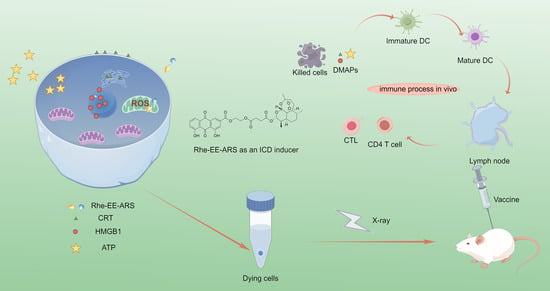
The Conjugate of Rhein–Artesunate for Inducing Immunogenic Cell Death to Prepare Cancer Vaccine and Suppress Tumor Growth
Abstract
:1. Introduction
2. Materials and Methods
3. Results
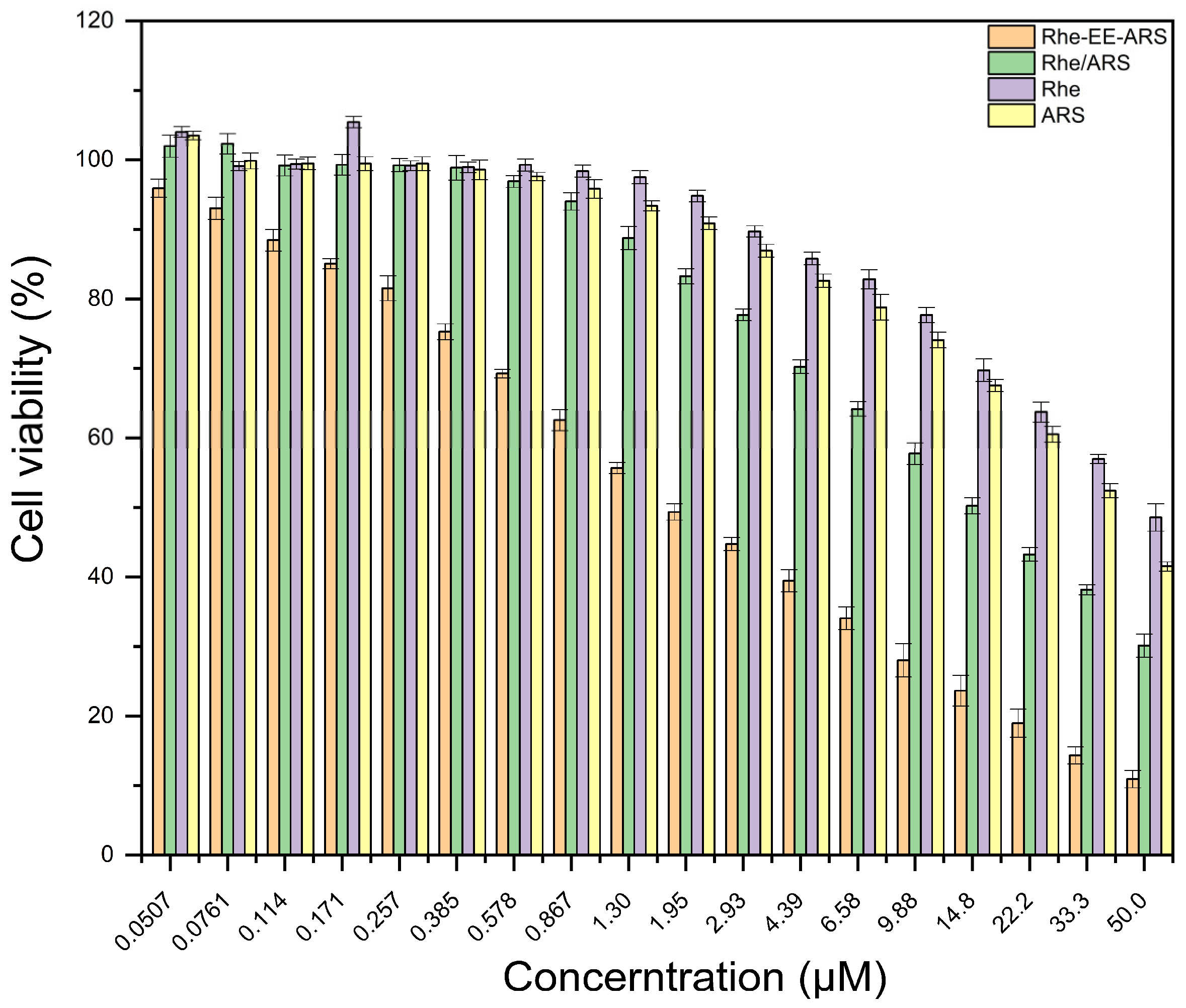
3.1. Cell Viability Test In Vitro
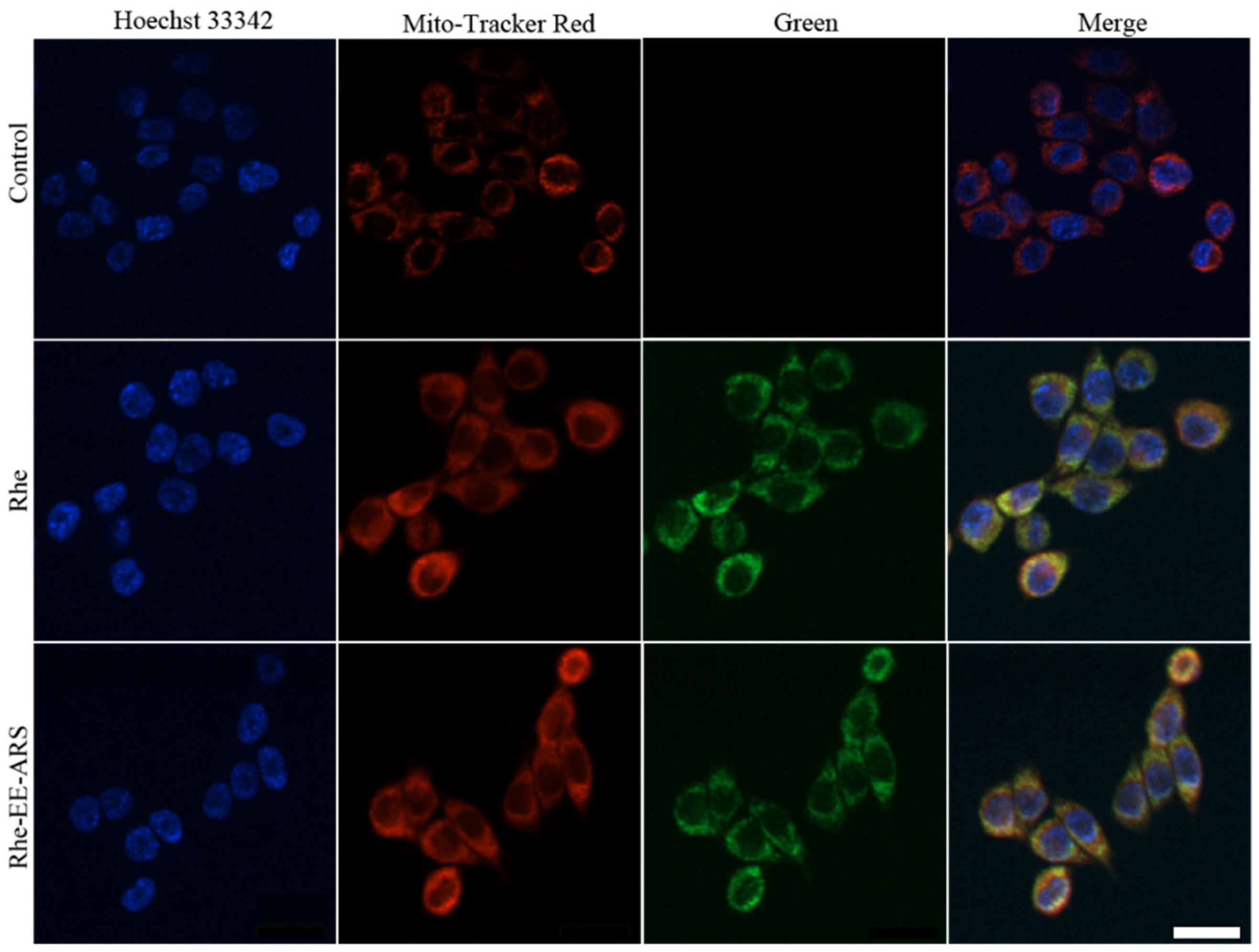
3.2. Mitochondrial Colocalization
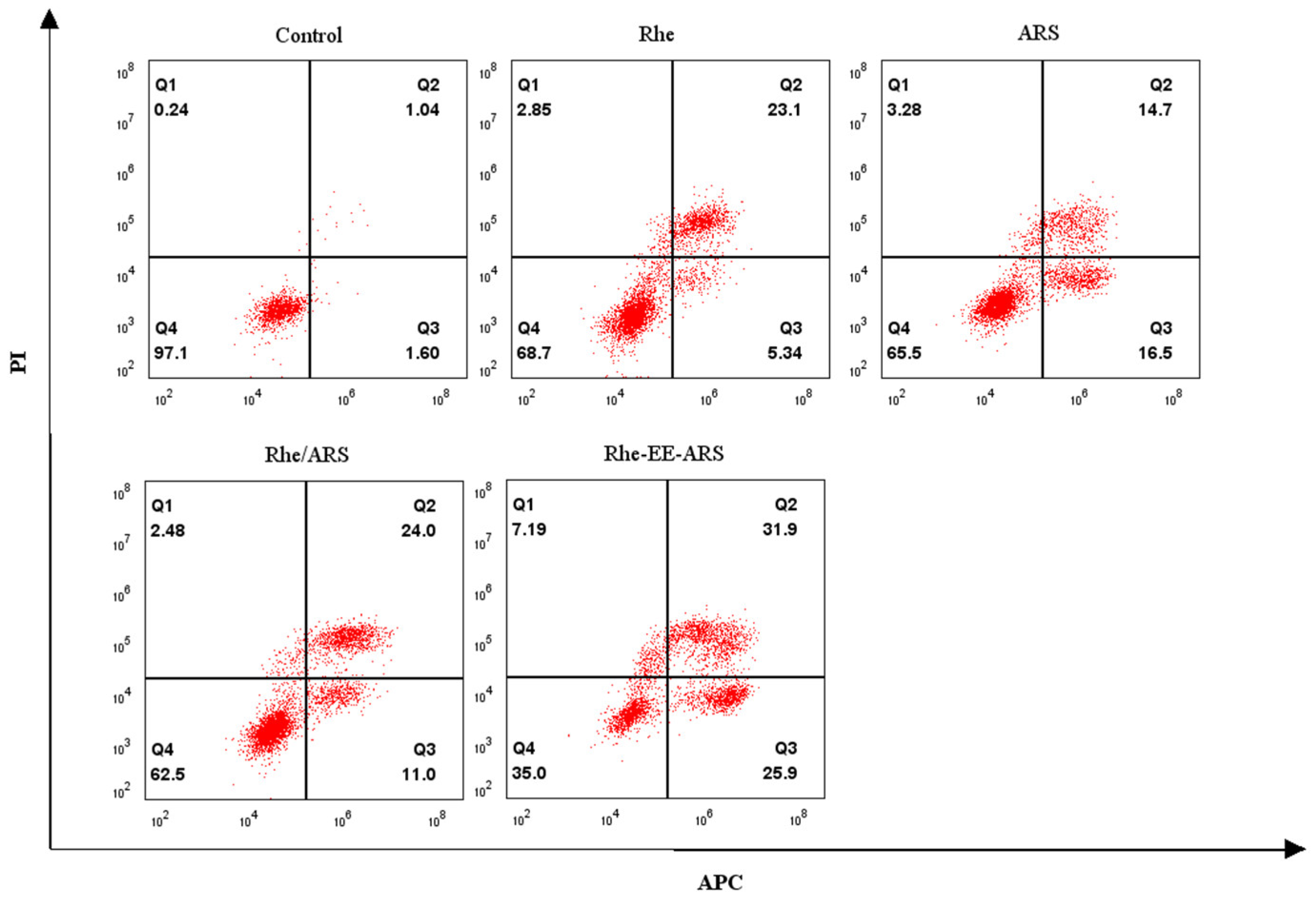
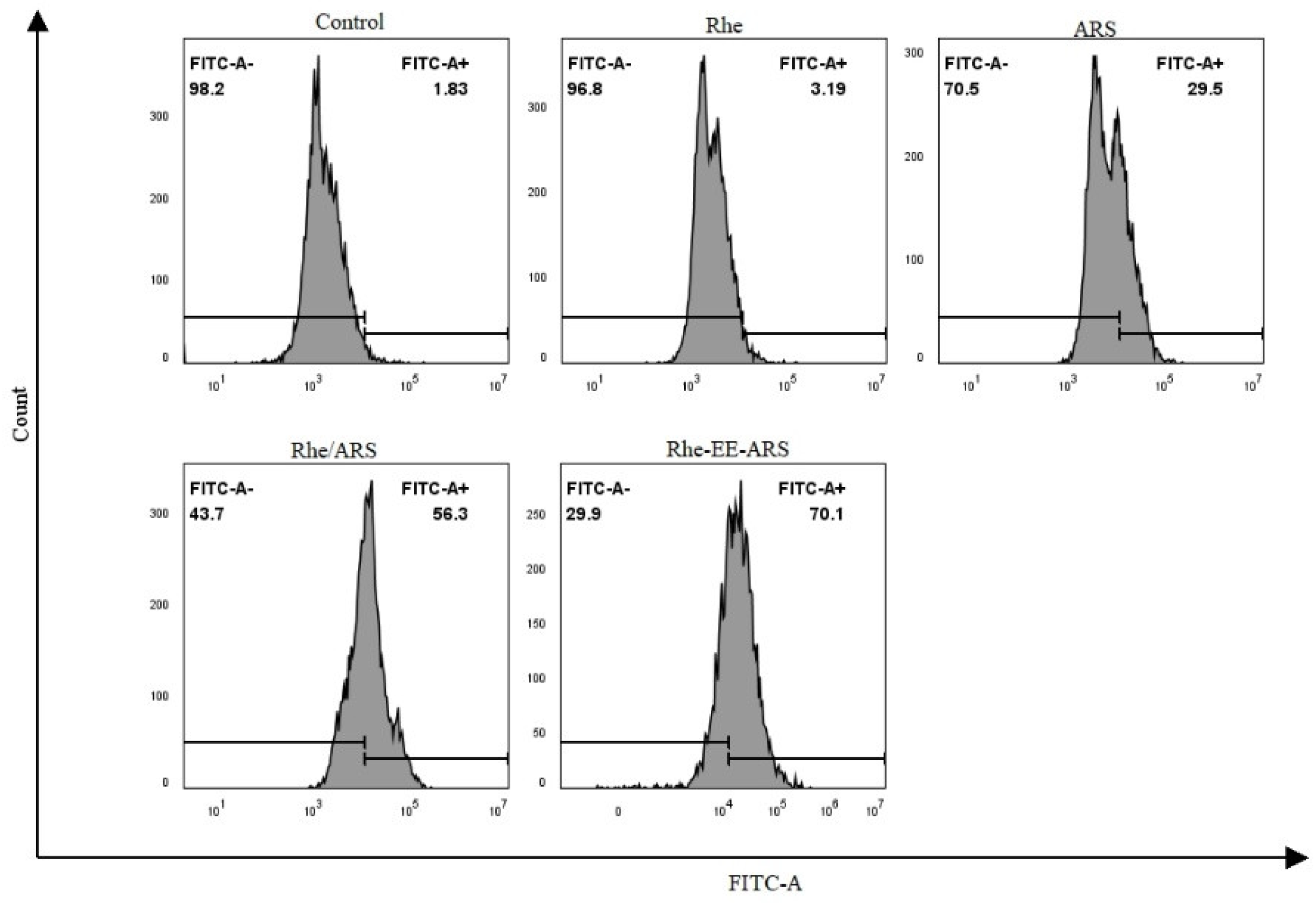
3.3. Detection of Calreticulin
3.4. Analysis of Reactive Oxygen Species Generation
3.5. Evaluation of Mitochondrial Depolarization
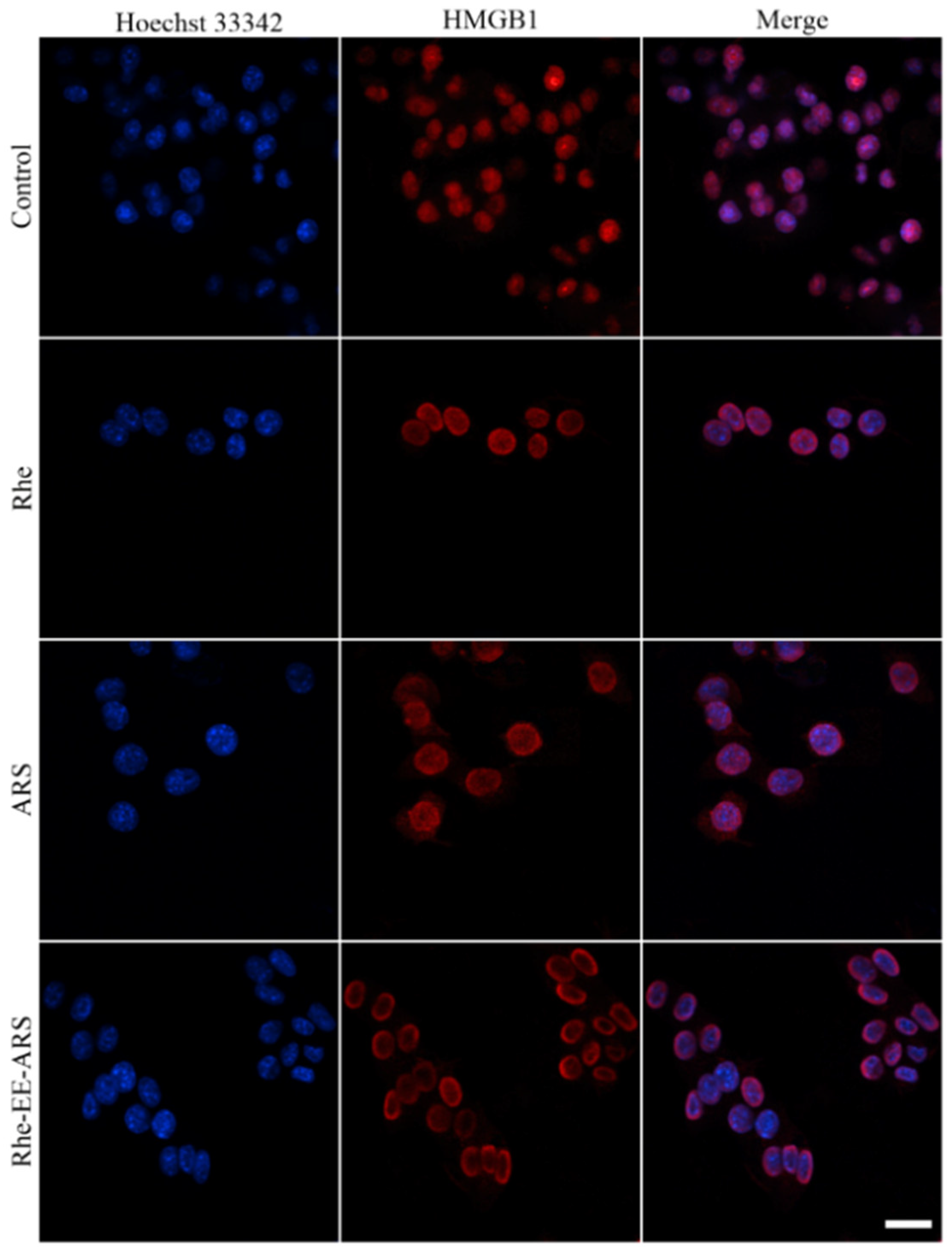
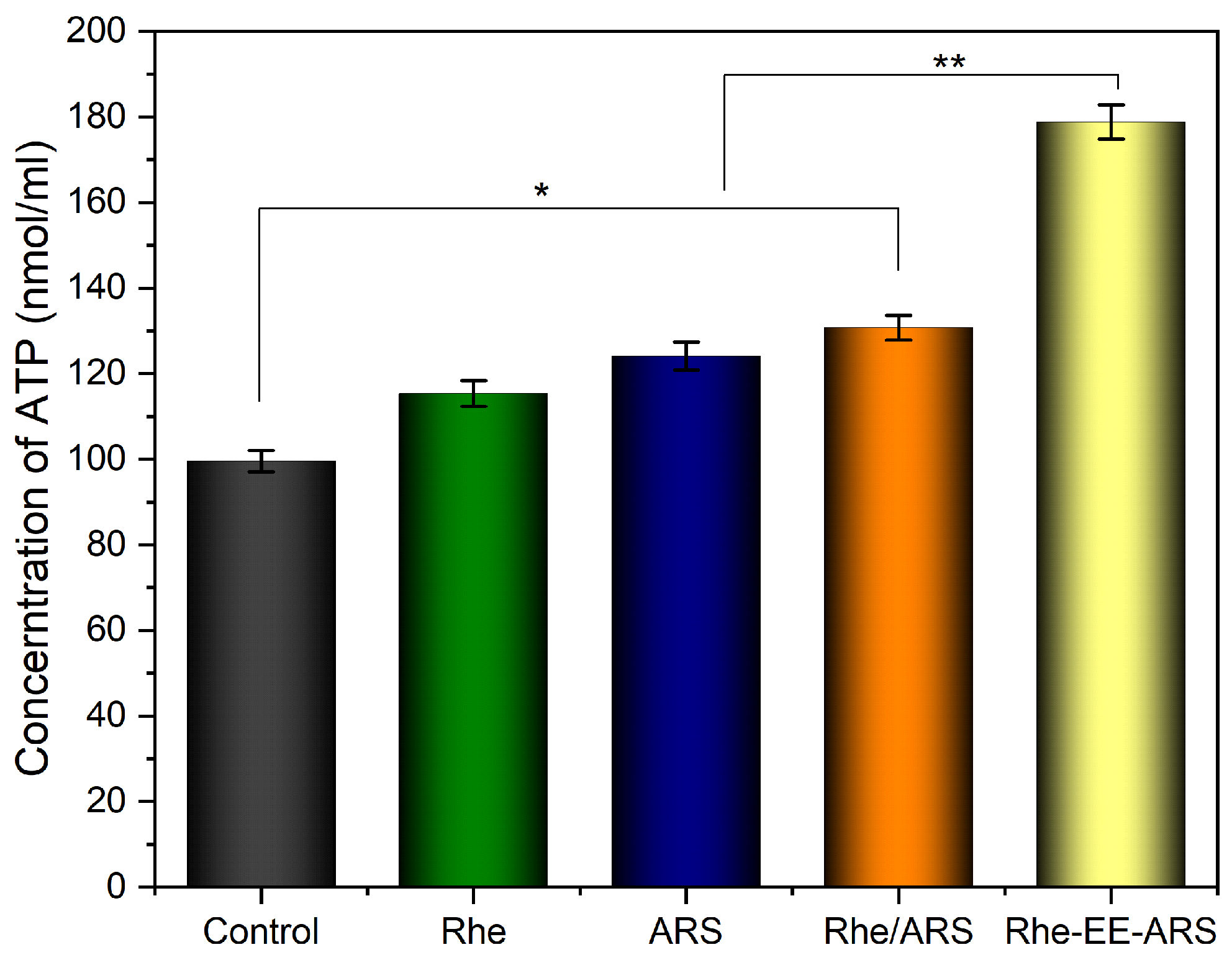
3.6. Detection of ATP and HMGB1
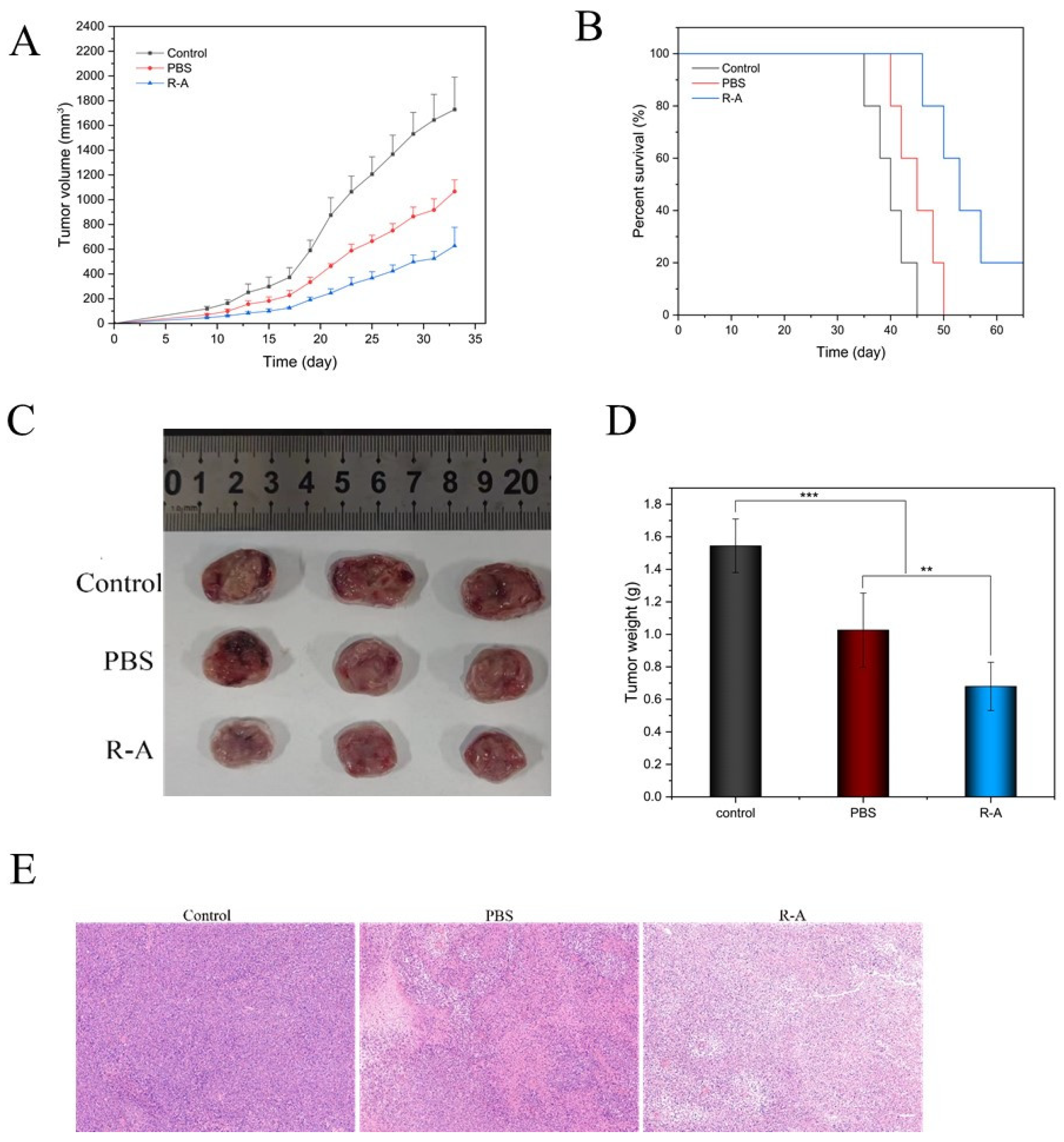
3.7. Tumor Prevention Effect
3.8. Immune Response In Vivo
3.9. Biosafety of Vaccines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Casares, N.; Pequignot, M.O.; Tesniere, A.; Ghiringhelli, F.; Roux, S.; Chaput, N.; Schmitt, E.; Hamai, A.; Hervas-Stubbs, S.; Obeid, M.; et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J. Exp. Med. 2005, 202, 1691–1701. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Jiang, X. Advances in immunogenic cell death for cancer immunotherapy. Small Methods 2023, 7, 2300354. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Dudek-Peric, A.; Romano, E.; Agostinis, P. Immunogenic cell death. Int. J. Dev. Biol. 2015, 59, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Krysko, D.V.; Garg, A.D.; Kaczmarek, A.; Krysko, O.; Agostinis, P.; Vandenabeele, P. Immunogenic cell death and DAMPs in cancer therapy. Nat. Rev. Cancer 2012, 12, 860–875. [Google Scholar] [CrossRef] [PubMed]
- Schcolnik-Cabrera, A.; Oldak, B.; Juárez, M.; Cruz-Rivera, M.; Flisser, A.; Mendlovic, F. Calreticulin in phagocytosis and cancer: Opposite roles in immune response outcomes. Apoptosis 2019, 24, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.C.; Kang, R.; Tang, D.L. The mechanism of HMGB1 secretion and release. Exp. Mol. Med. 2022, 54, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.L.; Liu, Z.P.; Xu, Z.R.; Liu, J.; Zhang, J. High mobility group box 1 (HMGB1): A pivotal regulator of hematopoietic malignancies. J. Hematol. Oncol. 2020, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Hernández, Á.P.; Juanes-Velasco, P.; Landeira-Viñuela, A.; Bareke, H.; Montalvillo, E.; Góngora, R.; Fuentes, M. Restoring the immunity in the tumor microenvironment: Insights into immunogenic cell death in onco-therapies. Cancers 2021, 13, 2821. [Google Scholar] [CrossRef] [PubMed]
- Zapletal, E.; Vasiljevic, T.; Busson, P.; Glavan, T.M. Dialog beyond the grave: Necrosis in the tumor microenvironment and its contribution to tumor growth. Int. J. Mol. Sci. 2023, 24, 5278. [Google Scholar] [CrossRef]
- Li, Y.H.; Liu, X.H.; Zhang, X.; Pan, W.; Li, N.; Tang, B. Immunogenic cell death inducers for enhanced cancer immunotherapy. Chem. Commun. 2021, 57, 12087–12097. [Google Scholar] [CrossRef]
- Zhu, M.Q.; Yang, M.D.; Zhang, J.J.; Yin, Y.Z.; Fan, X.; Zhang, Y.; Qin, S.S.; Zhang, H.; Yu, F. Immunogenic cell death induction by ionizing radiation. Front. Immunol. 2021, 12, 9. [Google Scholar] [CrossRef]
- Decraene, B.; Yang, Y.H.; De Smet, F.; Garg, A.D.; Agostinis, P.; De Vleeschouwer, S. Immunogenic cell death and its therapeutic or prognostic potential in high-grade glioma. Genes Immun. 2022, 23, 244. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Yao, F.; Zhao, J.; Zhang, W.; Chen, L.; Wang, X.; Yang, P.; Tang, J.; Chi, Y. Unraveling mitochondria-targeting reactive oxygen species modulation and their implementations in cancer therapy by nanomaterials. Exploration 2023, 3, 20220115. [Google Scholar]
- Li, X.; Fang, P.; Mai, J.; Choi, E.T.; Wang, H.; Yang, X.F. Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers. J. Hematol. Oncol. 2013, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; An, Y.H.; Ren, M.L.; Wang, H.J.; Bai, J.; Du, W.L.; Kong, D.Z. The mechanisms of action of mitochondrial targeting agents in cancer: Inhibiting oxidative phosphorylation and inducing apoptosis. Front. Pharmacol. 2023, 14, 16. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, V.K.; Rubel, M.A.; Schurr, T.G. Mitochondrial genetic variation in human bioenergetics, adaptation, and adult disease. Am. J. Hum. Biol. 2022, 34, 25. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Xie, F.; Ma, S.W.; Ma, C.; Jiang, X.; Yi, Y.; Song, Y.F.; Liu, M.Y.; Zhao, P.X.; Ma, X.M. Mitochondria: One of the vital hubs for molecular hydrogen’s biological functions. Front. Cell Dev. Biol. 2023, 11, 15. [Google Scholar] [CrossRef]
- Zhang, Y.; Yao, J.Y.; Zhang, M.M.; Wang, Y.S.; Shi, X.J. Mitochondria-associated endoplasmic reticulum membranes (MAMs): Possible therapeutic targets in heart failure. Front. Cardiovasc. Med. 2023, 10, 7. [Google Scholar] [CrossRef]
- Yang, M.; Li, C.R.; Yang, S.K.; Xiao, Y.; Xiong, X.F.; Chen, W.; Zhao, H.; Zhang, Q.; Han, Y.C.; Sun, L. Mitochondria-associated er membranes—The origin site of autophagy. Front. Cell Dev. Biol. 2020, 8, 11. [Google Scholar] [CrossRef]
- Chang, A.Y.; Marshall, W.F. Organelles—Understanding noise and heterogeneity in cell biology at an intermediate scale. J. Cell Sci. 2017, 130, 819–826. [Google Scholar] [CrossRef]
- Woldemichael, T.; Rosania, G.R. The physiological determinants of drug induced lysosomal stress resistance. PLoS ONE 2017, 12, 22. [Google Scholar] [CrossRef]
- Vaidziulyte, K.; Coppey, M.; Schauer, K. Intracellular organization in cell polarity—Placing organelles into the polarity loop. J. Cell Sci. 2019, 132, 9. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Zhang, X.F.; Yang, X.; Li, Y.; Xiang, J.J.; Xiang, C.B.; Liu, Z.K.; Hai, L.; Huang, S.P.; Zhou, L.H.; et al. A mitochondria-targeting self-assembled carrier-free lonidamine nanodrug for redox-activated drug release to enhance cancer chemotherapy. J. Mater. Chem. B 2023, 11, 3951–3957. [Google Scholar] [CrossRef]
- Zeng, Z.; Fang, C.; Zhang, Y.; Chen, C.X.; Zhang, Y.F.; Zhang, K. Mitochondria-targeted nanocarriers promote highly efficient cancer therapy: A review. Front. Bioeng. Biotechnol. 2021, 9, 12. [Google Scholar] [CrossRef]
- Tu, Y.Y. Artemisinin-A gift from traditional Chinese medicine to the world (Nobel lecture). Angew. Chem. Int. Ed. 2016, 55, 10210–10226. [Google Scholar] [CrossRef] [PubMed]
- Adebayo, J.O.; Tijjani, H.; Adegunloye, A.P.; Ishola, A.A.; Balogun, E.A.; Malomo, S.O. Enhancing the antimalarial activity of artesunate. Parasitol. Res. 2020, 119, 2749–2764. [Google Scholar] [CrossRef]
- Khanal, P. Antimalarial and anticancer properties of artesunate and other artemisinins: Current development. Mon. Chem. 2021, 152, 387–400. [Google Scholar] [CrossRef]
- Xie, D.Q.; Wang, Q.F.; Wu, G.Z. Research progress in inducing immunogenic cell death of tumor cells. Front. Immunol. 2022, 13, 27. [Google Scholar] [CrossRef]
- Catanzaro, E.; Feron, O.; Skirtach, A.G.; Krysko, D.V. Immunogenic cell death and role of nanomaterials serving as therapeutic vaccine for personalized cancer immunotherapy. Front. Immunol. 2022, 13, 18. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Duan, Y.B.; Yu, J.; Chen, J.Q.; Chao, M.; Ji, M. Bone-targeting glycol and NSAIDS ester prodrugs of rhein: Synthesis, hydroxyapatite affinity, stability, anti-inflammatory, ulcerogenicity index and pharmacokinetics studies. Eur. J. Med. Chem. 2012, 55, 409–419. [Google Scholar] [CrossRef]
- Kim, D.Y.; Pyo, A.; Yun, M.; Thangam, R.; You, S.H.; Zhang, Y.; Jung, Y.R.; Nguyen, D.H.; Venu, A.; Kim, H.S.; et al. Imaging calreticulin for early detection of immunogenic cell death during anticancer treatment. J. Nucl. Med. 2021, 62, 37. [Google Scholar] [CrossRef]
- Liu, C.; Leclair, P.; Pedari, F.; Monajemi, M.; Sly, L.; Reid, G.R.; Lim, C. Integrin activity reduces immunogenic cell death by inhibiting cell surface presentation of ERp57 and calreticulin. Mol. Biol. Cell 2018, 29, 1. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Kumar, N.V.A.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Fokou, P.V.T.; Azzini, E.; Peluso, I.; et al. Lifestyle, oxidative stress, and antioxidants: Back and forth in the pathophysiology of chronic diseases. Front. Physiol. 2020, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, X.; Liu, Y.P.; Zhang, Z.H. Effects of exercise-induced ROS on the pathophysiological functions of skeletal muscle. Oxidative Med. Cell Longev. 2021, 2021, 5. [Google Scholar] [CrossRef]
- Sinenko, S.A.; Starkova, T.Y.; Kuzmin, A.A.; Tomilin, A.N. Physiological signaling functions of reactive oxygen species in stem cells: From flies to man. Front. Cell Dev. Biol. 2021, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, D.; Guo, F.; Xuan, C. Mitochondrial membrane potential and reactive oxygen species in cancer stem cells. Familial Cancer 2015, 14, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Joshi, D.C.; Bakowska, J.C. Determination of mitochondrial membrane potential and reactive oxygen species in live rat cortical neurons. J. Vis. Exp. 2011, 51, 2704. [Google Scholar]
- Sivandzade, F.; Bhalerao, A.; Cucullo, L. Analysis of the mitochondrial membrane potential using the cationic JC-1 dye as a sensitive fluorescent probe. Bio-Protocol 2019, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Mathur, A.; Hong, Y.; Kemp, B.K.; Barrientos, A.A.; Erusalimsky, J.D. Evaluation of fluorescent dyes for the detection of mitochondrial membrane potential changes in cultured cardiomyocytes. Cardiovasc. Res. 2000, 46, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Klune, J.R.; Dhupar, R.; Cardinal, J.; Billiar, T.R.; Tsung, A. HMGB1: Endogenous danger signaling. Mol. Med. 2008, 14, 476–484. [Google Scholar] [CrossRef]
- Bhatia, S.S.; Pillai, S.D. Ionizing radiation technologies for vaccine development-A mini review. Front. Immunol. 2022, 13, 9. [Google Scholar] [CrossRef]
- Wijewardana, V.; Ulbert, S.; Cattoli, G. Editorial: Irradiation technologies for vaccine development. Front. Immunol. 2022, 13, 1075335. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.R.A.; O’Koren, E.G.; Hotten, D.F.; Kan, M.J.; Kopin, D.; Nelson, E.R.; Que, L.; Gunn, M.D. A protocol for the comprehensive flow cytometric analysis of immune cells in normal and inflamed murine non-lymphoid tissues. PLoS ONE 2016, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, L.; Vina, E.; Asrir, A.; Tardiveau, C.; Coudert, J.; Laffont, R.; Tarroux, D.; Bettini, S.; Veerman, K.; Lafouresse, F.; et al. Flow cytometry analysis of endothelial cells and subsets of exhausted CD8+ T cells in murine tumor models. Star Protoc. 2022, 3, 19. [Google Scholar] [CrossRef] [PubMed]
- Manhas, K.R.; Blattman, J.N. Flow cytometry analysis of immune cell responses. Methods Mol. Biol. 2023, 2597, 105–120. [Google Scholar] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.-J.; Wang, W.; Huang, S.-W. The Conjugate of Rhein–Artesunate for Inducing Immunogenic Cell Death to Prepare Cancer Vaccine and Suppress Tumor Growth. Chemistry 2024, 6, 345-360. https://doi.org/10.3390/chemistry6030020
Xu Z-J, Wang W, Huang S-W. The Conjugate of Rhein–Artesunate for Inducing Immunogenic Cell Death to Prepare Cancer Vaccine and Suppress Tumor Growth. Chemistry. 2024; 6(3):345-360. https://doi.org/10.3390/chemistry6030020
Chicago/Turabian StyleXu, Zi-Jian, Wei Wang, and Shi-Wen Huang. 2024. "The Conjugate of Rhein–Artesunate for Inducing Immunogenic Cell Death to Prepare Cancer Vaccine and Suppress Tumor Growth" Chemistry 6, no. 3: 345-360. https://doi.org/10.3390/chemistry6030020
APA StyleXu, Z. -J., Wang, W., & Huang, S. -W. (2024). The Conjugate of Rhein–Artesunate for Inducing Immunogenic Cell Death to Prepare Cancer Vaccine and Suppress Tumor Growth. Chemistry, 6(3), 345-360. https://doi.org/10.3390/chemistry6030020