High Thermal Stability of Enzyme-MOF Composites at 180 °C
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of CALB-ZIF-8
2.3. Characterizations
2.4. Determination of Enzyme Immobilization Yield
2.5. Release of CALB from CALB-ZIF-8
2.6. Measurement of Enzyme Activity
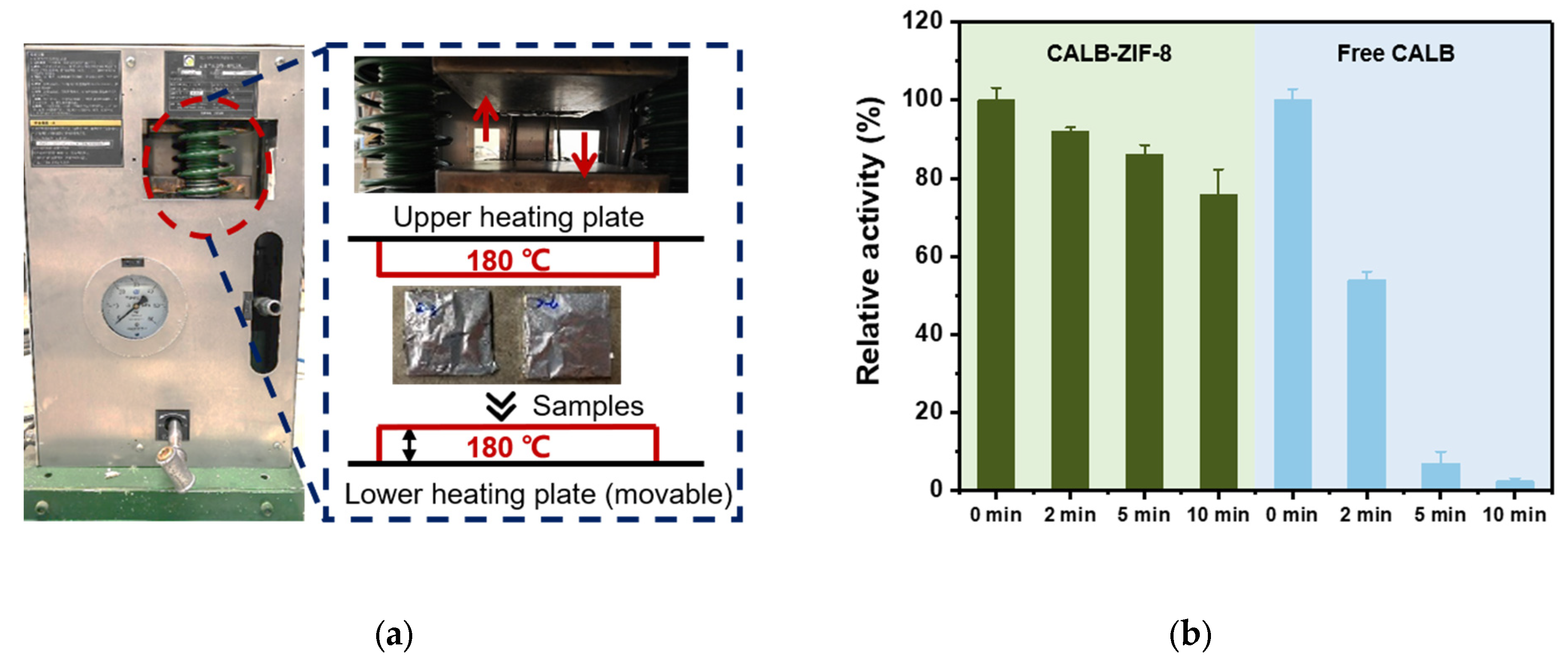
2.7. Measurement of Thermal Stability
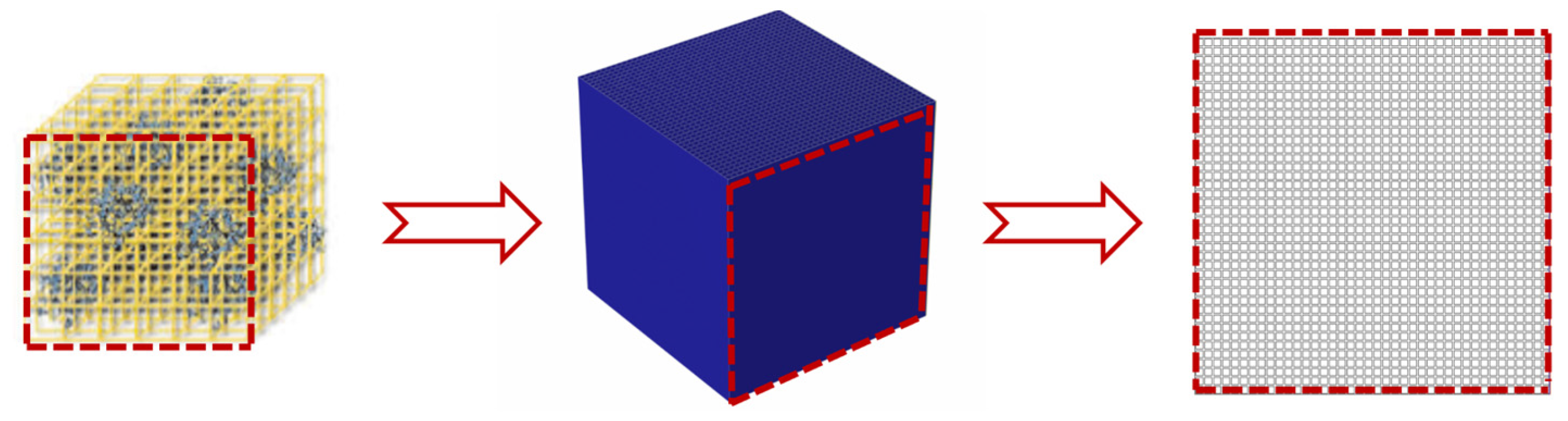
2.8. Simulation Models
- ✓ A ZIF-8 particle was composed of a number of repeating and regular representative volume elements (RVEs), the thermal conductivity of which is equal to the thermal conductivity of the material as a whole;
- ✓ ZIF-8 particles were tightly connected to each other by connections of RVEs, ignoring the air between ZIF-8 particles;
- ✓ Only heat transfer processes were considered in this model, ignoring the heat radiation and heat convection;
- ✓ A single RVE cell in this model was modeled as a 100 nm × 100 nm × 100 nm cube, which was close to a single ZIF-8 particle under SEM.
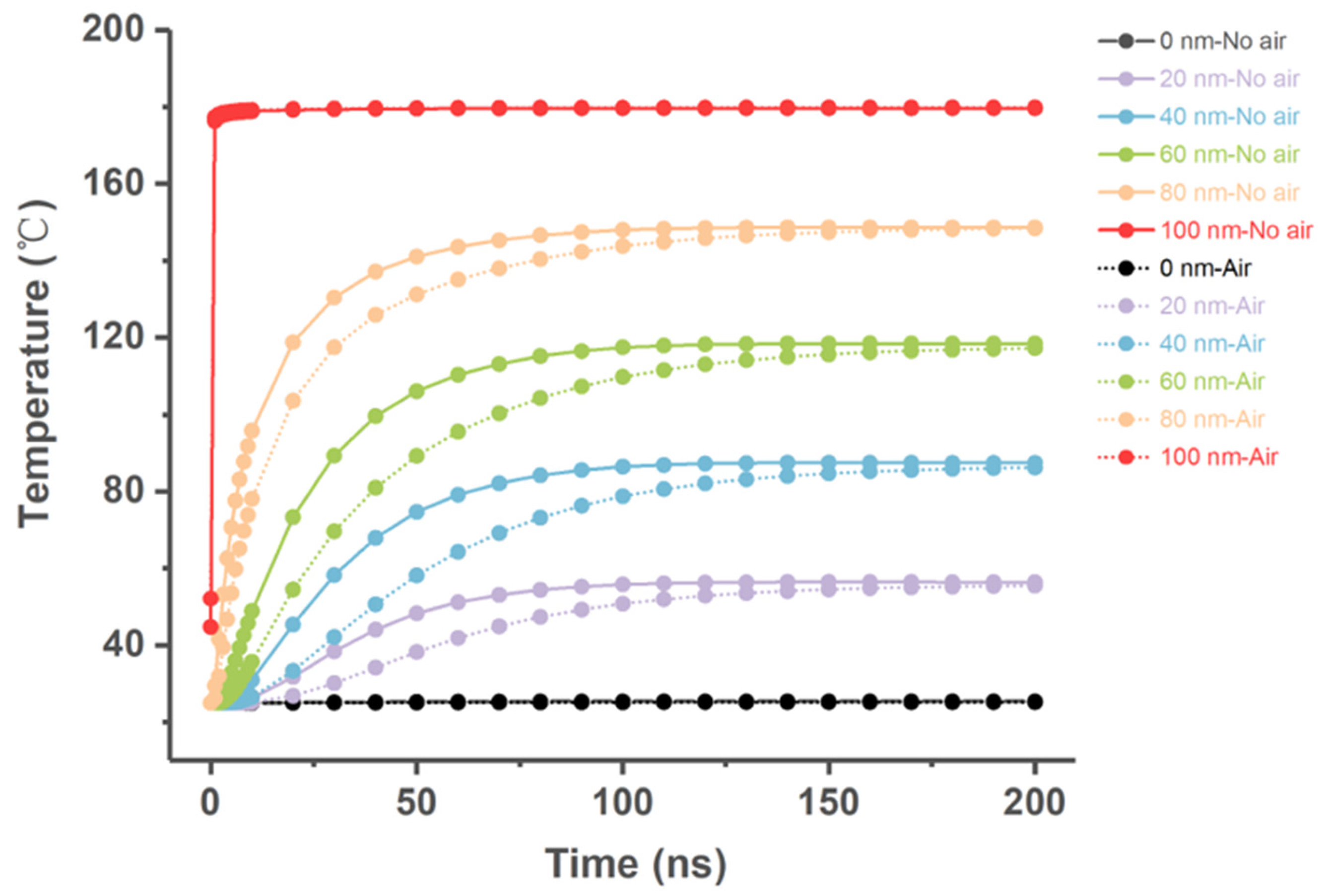
2.9. Simulation of Heat Transfer Process
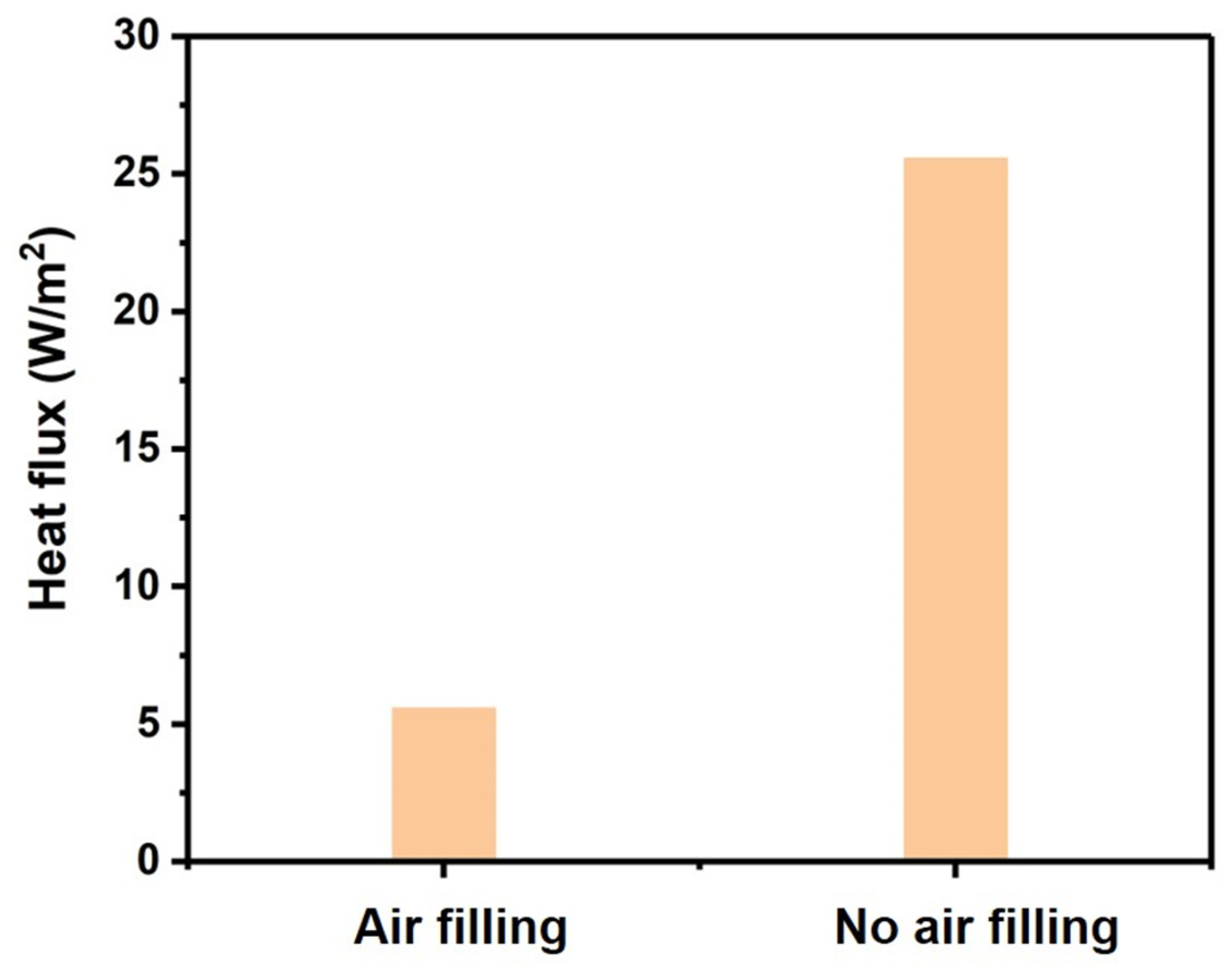
- ✓ Situation 1: air filling assumption, where cavities of the model were filled with air (which corresponded to the actual situation);
- ✓ Situation 2: no air filling assumption, where cavities of the model were filled with the same material as the wall;
3. Results and Discussion
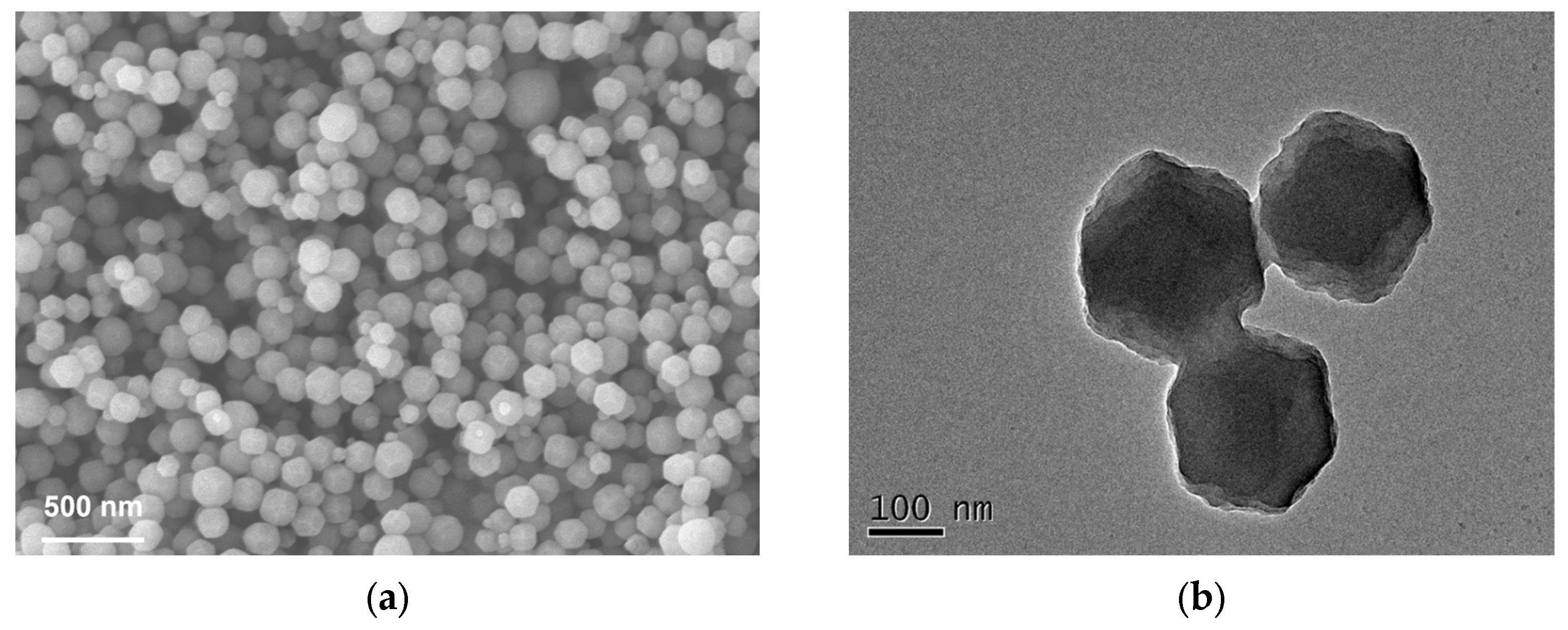
3.1. Synthesis and Characterization of CALB-ZIF-8
3.2. Thermal Stability of CALB-ZIF-8 under Extremely High Temperature
3.3. Finite Element Simulation of Heat Transfer within ZIF-8
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sun, H.; Zhang, H.; Ang, E.L.; Zhao, H. Biocatalysis for the synthesis of pharmaceuticals and pharmaceutical intermediates. Bioorgan. Med. Chem. 2018, 26, 1275–1284. [Google Scholar] [CrossRef]
- Choi, J.M.; Han, S.; Kim, H.S. Industrial applications of enzyme biocatalysis: Current status and future aspects. Biotechnol. Adv. 2015, 33, 1443–1454. [Google Scholar] [CrossRef]
- Sun, H.; Li, Y.; Yu, S.; Liu, J. Metal-organic frameworks (MOFs) for biopreservation: From biomacromolecules, living organisms to biological devices. Nano Today 2020, 35, 100985. [Google Scholar] [CrossRef]
- Panganiban, B.; Qiao, B.; Jiang, T.; DelRe, C.; Obadia, M.M.; Nguyen, T.D.; Smith, A.A.A.; Hall, A.; Sit, I.; Crosby, M.G.; et al. Random heteropolymers preserve protein function in foreign environments. Science 2018, 359, 1239–1243. [Google Scholar] [CrossRef] [PubMed]
- Wilner, O.I.; Weizmann, Y.; Gill, R.; Lioubashevski, O.; Freeman, R.; Willner, I. Enzyme cascades activated on topologically programmed DNA scaffolds. Nat. Nanotechnol. 2009, 4, 249–254. [Google Scholar] [CrossRef]
- Ge, J.; Lei, J.; Zare, R.N. Protein-inorganic hybrid nanoflowers. Nat. Nanotechnol. 2012, 7, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Wied, P.; Carraro, F.; Sumby, C.J.; Nidetzky, B.; Tsung, C.K.; Falcaro, P.; Doonan, C.J. Metal-organic framework-based enzyme biocomposites. Chem. Rev. 2021, 121, 1077–1129. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Ge, J. Hybrid enzyme catalysts synthesized by a de novo approach for expanding biocatalysis. Chin. J. Catal. 2021, 42, 1625–1633. [Google Scholar] [CrossRef]
- Cai, G.; Yan, P.; Zhang, L.; Zhou, H.; Jiang, H. Metal-organic framework-based hierarchically porous materials: Synthesis and applications. Chem. Rev. 2021, 121, 12278–12326. [Google Scholar] [CrossRef]
- Freund, R.; Zaremba, O.; Dinca, M.; Arnauts, G.; Ameloot, R.; Skorupskii, G.; Bavykina, A.; Gascon, J.; Ejsmont, A.; Goscianska, J.; et al. The current status of MOF and COF applications. Angew. Chem. Int. Edit. 2021, 60, 23975–24001. [Google Scholar] [CrossRef]
- Maurin, G.; Serre, C.; Cooper, A.; Fereyd, G. The new age of MOFs and of their porous-related solids. Chem. Soc. Rev. 2017, 46, 3104–3107. [Google Scholar] [CrossRef] [PubMed]
- Lyu, F.; Zhang, Y.; Zare, R.N.; Ge, J.; Liu, Z. One-pot synthesis of protein-embedded metal-organic frameworks with enhanced biological activities. Nano Lett. 2014, 14, 5761–5765. [Google Scholar] [CrossRef]
- Liang, K.; Coghlan, C.J.; Bell, S.G.; Doonan, C.; Falcaro, P. Enzyme encapsulation in zeolitic imidazolate frameworks: A comparison between controlled co-precipitation and biomimetic mineralisation. Chem. Commun. 2016, 52, 473–476. [Google Scholar] [CrossRef]
- Liang, K.; Ricco, R.; Doherty, C.M.; Styles, M.J.; Bell, S.; Kirby, N.; Mudie, S.; Haylock, D.; Hill, A.J.; Doonan, C.J.; et al. Biomimetic mineralization of metal-organic frameworks as protective coatings for biomacromolecules. Nat. Commun. 2015, 6, 7240. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yue, H.; Zhang, Y.; Gao, X.; Li, X.; Wang, L.; Cao, Y.; Hou, M.; An, H.; Zhang, L.; et al. Packaging and delivering enzymes by amorphous metal-organic frameworks. Nat. Commun. 2019, 10, 5165. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Huang, S.; Kou, X.; Zhu, F.; Ouyang, G. Embedding functional biomacromolecules within peptide-directed metal-organic framework (MOF) nanoarchitectures enables activity enhancement. Angew. Chem. Int. Edit. 2020, 59, 13947–13954. [Google Scholar] [CrossRef]
- Chen, G.; Kou, X.; Huang, S.; Tong, L.; Shen, Y.; Zhu, W.; Zhu, F.; Ouyang, G. Modulating the biofunctionality of metal-organic framework-encapsulated enzymes through controllable embedding patterns. Angew. Chem. Int. Edit. 2020, 59, 2867–2874. [Google Scholar] [CrossRef]
- Carraro, F.; Williams, J.D.; Linares-Moreau, M.; Parise, C.; Liang, W.; Amenitsch, H.; Doonan, C.; Kappe, C.O.; Falcaro, P. Continuous-flow synthesis of ZIF-8 biocomposites with tunable particle size. Angew. Chem. Int. Edit. 2020, 59, 8123–8127. [Google Scholar] [CrossRef]
- Hu, C.; Bai, Y.; Hou, M.; Wang, Y.; Wang, L.; Cao, X.; Chan, C.; Sun, H.; Li, W.; Ge, J.; et al. Defect-induced activity enhancement of enzyme-encapsulated metal-organic frameworks revealed in microfluidic gradient mixing synthesis. Sci. Adv. 2020, 6, eaax5785. [Google Scholar] [CrossRef]
- Wu, X.; Xiong, J.; Liu, S.; Zong, M.; Lou, W. A versatile competitive coordination strategy for tailoring bioactive zeolitic imidazolate framework composites. Small 2021, 17, 2007586. [Google Scholar] [CrossRef]
- Garcia-Garcia, P.; Muller, M.; Corma, A. MOF catalysis in relation to their homogeneous counterparts and conventional solid catalysts. Chem. Sci. 2014, 5, 2979–3007. [Google Scholar] [CrossRef]
- Delre, C.; Chang, B.; Jayapurna, I.; Hall, A.; Wang, A.; Zolkin, K.; Xu, T. Synergistic enzyme mixtures to realize near-complete depolymerization in biodegradable polymer/additive blends. Adv. Mater. 2021, 33, 2105707. [Google Scholar] [CrossRef]
- Delre, C.; Jiang, Y.; Kang, P.; Kwon, J.; Hall, A.; Jayapurna, I.; Ruan, Z.; Ma, L.; Zolkin, K.; Li, T.; et al. Near-complete depolymerization of polyesters with nano-dispersed enzymes. Nature 2021, 592, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Hiyama, M.; Kabe, T.; Kimura, S.; Iwata, T. Enzymatic self-biodegradation of poly(L-lactic acid) films by embedded heat-treated and immobilized Proteinase K. Biomacromolecules 2020, 21, 3301–3307. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cao, X.; Ge, J. Antioxidative composites based on multienzyme systems encapsulated in metal-organic frameworks. ACS Appl. Mater. Inter. 2021, 13, 46431–46439. [Google Scholar] [CrossRef]
- Velasquez-Hernandez, M.D.; Ricco, R.; Carraro, F.; Limpoco, F.T.; Linares-Moreau, M.; Leitner, E.; Wiltsche, H.; Rattenberger, J.; Schrottner, H.; Fruhwirt, P.; et al. Degradation of ZIF-8 in phosphate buffered saline media. Crystengcomm 2019, 21, 4538–4544. [Google Scholar] [CrossRef]
- Zhou, K.; Mousavi, B.; Luo, Z.X.; Phatanasri, S.; Chaemchuen, S.; Verpoort, F. Characterization and properties of Zn/Co zeolitic imidazolate frameworks vs. ZIF-8 and ZIF-67. J. Mater. Chem. A 2017, 5, 952–957. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, Y.; Zeng, G.; Zhao, L.; Lai, Z. Rapid synthesis of zeolitic imidazolate framework-8 (ZIF-8) nanocrystals in an aqueous system. Chem. Commun. 2011, 47, 2071–2073. [Google Scholar] [CrossRef]
- Shieh, F.K.; Wang, S.; Yen, C.; Wu, C.; Dutta, S.; Chou, L.; Morabito, J.V.; Hu, P.; Hsu, M.H.; Wu, K.C.W.; et al. Imparting functionality to biocatalysts via embedding enzymes into nanoporous materials by a de novo approach: Size-selective sheltering of catalase in metal-organic framework microcrystals. J. Am. Chem. Soc. 2015, 137, 4276–4279. [Google Scholar] [CrossRef]
- Maddigan, N.K.; Linder-Patton, O.M.; Falcaro, P.; Sumby, C.J.; Bell, S.G.; Doonan, C.J. Influence of the synthesis and storage conditions on the activity of candida antarctica lipase B ZIF-8 biocomposites. ACS Appl. Mater. Inter. 2021, 13, 51867–51875. [Google Scholar] [CrossRef]
- Butonova, S.A.; Ikonnikova, E.V.; Sharsheeva, A.; Chernyshov, I.Y.; Kuchur, O.A.; Mukhin, I.S.; Hey-Hawkins, E.; Vinogradov, A.V.; Morozov, M.I. Degradation kinetic study of ZIF-8 microcrystals with and without the presence of lactic acid. RSC Adv. 2021, 11, 39169–39176. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Cheng, J.; Zhang, J.; Xiong, Q.; Jin, M.; Zhao, J. pH-Responsive on-demand alkaloids release from core-shell ZnO@ZIF-8 nanosphere for synergistic control of bacterial wilt disease. ACS Nano 2022, 16, 2762–2773. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Kwon, H.T.; Jeong, H.K. High-flux zeolitic imidazolate framework membranes for propylene/propane separation by postsynthetic linker exchange. Angew. Chem. Int. Edit. 2018, 57, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Ying, P.; Zhang, J.; Zhang, X.; Zhong, Z. Impacts of functional group substitution and pressure on the thermal conductivity of ZIF-8. J. Phys. Chem. C 2020, 124, 6274–6283. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, J. Thermal conductivity of zeolitic imidazolate framework-8: A molecular simulation study. J. Phys. Chem. C 2013, 117, 18441–18447. [Google Scholar] [CrossRef]


| Code | Total Enzyme Employed (mg) 1 | Enzyme in Supernatant (mg) 2 | Averaged Immobilization Yield (%) |
|---|---|---|---|
| CALB-ZIF-8 | 1.004 ± 0.009 | 0.335 ± 0.015 | 66.2 ± 0.9 |
| Code | Total Activity Immobilized (U) 1 | Apparent Activity (U) 2 | After 6 h Release in PB Buffer | |
|---|---|---|---|---|
| Enzyme Release (%) | Activity Release (%) | |||
| CALB-ZIF-8 | 3640 ± 168 | 434 ± 21 | 38.2 ± 0.3 | 34.6 ± 1.7 |
| Properties | Unit | Value |
|---|---|---|
| Thermal conductivity | W·m−1·K−1 | 0.165 |
| Density 1 | g·cm−3 | 1.87 |
| Specific heat capacity 2 | J·g−1·C−1 | 2.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, S.; Ge, J. High Thermal Stability of Enzyme-MOF Composites at 180 °C. Chemistry 2023, 5, 2025-2037. https://doi.org/10.3390/chemistry5030137
Cui S, Ge J. High Thermal Stability of Enzyme-MOF Composites at 180 °C. Chemistry. 2023; 5(3):2025-2037. https://doi.org/10.3390/chemistry5030137
Chicago/Turabian StyleCui, Shitong, and Jun Ge. 2023. "High Thermal Stability of Enzyme-MOF Composites at 180 °C" Chemistry 5, no. 3: 2025-2037. https://doi.org/10.3390/chemistry5030137
APA StyleCui, S., & Ge, J. (2023). High Thermal Stability of Enzyme-MOF Composites at 180 °C. Chemistry, 5(3), 2025-2037. https://doi.org/10.3390/chemistry5030137







