Exploiting Supramolecular Synthons in Cocrystals of Two Racetams with 4-Hydroxybenzoic Acid and 4-Hydroxybenzamide Coformers
Abstract
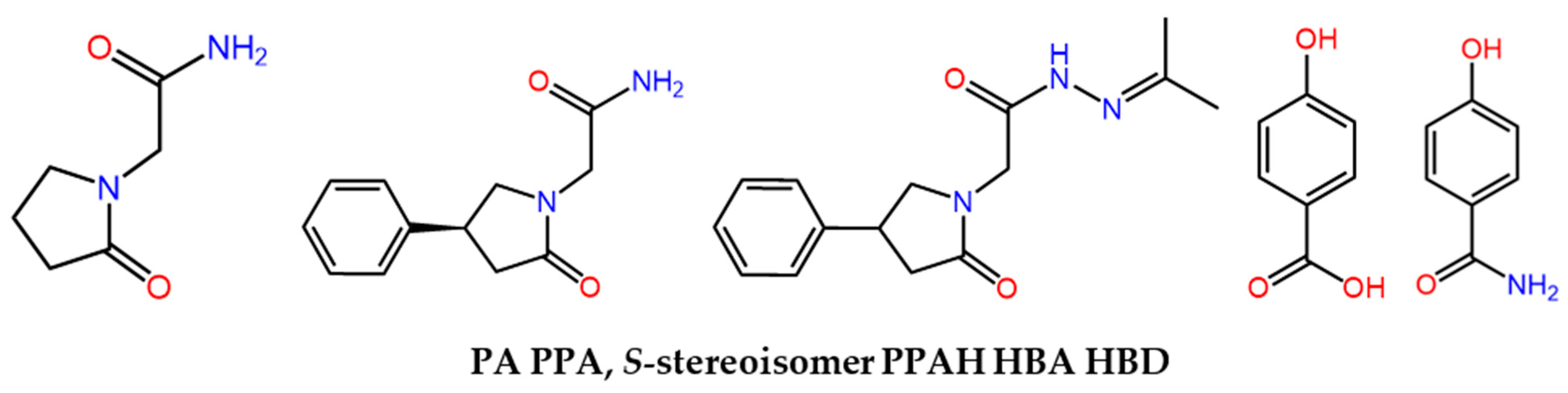
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Solvents
2.2. Synthesis
2.2.1. Synthesis of PPA·HBA (1:1) Cocrystal
2.2.2. Synthesis of PPA·2HBA (1:2) Cocrystal
2.2.3. Synthesis of PPAH·HBD Cocrystal
2.2.4. Synthesis of PPAH·HBD Cocrystal
2.3. Single-Crystal X-ray Diffraction Analysis
2.4. Quantum Chemical Calculations
3. Results
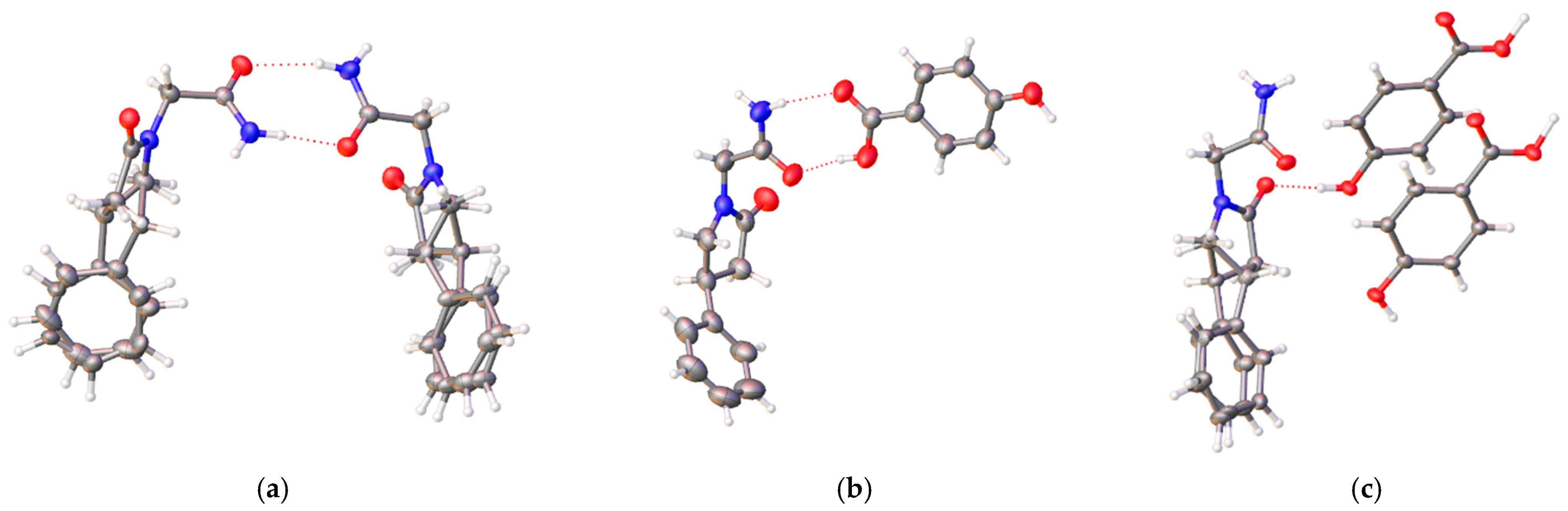
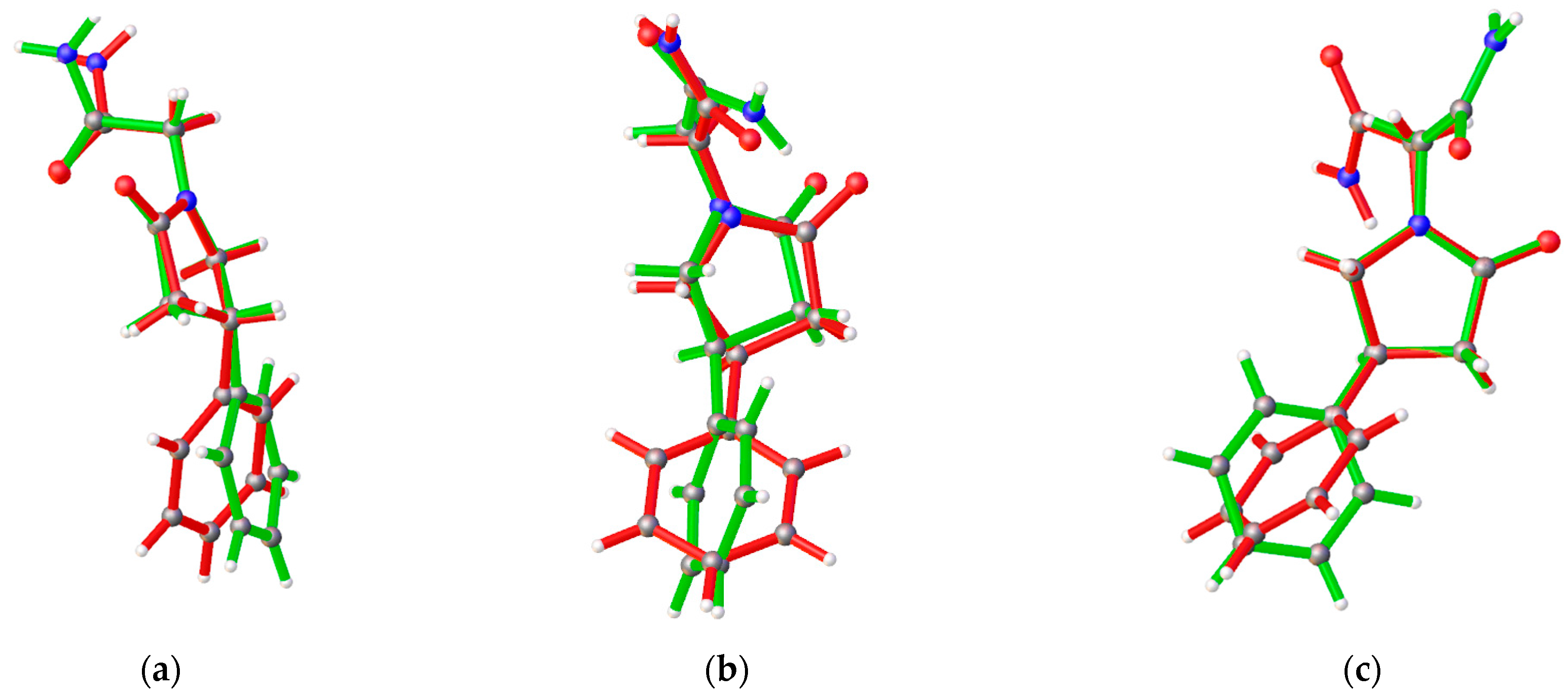
3.1. Crystal Structures of PPA Pure Form, PPA·HBA and PPA·2HBA Cocrystals
3.2. Crystal Structures of PPAH Pure Form and PPAH·HBD Cocrystal
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giurgea, C. The “Nootropic” Approach to the Pharmacology of the Integrative Activity of the Brain. Cond. Refl. 1973, 8, 108–115. [Google Scholar] [CrossRef]
- Gustafson, L.; Risberg, J.; Johanson, M.; Fransson, M.; Maximilian, V.A. Effects of piracetam on regional cerebral blood flow and mental functions in patients with organic dementia. PMC 1978, 56, 115–117. [Google Scholar] [CrossRef]
- Spignoli, G.; Pepeu, G. Interactions between oxiracetam, aniracetam and scopolamine on behavior and brain acetylcholine. Pharmacol. Biochem. Behav. 1987, 27, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Malykh, A.; Sadaie, M. Piracetam and Piracetam-Like Drugs from Basic Science to Novel Clinical Applications to CNS Disorders. Drugs 2010, 70, 287–312. [Google Scholar] [CrossRef] [PubMed]
- The Merck Index, 13th ed.; Merck & Co. Inc.: Whitehouse Station, NJ, USA, 2001; p. 1342. Available online: http://www.ucbpharma.com/ (accessed on 1 March 2022).
- Fabbiani, F.; Allan, D.; David, W.; Davidson, A.; Lennie, A.; Parsons, S.; Pulham, R.; Warren, J. High-Pressure Studies of Pharmaceuticals: An Exploration of the Behavior of Piracetam. Cryst. Growth Des. 2007, 7, 1115–1124. [Google Scholar] [CrossRef]
- Leng, F.; Robeyns, K.; Leyssens, T.; Shemchuk, O. Combining Racetams with a Sweetener through Complexation. Cryst. Growth Des. 2022, 22, 3016–3023. [Google Scholar] [CrossRef]
- Aitipamula, S.; Chow, P.; Tan, R. Structural, Spectroscopic and Thermal Analysis of Cocrystals of Carbamazepine and Piracetam with Hydroquinone. J. Chem. Crystallogr. 2011, 41, 1604–1611. [Google Scholar] [CrossRef]
- Durán-Palma, M.; Mendoza-Barraza, S.; Magaña-Vergara, N.; Martínez-Martínez, F.; González-González, J. Crystal structure of pharmaceutical cocrystals of 2,6-diaminopyridine with piracetam and theophylline. Acta Crystallogr. Sect. C Struct. Chem. 2017, C73, 767–772. [Google Scholar] [CrossRef]
- Viertelhaus, M.; Hilfiker, R.; Blatter, F.; Neuburger, M. Piracetam Co-Crystals with OH-Group Functionalized Carboxylic Acids. Cryst Growth Des. 2009, 9, 2220–2228. [Google Scholar] [CrossRef]
- Vishweshwar, P.; McMahon, J.; Peterson, M.; Hickey, M.; Shattock, T.; Zaworotko, M. Crystal engineering of pharmaceutical co-crystals from polymorphic active pharmaceutical ingredients. Chem. Commun. 2005, 4601–4603. [Google Scholar] [CrossRef]
- Abeysekera, A.M.; Averkiev, B.B.; Sinha, A.S.; Aakeröy, C.B. Evaluating structure–property relationship in a new family of mechanically flexible co-crystals. Chem. Commun. 2022, 58, 9480–9483. [Google Scholar] [CrossRef] [PubMed]
- Aakeröy, C.B.; Alicia, M.; Beatty, A.M.; Brian, A.; Helfrich, B.A. A High-Yielding Supramolecular Reaction. J. Am. Chem. Soc. 2002, 124, 14425–14432. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Desiraju, G.R. Acid···Amide Supramolecular Synthon in Cocrystals: From Spectroscopic Detection to Property Engineering. J. Am. Chem. Soc. 2018, 140, 6361–6373. [Google Scholar] [CrossRef]
- Bolla, G.; Sarma, B.; Nangia, A.K. Crystal Engineering of Pharmaceutical Cocrystals in the Discovery and Development of Improved Drugs. Chem. Rev. 2022, 122, 11514–11603. [Google Scholar] [CrossRef]
- Sowa, M.; Slepokura, K.; Matczak-Jon, E. A 1:1 pharmaceutical cocrystal of myricetin in combination with uncommon piracetam conformer: X-ray single crystal analysis and mechanochemical synthesis. J. Mol. Str. 2014, 1058, 114–121. [Google Scholar] [CrossRef]
- Springuel, G.; Norberg, B.; Robeyns, K.; Wouters, J.; Leyssens, T. Advances in Pharmaceutical Co-crystal Screening: Effective Cocrystal Screening through Structural Resemblance. Cryst. Growth Des. 2012, 12, 475–484. [Google Scholar] [CrossRef]
- Thomas, L.; Wales, C.; Wilson, C. Selective preparation of elusive and alternative single component polymorphic solid forms through multi-component crystallisation routes. Chem. Commun. 2016, 52, 7372–7375. [Google Scholar] [CrossRef]
- Gorodnicheva, N.V.; Vasil´eva, O.S.; Ostroglyadov, E.S.; Baichurin, R.I.; Makarenko, S.V.; Karamov, F.A.; Lodochnikova, O.A.; Litvinov, I.A. 2-[4-(Het)aryl-2-oxopyrrolidin-1-yl]acetohydrazides: Synthesis, structures, and reactions with carbonyl compounds. Russ. Chem. Bull. Int. Ed. 2020, 69, 996–1008. [Google Scholar] [CrossRef]
- Rekis, T.; Bērziņš, A.; Orola, L.; Holczbauer, T.; Actiņš, A.; Seidel-Morgenstern, A.; Lorenz, H. Single Enantiomer’s Urge to Crystallize in Centrosymmetric Space Groups: Solid Solutions of Phenylpiracetam. Cryst. Growth Des. 2017, 17, 1411–1418. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2016, B72, 171–179. [Google Scholar] [CrossRef]
- Zvejniece, L.; Svalbe, B.; Veinberg, G.; Grinberga, S.; Vorona, M.; Kalvinsh, I.; Dambrova, M. Investigation into Stereoselective Pharmacological Activity of Phenotropil. Basic Clin. Pharmacol. Toxicol. 2011, 109, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Childs, S.L.; Hardcastle, K.I. Cocrystals of Piroxicam with Carboxylic Acids. Cryst. Growth Des. 2007, 7, 1291–1304. [Google Scholar] [CrossRef]
- Sanphui, P.; Mishra, M.K.; Ramamurty, U.; Desiraju, G.R. Tuning Mechanical Properties of Pharmaceutical Crystals with Multicomponent Crystals: Voriconazole as a Case Study. Mol. Pharm. 2015, 12, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.N.; Reviglio, A.L.; Siedler, S.; Cardoso, S.G.; Linck, Y.G.; Monti, G.A.; Carvalho, A.M.G.; Resende, J.A.L.C.; Chaves, M.H.C.; Rocha, H.V.A.; et al. New Multicomponent Forms of the Antiretroviral Nevirapine with Improved Dissolution Performance. Cryst. Growth Des. 2020, 20, 688–698. [Google Scholar] [CrossRef]
- Gohel, S.K.; Palanisamy, V.; Sanphui, P.; Prakash, M.; Singh, G.P.; Chernyshev, V. Isostructural cocrystals of metaxalone with improved dissolution characteristics. RSC Adv. 2021, 11, 30689–30700. [Google Scholar] [CrossRef]
- Bruker. APEX 2 Software for Crystal Structure Data Collection, Refinement and Structure Solution; Bruker AXS Inc.: Madison, WI, USA, 2006. [Google Scholar]
- SADABS, version 2.10; Bruker Analytical X-ray Systems, Inc.: Madison, WI, USA, 2003.
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Adv. 2008, A64, 112–122. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. Sect. C Struct. Chem. 2015, C71, 9–18. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—new features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Alvarez, M.; Saavedra, E.; Olivella, M.; Suvire, F.; Zamora, M.; Enriz, R. Theoretical study of the conformational energy hypersurface of cyclotrisarcosyl. Open Chem. 2012, 10, 248–255. [Google Scholar] [CrossRef]
- Kitaigorodsky, A.I. Mixed Crystals; Springer Series in Solid-state Sciences; Springer: Berlin/Heidelberg, Germany, 1984; ISBN 13: 978-3-642-81674-1. [Google Scholar]
- Jacques, J.; Collet, A.; Wilen, S.H. Enantiomers, Racemates, and Resolutions; Krieger Publishing: Malabar, FL, USA, 1991. [Google Scholar]
- Brandel, C.; Petit, S.; Cartigny, Y.; Coquerel, G. Structural aspects of solid solutions of enantiomers. Curr. Pharm. Des. 2016, 22, 4929–4941. [Google Scholar] [CrossRef] [PubMed]
- Lodochnikova, O.A.; Kosolapova, L.S.; Saifina, A.F.; Gubaidullin, A.T.; Fayzullin, R.R.; Khamatgalimov, A.R.; Litvinov, I.A.; Kurbangalieva, A.R. Structural aspects of partial solid solution formation: Two crystalline modifications of a chiral derivative of 1,5-dihydro-2H-pyrrol-2-one under consideration. CrystEngComm 2017, 19, 7277–7286. [Google Scholar] [CrossRef]
- Castañeda, R.; Lindeman, S.V.; Krivoshein, A.V.; Metta-Magaña, A.J.; Chen, Y.; Timofeeva, T.V. Remarkable Similarity of Molecular Packing in Crystals of Racemic and Enantiopure 2-Phenylpropionamide: Z′ = 4 Structures, Molecular Disorder, and the Formation of a Partial Solid Solution. Cryst. Growth Des. 2022, 22, 4592–4600. [Google Scholar] [CrossRef]
- Samipillai, M.; Rohani, S. The role of higher coformer stoichiometry ratio in pharmaceutical cocrystals for improving their solid-state properties: The cocrystals of progesterone and 4-hydroxybenzoic acid. J. Cryst. Growth 2019, 507, 270–282. [Google Scholar] [CrossRef]
- Saikia, B.; Pathaka, D.; Sarma, B. Variable stoichiometry cocrystals: Occurrence and significance. CrystEngComm 2021, 23, 4583–4606. [Google Scholar] [CrossRef]
- Bernstein, J.; Davis, R.E.; Shimoni, L.; Chang, N.-L. Patterns in Hydrogen Bonding: Functionality and Graph Set Analysis in Crystals. Angew. Chem. Int. Ed. 1995, 34, 1555–1573. [Google Scholar] [CrossRef]
- Palanisamy, V.; Sanphui, P.; Bolla, G.; Narayan, A.; Seaton, C.C.; Vangala, V.R. Intriguing High Z″ Cocrystals of Emtricitabine. Cryst. Growth Des. 2020, 20, 4886–4891. [Google Scholar] [CrossRef]
- Arenas-García, J.I.; Herrera-Ruiz, D.; Mondragón-Vásquez, K.; Morales-Rojas, H.; Höpfl, H. Co-Crystals of Active Pharmaceutical Ingredients—Acetazolamide. Cryst. Growth Des. 2010, 10, 3732–3742. [Google Scholar] [CrossRef]
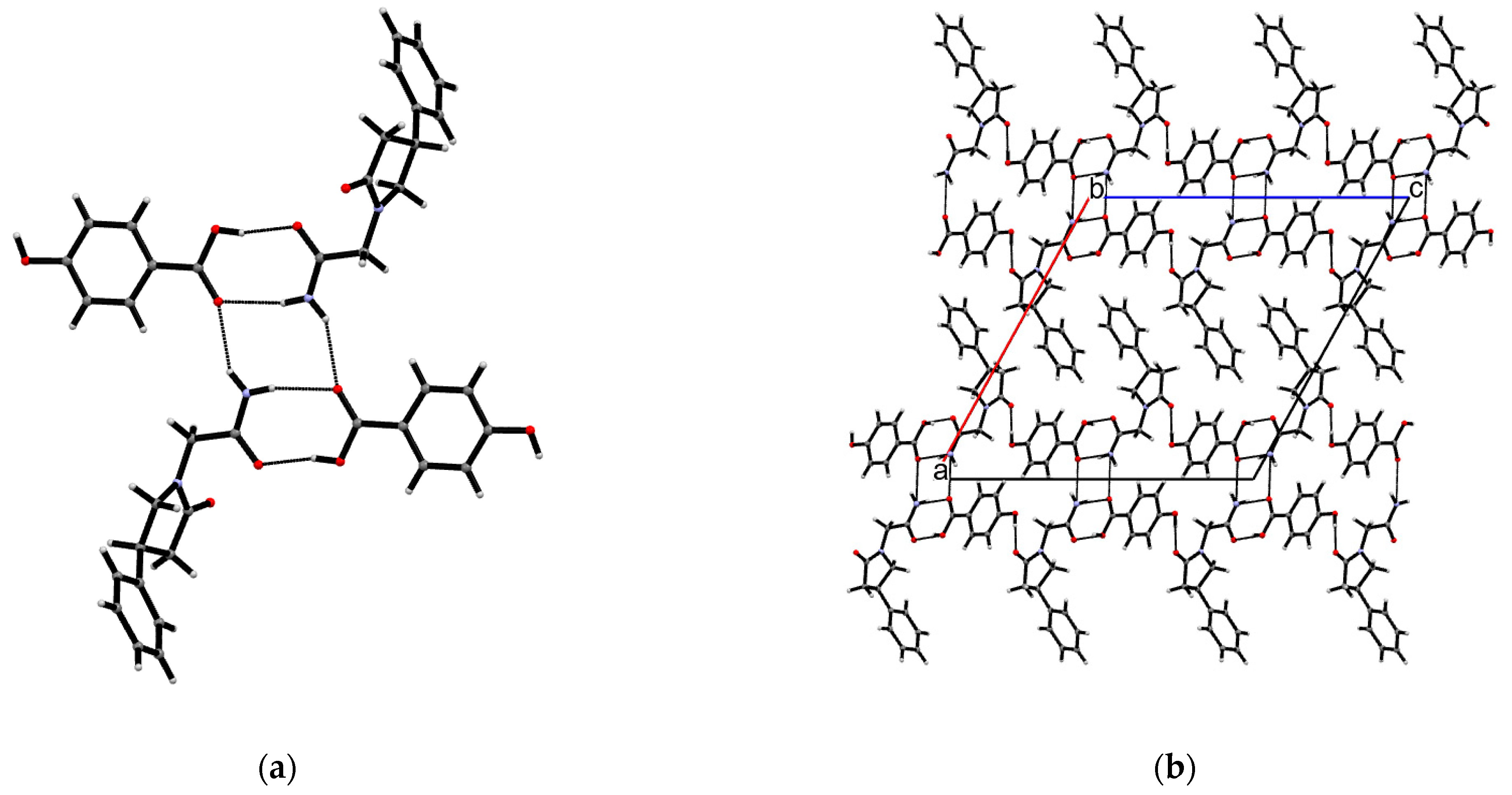
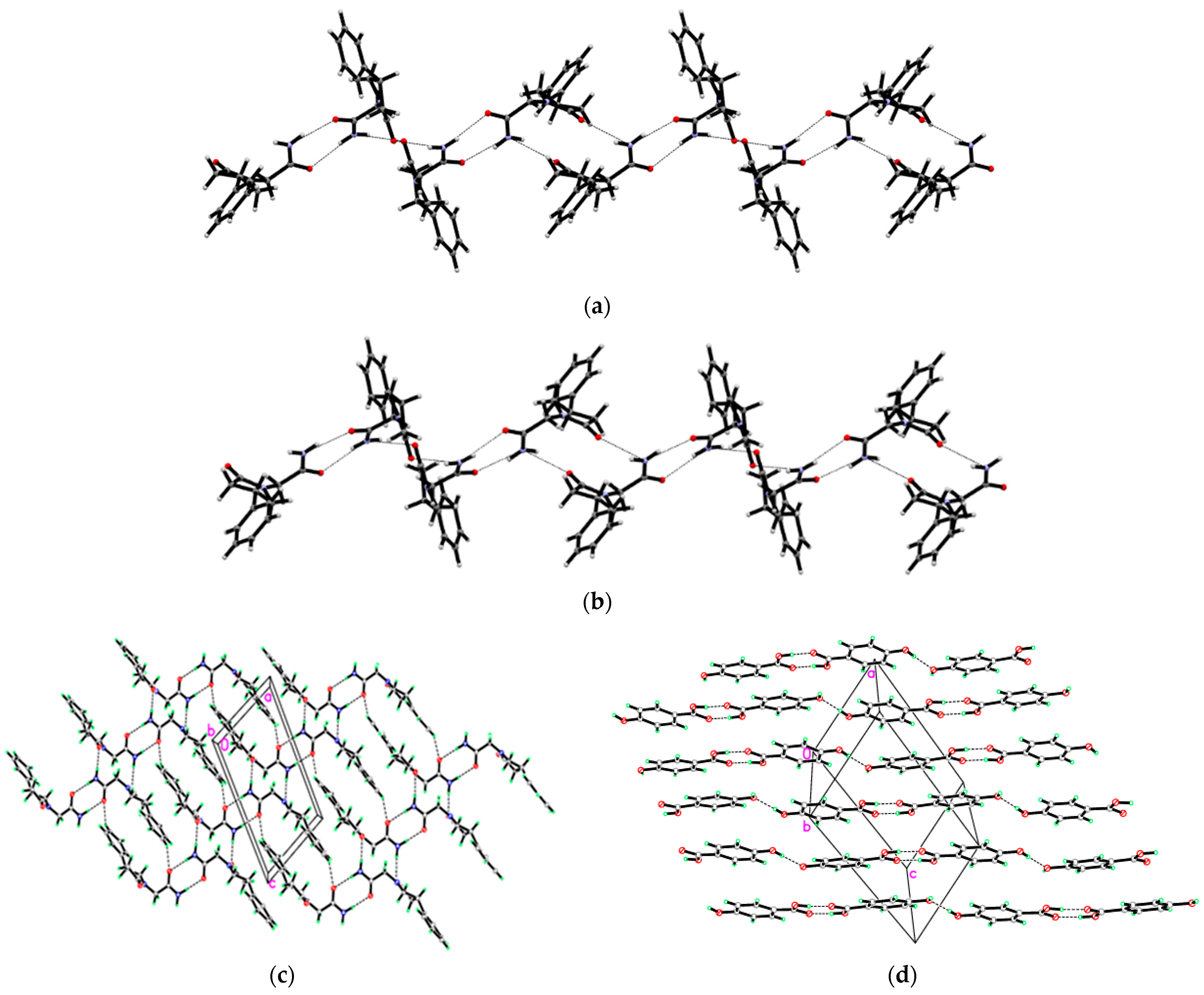
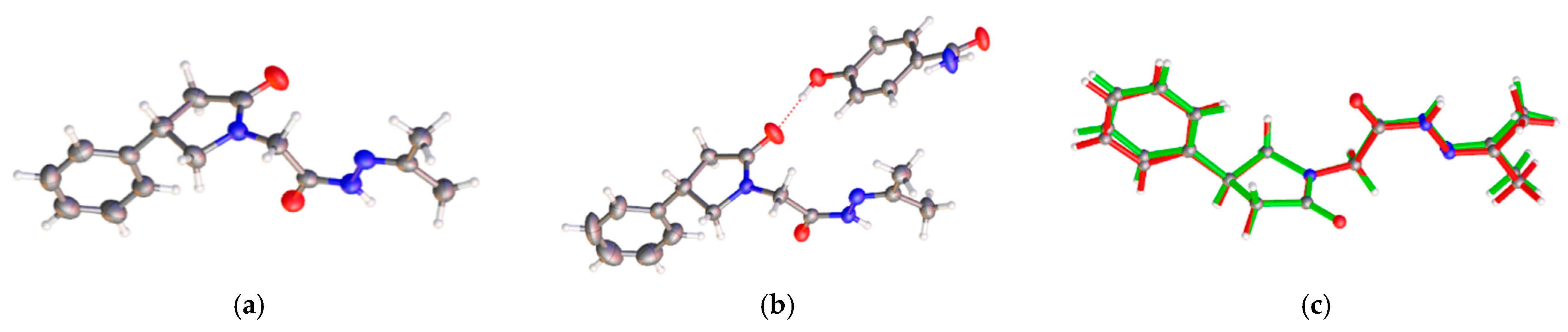

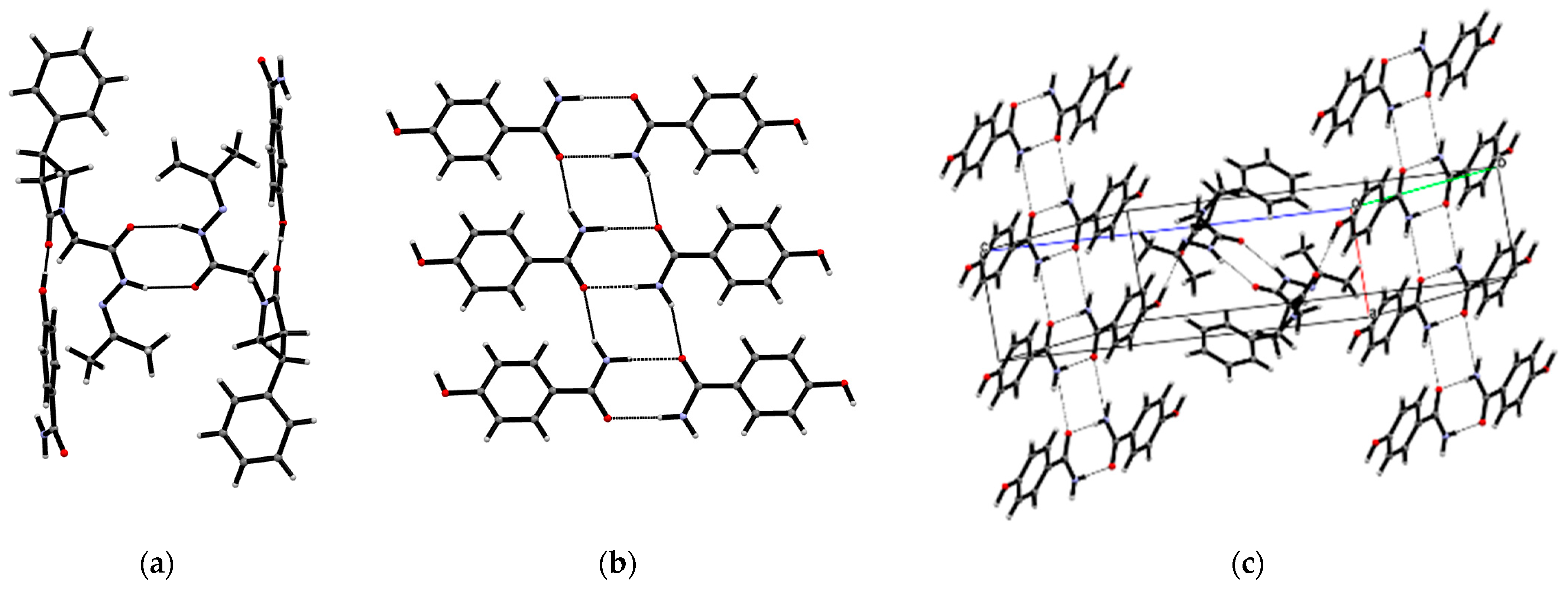
| Compound | PPA | PPA·HBA | PPA·2HBA | PPAH | PPAH·HBD a |
|---|---|---|---|---|---|
| CCDC number | 2247135 | 2247137 | 2247134 | 2247133 | 2247136 |
| Empirical formula | C12H14N2O2 | C19H20N2O5 | C26H26N2O8 | C15H19N3O2 | C25H32N4O5 |
| Formula weight | 218.25 | 356.37 | 494.49 | 273.337 | 468.54 |
| Temperature/K | 150(2) | 293(2) | 150(2) | 100(2) | 173(2) |
| Crystal system | triclinic | monoclinic | triclinic | monoclinic | triclinic |
| Space group | P-1 | P21/c | P-1 | P21/c | P-1 |
| a/Å | 6.0917(17) | 18.9326(3) | 7.9851(8) | 5.5613(8) | 5.2285(3) |
| b/Å | 10.782(3) | 5.58410(10) | 12.6900(12) | 14.190(2) | 13.2516(7) |
| c/Å | 17.502(5) | 18.7881(4) | 13.6456(13) | 18.170(3) | 18.4515(9) |
| α/° | 76.700(4) | 90 | 107.579(3) | 90 | 104.612(4) |
| β/° | 85.742(5) | 118.9220(10) | 104.040(3) | 91.034(5) | 92.217(3) |
| γ/° | 81.827(5) | 90 | 107.749(3) | 90 | 92.539(3) |
| Volume/Å3 | 1106.3(5) | 1738.57(6) | 1167.0(2) | 1433.7(4) | 1234.22(12) |
| Z | 4 | 4 | 2 | 4 | 2 |
| ρcalc g/cm3 | 1.310 | 1.361 | 1.407 | 1.266 | 1.261 |
| μ/mm−1 | 0.091 | 0.825 | 0.105 | 0.086 | 0.726 |
| F(000) | 464 | 752 | 520 | 584 | 500 |
| Crystal size/mm3 | 0.6 × 0.2 × 0.2 | 0.34 × 0.34 × 0.06 | 0.3 × 0.2 × 0.2 | 0.3 × 0.15 × 0.05 | 0.21 × 0.1 × 0.08 |
| Reflections collected | 52871 | 39609 | 34754 | 2220 | 16835 |
| Independent reflections | 6646 [Rint = 0.0958] | 2646 [Rint = 0.0595] | 4124 [Rint = 0.0842] | 2220 | 4272 [Rint = 0.0451] |
| Data/restraints/parameters | 6646/254/406 | 2646/0/239 | 4124/190/452 | 2220/36/184 | 4272/0/277 |
| Goodness-of-fit on F2 | 1.028 | 1.059 | 1.044 | 1.034 | 0.857 |
| Final R indexes R1, wR2 [I ≥ 2σ (I)] | 0.0484, 0.1090 | 0.0399, 0.1081 | 0.0438, 0.1033 | 0.0473, 0.1263 | 0.0519, 0.1426 |
| Final R indexes [all data] R1, wR2 | 0.0957, 0.1300 | 0.0422, 0.1102 | 0.0787, 0.1189 | 0.0767, 0.1379 | 0.0582, 0.1523 |
| Largest diff. peak/hole/e Å−3 | 0.25/−0.25 | 0.32/−0.19 | 0.30/−0.20 | 0.356/−0.196 | 0.64/−0.28 |
| D-H···A | H···A/Å | d(D···A)/Å | ∠D-H···A/° | Symmetry Transformation for Acceptor |
|---|---|---|---|---|
| PPA·HBA | ||||
| O(5)-H(5)···O(1) | 1.84 | 2.6638(18) | 178.9 | x, 5/2 − y, z + 1/2 |
| N(2)-H(2A)···O(4) | 2.10 | 2.936(2) | 163.6 | x, y + 1, z |
| N(2)-H(2B)···O(4) | 2.23 | 2.9751(19) | 144.9 | −x, 1 − y, 1 − z |
| O(3)-H(3)···O(2) | 1.83 | 2.6329(17) | 166.0 | x, y − 1, z |
| PPA·2HBA | ||||
| N(2)-H(2A)···O(1) | 1.97(3) | 2.906(3) | 174(2) | 2 − x, 1 − y, 1 − z |
| N(2)-H(2B)···O(2) | 2.12(3) | 2.999(3) | 169(2) | 1 − x, 1 − y, 1 − z |
| O(3)-H(3B)···O(2) | 1.78(3) | 2.671(2) | 176(2) | 3 − x, 2 − y, 1 − z |
| O(5)-H(5B)···O(6) | 1.710(17) | 2.599(2) | 175(2) | x, y, z |
| O(7)-H(7B)···O(4) | 1.745(17) | 2.636(2) | 174(2) | 3 − x, 2 − y, 1 − z |
| O(8)-H(8)···O(3) | 1.95(3) | 2.820(2) | 174(2) | 1 − x, 1 − y, −z |
| C(11)-H(11A)···O(6) | 2.58 | 3.407(3) | 142.6 | 2 − x, 1 − y, 1 − z |
| PPAH | ||||
| N(2)-H(2)···O(2) | 2.09 | 2.948(2) | 163.3 | 2 − x, 1 − y, 1 − z |
| C(10)-H(10B)···O(2) | 2.53 | 3.474(3) | 159.7 | x − 1, y, z |
| C(15)-H(15B)···O(1) | 2.53 | 3.387(3) | 146.1 | 2 − x, 1/2 + y, 3/2 − z |
| PPAH·HBD | ||||
| N(1)-H(1A)···O(2) | 2.07 | 2.940(4) | 172.5 | 2 − x, 1 − y, 2 − z |
| N(1)-H(1B)···O(2) | 2.26 | 3.064(4) | 151.2 | x − 1, y, z |
| N(3)-H(3)···O(4) | 2.07 | 2.924(4) | 161.9 | 1 − x, 1 − y, 1 − z |
| O(1)-H(6)···O(3) | 1.83(5) | 2.665(4) | 170(5) | x, y, z |
| C(2)-H(2)···O(3) | 2.53 | 3.217(5) | 129.2 | x, y, z |
| C(17)-H(17B)···O(4) | 2.53 | 3.423(4) | 150.2 | x − 1, y, z |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marquez, J.; Novikov, E.; Rigin, S.; Fonari, M.S.; Castañeda, R.; Kornilova, T.; Timofeeva, T.V. Exploiting Supramolecular Synthons in Cocrystals of Two Racetams with 4-Hydroxybenzoic Acid and 4-Hydroxybenzamide Coformers. Chemistry 2023, 5, 1089-1100. https://doi.org/10.3390/chemistry5020074
Marquez J, Novikov E, Rigin S, Fonari MS, Castañeda R, Kornilova T, Timofeeva TV. Exploiting Supramolecular Synthons in Cocrystals of Two Racetams with 4-Hydroxybenzoic Acid and 4-Hydroxybenzamide Coformers. Chemistry. 2023; 5(2):1089-1100. https://doi.org/10.3390/chemistry5020074
Chicago/Turabian StyleMarquez, Jason, Egor Novikov, Sergei Rigin, Marina S. Fonari, Raúl Castañeda, Tatiana Kornilova, and Tatiana V. Timofeeva. 2023. "Exploiting Supramolecular Synthons in Cocrystals of Two Racetams with 4-Hydroxybenzoic Acid and 4-Hydroxybenzamide Coformers" Chemistry 5, no. 2: 1089-1100. https://doi.org/10.3390/chemistry5020074
APA StyleMarquez, J., Novikov, E., Rigin, S., Fonari, M. S., Castañeda, R., Kornilova, T., & Timofeeva, T. V. (2023). Exploiting Supramolecular Synthons in Cocrystals of Two Racetams with 4-Hydroxybenzoic Acid and 4-Hydroxybenzamide Coformers. Chemistry, 5(2), 1089-1100. https://doi.org/10.3390/chemistry5020074