Abstract
Applied electrochemistry (AE) plays today an important role in a wide range of fields, including energy conversion and storage, processes, environment, (bio)analytical chemistry, and many others. Electrochemical synthesis is now proven as a promising pathway to avoid all disadvantages in terms of high energy consumption and high pollution, while electrochemical modeling becomes a powerful tool to understand complex systems and predict and optimize the electrochemical devices under various conditions, which reduce study time and cost. The vital role of electrochemistry will greatly be considered in the upcoming years, aiming to reduce carbon footprints and supporting the transition towards a green and more sustainable energy framework. This review article summarizes the recent advances in applied electrochemistry. It shows how this field has become an indispensable tool for innovation, progress, problem-solving in the modern world, and addressing societal challenges across diverse fields.
1. Introduction
Applied electrochemistry (AE) is a leading modern science that addresses societal challenges across diverse fields, including energy conversion and storage, processes, environment, (bio)analytical chemistry, and many others [1,2,3]. In the energy sector, electrochemical processes are used for energy conversion and storage. This enables the development of productive and sustainable technologies, such as batteries [4,5], fuel cells [6,7], and electrolyzes [8,9]. Therefore, these advancements help in the integration of renewable energy sources and support the transition towards green and more sustainable energy sources.
In the environmental field, electrochemistry provides novel solutions for pollution control and water treatment. Electrochemical processes, such as direct and indirect electrochemical oxidation processes and advanced oxidation processes, are used for the degradation of organic pollutants [10], removal of heavy metals [11,12,13], and enhancement of effluents’ biodegradability [14,15]. These technologies offer efficient and cost-effective approaches to address environmental challenges, protect ecosystems, and improve water quality.
In the manufacturing sector, electrochemistry plays a vital role in electrosynthesis, materials manufacturing, and surface modification. Electrochemical techniques, such as electroplating [16], electroforming [17], and electrochemical machining [18], are used to produce functional and protective coatings and enhance the performances of materials and components. These processes allow for the production of high-quality products with improved material properties and high durability. For example, the electrochemical treatments of material surfaces in metallurgical industries aim, first, to create porous materials that have a higher geometric surface area and, second, to enable the creation of a thick layer of metal oxide that protects and stabilizes the nanostructure of the metal or the semiconductor [19,20].
Additionally, electroanalysis is employed to measure antioxidant activity [21,22], for analysis [23,24], and to characterize various compounds and materials [25,26]. Typically, these methods rely on methods such as voltammetry [27], amperometry [28], potentiometry [29], and impedance spectroscopy. Electrochemical sensors and biosensors are used for the detection of biomolecules [30], pollution in the environment [31,32,33], pharmaceuticals [34,35], and clinical diagnostics [36]. These devices provide various advantages (cheap technologies and rapid and sensitive devices) that make them valuable tools for research, quality control, and the real-time monitoring of processes.
On the other hand, the European Union (EU) has set goals regarding the neutrality of the climate [37] and adopted in April 2021 the European climate law [38]. This is central to making the EU’s economy sustainable and reducing its environmental footprint [39,40]. This aims to address many changes and incorporate a wide range of initiatives that aim at transforming key sectors, such as energy [41,42], industry [43,44], transport [45], and agriculture [46], to reach climate neutrality and push towards a more sustainable economy. In this context, electrochemistry emerges as a keystone for industry decarbonization and transitioning towards sustainable manufacturing processes, reducing carbon footprints [47,48]. This includes the use of clean, renewable, and more sustainable energy sources instead of thermal energy sources via electrification powered by low-carbon electricity sources [49,50] and the use of fuel cells (for hydrogen production) [51,52]. Carbon dioxide capture offers a pathway for industries to mitigate CO2 emissions and simultaneously produce value-added products (e.g., methane production) [53,54].
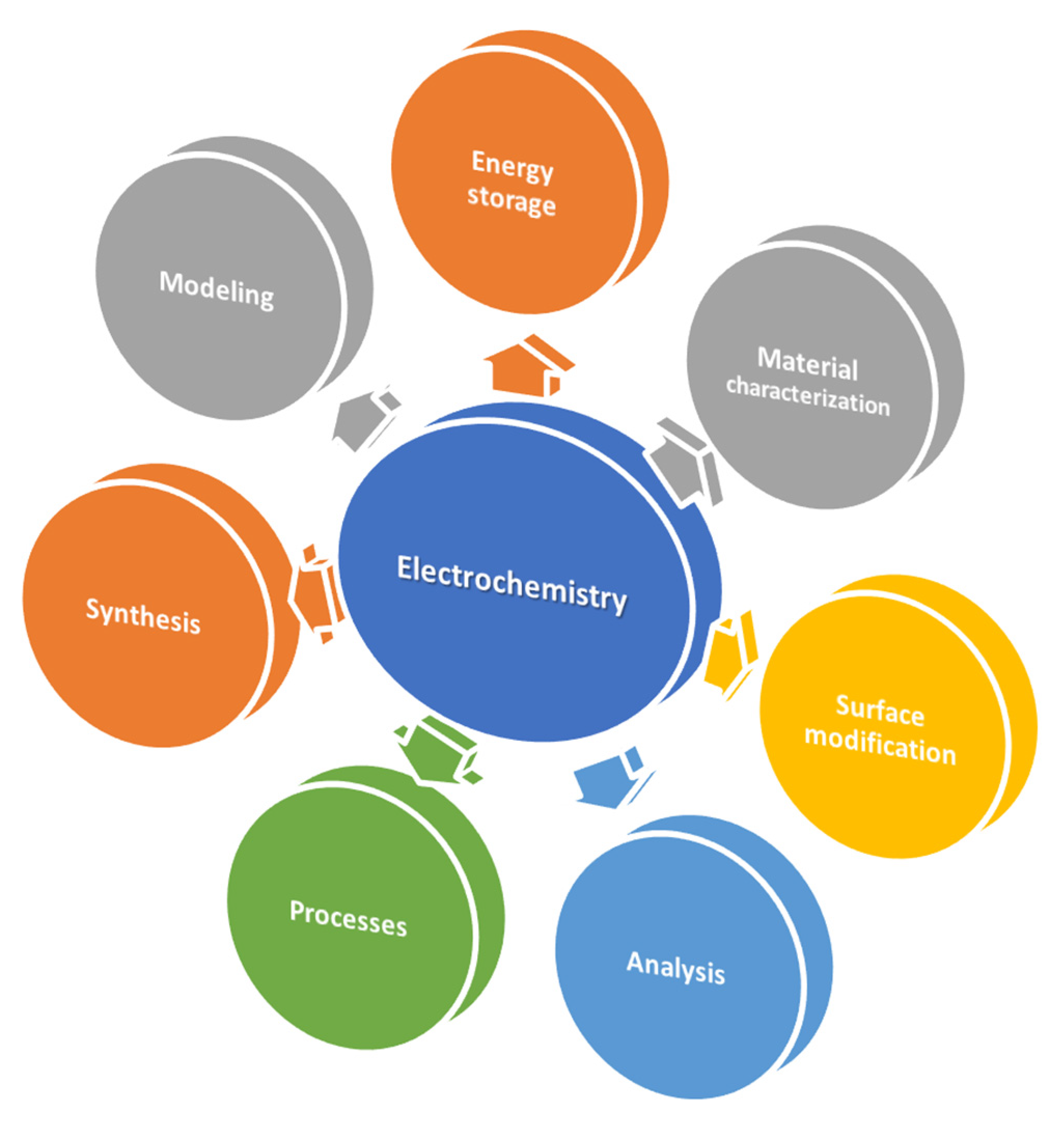
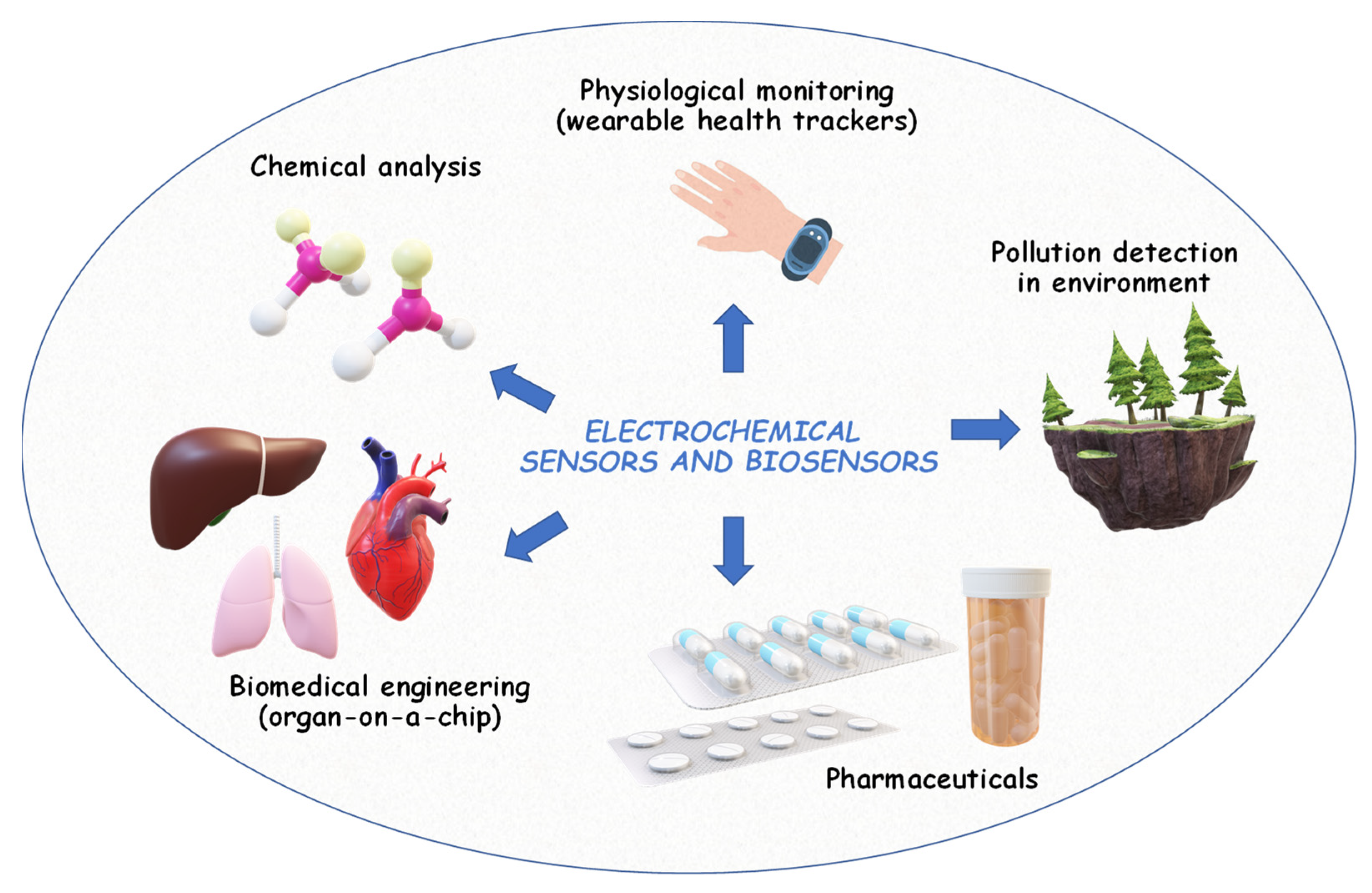
This review article summarizes the recent advances in applied electrochemistry. It shows how this field has become an indispensable tool for innovation, progress, and problem-solving in the modern world and addresses societal challenges across diverse fields (Figure 1).
Figure 1.
Main application of electrochemistry.
2. Application Field
2.1. Energy Conversion and Storage
Energy surrounds us all the time, fueling our activities day and night. We often take for granted the convenience of accessing energy to power our gadgets, appliances, machines, and vehicles. However, it is crucial to think about how we store this energy for use [55]. Unlike fossil fuels, which can be easily stored and transported in their natural state, renewable energy sources, such as sunlight and wind, require an intermediary storage method due to their intermittent nature. As a result, batteries are considered to be the only solution for storing this energy, so that it can be used when needed, providing a crucial bridge between energy generation and consumption [56,57].
There are two types of batteries, namely those storing energy for a single use, like non-rechargeable batteries, and those for multiple uses, exemplified by rechargeable batteries. Our focus is on batteries known for their ability to store and release energy repeatedly, leading to cost-effectiveness and eco-friendliness across various applications [58]. The batteries’ applications fall into three primary categories: transportation and automotive, including electric vehicles (EVs); portable electronics; and stationary power storage, with each type demanding unique specifications. Table 1 summarizes the different types of commercial batteries used for energy storage and their main applications, advantages, and disadvantages.
In fact, the progress of EVs heavily depends on the improvement of battery technology, which encounters various obstacles, such as underdeveloped batteries and challenges in practical applications [59]. Lead acid batteries (Pb-A) have historically dominated the rechargeable battery market, particularly within the automotive sector, owing to their significant market share in terms of sales value and energy production. However, Pb-A batteries come with inherent limitations, including a relatively short cycle life, low energy density, susceptibility to acid stratification and leakage if damaged, and challenges in downsizing due to concerns related to lead production. Moreover, the environmental impacts associated with lead acid batteries are well-documented [60,61], necessitating extensive recycling efforts to mitigate their adverse effects. Consequently, lithium-ion batteries (LIBs) have emerged as a promising alternative, gaining traction due to their numerous advantages, including the high storage efficiency of close to 100% and offering a diverse range of chemistries, making them suitable for various sustainable applications [62]. In the most common designs of LIBs, the cells consist of a negative electrode called an anode and a positive electrode called a cathode separated by an isolating separator and surrounded by an electrolyte. During discharge, lithium ions are transported from the anode, through the separator to the cathode and bound to the active material. Simultaneously, electrons are released and conducted via an external circuit from the anode to the cathode. When charging the LIB, the movements of lithium ions and electrons are reversed by a connected power supply [63].
In recent years, there has been a notable focus on advancing the development of cathodes to be more sustainable and safer. These efforts have resulted in the commercialization of different cathode materials in the EV market, including LiNixCoyAl1−x−yO2 (NCA), LiMn2O4 (LMO), LiNi0.5Mn1.5O4 (LNMO) [64], LiFePO4 (LFP), and LiNixMnyCo1−x−yO2 (NMC)-based batteries. Each of these materials offers its own set of advantages over the others [65]. NCA, LFP, and NMC-based batteries are prominently utilized in electric vehicles produced by companies like BMW, Chevrolet, Nissan, Tesla, etc. [66]. While Li-air and Li-S batteries have been manufactured, they are not yet ready for car applications. However, sodium-ion batteries are emerging as a potential alternative to lithium-ion batteries.
On the other hand, personal devices, including smartphones, laptops, tablets, cameras, and wearable technology, heavily depend on energy storage to function effectively within compact designs. Batteries serve as the primary power source for these devices, requiring relatively small storage capacities in limited volumes and lightweight formats. Among the battery types, LIBs, especially those utilizing LMO and LiCoO2, stand out as particularly well-suited for small-scale electronics. They serve as the primary power cathode in a variety of devices, from smartphones and computers to power tools. Moreover, the utility of LIBs extends beyond powering portable electronics and transportation. They now play vital roles in supporting the electricity grid. This expansion enables the integration of variable renewable energy sources, ultimately improving efficiency in transmission and distribution systems [67].


Table 1.
Summary of the energy-storage devices (batteries), with their applications, advantages, and disadvantages.
Table 1.
Summary of the energy-storage devices (batteries), with their applications, advantages, and disadvantages.
| Type of Battery | Chemistry of Battery | Main Application | Specific Energy (Wh/Kg) | Advantages | Disadvantages |
|---|---|---|---|---|---|
| Lead Acid [58] | Pb-A | Automotive industry (EV) and industrial use | 30–50 | - Low cost - Well-established manufacturing processes and widely available - High-rate performance in various conditions | - Limited energy density - Short cycle life (500–1000 cycles) - Maintenance requirements involving issues such as acid stratification and leakage -Environmental concerns associated with the use of lead (heavy metal) - Limited use in applications where weight is a concern |
| Lithium-Ion batteries (LIBs) (Many possible chemistries), [63,65] LIBs have a higher energy density allowing for lighter-weight designs, higher stability, and durability compared to Pb-A. Sensitive to Low temperatures, but improvements ongoing, and becoming more cost-competitive with advancements. | Lithium Manganese oxide (LMO) | Portable electronics (e.g., smartphones, laptops)—Power tools | 100–150 | - Lower cost - Improved environmental friendliness - Better thermal stability and safety compared to LCO, making them suitable for some electric vehicles | - Lower energy density - Reduced cycle life (300–700 cycles) |
| Lithium Cobalt oxide (LCO) | 150–250 | -Higher energy density. -Longer cycle life (500–1000 cycles) | - Higher cost - Environmental concerns due to cobalt mining | ||
| Lithium Iron phosphate (LFP) | Power tools and EV | 90–120 | - Safest - Better Thermal stability | - Lowest energy density among the three cathodes | |
| Lithium Nickel Cobalt Aluminum oxide (NCA) | Grid storage and EV | 155–260 | - Highest Energy density | - Lower thermal stability - Battery thermal Management Systems (BTMS) requirements | |
| Lithium Nickel Manganese Cobalt Oxide (NMC) | Power tools, EV | 150–200 | - Moderate energy density and safety | - BTMS requirements | |
| Polymer-Based Batteries, [68,69] Company: Evonik - Employ unique processing techniques such as printing | Redox active polymers for either the cathode, anode, or both electrodes. | Medical and Logistics fields | 50–200 | - Fabrication of thin and flexible batteries - High-rate capability - Metal free and Recyclability | - Limitations in terms of discharge capacities and voltage outputs - Restricted applicability to low-power systems |
| Biofuel [70,71,72,73,74] | Enzymatic Fuel cells | Ideal for wearables and implants: - Compact - Integrable - Biosafe | 20–300 | - Inexpensive - Lightweight - Flexible - Eco-friendly - Biodegradable - Capable of generating electricity from various kinds of organic matters | - Low energy density - Short-time stability (decreasing power output over time) |
| Microbial Fuel cells | Focused on macro-scale power generation from surroundings - Suitable for Minimal power demand applications (ex: wastewater treatment) |
An alternative strategy on the verge of commercialization, in addition to cathode development, involves transitioning from graphite anodes to silicon anodes. Silicon, an abundant, non-toxic, and cost-effective material, offers significantly higher storage capacity. Initial attempts to create anodes solely from pure silicon were unsuccessful. However, a more promising approach, now further developed by the industry, involves incorporating porous or other carbon additives into the anode [75]. Currently, various manufacturers, such as Varta, Sila Nanotechnologies, Enovix Corporation, Gotion High-Tech, and others, are actively working on composite anodes with varying, yet increasing, proportions of silicon and/or SiOx or TiSi [76]. Substituting conventional electrodes like graphite or silicon with graphene can enhance battery stability and lifespan, while also providing higher energy density at a lower cost. However, the structural constraints of graphene limit battery size, thereby restricting the energy-storage capacity primarily to small devices, rendering them unsuitable for large battery packs, including those for EVs [77,78].
Current advancements in nanotechnology focus on the miniaturization of electronic devices to provide power on demand. The lithium ion-based micro/nano-batteries are excellent candidates for this purpose, which feature small size, light weight, high capacity, and long cycle life, and they also offer stability and safety, making them suitable for energy storage in microdevices and wearable applications [79]. In addition to the development of metal-based batteries, organic batteries, also known as polymer-based batteries, feature several advantages over their common metal-based counterparts: they do not contain any toxic and rare heavy metals, their organic raw materials can potentially be obtained from renewable resources, and at the end of the life cycle, they can be disposed of by incineration without toxic leftovers. In the early 2000s, different potential applications were explored for these batteries, which are more environmentally friendly. Nevertheless, these systems have not found commercial applications until today, with Evonik Industries currently at the forefront in supplying materials for printable polymer-based batteries, which can be used in thin and flexible devices [69,80]. Biobatteries based on biofuel cells are also emerging as a net-zero CO2 emissions solution. While still in the development phase, these biobatteries provide clean, safe, durable, and efficient energy storage that aligns with future climate objectives. They use enzymes, organelles, or microorganisms as eco-friendly biocatalysts to convert chemical or biological energy into electrical energy, enabling sustainable power generation for portable, wearable, implantable, or ingestible devices, as well as offering long-term solutions for unattended environmental electronics [70,81]. The BeFC (Bioenzymatic Fuel Cells) company has developed the first cost-effective and efficient paper biofuel cells. These cells are metal-free, organic, recyclable, compostable, safe, and sustainable.
With the expected rise in battery demand by 2050, Verkor, headquartered in France, has launched a battery gigafactory. Their objective is to manufacture low-carbon, high-performance electric batteries, primarily LIBs, to support sustainable mobility efforts. This initiative aims to reduce reliance on Chinese battery manufacturers and create more job opportunities.
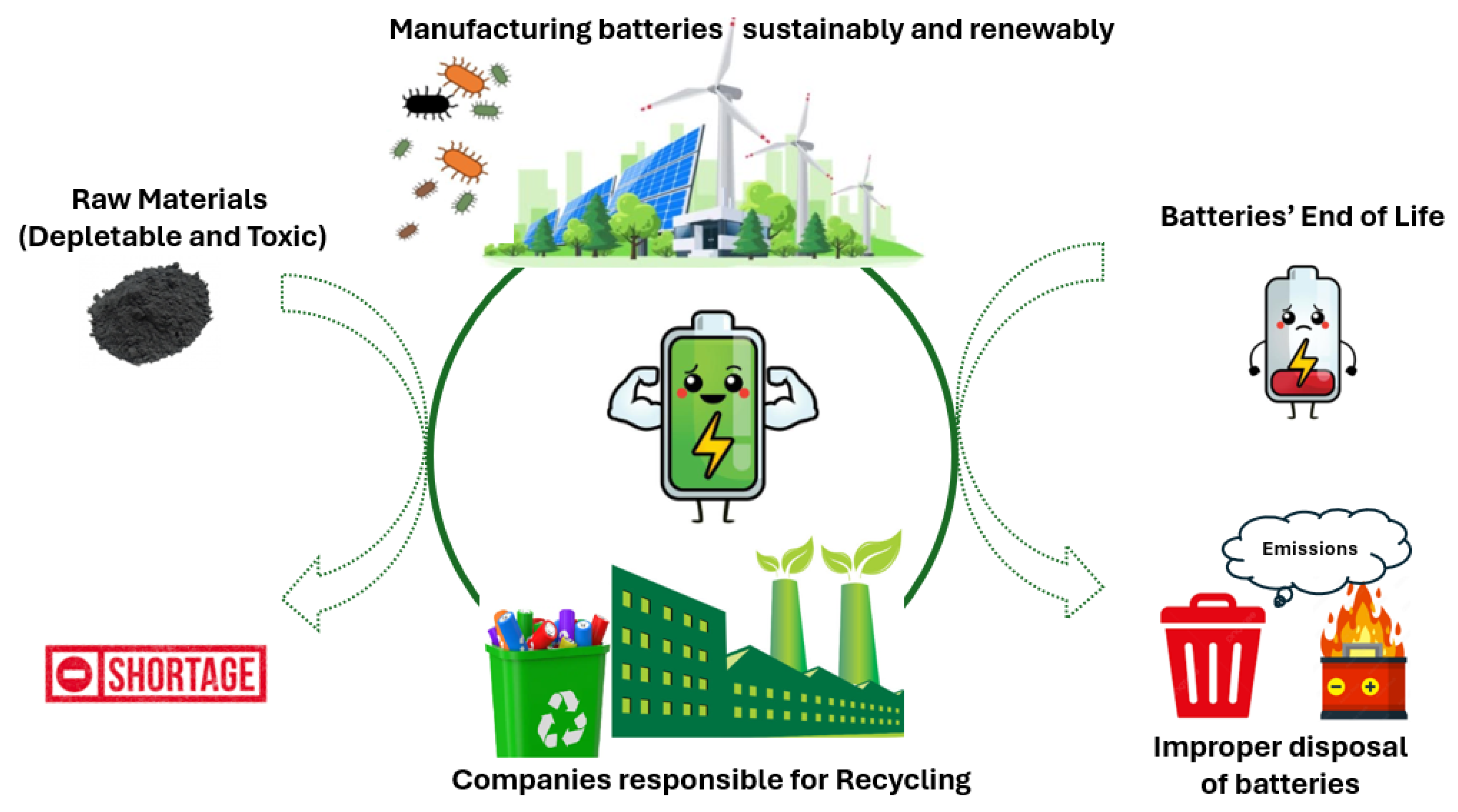
Apart from employing batteries with zero net CO2 emissions, battery recycling is crucial [58]. As most battery materials are recyclable, investing in sustainable practices is essential to mitigate environmental impact and meet growing energy needs. Recognizing this necessity, the Swedish battery company NorthVolt and its subsidiary Revolt, which handles recycling, have been established. These facilities are dedicated to not only producing high-quality batteries but also implementing comprehensive recycling strategies. From the initial selection of components (batteries devoid of Li or Co) to the end-of-life recycling process, these companies play a pivotal role in minimizing resource depletion and reducing carbon emissions associated with battery production and disposal (Figure 2).
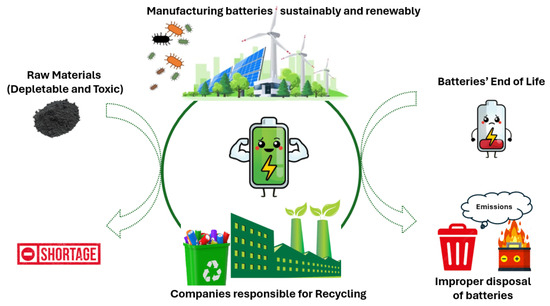
Figure 2.
Illustration of the fabrication of batteries using raw materials, such as Pb, Li, and Co, and their tendency for shortage in the long term (left-hand side), followed by their end-of-life phase after usage and improper disposal, which poses hazards (right-hand side). In the center, the trend among recent and future companies is to recycle batteries and manufacture new ones in a sustainable manner, utilizing renewable resources, such as solar energy, wind power, bacteria, and enzymes.
2.2. Material Characterization
Electrochemical characterization points to a range of techniques within the field of electrochemistry that are used to analyze and understand the properties and behaviors of materials or systems. These techniques include applying electrical stimuli and measuring the resulting electrical or electrochemical signals to achieve insights into various properties, such as conductivity, surface reactivity [82], corrosion resistance [83], catalytic activity [84], and ion transport [85]. Electrochemical characterization techniques are valuable tools for the analysis of both species in solution and solid states.
For species dissolved in a solution, common techniques, such as cyclic voltammetry, chronoamperometry, potentiometry, and impedance spectroscopy, are employed for the electrochemical characterization. However, for solid materials, cavity microelectrodes are typically used. To characterize the electroactive species, solids are inserted in the cavity of the microelectrode, and electrochemical characterization is performed. In this part, each technique is briefly described, and examples are given.
Cyclic voltammetry (CV) is a powerful and popular electrochemical method commonly used to explore the reduction and oxidation processes of molecular species. CV is also valuable for studying chemical reactions initiated by electron transfer, including catalysis, providing insights into catalytic processes, and facilitating the understanding of redox mechanisms in various systems [27]. Cyclic voltammetry characterizes electrochemical systems by measuring the current response (i) as a function of applied voltage (E) (i vs. E), providing information on redox reactions, electron transfer kinetics, and stability of electroactive species [86]. This technique involves linearly and cyclically sweeping the voltage while monitoring the resulting current response, allowing peak potentials, peak currents, and other electrochemical parameters to be determined [87]. In CV, the Butler–Volmer equation (Equation (1)) is often used to model the kinetics of electron transfer at the electrode interface as follows:
where i is the current density, i0 is the exchange current density (in A/m2), α is the charge transfer coefficient (dimensionless), n is the number of electrons involved in the electrode reaction, R and F are, respectively, the perfect gas and the Faraday constants, T is the temperature, and η is the overpotential, defined as the difference between the applied electrode potential and the equilibrium potential for the electrode reaction.
On the other hand, chronoamperometry is an electrochemical technique that measures the electric current (i) as a function of time (i vs. t) when a constant electric potential (E) is applied to an electrode. Unlike cyclic voltammetry, which involves applying a potential that varies cyclically, chronoamperometry maintains a constant potential and measures the resulting electric current over time according to Cottrell’s law (Equation (2)). This technique is often used to study electrochemical reactions with specific electrode materials and to determine the kinetic constants of electrochemical reactions [88,89].
where i is the current, n is the number of electrons involved in the electrochemical reaction, F is Faraday’s constant, A is the area of the electrode, D is the diffusion coefficient of the electroactive species, C is the concentration of the electroactive species, and t is time (in seconds).
Steady-state amperometry using microelectrodes and based on potential step experiments is widely considered to offer superior accuracy for monitoring the state of charge (SOC) in redox flow battery (RFB) electrolytes. A novel analytical method for amperometric state-of-charge (SOC) and state-of-health (SOH) measurements in individual redox flow battery (RFB) electrolytes was developed, focusing on transient current signals obtained in chronoamperometric potential step experiments [90].
On the other hand, potentiometric measurement is a technique used to determine the concentration of ions in a solution by measuring the difference in electrical potential (ΔE) between a reference electrode (typically Ag/AgCl) or a pseudo-reference electrode and a working electrode [91,92,93] as a function of time (ΔE vs. t). This method is based on the Nernst equation (Equation (3)), which relates the measured potential to the concentration of ions in the solution. It is characterized by its simplicity and sensitivity; however, the presence of different ions in solution conducts to measure a mixed potential that is proportional to the ion’s concentration.
where R and F are, respectively, the perfect gas and the Faraday constants, n is the number of electrons, T is the temperature, and E0 is the standard potential of the target analyte.
In the battery field, the entropy coefficient (dUOC/dT) serves as a crucial parameter for predicting the heat generation in lithium-ion batteries (LiBs), particularly at low-rate conditions. Although the potentiometric method is commonly used for entropy coefficient measurement, its accuracy comes at the expense of time. Consequently, there is a critical need for an efficient and accurate entropy measurement technique. The proposed rapid and precise improved potentiometric method, known as positive adjustment (PA), reduces the relaxation time to 10 min. Commercial 18650 lithium-ion cells are used to validate the PA method. A comparison between the entropy profiles obtained by the PA method and conventional potentiometric method (CPM) indicates a comparable accuracy, with an average error of ±0.01 mV/K. Even when contrasted with recent alternative methods, the PA method demonstrates notable advantages in measurement efficiency [94].
Conversely, impedance (Z) measurement is a technique used to characterize the electrical behavior of a system in response to an alternating electrical signal (for a fixed frequency range) [95]. Impedance is a measure of the resistance of an electrical circuit to alternating current (as per Ohm’s Law) and can include components of resistance, capacitance, and inductance. There are several impedance measurement techniques, such as electrochemical impedance spectroscopy (EIS), which is widely used to study electrochemical processes in batteries [96,97], sensors [98,99], and other electrochemical systems (Figure 3). A time-domain measurement technique utilizing a preset equivalent circuit model, comprising numerous series-connected resistor and capacitor parallel elements, was explored as a means of measuring the electrochemical impedance spectrum of a lithium-ion battery, while excluding the apparent impedances resulting from open circuit voltage changes in the low-frequency range. Initially, an extensive experimental investigation was conducted to determine the optimal conditions for the applied signal suitable for this technique. It was established that an impedance spectrum ranging from several tens of microhertz to several tens of millihertz could be accurately measured by selecting a suitable, low-rate, and long-duration constant current charge or discharge as the applied signal. Subsequently, impedance spectra, excluding the apparent impedances resulting from open circuit voltage changes, were measured under various conditions using this technique. The fundamental characteristics of the impedances associated with the solid-state diffusion processes of lithium within the corresponding low-frequency range were examined. It was revealed that the impedance spectrum, excluding the apparent impedances resulting from open-circuit voltage changes in the low-frequency range (ranging from several tens of microhertz to several tens of millihertz), could be reasonably separated into two finite-length Warburg impedances. These impedances could be clearly characterized by differences in their diffusion time-constant values and the state-of-charge dependence of their diffusion resistances. Furthermore, an Arrhenius-type temperature dependence was confirmed for both of their diffusion resistances. A time-domain measurement technique using a preset equivalent circuit model (ECM) was examined for electrochemical impedance spectrum analysis in lithium-ion batteries (LIBs). Experimental studies determined optimal signal conditions, allowing accurate measurements in the low-frequency range. Impedance spectra, excluding apparent impedances from open circuit voltage changes, were analyzed, revealing distinct characteristics related to SOC and battery temperature. This technique enabled the separation of two finite-length Warburg impedances with clear diffusion time-constant values and SOC dependencies. Minimal variations were observed in the time constants of these impedances, showcasing their potential for accurate impedance analysis in LIBs and reducing the resistance for the use of electrochemical impedance spectroscopy analysis in materials chemistry.
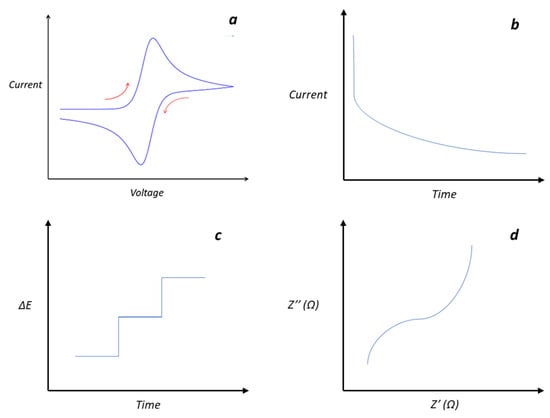
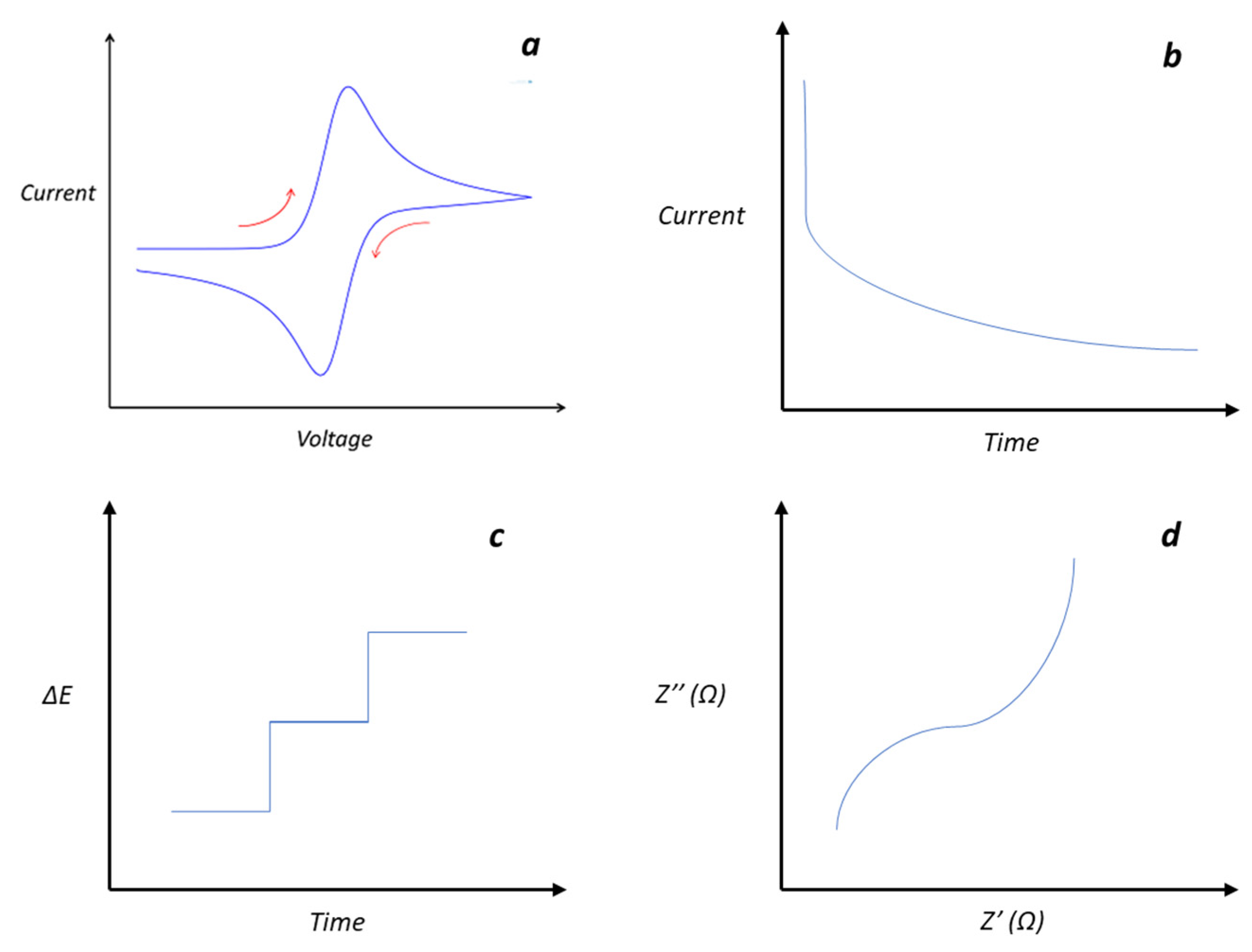
Figure 3.
Typical responses of electrochemical methods commonly used in materials characterization (a) cyclic voltammetry, (b) chronoamperometry, (c) potentiometry, and (d) impedance spectroscopy.
Cavity microelectrodes (CMEs) are used in various areas of scientific research [100], including electrophysiology, neuroscience, material [101,102] analytical chemistry, and even batteries [103]. They are generally made of a conductive material, often metallic, with a small cavity at the end (tens of µm depth and larger). These microelectrodes are used to measure electrical signals on a very small scale. The cavity at the end of the electrode allows better electrical conduction between the electrode and the medium in which it is inserted. This feature improves the sensitivity of electrophysiological or electrochemical measurements. When it comes to batteries, cavity microelectrodes are used to study the electrochemical processes that occur inside the battery. They make it possible to precisely measure electric currents and electrochemical potentials at very fine scales, thus contributing to the understanding of the performance and durability of batteries. Cavity microelectrodes in the field of batteries are characterized by their small size, high energy density, rapid response, stability, and durability, as well as their ability to enable precise control of electrochemical processes. They offer the potential for new battery architectures that can lead to improved performance and better energy efficiency. Cavity microelectrodes (CMEs) provide a valuable platform for evaluating the electrocatalytic performance of micro- and nanoparticle materials. The technical factors and physicochemical processes affecting the electrochemical response of CMEs need to be recognized, particularly the accessibility of redox species on the surface of the electrocatalyst. The voltammetric response of cavity microelectrodes (CMEs) is explored using a combined experimental and theoretical approach. This includes a comparative examination of cyclic voltammetry and square-wave voltammetry (SWV) techniques. The results demonstrate a capacitive response distortion, increasing with the powder surface area, but with a Faradaic response analogous to that of embedded microdisks, indicating that electrochemical reactions occur primarily on the first layer of the powder filler. Furthermore, it is demonstrated that the SWV is well suited to discriminate Faradic processes at CMEs, and precise mathematical expressions are presented to describe it. These results provide guidelines for the design and analysis of the voltammetric measurements of CMEs [104].
In summary, while cyclic voltammetry, impedance spectroscopy, potentiometry, and chronoamperometry are all electrochemical techniques used in battery research, they each have distinct principles, applications, advantages, and limitations. The choice of technique depends on the specific aspects of the battery behavior being studied and the desired level of detail and complexity in the analysis.
2.3. Surface Modification
The surface modification by electrochemical methods takes various forms through different processes, such as electroplating, electrodeposition, anodization, surface etching, and electrochemical functionalization. This section briefly summarizes the concept of each of the above-cited processes and focuses on the main applications that are related to each method.
Electroplating and electrodeposition processes are sometimes used interchangeably. However, in technical contexts, they refer to different processes. Electroplating refers to the process of depositing a thin layer of metal onto a substrate surface using an electrochemical reaction [16,105]. It is widely used in metallurgical industries, such as automotive, electronics, and jewelry manufacturing (e.g., nickel coatings, platinum coatings [106], gold coatings [107], copper coatings [108], etc.), with the objective of producing protective coatings to preserve surfaces from aggressive conditions, thereby increasing the performance of materials and enhancing the physical properties of components (brightness, shape, etc.). Conversely, electrodeposition refers to the process of depositing any material onto a substrate surface using electrochemical methods. Electrodeposition enables the production of high-quality products with improved material properties and high durability. It allows for performing the electrodeposition of alloys, composites, or non-metallic materials (e.g., deposition of glasses [109], silicone [110], and conductive polymers like polypyrrole [111], polyaniline [112,113], PEDOT [114], etc.). To summarize, electroplating is a type of electrodeposition focused on depositing metal coatings.
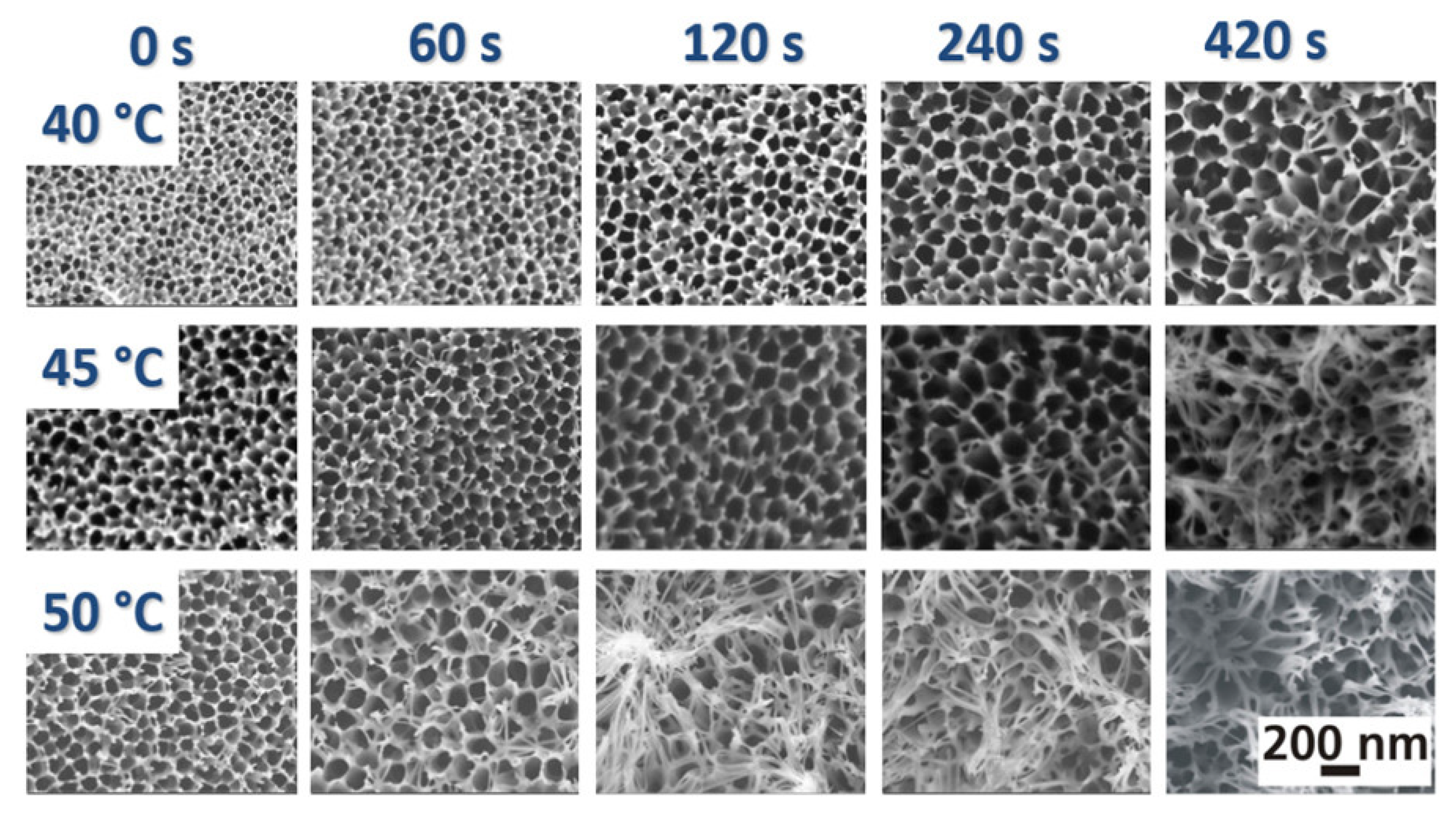
Nevertheless, the anodization process is commonly used to improve corrosion resistance, hardness, and adhesion of the substrate [115]. This technique involves applying a positive potential to the substrate surface in an electrolyte solution, leading to the formation of a stable oxide layer [116,117]. It is primarily used on aluminum and its alloys to enhance surface properties and provide various benefits (durability, enhanced aesthetics since anodizing has an impact on the surface color, electrical insulation required for some applications, and improved adhesion as anodizing creates a porous surface that enhances the adhesion of paints and other coatings) [118,119] (Figure 4).
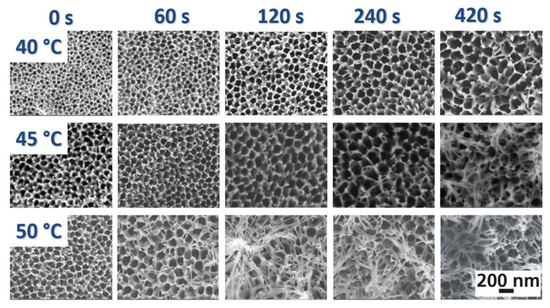
Figure 4.
SEM images of the surface morphology of oxide layers anodized in phosphoric acid at 50 V in dependency on the dwell time and the bath temperature (reproduced with permission from the publisher under license number 5777000505982) [120].
Electrochemical etching or wet etching (also known as electrochemical machining) is a process that uses an electrical current and an electrolyte solution to selectively remove materials from a conductive substrate (metal or semi-conductor) to perform etching [121,122]. This can be used to create surface textures, patterns, or microstructures for various applications. It is somehow the reversible process of electrodeposition where a thin layer of metal is coated. The process consists, in most cases, of immersing the metal (or the semiconductor) in acid baths (electrolyte) during a specific time to create, first, porous nanostructures onto the substrate surfaces and, second, to enable the creation of a thin layer of metal oxide (by anodization) that protects and stabilizes the nanostructures [19,20]. By this, the surface area increases as the structure of the substrate is converted from 2D surfaces to 3D surfaces [123]. Conceptively, many parameters affect the process and should be controlled, e.g., the concentration of the acidic solutions, the temperature of the baths, the presence of a catalyst that has an essential role in the initiation of material nucleation (e.g., copper ions), and, finally, the thickness of the substrate. On the other hand, etching may affect the mechanical properties of the etched substrate. For this reason, many physical properties are monitored during the process by relying on routine testing of the substrate (e.g., Bursting strength tester for foil substrates, etc.). To obtain the best compromise between the surface etching while conserving good and acceptable physical properties, the contact time (residency time of the substrate in the electrolytes) should be considered [124,125]. The surface etching can take various forms depending on the bath composition. Consequently, it can create tunnels crossing the material surfaces [126,127], and it can create nanopores (also named pore nucleation) on the substrate surfaces by increasing the surface area [128].
The widespread application of electrochemical etching is in the preparation of raw materials (anode and cathode components) used in the fabrication of electrochemical capacitors, also known as supercapacitors [129]. In this process, the aluminum foils (high purity ≥ 99%) are treated by electrochemical etching and then formed. Depending on the capacitance measurements, sheets may be classified and used for different applications, e.g., DC capacitor high voltage, AC capacitor motor start, etc. The higher capacitance values can indicate certain desirable properties, such as increased surface area or improved electrode performance [130]. Therefore, they should be interpreted in conjunction with other characterization techniques and performance metrics to assess overall foil quality accurately.
2.4. Electroanalysis
Electrochemical analysis (or electroanalysis) relies on the use of electrochemical methods to quantitively determine the concentration of an analyte in a sample. Typically, these methods include voltammetry [27], amperometry [28], potentiometry [29], and impedance spectroscopy [131] (the principle of these methods is detailed in § 2.2). These methods are employed to measure antioxidant activity [21,22] and analyze [24], and characterize various compounds and materials [25,26], as well as to develop reference methods [132]. Electrochemical sensors and biosensors are among the most common applications of electroanalysis. They are used for the detection of biomolecules [30,133], pollutants [31], and analytes in environmental samples [32,33], food [134], pharmaceuticals [34,35], and clinical and biochemical diagnostics [23,36,135]. These devices offer rapid, sensitive, and relatively selective measurements, making them valuable tools for research, quality control, and real-time process monitoring (Figure 5).
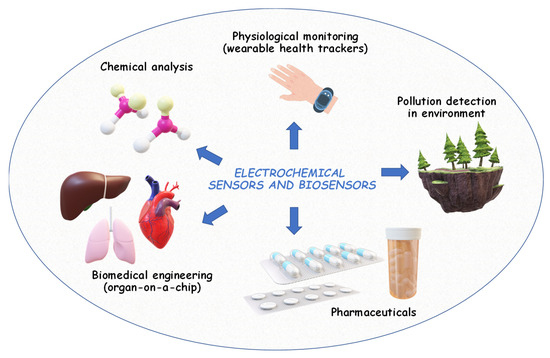
Figure 5.
Common applications of electroanalysis used for the detection of biomolecules, pollutants, and analytes in environmental samples, pharmaceuticals, and clinical and biochemical diagnostics.
In this section, two examples of electroanalysis are investigated based on their importance and widespread applications: the pH electrode and the glucose biosensor. In fact, pH monitoring is of high interest in many chemical and biochemical processes. The term pH stands for the potential of hydrogen and allows it to express its power. The pH measurements express the acidity and alkalinity of solutions and vapors and help in controlling and optimizing industrial processes. The measure of pH using a redox electrode probe consists of potentiometrically determining the concentration of hydrogen ions (H⁺) in a solution based on the Nernst equation [136,137]. Recently, many miniaturized pH electrodes have been reported in the literature. These devices are designed to be small and compact and are essentially important in many applications that require small-scale systems for integration (e.g., lab-on-a-chip systems [23,30], point-of-care diagnostics, etc.) or dedicated for portable devices (for physiological monitoring or wearable health trackers) [138,139].
A glucose biosensor is a device that incorporates a biological recognition element as a sensing component (enzymes, e.g., glucose oxidase (GOx) or glucose dehydrogenase (GDH) and its cofactor). The enzymatic reaction byproducts diffuse through the sensing membrane and are then oxidized or reduced on the electrode surface at a fixed potential that depends on the electrode material [140,141]. Typically, in the case of first-generation enzymatic glucose biosensors, the enzymatic oxidation of glucose by GOx leads to the production of hydrogen peroxide (H2O2), which is oxidized at a positive potential of +0.7 V vs. a Ag/AgCl reference electrode or reduced at negative potentials (between −0.2 and 0 V) using mediators that lower the detection potentials and prevent interference problems [142]. Glucose biosensors are devices commonly used to monitor blood glucose levels in diabetic patients and represent one of the most widely used applications for glucose monitoring worldwide [143].
2.5. Electrochemical Processes for Depollution and Water Remediation
Providing clean water for industrial or drinking purposes is challenging because of the wide range of chemicals generated and used on a regular basis that eventually contaminate water streams [144]. Huge pollutant fluxes from industrial and agricultural processes unintentionally affect the quality of water. In fact, it is well known that the world’s largest polluters are the manufacturers of synthetic organic chemicals (>400 million tons annually), fertilizer use (~200 million tons annually), and pesticide use (~3 million tons annually) [145]. Water-pollution management is still more important than ever, given the fact that over 30% of Earth’s usable freshwater is used for industrial processes, energy production, and agriculture.
Many ways to find more sustainable solutions for water-treatment applications are offered by electrochemical processes [146,147]. In most cases, electricity is used as the main energy source that facilitates the electrochemical reactions that occur and conducts these processes. Among well-developed electrochemical water-treatment processes, such as electrocoagulation, electroflotation, electrodialysis, electrochemical oxidation, and reduction, there are emerging processes that show good prerequisites for their use in industrial-scale applications. These processes tend to increase the rate of pollution removal, eliminate disadvantages, and expand the applicability of existing electrochlorinated water-treatment techniques to improve cost effectiveness. The following emerging and combined electrochemical processes, such as electrodeionization [148], capacitive deionization [149], electro-Fenton [150], microbial fuel cell treatment [151], photo- and sonoelectrocatalyses, are showing impressive results in water depollution, especially at lab scale [152]. However, the main concern in the use of these processes remains the scale-up to industrial scale which is a very challenging step to apply these processes.
Electrodeionization is a combination of two desalination processes, electrodialysis and ion exchange, that result in even deeper demineralization rates. Capacitive deionization allows for the simultaneous desalination of water and recovery of electrical energy, in an efficient manner. Electro-Fenton allows the generation of catalysts in situ, thereby reducing sludge formation. In addition to water treatment, microbial fuel cells allow electrical energy to be recovered, thus reducing the maintenance costs required for this process. The increased separation of charge carriers and the suppression of electron–hole recombination in an electrical field make photoelectrocatalysis provide better removal efficiencies than a single photocatalyst. Sonoelectrocatalysis increases the rate at which reactive substances are transferred, inhibits the polarization of electrodes, and plays an important role in hydroxyl radical production to improve treatment efficiency compared to single electrolyte oxidation.
An example of the oldest industry with complex processes and high chemical consumption is the textile sector. Due to the ever-increasing demand for clothing from the world’s population and changes in the fashion industry, it continues to grow in variety and number [153]. This may contribute to the economic benefit of the population, but the negative impact on the environment is often reported, in particular because of the discharge of wastewater [154]. Due to the potential toxicity and risks to human health, textile wastewater is a concern and must be treated before being discharged into the water supply.
In the treatment of textile waste, conventional processes are most applied, such as integrating chemical, biological, or physical methods. Aluminum sulfate, ferrous sulfate, and PAC (polyaluminum chloride) are generally used as coagulants in textile wastewater treatment [155]. These coagulants are easy to agglomerate with pollutants and form tiny particulate matter and flocculants in wastewater. Flocculants, in turn, improve the size and properties of the floc, particularly its stability [156]. However, for dye wastewater, this process has a limited COD (chemical oxygen demand) removal rate of 10%, which may lead to sludge production. To improve treatment performance, activated sludge, moving bioreactors (MBR), and biofilters are usually used after coagulation–flocculation. However, the quality of the effluents often does not meet the required quality standards. Thus, it needs a finishing process (sedimentation). Physical processes, such as adsorption, are reported for their potential application as a finishing treatment. However, it has a relatively short life span (less sustainable) and may produce spent adsorbent as a byproduct. The integration of chemical, biological, and physical processes generally requires more space, longer retention time (1–4 days), higher operational cost, and needs a sludge handling and disposal system. As a stand-alone technology or combined with aerobic processes, biological processes are often used. An integrated system of anaerobic and aerobic processes has become an option because of the large quantities of sludge generated in chemical processes [153]. The color-removal efficiency achieved in this process is, on the other hand, relatively small. In addition, high ammonium content, color, and non-degradable CODs are characteristics of printing textile wastewater. As a final step in removing persistent pollutants, including dyes, and improving the performance of biological wastewater-treatment plants, most textile industries use a decolorization agent (DCA) [157]. DCA is a cationic organic polymer with a dicyandiamide formaldehyde resin base with high adsorption efficiency and settleability, the ability to neutralize the electric charge of the particle surface, more stable flocs, and the ability to remove dissolved dyes, such as direct, reactive, disperse, and acid dyes [158]. However, DCA is often not the appropriate solution in every situation due to its high cost, low availability, and requirement for sludge disposal. The fact that sludge produced as a byproduct in this case may have significant environmental impacts and be of high risk to humans and the ecosystem is also worth noting.
Over the last decade, intensive research has been carried out on the removal of color in modern oxidation processes. It can be created by photocatalysts [159] ozonation, Fenton’s reagent [160], and electrocatalytic processes [161]. The obvious advantages of photocatalytic oxidation are the low temperature and pressure requirement and being biologically inert and soluble in water, which is widely available, highly photoactive, less toxic, and environmentally friendly. However, major limitations remain in terms of application potential as regards TiO2 catalyst morphology and crystallinity, metal doping requirements, high selectivity to the specific character of pollutants, and close contact with light sources.
Electrocatalytic oxidation, commonly known as EAOP (electrochemical advanced oxidation process), uses electrical energy in pollutant degradation. These processes rely on the in situ formation of hydroxyl radicals (OH●) known to non-selectively attack and oxidize the pollutants until the achievement of biodegradability or the total mineralization of the effluent. Its high performances in color and COD removals were reported in dyeing textile wastewater and printing textile wastewater [162]. A new hybrid Fenton electrochemical system and reactor are proposed to be an effective post-treatment option for textile effluents and many others [10,163]. In terms of color, COD, and ammonium removal, this process is highly effective. As they are cheaper than TiPtIr and more economically feasible than GDL (gas-diffusion layer) carbon [164], mesh TiRuO2 and graphite carbon rods have been used as electrodes.
Applied in the industrial sector, a unique system with a cylindrical stainless-steel reactor with pairs of electrodes (mesh cylinder Ti/RuO2/anode and graphite carbon rod cathode) can be proposed to degrade the complex pollutants of real textile wastewater. The internal circulation system provides considerable advantages for the operation of this system, e.g., increased volume loads of up to 400%. This method can be a promising alternative for textile wastewater after treatment, as pollutants have been degrading simultaneously using electro-Fenton oxidation. In a short time, the removal of color, COD, and ammonium was significant. Due to the longer contact between the generated reactive species and the pollutants in the wastewater, the circulating system was more advantageous than the noncirculating system. The removal efficiency of pollutants, color, and COD, the oxidability index, and operational costs have confirmed and supported this technique. Electrical consumption was about 3 kwh/m3 of Rp 4.200/m3 for the non-circulated system, and it was reduced significantly to 1.1 kwh/m3 or Rp1.540/m3 for the circulated system. A high potential system can be found in the hybrid Fenton electrochemical, and it is possible to investigate this through a scaleup reactor with direct integration into an existing anaerobic–aerobic unit of textile [165].
Finally, a variety of treatment techniques, such as biological remediation, physicochemical treatment, and electrochemical techniques like electrochemical/electrocatalytic reduction, oxidation, and electrocoagulation, will be needed due to the diversity and complexity of chemical water pollutants. The use of electrocatalytic treatment with contaminated water is becoming more common due to the falling cost of renewable energy sources and the growing need to transform harmful substances into harmless or beneficial compounds. While electrocatalytic reduction can handle streams containing oxidized species like nitrate and nitrite produced by fertilizer runoff, electrocatalytic oxidation can handle a variety of industrial waste streams, including textile and food effluents. Because of these benefits and their small-scale suitability, electrocatalytic processes are perfect for decentralized water treatment. Electrocatalysis has the potential to have a sustainable impact on converting harmful water pollutants into valuable or harmless substances with the increasing availability of cheap electricity from renewable sources.
2.6. Electrosynthesis
Electrochemical organic synthesis (or electrosynthesis) has now become a tool to synthesize new compounds via green chemistry [166]. They can function at room temperature and pressure and mainly do not require auxiliary chemicals. Typically, the process of electrosynthesis relies on the use of an electrochemical reactor supplied by a power source [167]. Two parameters are keystones for obtaining high efficiency of electrosynthesis. First, the reactor design is crucial for obtaining a high production yield. Second, electrode characteristics (material, number, size, geometry, and surface area) are an essential consideration for the quantity of electrogenerated compounds, since electrodes are the site hosting the electrochemical reactions [168].
Alternatively, the use of electrical current through a reaction to activate organic molecules by means of the addition or removal of electrons possesses several advantages, such as the simplicity and high selectivity of reactions and the availability of the synthetic materials (low cost, simplicity of uses, needleless for a separation method, etc.). In addition, electrosynthesis can be modular and scalable, allowing for flexibility in production capacity [169]. Electrooxidation and electrosynthesis methodologies have been developed for the selective functionalization of organic molecules, including C-H activation, C-C bond formation, and asymmetric synthesis. This field is important in many major industrial processes such as chlorine generation [170], aluminum manufacturing [171], production of decarbonized nitrogen-based fertilizers [172], drug synthesis [173], and many others [174].
This part shows a few examples dealing with the synthesis of new organic molecules by electrochemical pathways. The first example focuses on the synthesis of nitrogen-based fertilizers (NBFs) by electrochemical methods. The synthesis of NBFs, such as ammonia and urea, was successfully realized by using waste compounds, such as carbon dioxide and nitrates [175]. The work largely focused on understanding the catalytically active sites for urea electrosynthesis. In fact, selectivity, and activity dependence on the relative composition of the copper and zinc oxide catalyst was found and assigned to a synergetic electronic effect. On the other hand, amorphous nanomaterials were used as catalysts, with the reaction involving electrochemical synthesis of N-containing compounds from a variety of abundant N-containing small molecules (N2, NO, NO3− etc.…). The results show that these materials can simplify (break) the C-N coupling bond, leading to the synthesis of urea [176].
Practically, producing decarbonized nitrogen-based fertilizers (NBFs) was developed by CASFER technologies by bringing together nanotechnology and electrochemical science. In fact, they were able to develop precise, commercial-like NBFs from wastestreams. They used an organic synthetic approach (OSA) to produce NBFs, with ingredients predictability and reliability designed to stimulate plant growth [177].
Moreover, chlor–alkali electrolysis is one of the oldest and most implemented processes and has the most significant applications of electrochemistry in industry. This process consists of producing chlorine and sodium hydroxide (NaOH), which are commodity chemicals required by industry. Here, electrolysis was identified as a green method for molecular transfer. The electrons in these electrochemical reactions are waste-free when generated by solar energy and wind.
The importance of electrosynthesis was also implemented for the functionalization of molecules in a sustainable way, minimizing the use of toxic reagents and byproducts. This is implemented by alkene difunctionalization, stereoselective heterocyclic synthesis, and carboxylation reaction [178].
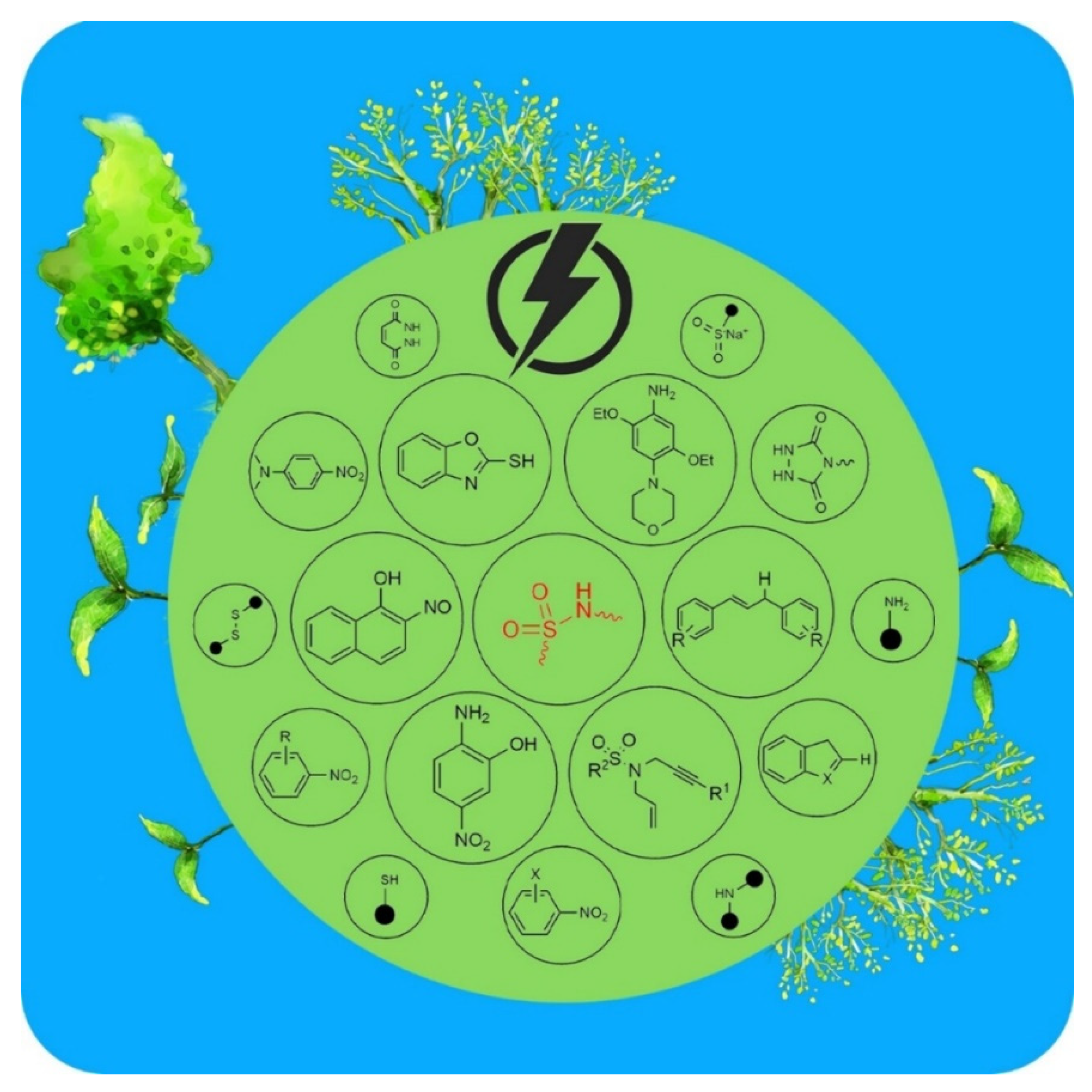
In addition, a very recent study by Talebi and co. described the electrochemical synthesis of sulfonamide derivatives known among the most widely used antibiotics in the world [179]. The electrosynthesis conditions and reaction pathways were studied. The optimal values of the operating parameters (pH, solvent, electrodes…) were evaluated. By optimizing the conditions, the control of the reaction kinetically and thermodynamically using the electrode potential was promising (Figure 6). In fact, this method was able to show the selectivity to oxidize or to reduce a given compound, preventing, then, the oxidation/reduction of intermediates. This electrochemical method of synthesis of sulfonamides over classical ways has many advantages:
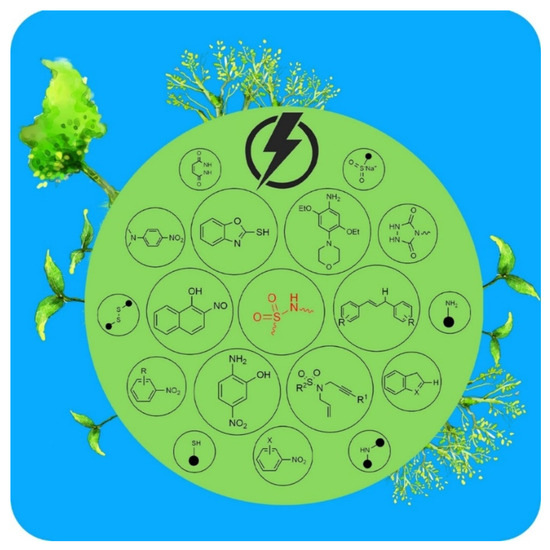
Figure 6.
Electrochemical synthesis of sulfonamide derivatives by electrochemical pathway (Creative Commons CC by license) [179].
- -
- Allow the use of green oxidants, which reduces the use of toxic compounds/solvents and prevents environmental risks;
- -
- Rely on electrification as an energy source, which decreases the amount and cost of the energy consumed;
- -
- Prevent the uses of additional catalysts;
- -
- Rely on a less complicated setup for the simplicity of the technical procedure.
Hydrogen peroxide (H2O2) can also be synthetized using electrochemistry. In fact, the cathodic two-electron (2e−) oxygen reduction reaction (ORR) conducts the electrosynthesis of H2O2. This reaction typically occurs under alkaline conditions and requires a suitable catalyst to enhance the efficiency of the process. Various catalysts, including metal complexes and metal oxides, have been investigated for this purpose. Some studies show the importance of a free Fe motif-based electrocatalyst for hydrogen peroxide synthesis [180]. This use of isolated Fe led to high activity, selectivity, and stability due to high binding energy with the intermediates to break the peroxyl bond into H2O [181].
In conclusion, electrochemical synthesis is now proven as a promising pathway to avoid all the disadvantages, in terms of high energy consumption and the large amount of pollution generated by the classical method of chemical synthesis.
2.7. Electrochemical Protection
Metal structures exposed to aggressive environments (hostile, corrosive, marine environments, etc.) require continuous preventive maintenance to ensure prolonged and safe operation [182]. Corrosion is identified as it occurs above water, in the splash zone, and subsea. In this part, we will discuss electrochemical metal corrosion protection to prevent corrosion in offshore gas platforms and underwater metal piping [183,184].
Electrochemical metal corrosion protection is the most appropriate method used and includes anodic and cathodic protections. Both methods rely on manipulating the electrochemical reactions at the metal’s surface to prevent corrosion and extend the lifespans of steel structures in harsh marine environments [185].
The anodic protection (AP) system maintains the surface in an actively oxidizing state. This method involves applying an external electrical current to the metal, making it the anode in an electrochemical cell, which polarizes the surface to a more positive potential, thus inhibiting and preventing corrosion reactions [186,187]. Another way to apply this method is by attaching sacrificial anodes made of more reactive metals that have higher electrical potentials, such as zinc or magnesium, to the metal structure. The sacrificial anodes corrode preferentially, protecting the structure from corrosion by sacrificing themselves. In some systems to maximize the protection level both ways can be used together [188].
The cathodic protection (CP) systems are another important method to reduce or arrest the corrosion of metal structures by lowering the metal potential using a cathodic current supplied by an anodic system [189,190]. Two main types of CP systems are widely used: sacrificial anode cathodic protection (SACP) and impressed current cathodic protection (ICCP). Sacrificial anode cathodic protection (SACP) is mainly used for smaller offshore structures or locations with low-to-moderate corrosion rates. In SACP systems, the structure to be protected becomes the cathode of an electrochemical cell, while sacrificial anodes made of more reactive metals, such as zinc or aluminum, are attached to the steel monopiles [191]. These sacrificial anodes corrode preferentially, effectively sacrificing themselves to protect the steel structure by providing a source of electrons that suppresses the oxidation of the steel. As a result, the sacrificial anodes need to be periodically replaced as they are consumed over time. However, ICCP systems are used in bigger offshore structures or located in areas with high corrosion rates. In fact, inert anodes composed of mixed metal oxides or platinized titanium are connected to an external power source [192]. This power source applies a controlled electrical current to the steel structure, creating a protective cathodic potential that suppresses corrosion [193]. ICCP systems offer precise control over the cathodic protection process.
2.8. Tools for Electrochemical Modeling
The field of electrochemical modeling has seen continuous advancements through improvements in computational techniques, software tools, and methodologies. Examples of this include COMSOL MUTIPHYSICS [194], MATLAB with Simulink [195], Battery Design Studio [196], Canton, DigiElch [197,198], Python [199], and others. These advances are crucial for the development of batteries, sensors and biosensors, fuel cells, and many other electrochemical applications [200]. In recent years, electrochemical modeling has shown an increasing demand and has become a powerful tool for researchers and scientists. In fact, it allows for an understanding of complex systems and helps in the prediction and optimization of electrochemical devices under various conditions, and as a result, it reduces the study time and the cost (Figure 7). In addition, electrochemical modeling enables the virtual prototyping of devices and systems, allowing engineers to simulate performance, troubleshoot potential issues, and iterate designs before physical fabrication [201,202]. Recently, to reduce animal testing, it became mandatory to model (bio)medical devices (including bioelectrochemical devices) to get Food and Drug Administration (FDA) approval, which highlights, again, the importance of electrochemical modeling [203,204,205].
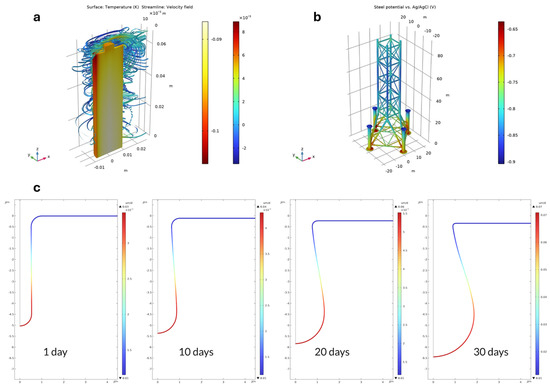
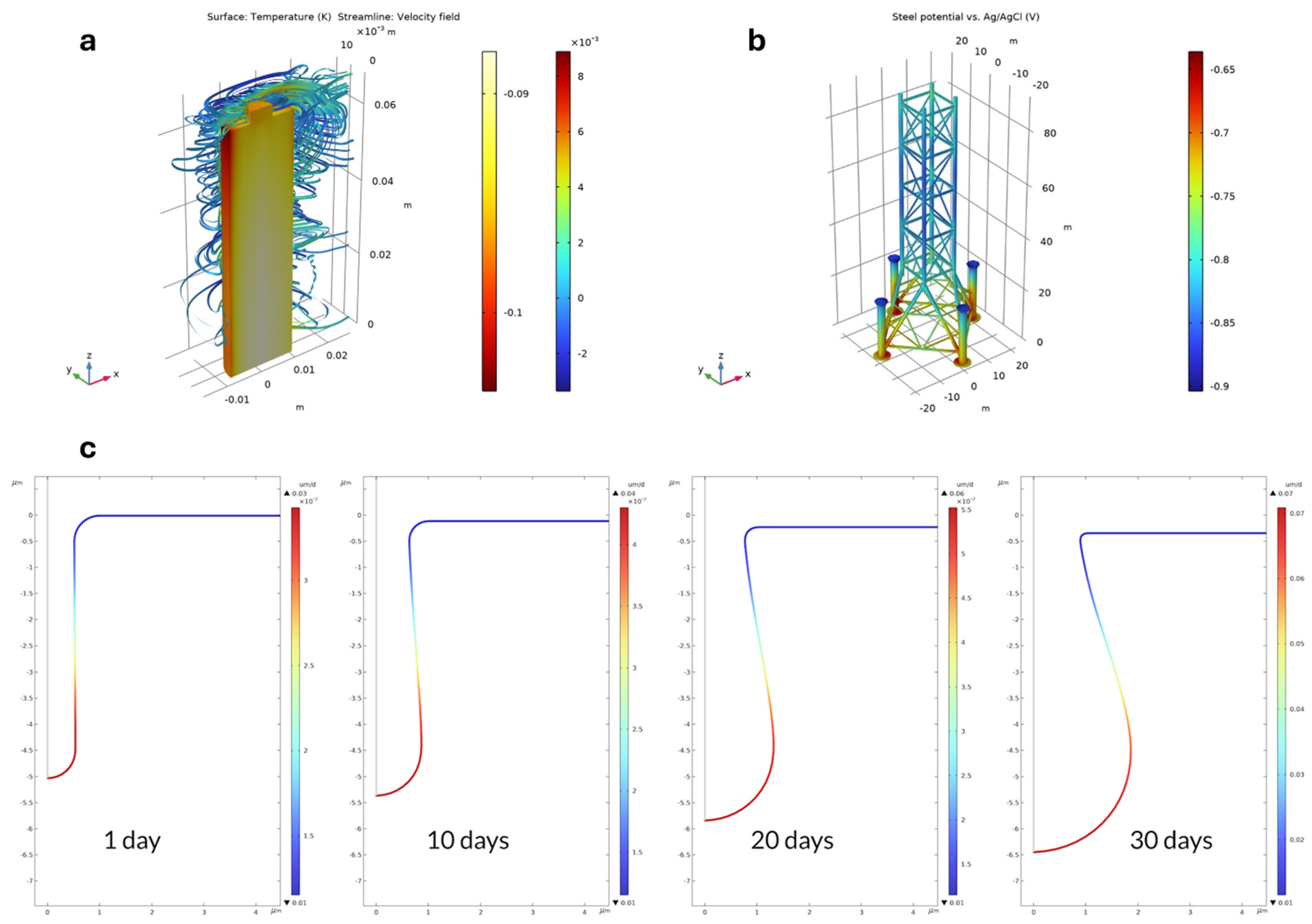
Figure 7.
(a) Difference in battery temperature and airflow streamlines between the coupled solution and the one-way solution after 2100 s, (b) electrochemical modeling of corrosion protection of an oil platform using sacrificial anodes. The figure shows the potential of the steel surface, the parts of the steel surface with the highest (most anodic) values in this plot are the least protected, and (c) corrosion rate in μm/day four times. The figure shows the maximum corrosion rate at the bottom of the pit, which is 2.3 higher after 30 days compared to the corrosion rate after 1 day. This is in line with the proton concentration being 2.2 higher after 30 days compared to 1 day (adapted from COMSOL MULTIPHYSICS website: www.comsol.com accessed on 16 April 2024).
In this part, we will give some examples of the recent research involving electrochemical modeling divided by application field. In the field of organic electrochemistry, Sigman et al. implemented statistical modeling tools for the design of redox-active organic molecules for the application as electrolytes in nonaqueous redox flow batteries [206]. In the field of electrochemical reactors, the modeling and simulation of electrochemical reactors (ECRs) by computational fluid dynamics (CFD) techniques have been of crucial importance due to their main applications: electrosynthesis of chemicals and drugs, electrowinning of metals, chlor–alkali, redox flow batteries, water treatment, and fuel cells [207]. In the field of nanoconfinement, Long et al. studied three nano-electrochemical techniques involving modeling to study multiphase chemistry under nanoconfinement: stochastic collision electrochemistry, single nanodroplet electrochemistry, and nanopore electrochemistry [208]. In the field of hydrogen production using a proton-exchange membrane (PEM) electrolyzer, a modeling tool based on computational fluid dynamics (CFD) software, ANSYS/Fluent was used [209]. In the field of fuel cells, which are a promising source of clean energy, COMSOL Multiphysics was used to incorporate a range of physical phenomena to simulate the performance of solid oxide fuel cells (SOFCs) [210]. In another study, a numerical simulation of a three-dimensional model with a single flow channel was constructed. It provides a scientific basis for the control strategy and structural design of SOFCs [211]. In the field of batteries, a study shows how the current implementation of the Doyle model in COMSOL in Li-ion battery electrodes can enhance the electrochemical dynamic of these batteries [212].
Modeling was also used for advancement in many other electrochemical applications; in thermo-electrochemical cells, the TEC Multiphysics model is constructed to provide a deeper understanding of the interplays between heat/mass transport and electrochemical reactions, with the objective of converting waste heat to electricity [213,214]. In gas-diffusion electrodes, modeling and numerical investigation were used in the performance of the electrochemical reduction of carbon dioxide to methanol. A model was built to investigate the role of Cu2O-/ZnO-based gas-diffusion electrodes in enhancing the reduction of carbon dioxide into methanol inside an electrochemical cell. The model was simulated using COMSOL Multiphysics software and validated using the experimental results [215]. In electrochemical machining (ECM), COMSOL Multiphysics software is used to optimize many parameters [216].
2.9. Electrochemistry in Education
Electrochemistry’s importance in different scientific and technological fields has led to its inclusion in educational curricula. The incorporation of electrochemistry into education has evolved to be more interdisciplinary and aligned with current scientific and technological advancements. Here are two examples involving electrochemistry and education.
Potentiostats are crucial for research development in electrochemistry, but their cost is the principal drawback to their massive use. With the aim to provide an affordable alternative for resource-constrained communities, a low-cost, portable electrochemical workstation that integrates an open-source potentiostat based on Arduino and a smartphone application was adopted in graduate teaching and research. It can perform the most used electrochemical techniques of cyclic and linear voltammetry and chronoamperometry [217].
On the other hand, electrochemistry is difficult to learn due to its abstract concepts involving macroscopic, microscopic, and symbolic representation levels. Studies have shown that students can visualize and improve their understanding of chemistry by using interactive computer animation and simulation. This study reports on the effect of the interactive computer animation and simulation module named “Interactive Electrolysis of Aqueous Solution” (IEAS) which was developed to aid students in learning electrolysis [218]. Thus, it can be concluded that IEAS has an impact on enhancing students’ understanding of the electrolysis concept, and the students are more motivated to learn electrochemistry.
Finally, hands-on experimentation with electrochemical cells, electrodes, electrolytes, and measurement devices remains the best choice to provide students with practical experience and reinforce theoretical concepts. Today, there are many manufacturers that can supply and provide academic institutions with an “Educational kit”. This contains electrodes, electrolytes, and instructional materials, making it easier for instructors to incorporate electrochemistry into their curriculum (refer to the subsection to know the companies that might deliver these kits). These kits often include experiments with clear procedures that facilitate the integration of applied electrochemistry learning in the educational system. Herein, it is worth noticing that the MENA region faces a lack of institutions providing higher education programs in electrochemistry, despite the availability of diverse academic offerings. This deficit is particularly concerning, given the growing demand in numerous sectors of the job market for professionals equipped with electrochemical knowledge. As industries increasingly recognize the importance of electrochemistry in various applications, the absence of relevant educational opportunities underscores a critical gap that needs to be addressed. In the coming years, integrating electrochemical expertise into educational curricula across the region will be imperative to meet the evolving demands of the job market and foster innovation and growth in key sectors.
2.10. Electrochemical Companies
Many Electrochemical companies are found around the world. We represent some of them according to their locations.
Europe’s electrochemical industry is characterized by its focus on sustainability and the circular economy, with significant investments in clean energy, battery technology, and recycling processes. The key players and initiatives include Northvolt (Stockholm, Sweden), Umicore (Brussels, Belgium), BASF (Ludwigshafen, Germany), ITM Power (Sheffield, United Kingdom), SOLVAY (Brussels, Belgium), etc. The electrochemical sector in the USA is diverse, with a strong emphasis on innovation and technology development, particularly in the areas of batteries and renewable energy-storage solutions. Some key players and areas of focus include Tesla Inc. (Texas, United States), 3M (Maplewood, United States), Dow Chemical Company (Michigan, United States), General Electric (Boston, United States), Albemarle Corporation (North Carolina, United States), etc. The MENA region’s participation in the electrochemical sector is growing, particularly in renewable energy and related technologies, like green hydrogen production. Some key players are ACWA Power (Riyadh, Saudi Arabia), MASDAR (Abu Dhabi, United Arab Emirates), SABIC (Riyadh, Saudi Arabia), OQ (Muscat, Oman), etc.
On the other side, there are several companies that provide instruments and accessories for electrochemical experimentation, like mini- and standard-type potentiostats (single- or multichannel devices and portable devices), electrodes (classical, rotating-disk electrodes, screen-printed…), electrochemical cells, educational kits, etc. The key players are Metrohm (Herisau, Switzerland), Bio-Logic Sciences instruments (Seyssinet-Pariset, France), PalmSens (Houten, Netherlands), Solartron (Bognor Regis, UK), Hanna Instruments (Smithfield, VA, USA), Gamry instruments (Warminster, PA, United States), Sciospec (Bennewitz, Germany), Princeton Applied Research (Bognor Regis, UK), PINE RESEARCH (Durham, NC, USA), Texas Instruments (Dallas, TX, USA), Kanopytech (Uttar Pradesh, India), Ivium Technologies (Eindhoven, Netherlands), Corrtest Instruments (Wuhan, China), Xiamen Tob New Energy Technology (Xiamen City, China), Phadke Instruments (Mumbai, India), etc. (Figure 8). This list is not exhaustive and reflects a snapshot of a rapidly advancing field (the websites of the providers are listed in Table 2).

Figure 8.
Main suppliers of electrochemical instruments and accessories worldwide.

Table 2.
Websites of electrochemical instruments and accessories providers.
Recently, Electrochemistry Consulting and Services (E2CS) (Tripoli, Lebanon) was launched as a new and emerging entity in the MENA region that provides consultancy and technical support to a wide range of clients in the field of applied electrochemistry, including those in the energy, environmental, and manufacturing sectors.
Finally, the electrochemical industry is undergoing rapid growth, driven by trends such as sustainability and circular economy, energy storage, electrification of transportation, and innovation in material science. The future of electrochemical companies appears promising in the upcoming years; however, success will depend on companies’ ability to innovate, adapt to changing market dynamics, and address emerging challenges effectively.
3. Conclusions
Applied electrochemistry plays an important role in advancing technology, promoting sustainability, and addressing societal challenges across diverse fields. Its versatility, efficiency, and reliability make it an indispensable tool for innovation, progress, and problem-solving in the modern world. Over the next several years, it is expected that electrochemistry will occupy a solid position in many sectors and become a reference science for many researchers. This requires special attention for integrating electrochemical expertise into educational curricula worldwide to meet the evolving demands of the job market and foster innovation and growth in key sectors. On the other hand, the future of electrochemical companies appears encouraging; however, companies should adapt to the market changes and address emerging challenges effectively to follow the evolution of electrochemical technology.
Funding
This research received no external funding.
Conflicts of Interest
Author Ayman Chmayssem is the founder of the company Electrochemistry Consulting & Services (E2CS). The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Singh, V.G. Applied Electrochemistry; Nova Science Publisher Inc.: New York, NY, USA, 2010. [Google Scholar] [CrossRef]
- Wragg, A.A. Journal of Applied Electrochemistry; Springer Nature: Berlin, Germany, 2009; Volume 39, p. 467. [Google Scholar] [CrossRef]
- Bevan, K.H.; Foong, Y.W.; Shirani, J.; Yuan, S.; Abi Farraj, S. Physics Applied to Electrochemistry: Tunneling Reactions. J. Appl. Phys. 2021, 129, 090901. [Google Scholar] [CrossRef]
- Duan, J.; Tang, X.; Dai, H.; Yang, Y.; Wu, W.; Wei, X.; Huang, Y. Building Safe Lithium-Ion Batteries for Electric Vehicles: A Review. Electrochem. Energy Rev. 2020, 3, 1–42. [Google Scholar] [CrossRef]
- Brinkert, K.; Mandin, P. Fundamentals and Future Applications of Electrochemical Energy Conversion in Space. npj Microgravity 2022, 8, 52. [Google Scholar] [CrossRef] [PubMed]
- Santoro, C.; Arbizzani, C.; Erable, B.; Ieropoulos, I. Microbial Fuel Cells: From Fundamentals to Applications. A Review. J. Power Sources 2017, 356, 225–244. [Google Scholar] [CrossRef] [PubMed]
- Sazali, N.; Salleh, W.N.W.; Jamaludin, A.S.; Razali, M.N.M. New Perspectives on Fuel Cell Technology: A Brief Review. Membranes 2020, 10, 99. [Google Scholar] [CrossRef] [PubMed]
- Esposito, D.V. Membraneless Electrolyzers for Low-Cost Hydrogen Production in a Renewable Energy Future. Joule 2017, 1, 651–658. [Google Scholar] [CrossRef]
- Abbasi, R.; Setzler, B.P.; Lin, S.; Wang, J.; Zhao, Y.; Xu, H.; Pivovar, B.; Tian, B.; Chen, X.; Wu, G.; et al. A Roadmap to Low-Cost Hydrogen with Hydroxide Exchange Membrane Electrolyzers. Adv. Mater. 2019, 31, e1805876. [Google Scholar] [CrossRef] [PubMed]
- Chmayssem, A.; Taha, S.; Hauchard, D. Scaled-up Electrochemical Reactor with a Fixed Bed Three-Dimensional Cathode for Electro-Fenton Process: Application to the Treatment of Bisphenol A. Electrochim. Acta 2017, 225, 435–442. [Google Scholar] [CrossRef]
- Yu, Y.; Zhong, Y.; Sun, W.; Xie, J.; Wang, M.; Guo, Z. A Novel Electrocoagulation Process with Centrifugal Electrodes for Wastewater Treatment: Electrochemical Behavior of Anode and Kinetics of Heavy Metal Removal. Chemosphere 2023, 310, 136862. [Google Scholar] [CrossRef]
- Tran, T.K.; Chiu, K.F.; Lin, C.Y.; Leu, H.J. Electrochemical Treatment of Wastewater: Selectivity of the Heavy Metals Removal Process. Int. J. Hydrogen Energy 2017, 42, 27741–27748. [Google Scholar] [CrossRef]
- Shrestha, R.; Ban, S.; Devkota, S.; Sharma, S.; Joshi, R.; Tiwari, A.P.; Kim, H.Y.; Joshi, M.K. Technological Trends in Heavy Metals Removal from Industrial Wastewater: A Review. J. Environ. Chem. Eng. 2021, 9, 105688. [Google Scholar] [CrossRef]
- Yue, H.; Xue, L.; Chen, F. Efficiently Electrochemical Removal of Nitrite Contamination with Stable RuO2-TiO2/Ti Electrodes. Appl. Catal. B 2017, 206, 683–691. [Google Scholar] [CrossRef]
- Dao, K.C.; Yang, C.C.; Chen, K.F.; Tsai, Y.P. Recent Trends in Removal Pharmaceuticals and Personal Care Products by Electrochemical Oxidation and Combined Systems. Water 2020, 12, 1043. [Google Scholar] [CrossRef]
- Bhat, R.S.; Shetty, S.M.; Kumar, N.A. Electroplating of Zn-Ni Alloy Coating on Mild Steel and Its Electrochemical Studies. J. Mater. Eng. Perform. 2021, 30, 8188–8195. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, N.; Gilchrist, M.; Fang, F. Advances in Precision Micro/Nano-Electroforming: A State-of-the-Art Review. J. Micromech. Microeng. 2020, 30, 103002. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, Y. Electrochemical Machining of Complex Components of Aero-Engines: Developments, Trends, and Technological Advances. Chin. J. Aeronaut. 2019, 34, 28–53. [Google Scholar] [CrossRef]
- Zhang, Y.; Lan, J.; Xu, Y.; Yan, Y.; Liu, W.; Liu, X.; Gu, S.; Zhou, J.; Wang, M. Ultrafine PtCo Alloy by Pyrolysis Etching-Confined Pyrolysis for Enhanced Hydrogen Evolution. J. Colloid. Interface Sci. 2024, 660, 997–1009. [Google Scholar] [CrossRef]
- Zhan, Y.; Zhang, G.; Shen, J.; Zhou, B.; Zhao, C.; Guo, J.; Wen, M.; Tan, Z.; Zheng, L.; Lu, J.; et al. Facile Electrochemical Surface-Alloying and Etching of Au Wires to Enable High-Performance Substrates for Surface Enhanced Raman Scattering. Nano Mater. Sci. 2023. [Google Scholar] [CrossRef]
- René, A.; Abasq, M.L.; Hauchard, D.; Hapiot, P. How Do Phenolic Compounds React toward Superoxide Ion? A Simple Electrochemical Method for Evaluating Antioxidant Capacity. Anal. Chem. 2010, 82, 8703–8710. [Google Scholar] [CrossRef]
- Keyrouz, R.; Abasq, M.L.; Le Bourvellec, C.; Blanc, N.; Audibert, L.; Argall, E.; Hauchard, D. Total Phenolic Contents, Radical Scavenging and Cyclic Voltammetry of Seaweeds from Brittany. Food Chem. 2011, 126, 831–836. [Google Scholar] [CrossRef]
- Chmayssem, A.; Verplanck, N.; Boizot, F.; Alessio, M.; Santos, L.; Mourier, V.; Vignoud, S.; Navarro, F.; Mailley, P. Microfluidic platform of electrochemical biosensors for organ-on-chip applications. In Proceedings of the MicroTAS 2021—25th International Conference on Miniaturized Systems for Chemistry and Life Sciences, Palm Springs, CA, USA, 10–14 October 2021. [Google Scholar]
- Chmayssem, A.; Hauchard, D. New Detection Method for Alkylphenol Traces in Water Based on an Integrated Electrochemical Cell Sensor. Rev. Sci. l’Eau 2015, 28, 35–40. [Google Scholar] [CrossRef]
- Hauchard, D.; Cassir, M.; Chivot, J.; Baudry, D.; Ephritikhine, M. Electrochemical Study of Uranium (IV) and (III) Organometallic Compounds in Tetrahydrofuran by Means of Conventional Microelectrodes and Ultramicroelectrodes. Part II. Application to Borohydride Compounds-Study of the Stability of CP2U(BH4)2. J. Electroanal. Chem. 1993, 347, 399–407. [Google Scholar] [CrossRef]
- Terbouche, A.; Lameche, S.; Ait-Ramdane-Terbouche, C.; Guerniche, D.; Lerari, D.; Bachari, K.; Hauchard, D. A New Electrochemical Sensor Based on Carbon Paste Electrode/Ru(III) Complex for Determination of Nitrite: Electrochemical Impedance and Cyclic Voltammetry Measurements. Measurement 2016, 92, 524–533. [Google Scholar] [CrossRef]
- Shin, K.Y.; Oum, W.; Yu, D.J.; Kang, S.; Kim, E.B.; Kim, H.W. Fundamentals of Cyclic Voltammetry. J. Sens. Sci. Technol. 2021, 30, 384–387. [Google Scholar] [CrossRef]
- Inzelt, G. Chronoamperometry, Chronocoulometry, and Chronopotentiometry. In Encyclopedia of Applied Electrochemistry; Springer Nature: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Bakker, E.; Pretsch, E. Modern Potentiometry. Angew. Chem.—Int. Ed. 2007, 46, 5660–5668. [Google Scholar] [CrossRef] [PubMed]
- Chmayssem, A.; Verplanck, N.; Tanase, C.E.; Costa, G.; Monsalve-Grijalba, K.; Amigues, S.; Alias, M.; Gougis, M.; Mourier, V.; Vignoud, S.; et al. Development of a Multiparametric (Bio)Sensing Platform for Continuous Monitoring of Stress Metabolites. Talanta 2021, 229, 122275. [Google Scholar] [CrossRef]
- Chmayssem, A.; Hauchard, D. Direct Ultra-Trace Detection of Alkylphenols in Water Using a Cavity Carbon-Paste Microelectrode Sensor. Desalination Water Treat. 2017, 83, 321–326. [Google Scholar] [CrossRef]
- Liu, Y.; Xue, Q.; Chang, C.; Wang, R.; Liu, Z.; He, L. Recent Progress Regarding Electrochemical Sensors for the Detection of Typical Pollutants in Water Environments. Anal. Sci. 2022, 38, 55–70. [Google Scholar] [CrossRef]
- Karthik, V.; Selvakumar, P.; Senthil Kumar, P.; Satheeskumar, V.; Godwin Vijaysunder, M.; Hariharan, S.; Antony, K. Recent Advances in Electrochemical Sensor Developments for Detecting Emerging Pollutant in Water Environment. Chemosphere 2022, 304, 135331. [Google Scholar] [CrossRef]
- Boumya, W.; Taoufik, N.; Achak, M.; Bessbousse, H.; Elhalil, A.; Barka, N. Electrochemical Sensors and Biosensors for the Determination of Diclofenac in Pharmaceutical, Biological and Water Samples. Talanta Open 2020, 3, 100026. [Google Scholar] [CrossRef]
- Sohrabi, H.; Dezhakam, E.; Khataee, A.; Nozohouri, E.; Majidi, M.R.; Mohseni, N.; Trofimov, E.; Yoon, Y. Recent Trends in Layered Double Hydroxides Based Electrochemical and Optical (Bio)Sensors for Screening of Emerging Pharmaceutical Compounds. Environ. Res. 2022, 211, 113068. [Google Scholar] [CrossRef] [PubMed]
- Malzahn, K.; Windmiller, J.R.; Valdés-Ramírez, G.; Schöning, M.J.; Wang, J. Wearable Electrochemical Sensors for in Situ Analysis in Marine Environments. Analyst 2011, 136, 2912–2917. [Google Scholar] [CrossRef] [PubMed]
- European Commission. An EU Strategy to Harness the Potential of Offshore Renewable Energy for a Climate Neutral Future; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- Dong, J.; Zeng, J.; Yang, Y.; Wang, H. A Review of Law and Policy on Decarbonization of Shipping. Front. Mar. Sci. 2022, 9, 1076352. [Google Scholar] [CrossRef]
- Madurai Elavarasan, R.; Pugazhendhi, R.; Irfan, M.; Mihet-Popa, L.; Khan, I.A.; Campana, P.E. State-of-the-Art Sustainable Approaches for Deeper Decarbonization in Europe—An Endowment to Climate Neutral Vision. Renew. Sustain. Energy Rev. 2022, 159, 112204. [Google Scholar] [CrossRef]
- Beccarello, M.; Di Foggia, G. Review and Perspectives of Key Decarbonization Drivers to 2030. Energies 2023, 16, 1345. [Google Scholar] [CrossRef]
- Nesterenko, N.; Medeiros-Costa, I.C.; Clatworthy, E.B.; Cruchade, H.; Konnov, S.V.; Dath, J.P.; Gilson, J.P.; Mintova, S. Methane-to-Chemicals: A Pathway to Decarbonization. Natl. Sci. Rev. 2023, 10, nwad116. [Google Scholar] [CrossRef]
- Papadis, E.; Tsatsaronis, G. Challenges in the Decarbonization of the Energy Sector. Energy 2020, 205, 118025. [Google Scholar] [CrossRef]
- Tautorat, P.; Lalin, B.; Schmidt, T.S.; Steffen, B. Directions of Innovation for the Decarbonization of Cement and Steel Production—A Topic Modeling-Based Analysis. J. Clean. Prod. 2023, 407, 137055. [Google Scholar] [CrossRef]
- Zier, M.; Stenzel, P.; Kotzur, L.; Stolten, D. A Review of Decarbonization Options for the Glass Industry. Energy Convers. Manag. X 2021, 10, 100083. [Google Scholar] [CrossRef]
- Ghisolfi, V.; Tavasszy, L.A.; Correia, G.H.d.A.; Chaves, G.d.L.D.; Ribeiro, G.M. Freight Transport Decarbonization: A Systematic Literature Review of System Dynamics Models. Sustainability 2022, 14, 3625. [Google Scholar] [CrossRef]
- Raza, M.Y.; Zhongpan, Q.; Pengju, W. Agriculture-Related Energy Consumption, Food Policy, and CO2 Emission Reduction: New Insights from Pakistan. Front. Environ. Sci. 2023, 10, 1099813. [Google Scholar] [CrossRef]
- Palou-Rivera, I.; Grieco, W. Electrochemical Processes Role in the Drive for Industrial Decarbonization. ECS Meet. Abstr. 2022, MA2022-01, 2338. [Google Scholar] [CrossRef]
- Griffiths, S.; Sovacool, B.K.; Kim, J.; Bazilian, M.; Uratani, J.M. Industrial Decarbonization via Hydrogen: A Critical and Systematic Review of Developments, Socio-Technical Systems and Policy Options. Energy Res. Soc. Sci. 2021, 80, 102208. [Google Scholar] [CrossRef]
- Schiffer, Z.J.; Manthiram, K. Electrification and Decarbonization of the Chemical Industry. Joule 2017, 1, 10–14. [Google Scholar] [CrossRef]
- Mallapragada, D.S.; Dvorkin, Y.; Modestino, M.A.; Esposito, D.V.; Smith, W.A.; Hodge, B.M.; Harold, M.P.; Donnelly, V.M.; Nuz, A.; Bloomquist, C.; et al. Decarbonization of the Chemical Industry through Electrification: Barriers and Opportunities. Joule 2023, 7, 23–41. [Google Scholar] [CrossRef]
- Brannstrom, C. The Emerging Energy Future(s) of Renewable Power and Electrochemistry: Advancing or Undermining Energy Democracy? In Energy Democracies for Sustainable Futures; Academic Press: Cambridge, MA, USA, 2022. [Google Scholar] [CrossRef]
- Xia, R.; Overa, S.; Jiao, F. Emerging Electrochemical Processes to Decarbonize the Chemical Industry. JACS Au 2022, 2, 1054–1070. [Google Scholar] [CrossRef] [PubMed]
- Brimley, P.; Almajed, H.; Alsunni, Y.; Alherz, A.W.; Bare, Z.J.L.; Smith, W.A.; Musgrave, C.B. Electrochemical CO2Reduction over Metal-/Nitrogen-Doped Graphene Single-Atom Catalysts Modeled Using the Grand-Canonical Density Functional Theory. ACS Catal. 2022, 12, 10161–10171. [Google Scholar] [CrossRef]
- Lvov, S.N.; Beck, J.R.; LaBarbera, M.S. Electrochemical Reduction of CO2 to Fuels. In Carbon-Neutral Fuels and Energy Carriers; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar] [CrossRef]
- Pomerantseva, E.; Bonaccorso, F.; Feng, X.; Cui, Y.; Gogotsi, Y. Energy Storage: The Future Enabled by Nanomaterials. Science 2019, 366, eaan8285. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Jung, S.; Lee, M.; Hong, T. How to Better Share Energy towards a Carbon-Neutral City? A Review on Application Strategies of Battery Energy Storage System in City. Renew. Sustain. Energy Rev. 2022, 157, 112113. [Google Scholar] [CrossRef]
- Sufyan, M.; Rahim, N.A.; Aman, M.M.; Tan, C.K.; Raihan, S.R.S. Sizing and Applications of Battery Energy Storage Technologies in Smart Grid System: A Review. J. Renew. Sustain. Energy 2019, 11, 014105. [Google Scholar] [CrossRef]
- Dehghani-Sanij, A.R.; Tharumalingam, E.; Dusseault, M.B.; Fraser, R. Study of Energy Storage Systems and Environmental Challenges of Batteries. Renew. Sustain. Energy Rev. 2019, 104, 192–208. [Google Scholar] [CrossRef]
- Li, Y.; Yang, J.; Song, J. Design Structure Model and Renewable Energy Technology for Rechargeable Battery towards Greener and More Sustainable Electric Vehicle. Renew. Sustain. Energy Rev. 2017, 74, 19–25. [Google Scholar] [CrossRef]
- Rydh, C.J. Environmental Assessment of Vanadium Redox and Lead-Acid Batteries for Stationary Energy Storage. J. Power Sources 1999, 80, 21–29. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, J.; Qu, R.; Xiao, G. Environmental Impact Assessment of the Dismantled Battery: Case Study of a Power Lead–Acid Battery Factory in China. Processes 2023, 11, 2119. [Google Scholar] [CrossRef]
- Divya, K.C.; Østergaard, J. Battery Energy Storage Technology for Power Systems-An Overview. Electr. Power Syst. Res. 2009, 79, 511–520. [Google Scholar] [CrossRef]
- Abraham, K.M. Prospects and Limits of Energy Storage in Batteries. J. Phys. Chem. Lett. 2015, 6, 830–844. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Dong, Y.; Li, S.; Lee, J.; Wang, C.; Zhu, Z.; Xue, W.; Li, Y.; Li, J. Lithium Manganese Spinel Cathodes for Lithium-Ion Batteries. Adv. Energy Mater. 2020, 11, 2000997. [Google Scholar] [CrossRef]
- Widyantara, R.D.; Zulaikah, S.; Juangsa, F.B.; Budiman, B.A.; Aziz, M. Review on Battery Packing Design Strategies for Superior Thermal Management in Electric Vehicles. Batteries 2022, 8, 287. [Google Scholar] [CrossRef]
- Schöberl, J.; Ank, M.; Schreiber, M.; Wassiliadis, N.; Lienkamp, M. Thermal Runaway Propagation in Automotive Lithium-Ion Batteries with NMC-811 and LFP Cathodes: Safety Requirements and Impact on System Integration. eTransportation 2024, 19, 100305. [Google Scholar]
- Trahey, L.; Brushett, F.R.; Balsara, N.P.; Ceder, G.; Cheng, L.; Chiang, Y.M.; Hahn, N.T.; Ingram, B.J.; Minteer, S.D.; Moore, J.S.; et al. Energy Storage Emerging: A Perspective from the Joint Center for Energy Storage Research. Proc. Natl. Acad. Sci. USA 2020, 117, 12550–12557. [Google Scholar] [CrossRef]
- Barbosa, J.C.; Fidalgo-Marijuan, A.; Dias, J.C.; Gonçalves, R.; Salado, M.; Costa, C.M.; Lanceros-Méndez, S. Molecular Design of Functional Polymers for Organic Radical Batteries. Energy Storage Mater. 2023, 60, 102841. [Google Scholar] [CrossRef]
- Hager, M.D.; Esser, B.; Feng, X.; Schuhmann, W.; Theato, P.; Schubert, U.S. Polymer-Based Batteries—Flexible and Thin Energy Storage Systems. Adv. Mater. 2020, 32, e2000587. [Google Scholar] [CrossRef] [PubMed]
- Choi, S. Biofuel Cells and Biobatteries: Misconceptions, Opportunities, and Challenges. Batteries 2023, 9, 119. [Google Scholar] [CrossRef]
- Patra, S.; Verma, J.; Mishra, Y.K.; Kurinec, S.; Wang, Q.; Syväjärvi, M.; Tiwari, A. The Positioning of Biofuel Cells-Based Biobatteries for Net-Zero Energy Future. J. Energy Storage 2023, 72, 107919. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Fraiwan, A.; Choi, S. Paper-Based Batteries: A Review. Biosens. Bioelectron. 2014, 54, 640–649. [Google Scholar] [CrossRef]
- Yazdi, A.A.; Preite, R.; Milton, R.D.; Hickey, D.P.; Minteer, S.D.; Xu, J. Rechargeable Membraneless Glucose Biobattery: Towards Solid-State Cathodes for Implantable Enzymatic Devices. J. Power Sources 2017, 343, 103–108. [Google Scholar] [CrossRef]
- Garland, N.T.; Kaveti, R.; Bandodkar, A.J. Biofluid-Activated Biofuel Cells, Batteries, and Supercapacitors: A Comprehensive Review. Adv. Mater. 2023, 35, e2303197. [Google Scholar] [CrossRef]
- Li, P.; Kim, H.; Myung, S.T.; Sun, Y.K. Diverting Exploration of Silicon Anode into Practical Way: A Review Focused on Silicon-Graphite Composite for Lithium Ion Batteries. Energy Storage Mater. 2020, 35, 550–576. [Google Scholar] [CrossRef]
- Fichtner, M. Recent Research and Progress in Batteries for Electric Vehicles. Batter. Supercaps 2022, 5, e20210022. [Google Scholar] [CrossRef]
- Li, Y.; Yang, J.; Song, J. Nano Energy System Model and Nanoscale Effect of Graphene Battery in Renewable Energy Electric Vehicle. Renew. Sustain. Energy Rev. 2016, 69, 652–663. [Google Scholar] [CrossRef]
- Raccichini, R.; Varzi, A.; Passerini, S.; Scrosati, B. The Role of Graphene for Electrochemical Energy Storage. Nat. Mater. 2015, 14, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Wang, X. Advances in Micro Lithium-Ion Batteries for on-Chip and Wearable Applications. J. Micromech. Microeng. 2021, 31, 114002. [Google Scholar] [CrossRef]
- Muench, S.; Burges, R.; Lex-Balducci, A.; Brendel, J.C.; Jäger, M.; Friebe, C.; Wild, A.; Schubert, U.S. Adaptation of Electrodes and Printable Gel Polymer Electrolytes for Optimized Fully Organic Batteries. J. Polym. Sci. 2021, 59, 494–501. [Google Scholar] [CrossRef]
- Winter, M.; Brodd, R.J. What Are Batteries, Fuel Cells, and Supercapacitors? Chem. Rev. 2004, 104, 4245–4270. [Google Scholar] [CrossRef] [PubMed]
- Isa, N.N.C.; Mohd, Y.; Zaki, M.H.M.; Mohamad, S.A.S. Characterization of Copper Coating Electrodeposited on Stainless Steel Substrate. Int. J. Electrochem. Sci. 2017, 12, 6010–6021. [Google Scholar] [CrossRef]
- Trentin, A.; Pakseresht, A.; Duran, A.; Castro, Y.; Galusek, D. Electrochemical Characterization of Polymeric Coatings for Corrosion Protection: A Review of Advances and Perspectives. Polymers 2022, 14, 2306. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.; Esteves, M.F.; Fernández, J.; Bonastre, J.; Cases, F. Polyaniline Coated Conducting Fabrics. Chemical and Electrochemical Characterization. Eur. Polym. J. 2011, 47, 2003–2015. [Google Scholar] [CrossRef]
- Stangl, A.; Muñoz-Rojas, D.; Burriel, M. In Situ and Operando Characterisation Techniques for Solid Oxide Electrochemical Cells: Recent Advances. J. Phys. Energy 2021, 3, 012001. [Google Scholar] [CrossRef]
- Rafiee, M.; Abrams, D.J.; Cardinale, L.; Goss, Z.; Romero-Arenas, A.; Stahl, S.S. Cyclic Voltammetry and Chronoamperometry: Mechanistic Tools for Organic Electrosynthesis. Chem. Soc. Rev. 2024, 53, 566–585. [Google Scholar] [CrossRef]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A Practical Beginner’s Guide to Cyclic Voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
- Guziejewski, D.; Stojanov, L.; Zwierzak, Z.; Compton, R.G.; Mirceski, V. Electrode Kinetics from a Single Experiment: Multi-Amplitude Analysis in Square-Wave Chronoamperometry. Phys. Chem. Chem. Phys. 2022, 24, 24419–24428. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.Y.; Park, J.H.; Chang, Y.W.; Cho, S.; Kang, M.J.; Pyun, J.C. Chronoamperometry-Based Redox Cycling for Application to Immunoassays. ACS Sens. 2018, 3, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Volodin, I.A.; Stolze, C.; Casas Mesa, C.; Haagen, U.; Terechin, C.; Hager, M.D.; Schubert, U.S. Beyond Steady-State Conditions: Chronoamperometric State-of-Charge and State-of-Health Measurements in Flow Battery Electrolytes. Sens. Actuators B Chem. 2024, 403, 135101. [Google Scholar] [CrossRef]
- Parrilla, M.; Cuartero, M.; Crespo, G.A. Wearable Potentiometric Ion Sensors. TrAC—Trends Anal. Chem. 2019, 110, 303–320. [Google Scholar] [CrossRef]
- Düzgün, A.; Zelada-Guillén, G.A.; Crespo, G.A.; MacHo, S.; Riu, J.; Rius, F.X. Nanostructured Materials in Potentiometry. Anal. Bioanal. Chem. 2011, 399, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Qin, W. Recent Advances in Potentiometric Biosensors. TrAC—Trends Anal. Chem. 2020, 124, 115803. [Google Scholar] [CrossRef]
- Lin, Z.; Wu, D.; Du, C.; Ren, Z. An Improved Potentiometric Method for the Measurement of Entropy Coefficient of Lithium-Ion Battery Based on Positive Adjustment. Energy Rep. 2022, 8, 54–63. [Google Scholar] [CrossRef]
- Chmayssem, A.; Tanase, C.E.; Verplanck, N.; Gougis, M.; Mourier, V.; Zebda, A.; Ghaemmaghami, A.M.; Mailley, P. New Microfluidic System for Electrochemical Impedance Spectroscopy Assessment of Cell Culture Performance: Design and Development of New Electrode Material. Biosensors 2022, 12, 452. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.; Li, D.; Cui, X.; Wang, L.; Li, L.; Wang, K. Electrochemical Impedance Spectroscopy: A New Chapter in the Fast and Accurate Estimation of the State of Health for Lithium-Ion Batteries. Energies 2023, 16, 1599. [Google Scholar] [CrossRef]
- Choi, W.; Shin, H.C.; Kim, J.M.; Choi, J.Y.; Yoon, W.S. Modeling and Applications of Electrochemical Impedance Spectroscopy (Eis) for Lithium-Ion Batteries. J. Electrochem. Sci. Technol. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Brett, C.M.A. Electrochemical Impedance Spectroscopy in the Characterisation and Application of Modified Electrodes for Electrochemical Sensors and Biosensors. Molecules 2022, 27, 1497. [Google Scholar] [CrossRef]
- Magar, H.S.; Hassan, R.Y.A.; Mulchandani, A. Electrochemical Impedance Spectroscopy (Eis): Principles, Construction, and Biosensing Applications. Sensors 2021, 21, 6578. [Google Scholar] [CrossRef] [PubMed]
- Cachet-Vivier, C.; Keddam, M.; Vivier, V.; Yu, L.T. Development of Cavity Microelectrode Devices and Their Uses in Various Research Fields. J. Electroanal. Chem. 2013, 688, 12–19. [Google Scholar] [CrossRef]
- Tremblay, M.L.; Martin, M.H.; Lebouin, C.; Lasia, A.; Guay, D. Determination of the Real Surface Area of Powdered Materials in Cavity Microelectrodes by Electrochemical Impedance Spectroscopy. Electrochim. Acta 2010, 55, 6283–6291. [Google Scholar] [CrossRef]
- Cachet-Viviera, C.; Vivier, V.; Cha, C.S.; Nedelec, J.Y.; Yu, L.T. Electrochemistry of Powder Material Studied by Means of the Cavity Microelectrode (CME). Electrochim. Acta 2001, 47, 181–189. [Google Scholar] [CrossRef]
- Vivier, V.; Cachet-Vivier, C.; Cha, C.S.; Nedelec, J.Y.; Yu, L.T. Cavity Microelectrode for Studying Battery Materials: Application to Polyaniline Powder. Electrochem. Commun. 2000, 2, 180–185. [Google Scholar] [CrossRef]
- Hasegawa, N.; Shoji, K. Microcavity Volume Control on a Tip of Ag/AgCl Electrodes for Stable Channel Current Measurements of Biological Nanopores. Analyst 2022, 147, 1191–1198. [Google Scholar] [CrossRef]
- Huang, X.; Shi, X.; Zeng, H. How Far Does the Copper/Nickle Recovery from the Practical Application in the Electroplating Wastewater? Resour. Conserv. Recycl. Adv. 2023, 19, 200170. [Google Scholar] [CrossRef]
- Mohajeri, R.; Alipour, Z.; Hajihosseini, S.; Mirzaei, S.I.; Fardmanesh, M. Characterization of Platinum Coated Electrodes Encapsulated in Microfluidic Channel for Impedancemetry Applications. In Proceedings of the 2021 28th National and 6th International Iranian Conference on Biomedical Engineering (ICBME 2021), Tehran, Iran, 25–26 November 2021. [Google Scholar] [CrossRef]
- Solonin, M.D.; Asnis, N.A.; Grigoryan, N.S.; Vagramyan, T.A. Cleaning of fine tungsten wire before gold electroplating. Tsvetnye Met. 2023, 2023, 58–63. [Google Scholar] [CrossRef]
- Menk, L.A.; Baca, E.D.; Hollowell, A.E. Copper Electrodeposition in Blind Mesoscale Through-Silicon-Vias. ECS Meet. Abstr. 2018, MA2018-02, 823. [Google Scholar] [CrossRef]
- Mochizuki, C.; Senga, T.; Shibata, M. Electrodeposition of Pd-Ni-P Metallic Glass Films. Electrochemistry 2011, 79, 249–251. [Google Scholar] [CrossRef]
- Krywko-Cendrowska, A.; Strawski, M.; Szklarczyk, M. Low Temperature Electrodeposition of SiOx Films Photoactive in Water Solution. Electrochim. Acta 2013, 108, 112–117. [Google Scholar] [CrossRef]
- Liu, A.S.; Oliveira, M.A.S. Electrodeposition of Polypyrrole Films on Aluminum from Tartrate Aqueous Solution. J. Braz. Chem. Soc. 2007, 18, 143–152. [Google Scholar] [CrossRef]
- Sayah, A.; Habelhames, F.; Bahloul, A.; Boudjadi, A. The Effect of Electrodeposition Applied Potential on the Electrochemical Performance of Polyaniline Films. J. Mater. Sci. Mater. Electron. 2021, 32, 10692–10701. [Google Scholar] [CrossRef]
- Lata, S.; Batra, B.; Kumar, P.; Pundir, C.S. Construction of an Amperometric D-Amino Acid Biosensor Based on d-Amino Acid Oxidase/Carboxylated Mutliwalled Carbon Nanotube/Copper Nanoparticles/Polyalinine Modified Gold Electrode. Anal. Biochem. 2013, 437, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Zhu, X.; Han, D.; Li, M.; Zhang, Q.; Shu, Y.; Cheng, Z.; Zhang, W.; Hua, E.; Sang, S. AC Electrodeposition of PEDOT Films in Protic Ionic Liquids for Long-Term Stable Organic Electrochemical Transistors. Molecules 2019, 24, 4105. [Google Scholar] [CrossRef]
- Martínez-Viademonte, M.P.; Abrahami, S.T.; Hack, T.; Burchardt, M.; Terryn, H. A Review on Anodizing of Aerospace Aluminum Alloys for Corrosion Protection. Coatings 2020, 10, 1106. [Google Scholar] [CrossRef]
- Iwasaki, M. Titanium Anodizing. J. Surf. Finish. Soc. Jpn. 2022, 73, 28–32. [Google Scholar] [CrossRef]
- de Sousa Araujo, J.V.; da Silva, R.M.P.; Klumpp, R.E.; Costa, I. The Anodizing Process of Aluminum and Its Alloys: A Historical and Electrochemical Approach. Quim. Nova 2021, 44, 999–1011. [Google Scholar] [CrossRef]
- Schneider, M.; Fürbeth, W. Anodizing—The Pore Makes the Difference. Mater. Corros. 2022, 73, 1752–1765. [Google Scholar] [CrossRef]
- Tsangaraki-Kaplanoglou, I.; Theohari, S.; Dimogerontakis, T.; Wang, Y.M.; Kuo, H.H.; Kia, S. Effect of Alloy Types on the Anodizing Process of Aluminum. Surf. Coat. Technol. 2006, 200, 2634–2641. [Google Scholar] [CrossRef]
- Schneider, M.; Kremmer, K.; Weidmann, S.K.; Fürbeth, W. Interplay between Parameter Variation and Oxide Structure of a Modified PAA Process. Surf. Interface Anal. 2013, 45, 1503–1509. [Google Scholar] [CrossRef]
- Kuntyi, O.; Zozulya, G.; Shepida, M. Porous Silicon Formation by Electrochemical Etching. Adv. Mater. Sci. Eng. 2022, 2022, 1482877. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, D.; Deng, H. Study on Material Removal Mechanisms in Electrochemical Etching-Enhanced Polishing of GaN. J. Manuf. Process 2022, 73, 903–913. [Google Scholar] [CrossRef]
- Fiuczek, N.; Sawicka, M.; Feduniewicz-Żmuda, A.; Siekacz, M.; Żak, M.; Nowakowski-Szkudlarek, K.; Muzioł, G.; Wolny, P.; Kelly, J.J.; Skierbiszewski, C. Electrochemical Etching of P-Type GaN Using a Tunnel Junction for Efficient Hole Injection. Acta Mater. 2022, 234, 118018. [Google Scholar] [CrossRef]
- Sun, W.; Shah, S.A.; Chen, Y.; Tan, Z.; Gao, H.; Habib, T.; Radovic, M.; Green, M.J. Electrochemical Etching of Ti2AlC to Ti2CT:X (MXene) in Low-Concentration Hydrochloric Acid Solution. J. Mater. Chem. A Mater. 2017, 5, 21663–21668. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, L.; Meunier, M.; Mirsaidov, U. Formation Pathways of Porous Alloy Nanoparticles through Selective Chemical and Electrochemical Etching. Small 2021, 17, 2006953. [Google Scholar] [CrossRef] [PubMed]
- Peng, N.; He, Y.; Yang, H. Effects of H2SO4 Content on Electrochemical Activation of Etched Tunnels on Aluminum Foil. Corrosion 2018, 74, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Peng, N.; Wen, Y.; He, Y. Improved Distribution of Etched Tunnels on Aluminum Foil with Silane Treatment. Prog. Org. Coat. 2019, 127, 151–156. [Google Scholar] [CrossRef]
- Kaya, D.; Keçeci, K. Review—Track-Etched Nanoporous Polymer Membranes as Sensors: A Review. J. Electrochem. Soc. 2020, 167, 037543. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, P.; Zhong, Y.; Dong, S.; Hou, M.; Liu, H.; Chen, X.; Gao, J.; Wong, C.-P. Review—Progress in Electrochemical Etching of Third-Generation Semiconductors. ECS J. Solid State Sci. Technol. 2023, 12, 045004. [Google Scholar] [CrossRef]
- Lin, J.C.; Liu, Y.C.; Lu, S.H.; Yen, H.N.; Settu, K. Enhancing the Specific Capacitance of a Porous Silicon-Based Capacitor by Embedding Graphene Combined with Three-Dimensional Electrochemical Etching. Electrochem. Commun. 2023, 154, 107555. [Google Scholar] [CrossRef]
- Lazanas, A.C.; Prodromidis, M.I. Electrochemical Impedance Spectroscopy—A Tutorial. ACS Meas. Sci. Au 2023, 3, 162–193. [Google Scholar] [CrossRef] [PubMed]
- Chmayssem, A.; Monsalve-Grijalba, K.; Alias, M.; Mourier, V.; Vignoud, S.; Scomazzon, L.; Muller, C.; Barthes, J.; Vrana, N.E.; Mailley, P. Reference Method for Off-Line Analysis of Nitrogen Oxides in Cell Culture Media by an Ozone-Based Chemiluminescence Detector. Anal. Bioanal. Chem. 2021, 413, 1383–1393. [Google Scholar] [CrossRef] [PubMed]
- Chmayssem, A.; Petit, L.; Verplanck, N.; Mourier, V.; Vignoud, S.; Vrana, N.E.; Mailley, P. Characterization of the Impact of Classical Cell-Culture Media on the Response of Electrochemical Sensors. Electroanalysis 2022, 34, 1201–1211. [Google Scholar] [CrossRef]
- Escarpa, A. Food Electroanalysis: Sense and Simplicity. Chem. Rec. 2012, 12, 72–91. [Google Scholar] [CrossRef] [PubMed]
- Bussooa, A.; Tubbs, E.; Revol-Cavalier, F.; Chmayssem, A.; Alessio, M.; Cosnier, M.-L.; Verplanck, N. Real-Time Monitoring of Oxygen Levels within Thermoplastic Organ-on-Chip Devices. SSRN Electron. J. 2022, 11, 4090175. [Google Scholar] [CrossRef]
- Bühlmann, P.; Pretsch, E.; Bakker, E. Carrier-Based Ion-Selective Electrodes and Bulk Optodes. 2. Ionophores for Potentiometric and Optical Sensors. Chem. Rev. 1998, 98, 1593–1688. [Google Scholar] [CrossRef]
- Lonsdale, W. Development, Manufacture and Application of a Solid-State PH Sensor Using Ruthenium Oxide; Edith Cowan University: Joondalup, WA, Australia, 2018. [Google Scholar]
- Liu, Y.; Pharr, M.; Salvatore, G.A. Lab-on-Skin: A Review of Flexible and Stretchable Electronics for Wearable Health Monitoring. ACS Nano 2017, 11, 9614–9635. [Google Scholar] [CrossRef]
- Gao, W.; Emaminejad, S.; Nyein, H.Y.Y.; Challa, S.; Chen, K.; Peck, A.; Fahad, H.M.; Ota, H.; Shiraki, H.; Kiriya, D.; et al. Fully Integrated Wearable Sensor Arrays for Multiplexed in Situ Perspiration Analysis. Nature 2016, 529, 509–514. [Google Scholar] [CrossRef]
- Soranzo, T.; Ben Tahar, A.; Chmayssem, A.; Zelsmann, M.; Vadgama, P.; Lenormand, J.L.; Cinquin, P.; Martin, D.K.; Zebda, A. Electrochemical Biosensing of Glucose Based on the Enzymatic Reduction of Glucose. Sensors 2022, 22, 7105. [Google Scholar] [CrossRef] [PubMed]
- Chmayssem, A.; Shalayel, I.; Marinesco, S.; Zebda, A. Investigation of GOx Stability in a Chitosan Matrix: Applications for Enzymatic Electrodes. Sensors 2023, 23, 465. [Google Scholar] [CrossRef] [PubMed]
- Chmayssem, A.; Nadolska, M.; Tubbs, E.; Sadowska, K.; Vadgma, P.; Shitanda, I.; Tsujimura, S.; Lattach, Y.; Peacock, M.; Tingry, S.; et al. Insight into Continuous Glucose Monitoring: From Medical Basics to Commercialized Devices. Microchim. Acta 2023, 190, 177. [Google Scholar] [CrossRef] [PubMed]
- Yoo, E.H.; Lee, S.Y. Glucose Biosensors: An Overview of Use in Clinical Practice. Sensors 2010, 10, 4558–4576. [Google Scholar] [CrossRef]
- Singh, N.; Goldsmith, B.R. Role of Electrocatalysis in the Remediation of Water Pollutants. ACS Catal. 2020, 10, 3365–3371. [Google Scholar] [CrossRef]
- Naidu, R.; Biswas, B.; Willett, I.R.; Cribb, J.; Kumar Singh, B.; Paul Nathanail, C.; Coulon, F.; Semple, K.T.; Jones, K.C.; Barclay, A.; et al. Chemical Pollution: A Growing Peril and Potential Catastrophic Risk to Humanity. Environ. Int. 2021, 156, 106616. [Google Scholar] [CrossRef]
- Isarain-Chávez, E.; De La Rosa, C.; Godínez, L.A.; Brillas, E.; Peralta-Hernández, J.M. Comparative Study of Electrochemical Water Treatment Processes for a Tannery Wastewater Effluent. J. Electroanal. Chem. 2014, 713, 62–69. [Google Scholar] [CrossRef]
- Feng, A.; Feng, J.; Xing, W.; Jiang, K.; Tang, W. Versatile Applications of Electrochemical Flow-through Systems in Water Treatment Processes. Chem. Eng. J. 2023, 473, 145400. [Google Scholar] [CrossRef]
- Alvarado, L.; Chen, A. Electrodeionization: Principles, Strategies and Applications. Electrochim. Acta 2014, 132, 583–597. [Google Scholar] [CrossRef]
- Salari, K.; Zarafshan, P.; Khashehchi, M.; Chegini, G.; Etezadi, H.; Karami, H.; Szulżyk-Cieplak, J.; Łagód, G. Knowledge and Technology Used in Capacitive Deionization of Water. Membranes 2022, 12, 459. [Google Scholar] [CrossRef]
- Wang, K.; Li, H.; Yang, Y.; Wang, P.; Zheng, Y.; Song, L. Making Cathode Composites More Efficient for Electro-Fenton and Bio-Electro-Fenton Systems: A Review. Sep. Purif. Technol. 2022, 304, 122302. [Google Scholar] [CrossRef]
- He, L.; Du, P.; Chen, Y.; Lu, H.; Cheng, X.; Chang, B.; Wang, Z. Advances in Microbial Fuel Cells for Wastewater Treatment. Renew. Sustain. Energy Rev. 2017, 71, 388–403. [Google Scholar] [CrossRef]
- Sillanpää, M.; Shestakova, M. Emerging and Combined Electrochemical Methods. In Electrochemical Water Treatment Methods: Fundamentals, Methods and Full Scale Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2017; Chapter 3; pp. 131–225. [Google Scholar] [CrossRef]
- Azanaw, A.; Birlie, B.; Teshome, B.; Jemberie, M. Textile Effluent Treatment Methods and Eco-Friendly Resolution of Textile Wastewater. Case Stud. Chem. Environ. Eng. 2022, 6, 100230. [Google Scholar] [CrossRef]
- Vineta, S.; Silvana, Z.; Sanja, R.; Golomeova, S. Methods for Waste Waters Treatment in Textile Industry. In Proceedings of the International Scientific Conference “UNITECH 2014”, Gabrovo, Bulgaria, 21–22 November 2014. [Google Scholar]
- Behera, M.; Nayak, J.; Banerjee, S.; Chakrabortty, S.; Tripathy, S.K. A Review on the Treatment of Textile Industry Waste Effluents towards the Development of Efficient Mitigation Strategy: An Integrated System Design Approach. J. Environ. Chem. Eng. 2021, 9, 105277. [Google Scholar] [CrossRef]
- Samsami, S.; Mohamadi, M.; Sarrafzadeh, M.H.; Rene, E.R.; Firoozbahr, M. Recent Advances in the Treatment of Dye-Containing Wastewater from Textile Industries: Overview and Perspectives. Process Saf. Environ. Prot. 2020, 143, 138–163. [Google Scholar] [CrossRef]
- Labidi, A.; Salaberria, A.M.; Fernandes, S.C.M.; Labidi, J.; Abderrabba, M. Functional Chitosan Derivative and Chitin as Decolorization Materials for Methylene Blue and Methyl Orange from Aqueous Solution. Materials 2019, 12, 361. [Google Scholar] [CrossRef]
- Meng, X.L.; Nie, Y.; Sun, J.; Cheng, W.G.; Wang, J.Q.; He, H.Y.; Zhang, S.J. Functionalized Dicyandiamide-Formaldehyde Polymers as Efficient Heterogeneous Catalysts for Conversion of CO2 into Organic Carbonates. Green. Chem. 2014, 16, 2771–2778. [Google Scholar] [CrossRef]
- Alinsafi, A.; Evenou, F.; Abdulkarim, E.M.; Pons, M.N.; Zahraa, O.; Benhammou, A.; Yaacoubi, A.; Nejmeddine, A. Treatment of Textile Industry Wastewater by Supported Photocatalysis. Dyes Pigments 2007, 74, 439–445. [Google Scholar] [CrossRef]
- Ilhan, F.; Ulucan-Altuntas, K.; Dogan, C.; Kurt, U. Treatability of Raw Textile Wastewater Using Fenton Process and Its Comparison with Chemical Coagulation. Desalination Water Treat. 2019, 162, 142–148. [Google Scholar] [CrossRef]
- Kaur, P.; Kushwaha, J.P.; Sangal, V.K. Electrocatalytic Oxidative Treatment of Real Textile Wastewater in Continuous Reactor: Degradation Pathway and Disposability Study. J. Hazard. Mater. 2018, 346, 242–252. [Google Scholar] [CrossRef]
- Mukimin, A.; Wijaya, K.; Kuncaka, A. Oxidation of Remazol Brilliant Blue r (RB.19) with in Situ Electro-Generated Active Chlorine Using Ti/PbO2 Electrode. Sep. Purif. Technol. 2012, 95, 1–9. [Google Scholar] [CrossRef]
- Chmayssem, A.; AlChoubassi, G.; Taha, S.; Hauchard, D. Electro-Fenton Process at Semi-Pilot Scale. A Study to Scale up the Reactor for Industrial Applications. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Park, S.; Lee, J.W.; Popov, B.N. A Review of Gas Diffusion Layer in PEM Fuel Cells: Materials and Designs. Int. J. Hydrogen Energy 2012, 37, 5850–5865. [Google Scholar] [CrossRef]
- Mukimin, A.; Vistanty, H.; Harihastuti, N.; Setianingsih, N.I.; Djayanti, S.; Nilawati; Astuti, Y. Hybrid Fenton-Electrochemical Reactor and System as Post-Treatment of Textile Wastewater. J. Water Process Eng. 2024, 59, 105028. [Google Scholar] [CrossRef]
- Zhu, C.; Ang, N.W.J.; Meyer, T.H.; Qiu, Y.; Ackermann, L. Organic Electrochemistry: Molecular Syntheses with Potential. ACS Cent. Sci. 2021, 7, 415–431. [Google Scholar] [CrossRef] [PubMed]
- Leech, M.C.; Lam, K. A Practical Guide to Electrosynthesis. Nat. Rev. Chem. 2022, 6, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Botte, G.G. Electrochemical Manufacturing in the Chemical Industry. Electrochem. Soc. Interface 2014, 23, 49–55. [Google Scholar] [CrossRef]
- Wang, F.; Li, W.; Wang, R.; Guo, T.; Sheng, H.; Fu, H.C.; Stahl, S.S.; Jin, S. Modular Electrochemical Synthesis Using a Redox Reservoir Paired with Independent Half-Reactions. Joule 2020, 5, 149–165. [Google Scholar] [CrossRef]
- Cui, B.; Shi, Y.; Li, G.; Chen, Y.; Chen, W.; Deng, Y.; Hu, W. Challenges and Opportunities for Seawater Electrolysis: A Mini-Review on Advanced Materials in Chlorine-Involved Electrochemistry. Wuli Huaxue Xuebao/Acta Phys.—Chim. Sin. 2022, 38, 2106010. [Google Scholar] [CrossRef]
- Li, Q.; Jensen, J.O.; Bjerrum, N.J. Chemistry, Electrochemistry, and Electrochemical Applications|Aluminum. In Encyclopedia of Electrochemical Power Sources; Elsevier Inc.: Amsterdam, The Netherlands, 2009. [Google Scholar] [CrossRef]
- Comer, B.M.; Fuentes, P.; Dimkpa, C.O.; Liu, Y.H.; Fernandez, C.A.; Arora, P.; Realff, M.; Singh, U.; Hatzell, M.C.; Medford, A.J. Prospects and Challenges for Solar Fertilizers. Joule 2019, 3, 1578–1605. [Google Scholar] [CrossRef]
- Fu, N.; Sauer, G.S.; Lin, S. A General, Electrocatalytic Approach to the Synthesis of Vicinal Diamines. Nat. Protoc. 2018, 13, 1725–1743. [Google Scholar] [CrossRef]
- Zheng, S.; Yan, J.; Wang, K. Engineering Research Progress of Electrochemical Microreaction Technology—A Novel Method for Electrosynthesis of Organic Chemicals. Engineering 2021, 7, 22–32. [Google Scholar] [CrossRef]
- Anastasiadou, D. Cu-Based Electrodes for Ammonia and Urea Electrosynthesis. Ph.D. Thesis, Eindhoven University of Technology, Eindhoven, The Netherlands, 2023. [Google Scholar]
- Chen, X.; Lv, S.; Su, Z.; Yang, X.; Cui, H.; Yang, Z.; Xu, Z.; Teobaldi, G.; Kang, J.; Liu, L.-M.; et al. Recent Progress in Amorphous Nanomaterials for Electrochemical Synthesis of N-Containing Compounds. Chem. Catal. 2024, 100871. [Google Scholar] [CrossRef]
- Botte, G.G. Transitioning Electrochemical Technologies into Agriculture via the National Science Foundation Engineering Research Center Model. Electrochem. Soc. Interface 2023, 32, 69–73. [Google Scholar] [CrossRef]
- Meyer, T.H.; Choi, I.; Tian, C.; Ackermann, L. Powering the Future: How Can Electrochemistry Make a Difference in Organic Synthesis? Chem 2020, 6, 2484–2496. [Google Scholar] [CrossRef]
- Talebi, M.R.; Nematollahi, D. Electrochemical Synthesis of Sulfonamide Derivatives: Electrosynthesis Conditions and Reaction Pathways. ChemElectroChem 2024, 11, e202300728. [Google Scholar] [CrossRef]
- Zuo, S.; Xue, W.; Zhang, Y.; Chen, J.; Lin, Z. Front Cover: Atomically Dispersed Fe Motif-based Electrocatalysts for Hydrogen Peroxide Synthesis (ChemNanoMat 2/2024). ChemNanoMat 2024, 10, 202400093. [Google Scholar] [CrossRef]
- Zuo, S.; Xue, W.; Zhang, Y.; Chen, J.; Lin, Z. Atomically Dispersed Fe Motif-based Electrocatalysts for Hydrogen Peroxide Synthesis. ChemNanoMat 2024, 10, 202300476. [Google Scholar] [CrossRef]
- Schremp, F.W. Corrosion prevention for offshore platforms. JPT J. Pet. Technol. 1984, 36, 605–612. [Google Scholar] [CrossRef]
- Chen, Y.; He, M.; Zong, Y.; Dong, S.; Yan, Y.; Zhang, Y.; Liu, H.; Cao, J. Research Progress on Failure Modes of Unbonded Flexible Pipe and Their Control Measures. Nat. Gas. Ind. 2021, 41, 122–131. [Google Scholar] [CrossRef]
- Mahajanam, S.P.V.; Joosten, M.W. Guidelines for Filler-Material Selection to Minimize Preferential Weld Corrosion in Pipeline Steels. SPE Proj. Facil. Constr. 2011, 6, 5–12. [Google Scholar] [CrossRef]
- Erdogan, C.; Swain, G. Conceptual Sacrificial Anode Cathodic Protection Design for Offshore Wind Monopiles. Ocean Eng. 2021, 235, 109339. [Google Scholar] [CrossRef]
- Popov, B.N. Corrosion Engineering: Principles and Solved Problems; Elsevier: Amsterdam, Netherlands, 2015. [Google Scholar] [CrossRef]
- Ahmad, Z. Principles of Corrosion Engineering and Corrosion Control; Elsevier: Burlington, MA, USA, 2006. [Google Scholar] [CrossRef]
- Cicek, V. Corrosion Engineering; Wiley: Hoboken, NJ, USA, 2014; ISBN 9781118720899. [Google Scholar] [CrossRef]
- Pyun, S.-I. Strategies of Metal Corrosion Protection. ChemTexts 2020, 7, 2. [Google Scholar] [CrossRef]
- Ducasse-Lapeyrusse, J.; Bouteiller, V.; Marie-Victoire, E.; Bouichou, M.; Damien, G.; Martinet, V.; Annede-Villeau, C.; Lesieutre, O. Assessment of the Impressed Current Cathodic Protection System after 4 Years Operation: Case Study of the Saint-Cloud Viaduct (France). Case Stud. Constr. Mater. 2023, 18, e02023. [Google Scholar] [CrossRef]
- Xu, J.; Yang, M.; Li, S.; Kainuma, S.; Ji, B.; Murayama, S. Application of Cathodic Protection Method on Steel Structures Using Sacrificial Anode and Sodium Polyacrylate-Sodium Carboxymethyl Cellulose (PANa–CMC) Hydrogel Electrolyte. Case Stud. Constr. Mater. 2024, 20, e02742. [Google Scholar] [CrossRef]
- Ramanavasu, R.; Vijayakumar, K.; Fernandez, S.G. Integrated Nanogrid for the Impressed Current Cathodic Protection System in Desalination Plant. Sustainability 2023, 15, 7088. [Google Scholar] [CrossRef]
- Yao, G.; He, X.; Liu, J.; Guo, Z.; Chen, P. Test Study of the Bridge Cable Corrosion Protection Mechanism Based on Impressed Current Cathodic Protection. Lubricants 2023, 11, 30. [Google Scholar] [CrossRef]
- Dickinson, E.J.F.; Ekström, H.; Fontes, E. COMSOL Multiphysics®: Finite Element Software for Electrochemical Analysis. A Mini-Review. Electrochem. Commun. 2014, 40, 71–74. [Google Scholar] [CrossRef]
- Jalalvand, A.R.; Roushani, M.; Goicoechea, H.C.; Rutledge, D.N.; Gu, H.W. MATLAB in Electrochemistry: A Review. Talanta 2018, 194, 205–225. [Google Scholar] [CrossRef]
- Weddle, P.; Vincent, T.; Kee, R.J. Identifying and Applying State-Space Models Derived from High-Fidelity Physical Models of Li-Ion Batteries. ECS Meet. Abstr. 2017, MA2017-01. [Google Scholar] [CrossRef]
- Bennett, B.; Chang, J.; Bard, A.J. Mechanism of the Br−/Br2 Redox Reaction on Platinum and Glassy Carbon Electrodes in Nitrobenzene by Cyclic Voltammetry. Electrochim. Acta 2016, 219, 1–9. [Google Scholar] [CrossRef]
- Habekost, A. Simulation and Fitting of Cyclic Voltammetry and Chronoamperometry Curves of Electrochemical Reactions with Different Mechanisms—A Didactic Perspective. World J. Chem. Educ. 2019, 7, 53–64. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z. Animated Electrochemistry Simulation Modules. J. Chem. Educ. 2022, 99, 752–758. [Google Scholar] [CrossRef]
- Jaugstetter, M.; Blanc, N.; Kratz, M.; Tschulik, K. Electrochemistry under Confinement. Chem. Soc. Rev. 2022, 51, 2491–2543. [Google Scholar] [CrossRef]
- Weiß, L.J.K.; Lubins, G.; Music, E.; Rinklin, P.; Banzet, M.; Peng, H.; Terkan, K.; Mayer, D.; Wolfrum, B. Single-Impact Electrochemistry in Paper-Based Microfluidics. ACS Sens. 2022, 7, 884–892. [Google Scholar] [CrossRef]
- Zhou, Y.; Qi, J.; Engelberg, D.L. On the Application of Bipolar Electrochemistry for Simulating Galvanic Corrosion Behaviour of Dissimilar Stainless Steels. Electrochem. Commun. 2021, 126, 107023. [Google Scholar] [CrossRef]
- Tettey, F.; Parupelli, S.K.; Desai, S. A Review of Biomedical Devices: Classification, Regulatory Guidelines, Human Factors, Software as a Medical Device, and Cybersecurity. Biomed. Mater. Devices 2023, 2, 316–341. [Google Scholar] [CrossRef]
- Marcus, H.J.; Payne, C.J.; Hughes-Hallett, A.; Marcus, A.P.; Yang, G.Z.; Darzi, A.; Nandi, D. Regulatory Approval of New Medical Devices: Cross Sectional Study. BMJ 2016, 353, i2587. [Google Scholar] [CrossRef]
- Wagner, M.V.; Schanze, T. Comparison of Approval Procedures for Medical Devices in Europe and the USA. Curr. Dir. Biomed. Eng. 2019, 5, 605–608. [Google Scholar] [CrossRef]
- Robinson, S.G.; Sigman, M.S. Integrating Electrochemical and Statistical Analysis Tools for Molecular Design and Mechanistic Understanding. Acc. Chem. Res. 2020, 53, 289–299. [Google Scholar] [CrossRef]
- Rivera, F.F.; Pérez, T.; Castañeda, L.F.; Nava, J.L. Mathematical Modeling and Simulation of Electrochemical Reactors: A Critical Review. Chem. Eng. Sci. 2021, 239, 116622. [Google Scholar] [CrossRef]
- Lu, S.-M.; Vannoy, K.J.; Dick, J.E.; Long, Y.-T. Multiphase Chemistry under Nanoconfinement: An Electrochemical Perspective. J. Am. Chem. Soc. 2023, 145, 25043–25055. [Google Scholar] [CrossRef]
- Ma, Z.; Witteman, L.; Wrubel, J.A.; Bender, G. A Comprehensive Modeling Method for Proton Exchange Membrane Electrolyzer Development. Int. J. Hydrogen Energy 2021, 46, 17627–17643. [Google Scholar] [CrossRef]
- Iliev, I.K.; Gizzatullin, A.R.; Filimonova, A.A.; Chichirova, N.D.; Beloev, I.H. Numerical Simulation of Processes in an Electrochemical Cell Using COMSOL Multiphysics. Energies 2023, 16, 7265. [Google Scholar] [CrossRef]
- Yaoxuan, Q.; Cheng, F.; Kening, S. Multiphysics Simulation of a Solid Oxide Fuel Cell Based on Comsol Method. In E3S Web of Conferences; EDP Sciences: Paris, France, 2021; Volume 245. [Google Scholar] [CrossRef]
- Goel, V.; Thornton, K. Enabling the Electrochemical Simulation of Li-Ion Battery Electrodes with Anisotropic Tortuosity in COMSOL Multiphysics®. MethodsX 2021, 8, 101425. [Google Scholar] [CrossRef]
- Salazar, P.F.; Kumar, S.; Cola, B.A. Design and Optimization of Thermo-Electrochemical Cells. J. Appl. Electrochem. 2013, 44, 325–336. [Google Scholar] [CrossRef]
- Yang, W.; Sun, L.; Bao, J.; Mo, Z.; Du, M.; Xu, Y.; Zhang, J. Multiphysics Modeling of Mass and Heat Transfer in a Thermo-Electrochemical Cell. Ind. Eng. Chem. Res. 2023, 62, 12345–12355. [Google Scholar] [CrossRef]
- El-Shafie, O.A.; El-Maghraby, R.M.; Albo, J.; Fateen, S.E.K.; Abdelghany, A. Modeling and Numerical Investigation of the Performance of Gas Diffusion Electrodes for the Electrochemical Reduction of Carbon Dioxide to Methanol. Ind. Eng. Chem. Res. 2020, 59, 20929–20942. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, J.; Guo, C.; Zhao, S. Investigation of Electrochemical Machining for Gradual Change Special-Shaped Deep Spiral Hole Based on COMSOL. Int. J. Adv. Manuf. Technol. 2020, 108, 2717–2725. [Google Scholar] [CrossRef]
- Cordova-Huaman, A.V.; Jauja-Ccana, V.R.; La Rosa-Toro, A. Low-Cost Smartphone-Controlled Potentiostat Based on Arduino for Teaching Electrochemistry Fundamentals and Applications. Heliyon 2021, 7, e06259. [Google Scholar] [CrossRef]
- Ahmad, N.J.; Yakob, N.; Bunyamin, M.A.H.; Winarno, N.; Akmal, W.H. The Effect of Interactive Computer Animation and Simulation on Students’ Achievement and Motivation in Learning Electrochemistry. J. Pendidik. IPA Indones. 2021, 10, 311–324. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).