UiO-66 MOF-Derived Ru@ZrO2 Catalysts for Photo-Thermal CO2 Hydrogenation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of Ru-Loaded UiO-66
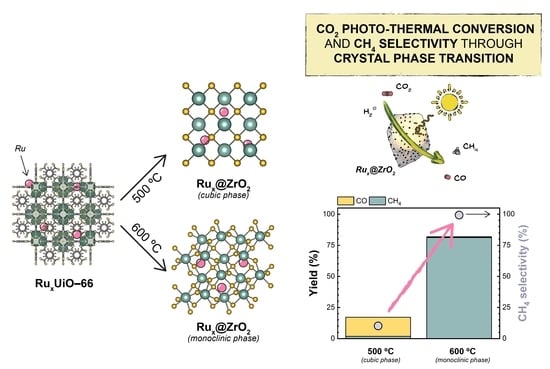
2.2. Preparation and Characterization of MOF-Derived Ru@ZrO2 Catalysts
2.3. Catalytic Tests
3. Materials and Methods
3.1. Materials
3.2. MOF Synthesis
3.3. Thermal Treatment
3.4. Characterization Techniques
3.5. Catalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Intergovernmental Panel on Climate Change, IPCC. Climate Change 2014: Mitigation of Climate Change; Cambridge University Press: Cambridge, UK, 2015; ISBN 9781107058217. [Google Scholar]
- Álvarez, A.; Bansode, A.; Urakawa, A.; Bavykina, A.V.; Wezendonk, T.A.; Makkee, M.; Gascon, J.; Kapteijn, F. Challenges in the Greener Production of Formates/Formic Acid, Methanol, and DME by Heterogeneously Catalyzed CO2 Hydrogenation Processes. Chem. Rev. 2017, 117, 9804–9838. [Google Scholar] [CrossRef] [PubMed]
- Goud, D.; Gupta, R.; Maligal-Ganesh, R.; Peter, S.C. Review of Catalyst Design and Mechanistic Studies for the Production of Olefins from Anthropogenic CO2. ACS Catal. 2020, 10, 14258–14282. [Google Scholar] [CrossRef]
- Ye, R.P.; Ding, J.; Gong, W.; Argyle, M.D.; Zhong, Q.; Wang, Y.; Russell, C.K.; Xu, Z.; Russell, A.G.; Li, Q.; et al. CO2 Hydrogenation to High-Value Products via Heterogeneous Catalysis. Nat. Commun. 2019, 10, 5698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corma, A.; Garcia, H. Photocatalytic Reduction of CO2 for Fuel Production: Possibilities and Challenges. J. Catal. 2013, 308, 168–175. [Google Scholar] [CrossRef]
- Ramirez, A.; Sarathy, S.M.; Gascon, J. CO2 Derived E-Fuels: Research Trends, Misconceptions, and Future Directions. Trends Chem. 2020, 2, 785–795. [Google Scholar] [CrossRef]
- Senderens, J.B.; Sabatier, P. Nouvelles Synthèses Du Méthane. Comptes. Rendus. Acad. Sci. 1902, 82, 514–516. [Google Scholar]
- Vogt, C.; Monai, M.; Kramer, G.J.; Weckhuysen, B.M. The Renaissance of the Sabatier Reaction and Its Applications on Earth and in Space. Nat. Catal. 2019, 2, 188–197. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Y.H.; Qi, M.Y.; Yamada, Y.M.A.; Anpo, M.; Tang, Z.R.; Xu, Y.J. Photothermal Catalytic CO2 Reduction over Nanomaterials. Chem. Catal. 2021, 1, 272–297. [Google Scholar] [CrossRef]
- Song, C.; Wang, Z.; Yin, Z.; Xiao, D.; Ma, D. Principles and Applications of Photothermal Catalysis. Chem. Catal. 2022, 2, 52–83. [Google Scholar] [CrossRef]
- Meng, X.; Wang, T.; Liu, L.; Ouyang, S.; Li, P.; Hu, H.; Kako, T.; Iwai, H.; Tanaka, A.; Ye, J. Photothermal Conversion of CO2 into CH4 with H2 over Group VIII Nanocatalysts: An Alternative Approach for Solar Fuel Production. Angew. Chem. 2014, 126, 11662–11666. [Google Scholar] [CrossRef]
- Ghoussoub, M.; Xia, M.; Duchesne, P.N.; Segal, D.; Ozin, G. Principles of Photothermal Gas-Phase Heterogeneous CO2 Catalysis. Energy Environ. Sci. 2019, 12, 1122–1142. [Google Scholar] [CrossRef]
- Fang, S.; Hu, Y.H. Thermo-Photo Catalysis: A Whole Greater than the Sum of Its Parts. Chem. Soc. Rev. 2022, 51, 3609–3647. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Bai, X.; Ning, S.; Song, C.; Guan, Q.; Liu, B.; Li, Y.; Ye, J. Nanostructured Materials for Photothermal Carbon Dioxide Hydrogenation: Regulating Solar Utilization and Catalytic Performance. ACS Nano 2022, 17, 1725–1738. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Shi, R.; Zhao, J.; Zhang, T. Ni-Based Catalysts Derived from Layered-Double-Hydroxide Nanosheets for Efficient Photothermal CO2 Reduction under Flow-Type System. Nano Res. 2021, 14, 4828–4832. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Jaroniec, M.; Chen, X. Cocatalysts for Selective Photoreduction of CO 2 into Solar Fuels. Chem. Rev. 2019, 119, 3962–4179. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Z.; Rao, Z.; Yu, F.; Bao, W.; Tang, Y.; Zhao, H.; Zhang, J.; Wang, Z.; Li, J.; et al. Experimental and Theoretical Insights into an Enhanced CO2 Methanation Mechanism over a Ru-Based Catalyst. Appl. Catal. B Environ. 2022, 319, 121903. [Google Scholar] [CrossRef]
- Ullah, S.; Lovell, E.C.; Wong, R.J.; Tan, T.H.; Scott, J.; Amal, R. Light-Enhanced CO2 Reduction to CH4 Using Nonprecious Transition-Metal Catalysts. ACS Sustain. Chem. Eng. 2020, 8, 5056–5066. [Google Scholar] [CrossRef]
- Ulmer, U.; Dingle, T.; Duchesne, P.N.; Morris, R.H.; Tavasoli, A.; Wood, T.; Ozin, G.A. Fundamentals and Applications of Photocatalytic CO2 Methanation. Nat. Commun. 2019, 10, 3169. [Google Scholar] [CrossRef] [Green Version]
- Kho, E.T.; Jantarang, S.; Zheng, Z.; Scott, J.; Amal, R. Harnessing the Beneficial Attributes of Ceria and Titania in a Mixed-Oxide Support for Nickel-Catalyzed Photothermal CO2 Methanation. Engineering 2017, 3, 393–401. [Google Scholar] [CrossRef]
- Albero, J.; Garcia, H.; Corma, A. Temperature Dependence of Solar Light Assisted CO2 Reduction on Ni Based Photocatalyst. Top. Catal. 2016, 59, 787–791. [Google Scholar] [CrossRef] [Green Version]
- González-Rangulan, V.V.; Reyero, I.; Bimbela, F.; Romero-Sarria, F.; Daturi, M.; Gandía, L.M. CO2 Methanation over Nickel Catalysts: Support Effects Investigated through Specific Activity and Operando IR Spectroscopy Measurements. Catalysts 2023, 13, 448. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Zhou, J.; Wang, L. Metal-Organic Framework-Derived Multifunctional Photocatalysts. Chin. J. Catal. 2022, 43, 971–1000. [Google Scholar] [CrossRef]
- Oar-Arteta, L.; Wezendonk, T.; Sun, X.; Kapteijn, F.; Gascon, J. Metal Organic Frameworks as Precursors for the Manufacture of Advanced Catalytic Materials. Mater. Chem. Front. 2017, 1, 1709–1745. [Google Scholar] [CrossRef] [Green Version]
- Khan, I.S.; Garzon-Tovar, L.; Mateo, D.; Gascon, J. Metal-Organic-Frameworks and Their Derived Materials in Photo-Thermal Catalysis. Eur. J. Inorg. Chem. 2022, 2022, e202200316. [Google Scholar] [CrossRef]
- Khan, I.S.; Mateo, D.; Shterk, G.; Shoinkhorova, T.; Poloneeva, D.; Garzón-Tovar, L.; Gascon, J. An Efficient Metal–Organic Framework-Derived Nickel Catalyst for the Light Driven Methanation of CO2. Angew. Chem. Int. Ed. 2021, 60, 26476–26482. [Google Scholar] [CrossRef]
- Gong, X.; Wang, W.W.; Fu, X.P.; Wei, S.; Yu, W.Z.; Liu, B.; Jia, C.J.; Zhang, J. Metal-Organic-Framework Derived Controllable Synthesis of Mesoporous Copper-Cerium Oxide Composite Catalysts for the Preferential Oxidation of Carbon Monoxide. Fuel 2018, 229, 217–226. [Google Scholar] [CrossRef]
- Zheng, L.; Li, X.; Du, W.; Shi, D.; Ning, W.; Lu, X.; Hou, Z. Metal-Organic Framework Derived Cu/ZnO Catalysts for Continuous Hydrogenolysis of Glycerol. Appl. Catal. B Environ. 2017, 203, 146–153. [Google Scholar] [CrossRef]
- Zeng, L.; Wang, Y.; Li, Z.; Song, Y.; Zhang, J.; Wang, J.; He, X.; Wang, C.; Lin, W. Highly Dispersed Ni Catalyst on Metal-Organic Framework-Derived Porous Hydrous Zirconia for CO2 Methanation. ACS Appl. Mater. Interfaces 2020, 12, 17436–17442. [Google Scholar] [CrossRef]
- Shearer, G.C.; Chavan, S.; Ethiraj, J.; Vitillo, J.G.; Svelle, S.; Olsbye, U.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. Tuned to Perfection: Ironing out the Defects in Metal-Organic Framework UiO-66. Chem. Mater. 2014, 26, 4068–4071. [Google Scholar] [CrossRef]
- Lippi, R.; D’Angelo, A.M.; Li, C.; Howard, S.C.; Madsen, I.C.; Wilson, K.; Lee, A.F.; Sumby, C.J.; Doonan, C.J.; Patel, J.; et al. Unveiling the Structural Transitions during Activation of a CO2 Methanation Catalyst Ru0/ZrO2 Synthesised from a MOF Precursor. Catal. Today 2021, 368, 66–77. [Google Scholar] [CrossRef]
- Xu, W.; Dong, M.; Di, L.; Zhang, X. A Facile Method for Preparing UiO-66 Encapsulated Ru Catalyst and Its Application in Plasma-Assisted CO2 Methanation. Nanomaterials 2019, 9, 1432. [Google Scholar] [CrossRef] [Green Version]
- Fereja, S.L.; Zhang, Z.; Fang, Z.; Guo, J.; Zhang, X.; Liu, K.; Li, Z.; Chen, W. High-Entropy Oxide Derived from Metal-Organic Framework as a Bifunctional Electrocatalyst for Efficient Urea Oxidation and Oxygen Evolution Reactions. ACS Appl. Mater. Interfaces 2022, 14, 38727–38738. [Google Scholar] [CrossRef] [PubMed]
- Pourkhosravani, M.; Dehghanpour, S.; Farzaneh, F. Palladium Nanoparticles Supported on Zirconium Metal Organic Framework as an Efficient Heterogeneous Catalyst for the Suzuki-Miyaura Coupling Reaction. Catal. Lett. 2016, 146, 499–508. [Google Scholar] [CrossRef]
- Akilandeswari, S.; Rajesh, G.; Govindarajan, D.; Thirumalai, K.; Swaminathan, M. Efficacy of Photoluminescence and Photocatalytic Properties of Mn Doped ZrO2 Nanoparticles by Facile Precipitation Method. J. Mater. Sci. Mater. Electron. 2018, 29, 18258–18270. [Google Scholar] [CrossRef]
- Han, Y.; Zhu, J. Surface Science Studies on the Zirconia-Based Model Catalysts. Top. Catal. 2013, 56, 1525–1541. [Google Scholar] [CrossRef]
- Sadati, S.M.; Feghhi, S.A.H.; Mohammadi, K. Effect of Time Exposure on Themoluminiscend Glow Curve For UV-Induced ZrO2:Mg Phosphor. Radiat. Prot. Dosim. 2017, 173, 333–337. [Google Scholar]
- Prakashbabu, D.; Hari Krishna, R.; Nagabhushana, B.M.; Nagabhushana, H.; Shivakumara, C.; Chakradar, R.P.S.; Ramalingam, H.B.; Sharma, S.C.; Chandramohan, R. Low Temperature Synthesis of Pure Cubic ZrO2 Nanopowder: Structural and Luminescence Studies. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 122, 216–222. [Google Scholar] [CrossRef]
- Nagase, H.; Naito, R.; Tada, S.; Kikuchi, R.; Fujiwara, K.; Nishijima, M.; Honma, T. Ru Nanoparticles Supported on Amorphous ZrO2 for CO2 Methanation. Catal. Sci. Technol. 2020, 10, 4522–4531. [Google Scholar] [CrossRef]
- Gao, M.; Zhang, J.; Zhu, P.; Liu, X.; Zheng, Z. Unveiling the Origin of Alkali Metal Promotion in CO2 Methanation over Ru/ZrO2. Appl. Catal. B Environ. 2022, 314, 121476. [Google Scholar] [CrossRef]
- Alves, L.M.N.C.; Almeida, M.P.; Ayala, M.; Watson, C.D.; Jacobs, G.; Rabelo-Neto, R.C.; Noronha, F.B.; Mattos, L.V. CO2 Methanation over Metal Catalysts Supported on ZrO2: Effect of the Nature of the Metallic Phase on Catalytic Performance. Chem. Eng. Sci. 2021, 239, 116604. [Google Scholar] [CrossRef]
- Ilsemann, J.; Murshed, M.M.; Gesing, T.M.; Kopyscinski, J.; Bäumer, M. On the Support Dependency of the CO2 Methanation—Decoupling Size and Support Effects. Catal. Sci. Technol. 2021, 11, 4098–4114. [Google Scholar] [CrossRef]
- Mateo, D.; Cerrillo, J.L.; Durini, S.; Gascon, J. Fundamentals and Applications of Photo-Thermal Catalysis. Chem. Soc. Rev. 2021, 50, 2173–2210. [Google Scholar] [CrossRef]
- Mateo, D.; Maity, P.; Shterk, G.; Mohammed, O.F.; Gascon, J. Tunable Selectivity in CO2 Photo-Thermal Reduction by Perovskite-Supported Pd Nanoparticles. ChemSusChem 2021, 14, 5525–5533. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, J.; Everitt, H.O. Untangling Thermal and Nonthermal Effects in Plasmonic Photocatalysis. In Plasmonic Catalysis: From Fundamentals to Applications; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2021; pp. 191–230. ISBN 9783527347506. [Google Scholar]
- Suoranta, T.; Niemelä, M.; Perämäki, P. Comparison of Digestion Methods for the Determination of Ruthenium in Catalyst Materials. Talanta 2014, 119, 425–429. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almazán, F.; Lafuente, M.; Echarte, A.; Imizcoz, M.; Pellejero, I.; Gandía, L.M. UiO-66 MOF-Derived Ru@ZrO2 Catalysts for Photo-Thermal CO2 Hydrogenation. Chemistry 2023, 5, 720-729. https://doi.org/10.3390/chemistry5020051
Almazán F, Lafuente M, Echarte A, Imizcoz M, Pellejero I, Gandía LM. UiO-66 MOF-Derived Ru@ZrO2 Catalysts for Photo-Thermal CO2 Hydrogenation. Chemistry. 2023; 5(2):720-729. https://doi.org/10.3390/chemistry5020051
Chicago/Turabian StyleAlmazán, Fernando, Marta Lafuente, Amaya Echarte, Mikel Imizcoz, Ismael Pellejero, and Luis M. Gandía. 2023. "UiO-66 MOF-Derived Ru@ZrO2 Catalysts for Photo-Thermal CO2 Hydrogenation" Chemistry 5, no. 2: 720-729. https://doi.org/10.3390/chemistry5020051
APA StyleAlmazán, F., Lafuente, M., Echarte, A., Imizcoz, M., Pellejero, I., & Gandía, L. M. (2023). UiO-66 MOF-Derived Ru@ZrO2 Catalysts for Photo-Thermal CO2 Hydrogenation. Chemistry, 5(2), 720-729. https://doi.org/10.3390/chemistry5020051