22, 319. [Google Scholar] [CrossRef]
- Oltolina, F.; Peigneux, A.; Colangelo, D.; Clemente, N.; D’Urso, A.; Valente, G.; Iglesias, G.R.; Jiménez-Lopez, C.; Prat, M. Biomimetic Magnetite Nanoparticles as Targeted Drug Nanocarriers and Mediators of Hyperthermia in an Experimental Cancer Model. Cancers 2020, 12, 2564. [Google Scholar] [CrossRef] [PubMed]
- Seynhaeve, A.L.B.; Amin, M.; Haemmerich, D.; van Rhoon, G.C.; ten Hagen, T.L.M. Hyperthermia and smart drug delivery systems for solid tumor therapy. Adv. Drug Deliv. Rev. 2020, 163–164, 125–144. [Google Scholar] [CrossRef]
- Martinez-Boubeta, C.; Simeonidis, K.; Oró, J.; Makridis, A.; Serantes, D.; Balcells, L. Finding the Limits of Magnetic Hyperthermia on Core-Shell Nanoparticles Fabricated by Physical Vapor Methods. Magnetochemistry 2021, 7, 49. [Google Scholar] [CrossRef]
- Yu, X.; Ding, S.; Yang, R.; Wu, C.; Zhang, W. Research progress on magnetic nanoparticles for magnetic induction hyperthermia of malignant tumor. Ceram. Int. 2021, 47, 5909–5917. [Google Scholar] [CrossRef]
- Szczȩch, M.; Orsi, D.; Łopuszyńska, N.; Cristofolini, L.; Jasiński, K.; Wȩglarz, W.ł.P.; Albertini, F.; Kereïche, S.; Szczepanowicz, K. Magnetically responsive polycaprolactone nanocarriers for application in the biomedical field: Magnetic hyperthermia, magnetic resonance imaging, and magnetic drug delivery. RSC Adv. 2020, 10, 43607–43618. [Google Scholar] [CrossRef]
- Didarian, R.; Vargel, I. Treatment of tumour tissue with radiofrequency hyperthermia (using antibody-carrying nanoparticles). IET Nanobiotechnol. 2021, 15, 639–653. [Google Scholar] [CrossRef]
- Veres, T.; Voniatis, C.; Molnár, K.; Nesztor, D.; Fehér, D.; Ferencz, A.; Gresits, I.; Thuróczy, G.; Márkus, B.G.; Simon, F.; et al. An Implantable Magneto-Responsive Poly(aspartamide) Based Electrospun Scaffold for Hyperthermia Treatment. Nanomaterials 2022, 12, 1476. [Google Scholar] [CrossRef]
- Yin, P.; Wei, C.; Jin, X.; Yu, X.; Wu, C.; Zhang, W. Magnetic polyvinyl alcohol microspheres with self-regulating temperature hyperthermia and CT/MR imaging for arterial embolization. Polym. Bull. 2022. [Google Scholar] [CrossRef]
- Ferreira, L.P.; Reis, C.P.; Robalo, T.T.; Melo Jorge, M.E.; Ferreira, P.; Gonçalves, J.; Hajalilou, A.; Cruz, M.M. Assisted Synthesis of Coated Iron Oxide Nanoparticles for Magnetic Hyperthermia. Nanomaterials 2022, 12, 1870. [Google Scholar] [CrossRef]
- Buschmann, H.M. Critical review of heat transfer experiments in ferrohydrodynamic pipe flow utilising ferronanofluids. Int. J. Therm. Sci. 2020, 157, 106426. [Google Scholar] [CrossRef]
- Socoliuc, V.; Avdeev, M.V.; Kuncser, V.; Turcu, R.; Tombácz, E.; Vékás, L. Ferrofluids and bio-ferrofluids: Looking back and stepping forward. Nanoscale 2022, 14, 4786–4886. [Google Scholar] [CrossRef] [PubMed]
- Mortezaee, K.; Narmani, A.; Salehi, M.; Bagheri, H.; Farhood, B.; Haghi-Aminjan, H.; Najafi, M. Synergic effects of nanoparticles-mediated hyperthermia in radiotherapy/chemotherapy of cancer. Life Sci. 2021, 269, 119020. [Google Scholar] [CrossRef] [PubMed]
- Maksoud, M.I.A.A.; Ghobashy, M.M.; Kodous, A.S.; Fahim, R.A.; Osman, A.I.; Al-Muhtaseb, A.H.; Rooney, D.W.; Mamdouh, M.A.; Nady, N.; Ashour, A.H. Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications. Nanotechnol. Rev. 2022, 11, 372–413. [Google Scholar] [CrossRef]
- Papadopoulos, C.; Kolokithas-Ntoukas, A.; Moreno, R.; Fuentes, D.; Loudos, G.; Loukopoulos, V.C.; Kagadis, G.C. Using kinetic Monte Carlo simulations to design efficient magnetic nanoparticles for clinical hyperthermia. Med. Phys. 2022, 49, 547–567. [Google Scholar] [CrossRef]
- Weeber, R.; Kreissl, P.; Holm, C. Magnetic field controlled behavior of magnetic gels studied using particle-based simulations. Phys. Sci. Rev. 2021. [Google Scholar] [CrossRef]
- Ilg, P.; Kroger, M. Dynamics of interacting magnetic nanoparticles: Effective behavior from competition between Brownian and Néel relaxation. Phys. Chem. Chem. Phys. 2020, 22, 22244–22259. [Google Scholar] [CrossRef]
- Jin, D.; Kim, S.H.; Kim, H. Concentration and Magnetic Field Effects on Thermal Fluctuation of Magnetic Weight of Magnetite Nanoparticles During Agglomeration Under Magnetic Field. Bull. Korean Chem. Soc. 2020, 41, 628–633. [Google Scholar] [CrossRef]
- Can, M.M.; Bairam, C.; Aksoy, S.; Kuruca, D.S.; Kaneko, S.; Aktaş, Z.; Öncül, M.O. Effect of Ti Atoms on Néel Relaxation Mechanism at Magnetic Heating Performance of Iron Oxide Nanoparticles. Coatings 2022, 12, 481. [Google Scholar] [CrossRef]
- Ge, M.; Li, Y.; Zhu, C.; Liang, G.; Jahangir Alam, S.M.; Hu, G.; Gui, Y.; Rashid, M.J. Preparation of organic-modified magadiite–magnetic nanocomposite particles as an effective nanohybrid drug carrier material for cancer treatment and its properties of sustained release mechanism by Korsmeyer–Peppas kinetic model. J. Mater. Sci. 2021, 56, 14270–14286. [Google Scholar] [CrossRef]
- Köhler, T.; Feoktystov, A.; Petracic, O.; Kentzinger, E.; Bhatnagar-Schöffmann, T.; Feygenson, M.; Nandakumaran, N.; Landers, J.; Wende, H.; Cervellino, A.; et al. Mechanism of magnetization reduction in iron oxide nanoparticles. Nanoscale 2021, 13, 6965–6976. [Google Scholar] [CrossRef] [PubMed]
- Shreyash, N.; Sonker, M.; Bajpai, S.; Tiwary, S.K. Review of the Mechanism of Nanocarriers and Technological Developments in the Field of Nanoparticles for Applications in Cancer Theragnostics. ACS Appl. Bio Mater. 2021, 4, 2307–2334. [Google Scholar] [CrossRef] [PubMed]
- Iacob, N.; Kuncser, A.; Comanescu, C.; Palade, P.; Kuncser, V. Optimization of magnetic fluid hyperthermia with respect to nanoparticle shape-related parameters: Case of magnetite ellipsoidal nanoparticles. J. Nanopart. Res. 2020, 22, 138. [Google Scholar] [CrossRef]
- Stoicescu, C.S.; Culita, D.; Stanica, N.; Papa, F.; State, R.N.; Munteanu, G. Temperature programmed reduction of a core-shell synthetic magnetite: Dependence on the heating rate of the reduction mechanism. Thermochim. Acta 2022, 709, 179146. [Google Scholar] [CrossRef]
- Liao, L.; Cen, D.; Fu, Y.; Liu, B.; Fang, C.; Wang, Y.; Cai, X.; Li, X.; Wu, H.B.; Han, G. Biodegradable MnFe-hydroxide nanocapsules to enable multi-therapeutics delivery and hypoxia-modulated tumor treatment. J. Mater. Chem. B 2020, 8, 3929–3938. [Google Scholar] [CrossRef]
- Zhou, H.; Guo, M.; Li, J.; Qin, F.; Wang, Y.; Liu, T.; Liu, J.; Sabet, Z.F.; Wang, Y.; Liu, Y.; et al. Hypoxia-Triggered Self-Assembly of Ultrasmall Iron Oxide Nanoparticles to Amplify the Imaging Signal of a Tumor. J. Am. Chem. Soc. 2021, 143, 1846–1853. [Google Scholar] [CrossRef]
- Farinha, P.; Coelho, J.M.P.; Reis, C.P.; Gaspar, M.M. A comprehensive updated review on magnetic nanoparticles in diagnostics. Nanomaterials 2021, 11, 3432. [Google Scholar] [CrossRef]
- Avolio, M.; Innocenti, C.; Lascialfari, A.; Mariani, M.; Sangregorio, C. Medical Applications of Magnetic Nanoparticles. Springer Ser. Mater. Sci. 2021, 308, 327–351. [Google Scholar] [CrossRef]
- Aisida, S.O.; Akpa, P.A.; Ahmad, I.; Zhao, T.-K.; Maaza, M.; Ezema, F.I. Bio-inspired encapsulation and functionalization of iron oxide nanoparticles for biomedical applications. Eur. Polym. J. 2020, 122, 109371. [Google Scholar] [CrossRef]
- Kolishetti, N.; Vashist, A.; Arias, A.Y.; Atluri, V.; Dhar, S.; Nair, M. Recent advances, status, and opportunities of magneto-electric nanocarriers for biomedical applications. Mol. Asp. Med. 2022, 83, 101046. [Google Scholar] [CrossRef]
- Singh, R.; Pal, D.; Chattopadhyay, S. Target-Specific Superparamagnetic Hydrogel with Excellent pH Sensitivity and Reversibility: A Promising Platform for Biomedical Applications. ACS Omega 2020, 5, 21768–21780. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Du, J. Superparamagnetic nanoparticles for biomedical applications. J. Mater. Chem. B 2020, 8, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Dash, P.; Raut, S.; Jena, M.; Nayak, B. Harnessing the biomedical properties of ferromagnetic α-Fe2O3 NPs with a plausible formation mechanism. Ceram. Int. 2020, 46, 26190–26204. [Google Scholar] [CrossRef]
- Sheikhpour, M.; Arabi, M.; Kasaeian, A.; Rabei, A.R.; Taherian, Z. Role of nanofluids in drug delivery and biomedical technology: Methods and applications. Nanotechnol. Sci. Appl. 2020, 13, 47–59. [Google Scholar] [CrossRef]
- Yang, H.Y.; Li, Y.; Lee, D.S. Functionalization of Magnetic Nanoparticles with Organic Ligands toward Biomedical Applications. Adv. NanoBio Res. 2021, 1, 2000043. [Google Scholar] [CrossRef]
- Monteserín, M.; Larumbe, S.; Martínez, A.V.; Burgui, S.; Martín, L.F. Recent Advances in the Development of Magnetic Nanoparticles for Biomedical Applications. J. Nanosci. Nanotechnol. 2021, 21, 2705–2741. [Google Scholar] [CrossRef]
- Baryeh, K.; Attia, M.; Ulloa, J.C.; Ye, J.Y. Magnetic nanoparticle-based hybrid materials in the biomedical field: Fundamentals and applications. In Magnetic Nanoparticle-Based Hybrid Materials; Woodhead Publishing: Cambridge, UK, 2021; pp. 387–423. [Google Scholar] [CrossRef]
- Saadatmand, S.E.; Kavousi, S.M.; Alam, N.R. The investigation of conductivity of magnetic nanoparticles in the vascular network by DCC method and the effect of forces on the efficiency of targeted magnetic drug delivery. Front. Biomed. Technol. 2021, 8, 26–36. [Google Scholar] [CrossRef]
- Canaparo, R.; Foglietta, F.; Limongi, T.; Serpe, L. Biomedical applications of reactive oxygen species generation by metal nanoparticles. Materials 2021, 14, 53. [Google Scholar] [CrossRef]
- Almeida, A.F.; Vinhas, A.; Gonçalves, A.I.; Miranda, M.S.; Rodrigues, M.T.; Gomes, M.E. Magnetic triggers in biomedical applications—Prospects for contact free cell sensing and guidance. J. Mater. Chem. B 2021, 9, 1259–1271. [Google Scholar] [CrossRef]
- Anik, M.I.; Hossain, M.K.; Hossain, I.; Ahmed, I.; Doha, R.M. Biomedical applications of magnetic nanoparticles. Magn. Nanoparticle-Based Hybrid Mater. Fundam. Appl. 2021, 463–497. [Google Scholar] [CrossRef]
- Jiang, K.; Zhang, L.; Bao, G. Magnetic iron oxide nanoparticles for biomedical applications. Curr. Opin. Biomed. Eng. 2021, 20, 100330. [Google Scholar] [CrossRef] [PubMed]
- Căpraru, A.; Moacă, E.-A.; Păcurariu, C.; Ianoş, R.; Lazău, R.; Barbu-Tudoran, L. Development and characterization of magnetic iron oxide nanoparticles using microwave for the combustion reaction ignition, as possible candidates for biomedical applications. Powder Technol. 2021, 394, 1026–1038. [Google Scholar] [CrossRef]
- Papadopoulou, S.; Kolokithas-ntoukas, A.; Salvanou, E.-A.; Gaitanis, A.; Xanthopoulos, S.; Avgoustakis, K.; Gazouli, M.; Paravatou-petsotas, M.; Tsoukalas, C.; Bakandritsos, A.; et al. Chelator-free/chelator-mediated radiolabeling of colloidally stabilized iron oxide nanoparticles for biomedical imaging. Nanomaterials 2021, 11, 1677. [Google Scholar] [CrossRef]
- Li, D.; Luo, Y.; Onidas, D.; He, L.; Jin, M.; Gazeau, F.; Pinson, J.; Mangeney, C. Surface functionalization of nanomaterials by aryl diazonium salts for biomedical sciences. Adv. Colloid Interface Sci. 2021, 294, 102479. [Google Scholar] [CrossRef]
- Han, W.J.; Lee, J.H.; Lee, J.; Choi, H.J. Remote-controllable, tough, ultrastretchable, and magneto-sensitive nanocomposite hydrogels with homogeneous nanoparticle dispersion as biomedical actuators, and their tuned structure, properties, and performances. Compos. Part B Eng. 2022, 236, 109802. [Google Scholar] [CrossRef]
- Farzin, A.; Etesami, S.A.; Quint, J.; Memic, A.; Tamayol, A. Magnetic Nanoparticles in Cancer Therapy and Diagnosis. Adv. Healthc. Mater. 2020, 9. Available online: https://onlinelibrary.wiley.com/doi/10.1002/adhm.201901058 (accessed on 30 June 2022). [CrossRef]
- Chi, X.; Liu, K.; Luo, X.; Yin, Z.; Lin, H.; Gao, J. Recent advances of nanomedicines for liver cancer therapy. J. Mater. Chem. B 2020, 8, 3747–3771. [Google Scholar] [CrossRef] [PubMed]
- Soares, P.I.; Romão, J.; Matos, R.; Silva, J.C.; Borges, J.P. Design and engineering of magneto-responsive devices for cancer theranostics: Nano to macro perspective. Prog. Mater. Sci. 2020, 116, 100742. [Google Scholar] [CrossRef]
- Naud, C.; Thébault, C.; Carrière, M.; Hou, Y.; Morel, R.; Berger, F.; Diény, B.; Joisten, H. Cancer treatment by magneto-mechanical effect of particles, a review. Nanoscale Adv. 2020, 2, 3632–3655. [Google Scholar] [CrossRef]
- Indoria, S.; Singh, V.; Hsieh, M.-F. Recent advances in theranostic polymeric nanoparticles for cancer treatment: A review. Int. J. Pharm. 2020, 582, 119314. [Google Scholar] [CrossRef]
- Gillson, J.; Ramaswamy, Y.; Singh, G.; Gorfe, A.A.; Pavlakis, N.; Samra, J.; Mittal, A.; Sahni, S. Small molecule KRAS inhibitors: The future for targeted pancreatic cancer therapy? Cancers 2020, 12, 1341. [Google Scholar] [CrossRef]
- Magro, M.; Venerando, A.; Macone, A.; Canettieri, G.; Agostinelli, E.; Vianello, F. Nanotechnology-based strategies to develop new anticancer therapies. Biomolecules 2020, 10, 735. [Google Scholar] [CrossRef] [PubMed]
- Bloise, N.; Massironi, A.; Della Pina, C.; Alongi, J.; Siciliani, S.; Manfredi, A.; Biggiogera, M.; Rossi, M.; Ferruti, P.; Ranucci, E.; et al. Extra-Small Gold Nanospheres Decorated with a Thiol Functionalized Biodegradable and Biocompatible Linear Polyamidoamine as Nanovectors of Anticancer Molecules. Front. Bioeng. Biotechnol. 2020, 8, 132. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento, T.; Todeschini, A.R.; Santos-Oliveira, R.; Monteiro, M.S.S.B.; Souza, V.T.; Ricci-Júnior, E. Trends in nanomedicines for cancer treatment. Curr. Pharm. Des. 2020, 26, 3579–3600. [Google Scholar] [CrossRef] [PubMed]
- Pandey, N.; Menon, J.U.; Takahashi, M.; Hsieh, J.-T.; Yang, J.; Nguyen, K.T.; Wadajkar, A.S. Thermo-responsive fluorescent nanoparticles for multimodal imaging and treatment of cancers. Nanotheranostics 2020, 4, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-Y.; Huang, L.; Zhang, Z.-P.; Fu, D.-H. Strategies for Precise Engineering and Conjugation of Antibody Targeted-nanoparticles for Cancer Therapy. Curr. Med. Sci. 2020, 40, 463–473. [Google Scholar] [CrossRef]
- Mukherjee, S.; Liang, L.; Veiseh, O. Recent advancements of magnetic nanomaterials in cancer therapy. Pharmaceutics 2020, 12, 147. [Google Scholar] [CrossRef] [PubMed]
- Avval, Z.M.; Malekpour, L.; Raeisi, F.; Babapoor, A.; Mousavi, S.M.; Hashemi, S.A.; Salari, M. Introduction of magnetic and supermagnetic nanoparticles in new approach of targeting drug delivery and cancer therapy application. Drug Metab. Rev. 2020, 52, 157–184. [Google Scholar] [CrossRef]
- Majidzadeh, H.; Araj-Khodaei, M.; Ghaffari, M.; Torbati, M.; Ezzati Nazhad Dolatabadi, J.; Hamblin, M.R. Nano-based delivery systems for berberine: A modern anti-cancer herbal medicine. Colloids Surf. B Biointerfaces 2020, 194, 111188. [Google Scholar] [CrossRef]
- Gorbet, M.-J.; Ranjan, A. Cancer immunotherapy with immunoadjuvants, nanoparticles, and checkpoint inhibitors: Recent progress and challenges in treatment and tracking response to immunotherapy. Pharmacol. Ther. 2020, 207, 107456. [Google Scholar] [CrossRef]
- Mukherjee, B.; Al Hoque, A.; Dutta, D.; Paul, B.; Mukherjee, A.; Mallick, S. Nanoformulated drug delivery of potential betulinic acid derivatives: A promising approach toward cancer therapy. In Nanomedicine for Bioactives: Healthcare Applications; Springer: Singapore, 2020; pp. 127–153. [Google Scholar] [CrossRef]
- Cheng, H.-W.; Tsao, H.-Y.; Chiang, C.-S.; Chen, S.-Y. Advances in Magnetic Nanoparticle-Mediated Cancer Immune-Theranostics. Adv. Healthcare Mater. 2021, 10, 2001451. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Zhang, Y.; Wei, J.; Wang, W.; Dong, C.; Xue, Y.; Liu, M.; Pei, R. Engineered Fe3O4-based nanomaterials for diagnosis and therapy of cancer. New J. Chem. 2021, 45, 7918. [Google Scholar] [CrossRef]
- Rahman, M.; Alam, K.; Hafeez, A.; Ilyas, R.; Beg, S. Metallic nanoparticles in drug delivery and cancer treatment. In Nanoformulation Strategies for Cancer Treatment; Elsevier: Amsterdam, The Netherlands, 2021; pp. 107–119. [Google Scholar] [CrossRef]
- Jayaraman, M.; Dutta, P.; Telang, J.; Krishnan, B.B.S. Nanoparticles for Cancer Therapy. In Nanomedicine for Cancer Diagnosis and Therapy; Springer: Singapore, 2021; pp. 1–45. [Google Scholar] [CrossRef]
- Nedyalkova, M.; Todorov, B.; Barazorda-Ccahuanac, H.L.; Madurga, S. Iron oxide Nanoparticles in Anticancer Drug Delivery and Imaging Diagnostics. In Magnetic Nanoparticles in Human Health and Medicine; Wiley: Hoboken, NJ, USA, 2021; pp. 151–163. [Google Scholar]
- Chen, W.; Sun, Z.; Lu, L. Targeted Engineering of Medicinal Chemistry for Cancer Therapy: Recent Advances and Perspectives. Angew. Chem. Int. Ed. 2021, 60, 5626–5643. [Google Scholar] [CrossRef] [PubMed]
- Alromi, D.A.; Madani, S.Y.; Seifalian, A. Emerging application of magnetic nanoparticles for diagnosis and treatment of cancer. Polymers 2021, 13, 4146. [Google Scholar] [CrossRef] [PubMed]
- Lorkowski, M.E.; Atukorale, P.U.; Ghaghada, K.B.; Karathanasis, E. Stimuli-Responsive Iron Oxide Nanotheranostics: A Versatile and Powerful Approach for Cancer Therapy. Adv. Healthc. Mater. 2021, 10, 2001044. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhang, S.; He, J.; Nie, Z. Polymers and inorganic nanoparticles: A winning combination towards assembled nanostructures for cancer imaging and therapy. Nano Today 2021, 36, 101046. [Google Scholar] [CrossRef]
- Lafuente-Gómez, N.; Milán-Rois, P.; García-Soriano, D.; Luengo, Y.; Cordani, M.; Alarcón-Iniesta, H.; Salas, G.; Somoza, Á. Smart modification on magnetic nanoparticles dramatically enhances their therapeutic properties. Cancers 2021, 13, 4095. [Google Scholar] [CrossRef]
- Cerqueira, M.; Belmonte-Reche, E.; Gallo, J.; Baltazar, F.; Bañobre-López, M. Magnetic Solid Nanoparticles and Their Counterparts: Recent Advances towards Cancer Theranostics. Pharmaceutics 2022, 14, 506. [Google Scholar] [CrossRef]
- Ahmed, F.; Khan, M.A.; Haider, N.; Ahmad, M.Z.; Ahmad, J. Recent Advances in Theranostic Applications of Nanomaterials in Cancer. Curr. Pharm. Des. 2022, 28, 133–150. [Google Scholar] [CrossRef]
- Morsink, M.; Parente, L.; Silva, F.; Abrantes, A.; Ramos, A.; Primo, I.; Willemen, N.; Sanchez-Lopez, E.; Severino, P.; Souto, E.B. Nanotherapeutics and Nanotheragnostics for Cancers: Properties, Pharmacokinetics, Biopharmaceutics, and Biosafety. Curr. Pharm. Des. 2022, 28, 104–115. [Google Scholar] [CrossRef]
- Du, H.; Akakuru, O.U.; Yao, C.; Yang, F.; Wu, A. Transition metal ion-doped ferrites nanoparticles for bioimaging and cancer therapy. Transl. Oncol. 2022, 15, 101264. [Google Scholar] [CrossRef]
- Maffei, M.E. Magnetic Fields and Cancer: Epidemiology, Cellular Biology, and Theranostics. Int. J. Mol. Sci. 2022, 23, 1339. [Google Scholar] [CrossRef] [PubMed]
- Boutopoulos, I.D.; Lampropoulos, D.S.; Bourantas, G.C.; Miller, K.; Loukopoulos, V.C. Two-Phase Biofluid Flow Model for Magnetic Drug Targeting. Symmetry 2020, 12, 1083. [Google Scholar] [CrossRef]
- Majee, S.; Shit, G.C. Modeling and simulation of blood flow with magnetic nanoparticles as carrier for targeted drug delivery in the stenosed artery. Eur. J. Mech. B/Fluids 2020, 83, 42–57. [Google Scholar] [CrossRef]
- Tabaei, A.; Sadeghi, S.; Hosseinzadeh, S.; Bidabadi, M.; Xiong, Q.; Karimi, N. A simplified mathematical study of thermochemical preparation of particle oxide under counterflow configuration for use in biomedical applications. J. Therm. Anal. Calorim. 2020, 139, 2769–2779. [Google Scholar] [CrossRef]
- Znoyko, S.L.; Orlova, A.V.; Bragina, V.A.; Nikitin, M.P.; Nikitin, P.I. Nanomagnetic lateral flow assay for high-precision quantification of diagnostically relevant concentrations of serum TSH. Talanta 2020, 216, 120961. [Google Scholar] [CrossRef] [PubMed]
- Lanier, O.L.; Velez, C.; Arnold, D.P.; Dobson, J. Model of Magnetic Particle Capture Under Physiological Flow Rates for Cytokine Removal During Cardiopulmonary Bypass. IEEE Trans. Biomed. Eng. 2021, 68, 1198–1207. [Google Scholar] [CrossRef]
- Zahn, D.; Klein, K.; Radon, P.; Berkov, D.; Erokhin, S.; Nagel, E.; Eichhorn, M.; Wiekhorst, F.; Dutz, S. Investigation of magnetically driven passage of magnetic nanoparticles through eye tissues for magnetic drug targeting. Nanotechnology 2020, 31, 495101. [Google Scholar] [CrossRef]
- Forouzandehmehr, M.; Ghoytasi, I.; Shamloo, A.; Ghosi, S. Particles in coronary circulation: A review on modelling for drug carrier design. Mater. Des. 2022, 216, 110511. [Google Scholar] [CrossRef]
- Eslami, P.; Albino, M.; Scavone, F.; Chiellini, F.; Morelli, A.; Baldi, G.; Cappiello, L.; Doumett, S.; Lorenzi, G.; Ravagli, C.; et al. Smart Magnetic Nanocarriers for Multi-Stimuli On-Demand Drug Delivery. Nanomaterials 2022, 12, 303. [Google Scholar] [CrossRef]
- Kuznetsova, O.V.; Timerbaev, A.R. Magnetic nanoparticles for highly robust, facile and efficient loading of metal-based drugs. J. Inorg. Biochem. 2022, 227, 111685. [Google Scholar] [CrossRef]
- Im, G.-B.; Jung, E.; Kim, Y.H.; Kim, Y.-J.; Kim, S.-W.; Jeong, G.-J.; Lee, T.-J.; Kim, D.-I.; Kim, J.; Hyeon, T.; et al. Endosome-triggered ion-releasing nanoparticles as therapeutics to enhance the angiogenic efficacy of human mesenchymal stem cells. J. Control. Release 2020, 324, 586–597. [Google Scholar] [CrossRef]
- Real, D.A.; Bolaños, K.; Priotti, J.; Yutronic, N.; Kogan, M.J.; Sierpe, R.; Donoso-González, O. Cyclodextrin-modified nanomaterials for drug delivery: Classification and advances in controlled release and bioavailability. Pharmaceutics 2021, 13, 2131. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Nguyen, N.-M.; Vu, D.-M.; Tran, M.-D.; Ta, V.-T. On-demand release of drug from magnetic nanoparticle-loaded alginate beads. J. Anal. Methods Chem. 2021, 2021, 5576283. [Google Scholar] [CrossRef] [PubMed]
- de Freitas e Castro, M.; Mendonça, T.T.; da Silva, L.F.; Gomez, J.G.C.; Sanchez Rodriguez, R.J. Carriers based on poly-3-hydroxyalkanoates containing nanomagnetite to trigger hormone release. Int. J. Biol. Macromol. 2021, 166, 448–458. [Google Scholar] [CrossRef]
- Neves, R.P.; Bronze-Uhle, E.S.; Santos, P.L.; Lisboa-Filho, P.N.; Magdalena, A.G. Salicylic acid incorporation in Fe3O4-BSA nanoparticles for drug release. Quim. Nova 2021, 44, 824–829. [Google Scholar] [CrossRef]
- Lu, C.-H.; Yeh, Y.-C. Fabrication of Multiresponsive Magnetic Nanocomposite Double-Network Hydrogels for Controlled Release Applications. Small 2021, 17, 2105997. [Google Scholar] [CrossRef] [PubMed]
- Mandić, L.; Sadžak, A.; Erceg, I.; Baranović, G.; Šegota, S. The fine-tuned release of antioxidant from superparamagnetic nanocarriers under the combination of stationary and alternating magnetic fields. Antioxidants 2021, 10, 1212. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Huang, J.; Yan, Y.; Chen, W.; Wen, J.; Wu, X.; Liu, J.; Liu, H.; Huang, C. Ferroferric oxide loaded near-infrared triggered photothermal microneedle patch for controlled drug release. J. Colloid Interface Sci. 2022, 617, 718–729. [Google Scholar] [CrossRef]
- Gil, C.J.; Li, L.; Hwang, B.; Cadena, M.; Theus, A.S.; Finamore, T.A.; Bauser-Heaton, H.; Mahmoudi, M.; Roeder, R.K.; Serpooshan, V. Tissue engineered drug delivery vehicles: Methods to monitor and regulate the release behavior. J. Control. Release 2022, 349, 143–155. [Google Scholar] [CrossRef]
- Andjelković, L.; Jeremić, D.; Milenković, M.R.; Radosavljević, J.; Vulić, P.; Pavlović, V.; Manojlović, D.; Nikolić, A.S. Synthesis, characterization and in vitro evaluation of divalent ion release from stable NiFe2O4, ZnFe2O4 and core-shell ZnFe2O4@NiFe2O4 nanoparticles. Ceram. Int. 2020, 46, 3528–3533. [Google Scholar] [CrossRef]
- Ranjbary, A.G.; Saleh, G.K.; Azimi, M.; Karimian, F.; Mehrzad, J.; Zohdi, J. Superparamagnetic Iron Oxide Nanoparticles Induce Apoptosis in HT-29 Cells by Stimulating Oxidative Stress and Damaging DNA. Biol. Trace Elem. Res. 2022. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Pua, C.H.; Misran, M.; Lee, P.F. The rotational effect of magnetic particles on cellular apoptosis based on four electromagnet feedback control system. Biomed. Eng. Appl. Basis Commun. 2021, 33, 459. [Google Scholar] [CrossRef]
- Ferraz, F.S.; López, J.L.; Lacerda, S.M.S.N.; Procópio, M.S.; Figueiredo, A.F.A.; Martins, E.M.N.; Guimarães, P.P.G.; Ladeira, L.O.; Kitten, G.T.; Dias, F.F.; et al. Biotechnological approach to induce human fibroblast apoptosis using superparamagnetic iron oxide nanoparticles. J. Inorg. Biochem. 2020, 206, 111017. [Google Scholar] [CrossRef]
- Yadav, S.K.; Khan, Z.A.; Mishra, B.; Bahadur, S.; Kumar, A.; Yadav, B. The Toxic Side of Nanotechnology: An Insight into Hazards to Health and the Ecosystem. Micro Nanosyst. 2022, 14, 21–33. [Google Scholar] [CrossRef]
- Fernández-Bertólez, N.; Costa, C.; Brandão, F.; Teixeira, J.P.; Pásaro, E.; Valdiglesias, V.; Laffon, B. Toxicological Aspects of Iron Oxide Nanoparticles. Adv. Exp. Med. Biol. 2022, 1357, 303–350. [Google Scholar] [CrossRef] [PubMed]
- Jahanban-Esfahlan, R.; Derakhshankhah, H.; Haghshenas, B.; Massoumi, B.; Abbasian, M.; Jaymand, M. A bio-inspired magnetic natural hydrogel containing gelatin and alginate as a drug delivery system for cancer chemotherapy. Int. J. Biol. Macromol. 2020, 156, 438–445. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, J.; Cui, X.; Wang, X.; Zhang, L.; Tang, P. Recent Advances on Magnetic Sensitive Hydrogels in Tissue Engineering. Front. Chem. 2020, 8, 124. [Google Scholar] [CrossRef]
- Chen, F.; Bian, M.; Nahmou, M.; Myung, D.; Goldberg, J.L. Fusogenic liposome-enhanced cytosolic delivery of magnetic nanoparticles. RSC Adv. 2021, 57, 35796–35805. [Google Scholar] [CrossRef]
- Nuñez-Magos, L.; Lira-Escobedo, J.; Rodríguez-López, R.; Muñoz-Navia, M.; Castillo-Rivera, F.; Viveros-Méndez, P.X.; Araujo, E.; Encinas, A.; Saucedo-Anaya, S.A.; Aranda-Espinoza, S. Effects of DC Magnetic Fields on Magnetoliposomes. Front. Mol. Biosci. 2021, 8, 703417. [Google Scholar] [CrossRef]
- Sriwidodo Umar, A.K.; Wathoni, N.; Zothantluanga, J.H.; Das, S.; Luckanagul, J.A. Liposome-polymer complex for drug delivery system and vaccine stabilization. Heliyon 2022, 8, e08934. [Google Scholar] [CrossRef]
- Kumar, N.; Tyeb, S.; Verma, V. Recent advances on Metal oxide-polymer systems in targeted therapy and diagnosis: Applications and toxicological perspective. J. Drug Deliv. Sci. Technol. 2021, 66, 102814. [Google Scholar] [CrossRef]
- Ezealigo, U.S.; Ezealigo, B.N.; Aisida, S.O.; Ezema, F.I. Iron oxide nanoparticles in biological systems: Antibacterial and toxicology perspective. JCIS Open 2021, 4, 100027. [Google Scholar] [CrossRef]
- Novak, E.V.; Pyanzina, E.S.; Gupalo, M.A.; Mauser, N.J.; Kantorovich, S.S. Structural transitions and magnetic response of supramolecular magnetic polymerlike structures with bidisperse monomers. Phys. Rev. E 2022, 105, 054601. [Google Scholar] [CrossRef]
- Iliasov, A.R.; Nizamov, T.R.; Naumenko, V.A.; Garanina, A.S.; Vodopyanov, S.S.; Nikitin, A.A.; Pershina, A.G.; Chernysheva, A.A.; Kan, Y.; Mogilnikov, P.S.; et al. Non-magnetic shell coating of magnetic nanoparticles as key factor of toxicity for cancer cells in a low frequency alternating magnetic field. Colloids Surf. B 2021, 206, 111931. [Google Scholar] [CrossRef]
- Malhotra, N.; Lee, J.-S.; Liman, R.A.D.; Ruallo, J.M.S.; Villaflore, O.B.; Ger, T.-R.; Hsiao, C.-D. Potential toxicity of iron oxide magnetic nanoparticles: A review. Molecules 2020, 25, 3159. [Google Scholar] [CrossRef]
- Kefeni, K.K.; Msagati, T.A.M.; Nkambule, T.T.; Mamba, B.B. Spinel ferrite nanoparticles and nanocomposites for biomedical applications and their toxicity. Mater. Sci. Eng. C 2020, 107, 110314. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, O.; Casillas, N.; Knauth, P.; Lopez, Z.; Virgen-Ortiz, A.; Lozano, O.; Delgado-Enciso, I.; Sámano, A.H.; Rosales, S.; Martinez-Ceseña, L.; et al. An easily prepared ferrofluid with high power absorption density and low cytotoxicity for biomedical applications. Mater. Chem. Phys. 2020, 245, 122752. [Google Scholar] [CrossRef]
- Toropova, Y.G.; Motorina, D.S.; Gorshkova, M.; Gareev, K.G.; Korolev, D.V.; Muzhikyan, A.A. The effect of intravenous administration to rats of magnetite nanoparticles with various shells on the functional state and morphology of the endothelium and on antioxidant status. Transl. Med. 2020, 7, 52–64. [Google Scholar] [CrossRef]
- Srikanth, K.; Nutalapati, V. Copper ferrite nanoparticles induced cytotoxicity and oxidative stress in Channel catfish ovary cells. Chemosphere 2022, 287, 132166. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Tan, W.; Nie, K.; Xu, X. Protective effect of Fe3O4 nanoparticles on cadmium chloride-induced toxicity in the small intestine of mice. J. Univ. Sci. Technol. China 2020, 50, 887–893. [Google Scholar] [CrossRef]
- Garcia-Fernandez, J.; Turiel, D.; Bettmer, J.; Jakubowski, N.; Panne, U.; Rivas García, L.; Llopis, J.; Sánchez González, C.; Montes-Bayón, M. In vitro and in situ experiments to evaluate the biodistribution and cellular toxicity of ultrasmall iron oxide nanoparticles potentially used as oral iron supplements. Nanotoxicology 2020, 14, 388–403. [Google Scholar] [CrossRef] [PubMed]
- Radeloff, K.; Radeloff, A.; Tirado, M.R.; Scherzad, A.; Hagen, R.; Kleinsasser, N.H.; Hackenberg, S. Toxicity and functional impairment in human adipose tissue-derived stromal cells (Hascs) following long-term exposure to very small iron oxide particles (vsops). Nanomaterials 2020, 10, 741. [Google Scholar] [CrossRef]
- Radeloff, K.; Tirado, M.R.; Haddad, D.; Breuer, K.; Müller, J.; Hochmuth, S.; Hackenberg, S.; Scherzad, A.; Kleinsasser, N.; Radeloff, A. Superparamagnetic iron oxide particles (VSOPS) show genotoxic effects but no functional impact on human adipose tissue-derived stromal cells (ASCS). Materials 2021, 14, 263. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Chang, K.-A. Therapeutic potential of magnetic nanoparticle-based human adipose-derived stem cells in a mouse model of parkinson’s disease. Int. J. Mol. Sci. 2021, 22, 654. [Google Scholar] [CrossRef] [PubMed]
- Bag, J.; Mukherjee, S.; Ghosh, S.K.; Das, A.; Mukherjee, A.; Sahoo, J.K.; Tung, K.S.; Sahoo, H.; Mishra, M. Fe3O4 coated guargum nanoparticles as non-genotoxic materials for biological application. Int. J. Biol. Macromol. 2020, 165, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Janßen, H.C.; Angrisani, N.; Kalies, S.; Hansmann, F.; Kietzmann, M.; Warwas, D.P.; Behrens, P.; Reifenrath, J. Biodistribution, biocompatibility and targeted accumulation of magnetic nanoporous silica nanoparticles as drug carrier in orthopedics. J. Nanobiotechnol. 2020, 18, 14. [Google Scholar] [CrossRef]
- Zandi, M.; Zare, M.; Nazari, H.; Ashjaee, M.; Zale, A.A. Investigating the effect of injection rate on the capture efficiency of nanoparticles in different geometries of stenosed vessel. J. Magn. Magn. Mater. 2022, 544, 168665. [Google Scholar] [CrossRef]
- Liu, J.F.; Lan, Z.; Ferrari, C.; Stein, J.M.; Higbee-Dempsey, E.; Yan, L.; Amirshaghaghi, A.; Cheng, Z.; Issadore, D.; Tsourkas, A. Use of Oppositely Polarized External Magnets to Improve the Accumulation and Penetration of Magnetic Nanocarriers into Solid Tumors. ACS Nano 2020, 14, 142–152. [Google Scholar] [CrossRef]
- Próspero, A.G.; Soares, G.A.; Moretto, G.M.; Quini, C.C.; Bakuzis, A.F.; De Arruda Miranda, J.R. Dynamic cerebral perfusion parameters and magnetic nanoparticle accumulation assessed by AC biosusceptometry. Biomed. Tech. 2020, 65, 343–351. [Google Scholar] [CrossRef]
- Dahaghin, A.; Emadiyanrazavi, S.; Haghpanahi, M.; Salimibani, M.; Bahreinizad, H.; Eivazzadeh-Keihan, R.; Maleki, A. A comparative study on the effects of increase in injection sites on the magnetic nanoparticles hyperthermia. J. Drug Deliv. Sci. Technol. 2021, 63, 102542. [Google Scholar] [CrossRef]
- Carter, T.J.; Agliardi, G.; Lin, F.; Ellis, M.; Jones, C.; Robson, M.; Richard-Londt, A.; Southern, P.; Lythgoe, M.; Thin, M.Z.; et al. Potential of Magnetic Hyperthermia to Stimulate Localized Immune Activation. Small 2021, 17, 2005241. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, R.P.; Janko, C.; Unterweger, H.; Lyer, S.; Alexiou, C. SPIONs and magnetic hybrid materials: Synthesis, toxicology and biomedical applicatiodddns. Magn. Hybrid-Mater. Multi-Scale Model. Synth. Appl. 2021, 739–768. [Google Scholar] [CrossRef]
- Khoei, A.J. Evaluation of potential immunotoxic effects of iron oxide nanoparticles (IONPs) on antioxidant capacity, immune responses and tissue bioaccumulation in common carp (Cyprinus carpio). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 244, 109005. [Google Scholar] [CrossRef] [PubMed]
- Tangra, A.K.; Singh, G. Investigation of cytotoxicity of superparamagnetic KFeO2 nanoparticles on MCF-7 cell lines for biomedical applications. J. Mater. Sci. Mater. Electron. 2021, 32, 11232–11242. [Google Scholar] [CrossRef]
- Khan, M.S.; Buzdar, S.A.; Hussain, R.; Afzal, G.; Jabeen, G.; Javid, M.A.; Iqbal, R.; Iqbal, Z.; Mudassir, K.B.; Saeed, S.; et al. Hematobiochemical, Oxidative Stress, and Histopathological Mediated Toxicity Induced by Nickel Ferrite (NiFe2O4) Nanoparticles in Rabbits. Oxidative Med. Cell. Longev. 2022, 2022, 5066167. [Google Scholar] [CrossRef]
- Senthilkumar, N.; Sharma, P.K.; Sood, N.; Bhalla, N. Designing magnetic nanoparticles for in vivo applications and understanding their fate inside human body. Coord. Chem. Rev. 2021, 445, 214082. [Google Scholar] [CrossRef]
- Timerbaev, A.R. How well can we characterize human serum transformations of magnetic nanoparticles? Analyst 2020, 145, 1103–1109. [Google Scholar] [CrossRef]
- Sheridan, E.; Vercellino, S.; Cursi, L.; Adumeau, L.; Behan, J.A.; Dawson, K.A. Understanding intracellular nanoparticle trafficking fates through spatiotemporally resolved magnetic nanoparticle recovery. Nanoscale Adv. 2021, 3, 2397–2410. [Google Scholar] [CrossRef]
- Sun, C.; Lee, J.S.H.; Zhang, M. Magnetic nanoparticles in MR imaging and drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1252–1265. [Google Scholar] [CrossRef] [Green Version]
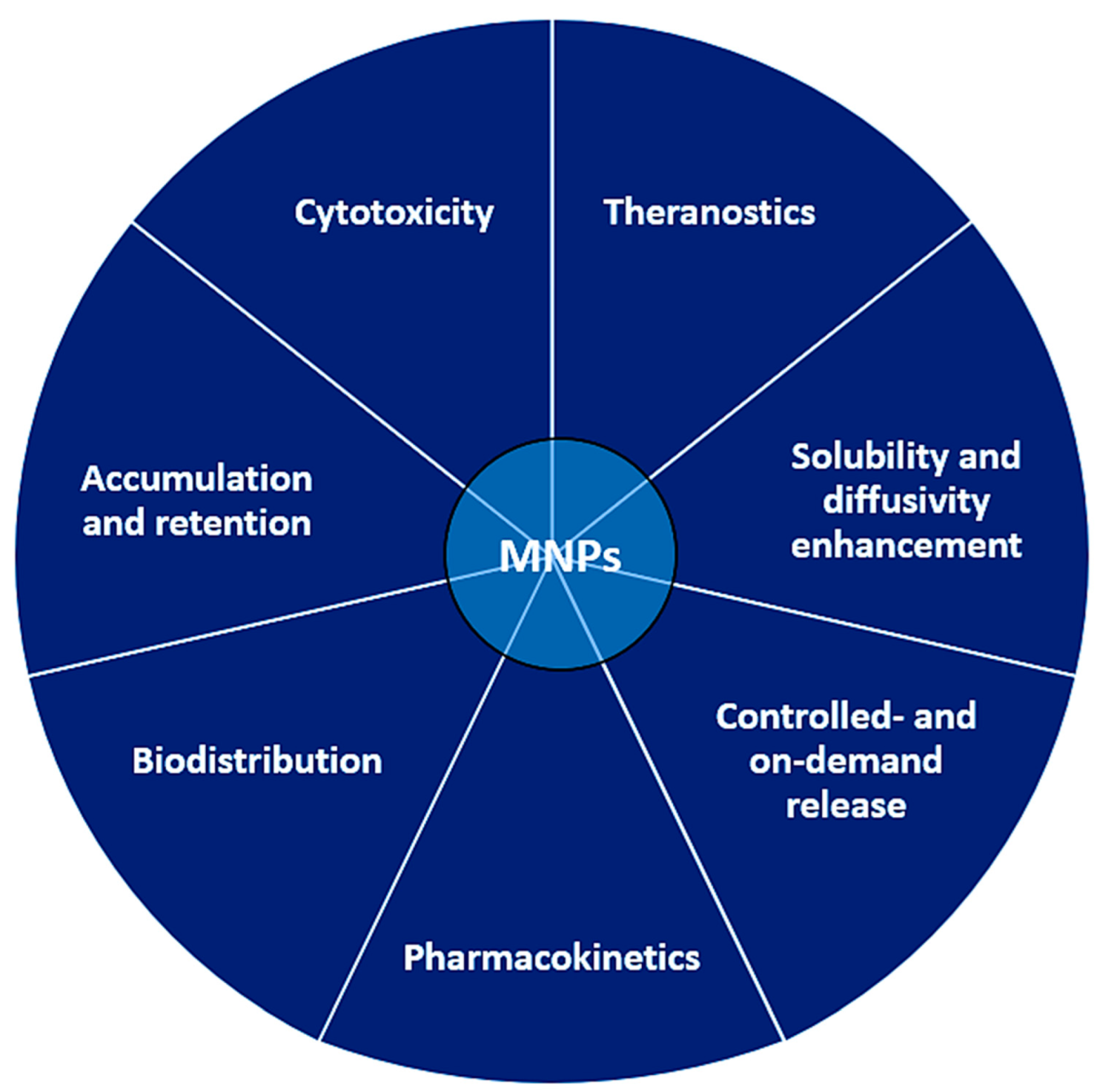
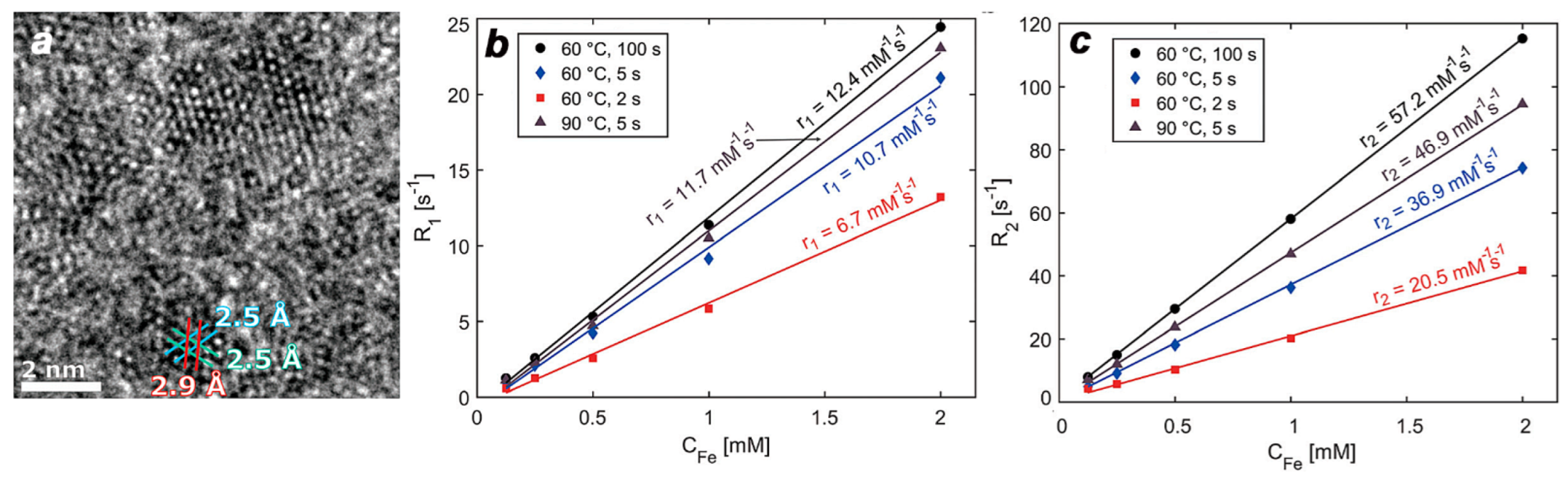

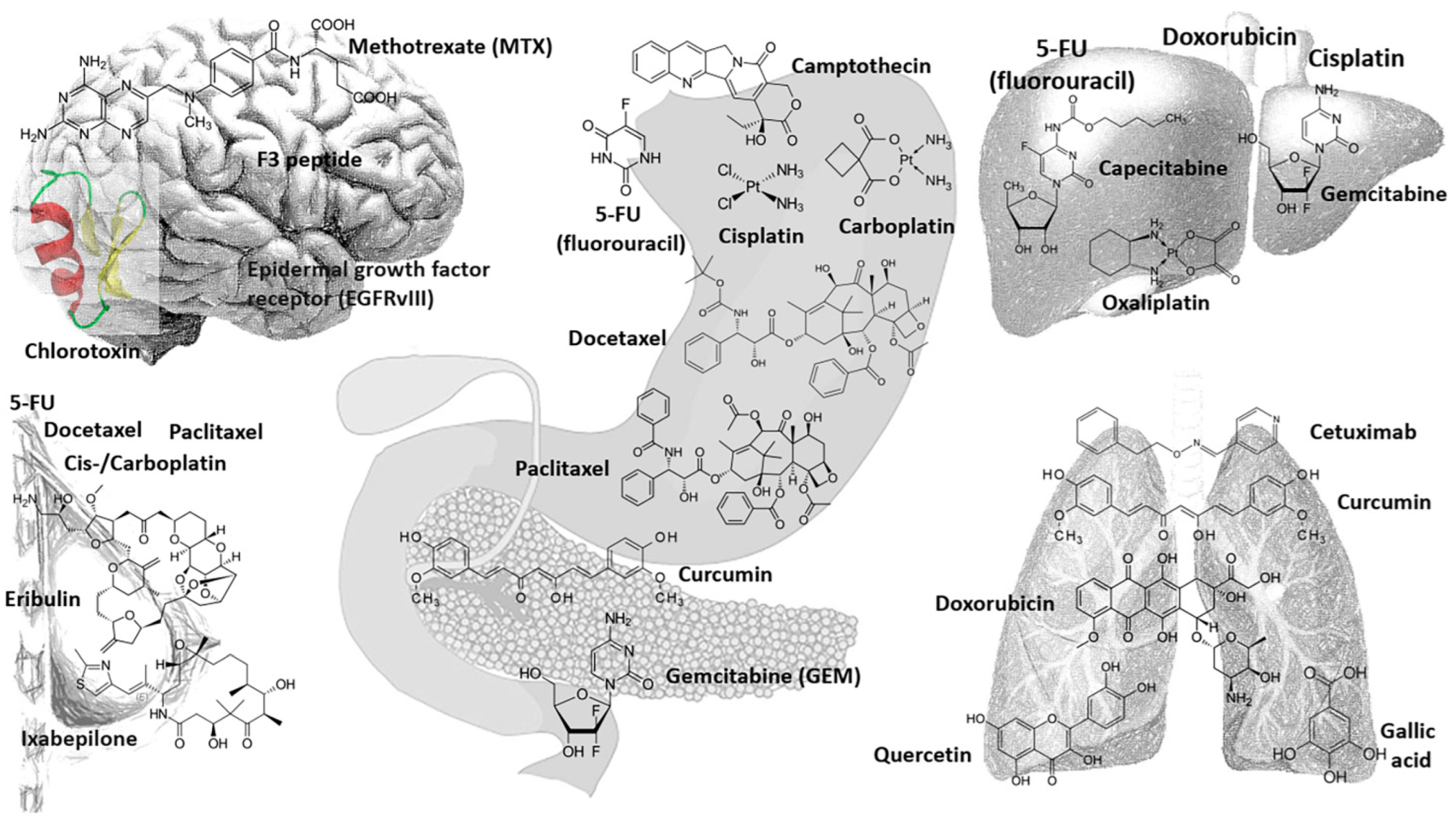
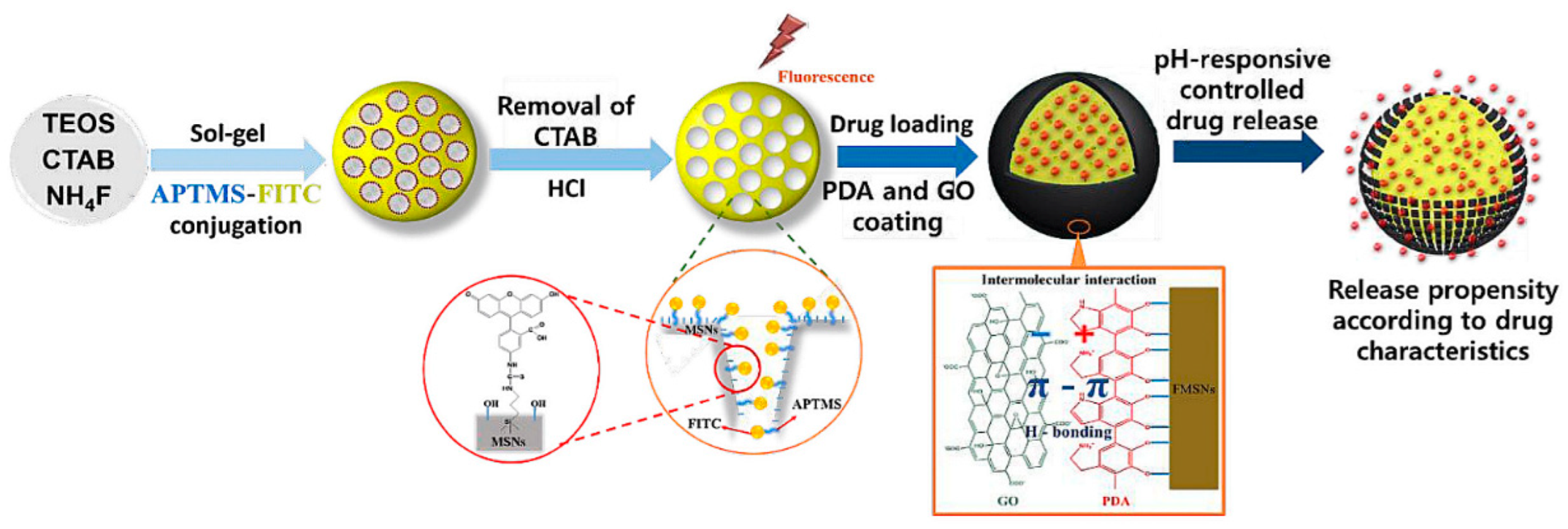
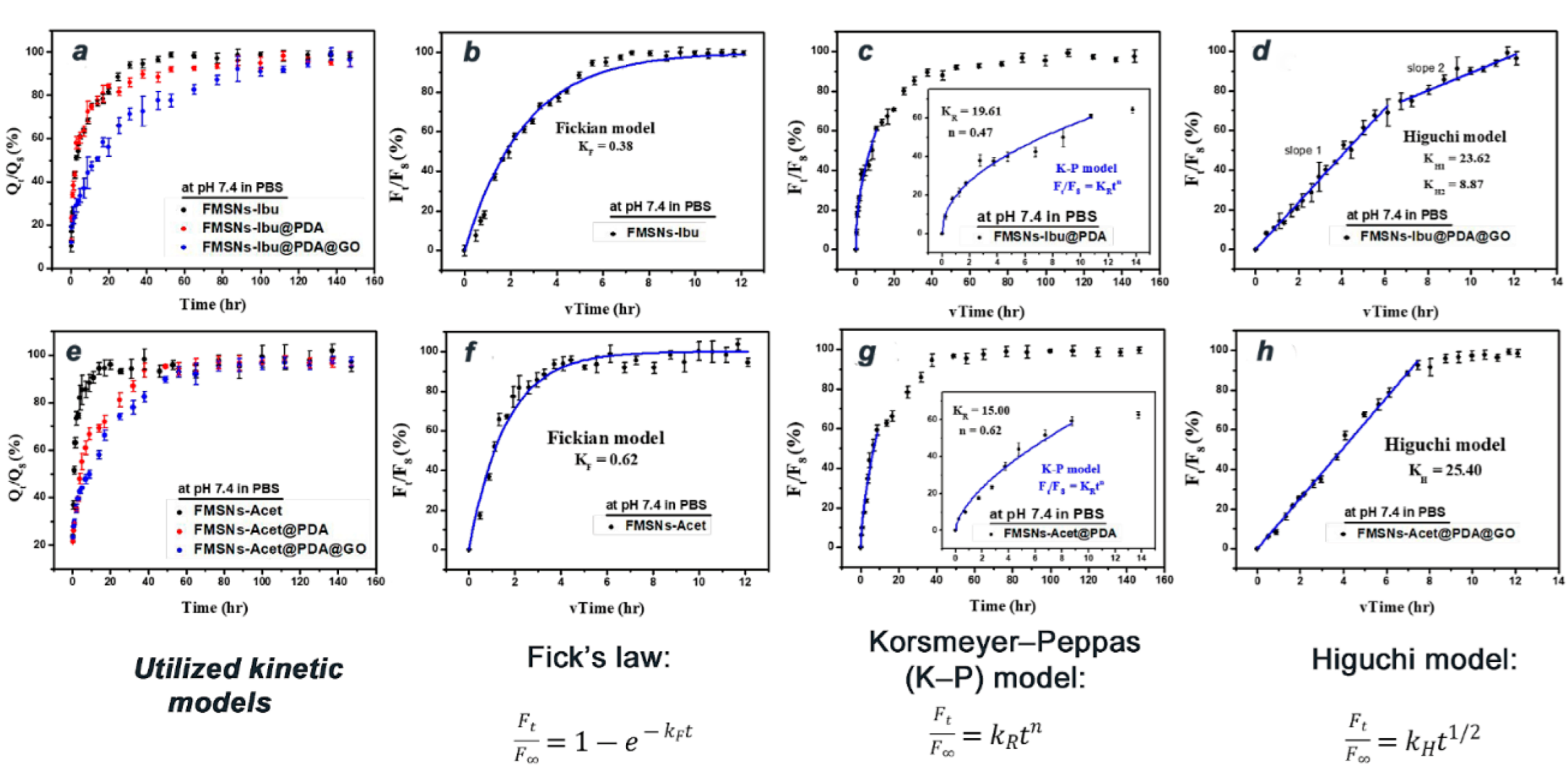



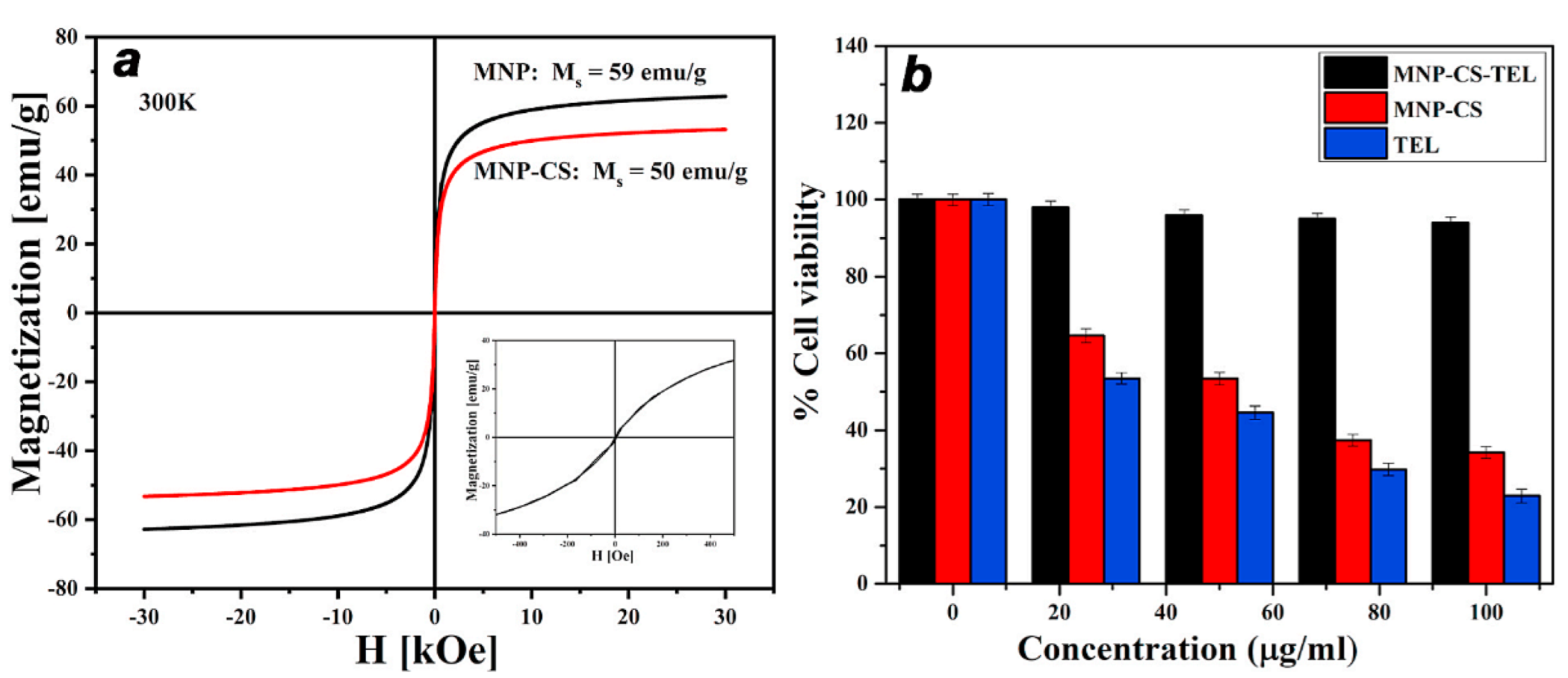
| Type of NP | Synthesis of MNPs/Coating | Surface Functionalization | Type of Drug/Molecule | Targeted Disease/Application | Ref. |
|---|---|---|---|---|---|
| Fe3O4 | Tripolyphosphate (TPP) and glutaraldehyde stabilizers | polyvinyl alcohol/collagen | BSA protein | drug release system | [65] |
| NixCu1−x-silica nanoparticles (x = 0.675) | sol–gel method | SiO2 silica from tetraethyl-orthosilicate (TEOS, Si(OC2H5)4) hydrolysis and condensation | PTX (C13H18N4O3), BPC (C18H28N2O), PCM (C8H9NO2) | Skin cancer-Humanskin fibroblasts (ATCC-CCL-110, Detroit 551) | [126] |
| Multifunctional Fe3O4@SiO2-APTES-DOTA | sol–gel | SiO2 with amino-functionality by aminopropyltriethoxysilane (APTES) usage; and 1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) | Teniposide, anticancer drug | brain tumors, acute lymphocytic leukemia (ALL) | [258] |
| MnFe2O4 and Cr2Fe6O12 nanocarriers | combustion/calcination of PVP stabilized metal salt complexes | N/A; curcumin loading by precipitation | curcumin (CUR) release to MCF-7 cells; anti-inflammatory, anti-oxidant, antimicrobial, antispasmodic and antiproliferative activity; (release was pH-dependent) | photosensitizer for photodynamic therapy (PDT); drug delivery | [259] |
| superparamagnetic iron oxide (Fe3O4) nanoparticles (SPIONs) | co-precipitation; Dextran (DEX) stabilization | Folate (FA)-modification by conjugation to MNPs | camptothecin (CPT) action on AT3B-1 cancer cells | prostate cancer | [260] |
| ZnFe2O4 zinc ferrite nano-hollowspheres (NHSs) | solvothermal method | Sodium folate ligand modification for biocompatibility (folic acid small molecule vitamin) | Doxorubicin | cancer treatment | [318] |
| iron oxides (hematite, magnetite); carbonyl iron (due to its low size) | various (precipitation etc.) | polyethylene glycol (PEG); Magnetic liposomes; biodegradable polymers as stabilizers against oxidation (celluloseacetate; hydrogen phthalate) | Paclitaxel; Celecoxib; (Doxorubicin—poor distribution through BBB); Ferrocenyl diphenoltamoxifen; Gadd 153 | Glioblastoma Multiforme management | [319] |
| SPION (magnetite, Fe3O4 nanospheres) | emulsion solvent evaporation method; polymer (ethylcellulose) coating | ascorbic acid (Vitamin C) capping | Carboplatin (CPt) | Breast cancer | [320] |
| multifunctional mesoporous silica nanoparticles (MSNs) | coating: Polydopamine (PDA) and graphene oxide (GO) double layer | fluorescent conjugates to yield FMSNs | ibuprofen and acetaminophen | Drug release | [321] |
| Fe3O4 | dextran, PEG, Hyaluronic acid, Human serum albumin conjugate | polyvinyl alcohol (PVA) and PEG-derivatives/functional ligands: PEG(5)-nitrodopamine, PEG(5)-dopamine, PEG(5)-hydroxipyridine | Cetuximab and doxorubicin, Gallic acid, Erotinib, Actein, Quercetin | Lung cancer | [263] |
| Fe3O4-Dex-MA-g-P(NVI/NVCL) (pH-sensitive multi-functional magnetic nanocomposite) | Dex-MA (dextran modified by Glycidyl methacrylate) | Poly(N-vinylcaprolactam) (PNVCL), a temperature-sensitive biocompatible polymer | 5-FLU (5-fluorouracil or 5-Fluoro-2,4-pyrimidinedione) | cancer | [322] |
| magnetic microspheres (MMS) based on Fe3O4 | co-precipitation and water-in-oil-in-water (W1/O/W2) ternary emulsion solvent evaporation process | PLGA (poly-(D, L-lactide-co-glycolic acid)) microspheres; polymer coating tuned for required drug release rate | 5-fluorouracil | cancer therapy, drug release | [323] |
| carbon-coated iron magnetic NPs; USPIOs; magnetite-gold nanocluster Fe3O4@AuNCs@ERL nanocomposite | various (precipitation followed by magnetic separation) | carbon coating; Au/polymeric coating | Erlotinib. ERL: N-(3-ethynylphenyl)-6,7-bis(2-methoxyethoxy)-4-quinazolinamine (epidermal growth factor receptor (EGFR) inhibitor) | metastatic non-small cell lung cancer; aggressive pancreatic cancer | [324] |
| magnetite nanoparticles (MNPs) | modified co-precipitation method (MNPs); glutaraldehyde (GA)/calcium chloride CaCl2 (crosslinker) | sodium alginate (SA)/polyvinylpyrrolidone-co-vinyl acetate (PVP-co-VAc) semi ipn microbeads | curcumin (CUR) (encapsulation by simple ionotropic gelation technique) | cancer treatment | [325] |
| SPIONs (Maghemite) | no organic dispersant | linking the keto-enol moiety of CUR with Fe atoms to form SPION@curcumin hybrids | curcumin | photodynamic therapy (PDT): photodynamic action against S. aureus using blue LED light. | [326] |
| iron oxide nanoparticles (bare Fe3O4 NPs) | Micellar-assisted aqueous stabilization: micelles (dhydrodynamic =120 nm): sodium dodecyl sulfate (SDS) and aniline hydrochloride (AHC) | - | curcumin | hyperthermia therapy (under AC magnetic field); drug (curcumin) delivery | [327] |
| multicore magnetic nanoparticles: magnetite (Fe3O4) and/or maghemite (γ-Fe2O3) | coprecipitation method; coating with SiO2 silica using TEOS, in a modified Stöber method | SiO2 coating of MNPs yields MNP@SiO2 (centrifugation) | Curcuminoids (CC) extracted from turmeric: curcumin (>50%), desmethoxycurcumin, and bisdemethoxycurcumin | theranostic nanoplatform; drug release; hyperthermia candidate | [328] |
| Fe3O4 | chemical co-precipitation; folic acid labeling of MNPs | Polyethylenimine-graft-poly (maleic anhydride-alt-1-octadecene) coated, to yield Fe3O4@PIMF | curcumin (effect on MCF-7 and Helacells) | Drug delivery, MRI (negative signal enhancement in MRI) | [329] |
| NiFe2O4 in x(NiFe2O4)@(100−x)SiO2@HKUST-1 (10 ≤ x ≤ 60 wt.%) | Core-shell strategy; trichloroacetic acid (BTC) as the organic binding for MOF | Silica coating (by TEOS) and MOF functionalization; NiFe2O4@SiO2@HKUST-1 as Novel Magnetic Metal-Organic Framework Nanocomposites | Curcumin adsorption in mesoporous host | drug delivery | [330] |
| Magnetite Fe3O4 in PEGylated Fe3O4/hydroxyapatite (PMHA) nanocomposite | PEG coating | Hydroxyapatite (shell) | Curcumin (effect on A549, MCF-7, and MRC-5 cells) (1.9 mg/g loading, pH-dependent release) | MRI; drug release | [331] |
| Yttrium Y3+-Doped Iron Oxide Fe3O4 Nanoparticles | Co-precipitation (Fe3O4 and Y3+ xFe2+Fe3+2 − xO4) | - | (effect on 4T1 cells-mice mammary gland cancer cells; ATCC CRL2539) | Hyperthermia | [332] |
| Mesoporous Fe3O4 nanoparticles (SPIONs) | solvothermal method (PEG-diamine, hydrazine; for SPIONs); Folate encapsulation in PEG-diamine grafted NPs | Multifunctional polyethyleneglycol-diamine functionalized; 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride/Nhydroxysuccinimide | doxorubicin (DOX) (effect on breast cancer cells MCF-7); through electrostatic attachment to daunosamine (NH3+) | (breast) cancer treatment | [333] |
| iron oxides maghemite (γ-Fe2O3) and magnetite (Fe3O4) nanoparticles | ultrasonic irradiation assisted co-precipitation route (providing good dispersion) | - | - | Hyperthermia | [334] |
| Co/Li/Zn-mixed ferrites: Co0.76Zn0.24Fe2O4, Li0.375Zn0.25Fe2.375O4 and ZnFe2O4 mixed-structure ferrite | ‘dry gel’ formed by a sol–gel auto-combustion method | - | - | Magnetic hyperthermia | [335] |
| FeNiCo ternary alloy nanoparticles | [FeNi]100−xCox (2.5 ≤ x ≤ 50) by polyol method | - | - | Hyperthermia | [336] |
| Co2+-doped magnetite, CoxFe3−xO4–carboxymethylcellulose conjugate ferrofluids | Cox-Fe3O4; (x = 3, 5, and 10% mol of cobalt) | carboxymethylcellulose (biocompatible macromolecular ligand) ferrofluids | (effect of AC magnetic field on human brain cancer cells U87) | magnetic hyperthermia, cancer therapy | [337] |
| magnetic hydrogel based on Fe3O4 NPs | co-precipitation method; Hydrogel formation | Gelatin formulation; Functionalization with methacrylic anhydride (GelMA), then copolymerization with (2-dimethylaminoethyl) methacrylate (DMAEMA) monomer | Doxorubicin (Dox) | breast cancer, hyperthermia | [338] |
| Copper Ferrite Nanoparticles CuFe2O4 MNPs | Co-precipitation, then magnetic separation; Silica coating (TEOS tetraethyl orthosilicate and CPTMS (3-chloropropyl)- trimethoxysilane) | Aromatic Polyamide Chains by polymerization of diamino-benzenes and -naphtalene with terephthaloyl chloride. Final nanocomposite: CuFe2O4@SiO2-poly(p-phenylene Terephthalamide) star-like polymers | - | Hyperthermia evaluation- suitable for mild hyperthermia (ΔT~4 °C) | [339] |
| Fe3 − xCoxO4 (X = 0–1) spherical nanoparticles (7 nm) | thermal decomposition or organometallic precursors: Fe(acac)3 and Co(acac)2 in 1,2 hexadecanediol, oleic acid, and olylamine (polyol) | hydrophilization of hydrophobic Fe3−xCoxO4 by TMAH (tetramethyl ammonium hydroxide, caping agent) | - | Hyperthermia (maximum SAR for x = 0.75) | [340] |
| Fe3O4 | polyol synthesis to give Fe3O4@Au@Cu2 − xS dumbbell heterostructures | hydrophobic-to-hydrophilic by two-step procedure (ligand exchange); thiol-polyethylene glycol coordinate Au and Cu2-xS surfaces and polycatechol–polyethylene glycol bind Fe3O4 surface; 64CuCl2 radiolabeling | - | Photo-Magnetic Hyperthermia and 64Cu Radio-Insertion (Tri-Modal Therapy); suggested efficient for skin cancer treatment | [341] |
| Mg1 − xCoxFe2O4 (0 < x < 1; Δx = 0.1) | chemical co-precipitation method | surface-functionalized: chitosan and chitosan-coated MNPs reported biocompatible behavior | (effect on HeLa cells showed no cytotoxicity) | hyperthermia and in vivo MR imaging | [342] |
| Coated Iron Oxide Nanoparticles (IONPs) | cross-linking with the adsorbed model drug (DOX) | Gelatin-coated (biocompatible natural polymer) | Doxorubicin (DOX); effect on MG-63 osteosarcoma cells | Cancer Treatment; potential hyperthermia effect | [343] |
| Magnetic nanoparticles (MNPs) Iron oxide (Fe3O4) | co-precipitation synthesis of magnetite Fe3O4; coated with four types of primary surfactants, polyethylene glycol 2000 (PEG 2000), oleic acid (OA), Tween 20, and Tween 80 | - | Doxorubicin (high loading); effect on lung adenocarcinoma A549 cell line | cancer treatment | [344] |
| MNPs (Fe3+) | Mohr salt (NH4)2Fe(SO4)2(H2O)6 alkaline solution with sodium hypophosphite NaH2PO2 in the presence of NIPAM; Fe2+/PAA = 1/1 | polyacrylic acid (30%), N-isopropylacrylamide (70%) (NIPAM) nanogel of ~150 nm | Doxorubicin (DOX) | anticancer activity | [345] |
| Magnetite Fe3O4 | simple ionotropic gelationmethod (Gelatin-Coated) | Sodium Alginate (anionic polysaccharide)/Magnetite Nanoparticle Microbeads, doped with Mg2+and Al3+ ions | Doxorubicin (DOX) | drug delivery carriers and applications | [346] |
| MNPs—manganese ferrite MnFe2O4 nanoparticles | co-precipitation method (MnFe2O4) | citrate coating yielding Cit-MnFe2O4 | Doxorubicin (effect on 60 male Wistar rats) | kidney injury (in rats); Chronic kidney disease (CKD) | [347] |
| MNPs (Magnetite Fe3O4 NPs) | co-precipitation (product recovery by magnetic decantation); | PEG coating to produce Fe3O4@PEG; Fe3O4@PEG immersion in Graphene quantum dots solution (by pyrolysis of citric acid, and 24 h still) to yield Fe3O4@PEG@GQD dispersed in PBS, then Fe3O4@PEG@GQD-DOX (magnetic separation) | Doxorubicin (effect on breast cancer MCF7 cells) | anticancer activity, drug release (in vitro) | [348] |
| - | unseeded stable cavitation (ultrasound) | - | Doxorubicin (or Adriamycin); effect on 4T1 murine mammary carcinoma | Murine Mammary Tumor Cells | [349] |
| Magnetite-based magnetic gelatin microspheres | co-precipitation method; using FeCl2 instead of FeSO4 produces higher Ms (61.6 emu/g); gelatin coating | fructose, glucose, genipin (most efficient) and 1-ethyl-3(3-dimethylaminopropyl)carbodiimide (EDC) as crosslinking agents of gelatin | Doxorubicin | drug delivery | [350] |
| MgFe2O4 ferrite MNPs | glycol-thermal method | Chitosan (CHI), polyethylene glycol (PEG) and polyvinyl alcohol (PVA); CHI-MNPs have highest DOX encapsulation (84.28%) | Doxorubicin; effect on human embryonic kidney (HEK293), colorectal adenocarcinoma (Caco-2), and breast adenocarcinoma (SKBR-3) cell lines | (pH controlled) drug release, cancer treatment | [351] |
| Fe3O4@SiO2@SBA-15 | co-precipitation (and magnetic separation); PEG 400 coating | SiO2 silica coating (TEOS); PEI grafted | Doxorubicin | MCF-7 cell line drug delivery | [352] |
| MNPs | co-precipitation in alkaline media (NH4OH) of Fe2+/Fe3+/ethylene diamine (for introduction of -NH2 functionalization) | carboxymethyl chitosan (CMC) coating to yield MNPs-CMC-DOX | Doxorubicin | drug release | [353] |
| Superparamagnetic SPIONs (Fe3O4) | co-precipitation (using chloride sources); polymer-coated NPs, by polymerization of glycidyl methacrylate (GMA). | SiO2 and SiO2-NH2 functionalization with tetraethoxysilane (TEOS) and 3-(trimethoxysilyl) propyl methacrylate (TMSPM) | carboranes (by 1sopropyl-o-carborane immobilization) | boron neutron cancer therapy | [354] |
| MNPs magnetite Fe3O4 | co-precipitation; Silica coating | surface-modification with N- (phosphonomethyl) iminodiacetic acid (PMIDA) to Fe3O4@SiO2@PMIDA | anti-CD4 monoclonal antibody (by bioconjugation) | positive selection of peripheral blood T CD4+ lymphocytes | [355] |
| γ-Fe2O3 | bio-assisted method/aqueous co-precipitation; 3 morphologies of MNPs: nanospheres (NS), nanograsses (NG) and nanowires (NW) | green route: biosurfactant Furostanol Saponin (FS) from Fenugreek seeds extract | dopamine (DA) and uricacid (UA) | biosensors (molecular recognition platform for simultaneous detection of biomarkers) | [356] |
| Iron oxide MNPs | precipitation (under N2); coating and conjugation to yield Gem-PHB-MNPs hybrids | polyhydroxybutyrate coated | gemcitabine (effect on cell proliferation assay using SKBR-3 and MCF-7 breast cancer cell lines) | targeted drug delivery; treatment of breast cancer | [357] |
| SPION-type, reduced graphene oxide GO—Fe3O4 | co-precipitation; In situ surface functionalization; coating with Pluronic F-127 (PF) to reduce cytotoxicity | delivery via an oriental fungus-type Ganoderma lucidum(provides stabilization); after drug Que loading: rGO-Fe3O4-GL-PF | Quercetin (Que), natural polyphenolic flavonoid with anti-cancer properties | cancer therapy; targeted drug delivery | [358] |
| Magnetite Fe3O4 | precipitation in aqueous media with NH4OH of precursors, then oleic acid coating | PLGA–mPEG star-like block copolymers using biodegradable poly(lactic-co-glycolic acid) (PLGA) and methoxy poly(ethylene glycol) (mPEG) | Quercetin (conjugation to MNPs by dialysis method) | anticancer; nanocarrier for hydrophobic drugs | [359] |
| Magnetite Fe3O4 | microemulsion-assisted co-precipitation method (for MNPs) | PEG-ylation (coating) to PMNPs | gallic acid | cancer treatment | [360] |
| Paramagnetic Fe3O4 nanoparticles | Monte Carlo simulated annealing scheme; molecular dynamics (MD) | PEG-ylation | 5-fluorouracil | cancer treatment; drug delivery | [361] |
| MNPs (DEAE-FluidMAG; 5 mg, 200 nm, ChemicellTM) | enzyme encapsulation stable at 37 °C | - | CLytA-DAAO Chimeric Enzyme; effect against Hs766T, IMIM-PC-2 and RWP-1 pancreatic carcinoma cells, HT-29, SW-480and SW-620 colorectal carcinoma cell lines | cancer therapy (pancreatic and colorectal carcinoma and glioblastoma) | [362] |
| iron oxide nanoparticle | Quantum chemical analysis (B3LYP/6-31G(d,p) in aqueous solution; M06-2X dispersion correction) | - | 5-aminolevulinic acid (anticancer drug); drug binding via advanced hydrogen bonding | cancer treatment | [363] |
| self-assembled magnetic nanospheres (MNS) | solvothermal method (MNS); Nintedanib (NTD) conjugated with MNS-APTES through the acid liable imine bond | Aminopropyltriethoxysilane (APTES) monolayer coating and functionalization | Nintedanib (NTD); targets human lung cancer cells L-132 | anticancer | [364] |
| iron oxide (IO) | wet chemical co-precipitation (with enriched KNO3 content of FeSO4 solution prior to KOH precipitation) | APTES-Modified Nanohydroxyapatite (nHAp); Nanohydroxyapatite–Iron Oxide Composite (nHAp/IO) produces after APTES surface modification: nHAp/IO@APTES | effect on murine osteoblast precursor cell line (MC3T3-E1) and murine monocyte–macrophage cell line (RAW 264.7) | Early Osteogenesis, Reduces Inflammation and Inhibits Osteoclast Activity | [365] |
| Magnetic nanoparticles Fe3O4 | co-precipitation method (using chloride iron sources); drug was loaded on MNP-CS through an amide bond between -NH2 groups (chitosan) and -COOH groups (TEL) | Chitosan coating; their solutions shaken gently for 2 h at 25 °C to obtain chitosan coated MNPs (MNP-CS) | Telmisartan (TEL), a water-soluble anticancer drug | cancer treatment | [366] |
| Fe3O4 in superparamagnetic graphene oxide (SPMGO) nanocomposite | chemical precipitation method, graphene oxide/magnetite nanocomposite | cyanuric chloride (CC), used as linker; final nanocarriers: SPMGO and SPMGO/CC, to yield SPMGO/MTX and SPMGO/CC/MTX | methotrexate (MTX); tested against Caov-4, HeLa and MCF-7 cell lines | cancer treatment | [367] |
| Mn0.5Zn0.5DyxFe2−xO4 (x ≤ 0.1) NPs | ultrasonic irradiation method | sonication in LB (Luria Bertaini) to achieve the suspended broth-drug solution | tested against Escherchia coli ATCC35218 as Gram-negative and Staphlyloccocus aureus ATCC29213, as Gram-positive bacteria; and Human colorectal or colon carcinoma cells (HCT-116) | anticancer; antifungal activity (vs. Candida albicans ATCC 14053, yeast) | [368] |
| magnetic silk nanoparticles | microfluidic device using silk fibroin and MNPs | Peptide-functionalization of magnetic silk NPs, with Antitumor peptide G3-a cationic amphiphilic anticancer peptide, G(IIKK)3I-NH2 | Dimethylcurcumin (ASC-J9), androgen receptor inhibitor; tested against HCT 116 colorectal cancer cells | anticancer | [369] |
| metal ferrite NPs, MnFe2O4, CuFe2O4 | one-pot solvothermal method (270 °C, polyol method, in situ CD formation, ethanolamine 1-amino-2-hydroxy-ethane as source) | oleyl amine surface coating and functionalization; carbon dots-metal ferrite hybrids, CDs-MNPs: CDs@MnFe2O4, CDs@CuFe2O4 | (tested on HeLa cancer cells) | multipurpose marker agent of HeLa cancer cells | [370] |
| magnetic graphene oxide hybrid, based on MnFe2O4 magnetic core | nanocomposite (mGG3F) of graphene, MnFe2O4 NPs, poly(amidoamine) dendrons and folic acid | poly(amidoamine) dendron-functionalization | Pd(II) complex synthesized using Naphcon as a model drug, with entrapment efficiency (EE) 73.9% ± 0.08 | cancer therapy | [371] |
| SPIONs (Fe3O4) | superparamagnetic iron oxide nanoparticles | polyamidoamine PAMAM-modified mesoporous silica-coating of SPIONS | folic acid (effect on MCF-7 cells); Indocyanine green (ICG) a near-infrared dye was loaded in M-MSN-PAMAM nanocarriers | cancer (photodynamic) therapy | [372] |
| Methionine Magnetic Nanoparticles Ni1−xCoxFe2O4@Methionine@PEG NPs | Ni1−xCoxFe2O4 NP coated with methionine using the reflux method (under N2); 1 mg of Ni1 − xCoxFe2O4@Methionine@PEG NPs could load 0.51 mg naproxen | PEG-coating by 30 min vigorously stirring PEG-6000 powder in a phosphate-buffered saline (PBS) with Ni1−xCoxFe2O4@Methionine | Naproxen (most potent COX-1 and COX-2 inhibitors) | cancer growth inhibition; controlled drug release | [373] |
| iron oxide nanocubes (IONCs) | one pot synthesis, from Fe(acac)3, decanoic acid and dibenzylether (DBE) in squalene (SQ) at 310 °C, then magnetic separation and centrifugation yield IONCs (15 nm ± 1 nm and 23 nm ± 5 nm edge length) | polycaprolactone fibers (electrospinning process) | doxorubicin; tested against Mouse embryonic fibroblast cell line (NIH 3 T3 cells), DOXOsensitive HeLa-WT cervical cancer cells and the DOXO-resistant MCF7 breast cancer cells | hyperthermia and cancer treatment | [374] |
| Iron Oxide nanocomposites with Fe2O3 core | commercial | Polyurethane diol/Polycaprolactone to yield PUD/PCL-Fe2O3 nanocomposites | - | catalytic effect (potential alternative use in fuel cells) | [375] |
| Iron Oxide (Fe3O4) | Two-Step LASER Ablation in Aqueous Media; TiO2 (core-shell), in both Fe3O4 and TiO2 pressed into pellets (commercial sources) | organic binder material | cytotoxicity against lung cancer cell lines (A549), Escherichia coli and Staphylococcus aureus | Antimicrobial and Anticancer | [376] |
| Iron MNPs | Co-precipitation method (MNPs); Polymer coatings were synthesized by two-stage melt polycondensation using BD:ADA:TBT as catalyst (molar ratio of 1:1:0.1), under N2. | biofunctionalized with poly(butylene adipate-co-terephthalate) (PBAT), and poly(butylene adipate) (PBA). | Absorption of DOPh and DBPh from aqueous medium | Phthalate absorption | [377] |
| SPIONs | dextran-coated PEG-COOH functionalized super-paramagnetic ironoxide nanoparticles, SPIONs (micromod Partikeltechnologie, GmbH); carbodiimide chemistry producing FGF2-SPIONs | dextran-coated PEG-COOH functionalized super-paramagnetic ironoxide nanoparticles, SPIONs (micromod Partikeltechnologie, GmbH, Rostock, Germany) | Fibroblast growth factor 2 (FGF2); effect studied on normal and cirrhotic human livers, Human hepatic stellate cells (LX2 cells) | treatment of acute liver injury (in vivo) | [378] |
| Manganese ferrite MnFe2O4 magnetic core | Microwave Driven Solvothermal Synthesis | Functionalization by oxidation polymerization process to yield polyrhodanine manganese ferrite PRHD@MnFe2O4 binary hybrids | effect against specific cell lines: macrophages (RAW 264.7), osteosarcoma cells line (UMR-106), and stromal progenitor cells of adipose tissue (ASCs); Antimicrobial activity against Escherichia coli and Staphylococcus aureus. | protection against Fenton’s reactions, and generation of highly toxic radicals; antimicrobial therapy | [379] |
| Zn2+Doped Magnetite Fe3O4 Nanoparticles | low-cost method oleic acid/alcohol/water system to synthesize Zn0.4Fe2.6O4 NPs; dimercaptosuccinic acid coated Zn2+ doped magnetite nanoparticles (DMSA-Zn0.4Fe2.6O4) | dimercaptosuccinic coating providing -SH functionalization | Spleen accumulation through translocation of oral medicine; in vivo study (rats) | (oral) drug delivery, MRI; evidence of drug translocation from oral to organ (liver, spleen) in non-toxic forms | [380] |
| magnetic microspheres with γ-Fe2O3 magnetic core | core-shell synthesis; doping with Tb3+ ions could sensitize the fluorescence of Enr | silica coating with -NH2 grafted functionality, and MOF and CMC- sodium carboxymethyl cellulose functionalization; γ-Fe2O3@SiO2-NH2-CMC/MOF5 and γ-Fe2O3@SiO2-NH2-CMC/IRMOF3 magnetic MOF nanoparticles | Enrofloxacin Enr (fluoroquinolone antibiotic, brand name: Baytril®); best results for γ-Fe2O3@SiO2-NH2-CMC/IRMOF3 | treatment of bacterial infections | [381] |
| Ni(1−x)CoxFe2O4 NPs | reflux process (modified co-precipitation with NaOH under N2, reflux, then amino-acid addition) | Methionine (amino acid) coating during MNPs synthesis | Tetracycline (drug loading 0.33 mg in 1 mg of carrier); tested on Melanoma cancer cell line (A375) and HFF normal cell, Staphylococcus aureus, Escherichia coli. | drug delivery | [382] |
| MNPs Fe3O4 | co-precipitation | SH functionalization via (3-Mercaptopropyl) and trimethoxysilane | Coenzyme Q0 (CoQ0, 2,3-dimethoxy-5-methyl-1,4-benzo-quinone); effect on Saos, MCF7 and Hela cell lines | antitumoral effect; anti-inflammatory, anticancer, and antioxidant | [383] |
| MNPs Fe3O4 | coprecipitation of iron sulfate salts in basic media; 2-step strategy for nanohybrid | APTES linker between MNPs and the stearyl moiety (amide bond) | (R)-9-Acetoxystearic Acid (9-HSA); | biomedical (antiproliferative agent active against different cancer cells) | [384] |
| iron oxide NPs | co-precipitation of Fe(III) and Fe(II) in alkaline medium (MNPs); ceftriaxone (CFT)-loaded Nʹ-methacryloylisonicotinohydrazide (MIH)-functionalized magnetic nanoparticles(CFT-MIH-MNPs) | high functionalization degree | ceftriaxone (oral administration, brand name Rocephin, a third-generation cephalosporin antibiotic) in vitro stability using simulated gastrointestinal tract (GIT) fluids | treatment of bacterial infections; high drug entrapment, gradual drug release; enhanced oral delivery of CFT. | [385] |
| α -Fe2O3/Gadofullerene (GdF) Hybrid | simple chemical precipitation method | chitosan | chitosan-α-Fe2O3/GdF hybrid composites-antibacterial resistance against Escherichia coli, Pseudomonas aeruginosa, Bacilus subtilis, and Staphylococcus aereus, and P. aeruginosa (inducing pneumonia) | Treatment of Antibiotic-Resistant Bacterial Pneumonia | [386] |
| α-Fe2O3 | chemical precipitation method | chitosan | Chitosan/α-Fe2O3 nanocomposite; antibacterial activity against Staphylococcus aureus and Escherichia coli. | antibacterial treatment | [387] |
| SPION Fe3O4 | co-precipitation with sonication in thermostatic bath (under Ar) | chitosan coating, collagen functionalization | - | biomedical and technological applications, scaffolds for tissue regeneration | [388] |
| Fe2O3 | chitosan coating | Fe2O3/chitosan/montmorillonite (MMT, polymer layered silicate); after encapsulation, QC release is pH-dependent and follows Weibullkinetic model. | Quercetin (QC) delivery (potential adjuvant in COVID-19 medication); Fe2O3/CS/MMT NPs tested against MCF-7 cells | drug delivery; cancer treatment | [389] |
| Fe3O4@PAA@MIL-100(Cr) metal-organic framework | 50 wt% drug CIP encapsulation in Fe3O4@PAA@MIL-100(Cr)@CIP | PAA@MIL-100(Cr) | ciprofloxacin (CIP)(fluoroquinolone antibiotic); tested by disk diffusion method against Escherichia coli and Staphylococcus aureus | antibacterial | [390] |
| Ag-coated MNPs; Fe3O4/Ag and Fe3O4@SiO2/Ag (33.2–35.1 nm) | chemical reduction method (co-precipitation) | Ag coating; -NH2 functionalization via APTES | trimethoprim (antibiotic); sulfamethoxazole; effect on Escherichia coli and Staphylococcus aureus | antibiotic treatment; drug release | [391] |
| CoFe2O4–BaTiO3, CoFe2O4–Bi4Ti3O12 and Fe3O4–BaTiO3 core-shell magnetoelectric nanoparticles | core-shell type magnetoelectric nanoparticles | PNIPAm. -functionalized | methotrexate MTX (model drug; its adsorption best described by Freundlich model) | drug delivery | [392] |
| magnetic nanoparticles MNPs | Co-precipitation of Fe2+/Fe3+ = 1/2, with NH4OH | Tryptophan (amino acid involved in metabolic functions); 99mTc labeling afforded evaluation of the biodistribution and the blood kinetics | indoleamine 2,3 dioxygenase (IDO) and L-type amino acid transporter (effect on cell lines A-549, MCF-7) | tumor treatment; cancer treatment (ovarian, lung, colorectal) | [393] |
| Maghemite (γ-Fe2O3) | core-shell magnetic nanoparticles; | γ-Fe2O3@SiO2, γ-Fe2O3@SiO2-NH2 and γ-Fe2O3@SiO2-NH2-COOH MNPs via TEOS, APTMS and glutaric anhydride (GA). Further functionalization: Core-Shell package of Tb-BDC-NH2 and Tb-BDC, with ligands 2-aminoterephthalic acid (H2BDC-NH2) and terephthalic acid (H2BDC). | norfloxacin (Nor); via coordination between γ-Fe2O3@SiO2-NH2-COOH/Tb-BDC and Nor | antibiotic | [394] |
| MNPs (Fe) | For C-coating, Fe is more biocompatible and less toxic than Fe3O4 | Graphene-encapsulated to produce Fe@C (biocompatible graphene shell) | ferulic acid (pharmaceutical ingredient found in the traditional Chinese herb Angelica sinensis) | diabet (mice); (controlled) drug release | [395] |
| Ionic magnetic Fe2O3 core-shell nanoparticles | coprecipitation of Fe3+ and Fe2+ ions at a molar ratio of 2:1. with NH4OH. Oxidation occurs due to air exposure (24 h) | silica shell and functionalized with alkylimidazolium organic halide: Guerbet imidazoles (to yield NpFeSi MNPs) | DNA extraction and stabilization against fragmentation | promising platform for therapeutic delivery; DNA extraction | [396] |
| superparamagnetic iron oxide nanoparticles Fe3O4 (APTMS@SPIONs) | co-precipitation method from iron salts Fe3+/Fe2+: 2/1 (mol ratio) using NH4OH in presence of APTMS at 85 °C (Ar flow) | 3-aminopropylsilane coating (APTMS) for cationic APTMS@SPIONs; after ICG encapsulation, hydrodynamic size increased from 18 to 35 nm for ICG-APTMS@SPIONs | indocyanine green (ICG) (25 μg mL−1); effect evaluated on planktonic cells and biofilms of Gram-negative (E. coli, K. pneumoniae, P. aeruginosa) and Gram-positive (S. epidermis) bacteria | Antimicrobial photodynamic therapy (aPDT) and antimicrobial photothermal therapy (aPTT) | [397] |
| MNPs (Fe3O4) as Fe3O4@SIO2/SH/NH2 | Chemical co-precipitation for core-shell MNPs (under N2) | Silica-thiol coating; -NH2 functionalization via hydrolysis/condensation of APTES solution, CPTES and MPTES. | methotrexate (MTX) and cysteine (Cys); up to 65% drug absorption | drug release (tested at 37° and 25 °C) | [398] |
| Magnetite Fe3O4 | (commercial powder, from Sigma-Aldrich Ltd., ≥97% trace metal basis, particle size 50–100 nm.) | chemical-free, pulsed laser ablation (PLAL) to give ibuprofen:magnetite composites 4:1, 3:1 and 2:1 (wt) | ibuprofen | inflammation and pain management; targeted drugdelivery | [399] |
| MNPs Fe6(OH)18(H2O)6 | quantum chemical study (using GAUSSIAN 09 and LANL2DZ basis set) | drug approaches TPZ via NH2 (MNP/TPZ1), NO (MNP/TPZ2-3) and intraring N-atom (MNP/TP4) functional groups/-NH2 mechanism leads to the thermodynamically- stable product via reaction to surface -OH groups (MNPs) | Tirapazamine (TPZ), experimental anticancer drug activated to a toxic radical only at hypoxia (low [O2]) | cancer treatment | [400] |
| core-shell magnetic nanoparticles (NPs): Fe3O4@SiO2/NH2 and Fe3O4@CS | co-precipitation under Ar atmosphere; chitosan coating | silica coating and -NH2 functionalization using APTES (3-(triethoxysilyl)-propylamine) | goat anti-HBsAg antibody (with NaIO4 activation procedure) | antibody immobilization; sensing nanoplatforms (detection of HBsAg) | [401] |
| iron oxide nanoparticles (Magnetite Fe3O4 with hydroxyl endings) | standard co-precipitation technique (MNPs); silane coating with APTES by silanization reaction; Galactosylated coating | lactobionic acid (LBA)-functionalized: MNP-LBA | ceftriaxone (CFT) | controlled drug release for the oral delivery of CFT | [402] |
| bare iron oxide NPs (IONs): magnetite (cubic) | - | - | lasioglossin III (short cationic peptide) from bee venom | drug delivery (Escherichia coli tests show higher antimicrobial activity of bound lasioglossin) | [403] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Comanescu, C. Magnetic Nanoparticles: Current Advances in Nanomedicine, Drug Delivery and MRI. Chemistry 2022, 4, 872-930. https://doi.org/10.3390/chemistry4030063
Comanescu C. Magnetic Nanoparticles: Current Advances in Nanomedicine, Drug Delivery and MRI. Chemistry. 2022; 4(3):872-930. https://doi.org/10.3390/chemistry4030063
Chicago/Turabian StyleComanescu, Cezar. 2022. "Magnetic Nanoparticles: Current Advances in Nanomedicine, Drug Delivery and MRI" Chemistry 4, no. 3: 872-930. https://doi.org/10.3390/chemistry4030063
APA StyleComanescu, C. (2022). Magnetic Nanoparticles: Current Advances in Nanomedicine, Drug Delivery and MRI. Chemistry, 4(3), 872-930. https://doi.org/10.3390/chemistry4030063







