Abstract
Quinoxaline is a fused heterocycle system of a benzene ring and pyrazine ring. It has earned considerable attention due to its importance in the field of medicinal chemistry. The system is of extensive importance due to its comprehensive array of biological activities. Quinoxaline derivatives have been used as anticancer, anticonvulsant, anti-inflammatory, antidiabetic, antioxidant, antibacterial, anti-TB, antimalarial, antiviral, anti-HIV, and many other uses. Variously substituted quinoxalines are significant therapeutic agents in the pharmaceutical industry. This review spotlights on the chemistry, physiochemical characters, synthesis, pharmaceutical products, and medicinal chemistry of various anticancer quinoxaline derivatives that were developed in the last period. It covers the period from 2016 to 2023.
1. Introduction
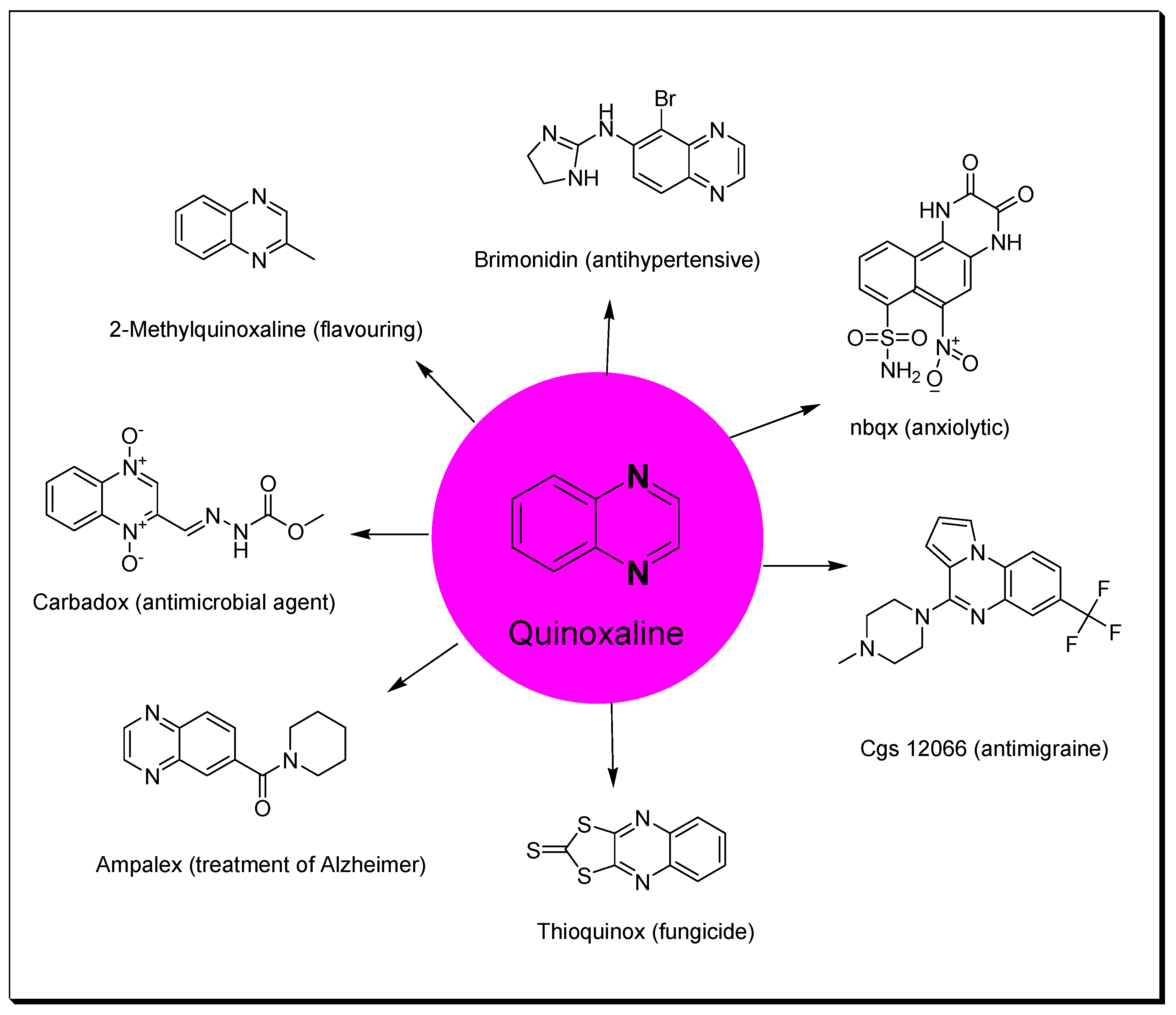
Heterocycles containing nitrogen have great importance in the pharmaceutical field including uses in drug discovery, synthesis, and development processes [1,2,3]. Diazine heterocycles are central components of several drug candidates [4,5]. The benzo-diazene systems of quinoxalines, cinnolines, quinazolines, phthalazines, naphthalenes, and quinolines are used in the preparation of various drugs [6,7]. They are also used in several research studies for the discovery of new drugs [8,9]. Among these heterocycles, quinoxaline plays an essential role in drug discovery and production [10,11,12]. Quinoxaline is a benzopyrazine system with the molecular formula C8H6N2 [13]. It is formed of a benzene ring fused to the six-membered pyrazine ring [14]. It is a low-melting solid (29–30 °C), soluble in water, and a weak base (pKa = 0.56) [15]. Several studies were performed and displayed a wide range of pharmacological activities for quinoxaline derivatives (Figure 1) [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30]. Additionally, quinoxalines are used for crop protection as a component of insecticides, herbicides, and fungicides [31,32,33,34,35]. Quinoxalines were linked to a metal center such as ruthenium or another heterocyclic moiety such as indole to be used as dyes in solar cell preparation, fluorescent materials, organic semiconductors, and inhibitors of corrosion in metals [36,37,38]. There are many commercially available quinoxalines that have an essential role in the pharmaceutical and industrial market [39,40]. The objective of this review is to gather the literature reported by researchers on quinoxaline derivatives, their preparations, and their structure–activity relationship (SAR) as anticancer agents.
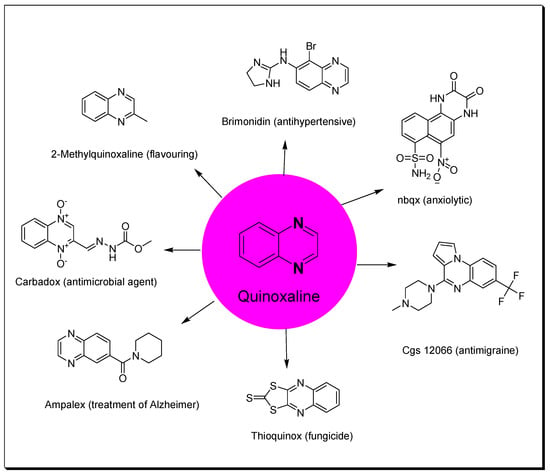
Figure 1.
Quinoxaline and examples of its pharmacological activities.
2. Chemical Characters of Quinoxalines
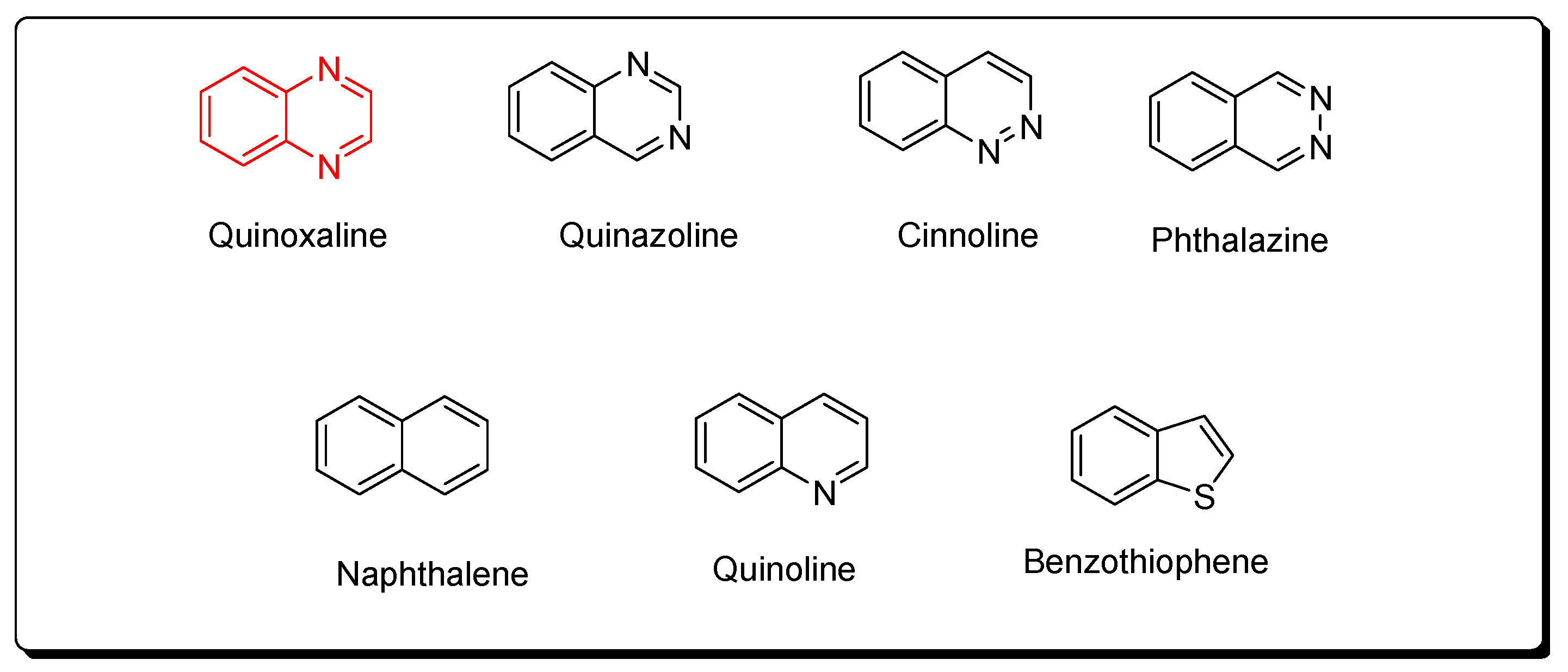
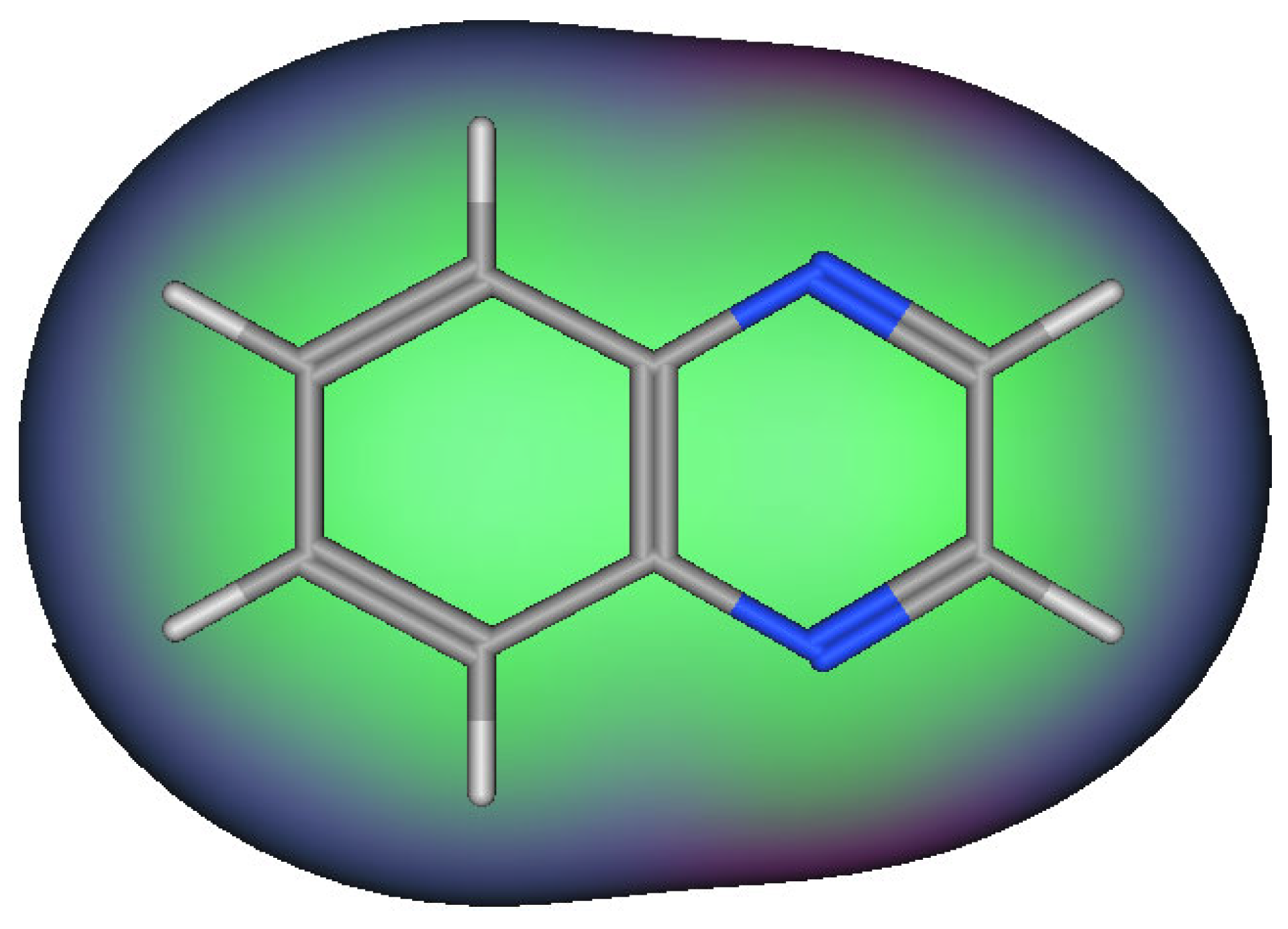
Quinoxaline molecules are named benzopyrazines or 1,4-benzodiazines [41,42,43]. There are four benzodiazines: quinoxaline, quinazolines, phthalazines, and cinnolines. In addition, the bioisosteres of benzodiazines are benzothiophenes, naphthalenes, and quinolines (Figure 2) [14]. These systems have an aromatic nature, so they have a chemical stability by resonance characters [44,45,46]. Quinoxaline is a white crystalline solid at room temperature. It presents two ionization states. The first and the second ionization states were calculated by photon electron spectroscopy, and they were 8.99 and 10.72 eV, respectively [47,48,49,50]. The past twenty years have witnessed huge progress in the synthesis of quinoxaline derivatives [51,52,53,54,55]. These synthetic processes focused on function groups and their tolerance, product variation, selective catalysis, and substrates [56,57,58]. They also gave a mechanistic insight to correct and explain the different types of reactions [59,60,61,62]. These continuous scientific efforts supported the production of many pharmaceutical products and helped in the treatment of various diseases and infections [63,64,65]. The quinoxaline molecule has a specific electrostatic potential that influences its hydrophobic and hydrophilic interactions with the different molecules (Figure 3) [66]. Table 1 displays the physicochemical characteristics of the quinoxaline system [67]. There are many synthetic methods used for the preparation of biologically active quinoxaline derivatives [68,69,70,71,72,73,74,75].
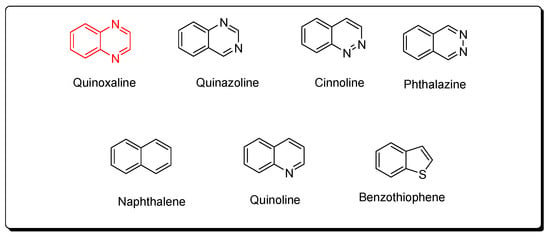
Figure 2.
Benzodiazines (quinoxaline, quinazoline, cinnoline, phthalazine) and their bioisosteres (naphthalene, quinoline, benzothiophene).
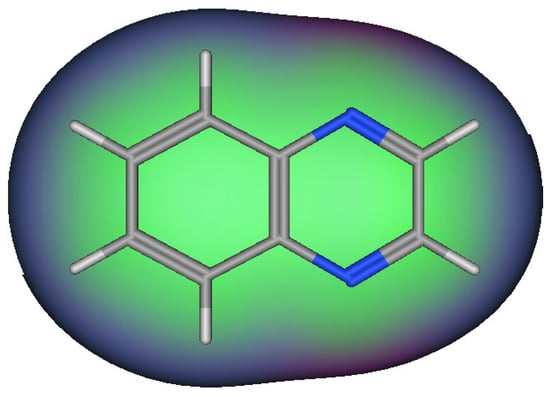
Figure 3.
Surface map for interactions in the quinoxaline system. The pink color shows hydrogen bonding area, the green color indicates hydrophobic area, and the blue color indicates a mild polar area.

Table 1.
The physicochemical characteristics of the quinoxaline system.
3. Methods of Preparation of Quinoxalines
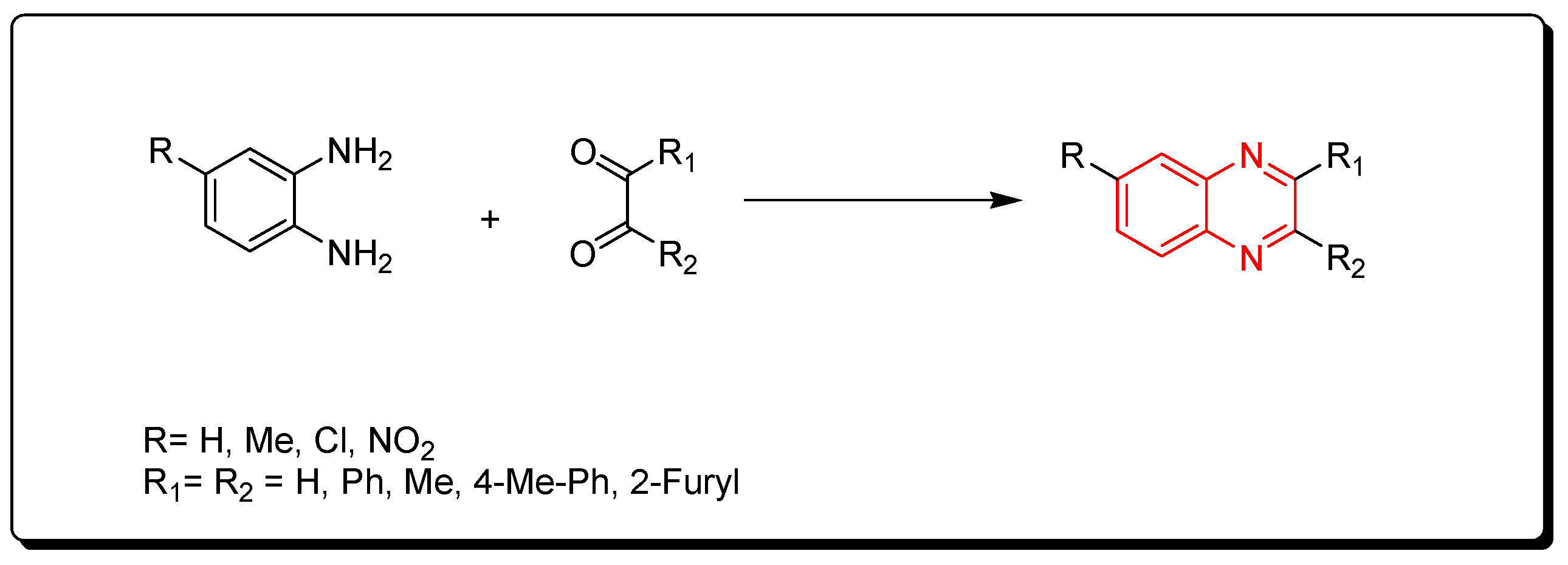
Due to the massive synthetic importance and the various therapeutic activities of quinoxaline derivatives, several attempts have been made by many researchers to prepare a library of these molecules [76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91]. The methods of preparation of quinoxalines can be divided into two pathways:
- The traditional chemistry pathway, which is based on the condensation between o-phenylenediamines and dicarbonyl compounds in the presence of special conditions such as organic solvents, high temperatures, long times, or strong catalysts. Additionally, the reaction yield may be low and side products may be produced. These types of reactions have negative effects on the environment.
- The green chemistry pathway, which is a cost-effective pathway through using green chemistry methodologies to produce quinoxalines. This pathway is characterized by using an environmentally friendly recyclable catalyst, a low cost, lower consumption of energy, one-pot synthesis, no side products, short time, and high yield. It can be performed in an aqueous medium at room temperature or by the microwave reactor.
3.1. Traditional Chemistry Pathway
3.1.1. Condensation of o-Phenylenediamine and 1,2-Dicarbonyl Derivatives
Korner and Hinsberg in 1884 synthesized the first derivative of quinoxaline through a condensation of o-phenylenediamine with a 1,2-dicarbonyl derivative. Various derivatives were obtained from this reaction (Scheme 1) [76].
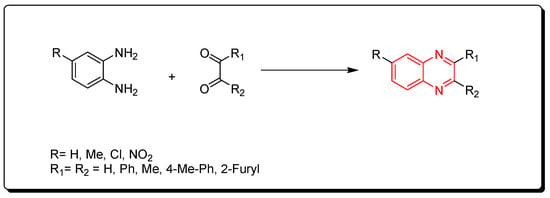
Scheme 1.
Synthesis of quinoxaline by the condensation technique: diamine (1 mmol), dicarbonyl (1 mmol), glycerol (5 mL), water (2 mL), 90 °C, 4–6 min, yield (85–91%).
3.1.2. O-Phenylenediamine and In Situ Produced 1,2-Dicarbonyls
Quinoxalines were synthesized via catalytic iodine, which was used to accelerate the oxidative cyclization cascade between different 1,2-diamino compounds and hydroxyl ketones (Scheme 2) [77].
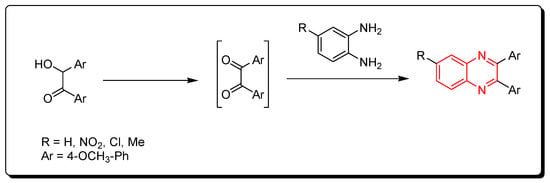
Scheme 2.
Synthesis of quinoxaline from o-phenylenediamine and in situ generated 1,2-dicarbonyl derivatives: o-phenylenediamine (1 mmol), hydroxyl ketone (1 mmol), I2 (0.25 mmol), DMSO (2 mL), RT, 12 h, yield (80–90%).
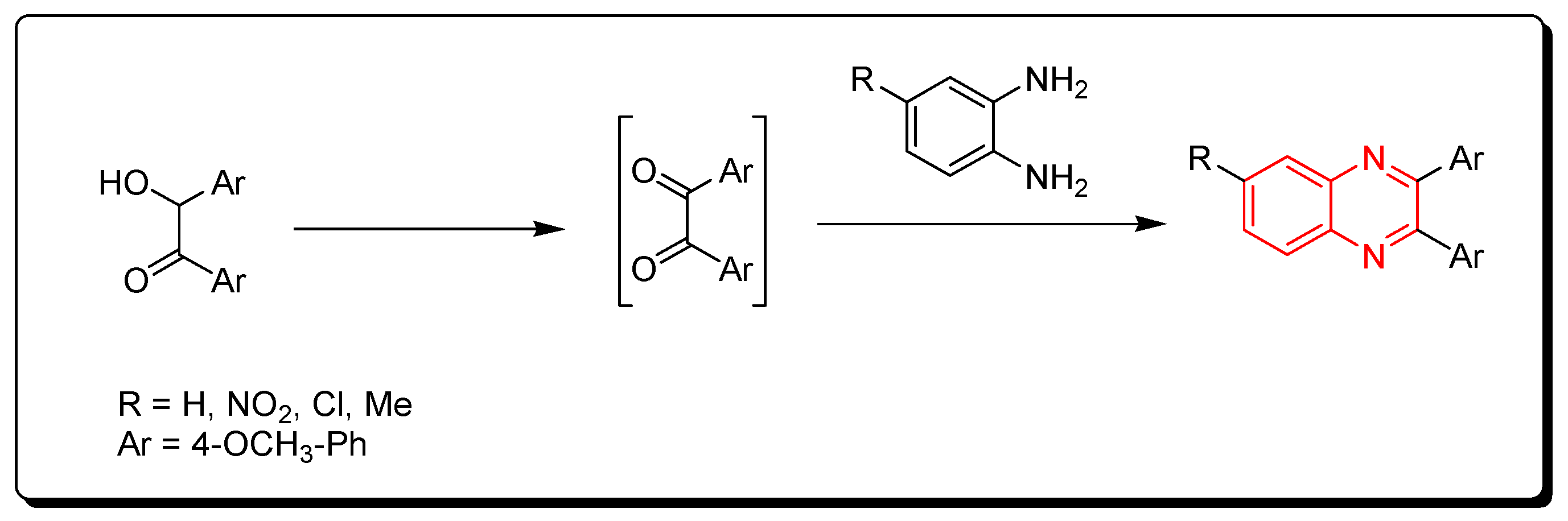
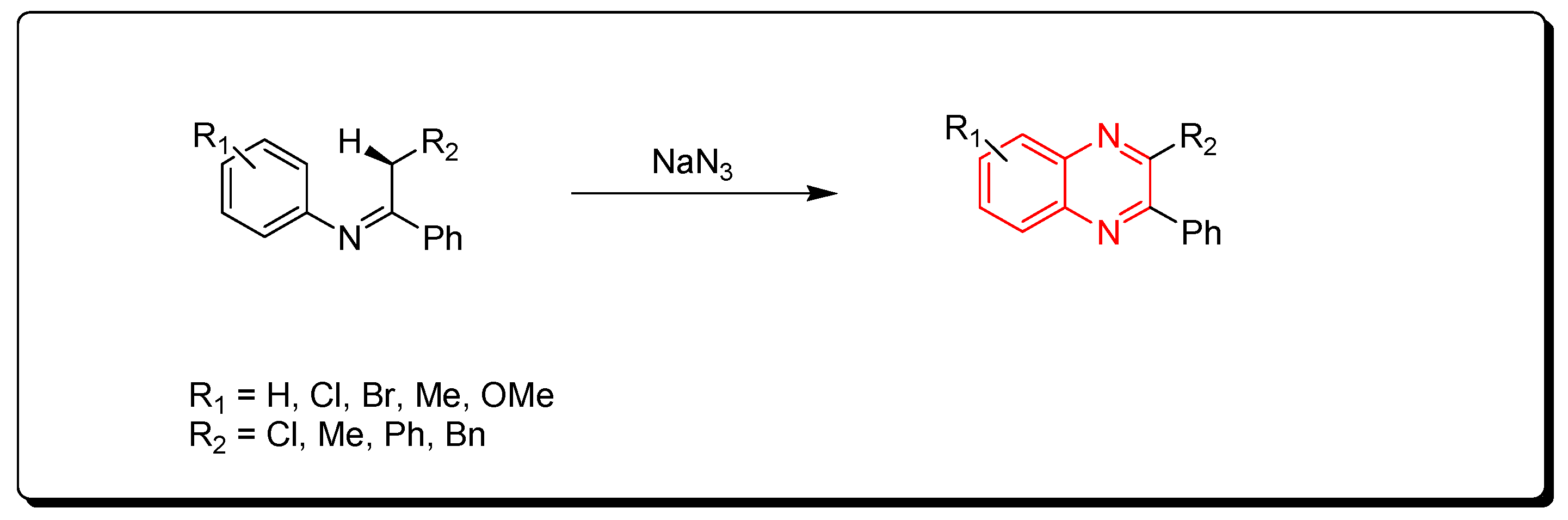
3.1.3. Metal-Catalyzed Cyclization of Imines and Azides
Ketimines and azides were used to create quinoxalines. This is a metal-catalyzed cyclization reaction that produces quinoxaline derivatives (Scheme 3) [78,79,80].
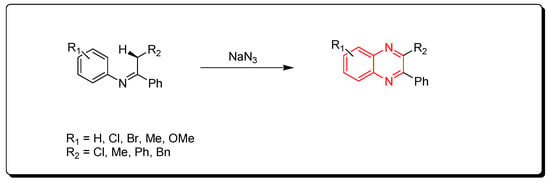
Scheme 3.
Synthesis of quinoxalines from imines and azides: imine (1 mmol), sodium azide (3 mmol), (diacetoxyiodo)benzene (3 mmol), CuO (1 mmol), ethylacetate, Rt, 16 h, yield (35–80%).
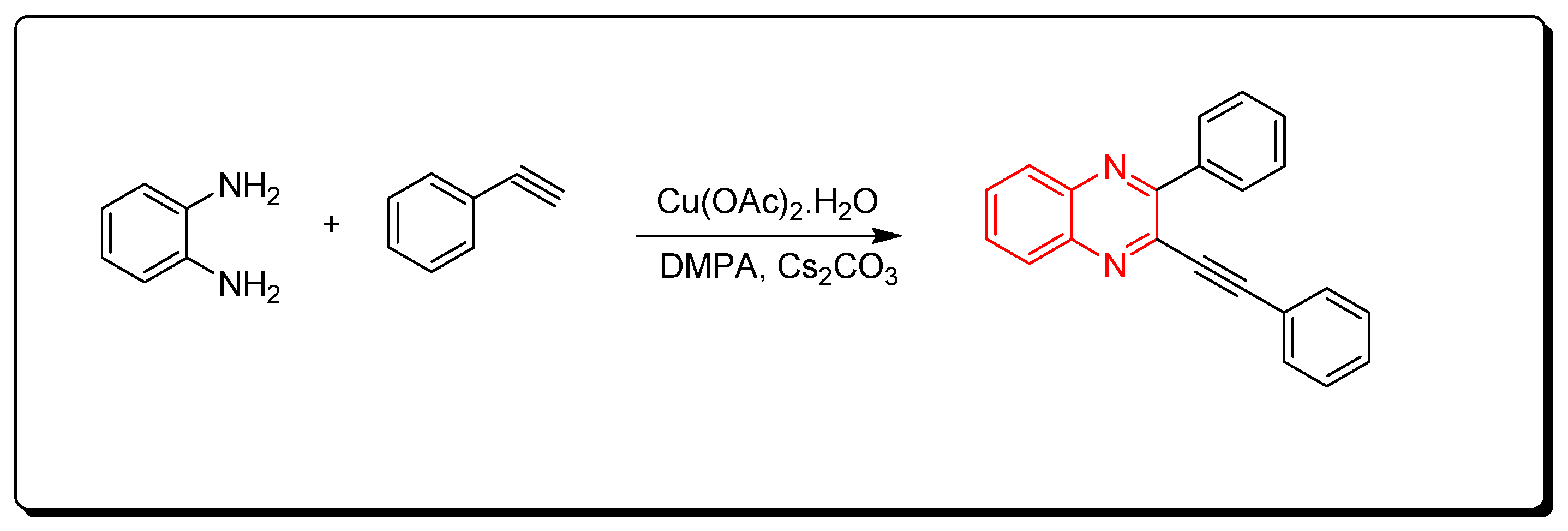
3.1.4. Cyclocondensation of o-Phenylenediamine and Aromatic Alkynes
Quinoxalines were synthesized via cyclocondensation of phenylene diamine and aromatic alkynes in the presence of Cu(OAc)2 as a catalyst (Scheme 4) [81].
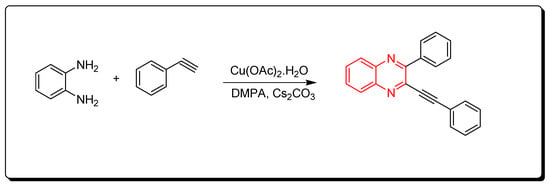
Scheme 4.
Synthesis of quinoxalines from aromatic alkynes and amines: o-phenylenedia mine (0.25 mmol) in toluene, phenyl acetylene (1 mmol), Cs2CO3 (0.75 mmol), Cu(OAc)2.H2O (10 mol % from the o-phenylenediamine), DMPA (0.75 mmol), 70 °C, 8 h, yield (86%).
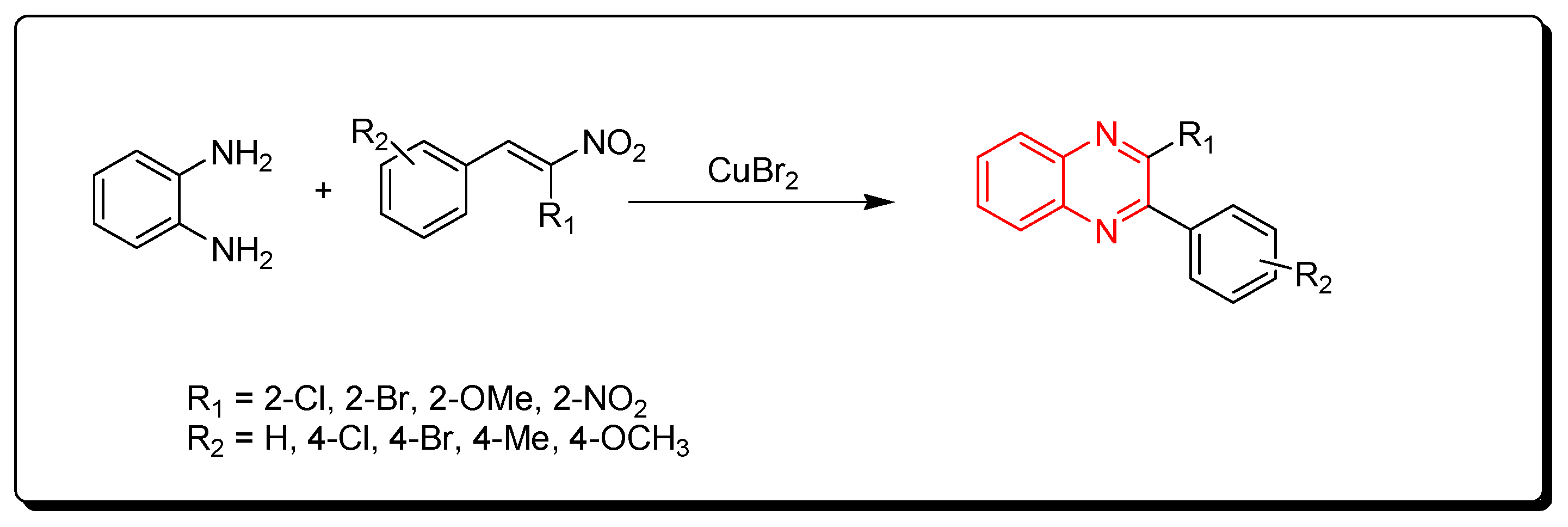
3.1.5. Cyclocondensation of o-Phenylenediamine and Nitro-Olefins
Using CuBr2 as a catalyst, phenylenediamine and nitro-olefins reacted to produce quinoxalines (Scheme 5) [82].
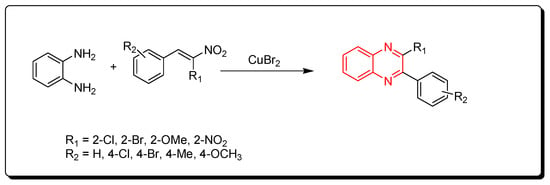
Scheme 5.
Synthesis of quinoxalines from nitro-olefins and amines: phenylenediamine (1 mmol), nitro-olefins (1 mmol), CuBr2 (1 mmol), ethanol, 110 °C, 2–4 h, yield (35–90%).
3.1.6. Cyclocondensation of Aromatic Amines and DMF
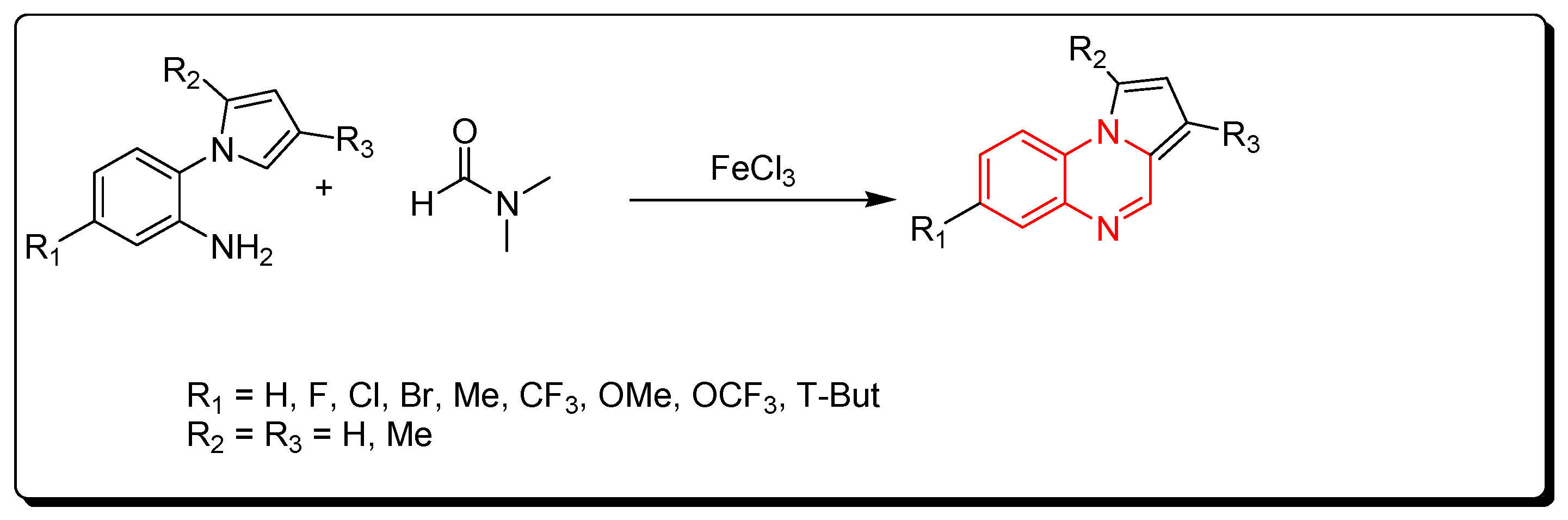
A new strategy for the preparation of pyrrol [1,2-a]quinoxaline derivatives was described by using ferric chloride as a Lewis acid and an initiator for a straightforward reaction. DMF solvent was used as a source of carbon (Scheme 6) [83].
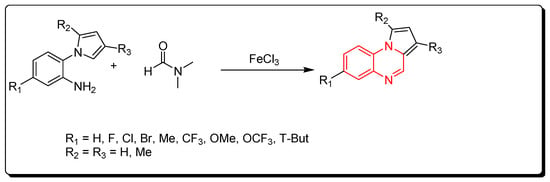
Scheme 6.
Synthesis of quinoxalines from amines and DMF in Fe-mediated catalyst: aniline derivative (0.3 mmol), DMF (2 mL), FeCl3 (0.3 mmol), TBPB (0.9 mmol), 120 °C, 5–12 h, yield (40–97%).
3.2. Green Chemistry Pathway
3.2.1. Clay-10 Based Method
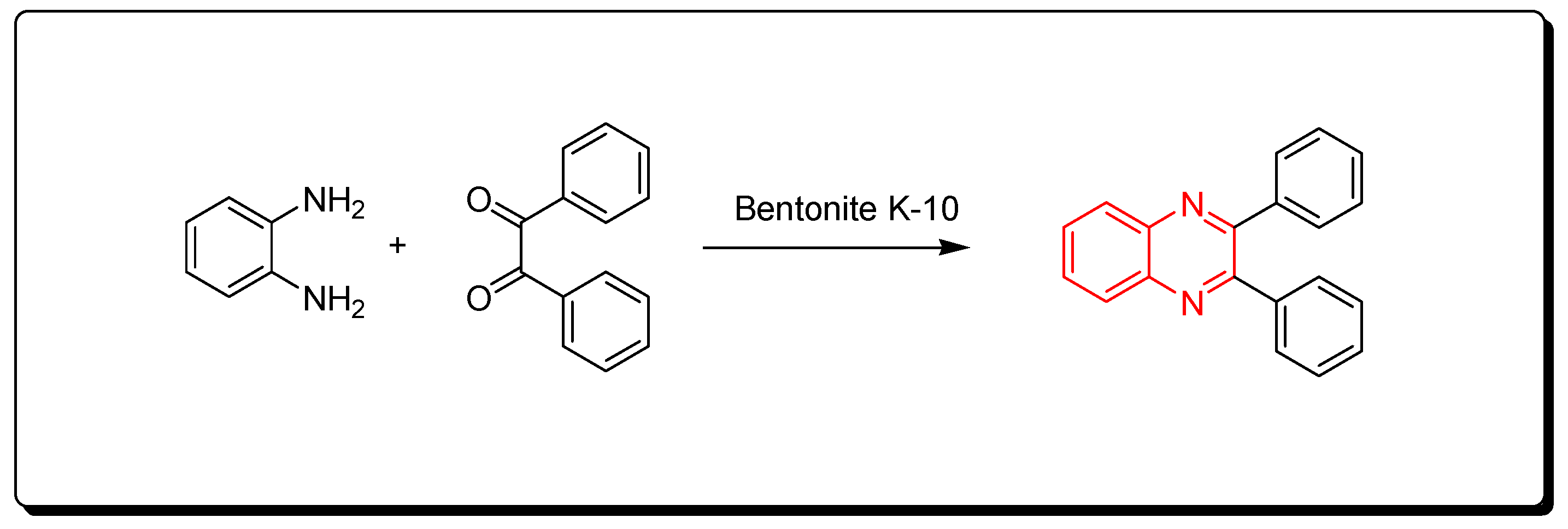
This is a green synthetic pathway for the synthesis of quinoxalines. It is environmentally friendly with no traditional limitations such as high temperature, expensive reagents, low yield, and contamination. Clay is a cheap material, green reagent, and is continuously available. This reaction is performed by mixing the two reagents with bentonite K-10 at room temperature, then it is flowed on a celite pad and ethanol. The mixture is concentrated to 5 mL and diluted with 10 mL of water. The reaction is allowed to stand for 1 h. The clay can be recovered after formation of the product as pure crystals and can be used five times again. This method agrees with the green chemistry protocol, and it is recommended for the synthesis of different quinoxaline derivatives to avoid the problems of the traditional pathway. The reaction is shown in Scheme 7 [84].
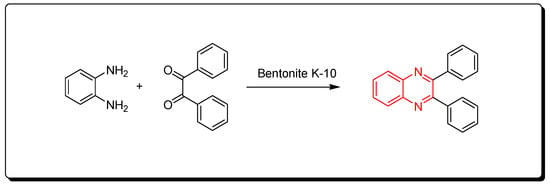
Scheme 7.
Synthesis of quinoxalines by one-pot cascade method: o-phenylene-diamine (1 mmol), benzil (1 mmol), bentonite K-10 (3 gm), ethanol 50 mL, RT, 20 min, yield (95%).
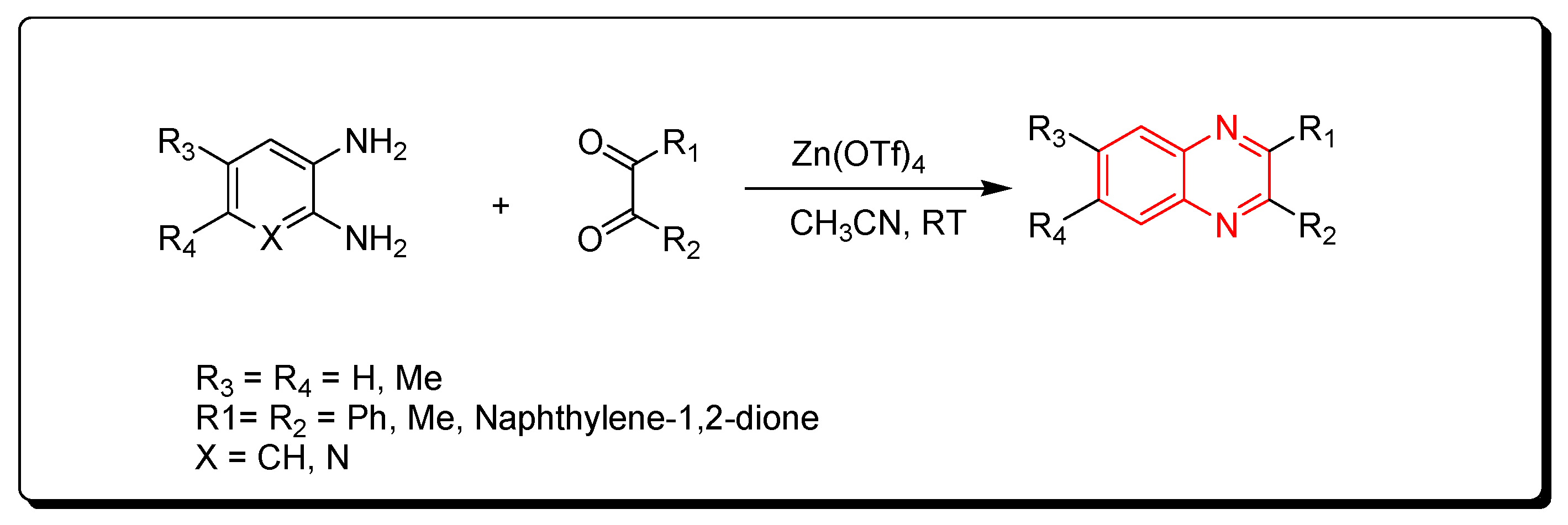
3.2.2. Zinc Triflate Catalyst
Zinc triflate is a zinc salt of trifluoromethanesulfonic acid. It is an ecologically friendly and highly effective catalyst. It is one of the green chemistry catalysts. The reactions performed by using zinc triflate catalyst can be completed without solvent (solvent-free) using a microwave-assisted reactor or by using acetonitrile solvent. Quinoxaline derivatives were prepared by the reaction of o-phenylenediamine and α-diketones using a zinc triflate catalyst at room temperature in acetonitrile. This reaction produced a yield up to 90% (Scheme 8) [85].
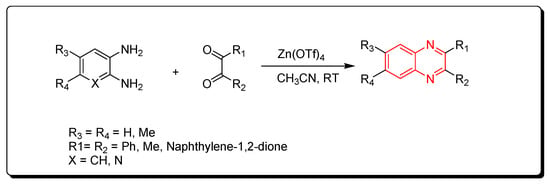
Scheme 8.
Synthesis of quinoxaline by using zinc triflate catalyst: diamine (1.1 mmol), dicarbonyl (1 mmol), Zn(OTf)4 (0.2 mmol), CH3CN (5 mL), RT, yield (85–91%).
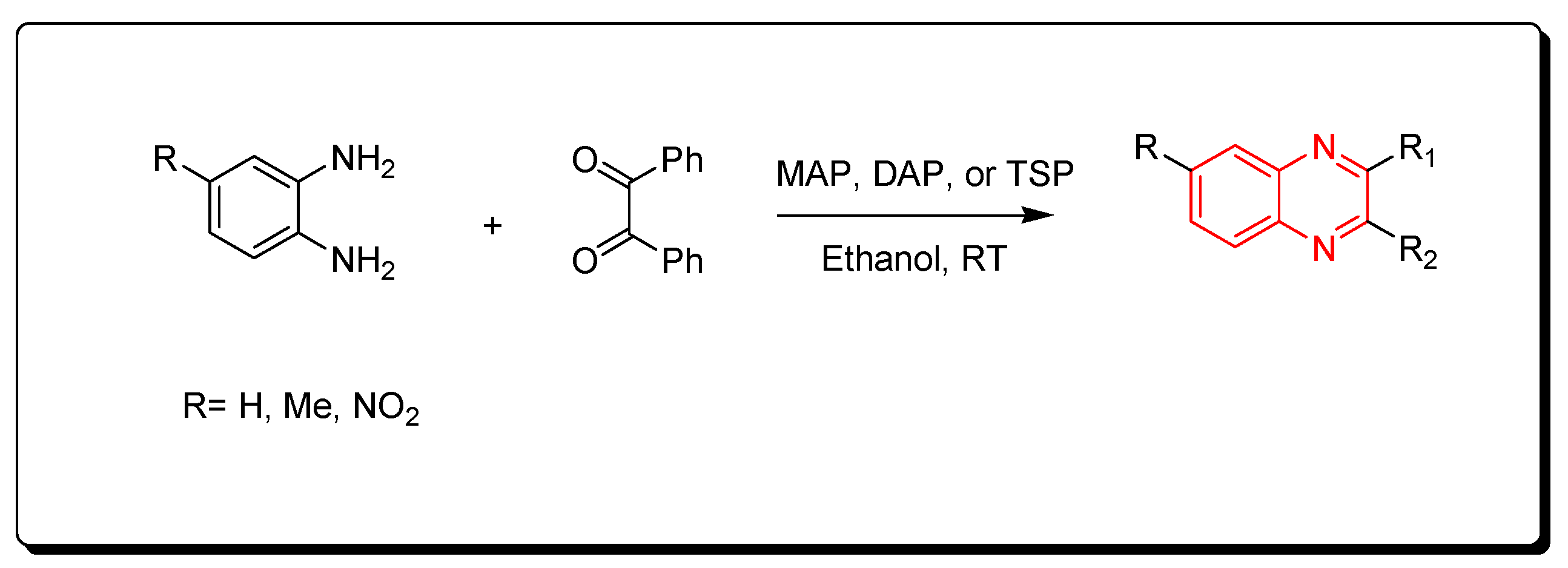
3.2.3. Phosphate-Based Catalyst
The phosphate catalysts include monoammonium phosphate (MAP), diammonium phosphate (DAP), and triple super phosphate (TSP), which is a constituent of fertilizer that mainly consists of monocalcium phosphate Ca(H2PO4)2. The needed amount from this type of catalyst is a minute amount (0.006 gm) for performing the one molar equivalent reaction. The resulting product is crystallized from ethanol while the catalyst is recovered from the reaction by simple filtration, washing with hot ethanol, and drying for 6 h (Scheme 9) [86].
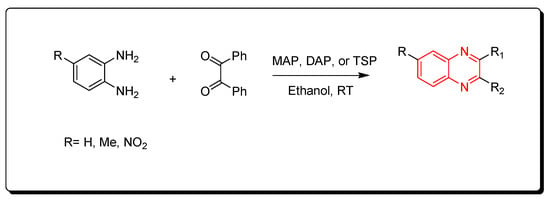
Scheme 9.
Synthesis of quinoxaline by using phosphate-based catalyst: amine (1 mmol), benzil (1 mmol), MAP, DAP, or TSP (0.0006 gm), ethanol, RT, yield (85–91%).
3.2.4. Lanthanide-Based Catalyst
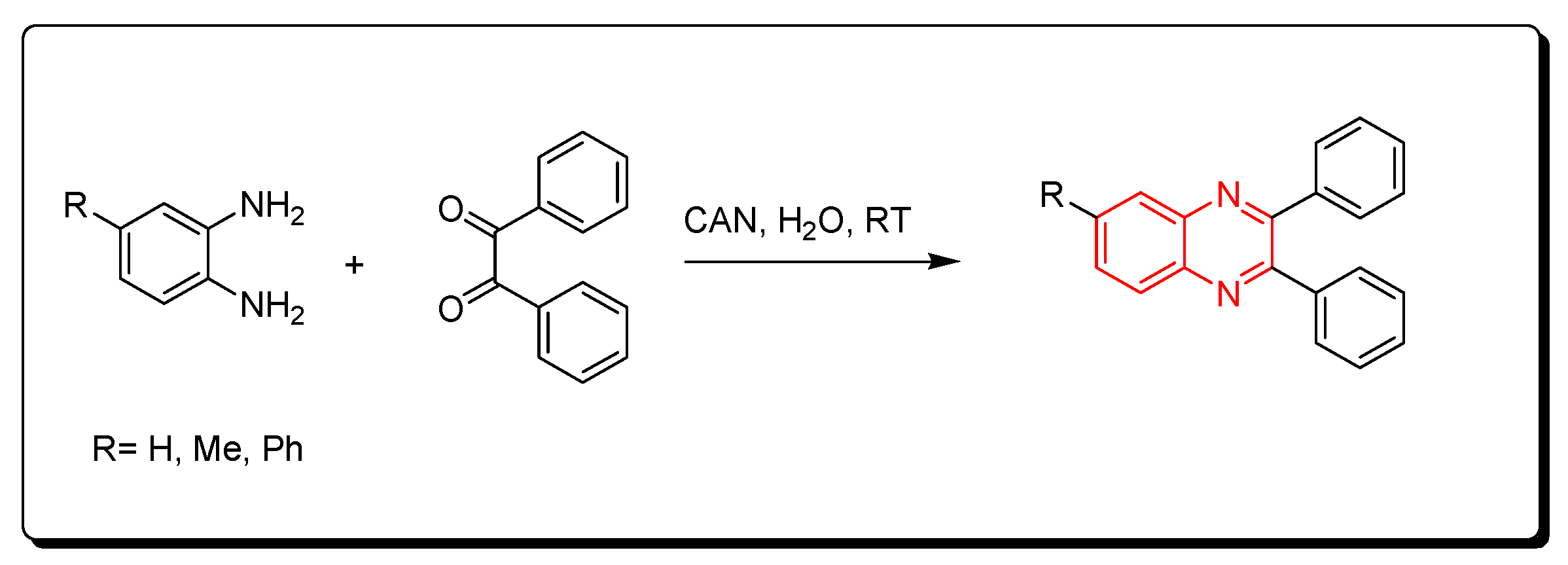
Cerium (IV) ammonium nitrate (CAN) is one of the lanthanide reagents. It has earned attention in synthetic reactions of organic chemistry due to its low cost, availability, miscibility in water, safety, and high reactivity. It is used in green chemistry due to its unique characters. The reaction between o-phenylenediamine and benzil derivatives in the presence of cerium (IV) ammonium nitrate (CAN) readily happens in 20 min without any side products at room temperature to produce a good yield reaching up to 98%. Additionally, it is performed in an aqueous medium. The CAN catalyst is mixed with acetonitrile or any aprotic solvent. It is one of the green chemistry protocols that are used for the synthesis of quinoxaline derivatives (Scheme 10) [87].
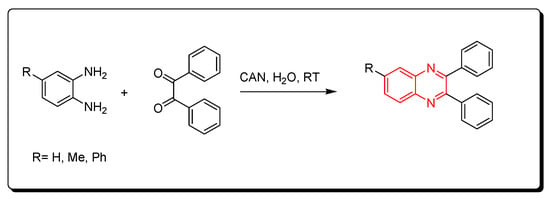
Scheme 10.
Synthesis of quinoxaline by using lanthanide-based catalyst. Amine (1 mmol), benzil (1 mmol), CAN (5 mol), acetonitrile, RT, 20 min, yield (80–98%).
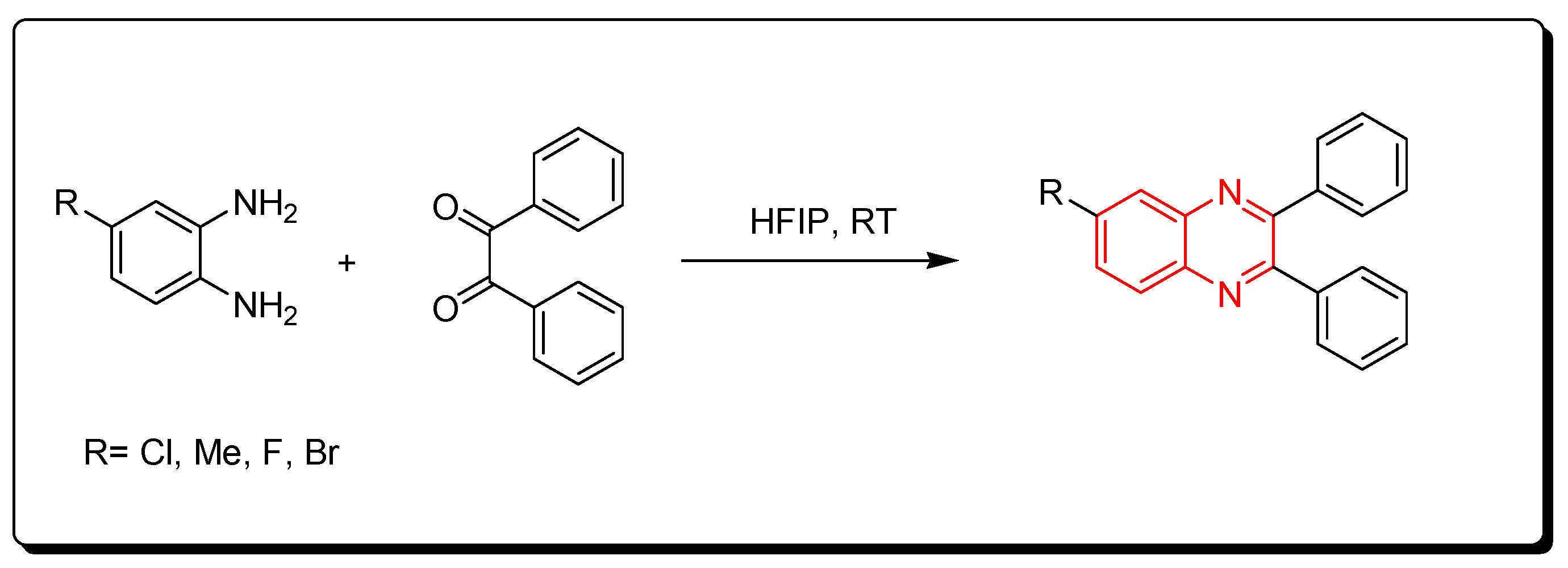
3.2.5. Fluorinated Alcohols Catalyst (HFIP)
Fluorinated alcohols are related to green chemistry catalysts. Recently, they have gained much attention due to their low nucleophilic characteristics, high polarity, their ability to strongly donate hydrogen bonds, and water solvation characteristics. They also can stabilize the helix conformation of proteins. The reaction between o-phenylenediamine and benzil derivatives in the presence of hexafluoroisopropanol (HFIP) was run at room temperature for one hour to produce quinoxaline derivatives with a 95% yield. It is a solvent-free reaction without side products and toxic solvents. Furthermore, the hexafluoroisopropanol can be recovered from the reaction without activity change. Therefore, it is a green chemistry pathway (Scheme 11) [87].
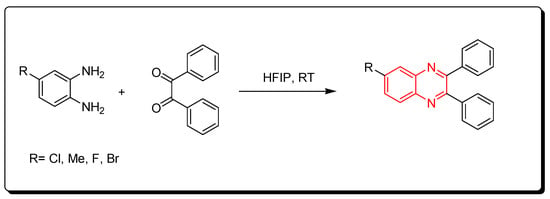
Scheme 11.
Synthesis of quinoxaline by using fluorinated alcohols catalyst: amine (1 mmol), benzil (1 mmol), HFIP (5 mol), RT, 20 min, yield (95%).
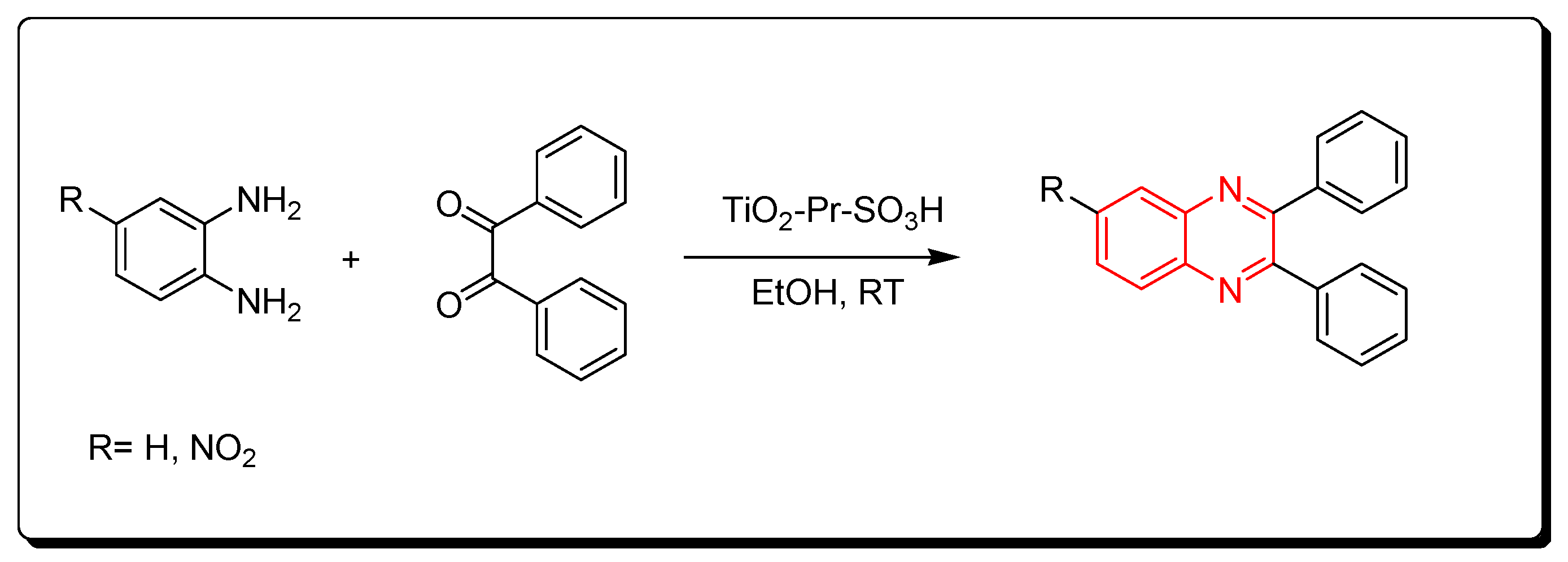
3.2.6. Solid Acid Catalyst (TiO2-Pr-SO3H)
Nanocrystalline titania-based sulfonic acid (TiO2-Pr-SO3H) is a green chemistry catalyst. It is a sulfonic acid nano porous titania resulting from the reaction of (3-mercaptopropyl) trimethoxysilane and titanium oxide. It can be recovered from the reaction without a change in the activity. It is used to catalyze the reaction between o-phenylenediamine and benzil derivatives at room temperature. The product yield was 95% and it needed only 10 min to be accomplished. This reaction can be performed in the presence of ethanol or in the absence of any solvent. It is a one-step reaction without side products (Scheme 12) [87].
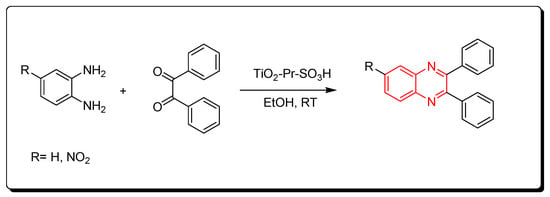
Scheme 12.
Synthesis of quinoxaline by using solid acid catalyst: amine (1 mmol), benzil (1 mmol), TiO2-Pr-SO3H (1 mol), RT, 10 min, yield (95%).
3.3. Reaction of Quinoxalines
3.3.1. Intramolecular Arylation Using Lewis Acid Catalyst
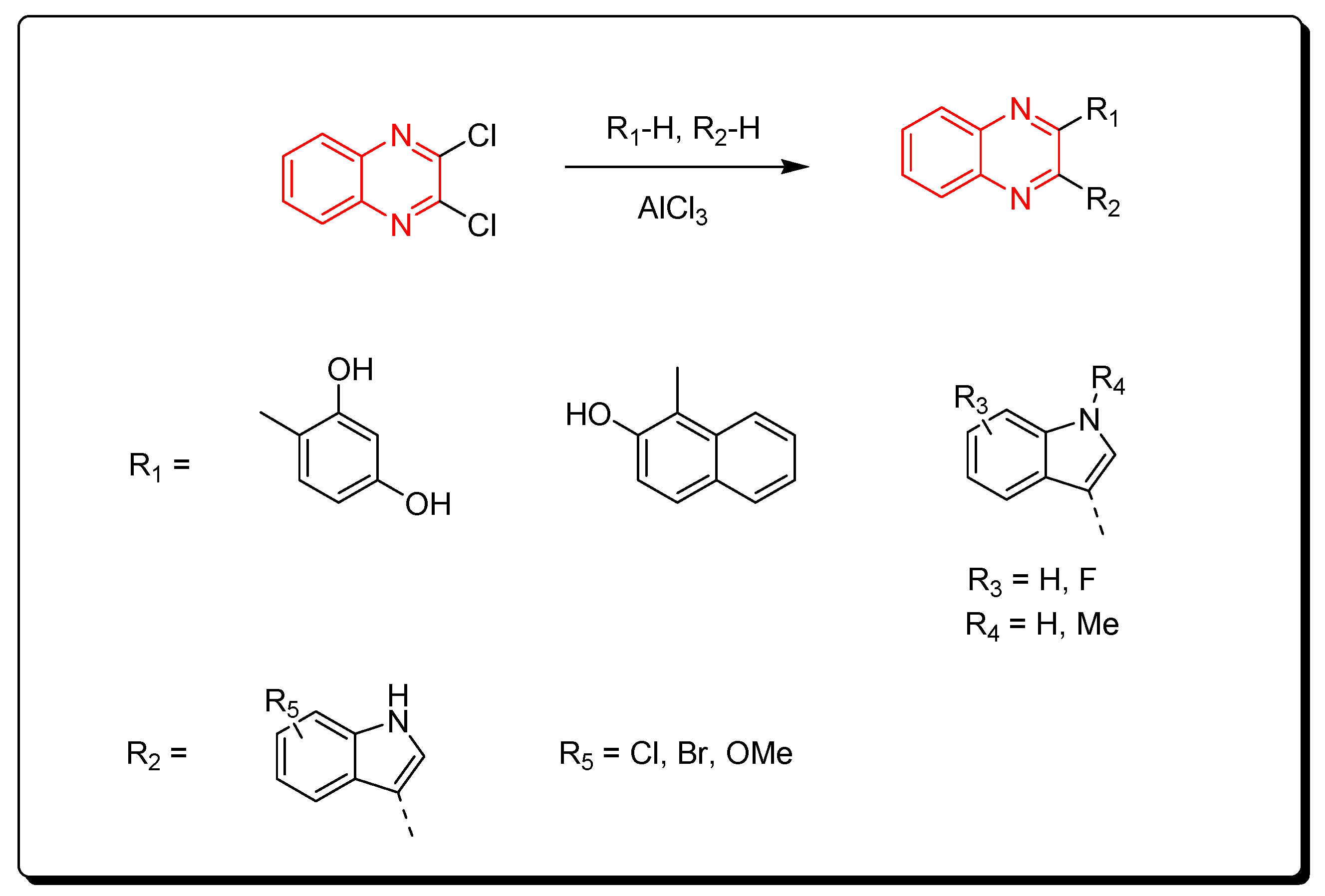
Aryl derivatives of quinoxalines were produced via the reaction between dichloroquinoxalines and aryl derivatives using a Lewis acid catalyst (AlCl3). The previous method induced arylation via C–C bond formation (Scheme 13) [88].
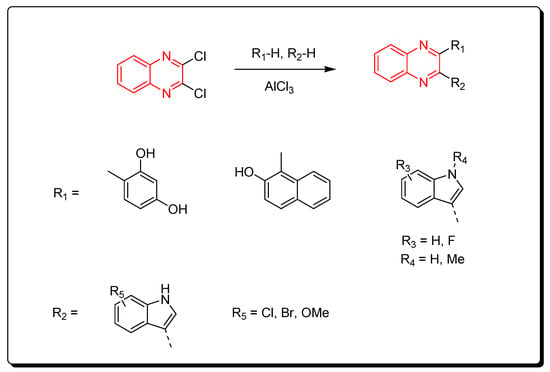
Scheme 13.
Synthesis of quinoxalines derivatives by AlCl3-induced arylation of dichloroquinoxalines: dichloroquinoxaline (1 mmol), R1-H (1 mmol), R2-H (1 mmol), AlCl3 (2.2 mmol), DCE, 80 °C, 60 min, yield (87–85%).
3.3.2. Intramolecular Cyclization of Quinoxalines
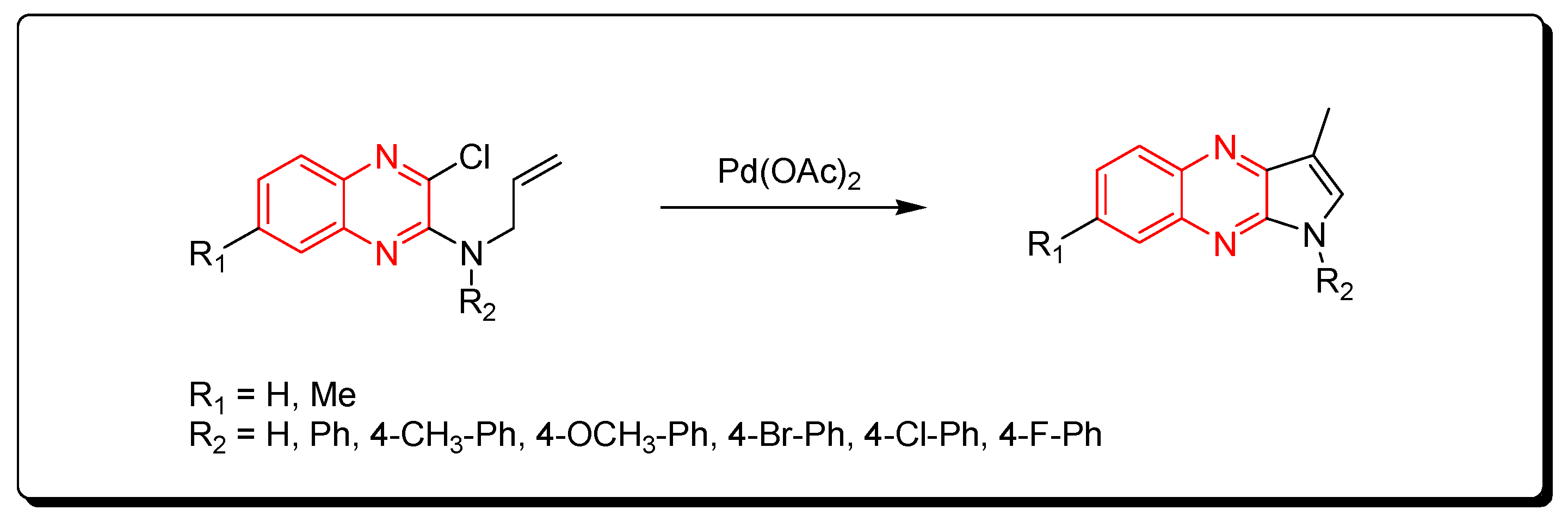
Substituted pyrrolo[2,3-b]quinoxaline from allyl-3-chloroquinoxaline-2-ylamine having terminal alkene and aromatic amine derivatives was prepared using a Pd-mediated catalyst Pd(OAc)2 (Scheme 14) [89].
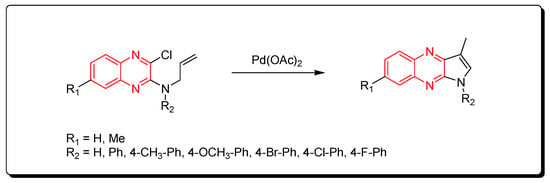
Scheme 14.
Synthesis of quinoxalines derivatives by Pd-mediated catalyst: amine (1 mmol), Pd(OAc)2 (0.015 mmol), K2CO3 (3 mmol), DMF, 100 °C, 2 h, yield (80–91%).
4. Pharmaceutical Products of Anticancer Quinoxalines
Many anticancer drugs containing quinoxaline were developed. While some of them are still undergoing clinical trials or have been discontinued, others have FDA approval and are marketed as pharmaceutical products. Examples of some common derivatives are shown in Table 2, along with their generic names, formulas, and molecular weights [14,90].
- Erdafitinib is an inhibitor of the subgroup tyrosine kinase fibroblast (FGFR). These receptors become unregulated and are exposed to angiogenesis, differentiation, and proliferation in certain types of tumors. Erdafitinib is used for the treatment of malignancy and some types of solid tumors. It has the brand name Balversa. It was discovered to overcome the toxicity profiles of other anticancer agents used for the treatment of gastric cancer, bile duct cancer, and lung cancer. It was invented for the first time by the Astex Pharmaceutical Company. The FDA approved it in 2018 for the management of urothelial tumors. In 2019 it was approved for the treatment of other types of tumors. It inhibits FGFR-1, FGFR-2, FGFR-3, and FGFR-4 with a strong IC50 = 1.2, 2.5, 3, and 5.7 nM, respectively [14].
- Chloroquinoxaline sulfonamide was listed as CQS, and it was used in the treatment of different types of tumors. It completed clinical trials (phase II) on colorectal and lung cancer cell lines. It works via the inhibition of topoisomerase IIα and topoisomerase IIβ. Therefore, it inhibits DNA replication. It showed a high toxicity profile, so it was discontinued after this phase II. It showed IC50 = 1.8 μM against B16 murine melanoma cells [14].
- Tyrophostin is a tyrosine kinase inhibitor. It was used for the treatment of resistant melanoma cell platelet-derived growth factor receptor kinase (PDGFR), activates apoptosis, and reduces capability and movement of resistant melanoma cells of skin cancer. It has no effect on the epidermal growth factor receptor (EGFR), but it strongly inhibits PDGFR with an IC50 = 0.3 to 0.5 μM. It also works via the activation of apoptosis in tumor cell lines. It is used in the treatment of melanoma [14].
- Pilaralisib is an effective and favorably selective inhibitor of class I phosphatidylinositol 3-kinase (PI3K). It inhibits the formation of PIP3 in the cell membrane, which leads to the inhibition of cell differentiation and proliferation. It was invented for the treatment of solid tumors by Sanofi and Exelixis. It significantly inhibited tumor growth but showed a high toxicity profile, so it was discontinued after phase II. It displayed an IC50 of 39, 383, 23, and 36 nM against PI3Kα, PI3Kβ, PI3Kγ, and PI3Kδ [14].
- 2-(4-Chlorophenyl)-5-Quinoxalinecarboxamide is an example of an antineoplastic agent that inhibits the poly(ADP-ribose) polymerase enzyme. This enzyme participates in the base excision repair (BER) pathway by facilitating the poly(ADP-ribosyl)action of a select few acceptor proteins that are important for DNA metabolism and chromatin architecture. The quinoxaline derivative is still in the experimental stage [90].
- PQ-10 is a quinoxaline and quinazoline derivative that works via the inhibition of cAMP and cAMP-inhibited cGMP 3′,5′-cyclic phosphodiesterase 10A. The latter enzyme controls the amount of cyclic nucleotides inside cells, which aids in signal transduction. It is capable of hydrolyzing both cAMP and cGMP but prefers cAMP more highly. This derivative is in the experimental stage as a new anticancer treatment with a quinoxaline system [90].

Table 2.
Some pharmaceutical products of anticancer quinoxalines.
Table 2.
Some pharmaceutical products of anticancer quinoxalines.
| Molecular Structure | Generic Name | Chemical Name | Molecular Formula/ Molecular Weight |
|---|---|---|---|
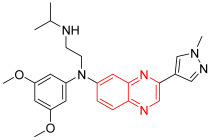 | Erdafitinib | N’-(3,5-dimethoxyphenyl)-N’-[3-(1-methylpyrazol-4-yl)quinoxalin-6-yl]-N-propan-2-ylethane-1,2-diamine | C25H30N6O2 446.2 |
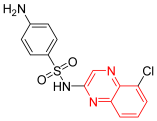 | Chloroquinoxaline sulfonamide | 4-amino-N-(5-chloro-2-quinoxalinyl)-benzenesulfonamide | C14H11ClN4O2S 334.03 |
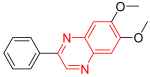 | Tyrophostin | 6,7-Dimethoxy-2-phenylquinoxaline | C16H14N2O2 266.1 |
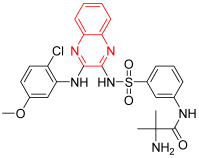 | Pilaralisib | 2-amino-N-[3-[[3-(2-chloro-5-methoxyanilino)quinoxalin-2-yl]sulfamoyl]phenyl]-2-methylpropanamide | C25H25ClN6O4S 540.13 |
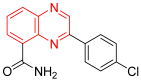 | NA | 2-(4-Chlorophenyl)-5-Quinoxalinecarboxamide | C15H10ClN3O 283.05 |
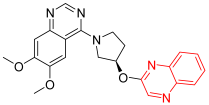 | PQ-10 | 6,7-Dimethoxy-4-[(3R)-3-(2-quinoxalinyloxy)-1-pyrrolidinyl]-quinazoline | C22H21N5O3 403.43 |
5. Anticancer Quinoxalines
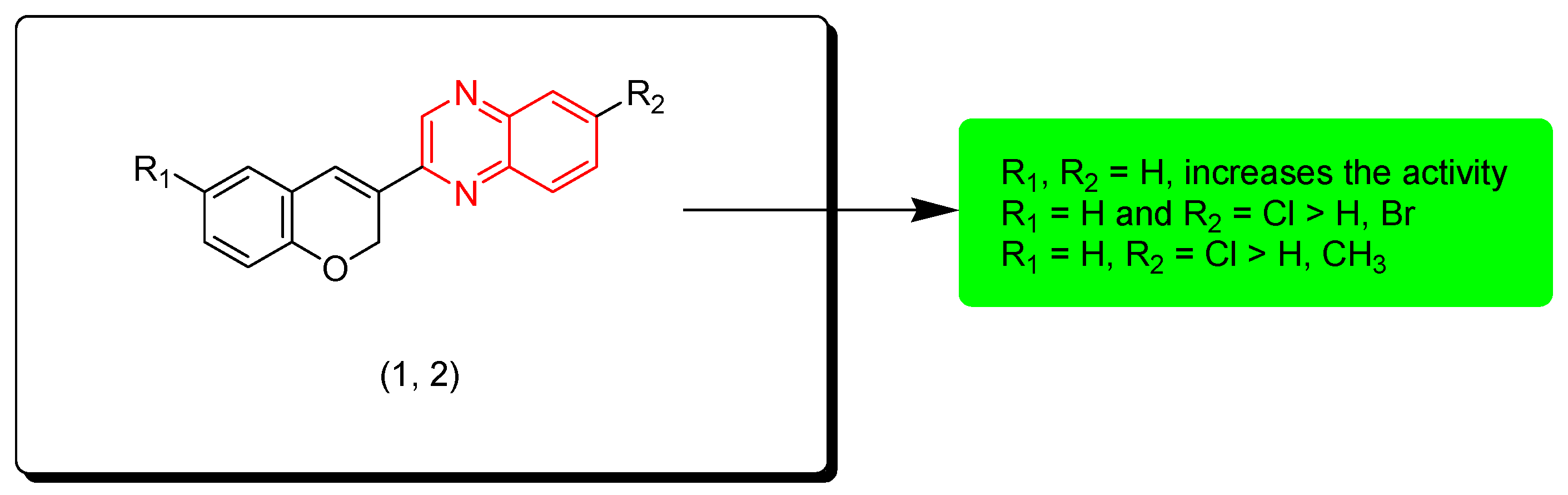
Kamble and colleagues (2016) created hybrid derivatives of quinoxaline molecules linked with coumarin to test their anticancer potential. Compounds 1 and 2 were tested against 60 cancer cell lines among these derivatives. Compound 1 showed a 55.75% growth inhibition (GI) against a melanoma (MALME-M) tumor cell line. The SAR of these derivatives showed that unsubstituted aromatic rings (R1, R2 = H) have a higher activity than other substituents while the electron withdrawing group (Cl) produces higher activity than the electron withdrawing group (Br) and the electron releasing group (CH3). Figure 4 shows the molecular structures of compounds 1, 2, and their SAR [91].
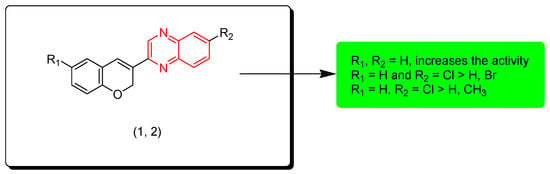
Figure 4.
Anticancer quinoxaline 1 (R1 = H, R2 = H), 2 (R1 H, R2 = Cl), and their SAR.
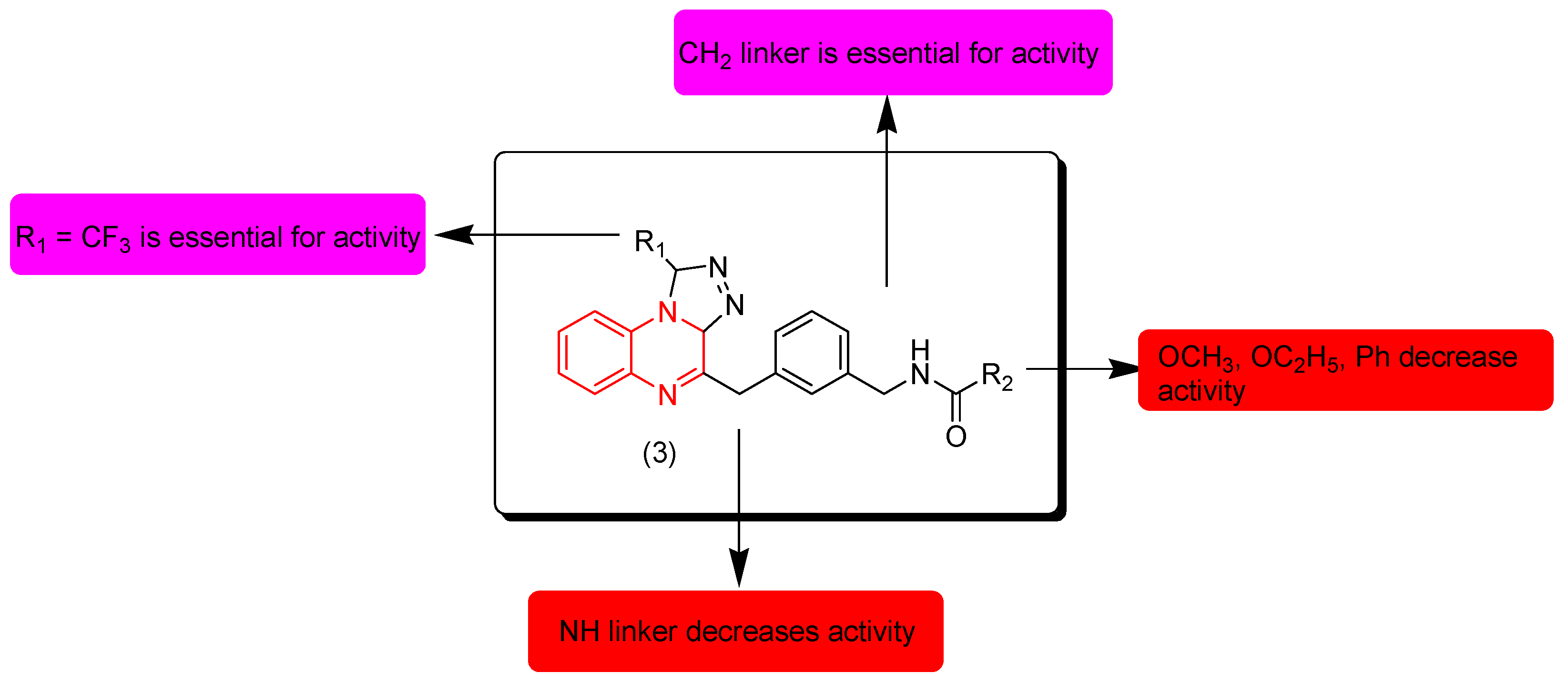
Ali and coworkers (2017) designed and synthesized some quinoxaline derivatives with a triazole ring. These derivatives were screened for their anticancer activity against leukemia cell lines Ty-82 and THP-1. Compound 3 was the highest active compound. It showed an excellent potency on the two cell lines Ty-82 (IC50 = 2.5 μM) and THP-1 (IC50 = 1.6 μM). The SAR of these derivatives showed that the aliphatic linker CH2 at the third position of quinoxaline is essential for the activity while N-linker decreases the activity. Electron releasing groups containing an oxygen atom (OCH3, OC2H5) and phenyl substituents at R2 decrease the activity while an isopropyl group (CH(CH3)2) increases the activity. Figure 5 shows the molecular structures of compound 3 and its SAR [92].
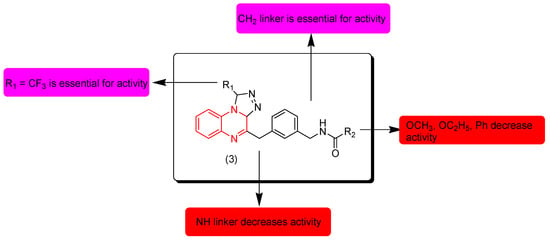
Figure 5.
Anticancer quinoxaline 3 (R1 = CF3, R2 = CH(CH3)2 and its SAR.
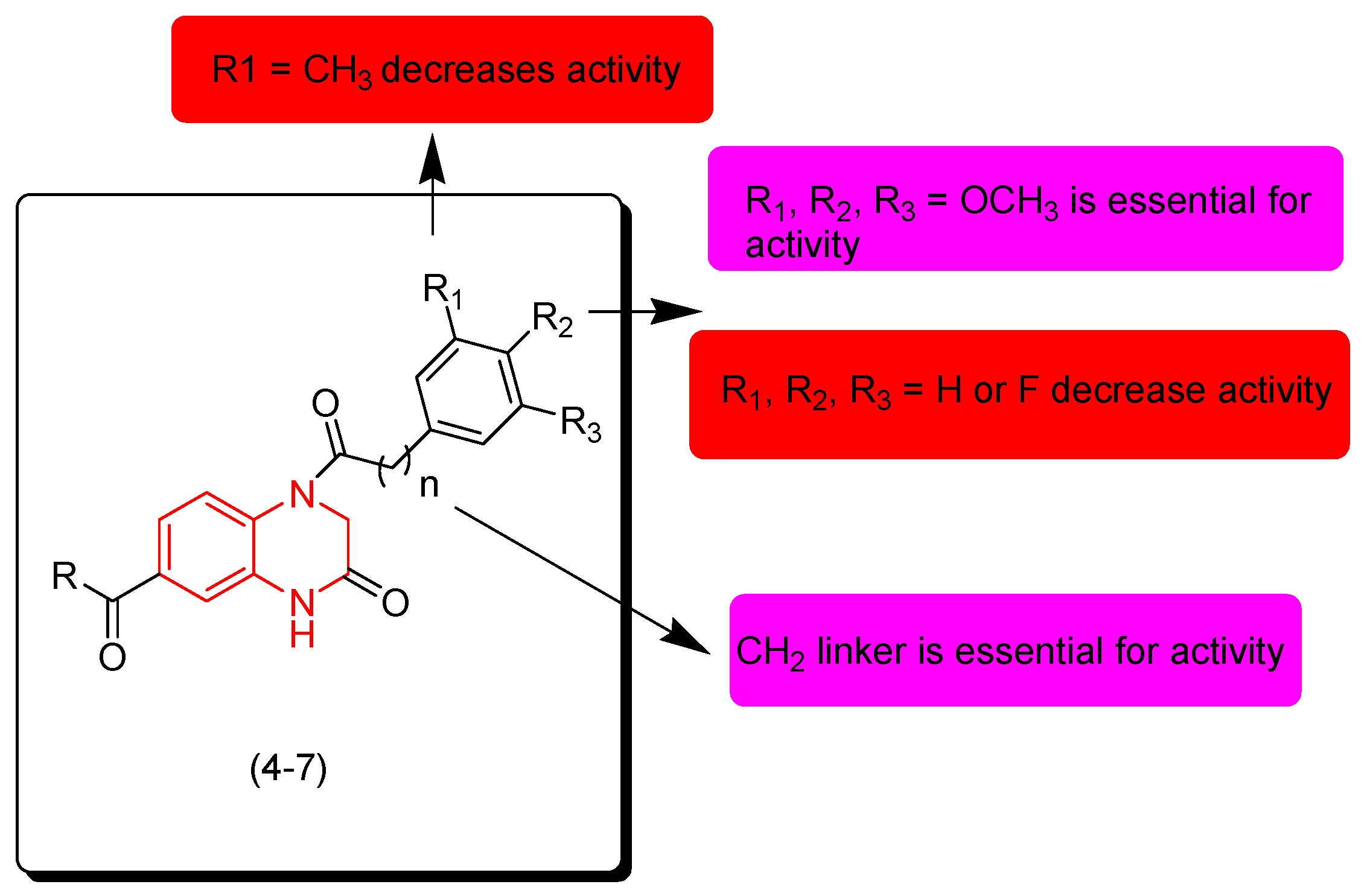
Dong and coworkers (2018) identified new derivatives of quinoxalines containing ester and amide groups (4–7). They were tested for their anticancer activity against cervical cancer (HeLa), human hepatoma cancer cells (SMMC-7721), and leukemia (K562). Some compounds 4, 6, and 7 showed moderate activity, while compound 5 showed excellent activity against HeLa (IC50 = 0.126 μM), SMMC-7721 (IC50 = 0.071 μM), and K562 (IC50 = 0.164 μM) compared to the reference doxorubicin. The SAR of these derivatives showed that the electron releasing group (OCH3) at R1, R2, and R3 is essential for the activity. Substitution of this group with an electron withdrawing group such as (F) decreases the activity while other electron releasing groups such as CH3 or C2H5 decrease the activity. It also showed that the aliphatic linker CH2 fused to the aromatic ring at the second position from the quinoxaline nucleus is essential for the activity. Figure 6 shows the molecular structures of compounds 4–7 and their SAR [93].
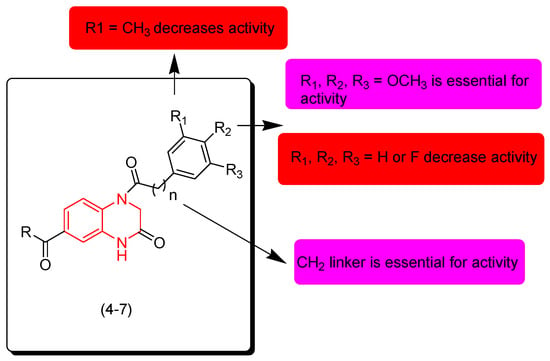
Figure 6.
Anticancer quinoxalines 4 (R = OMe, (R = OMe, R1 = OMe, R2 = OMe, R3 = H, n = 0, R1 = OMe, R2 = OMe, R3 = H), 5 (R = OMe, R1 = OMe, R2 = OMe, R3 = OMe, n = 0), 6 (R = OMe, R1 = OMe, R2 = OMe, R3 = OMe, n = 1), 7 (R = -NH-Bu, R1 = OMe, R2 = OMe, R3 = H, n = 0), and their SAR.
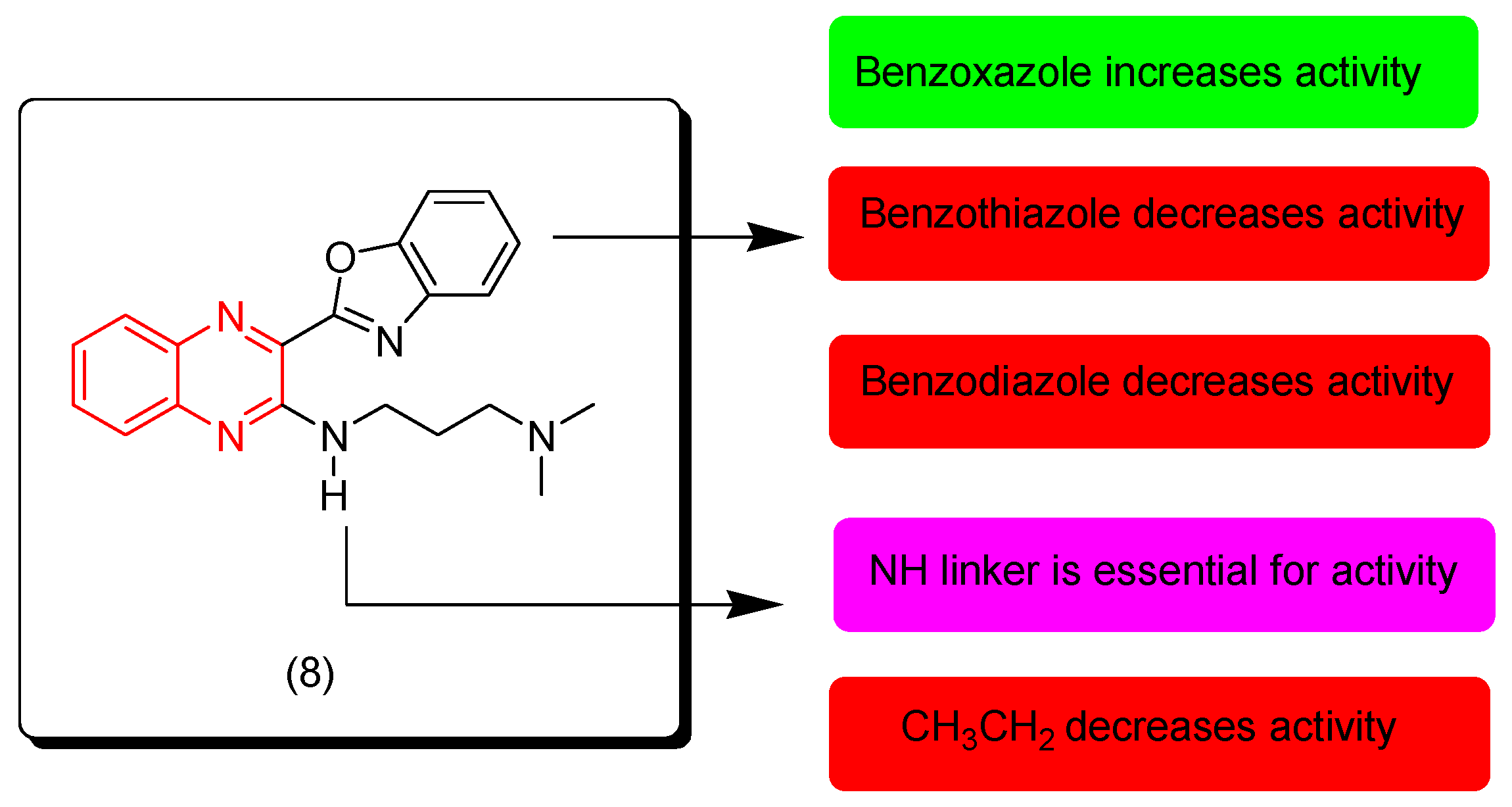
Liu and coworkers (2019) discovered new quinoxaline derivatives with benzoxazole, benzothiazole, and benzimidazole rings. They were evaluated for their anticancer activity against different cell lines (MGC-803, HepG-2, A549, Hela, and T24). Additionally, they were evaluated against normal cells (WI-38). Benzoxazole derivatives showed excellent activity while benzimidazole and benzothiazole were moderately active. Compound 8 displayed the highest activity among all compounds, including MGC-803 (IC50 = 1.49 ± 0.18 μM), Hep G2 (IC50 = 5.27 ± 0.72 μM), A549 (IC50 = 6.91 ± 0.84 μM), Hela (IC50 = 6.38 ± 0.81 μM), T-24 (IC50 = 4.49 ± 0.65 μM), and WI-38 (IC50 = 10.99 ± 1.06 μM). The SAR of these derivatives showed that the NH linker at the third position from the quinoxaline nucleus is essential for the activity while the aliphatic linkers decrease the activity. The benzoxazole moiety at the second position from the quinoxaline nucleus produced higher activity than other heterocyclic systems. Figure 7 shows the molecular structure of compound 8 and its SAR [94].
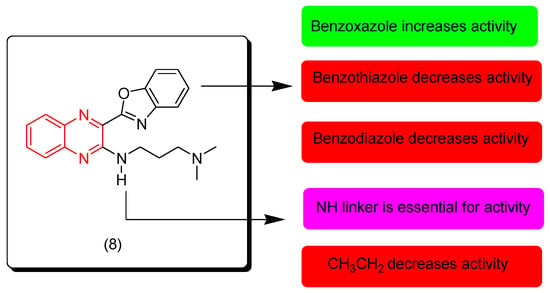
Figure 7.
Anticancer quinoxaline 8 and its SAR.
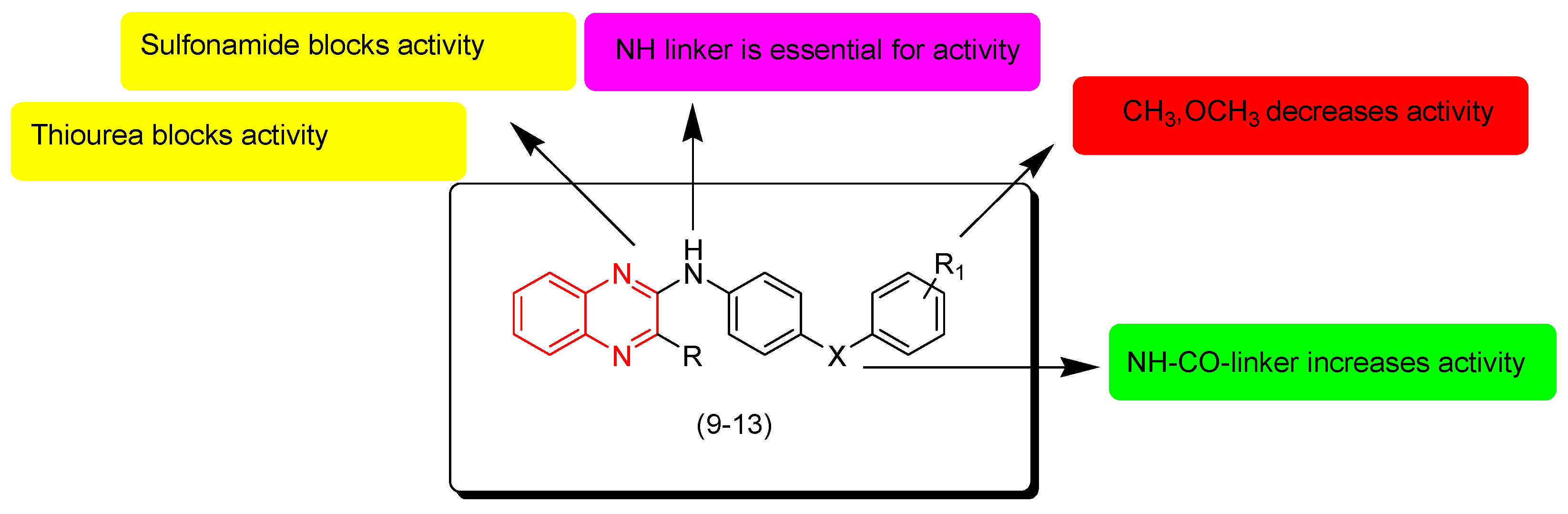
Newahie and coworkers (2019) designed new quinoxaline derivatives including urea, thiourea, amide, and sulfonamide groups (9–13). The anticancer evaluation of these derivatives was performed against the breast cancer cell line (MCF-7), liver hepatocellular carcinoma Hep G2, and human colon carcinoma HCT116. Compounds containing sulfonamide and thiourea 12 and 13 were inactive. The compound with an amide group (9) showed moderate activity while compound 12 that had thiourea showed good results against HCT116 (IC50 = 4.4 μM) and MCF-7 (IC50 = 4.4 μM). Compound 11, having a chloro-substitution at the fourth position from the phenyl ring, showed excellent activity against MCF-7 (IC50 = 9 μM) and HCT116 (IC50 = 2.5 μM). Doxorubicin was used as a standard drug to compare the activity. The SAR of these derivatives showed that the NH-CO linker at the second position from the quinoxaline nucleus increased the activity while aliphatic linkers decreased the activity. Electron releasing groups CH3 and OCH3 at R1 decreased the activity. Sulfonamide and thiourea systems at R1 blocked the activity. Figure 8 shows the molecular structures of compounds 9–13 and their SAR [95].
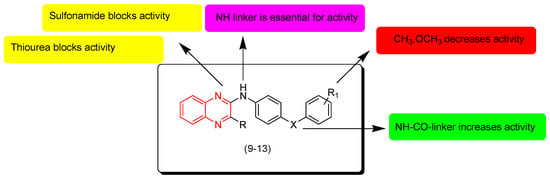
Figure 8.
Anticancer quinoxalines 9 (R = Me, R1 = 4-Me, X = -NHCO-), 10 (R = Cl, R1 = H, X = -NHCO-), 11 (R = Me, R1 = 4-Cl, X = -NHCONH-), 12 (R = Me, R1 = H, X = -NHCSNH-), and 13 (R = Me, R1 = 4-Me, X = -NHSO2-).
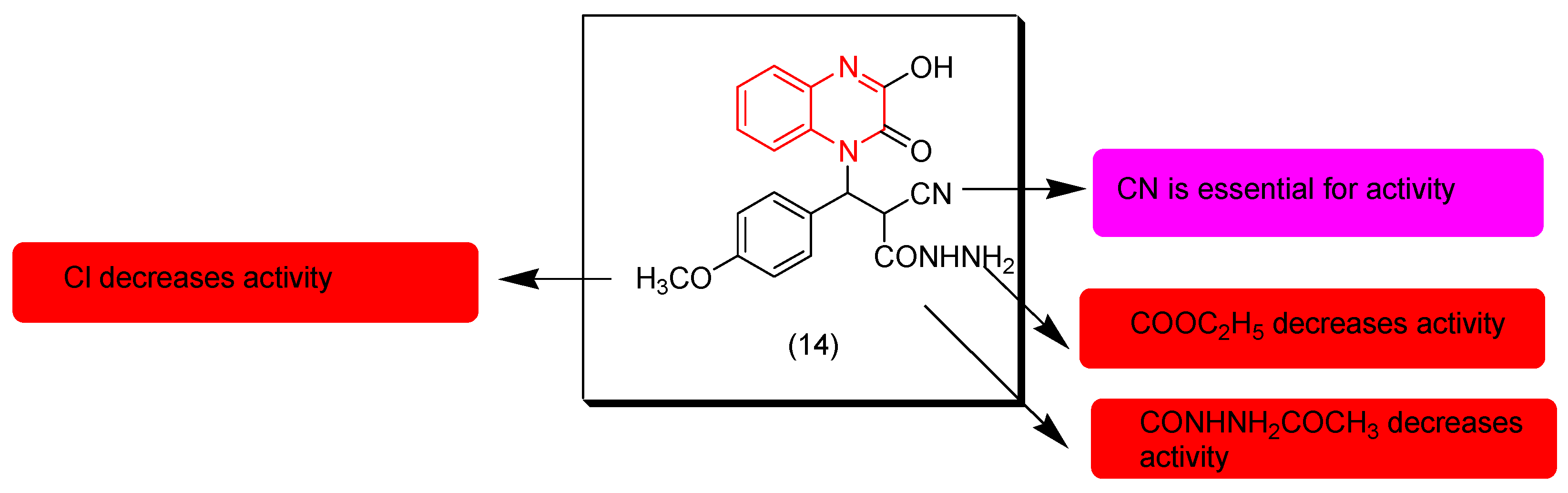
Cobouri and coworkers (2019) prepared some derivatives from 1-(N-substituted)-quinoxaline and tested their anticancer activity on a breast cancer cell line (MCF-7) and cervix cancer (Hela). Compound (14) was the most active among other derivatives with IC50 = 2.61 μM on MCF-7, while other derivatives showed comparable anticancer activity with the reference doxorubicin. The SAR of these derivatives showed that replacement of the electron releasing group OCH3 with an electron withdrawing group such as Cl decreases the activity. The CN group at the aliphatic chain fused to the nitrogen atom of the quinoxaline nucleus is essential for the activity. Replacement of the ester group COOC2H5 with the hydrazide group CONHNH2 decreases the activity. Figure 9 shows the molecular structures of compound 14 and its SAR [96].
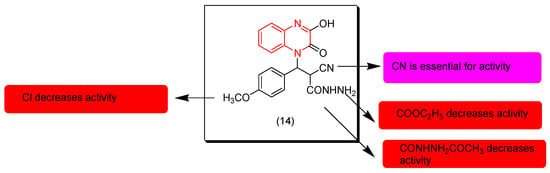
Figure 9.
Anticancer quinoxaline 14 and its SAR.
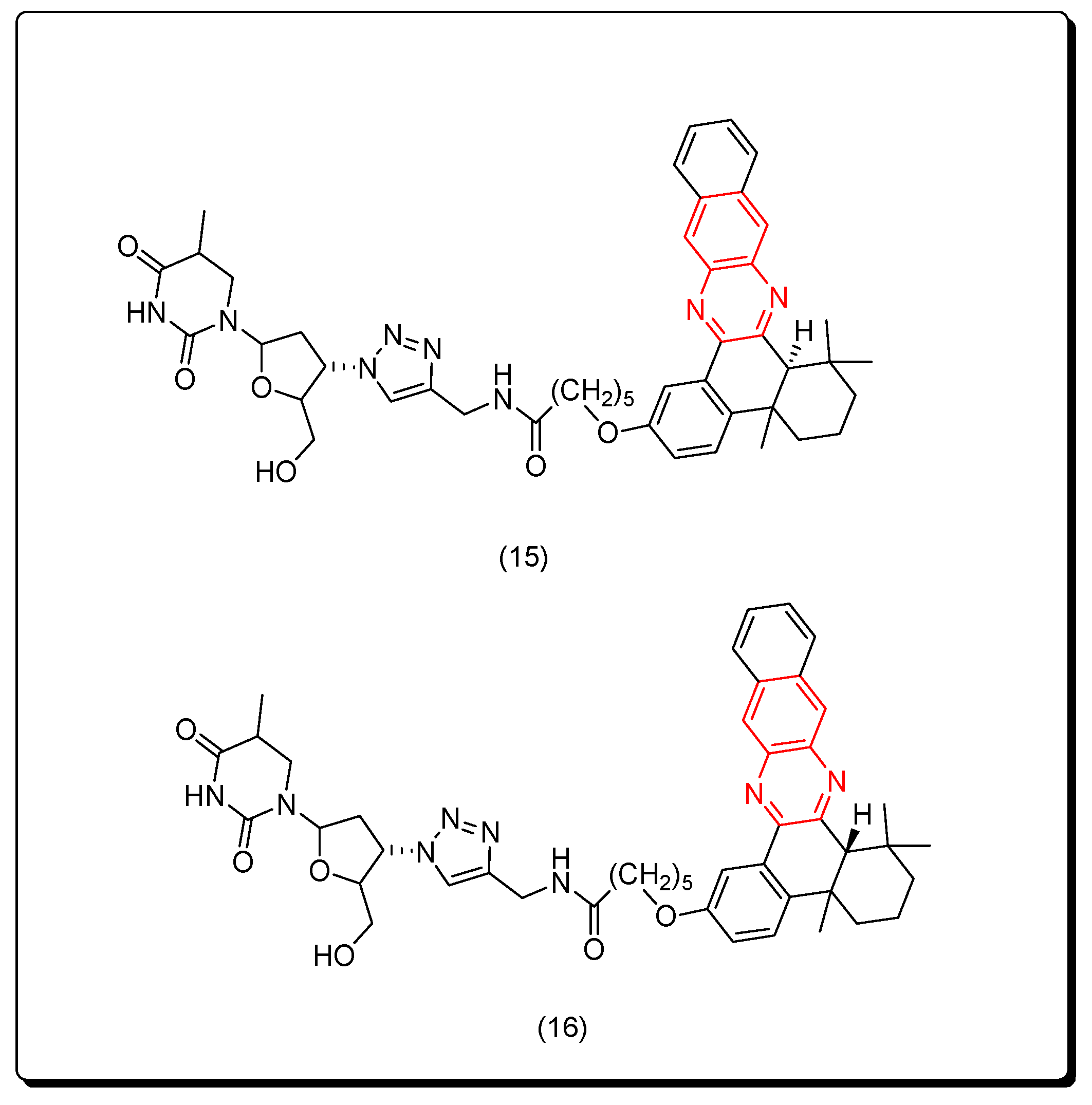
Yuan and coworkers (2019) prepared two forms of naphthyl quinoxaline thymidine conjugates cis (15) and trans (16) as a novel class of cytotoxic molecules that efficiently induced in vivo antitumor activity through the vaccination application. The two conjugates 15 and 16 exhibited a pronounced cytotoxicity post 400 nm UVA activation at 3 mW/cm2 for 20 min with the IC50 = 44.3 and 26.6 nM, respectively. This study connected the immunological effects and the antitumor activity of quinoxaline derivatives through this newly synthesized model. The structures of cis and trans isomers were separated by HPLC, and they were confirmed based on their chemical shifts. The measurement of antitumor activity was performed by measuring the immunogenic cell death markers after 2 h from UVA activation. The authors only synthesized two compounds, which was insufficient to study the SAR of these derivatives. The trans isomer was more active than the cis isomer. Figure 10 shows the molecular structures of compounds 15 and 16 [97].
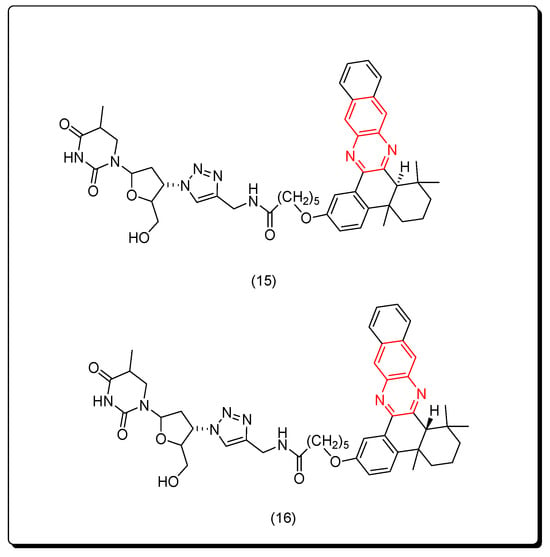
Figure 10.
Anticancer quinoxalines 15 and 16.
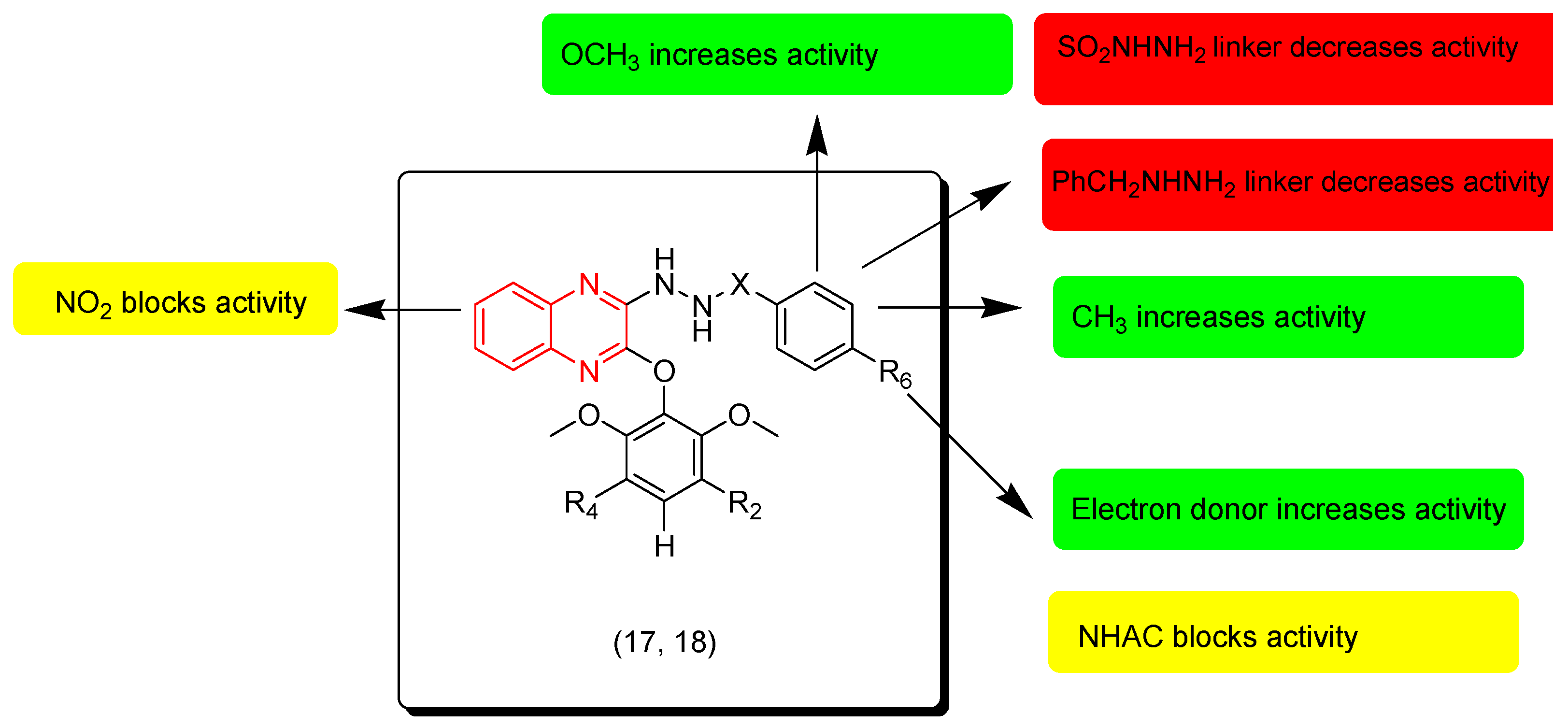
Jin and coworkers (2020) synthesized sulfono-hydrazide and benzo-hydrazide derivatives of quinoxaline. These derivatives were tested against lung cancer cells (A549), breast adenocarcinoma cells (MCF-7), and colon cancer cells (HCT-116). Compound 18 had the highest activity against MCF-7 (IC50 = 22.11 ± 13.3 μM) compared to the reference (IC50 = 11.77 ± 4.57 μM). Compound 17 displayed moderate activity on (A549) and (HCT-116) with IC50 = 46.6 ± 7.41 μM and 48 ± 8.79 μM, respectively. The SAR of these derivatives showed that the sulfonyl linker at the third position from the quinoxaline system decreases the activity while the benzyl linker increases the activity. The electron withdrawing group NO2 at the seventh position from the quinoxaline nucleus decreases the activity. Electron releasing groups at the aromatic ring fused to the second position from the quinoxaline system increase the activity while electron withdrawing groups decrease it. Figure 11 shows the molecular structures of compounds 17, 18, and their SAR [98].
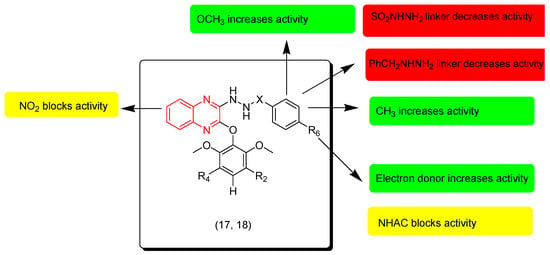
Figure 11.
Anticancer quinoxalines 17 (R2 = H, R4 = H, R6 = H, X = CO), 18 (R2 = H, R4 = H, R6 = Me, X = CO), and their SAR.
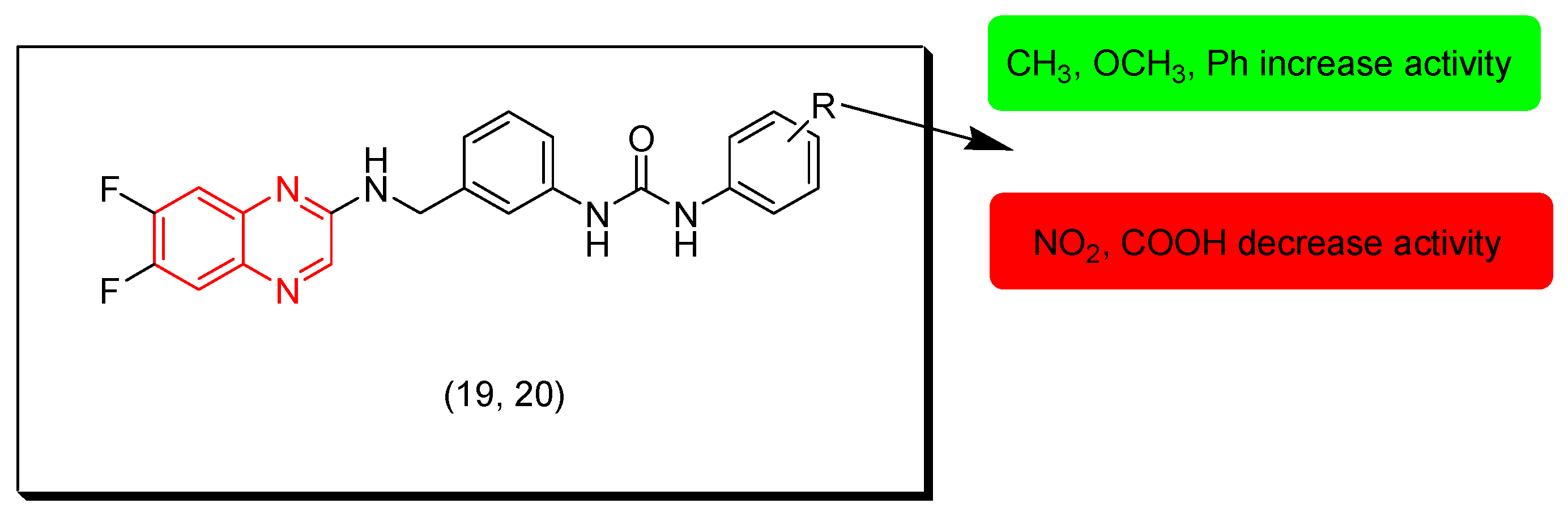
Li and coworkers (2021) designed and synthesized a group of 1,3-diphenylurea-quinoxaline compounds and investigated their in vitro cytotoxic activity against MGC-803, HeLa, NCI-H460, HepG2, SMMC-7721, T-24, and HL-7702 cancer cell lines. Most of these compounds showed good results while compounds 19 and 20 were the highest active derivatives with IC50 = 9, 12.3, 13.3, 30.4, 17.6, 27.5, and 80.9 μM for compound 19 and IC50 = 17.2, 12.3, 40.6, 46.8, 95.4, 8.9, and 86.8 μM for compound 20 against the tested cell lines, respectively. The SAR of these derivatives showed that the replacement of the R group with electron donating groups increases the activity while electron withdrawing groups decrease the activity. Figure 12 shows the molecular structures of compounds 19, 20, and their SAR [99].
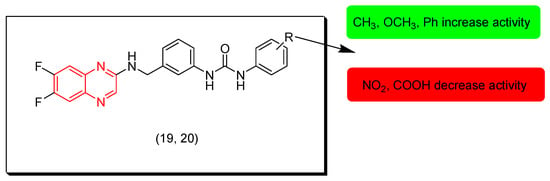
Figure 12.
Anticancer quinoxalines 19 (R = 4-O-Ph), 20 (R = (3,5-(Me)2), and their SAR.
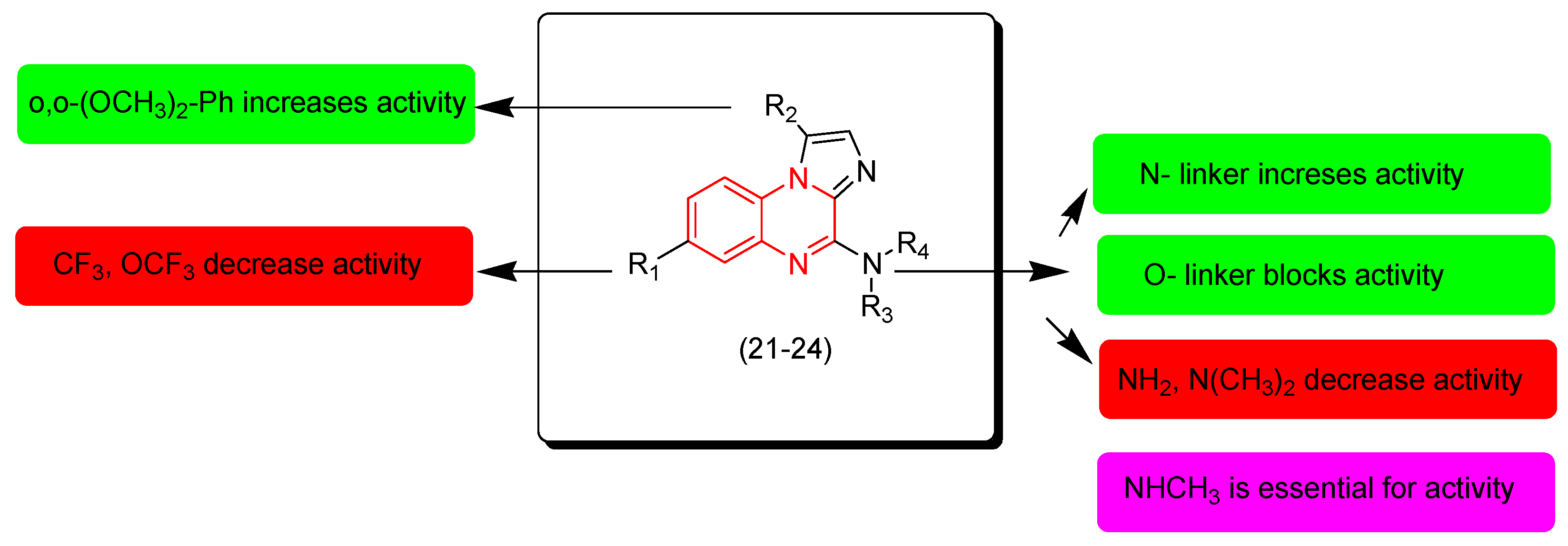
Pationate and coworkers (2021) investigated new quinoxaline derivatives with substituted imidazole-substitution. These derivatives were screened against the melanoma cells A375. Compound 24 showed an excellent activity (IC50 = 3 nM) 20-fold more potent than the reference Vemurafenib (IC50 = 139 nM). The other derivatives 21, 22, 23 showed moderate activities. The SAR of these derivatives showed that the o,o-dimethoxyphenyl group at the second position from the quinoxaline nucleus increases the activity while CF3 and OCF3 decrease the activity. The N-linker at the third position increases the activity while the O-linker decreases the activity. The secondary amine at the third position from the quinoxaline ring increases the activity while primary and tertiary amines decrease the activity. The presence of NH or NCH3 at the second position from the quinoxaline nucleus is essential for the activity. Figure 13 shows the molecular structures of compounds 21–24 and their SAR [100].
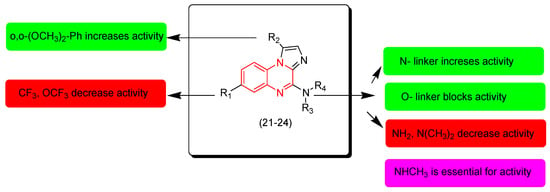
Figure 13.
Anticancer quinoxalines 21 (R1 = OCF3, R2 = H, R3 = H, R4 = Me), 22 (R1 = OCF3, R2 = o,o-(OCH3)2-Ph, R3 = H, R4 = Me), 23 (R1 = H, R2 = o,o-(OCH3)2-Ph, R3 = Me, R4 = Me), 24 (R1 = H, R2 = o,o-(OCH3)2-Ph, R3 = H, R4 = Me), and their SAR.
6. Quantitative Structure–Activity Relationship (QSAR) Modeling of Anticancer Quinoxalines
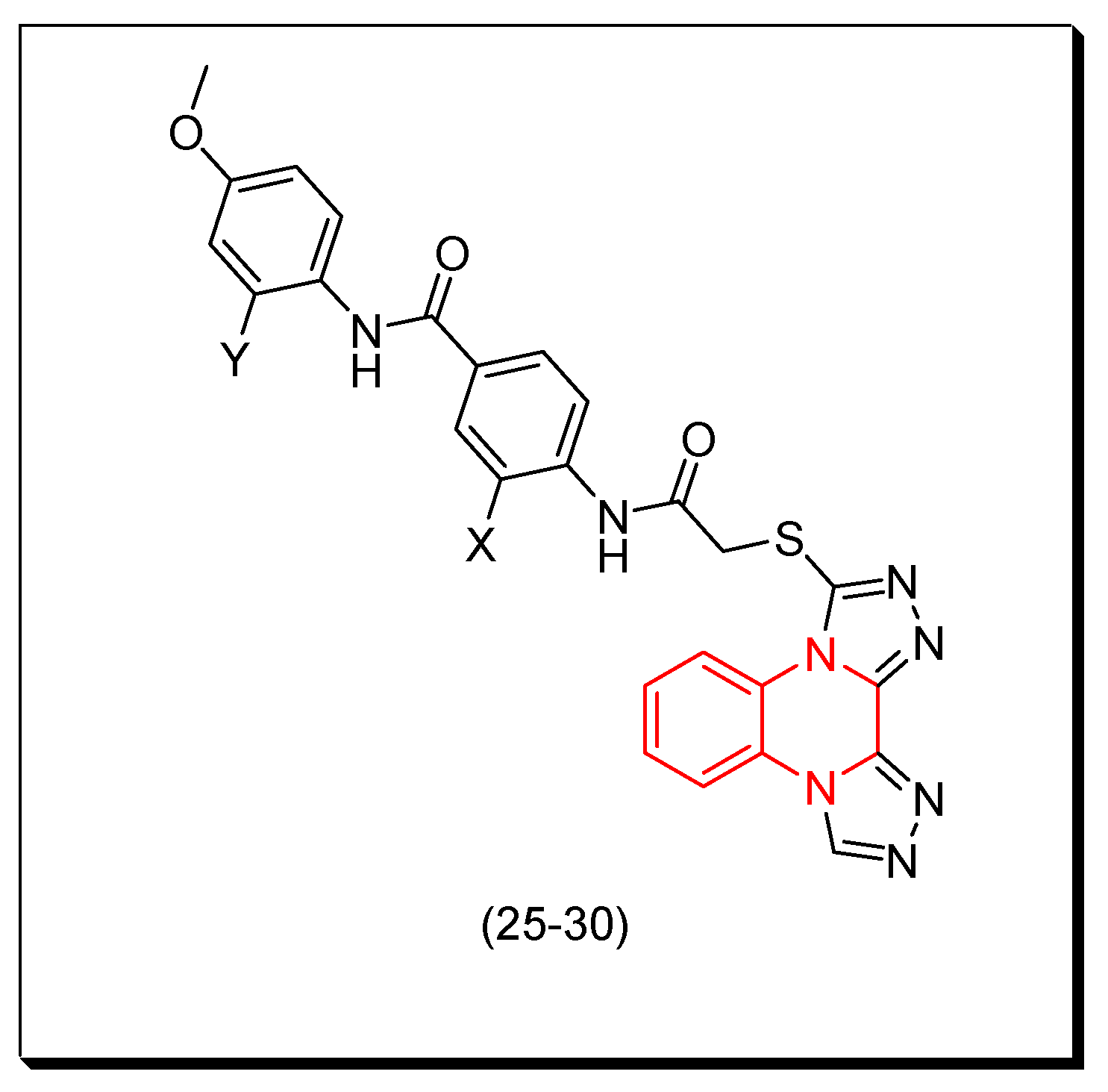
In a recent study, using some reported anticancer quinoxaline derivatives, a statistically verified 2D-QSAR model was developed by Abdullahi and coworkers (2023). By means of the created model, quinoxaline derivatives were virtually screened, and compound 25 with a high inhibiting capacity (pIC50 = 5.357) was chosen as the model for the design, and five potential better VEGFR-2 inhibitory compounds (26–30), having pIC50 values between 5.43 and 6.16, were created. The intended compounds were used as ligands in docking studies, and the active site residues of VEGFR-2 were discovered to have docking scores ranging from −171.384 to −182.241 kcal/mol, surpassing the score of −170.579 kcal/mol for the template ligand. MD simulation indicated that the ligands remained in the stable docked complex and that the molecules did not leave the VEGFR-2 active site during the 200 ns simulation. Figure 14 shows the molecular structure of the model 25 and the designed derivatives (26–30) while Table 3 shows the computed activities of these derivatives [101].
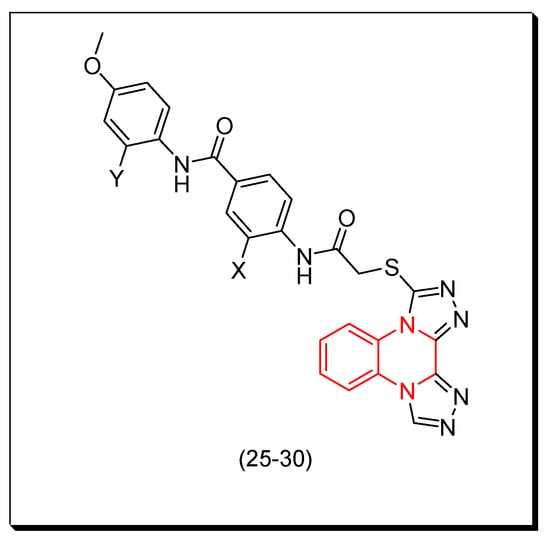
Figure 14.
The molecular structure of the model 25 and the designed derivatives (26–30): 25 (Y = H, X = H), 26 (Y = H, X = CH3), 27 (Y = H, X = OH), 28 (Y = H, X = OCH3), 29 (Y = H, X = NH2), 30 (Y = NH2, X = NH2).

Table 3.
The selected quinoxaline model (25), the designed quinoxaline derivatives (26–30), and their computed VEGFR-2 inhibitive activities. GATS5e (Geary autocorrelation—lag 5/weighted by Sanderson electronegativities), GATS3i (Geary autocorrelation—lag 3/weighted by Sanderson electronegativities), GATS8i (Geary autocorrelation—lag 8/weighted by Sanderson electronegativities), SpMax8_Bhs (the largest absolute eigenvalue of Burden modified matrix—n 8/weighted by relative I-state), VR2-Dt (normalized Randic-like eigenvector-based index from Detourn matrix).
7. SAR of Anticancer Quinoxalines
From the previously discussed examples, we can conclude the following SAR of anticancer quinoxalines:
- Quinoxaline moiety is an essential pharmacophore for the anticancer activity.
- The main sites of substitutions are first, second, and third, sixth, and/or seventh positions.
- The quinoxaline nucleus can be part of a hybrid molecule through a molecular hybridization process to potentiate the anticancer activity.
- The quinoxaline system can be joined with a polycyclic aromatic system at the (B) junction.
- There are two types of linkers that can be fused to the quinoxaline nucleus; in most cases the aliphatic linker is more reactive than the hetero-atomic linker.
- The third position from the quinoxaline nucleus can be fused to the heterocyclic system or aromatic system via an aliphatic linker.
- The aromatic ring of the quinoxaline nucleus can be substituted with halogens such as Cl or F at the sixth and/or seventh position/s to increase the activity.
8. Future Potentials
Heterocyclic molecules are an important class of derivatives with huge potential in medicinal activities. Quinoxaline is a heterocyclic system used in the design of many types of drugs. Many efficient methods were used for the synthesis of quinoxaline molecules. Chemists should use and develop green chemistry protocols as a new developed way for the future production of these molecules to avoid the problems of traditional reactions. The anticancer activity of quinoxaline compounds was reported in many studies, however the main problem associated with its anticancer activity was toxicity effects. Consequently, many quinoxaline derivatives are under development for lowering the toxicity profiles and increasing the activity. Quinoxaline pharmacophore possesses broad-spectrum pharmacological activities and still has many ranges to be discovered.
9. Conclusions
Drug development is a continuous job with limitless borders. It plays a major role in the progress of medicinal chemistry research. Nonstop research efforts in the field of drug discovery have resulted in the discovery of quinoxaline and its derivatives as versatile medicinal agents. A vast frame of scientific experiments proved the efficacy of quinoxalines in the treatment of many diseases and infections. Numerous molecules of quinoxalines were approved by the FDA and are available in the market. This review article sheds light on chemistry, physicochemical characteristics, methods of preparations, pharmaceutical products, molecular structures, and anticancer activities of some quinoxaline derivatives and their SAR characteristics. The study of SARs will be the major key for the development of efficient quinoxaline therapeutic agents. The improved quinoxaline derivatives will be identified via SAR-based studies. The structural optimization was performed with different strategies such as molecular hybridization or biological isosteric replacement to produce more potent anticancer molecules.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The author would like to extend his thanks to the Unit of Scientific Research, Fakeeh College for Medical Sciences, Jeddah, Saudi Arabia for providing valuable suggestions and moral support.
Conflicts of Interest
The author declares no conflict of interest, financial or otherwise.
References
- Mabrouk, R.R.; Abdallaha, A.E.; Mahdy, H.A.; El-Kalyoubi, S.A.; Kamal, O.J.; Abdelghany, T.M.; Zayed, M.F.; Alshaeri, H.K.; Alasmari, M.M.; El-Zahabi, M.A. Design, Synthesis, and Biological Evaluation of New Potential Unusual Modified Anticancer Immunomodulators for Possible Non-Teratogenic Quinazoline-Based Thalidomide Analogs. Int. J. Mol. Sci. 2023, 24, 12416. [Google Scholar] [CrossRef] [PubMed]
- Ihmaid, S.; Ahmed, H.E.A.; Zayed, M.F. The Design and Development of Potent Small Molecules as Anticancer Agents Targeting EGFR TK and Tubulin Polymerization. Int. J. Mol. Sci. 2018, 19, 408. [Google Scholar] [CrossRef] [PubMed]
- El-Helby, A.A.; Ayyad, A.R.; Zayed, M.F.; Abulkhir, S.H.; Elkady, H.; El-Adl, K. Design, synthesis, in silico ADMET profile and GABA-A docking of novel phthalazines as potent anticonvulsants. Arch. Pharm. 2019, 352, 1800373. [Google Scholar] [CrossRef] [PubMed]
- Zayed, M.F. Medicinal Chemistry of Quinazolines as Anticancer Agents Targeting Tyrosine Kinases. Sci. Pharm. 2023, 91, 18. [Google Scholar] [CrossRef]
- El-Zahabia, M.A.; Bamanie, H.F.; Ghareeb, S.; Alshaeri, K.H.; Alasmari, M.M.; Muostafa, M.; Al-Marzoki, Z.; Zayed, M.F. Design, Synthesis, Molecular Modeling andAnti-Hyperglycemic Evaluation of Quinazoline-Sulfonylurea Hybrids as Peroxisome Proliferator-Activated Receptor Gamma (PPAR) and Sulfonylurea Receptor (SUR) Agonists. Int. J. Mol. Sci. 2022, 23, 9605. [Google Scholar] [CrossRef] [PubMed]
- Zayed, M.F.; Ibrahim, S.; Habib, E.E.; Hassan, M.H.; Ahmed, S.; Rateb, H.S. Design, synthesis, antimicrobial and anti-biofilm evaluation, and molecular docking of new substituted fluoroquinazolinones. J. Med. Chem. 2019, 15, 657–673. [Google Scholar]
- Ghorab, M.M.; Abdel-Kader, M.S.; Alqahtani, A.S.; Soliman, A.M. Synthesis of some quinazolinones inspired from the natural alkaloid L-norephedrine as EGFR inhibitors and radiosensitizers. J. Enzym. Inhib. Med. Chem. 2021, 36, 218–237. [Google Scholar] [CrossRef] [PubMed]
- Zayed, M.F.; Ayyad, R.R. Some Novel Anticonvulsant Agents Derived from Phthalazinedione. Arzneimittelforschung 2012, 62, 532–536. [Google Scholar] [CrossRef]
- Zayed, M.F. Medicinal Chemistry of Quinazolines as Analgesic and Anti-Inflammatory Agents. ChemEngineering 2022, 6, 94. [Google Scholar] [CrossRef]
- Abulkhair, S.H.; Elmeligie, S.; Ghiaty, A.; El-Morsy, A.; Bayoumi, H.A.; Ahmed, A.E.H.; El-Adl, K.; Zayed, M.F.; Hassan, H.M.; Akl, E.N.; et al. In vivo- and in silico-driven identification of novel synthetic quinoxalines as anticonvulsants and AMPA inhibitors. Arch. Pharm. 2021, 354, 2000449. [Google Scholar] [CrossRef]
- Elhelby, A.A.; Ayyad, R.R.; Zayed, M.F. Synthesis and biological evaluation of some quinoxaline derivatives as anticonvulsant agents. Arzneimittelforschung 2011, 61, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Iazzetti, A.; Fabrizi, G.; Goggiamani, A.; Marrone, F.; Sferrazza, A.; Ullah, K. Synthesis of Functionalized 3H-pyrrolo-[1,2,3-de] Quinoxalines via Gold-Catalyzed Intramolecular Hydroamination of Alkynes. Molecules 2023, 28, 5831. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Deep, A.; Marwaha, M.; Marwaha, R.K. Quinoxaline: A chemical moiety with spectrum of interesting biological activities. Mini Rev. Med. Chem. 2022, 22, 927–948. [Google Scholar] [CrossRef] [PubMed]
- Suthar, K.S.; NChundawat, S.N.; Singh, P.G.; Padrón, M.J.; Jhala, K.Y. Quinoxaline: A comprehension of current pharmacological advancement in medicinal chemistry. Eur. J. Med. Chem. Rep. 2022, 5, 100040. [Google Scholar] [CrossRef]
- Montana, M.; Montero, V.; Khoumeri, O.; Vanelle, P. Quinoxaline Derivatives as Antiviral Agents: A Systematic Review. Molecules 2020, 25, 2784. [Google Scholar] [CrossRef] [PubMed]
- Patinote, C.; Raevens, S.; Baumann, A.; Pellegrin, E.; Bonnet, P.-A.; Deleuze-Masquéfa, C. [1,2,4]triazolo[4,3-a]quinoxaline as Novel Scaffold in the Imiqualines Family: Candidates with Cytotoxic Activities on Melanoma Cell Lines. Molecules 2023, 28, 5478. [Google Scholar] [CrossRef] [PubMed]
- Guillon, J.; Savrimoutou, S.; Albenque-Rubio, S.; Pinaud, N.; Fillová, N.; Moreau, S.; Baylot, V.; Desplat, V. Synthesis, Crystal Structure and Anti-Leukemic Activity of (E)-Pyrrolo[1,2-a]quinoxalin-4-yl)-1-(3,4,5-trimethoxyphenyl)prop-2-en-1-one. Molbank 2023, 2023, M1691. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, H.; Wang, X.; Lei, Y. Recent Developments in Direct C–H Functionalization of Quinoxalin-2(1H)-Ones via Heterogeneous Catalysis Reactions. Molecules 2023, 28, 5030. [Google Scholar] [CrossRef]
- Matveevskaya, V.V.; Pavlov, D.I.; Kovrizhina, A.R.; Sukhikh, T.S.; Sadykov, E.H.; Dorovatovskii, P.V.; Lazarenko, V.A.; Khlebnikov, A.I.; Potapov, A.S. Experimental and Computational Investigation of the Oxime Bond Stereochemistry in c-Jun N-terminal Kinase 3 Inhibitors 11H-Indeno[1,2-b]quinoxalin-11-one Oxime and Tryptanthrin-6-oxime. Pharmaceutics 2023, 15, 1802. [Google Scholar] [CrossRef]
- Dumur, F. Recent Advances on Quinoxaline-Based Photoinitiators of Polymerization. Catalysts 2023, 13, 718. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, B.; Wu, M.; Lei, Y.-Z. Recent Developments in Direct C–H Functionalization of Quinoxalin-2(1H)-Ones via Multi-Component Tandem Reactions. Molecules 2023, 28, 2513. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, A.K.; Singh, H.; Vijayan, V.; Kumar, D.; Naik, J.; Thareja, S.; Yadav, J.P.; Pathak, P.; Grishina, M.; et al. Nitrogen Containing Heterocycles as Anticancer Agents: A Medicinal Chemistry Perspective. Pharmaceuticals 2023, 16, 299. [Google Scholar] [CrossRef] [PubMed]
- Zhdankina, A.A.; Tikhonov, D.I.; Logvinov, S.V.; Plotnikov, M.B.; Khlebnikov, A.I.; Kolosova, N.G. Suppression of Age-Related Macular Degeneration-like Pathology by c-Jun N-Terminal Kinase Inhibitor IQ-1S. Biomedicines 2023, 11, 395. [Google Scholar] [CrossRef] [PubMed]
- Pooladian, F.; Crawford, P.W.; Kessler, J.M.; Casey, G.R.; Ragain, C.M. Reduction Potential Predictions for Thirty-Seven 1,4-di-N-Oxide Quinoxaline-2-Carboxamide Derivatives with Anti-Tuberculosis Activity. Compounds 2023, 3, 83–95. [Google Scholar] [CrossRef]
- Goel, K.K.; Hussain, A.; Altamimi, M.A.; Rajput, S.K.; Sharma, P.P.; Kharb, R.; Mahdi, W.A.; Imam, S.S.; Alshehri, S.; Alnemer, O.A.; et al. Identification of Potential Antitubulin Agents with Anticancer Assets from a Series of Imidazo[1,2-a]quinoxaline Derivatives: In Silico and In Vitro Approaches. Molecules 2023, 28, 802. [Google Scholar] [CrossRef] [PubMed]
- Maltsev, D.V.; Skripka, M.O.; Spasov, A.A.; Vassiliev, P.M.; Perfiliev, M.A.; Divaeva, L.N.; Zubenko, A.A.; Morkovnik, A.S.; Klimenko, A.I.; Miroshnikov, M.V.; et al. Design, Synthesis and Pharmacological Evaluation of Novel C2,C3-Quinoxaline Derivatives as Promising Anxiolytic Agents. Int. J. Mol. Sci. 2022, 23, 14401. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Liu, P.; Jiang, Y.; He, X.; Zhang, L.; Wang, L.; Yang, T. Discovery and SAR Study of Quinoxaline–Arylfuran Derivatives as a New Class of Antitumor Agents. Pharmaceutics 2022, 14, 2420. [Google Scholar] [CrossRef]
- González-González, A.; Sánchez-Sánchez, O.; Krauth-Siegel, R.L.; Bolognesi, M.L.; Gớmez-Escobedo, R.; Nogueda-Torres, B.; Vázquez-Jiménez, L.K.; Saavedra, E.; Encalada, R.; Espinoza-Hicks, J.C.; et al. In Vitro and In Silico Analysis of New n-Butyl and Isobutyl Quinoxaline-7-carboxylate 1,4-di-N-oxide Derivatives against Trypanosoma cruzi as Trypanothione Reductase Inhibitors. Int. J. Mol. Sci. 2022, 23, 13315. [Google Scholar] [CrossRef]
- Bouali, N.; Hammouda, M.B.; Ahmad, I.; Ghannay, S.; Thouri, A.; Dbeibia, A.; Patel, H.; Hamadou, W.S.; Hosni, K.; Snoussi, M.; et al. Multifunctional Derivatives of Spiropyrrolidine Tethered Indeno-Quinoxaline Heterocyclic Hybrids as Potent Antimicrobial, Antioxidant and Antidiabetic Agents: Design, Synthesis, In Vitro and In Silico Approaches. Molecules 2022, 27, 7248. [Google Scholar] [CrossRef]
- Aksenov, A.V.; Arutiunov, N.A.; Aksenov, D.A.; Samovolov, A.V.; Kurenkov, I.A.; Aksenov, N.A.; Aleksandrova, E.A.; Momotova, D.S.; Rubin, M. A Convenient Way to Quinoxaline Derivatives through the Reaction of 2-(3-Oxoindolin-2-yl)-2-phenylacetonitriles with Benzene-1,2-diamines. Int. J. Mol. Sci. 2022, 23, 11120. [Google Scholar] [CrossRef]
- Bhat, Z.R.; Kumar, M.; Sharma, N.; Yadav, U.P.; Singh, T.; Joshi, G.; Pujala, B.; Raja, M.; Chatterjee, J.; Tikoo, K.; et al. In Vivo Anticancer Evaluation of 6b, a Non-Covalent Imidazo[1,2-a]quinoxaline-Based Epidermal Growth Factor Receptor Inhibitor against Human Xenograft Tumor in Nude Mice. Molecules 2022, 27, 5540. [Google Scholar] [CrossRef] [PubMed]
- Syam, Y.M.; Anwar, M.M.; Abd El-Karim, S.S.; Elokely, K.M.; Abdelwahed, S.H. New Quinoxaline-Based Derivatives as PARP-1 Inhibitors: Design, Synthesis, Antiproliferative, and Computational Studies. Molecules 2022, 27, 4924. [Google Scholar] [CrossRef] [PubMed]
- Montero, V.; Montana, M.; Khoumeri, O.; Correard, F.; Estève, M.-A.; Vanelle, P. Synthesis, In Vitro Antiproliferative Activity, and In Silico Evaluation of Novel Oxiranyl-Quinoxaline Derivatives. Pharmaceuticals 2022, 15, 781. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Inwati, G.K.; Ahmed, E.M.; Lal, C.; Makwana, B.; Yadav, V.K.; Islam, S.; Ahn, H.-J.; Yadav, K.K.; Jeon, B.-H. Modified 7-Chloro-11H-indeno[1,2-b]quinoxaline Heterocyclic System for Biological Activities. Catalysts 2022, 12, 213. [Google Scholar] [CrossRef]
- Guillon, J.; Savrimoutou, S.; Albenque-Rubio, S.; Pinaud, N.; Moreau, S.; Desplat, V. Synthesis, Crystal Structure and Anti-Leukemic Activity of 1,3-Dihydro-1-{1-[4-(4-phenylpyrrolo[1,2-a]quinoxalin-3-yl)benzyl]piperidin-4-yl}-2H-benzimidazol-2-one. Molbank 2022, 2022, M1333. [Google Scholar] [CrossRef]
- Sharma, B.K.; Shaikh, A.M.; Chacko, S.; Kamble, M.R. Synthesis, spectral, electrochemical and theoretical investigation of indolo[2,3-b]quinoxaline dyes derived from anthraquinone for n–type materials. J. Chem. Sci. 2017, 129, 483–494. [Google Scholar] [CrossRef]
- Peppas, A.; Sokalis, D.; Perganti, D.; Schnakenburg, G.; Falaras, P.; Philippopoulos, I.A. Sterically demanding pyridine-quinoline anchoring ligands as building blocks for copper(i)-based dye-sensitized solar cell (DSSC) complexes. Dalton Trans. 2022, 51, 15049. [Google Scholar] [CrossRef] [PubMed]
- Abu-Hashem, A.A.; Al-Hussain, S.A. Design, Synthesis of New 1,2,4-Triazole/1,3,4-Thiadiazole with Spiroindoline, Imidazo[4,5-b]quinoxaline and Thieno[2,3-d]pyrimidine from Isatin Derivatives as Anticancer Agents. Molecules 2022, 27, 835. [Google Scholar] [CrossRef]
- Liakhov, S.A.; Schepetkin, I.A.; Karpenko, O.S.; Duma, H.I.; Haidarzhy, N.M.; Kirpotina, L.N.; Kovrizhina, A.R.; Khlebnikov, A.I.; Bagryanskaya, I.Y.; Quinn, M.T. Novel c-Jun N-Terminal Kinase (JNK) Inhibitors with an 11H-Indeno[1,2-b]quinoxalin-11-one Scaffold. Molecules 2021, 26, 5688. [Google Scholar] [CrossRef]
- Suwanhom, P.; Saetang, J.; Khongkow, P.; Nualnoi, T.; Tipmanee, V.; Lomlim, L. Synthesis, Biological Evaluation, and In Silico Studies of New Acetylcholinesterase Inhibitors Based on Quinoxaline Scaffold. Molecules 2021, 26, 4895. [Google Scholar] [CrossRef]
- Montana, M.; Montero, V.; Khoumeri, O.; Vanelle, P. Quinoxaline Moiety: A Potential Scaffold against Mycobacterium tuberculosis. Molecules 2021, 26, 4742. [Google Scholar] [CrossRef] [PubMed]
- Bouz, G.; Bouz, S.; Janďourek, O.; Konečná, K.; Bárta, P.; Vinšová, J.; Doležal, M.; Zitko, J. Synthesis, Biological Evaluation, and In Silico Modeling of N-Substituted Quinoxaline-2-Carboxamides. Pharmaceuticals 2021, 14, 768. [Google Scholar] [CrossRef] [PubMed]
- Amrane, D.; Arnold, C.-S.; Hutter, S.; Sanz-Serrano, J.; Collia, M.; Azqueta, A.; Paloque, L.; Cohen, A.; Amanzougaghene, N.; Tajeri, S.; et al. 2-Phenoxy-3-Trichloromethylquinoxalines Are Antiplasmodial Derivatives with Activity against the Apicoplast of Plasmodium falciparum. Pharmaceuticals 2021, 14, 724. [Google Scholar] [CrossRef] [PubMed]
- Irfan, A.; Ahmad, S.; Hussain, S.; Batool, F.; Riaz, H.; Zafar, R.; Kotwica-Mojzych, K.; Mojzych, M. Recent Updates on the Synthesis of Bioactive Quinoxaline-Containing Sulfonamides. Appl. Sci. 2021, 11, 5702. [Google Scholar] [CrossRef]
- El-Sayed, N.N.E.; Al-Otaibi, T.M.; Alonazi, M.; Masand, V.H.; Barakat, A.; Almarhoon, Z.M.; Ben Bacha, A. Synthesis and Characterization of Some New Quinoxalin-2(1H)one and 2-Methyl-3H-quinazolin-4-one Derivatives Targeting the Onset and Progression of CRC with SAR, Molecular Docking, and ADMET Analyses. Molecules 2021, 26, 3121. [Google Scholar] [CrossRef]
- Mehmood, R.; Rashid, N.; Ullah, S.; Amaldoss, M.J.N.; Sorrell, C.C. Synthesis and Structure-Chirality Relationship Analysis of Steroidal Quinoxalines to Design and Develop New Chiral Drugs. Chemistry 2021, 3, 402–410. [Google Scholar] [CrossRef]
- Kumar, M.; Joshi, G.; Arora, S.; Singh, T.; Biswas, S.; Sharma, N.; Bhat, Z.R.; Tikoo, K.; Singh, S.; Kumar, R. Design and Synthesis of Non-Covalent Imidazo[1,2-a]quinoxaline-Based Inhibitors of EGFR and Their Anti-Cancer Assessment. Molecules 2021, 26, 1490. [Google Scholar] [CrossRef]
- Khatoon, H.; Abdulmalek, E. Novel Synthetic Routes to Prepare Biologically Active Quinoxalines and Their Derivatives: A Synthetic Review for the Last Two Decades. Molecules 2021, 26, 1055. [Google Scholar] [CrossRef]
- Oyallon, B.; Brachet-Botineau, M.; Logé, C.; Robert, T.; Bach, S.; Ibrahim, S.; Raoul, W.; Croix, C.; Berthelot, P.; Guillon, J.; et al. New Quinoxaline Derivatives as Dual Pim-1/2 Kinase Inhibitors: Design, Synthesis and Biological Evaluation. Molecules 2021, 26, 867. [Google Scholar] [CrossRef]
- Nam, K.Y.; Damodar, K.; Lee, Y.; Park, L.S.; Gim, J.G.; Park, J.P.; Jeon, S.H.; Lee, J.T. Design and Synthesis of π-Extended Resveratrol Analogues and In Vitro Antioxidant and Anti-Inflammatory Activity Evaluation. Molecules 2021, 26, 646. [Google Scholar] [CrossRef]
- Giuglio-Tonolo, A.G.; Curti, C.; Terme, T.; Vanelle, P. A Survey of Synthetic Routes and Antitumor Activities for Benzo[g]quinoxaline-5,10-diones. Molecules 2020, 25, 5922. [Google Scholar] [CrossRef] [PubMed]
- Guillon, J.; Savrimoutou, S.; Rubio, S.; Moreau, S.; Pinaud, N.; Marchivie, M.; Desplat, V. 1-Phenyl-8-[[4-(pyrrolo[1,2-a]quinoxalin-4-yl)phenyl]methyl]-1,3,8-triazaspiro[4.5]decan-4-one: Synthesis, Crystal Structure and Anti-Leukemic Activity. Molbank 2020, 2020, M1113. [Google Scholar] [CrossRef]
- Palos, I.; Luna-Herrera, J.; Lara-Ramírez, E.E.; Loera-Piedra, A.; Fernández-Ramírez, E.; Aguilera-Arreola, M.G.; Paz-González, A.D.; Monge, A.; Wan, B.; Franzblau, S.; et al. Anti-Mycobacterium tuberculosis Activity of Esters of Quinoxaline 1,4-Di-N-Oxide. Molecules 2018, 23, 1453. [Google Scholar] [CrossRef] [PubMed]
- Alswah, M.; Bayoumi, A.H.; Elgamal, K.; Elmorsy, A.; Ihmaid, S.; Ahmed, H.E.A. Design, Synthesis and Cytotoxic Evaluation of Novel Chalcone Derivatives Bearing Triazolo[4,3-a]-quinoxaline Moieties as Potent Anticancer Agents with Dual EGFR Kinase and Tubulin Polymerization Inhibitory Effects. Molecules 2018, 23, 48. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Wang, S.; Jin, X.; Zhang, Y.; Hua, D.; Miao, T.; Tao, X.; Wang, S. Synthesis and Evaluation of New Quinoxaline Derivatives of Dehydroabietic Acid as Potential Antitumor Agents. Molecules 2017, 22, 1154. [Google Scholar] [CrossRef] [PubMed]
- Chacón-Vargas, K.F.; Nogueda-Torres, B.; Sánchez-Torres, L.E.; Suarez-Contreras, E.; Villalobos-Rocha, J.C.; Torres-Martinez, Y.; Lara-Ramirez, E.E.; Fiorani, G.; Krauth-Siegel, R.L.; Bolognesi, M.L.; et al. Trypanocidal Activity of Quinoxaline 1,4 Di-N-oxide Derivatives as Trypanothione Reductase Inhibitors. Molecules 2017, 22, 220. [Google Scholar] [CrossRef] [PubMed]
- Al-Marhabi, A.R.; Abbas, H.-A.S.; Ammar, Y.A. Synthesis, Characterization and Biological Evaluation of Some Quinoxaline Derivatives: A Promising and Potent New Class of Antitumor and Antimicrobial Agents. Molecules 2015, 20, 19805–19822. [Google Scholar] [CrossRef]
- Mickevičienė, K.; Baranauskaitė, R.; Kantminienė, K.; Stasevych, M.; Komarovska-Porokhnyavets, O.; Novikov, V. Synthesis and Antimicrobial Activity of N-Substituted-β-amino Acid Derivatives Containing 2-Hydroxyphenyl, Benzo[b]phenoxazine and Quinoxaline Moieties. Molecules 2015, 20, 3170–3189. [Google Scholar] [CrossRef]
- Radwan, A.A.; Abdel-Mageed, W.M. In Silico Studies of Quinoxaline-2-Carboxamide 1,4-di-N-Oxide Derivatives as Antimycobacterial Agents. Molecules 2014, 19, 2247–2260. [Google Scholar] [CrossRef]
- Gil, A.; Pabón, A.; Galiano, S.; Burguete, A.; Pérez-Silanes, S.; Deharo, E.; Monge, A.; Aldana, I. Synthesis, Biological Evaluation and Structure-Activity Relationships of New Quinoxaline Derivatives as Anti-Plasmodium falciparum Agents. Molecules 2014, 19, 2166–2180. [Google Scholar] [CrossRef]
- Barea, C.; Pabón, A.; Pérez-Silanes, S.; Galiano, S.; Gonzalez, G.; Monge, A.; Deharo, E.; Aldana, I. New Amide Derivatives of Quinoxaline 1,4-di-N-Oxide with Leishmanicidal and Antiplasmodial Activities. Molecules 2013, 18, 4718–4727. [Google Scholar] [CrossRef]
- Hu, Y.; Xia, Q.; Shangguan, S.; Liu, X.; Hu, Y.; Sheng, R. Synthesis and Biological Evaluation of 3-Aryl-quinoxaline-2-carbonitrile 1,4-Di-N-oxide Derivatives as Hypoxic Selective Anti-tumor Agents. Molecules 2012, 17, 9683–9696. [Google Scholar] [CrossRef] [PubMed]
- Barea, C.; Pabón, A.; Galiano, S.; Pérez-Silanes, S.; Gonzalez, G.; Deyssard, C.; Monge, A.; Deharo, E.; Aldana, I. Antiplasmodial and Leishmanicidal Activities of 2-Cyano-3-(4-phenylpiperazine-1-carboxamido) Quinoxaline 1,4-Dioxide Derivatives. Molecules 2012, 17, 9451–9461. [Google Scholar] [CrossRef]
- Ancizu, S.; Castrillo, N.; Pérez-Silanes, S.; Aldana, I.; Monge, A.; Delagrange, P.; Caignard, D.-H.; Galiano, S. New Quinoxaline Derivatives as Potential MT1 and MT2 Receptor Ligands. Molecules 2012, 17, 7737–7757. [Google Scholar] [CrossRef] [PubMed]
- Morales-Castellanos, J.J.; Ramírez-Hernández, K.; Gómez-Flores, N.S.; Rodas-Suárez, O.R.; Peralta-Cruz, J. Microwave-assisted Solvent-free Synthesis and in Vitro Antibacterial Screening of Quinoxalines and Pyrido[2,3b]pyrazines. Molecules 2012, 17, 5164–5176. [Google Scholar] [CrossRef] [PubMed]
- Molecular Operating Environment (MOE) Chemical Computing Group. Available online: http://www.chemcomp.com (accessed on 9 July 2023).
- Swiss Institute of Bioinformatics (SwissADME). Available online: http://www.swissADME.ch (accessed on 10 July 2023).
- Singh, D.P.; Deivedi, S.K.; Hashim, S.R.; Singhal, R.G. Synthesis and Antimicrobial Activity of Some New Quinoxaline Derivatives. Pharmaceuticals 2010, 3, 2416–2425. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, D.; Mukherjee, S.; Rodriguez, R.R.; Banik, B.K. An Effective Microwave-Induced Iodine-Catalyzed Method for the Synthesis of Quinoxalines via Condensation of 1,2-Diamines with 1,2-Dicarbonyl Compounds. Molecules 2010, 15, 4207–4212. [Google Scholar] [CrossRef] [PubMed]
- Vicente, E.; Charnaud, S.; Bongard, E.; Villar, R.; Burguete, A.; Solano, B.; Ancizu, S.; Pérez-Silanes, S.; Aldana, I.; Vivas, L.; et al. Synthesis and Antiplasmodial Activity of 3-Furyl and 3-Thienylquinoxaline-2-carbonitrile 1,4-Di-N-oxide Derivatives. Molecules 2008, 13, 69–77. [Google Scholar] [CrossRef] [PubMed]
- EI-Bendary, E.R.; Goda, F.E.; Maarouf, A.R.; Badria, F.A. Synthesis and antimicrobial evaluation of 3-hydrazino-quinoxaline derivatives and their cyclic analoaues. Sci. Pharm. 2004, 72, 175–185. [Google Scholar] [CrossRef][Green Version]
- Nasr, M.N.; Gineinaha, M.M.; Maarouf, A.R. Synthesis and Anticonvulsant Activity of Novel 2- and 3-14-(Trisubstituted Pyridy1)-phenylaminol- and 2-[3- and 4-(Trisubstituted Pyridy1)-phenoxylquinoxaline Derivatives. Sci. Pharm. 2003, 71, 9–18. [Google Scholar] [CrossRef]
- Waring, M.J.; Ben-Hadda, T.; Kotchevar, A.T.; Ramdani, A.; Touzani, R.; Elkadiri, S.; Hakkou, A.; Bouakka, M.; Ellis, T. 2,3-Bifunctionalized Quinoxalines: Synthesis, DNA Interactions and Evaluation of Anticancer, Anti-tuberculosis and Antifungal Activity. Molecules 2002, 7, 641–656. [Google Scholar] [CrossRef]
- Rodrigo, G.A.; Bekerman, D.G.; Robinsohn, A.E.; Fernández, B.M. Synthesis and Physicochemical Study of a Quinoxaline Derivative with Potencial Antineoplasic or Anti-HIV Activity. Molecules 2000, 5, 358–359. [Google Scholar] [CrossRef]
- Veisi, H. Molecular iodine: Recent application in heterocyclic synthesis. Curr. Org. Chem. 2011, 15, 2438–2468. [Google Scholar] [CrossRef]
- Aichhorn, S.; Himmelsbach, M.; Schofberger, W. Synthesis of quinoxalines or quinolin-8-amines from N-propargyl aniline derivatives employing tin and indium chlorides. Org. Biomol. Chem. 2015, 13, 9373–9380. [Google Scholar] [CrossRef]
- Heravi, M.M.; Bakhtiari, K.; Tehrani, M.H.; Javadi, N.M.; Oskooie, A.H. Facile synthesis of quinoxaline derivatives using o-iodoxybenzoic acid (IBX) at room temperature. ARKIVOC 2006, XVI, 16–22. [Google Scholar] [CrossRef]
- Jeganathan, M.; Dhakshinamoorthy, A.; Pitchumani, K. One-pot synthesis of 2- substituted quinoxalines using K10-montmorillonite as heterogeneous catalyst. Tetrahedron Lett. 2014, 55, 1616–1620. [Google Scholar] [CrossRef]
- Chen, T.; Chen, X.; Wei, J.; Lin, D.; Xie, Y.; Zeng, W. Copper-catalyzed cascade Cycloamination of α-csp3–H Bond of N-aryl Ketimines with azides: Access to Quinoxalines. Org. Lett. 2016, 18, 2078–2081. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Shen, Y.; Meng, X.; Zhao, M.; Chen, Y.; Chen, B. Copper-catalyzed synthesis of quinoxalines with o-phenylenediamine and terminal alkyne in the presence of bases. Org. Lett. 2011, 13, 4514–4517. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, K.; Zhao, M.; Li, Y.; Chen, B. Cu(II)-catalyzed synthesis of quinoxalines from o-phenylene diamines and nitroolefins. Tetrahedron Lett. 2013, 54, 1627–1630. [Google Scholar] [CrossRef]
- Wang, X.; Liu, H.; Xie, C.; Zhou, F.; Chen, M. Terminal methyl as a one-carbon synthon: Synthesis of quinoxaline derivatives via radical-type transformation. New J. Chem. 2020, 44, 2465–2470. [Google Scholar] [CrossRef]
- Hasaninejad, A.; Zare, A.; Shekouhy, M.; Moosavi-Zare, A.R. Bentonite clay K-10 as an efficient reagent for the synthesis of quinoxaline derivatives at room temperature. J. Chem. 2009, 6, S247–S253. [Google Scholar] [CrossRef]
- Anastas, P.; Warner, J. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 1998; Volume 30. [Google Scholar]
- Subrahmanyam, S.C.; Narayanan, S. Synthesis of Quinoxalines in Presence of Zinc Triflate. Asian J. Chem. 2011, 23, 1331–1333. [Google Scholar]
- An, Z.; Wu, M.; Ni, J.; Qi, Z.; Yu, G.; Yan, R.; Zhao, L.-B. FeCl3-Catalyzed synthesis of pyrrolo[1,2-a]quinoxaline derivatives from 1-(2-aminophenyl)pyrroles through annulation and cleavage of cyclic ethers. Chem. Commun. 2017, 53, 11572–11575. [Google Scholar] [CrossRef] [PubMed]
- Atghia, S.V.; Beigbaghlou, S.S. Nanocrystalline titania-based sulfonic acid (TiO2-Pr-SO3H) as a new, highly efficient, and recyclable solid acid catalyst for the preparation of quinoxaline derivatives. J. Nanostruct. Chem. 2013, 3, 38. [Google Scholar] [CrossRef]
- Kumar, K.S.; Rambabu, D.; Sandra, S.; Kapavarapu, R.; Krishna, R.G.G.; Rao, B.V.M.; Chatti, K.; Reddy, M.C.; Misra, P.; Pal, M. AlCl3 induced (hetero)arylation of 2,3-dichloroquinoxaline: A one-pot synthesis of mono/disubstituted quinoxalines as potential antitubercular agents. Bioorg. Med. Chem. 2012, 20, 1711–1722. [Google Scholar] [CrossRef] [PubMed]
- Babu, V.P.; Mukherjee, S.; Deora, S.G.; Chennubhotla, S.K.; Medisetti, R.; Yellanki, S.; Kulkarni, P.P.; Sripelly, S.; Parsa, L.V.K.; Chatti, K.; et al. Ligand/PTCfree intramolecular Heck reaction: Synthesis of pyrroloquinoxalines and their evaluation against PDE4/luciferase/oral cancer cell growth in vitro and zebrafish in vivo. Org. Biomol. Chem. 2013, 11, 6680. [Google Scholar] [CrossRef] [PubMed]
- DrugBank Online. Available online: https://go.drugbank.com/ (accessed on 28 September 2023).
- Kamble, A.A.; Kamble, R.R.; Kumbar, M.N.; Tegginamath, G. Pyridine-catalyzed synthesis of quinoxalines as anticancer and anti-tubercular agents. Med. Chem. Res. 2016, 25, 1163–1174. [Google Scholar] [CrossRef]
- Ali, I.; Lee, J.; Choi, G.G.A.; Lee, K. Discovery of novel [1,2,4]triazolo[4,3-a] quinoxaline aminophenyl derivatives as BET inhibitors for cancer treatment. Bioorg. J. Med. Chem. Lett. 2017, 27, 4606–4613. [Google Scholar] [CrossRef]
- Dong, H.Q.J.; Huang, J.; Zhang, S.; Niu, L.; Zhang, Y.; Wang, J. Synthesis and biological evaluation of N-substituted3-oxo-1,2,3,4-tetrahydro-quinoxaline-6- carboxylic acid derivatives as tubulin polymerization inhibitors. Eur. J. Med. Chem. 2018, 143, 8–20. [Google Scholar]
- Liu, J.; Wang, P.; Zhang, Z.; Xue, G.; Ahmed, A.E.; Mamdouh, F.A.; Omran, M.A.; Salah, H. Synthesis, EGFR-TK inhibition and anticancer activity of new quinoxaline derivatives. Synth. Commun. 2020, 50, 2924–2940. [Google Scholar]
- Newahie, E.S.M.A.; Nissan, M.; Ismail, M.S.N.; Ella, E.A.A.D.; Khojah, M.S.; Abouzid, M.A.K. Design and synthesis of new quinoxaline derivatives as anticancer agents and apoptotic inducers. Molecules 2019, 24, 1175. [Google Scholar] [CrossRef] [PubMed]
- Gobouri, A.A. Synthesis and biological evaluation of some N-substituted quinoxaline derivatives as antitumor agents. Russ. J. Bioorg. Chem. 2020, 46, 409–416. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, Z.; Yang, R.; Qian, T.; Zhou, Q. Naphthyl quinoxaline thymidine conjugate is a potent anticancer agent post UVA activation and elicits marked inhibition of tumor growth through vaccination. Eur. J. Med. Chem. 2019, 1, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Wang, P.; Zhang, Z.; Xue, G.; Zha, D.; Wang, J.; Xu, X. Facile synthesis and anti-proliferative activity evaluation of quinoxaline derivatives. Synth. Commun. 2020, 50, 823–830. [Google Scholar]
- Li, G.Z.; Ouyang, X.L.; Mo, Z.Y.; Ju, Y.; Cao, X.L.; Yang, L.; Tang, H.T.; Pan, Y.M. Synthesis and biological evaluation of novel 1,3-diphenylurea quinoxaline derivatives as potent anticancer agents. Med. Chem. Res. 2021, 30, 1496–1511. [Google Scholar] [CrossRef]
- Patinote, C.; Masquefa, D.C.; Kaddour, H.K.; Vincent, A.L.; Larive, R.; Zghaib, Z.; Guichou, F.J.; Assaf, D.M.; Cuq, P.; Bonnet, A.P. Imidazo[1,2-a]quinoxalines for melanoma treatment with original mechanism of action. Eur. J. Med. Chem. 2021, 212, 113031. [Google Scholar] [CrossRef] [PubMed]
- Abdullahi, H.S.; Uzairu, A.; Danazumi, U.A.; Finbarrs-Bello, E.; Umar, B.A.; Shallangwa, A.G.; Uba, S. Computational design of quinoxaline molecules as VEGFR-2 inhibitors: QSAR modelling, pharmacokinetics, molecular docking, and dynamics simulation studies. Biocatal. Agric. Biotechnol. 2023, 51, 102787. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).