Recent Advances in Applied Electrochemistry: A Review
Abstract
:1. Introduction
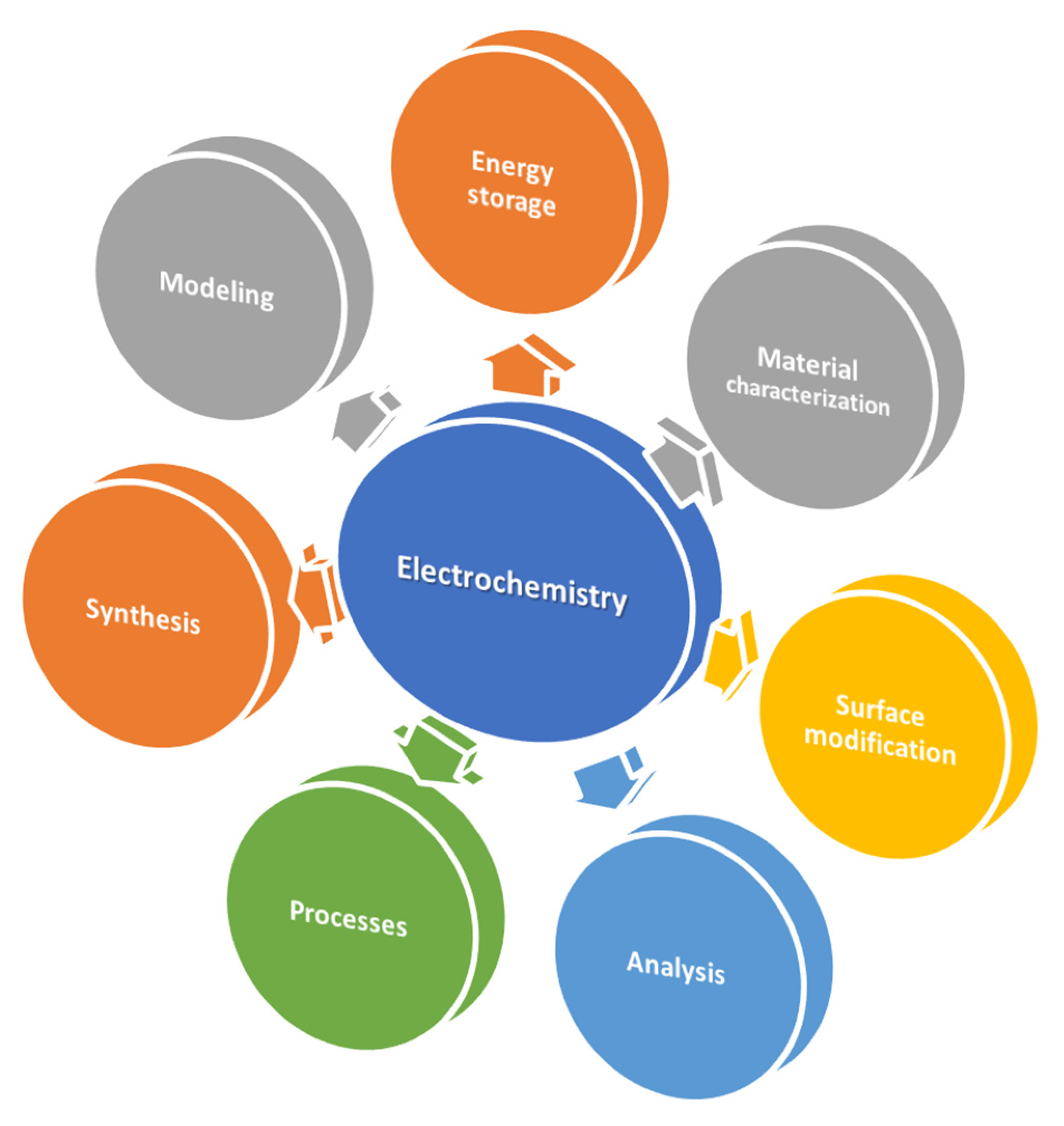
2. Application Field
2.1. Energy Conversion and Storage
| Type of Battery | Chemistry of Battery | Main Application | Specific Energy (Wh/Kg) | Advantages | Disadvantages |
|---|---|---|---|---|---|
| Lead Acid [58] | Pb-A | Automotive industry (EV) and industrial use | 30–50 | - Low cost - Well-established manufacturing processes and widely available - High-rate performance in various conditions | - Limited energy density - Short cycle life (500–1000 cycles) - Maintenance requirements involving issues such as acid stratification and leakage -Environmental concerns associated with the use of lead (heavy metal) - Limited use in applications where weight is a concern |
| Lithium-Ion batteries (LIBs) (Many possible chemistries), [63,65] LIBs have a higher energy density allowing for lighter-weight designs, higher stability, and durability compared to Pb-A. Sensitive to Low temperatures, but improvements ongoing, and becoming more cost-competitive with advancements. | Lithium Manganese oxide (LMO) | Portable electronics (e.g., smartphones, laptops)—Power tools | 100–150 | - Lower cost - Improved environmental friendliness - Better thermal stability and safety compared to LCO, making them suitable for some electric vehicles | - Lower energy density - Reduced cycle life (300–700 cycles) |
| Lithium Cobalt oxide (LCO) | 150–250 | -Higher energy density. -Longer cycle life (500–1000 cycles) | - Higher cost - Environmental concerns due to cobalt mining | ||
| Lithium Iron phosphate (LFP) | Power tools and EV | 90–120 | - Safest - Better Thermal stability | - Lowest energy density among the three cathodes | |
| Lithium Nickel Cobalt Aluminum oxide (NCA) | Grid storage and EV | 155–260 | - Highest Energy density | - Lower thermal stability - Battery thermal Management Systems (BTMS) requirements | |
| Lithium Nickel Manganese Cobalt Oxide (NMC) | Power tools, EV | 150–200 | - Moderate energy density and safety | - BTMS requirements | |
| Polymer-Based Batteries, [68,69] Company: Evonik - Employ unique processing techniques such as printing | Redox active polymers for either the cathode, anode, or both electrodes. | Medical and Logistics fields | 50–200 | - Fabrication of thin and flexible batteries - High-rate capability - Metal free and Recyclability | - Limitations in terms of discharge capacities and voltage outputs - Restricted applicability to low-power systems |
| Biofuel [70,71,72,73,74] | Enzymatic Fuel cells | Ideal for wearables and implants: - Compact - Integrable - Biosafe | 20–300 | - Inexpensive - Lightweight - Flexible - Eco-friendly - Biodegradable - Capable of generating electricity from various kinds of organic matters | - Low energy density - Short-time stability (decreasing power output over time) |
| Microbial Fuel cells | Focused on macro-scale power generation from surroundings - Suitable for Minimal power demand applications (ex: wastewater treatment) |
2.2. Material Characterization
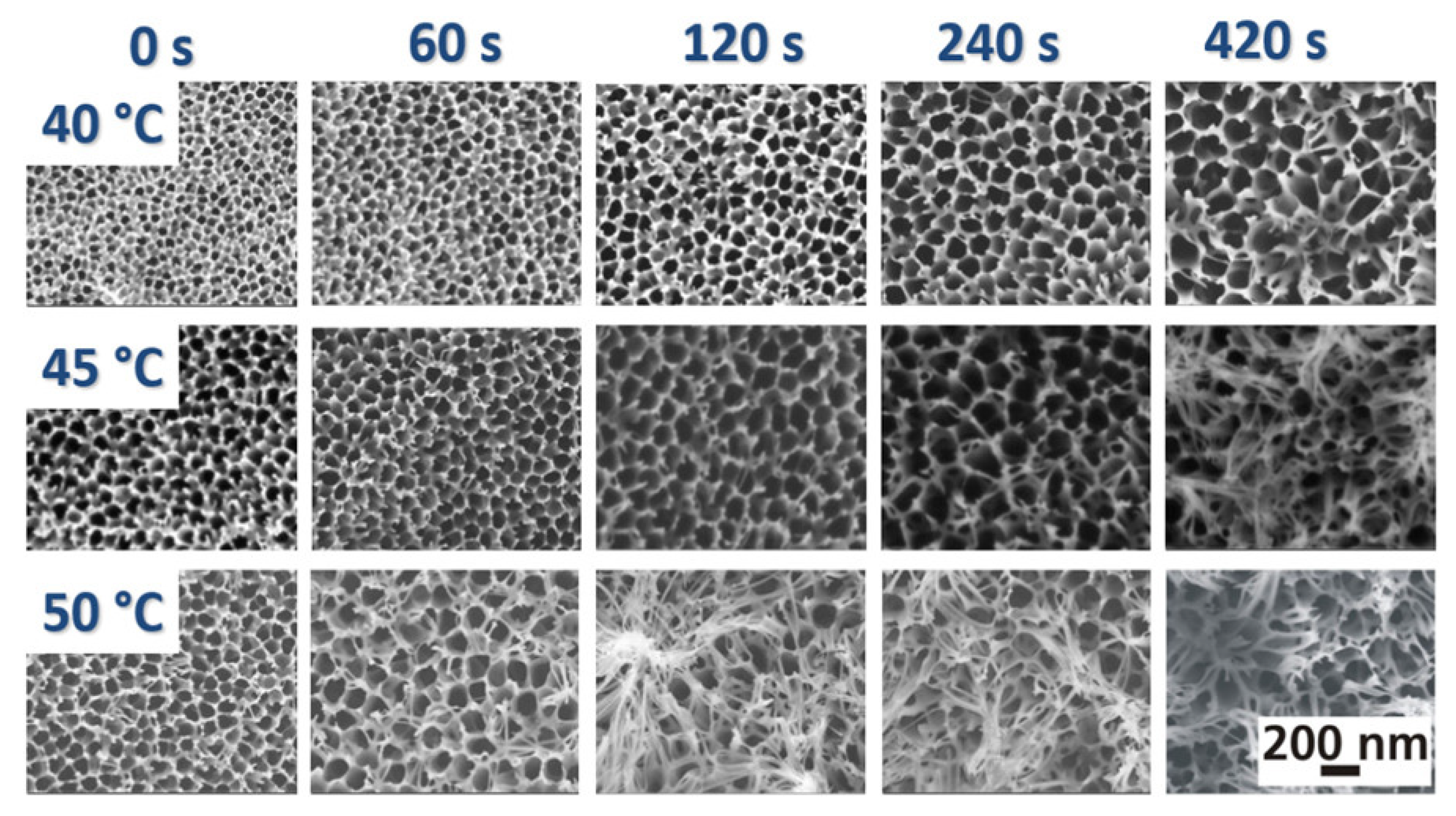
2.3. Surface Modification
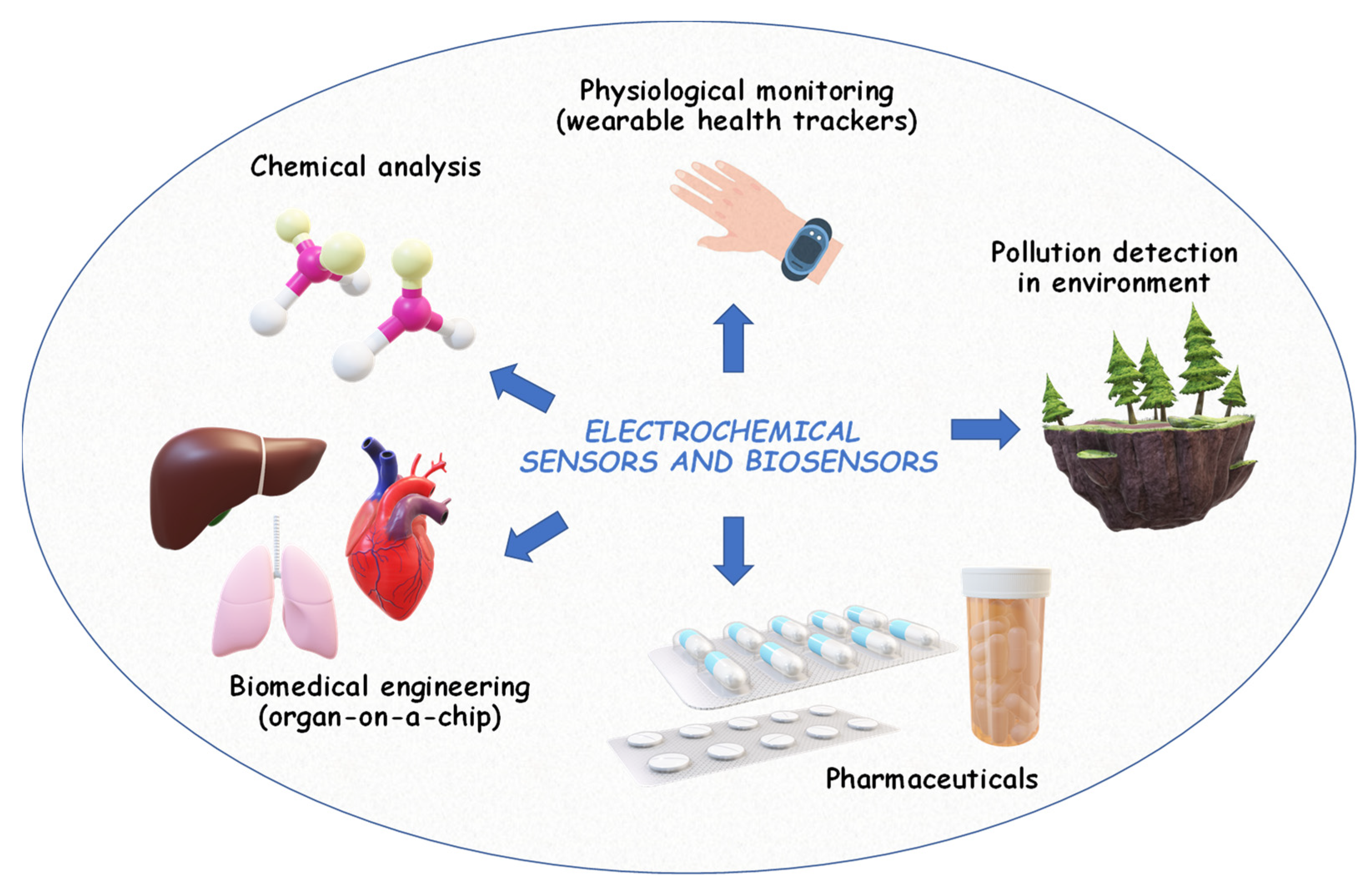
2.4. Electroanalysis
2.5. Electrochemical Processes for Depollution and Water Remediation
2.6. Electrosynthesis
- -
- Allow the use of green oxidants, which reduces the use of toxic compounds/solvents and prevents environmental risks;
- -
- Rely on electrification as an energy source, which decreases the amount and cost of the energy consumed;
- -
- Prevent the uses of additional catalysts;
- -
- Rely on a less complicated setup for the simplicity of the technical procedure.
2.7. Electrochemical Protection
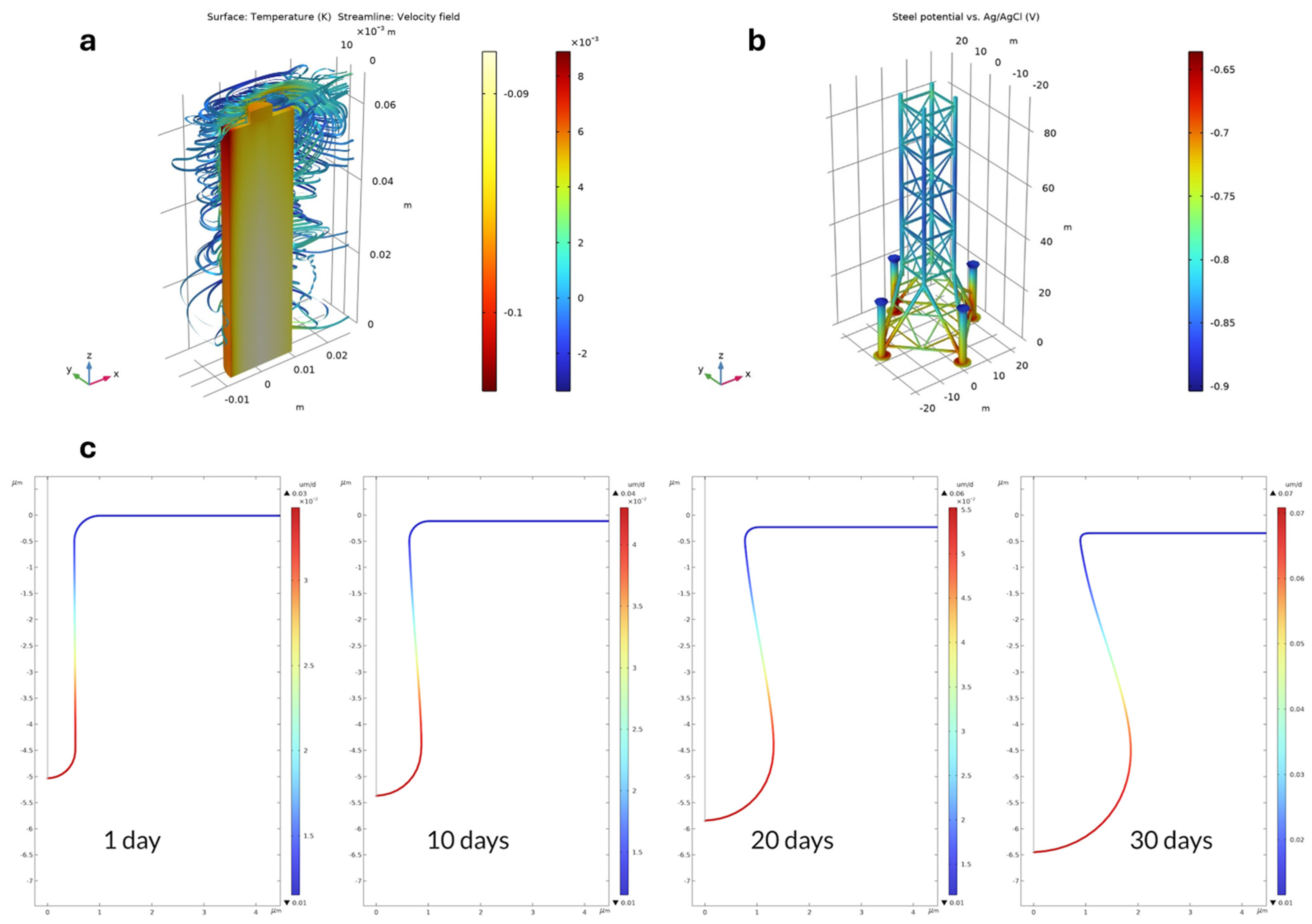
2.8. Tools for Electrochemical Modeling
2.9. Electrochemistry in Education
2.10. Electrochemical Companies
3. Conclusions
Funding
Conflicts of Interest
References
- Singh, V.G. Applied Electrochemistry; Nova Science Publisher Inc.: New York, NY, USA, 2010. [Google Scholar] [CrossRef]
- Wragg, A.A. Journal of Applied Electrochemistry; Springer Nature: Berlin, Germany, 2009; Volume 39, p. 467. [Google Scholar] [CrossRef]
- Bevan, K.H.; Foong, Y.W.; Shirani, J.; Yuan, S.; Abi Farraj, S. Physics Applied to Electrochemistry: Tunneling Reactions. J. Appl. Phys. 2021, 129, 090901. [Google Scholar] [CrossRef]
- Duan, J.; Tang, X.; Dai, H.; Yang, Y.; Wu, W.; Wei, X.; Huang, Y. Building Safe Lithium-Ion Batteries for Electric Vehicles: A Review. Electrochem. Energy Rev. 2020, 3, 1–42. [Google Scholar] [CrossRef]
- Brinkert, K.; Mandin, P. Fundamentals and Future Applications of Electrochemical Energy Conversion in Space. npj Microgravity 2022, 8, 52. [Google Scholar] [CrossRef] [PubMed]
- Santoro, C.; Arbizzani, C.; Erable, B.; Ieropoulos, I. Microbial Fuel Cells: From Fundamentals to Applications. A Review. J. Power Sources 2017, 356, 225–244. [Google Scholar] [CrossRef] [PubMed]
- Sazali, N.; Salleh, W.N.W.; Jamaludin, A.S.; Razali, M.N.M. New Perspectives on Fuel Cell Technology: A Brief Review. Membranes 2020, 10, 99. [Google Scholar] [CrossRef] [PubMed]
- Esposito, D.V. Membraneless Electrolyzers for Low-Cost Hydrogen Production in a Renewable Energy Future. Joule 2017, 1, 651–658. [Google Scholar] [CrossRef]
- Abbasi, R.; Setzler, B.P.; Lin, S.; Wang, J.; Zhao, Y.; Xu, H.; Pivovar, B.; Tian, B.; Chen, X.; Wu, G.; et al. A Roadmap to Low-Cost Hydrogen with Hydroxide Exchange Membrane Electrolyzers. Adv. Mater. 2019, 31, e1805876. [Google Scholar] [CrossRef] [PubMed]
- Chmayssem, A.; Taha, S.; Hauchard, D. Scaled-up Electrochemical Reactor with a Fixed Bed Three-Dimensional Cathode for Electro-Fenton Process: Application to the Treatment of Bisphenol A. Electrochim. Acta 2017, 225, 435–442. [Google Scholar] [CrossRef]
- Yu, Y.; Zhong, Y.; Sun, W.; Xie, J.; Wang, M.; Guo, Z. A Novel Electrocoagulation Process with Centrifugal Electrodes for Wastewater Treatment: Electrochemical Behavior of Anode and Kinetics of Heavy Metal Removal. Chemosphere 2023, 310, 136862. [Google Scholar] [CrossRef]
- Tran, T.K.; Chiu, K.F.; Lin, C.Y.; Leu, H.J. Electrochemical Treatment of Wastewater: Selectivity of the Heavy Metals Removal Process. Int. J. Hydrogen Energy 2017, 42, 27741–27748. [Google Scholar] [CrossRef]
- Shrestha, R.; Ban, S.; Devkota, S.; Sharma, S.; Joshi, R.; Tiwari, A.P.; Kim, H.Y.; Joshi, M.K. Technological Trends in Heavy Metals Removal from Industrial Wastewater: A Review. J. Environ. Chem. Eng. 2021, 9, 105688. [Google Scholar] [CrossRef]
- Yue, H.; Xue, L.; Chen, F. Efficiently Electrochemical Removal of Nitrite Contamination with Stable RuO2-TiO2/Ti Electrodes. Appl. Catal. B 2017, 206, 683–691. [Google Scholar] [CrossRef]
- Dao, K.C.; Yang, C.C.; Chen, K.F.; Tsai, Y.P. Recent Trends in Removal Pharmaceuticals and Personal Care Products by Electrochemical Oxidation and Combined Systems. Water 2020, 12, 1043. [Google Scholar] [CrossRef]
- Bhat, R.S.; Shetty, S.M.; Kumar, N.A. Electroplating of Zn-Ni Alloy Coating on Mild Steel and Its Electrochemical Studies. J. Mater. Eng. Perform. 2021, 30, 8188–8195. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, N.; Gilchrist, M.; Fang, F. Advances in Precision Micro/Nano-Electroforming: A State-of-the-Art Review. J. Micromech. Microeng. 2020, 30, 103002. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, Y. Electrochemical Machining of Complex Components of Aero-Engines: Developments, Trends, and Technological Advances. Chin. J. Aeronaut. 2019, 34, 28–53. [Google Scholar] [CrossRef]
- Zhang, Y.; Lan, J.; Xu, Y.; Yan, Y.; Liu, W.; Liu, X.; Gu, S.; Zhou, J.; Wang, M. Ultrafine PtCo Alloy by Pyrolysis Etching-Confined Pyrolysis for Enhanced Hydrogen Evolution. J. Colloid. Interface Sci. 2024, 660, 997–1009. [Google Scholar] [CrossRef]
- Zhan, Y.; Zhang, G.; Shen, J.; Zhou, B.; Zhao, C.; Guo, J.; Wen, M.; Tan, Z.; Zheng, L.; Lu, J.; et al. Facile Electrochemical Surface-Alloying and Etching of Au Wires to Enable High-Performance Substrates for Surface Enhanced Raman Scattering. Nano Mater. Sci. 2023. [Google Scholar] [CrossRef]
- René, A.; Abasq, M.L.; Hauchard, D.; Hapiot, P. How Do Phenolic Compounds React toward Superoxide Ion? A Simple Electrochemical Method for Evaluating Antioxidant Capacity. Anal. Chem. 2010, 82, 8703–8710. [Google Scholar] [CrossRef]
- Keyrouz, R.; Abasq, M.L.; Le Bourvellec, C.; Blanc, N.; Audibert, L.; Argall, E.; Hauchard, D. Total Phenolic Contents, Radical Scavenging and Cyclic Voltammetry of Seaweeds from Brittany. Food Chem. 2011, 126, 831–836. [Google Scholar] [CrossRef]
- Chmayssem, A.; Verplanck, N.; Boizot, F.; Alessio, M.; Santos, L.; Mourier, V.; Vignoud, S.; Navarro, F.; Mailley, P. Microfluidic platform of electrochemical biosensors for organ-on-chip applications. In Proceedings of the MicroTAS 2021—25th International Conference on Miniaturized Systems for Chemistry and Life Sciences, Palm Springs, CA, USA, 10–14 October 2021. [Google Scholar]
- Chmayssem, A.; Hauchard, D. New Detection Method for Alkylphenol Traces in Water Based on an Integrated Electrochemical Cell Sensor. Rev. Sci. l’Eau 2015, 28, 35–40. [Google Scholar] [CrossRef]
- Hauchard, D.; Cassir, M.; Chivot, J.; Baudry, D.; Ephritikhine, M. Electrochemical Study of Uranium (IV) and (III) Organometallic Compounds in Tetrahydrofuran by Means of Conventional Microelectrodes and Ultramicroelectrodes. Part II. Application to Borohydride Compounds-Study of the Stability of CP2U(BH4)2. J. Electroanal. Chem. 1993, 347, 399–407. [Google Scholar] [CrossRef]
- Terbouche, A.; Lameche, S.; Ait-Ramdane-Terbouche, C.; Guerniche, D.; Lerari, D.; Bachari, K.; Hauchard, D. A New Electrochemical Sensor Based on Carbon Paste Electrode/Ru(III) Complex for Determination of Nitrite: Electrochemical Impedance and Cyclic Voltammetry Measurements. Measurement 2016, 92, 524–533. [Google Scholar] [CrossRef]
- Shin, K.Y.; Oum, W.; Yu, D.J.; Kang, S.; Kim, E.B.; Kim, H.W. Fundamentals of Cyclic Voltammetry. J. Sens. Sci. Technol. 2021, 30, 384–387. [Google Scholar] [CrossRef]
- Inzelt, G. Chronoamperometry, Chronocoulometry, and Chronopotentiometry. In Encyclopedia of Applied Electrochemistry; Springer Nature: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Bakker, E.; Pretsch, E. Modern Potentiometry. Angew. Chem.—Int. Ed. 2007, 46, 5660–5668. [Google Scholar] [CrossRef] [PubMed]
- Chmayssem, A.; Verplanck, N.; Tanase, C.E.; Costa, G.; Monsalve-Grijalba, K.; Amigues, S.; Alias, M.; Gougis, M.; Mourier, V.; Vignoud, S.; et al. Development of a Multiparametric (Bio)Sensing Platform for Continuous Monitoring of Stress Metabolites. Talanta 2021, 229, 122275. [Google Scholar] [CrossRef]
- Chmayssem, A.; Hauchard, D. Direct Ultra-Trace Detection of Alkylphenols in Water Using a Cavity Carbon-Paste Microelectrode Sensor. Desalination Water Treat. 2017, 83, 321–326. [Google Scholar] [CrossRef]
- Liu, Y.; Xue, Q.; Chang, C.; Wang, R.; Liu, Z.; He, L. Recent Progress Regarding Electrochemical Sensors for the Detection of Typical Pollutants in Water Environments. Anal. Sci. 2022, 38, 55–70. [Google Scholar] [CrossRef]
- Karthik, V.; Selvakumar, P.; Senthil Kumar, P.; Satheeskumar, V.; Godwin Vijaysunder, M.; Hariharan, S.; Antony, K. Recent Advances in Electrochemical Sensor Developments for Detecting Emerging Pollutant in Water Environment. Chemosphere 2022, 304, 135331. [Google Scholar] [CrossRef]
- Boumya, W.; Taoufik, N.; Achak, M.; Bessbousse, H.; Elhalil, A.; Barka, N. Electrochemical Sensors and Biosensors for the Determination of Diclofenac in Pharmaceutical, Biological and Water Samples. Talanta Open 2020, 3, 100026. [Google Scholar] [CrossRef]
- Sohrabi, H.; Dezhakam, E.; Khataee, A.; Nozohouri, E.; Majidi, M.R.; Mohseni, N.; Trofimov, E.; Yoon, Y. Recent Trends in Layered Double Hydroxides Based Electrochemical and Optical (Bio)Sensors for Screening of Emerging Pharmaceutical Compounds. Environ. Res. 2022, 211, 113068. [Google Scholar] [CrossRef] [PubMed]
- Malzahn, K.; Windmiller, J.R.; Valdés-Ramírez, G.; Schöning, M.J.; Wang, J. Wearable Electrochemical Sensors for in Situ Analysis in Marine Environments. Analyst 2011, 136, 2912–2917. [Google Scholar] [CrossRef] [PubMed]
- European Commission. An EU Strategy to Harness the Potential of Offshore Renewable Energy for a Climate Neutral Future; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- Dong, J.; Zeng, J.; Yang, Y.; Wang, H. A Review of Law and Policy on Decarbonization of Shipping. Front. Mar. Sci. 2022, 9, 1076352. [Google Scholar] [CrossRef]
- Madurai Elavarasan, R.; Pugazhendhi, R.; Irfan, M.; Mihet-Popa, L.; Khan, I.A.; Campana, P.E. State-of-the-Art Sustainable Approaches for Deeper Decarbonization in Europe—An Endowment to Climate Neutral Vision. Renew. Sustain. Energy Rev. 2022, 159, 112204. [Google Scholar] [CrossRef]
- Beccarello, M.; Di Foggia, G. Review and Perspectives of Key Decarbonization Drivers to 2030. Energies 2023, 16, 1345. [Google Scholar] [CrossRef]
- Nesterenko, N.; Medeiros-Costa, I.C.; Clatworthy, E.B.; Cruchade, H.; Konnov, S.V.; Dath, J.P.; Gilson, J.P.; Mintova, S. Methane-to-Chemicals: A Pathway to Decarbonization. Natl. Sci. Rev. 2023, 10, nwad116. [Google Scholar] [CrossRef]
- Papadis, E.; Tsatsaronis, G. Challenges in the Decarbonization of the Energy Sector. Energy 2020, 205, 118025. [Google Scholar] [CrossRef]
- Tautorat, P.; Lalin, B.; Schmidt, T.S.; Steffen, B. Directions of Innovation for the Decarbonization of Cement and Steel Production—A Topic Modeling-Based Analysis. J. Clean. Prod. 2023, 407, 137055. [Google Scholar] [CrossRef]
- Zier, M.; Stenzel, P.; Kotzur, L.; Stolten, D. A Review of Decarbonization Options for the Glass Industry. Energy Convers. Manag. X 2021, 10, 100083. [Google Scholar] [CrossRef]
- Ghisolfi, V.; Tavasszy, L.A.; Correia, G.H.d.A.; Chaves, G.d.L.D.; Ribeiro, G.M. Freight Transport Decarbonization: A Systematic Literature Review of System Dynamics Models. Sustainability 2022, 14, 3625. [Google Scholar] [CrossRef]
- Raza, M.Y.; Zhongpan, Q.; Pengju, W. Agriculture-Related Energy Consumption, Food Policy, and CO2 Emission Reduction: New Insights from Pakistan. Front. Environ. Sci. 2023, 10, 1099813. [Google Scholar] [CrossRef]
- Palou-Rivera, I.; Grieco, W. Electrochemical Processes Role in the Drive for Industrial Decarbonization. ECS Meet. Abstr. 2022, MA2022-01, 2338. [Google Scholar] [CrossRef]
- Griffiths, S.; Sovacool, B.K.; Kim, J.; Bazilian, M.; Uratani, J.M. Industrial Decarbonization via Hydrogen: A Critical and Systematic Review of Developments, Socio-Technical Systems and Policy Options. Energy Res. Soc. Sci. 2021, 80, 102208. [Google Scholar] [CrossRef]
- Schiffer, Z.J.; Manthiram, K. Electrification and Decarbonization of the Chemical Industry. Joule 2017, 1, 10–14. [Google Scholar] [CrossRef]
- Mallapragada, D.S.; Dvorkin, Y.; Modestino, M.A.; Esposito, D.V.; Smith, W.A.; Hodge, B.M.; Harold, M.P.; Donnelly, V.M.; Nuz, A.; Bloomquist, C.; et al. Decarbonization of the Chemical Industry through Electrification: Barriers and Opportunities. Joule 2023, 7, 23–41. [Google Scholar] [CrossRef]
- Brannstrom, C. The Emerging Energy Future(s) of Renewable Power and Electrochemistry: Advancing or Undermining Energy Democracy? In Energy Democracies for Sustainable Futures; Academic Press: Cambridge, MA, USA, 2022. [Google Scholar] [CrossRef]
- Xia, R.; Overa, S.; Jiao, F. Emerging Electrochemical Processes to Decarbonize the Chemical Industry. JACS Au 2022, 2, 1054–1070. [Google Scholar] [CrossRef] [PubMed]
- Brimley, P.; Almajed, H.; Alsunni, Y.; Alherz, A.W.; Bare, Z.J.L.; Smith, W.A.; Musgrave, C.B. Electrochemical CO2Reduction over Metal-/Nitrogen-Doped Graphene Single-Atom Catalysts Modeled Using the Grand-Canonical Density Functional Theory. ACS Catal. 2022, 12, 10161–10171. [Google Scholar] [CrossRef]
- Lvov, S.N.; Beck, J.R.; LaBarbera, M.S. Electrochemical Reduction of CO2 to Fuels. In Carbon-Neutral Fuels and Energy Carriers; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar] [CrossRef]
- Pomerantseva, E.; Bonaccorso, F.; Feng, X.; Cui, Y.; Gogotsi, Y. Energy Storage: The Future Enabled by Nanomaterials. Science 2019, 366, eaan8285. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Jung, S.; Lee, M.; Hong, T. How to Better Share Energy towards a Carbon-Neutral City? A Review on Application Strategies of Battery Energy Storage System in City. Renew. Sustain. Energy Rev. 2022, 157, 112113. [Google Scholar] [CrossRef]
- Sufyan, M.; Rahim, N.A.; Aman, M.M.; Tan, C.K.; Raihan, S.R.S. Sizing and Applications of Battery Energy Storage Technologies in Smart Grid System: A Review. J. Renew. Sustain. Energy 2019, 11, 014105. [Google Scholar] [CrossRef]
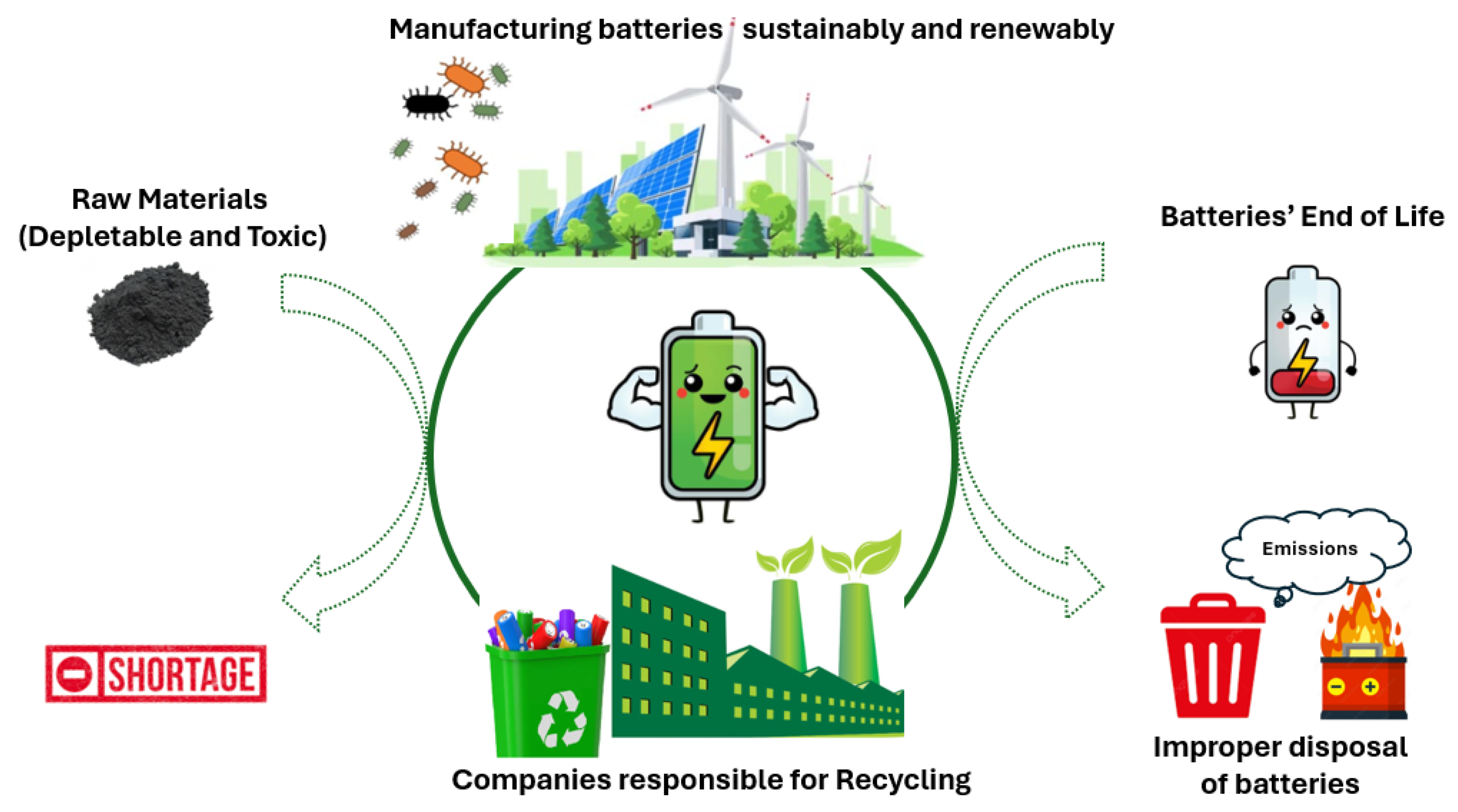
- Dehghani-Sanij, A.R.; Tharumalingam, E.; Dusseault, M.B.; Fraser, R. Study of Energy Storage Systems and Environmental Challenges of Batteries. Renew. Sustain. Energy Rev. 2019, 104, 192–208. [Google Scholar] [CrossRef]
- Li, Y.; Yang, J.; Song, J. Design Structure Model and Renewable Energy Technology for Rechargeable Battery towards Greener and More Sustainable Electric Vehicle. Renew. Sustain. Energy Rev. 2017, 74, 19–25. [Google Scholar] [CrossRef]
- Rydh, C.J. Environmental Assessment of Vanadium Redox and Lead-Acid Batteries for Stationary Energy Storage. J. Power Sources 1999, 80, 21–29. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, J.; Qu, R.; Xiao, G. Environmental Impact Assessment of the Dismantled Battery: Case Study of a Power Lead–Acid Battery Factory in China. Processes 2023, 11, 2119. [Google Scholar] [CrossRef]
- Divya, K.C.; Østergaard, J. Battery Energy Storage Technology for Power Systems-An Overview. Electr. Power Syst. Res. 2009, 79, 511–520. [Google Scholar] [CrossRef]
- Abraham, K.M. Prospects and Limits of Energy Storage in Batteries. J. Phys. Chem. Lett. 2015, 6, 830–844. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Dong, Y.; Li, S.; Lee, J.; Wang, C.; Zhu, Z.; Xue, W.; Li, Y.; Li, J. Lithium Manganese Spinel Cathodes for Lithium-Ion Batteries. Adv. Energy Mater. 2020, 11, 2000997. [Google Scholar] [CrossRef]
- Widyantara, R.D.; Zulaikah, S.; Juangsa, F.B.; Budiman, B.A.; Aziz, M. Review on Battery Packing Design Strategies for Superior Thermal Management in Electric Vehicles. Batteries 2022, 8, 287. [Google Scholar] [CrossRef]
- Schöberl, J.; Ank, M.; Schreiber, M.; Wassiliadis, N.; Lienkamp, M. Thermal Runaway Propagation in Automotive Lithium-Ion Batteries with NMC-811 and LFP Cathodes: Safety Requirements and Impact on System Integration. eTransportation 2024, 19, 100305. [Google Scholar]
- Trahey, L.; Brushett, F.R.; Balsara, N.P.; Ceder, G.; Cheng, L.; Chiang, Y.M.; Hahn, N.T.; Ingram, B.J.; Minteer, S.D.; Moore, J.S.; et al. Energy Storage Emerging: A Perspective from the Joint Center for Energy Storage Research. Proc. Natl. Acad. Sci. USA 2020, 117, 12550–12557. [Google Scholar] [CrossRef]
- Barbosa, J.C.; Fidalgo-Marijuan, A.; Dias, J.C.; Gonçalves, R.; Salado, M.; Costa, C.M.; Lanceros-Méndez, S. Molecular Design of Functional Polymers for Organic Radical Batteries. Energy Storage Mater. 2023, 60, 102841. [Google Scholar] [CrossRef]
- Hager, M.D.; Esser, B.; Feng, X.; Schuhmann, W.; Theato, P.; Schubert, U.S. Polymer-Based Batteries—Flexible and Thin Energy Storage Systems. Adv. Mater. 2020, 32, e2000587. [Google Scholar] [CrossRef] [PubMed]
- Choi, S. Biofuel Cells and Biobatteries: Misconceptions, Opportunities, and Challenges. Batteries 2023, 9, 119. [Google Scholar] [CrossRef]
- Patra, S.; Verma, J.; Mishra, Y.K.; Kurinec, S.; Wang, Q.; Syväjärvi, M.; Tiwari, A. The Positioning of Biofuel Cells-Based Biobatteries for Net-Zero Energy Future. J. Energy Storage 2023, 72, 107919. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Fraiwan, A.; Choi, S. Paper-Based Batteries: A Review. Biosens. Bioelectron. 2014, 54, 640–649. [Google Scholar] [CrossRef]
- Yazdi, A.A.; Preite, R.; Milton, R.D.; Hickey, D.P.; Minteer, S.D.; Xu, J. Rechargeable Membraneless Glucose Biobattery: Towards Solid-State Cathodes for Implantable Enzymatic Devices. J. Power Sources 2017, 343, 103–108. [Google Scholar] [CrossRef]
- Garland, N.T.; Kaveti, R.; Bandodkar, A.J. Biofluid-Activated Biofuel Cells, Batteries, and Supercapacitors: A Comprehensive Review. Adv. Mater. 2023, 35, e2303197. [Google Scholar] [CrossRef]
- Li, P.; Kim, H.; Myung, S.T.; Sun, Y.K. Diverting Exploration of Silicon Anode into Practical Way: A Review Focused on Silicon-Graphite Composite for Lithium Ion Batteries. Energy Storage Mater. 2020, 35, 550–576. [Google Scholar] [CrossRef]
- Fichtner, M. Recent Research and Progress in Batteries for Electric Vehicles. Batter. Supercaps 2022, 5, e20210022. [Google Scholar] [CrossRef]
- Li, Y.; Yang, J.; Song, J. Nano Energy System Model and Nanoscale Effect of Graphene Battery in Renewable Energy Electric Vehicle. Renew. Sustain. Energy Rev. 2016, 69, 652–663. [Google Scholar] [CrossRef]
- Raccichini, R.; Varzi, A.; Passerini, S.; Scrosati, B. The Role of Graphene for Electrochemical Energy Storage. Nat. Mater. 2015, 14, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Wang, X. Advances in Micro Lithium-Ion Batteries for on-Chip and Wearable Applications. J. Micromech. Microeng. 2021, 31, 114002. [Google Scholar] [CrossRef]
- Muench, S.; Burges, R.; Lex-Balducci, A.; Brendel, J.C.; Jäger, M.; Friebe, C.; Wild, A.; Schubert, U.S. Adaptation of Electrodes and Printable Gel Polymer Electrolytes for Optimized Fully Organic Batteries. J. Polym. Sci. 2021, 59, 494–501. [Google Scholar] [CrossRef]
- Winter, M.; Brodd, R.J. What Are Batteries, Fuel Cells, and Supercapacitors? Chem. Rev. 2004, 104, 4245–4270. [Google Scholar] [CrossRef] [PubMed]
- Isa, N.N.C.; Mohd, Y.; Zaki, M.H.M.; Mohamad, S.A.S. Characterization of Copper Coating Electrodeposited on Stainless Steel Substrate. Int. J. Electrochem. Sci. 2017, 12, 6010–6021. [Google Scholar] [CrossRef]
- Trentin, A.; Pakseresht, A.; Duran, A.; Castro, Y.; Galusek, D. Electrochemical Characterization of Polymeric Coatings for Corrosion Protection: A Review of Advances and Perspectives. Polymers 2022, 14, 2306. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.; Esteves, M.F.; Fernández, J.; Bonastre, J.; Cases, F. Polyaniline Coated Conducting Fabrics. Chemical and Electrochemical Characterization. Eur. Polym. J. 2011, 47, 2003–2015. [Google Scholar] [CrossRef]
- Stangl, A.; Muñoz-Rojas, D.; Burriel, M. In Situ and Operando Characterisation Techniques for Solid Oxide Electrochemical Cells: Recent Advances. J. Phys. Energy 2021, 3, 012001. [Google Scholar] [CrossRef]
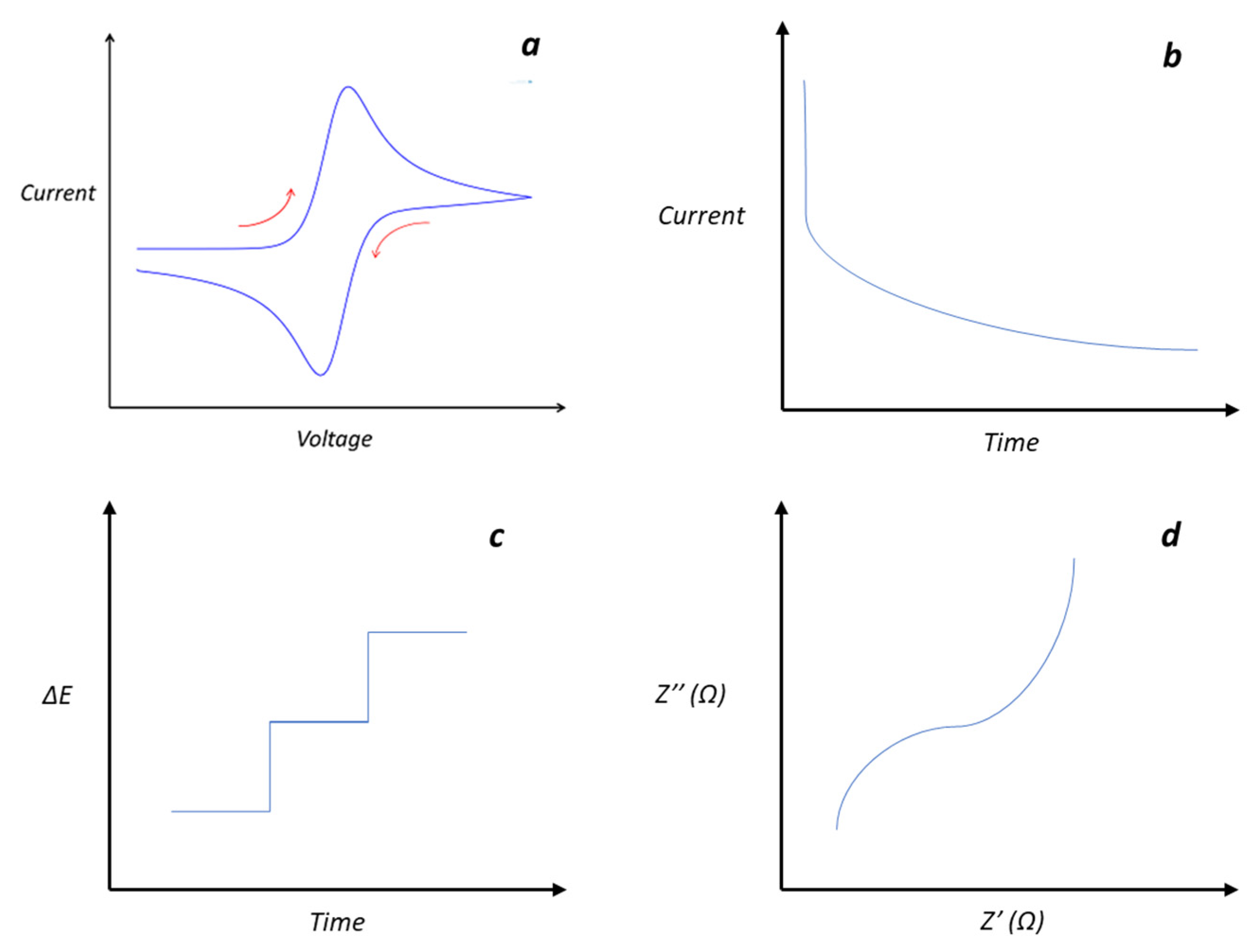
- Rafiee, M.; Abrams, D.J.; Cardinale, L.; Goss, Z.; Romero-Arenas, A.; Stahl, S.S. Cyclic Voltammetry and Chronoamperometry: Mechanistic Tools for Organic Electrosynthesis. Chem. Soc. Rev. 2024, 53, 566–585. [Google Scholar] [CrossRef]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A Practical Beginner’s Guide to Cyclic Voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
- Guziejewski, D.; Stojanov, L.; Zwierzak, Z.; Compton, R.G.; Mirceski, V. Electrode Kinetics from a Single Experiment: Multi-Amplitude Analysis in Square-Wave Chronoamperometry. Phys. Chem. Chem. Phys. 2022, 24, 24419–24428. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.Y.; Park, J.H.; Chang, Y.W.; Cho, S.; Kang, M.J.; Pyun, J.C. Chronoamperometry-Based Redox Cycling for Application to Immunoassays. ACS Sens. 2018, 3, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Volodin, I.A.; Stolze, C.; Casas Mesa, C.; Haagen, U.; Terechin, C.; Hager, M.D.; Schubert, U.S. Beyond Steady-State Conditions: Chronoamperometric State-of-Charge and State-of-Health Measurements in Flow Battery Electrolytes. Sens. Actuators B Chem. 2024, 403, 135101. [Google Scholar] [CrossRef]
- Parrilla, M.; Cuartero, M.; Crespo, G.A. Wearable Potentiometric Ion Sensors. TrAC—Trends Anal. Chem. 2019, 110, 303–320. [Google Scholar] [CrossRef]
- Düzgün, A.; Zelada-Guillén, G.A.; Crespo, G.A.; MacHo, S.; Riu, J.; Rius, F.X. Nanostructured Materials in Potentiometry. Anal. Bioanal. Chem. 2011, 399, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Qin, W. Recent Advances in Potentiometric Biosensors. TrAC—Trends Anal. Chem. 2020, 124, 115803. [Google Scholar] [CrossRef]
- Lin, Z.; Wu, D.; Du, C.; Ren, Z. An Improved Potentiometric Method for the Measurement of Entropy Coefficient of Lithium-Ion Battery Based on Positive Adjustment. Energy Rep. 2022, 8, 54–63. [Google Scholar] [CrossRef]
- Chmayssem, A.; Tanase, C.E.; Verplanck, N.; Gougis, M.; Mourier, V.; Zebda, A.; Ghaemmaghami, A.M.; Mailley, P. New Microfluidic System for Electrochemical Impedance Spectroscopy Assessment of Cell Culture Performance: Design and Development of New Electrode Material. Biosensors 2022, 12, 452. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.; Li, D.; Cui, X.; Wang, L.; Li, L.; Wang, K. Electrochemical Impedance Spectroscopy: A New Chapter in the Fast and Accurate Estimation of the State of Health for Lithium-Ion Batteries. Energies 2023, 16, 1599. [Google Scholar] [CrossRef]
- Choi, W.; Shin, H.C.; Kim, J.M.; Choi, J.Y.; Yoon, W.S. Modeling and Applications of Electrochemical Impedance Spectroscopy (Eis) for Lithium-Ion Batteries. J. Electrochem. Sci. Technol. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Brett, C.M.A. Electrochemical Impedance Spectroscopy in the Characterisation and Application of Modified Electrodes for Electrochemical Sensors and Biosensors. Molecules 2022, 27, 1497. [Google Scholar] [CrossRef]
- Magar, H.S.; Hassan, R.Y.A.; Mulchandani, A. Electrochemical Impedance Spectroscopy (Eis): Principles, Construction, and Biosensing Applications. Sensors 2021, 21, 6578. [Google Scholar] [CrossRef] [PubMed]
- Cachet-Vivier, C.; Keddam, M.; Vivier, V.; Yu, L.T. Development of Cavity Microelectrode Devices and Their Uses in Various Research Fields. J. Electroanal. Chem. 2013, 688, 12–19. [Google Scholar] [CrossRef]
- Tremblay, M.L.; Martin, M.H.; Lebouin, C.; Lasia, A.; Guay, D. Determination of the Real Surface Area of Powdered Materials in Cavity Microelectrodes by Electrochemical Impedance Spectroscopy. Electrochim. Acta 2010, 55, 6283–6291. [Google Scholar] [CrossRef]
- Cachet-Viviera, C.; Vivier, V.; Cha, C.S.; Nedelec, J.Y.; Yu, L.T. Electrochemistry of Powder Material Studied by Means of the Cavity Microelectrode (CME). Electrochim. Acta 2001, 47, 181–189. [Google Scholar] [CrossRef]
- Vivier, V.; Cachet-Vivier, C.; Cha, C.S.; Nedelec, J.Y.; Yu, L.T. Cavity Microelectrode for Studying Battery Materials: Application to Polyaniline Powder. Electrochem. Commun. 2000, 2, 180–185. [Google Scholar] [CrossRef]
- Hasegawa, N.; Shoji, K. Microcavity Volume Control on a Tip of Ag/AgCl Electrodes for Stable Channel Current Measurements of Biological Nanopores. Analyst 2022, 147, 1191–1198. [Google Scholar] [CrossRef]
- Huang, X.; Shi, X.; Zeng, H. How Far Does the Copper/Nickle Recovery from the Practical Application in the Electroplating Wastewater? Resour. Conserv. Recycl. Adv. 2023, 19, 200170. [Google Scholar] [CrossRef]
- Mohajeri, R.; Alipour, Z.; Hajihosseini, S.; Mirzaei, S.I.; Fardmanesh, M. Characterization of Platinum Coated Electrodes Encapsulated in Microfluidic Channel for Impedancemetry Applications. In Proceedings of the 2021 28th National and 6th International Iranian Conference on Biomedical Engineering (ICBME 2021), Tehran, Iran, 25–26 November 2021. [Google Scholar] [CrossRef]
- Solonin, M.D.; Asnis, N.A.; Grigoryan, N.S.; Vagramyan, T.A. Cleaning of fine tungsten wire before gold electroplating. Tsvetnye Met. 2023, 2023, 58–63. [Google Scholar] [CrossRef]
- Menk, L.A.; Baca, E.D.; Hollowell, A.E. Copper Electrodeposition in Blind Mesoscale Through-Silicon-Vias. ECS Meet. Abstr. 2018, MA2018-02, 823. [Google Scholar] [CrossRef]
- Mochizuki, C.; Senga, T.; Shibata, M. Electrodeposition of Pd-Ni-P Metallic Glass Films. Electrochemistry 2011, 79, 249–251. [Google Scholar] [CrossRef]
- Krywko-Cendrowska, A.; Strawski, M.; Szklarczyk, M. Low Temperature Electrodeposition of SiOx Films Photoactive in Water Solution. Electrochim. Acta 2013, 108, 112–117. [Google Scholar] [CrossRef]
- Liu, A.S.; Oliveira, M.A.S. Electrodeposition of Polypyrrole Films on Aluminum from Tartrate Aqueous Solution. J. Braz. Chem. Soc. 2007, 18, 143–152. [Google Scholar] [CrossRef]
- Sayah, A.; Habelhames, F.; Bahloul, A.; Boudjadi, A. The Effect of Electrodeposition Applied Potential on the Electrochemical Performance of Polyaniline Films. J. Mater. Sci. Mater. Electron. 2021, 32, 10692–10701. [Google Scholar] [CrossRef]
- Lata, S.; Batra, B.; Kumar, P.; Pundir, C.S. Construction of an Amperometric D-Amino Acid Biosensor Based on d-Amino Acid Oxidase/Carboxylated Mutliwalled Carbon Nanotube/Copper Nanoparticles/Polyalinine Modified Gold Electrode. Anal. Biochem. 2013, 437, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Zhu, X.; Han, D.; Li, M.; Zhang, Q.; Shu, Y.; Cheng, Z.; Zhang, W.; Hua, E.; Sang, S. AC Electrodeposition of PEDOT Films in Protic Ionic Liquids for Long-Term Stable Organic Electrochemical Transistors. Molecules 2019, 24, 4105. [Google Scholar] [CrossRef]
- Martínez-Viademonte, M.P.; Abrahami, S.T.; Hack, T.; Burchardt, M.; Terryn, H. A Review on Anodizing of Aerospace Aluminum Alloys for Corrosion Protection. Coatings 2020, 10, 1106. [Google Scholar] [CrossRef]
- Iwasaki, M. Titanium Anodizing. J. Surf. Finish. Soc. Jpn. 2022, 73, 28–32. [Google Scholar] [CrossRef]
- de Sousa Araujo, J.V.; da Silva, R.M.P.; Klumpp, R.E.; Costa, I. The Anodizing Process of Aluminum and Its Alloys: A Historical and Electrochemical Approach. Quim. Nova 2021, 44, 999–1011. [Google Scholar] [CrossRef]
- Schneider, M.; Fürbeth, W. Anodizing—The Pore Makes the Difference. Mater. Corros. 2022, 73, 1752–1765. [Google Scholar] [CrossRef]
- Tsangaraki-Kaplanoglou, I.; Theohari, S.; Dimogerontakis, T.; Wang, Y.M.; Kuo, H.H.; Kia, S. Effect of Alloy Types on the Anodizing Process of Aluminum. Surf. Coat. Technol. 2006, 200, 2634–2641. [Google Scholar] [CrossRef]
- Schneider, M.; Kremmer, K.; Weidmann, S.K.; Fürbeth, W. Interplay between Parameter Variation and Oxide Structure of a Modified PAA Process. Surf. Interface Anal. 2013, 45, 1503–1509. [Google Scholar] [CrossRef]
- Kuntyi, O.; Zozulya, G.; Shepida, M. Porous Silicon Formation by Electrochemical Etching. Adv. Mater. Sci. Eng. 2022, 2022, 1482877. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, D.; Deng, H. Study on Material Removal Mechanisms in Electrochemical Etching-Enhanced Polishing of GaN. J. Manuf. Process 2022, 73, 903–913. [Google Scholar] [CrossRef]
- Fiuczek, N.; Sawicka, M.; Feduniewicz-Żmuda, A.; Siekacz, M.; Żak, M.; Nowakowski-Szkudlarek, K.; Muzioł, G.; Wolny, P.; Kelly, J.J.; Skierbiszewski, C. Electrochemical Etching of P-Type GaN Using a Tunnel Junction for Efficient Hole Injection. Acta Mater. 2022, 234, 118018. [Google Scholar] [CrossRef]
- Sun, W.; Shah, S.A.; Chen, Y.; Tan, Z.; Gao, H.; Habib, T.; Radovic, M.; Green, M.J. Electrochemical Etching of Ti2AlC to Ti2CT:X (MXene) in Low-Concentration Hydrochloric Acid Solution. J. Mater. Chem. A Mater. 2017, 5, 21663–21668. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, L.; Meunier, M.; Mirsaidov, U. Formation Pathways of Porous Alloy Nanoparticles through Selective Chemical and Electrochemical Etching. Small 2021, 17, 2006953. [Google Scholar] [CrossRef] [PubMed]
- Peng, N.; He, Y.; Yang, H. Effects of H2SO4 Content on Electrochemical Activation of Etched Tunnels on Aluminum Foil. Corrosion 2018, 74, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Peng, N.; Wen, Y.; He, Y. Improved Distribution of Etched Tunnels on Aluminum Foil with Silane Treatment. Prog. Org. Coat. 2019, 127, 151–156. [Google Scholar] [CrossRef]
- Kaya, D.; Keçeci, K. Review—Track-Etched Nanoporous Polymer Membranes as Sensors: A Review. J. Electrochem. Soc. 2020, 167, 037543. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, P.; Zhong, Y.; Dong, S.; Hou, M.; Liu, H.; Chen, X.; Gao, J.; Wong, C.-P. Review—Progress in Electrochemical Etching of Third-Generation Semiconductors. ECS J. Solid State Sci. Technol. 2023, 12, 045004. [Google Scholar] [CrossRef]
- Lin, J.C.; Liu, Y.C.; Lu, S.H.; Yen, H.N.; Settu, K. Enhancing the Specific Capacitance of a Porous Silicon-Based Capacitor by Embedding Graphene Combined with Three-Dimensional Electrochemical Etching. Electrochem. Commun. 2023, 154, 107555. [Google Scholar] [CrossRef]
- Lazanas, A.C.; Prodromidis, M.I. Electrochemical Impedance Spectroscopy—A Tutorial. ACS Meas. Sci. Au 2023, 3, 162–193. [Google Scholar] [CrossRef] [PubMed]
- Chmayssem, A.; Monsalve-Grijalba, K.; Alias, M.; Mourier, V.; Vignoud, S.; Scomazzon, L.; Muller, C.; Barthes, J.; Vrana, N.E.; Mailley, P. Reference Method for Off-Line Analysis of Nitrogen Oxides in Cell Culture Media by an Ozone-Based Chemiluminescence Detector. Anal. Bioanal. Chem. 2021, 413, 1383–1393. [Google Scholar] [CrossRef] [PubMed]
- Chmayssem, A.; Petit, L.; Verplanck, N.; Mourier, V.; Vignoud, S.; Vrana, N.E.; Mailley, P. Characterization of the Impact of Classical Cell-Culture Media on the Response of Electrochemical Sensors. Electroanalysis 2022, 34, 1201–1211. [Google Scholar] [CrossRef]
- Escarpa, A. Food Electroanalysis: Sense and Simplicity. Chem. Rec. 2012, 12, 72–91. [Google Scholar] [CrossRef] [PubMed]
- Bussooa, A.; Tubbs, E.; Revol-Cavalier, F.; Chmayssem, A.; Alessio, M.; Cosnier, M.-L.; Verplanck, N. Real-Time Monitoring of Oxygen Levels within Thermoplastic Organ-on-Chip Devices. SSRN Electron. J. 2022, 11, 4090175. [Google Scholar] [CrossRef]
- Bühlmann, P.; Pretsch, E.; Bakker, E. Carrier-Based Ion-Selective Electrodes and Bulk Optodes. 2. Ionophores for Potentiometric and Optical Sensors. Chem. Rev. 1998, 98, 1593–1688. [Google Scholar] [CrossRef]
- Lonsdale, W. Development, Manufacture and Application of a Solid-State PH Sensor Using Ruthenium Oxide; Edith Cowan University: Joondalup, WA, Australia, 2018. [Google Scholar]
- Liu, Y.; Pharr, M.; Salvatore, G.A. Lab-on-Skin: A Review of Flexible and Stretchable Electronics for Wearable Health Monitoring. ACS Nano 2017, 11, 9614–9635. [Google Scholar] [CrossRef]
- Gao, W.; Emaminejad, S.; Nyein, H.Y.Y.; Challa, S.; Chen, K.; Peck, A.; Fahad, H.M.; Ota, H.; Shiraki, H.; Kiriya, D.; et al. Fully Integrated Wearable Sensor Arrays for Multiplexed in Situ Perspiration Analysis. Nature 2016, 529, 509–514. [Google Scholar] [CrossRef]
- Soranzo, T.; Ben Tahar, A.; Chmayssem, A.; Zelsmann, M.; Vadgama, P.; Lenormand, J.L.; Cinquin, P.; Martin, D.K.; Zebda, A. Electrochemical Biosensing of Glucose Based on the Enzymatic Reduction of Glucose. Sensors 2022, 22, 7105. [Google Scholar] [CrossRef] [PubMed]
- Chmayssem, A.; Shalayel, I.; Marinesco, S.; Zebda, A. Investigation of GOx Stability in a Chitosan Matrix: Applications for Enzymatic Electrodes. Sensors 2023, 23, 465. [Google Scholar] [CrossRef] [PubMed]
- Chmayssem, A.; Nadolska, M.; Tubbs, E.; Sadowska, K.; Vadgma, P.; Shitanda, I.; Tsujimura, S.; Lattach, Y.; Peacock, M.; Tingry, S.; et al. Insight into Continuous Glucose Monitoring: From Medical Basics to Commercialized Devices. Microchim. Acta 2023, 190, 177. [Google Scholar] [CrossRef] [PubMed]
- Yoo, E.H.; Lee, S.Y. Glucose Biosensors: An Overview of Use in Clinical Practice. Sensors 2010, 10, 4558–4576. [Google Scholar] [CrossRef]
- Singh, N.; Goldsmith, B.R. Role of Electrocatalysis in the Remediation of Water Pollutants. ACS Catal. 2020, 10, 3365–3371. [Google Scholar] [CrossRef]
- Naidu, R.; Biswas, B.; Willett, I.R.; Cribb, J.; Kumar Singh, B.; Paul Nathanail, C.; Coulon, F.; Semple, K.T.; Jones, K.C.; Barclay, A.; et al. Chemical Pollution: A Growing Peril and Potential Catastrophic Risk to Humanity. Environ. Int. 2021, 156, 106616. [Google Scholar] [CrossRef]
- Isarain-Chávez, E.; De La Rosa, C.; Godínez, L.A.; Brillas, E.; Peralta-Hernández, J.M. Comparative Study of Electrochemical Water Treatment Processes for a Tannery Wastewater Effluent. J. Electroanal. Chem. 2014, 713, 62–69. [Google Scholar] [CrossRef]
- Feng, A.; Feng, J.; Xing, W.; Jiang, K.; Tang, W. Versatile Applications of Electrochemical Flow-through Systems in Water Treatment Processes. Chem. Eng. J. 2023, 473, 145400. [Google Scholar] [CrossRef]
- Alvarado, L.; Chen, A. Electrodeionization: Principles, Strategies and Applications. Electrochim. Acta 2014, 132, 583–597. [Google Scholar] [CrossRef]
- Salari, K.; Zarafshan, P.; Khashehchi, M.; Chegini, G.; Etezadi, H.; Karami, H.; Szulżyk-Cieplak, J.; Łagód, G. Knowledge and Technology Used in Capacitive Deionization of Water. Membranes 2022, 12, 459. [Google Scholar] [CrossRef]
- Wang, K.; Li, H.; Yang, Y.; Wang, P.; Zheng, Y.; Song, L. Making Cathode Composites More Efficient for Electro-Fenton and Bio-Electro-Fenton Systems: A Review. Sep. Purif. Technol. 2022, 304, 122302. [Google Scholar] [CrossRef]
- He, L.; Du, P.; Chen, Y.; Lu, H.; Cheng, X.; Chang, B.; Wang, Z. Advances in Microbial Fuel Cells for Wastewater Treatment. Renew. Sustain. Energy Rev. 2017, 71, 388–403. [Google Scholar] [CrossRef]
- Sillanpää, M.; Shestakova, M. Emerging and Combined Electrochemical Methods. In Electrochemical Water Treatment Methods: Fundamentals, Methods and Full Scale Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2017; Chapter 3; pp. 131–225. [Google Scholar] [CrossRef]
- Azanaw, A.; Birlie, B.; Teshome, B.; Jemberie, M. Textile Effluent Treatment Methods and Eco-Friendly Resolution of Textile Wastewater. Case Stud. Chem. Environ. Eng. 2022, 6, 100230. [Google Scholar] [CrossRef]
- Vineta, S.; Silvana, Z.; Sanja, R.; Golomeova, S. Methods for Waste Waters Treatment in Textile Industry. In Proceedings of the International Scientific Conference “UNITECH 2014”, Gabrovo, Bulgaria, 21–22 November 2014. [Google Scholar]
- Behera, M.; Nayak, J.; Banerjee, S.; Chakrabortty, S.; Tripathy, S.K. A Review on the Treatment of Textile Industry Waste Effluents towards the Development of Efficient Mitigation Strategy: An Integrated System Design Approach. J. Environ. Chem. Eng. 2021, 9, 105277. [Google Scholar] [CrossRef]
- Samsami, S.; Mohamadi, M.; Sarrafzadeh, M.H.; Rene, E.R.; Firoozbahr, M. Recent Advances in the Treatment of Dye-Containing Wastewater from Textile Industries: Overview and Perspectives. Process Saf. Environ. Prot. 2020, 143, 138–163. [Google Scholar] [CrossRef]
- Labidi, A.; Salaberria, A.M.; Fernandes, S.C.M.; Labidi, J.; Abderrabba, M. Functional Chitosan Derivative and Chitin as Decolorization Materials for Methylene Blue and Methyl Orange from Aqueous Solution. Materials 2019, 12, 361. [Google Scholar] [CrossRef]
- Meng, X.L.; Nie, Y.; Sun, J.; Cheng, W.G.; Wang, J.Q.; He, H.Y.; Zhang, S.J. Functionalized Dicyandiamide-Formaldehyde Polymers as Efficient Heterogeneous Catalysts for Conversion of CO2 into Organic Carbonates. Green. Chem. 2014, 16, 2771–2778. [Google Scholar] [CrossRef]
- Alinsafi, A.; Evenou, F.; Abdulkarim, E.M.; Pons, M.N.; Zahraa, O.; Benhammou, A.; Yaacoubi, A.; Nejmeddine, A. Treatment of Textile Industry Wastewater by Supported Photocatalysis. Dyes Pigments 2007, 74, 439–445. [Google Scholar] [CrossRef]
- Ilhan, F.; Ulucan-Altuntas, K.; Dogan, C.; Kurt, U. Treatability of Raw Textile Wastewater Using Fenton Process and Its Comparison with Chemical Coagulation. Desalination Water Treat. 2019, 162, 142–148. [Google Scholar] [CrossRef]
- Kaur, P.; Kushwaha, J.P.; Sangal, V.K. Electrocatalytic Oxidative Treatment of Real Textile Wastewater in Continuous Reactor: Degradation Pathway and Disposability Study. J. Hazard. Mater. 2018, 346, 242–252. [Google Scholar] [CrossRef]
- Mukimin, A.; Wijaya, K.; Kuncaka, A. Oxidation of Remazol Brilliant Blue r (RB.19) with in Situ Electro-Generated Active Chlorine Using Ti/PbO2 Electrode. Sep. Purif. Technol. 2012, 95, 1–9. [Google Scholar] [CrossRef]
- Chmayssem, A.; AlChoubassi, G.; Taha, S.; Hauchard, D. Electro-Fenton Process at Semi-Pilot Scale. A Study to Scale up the Reactor for Industrial Applications. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Park, S.; Lee, J.W.; Popov, B.N. A Review of Gas Diffusion Layer in PEM Fuel Cells: Materials and Designs. Int. J. Hydrogen Energy 2012, 37, 5850–5865. [Google Scholar] [CrossRef]
- Mukimin, A.; Vistanty, H.; Harihastuti, N.; Setianingsih, N.I.; Djayanti, S.; Nilawati; Astuti, Y. Hybrid Fenton-Electrochemical Reactor and System as Post-Treatment of Textile Wastewater. J. Water Process Eng. 2024, 59, 105028. [Google Scholar] [CrossRef]
- Zhu, C.; Ang, N.W.J.; Meyer, T.H.; Qiu, Y.; Ackermann, L. Organic Electrochemistry: Molecular Syntheses with Potential. ACS Cent. Sci. 2021, 7, 415–431. [Google Scholar] [CrossRef] [PubMed]
- Leech, M.C.; Lam, K. A Practical Guide to Electrosynthesis. Nat. Rev. Chem. 2022, 6, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Botte, G.G. Electrochemical Manufacturing in the Chemical Industry. Electrochem. Soc. Interface 2014, 23, 49–55. [Google Scholar] [CrossRef]
- Wang, F.; Li, W.; Wang, R.; Guo, T.; Sheng, H.; Fu, H.C.; Stahl, S.S.; Jin, S. Modular Electrochemical Synthesis Using a Redox Reservoir Paired with Independent Half-Reactions. Joule 2020, 5, 149–165. [Google Scholar] [CrossRef]
- Cui, B.; Shi, Y.; Li, G.; Chen, Y.; Chen, W.; Deng, Y.; Hu, W. Challenges and Opportunities for Seawater Electrolysis: A Mini-Review on Advanced Materials in Chlorine-Involved Electrochemistry. Wuli Huaxue Xuebao/Acta Phys.—Chim. Sin. 2022, 38, 2106010. [Google Scholar] [CrossRef]
- Li, Q.; Jensen, J.O.; Bjerrum, N.J. Chemistry, Electrochemistry, and Electrochemical Applications|Aluminum. In Encyclopedia of Electrochemical Power Sources; Elsevier Inc.: Amsterdam, The Netherlands, 2009. [Google Scholar] [CrossRef]
- Comer, B.M.; Fuentes, P.; Dimkpa, C.O.; Liu, Y.H.; Fernandez, C.A.; Arora, P.; Realff, M.; Singh, U.; Hatzell, M.C.; Medford, A.J. Prospects and Challenges for Solar Fertilizers. Joule 2019, 3, 1578–1605. [Google Scholar] [CrossRef]
- Fu, N.; Sauer, G.S.; Lin, S. A General, Electrocatalytic Approach to the Synthesis of Vicinal Diamines. Nat. Protoc. 2018, 13, 1725–1743. [Google Scholar] [CrossRef]
- Zheng, S.; Yan, J.; Wang, K. Engineering Research Progress of Electrochemical Microreaction Technology—A Novel Method for Electrosynthesis of Organic Chemicals. Engineering 2021, 7, 22–32. [Google Scholar] [CrossRef]
- Anastasiadou, D. Cu-Based Electrodes for Ammonia and Urea Electrosynthesis. Ph.D. Thesis, Eindhoven University of Technology, Eindhoven, The Netherlands, 2023. [Google Scholar]
- Chen, X.; Lv, S.; Su, Z.; Yang, X.; Cui, H.; Yang, Z.; Xu, Z.; Teobaldi, G.; Kang, J.; Liu, L.-M.; et al. Recent Progress in Amorphous Nanomaterials for Electrochemical Synthesis of N-Containing Compounds. Chem. Catal. 2024, 100871. [Google Scholar] [CrossRef]
- Botte, G.G. Transitioning Electrochemical Technologies into Agriculture via the National Science Foundation Engineering Research Center Model. Electrochem. Soc. Interface 2023, 32, 69–73. [Google Scholar] [CrossRef]
- Meyer, T.H.; Choi, I.; Tian, C.; Ackermann, L. Powering the Future: How Can Electrochemistry Make a Difference in Organic Synthesis? Chem 2020, 6, 2484–2496. [Google Scholar] [CrossRef]
- Talebi, M.R.; Nematollahi, D. Electrochemical Synthesis of Sulfonamide Derivatives: Electrosynthesis Conditions and Reaction Pathways. ChemElectroChem 2024, 11, e202300728. [Google Scholar] [CrossRef]
- Zuo, S.; Xue, W.; Zhang, Y.; Chen, J.; Lin, Z. Front Cover: Atomically Dispersed Fe Motif-based Electrocatalysts for Hydrogen Peroxide Synthesis (ChemNanoMat 2/2024). ChemNanoMat 2024, 10, 202400093. [Google Scholar] [CrossRef]
- Zuo, S.; Xue, W.; Zhang, Y.; Chen, J.; Lin, Z. Atomically Dispersed Fe Motif-based Electrocatalysts for Hydrogen Peroxide Synthesis. ChemNanoMat 2024, 10, 202300476. [Google Scholar] [CrossRef]
- Schremp, F.W. Corrosion prevention for offshore platforms. JPT J. Pet. Technol. 1984, 36, 605–612. [Google Scholar] [CrossRef]
- Chen, Y.; He, M.; Zong, Y.; Dong, S.; Yan, Y.; Zhang, Y.; Liu, H.; Cao, J. Research Progress on Failure Modes of Unbonded Flexible Pipe and Their Control Measures. Nat. Gas. Ind. 2021, 41, 122–131. [Google Scholar] [CrossRef]
- Mahajanam, S.P.V.; Joosten, M.W. Guidelines for Filler-Material Selection to Minimize Preferential Weld Corrosion in Pipeline Steels. SPE Proj. Facil. Constr. 2011, 6, 5–12. [Google Scholar] [CrossRef]
- Erdogan, C.; Swain, G. Conceptual Sacrificial Anode Cathodic Protection Design for Offshore Wind Monopiles. Ocean Eng. 2021, 235, 109339. [Google Scholar] [CrossRef]
- Popov, B.N. Corrosion Engineering: Principles and Solved Problems; Elsevier: Amsterdam, Netherlands, 2015. [Google Scholar] [CrossRef]
- Ahmad, Z. Principles of Corrosion Engineering and Corrosion Control; Elsevier: Burlington, MA, USA, 2006. [Google Scholar] [CrossRef]
- Cicek, V. Corrosion Engineering; Wiley: Hoboken, NJ, USA, 2014; ISBN 9781118720899. [Google Scholar] [CrossRef]
- Pyun, S.-I. Strategies of Metal Corrosion Protection. ChemTexts 2020, 7, 2. [Google Scholar] [CrossRef]
- Ducasse-Lapeyrusse, J.; Bouteiller, V.; Marie-Victoire, E.; Bouichou, M.; Damien, G.; Martinet, V.; Annede-Villeau, C.; Lesieutre, O. Assessment of the Impressed Current Cathodic Protection System after 4 Years Operation: Case Study of the Saint-Cloud Viaduct (France). Case Stud. Constr. Mater. 2023, 18, e02023. [Google Scholar] [CrossRef]
- Xu, J.; Yang, M.; Li, S.; Kainuma, S.; Ji, B.; Murayama, S. Application of Cathodic Protection Method on Steel Structures Using Sacrificial Anode and Sodium Polyacrylate-Sodium Carboxymethyl Cellulose (PANa–CMC) Hydrogel Electrolyte. Case Stud. Constr. Mater. 2024, 20, e02742. [Google Scholar] [CrossRef]
- Ramanavasu, R.; Vijayakumar, K.; Fernandez, S.G. Integrated Nanogrid for the Impressed Current Cathodic Protection System in Desalination Plant. Sustainability 2023, 15, 7088. [Google Scholar] [CrossRef]
- Yao, G.; He, X.; Liu, J.; Guo, Z.; Chen, P. Test Study of the Bridge Cable Corrosion Protection Mechanism Based on Impressed Current Cathodic Protection. Lubricants 2023, 11, 30. [Google Scholar] [CrossRef]
- Dickinson, E.J.F.; Ekström, H.; Fontes, E. COMSOL Multiphysics®: Finite Element Software for Electrochemical Analysis. A Mini-Review. Electrochem. Commun. 2014, 40, 71–74. [Google Scholar] [CrossRef]
- Jalalvand, A.R.; Roushani, M.; Goicoechea, H.C.; Rutledge, D.N.; Gu, H.W. MATLAB in Electrochemistry: A Review. Talanta 2018, 194, 205–225. [Google Scholar] [CrossRef]
- Weddle, P.; Vincent, T.; Kee, R.J. Identifying and Applying State-Space Models Derived from High-Fidelity Physical Models of Li-Ion Batteries. ECS Meet. Abstr. 2017, MA2017-01. [Google Scholar] [CrossRef]
- Bennett, B.; Chang, J.; Bard, A.J. Mechanism of the Br−/Br2 Redox Reaction on Platinum and Glassy Carbon Electrodes in Nitrobenzene by Cyclic Voltammetry. Electrochim. Acta 2016, 219, 1–9. [Google Scholar] [CrossRef]
- Habekost, A. Simulation and Fitting of Cyclic Voltammetry and Chronoamperometry Curves of Electrochemical Reactions with Different Mechanisms—A Didactic Perspective. World J. Chem. Educ. 2019, 7, 53–64. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z. Animated Electrochemistry Simulation Modules. J. Chem. Educ. 2022, 99, 752–758. [Google Scholar] [CrossRef]
- Jaugstetter, M.; Blanc, N.; Kratz, M.; Tschulik, K. Electrochemistry under Confinement. Chem. Soc. Rev. 2022, 51, 2491–2543. [Google Scholar] [CrossRef]
- Weiß, L.J.K.; Lubins, G.; Music, E.; Rinklin, P.; Banzet, M.; Peng, H.; Terkan, K.; Mayer, D.; Wolfrum, B. Single-Impact Electrochemistry in Paper-Based Microfluidics. ACS Sens. 2022, 7, 884–892. [Google Scholar] [CrossRef]
- Zhou, Y.; Qi, J.; Engelberg, D.L. On the Application of Bipolar Electrochemistry for Simulating Galvanic Corrosion Behaviour of Dissimilar Stainless Steels. Electrochem. Commun. 2021, 126, 107023. [Google Scholar] [CrossRef]
- Tettey, F.; Parupelli, S.K.; Desai, S. A Review of Biomedical Devices: Classification, Regulatory Guidelines, Human Factors, Software as a Medical Device, and Cybersecurity. Biomed. Mater. Devices 2023, 2, 316–341. [Google Scholar] [CrossRef]
- Marcus, H.J.; Payne, C.J.; Hughes-Hallett, A.; Marcus, A.P.; Yang, G.Z.; Darzi, A.; Nandi, D. Regulatory Approval of New Medical Devices: Cross Sectional Study. BMJ 2016, 353, i2587. [Google Scholar] [CrossRef]
- Wagner, M.V.; Schanze, T. Comparison of Approval Procedures for Medical Devices in Europe and the USA. Curr. Dir. Biomed. Eng. 2019, 5, 605–608. [Google Scholar] [CrossRef]
- Robinson, S.G.; Sigman, M.S. Integrating Electrochemical and Statistical Analysis Tools for Molecular Design and Mechanistic Understanding. Acc. Chem. Res. 2020, 53, 289–299. [Google Scholar] [CrossRef]
- Rivera, F.F.; Pérez, T.; Castañeda, L.F.; Nava, J.L. Mathematical Modeling and Simulation of Electrochemical Reactors: A Critical Review. Chem. Eng. Sci. 2021, 239, 116622. [Google Scholar] [CrossRef]
- Lu, S.-M.; Vannoy, K.J.; Dick, J.E.; Long, Y.-T. Multiphase Chemistry under Nanoconfinement: An Electrochemical Perspective. J. Am. Chem. Soc. 2023, 145, 25043–25055. [Google Scholar] [CrossRef]
- Ma, Z.; Witteman, L.; Wrubel, J.A.; Bender, G. A Comprehensive Modeling Method for Proton Exchange Membrane Electrolyzer Development. Int. J. Hydrogen Energy 2021, 46, 17627–17643. [Google Scholar] [CrossRef]
- Iliev, I.K.; Gizzatullin, A.R.; Filimonova, A.A.; Chichirova, N.D.; Beloev, I.H. Numerical Simulation of Processes in an Electrochemical Cell Using COMSOL Multiphysics. Energies 2023, 16, 7265. [Google Scholar] [CrossRef]
- Yaoxuan, Q.; Cheng, F.; Kening, S. Multiphysics Simulation of a Solid Oxide Fuel Cell Based on Comsol Method. In E3S Web of Conferences; EDP Sciences: Paris, France, 2021; Volume 245. [Google Scholar] [CrossRef]
- Goel, V.; Thornton, K. Enabling the Electrochemical Simulation of Li-Ion Battery Electrodes with Anisotropic Tortuosity in COMSOL Multiphysics®. MethodsX 2021, 8, 101425. [Google Scholar] [CrossRef]
- Salazar, P.F.; Kumar, S.; Cola, B.A. Design and Optimization of Thermo-Electrochemical Cells. J. Appl. Electrochem. 2013, 44, 325–336. [Google Scholar] [CrossRef]
- Yang, W.; Sun, L.; Bao, J.; Mo, Z.; Du, M.; Xu, Y.; Zhang, J. Multiphysics Modeling of Mass and Heat Transfer in a Thermo-Electrochemical Cell. Ind. Eng. Chem. Res. 2023, 62, 12345–12355. [Google Scholar] [CrossRef]
- El-Shafie, O.A.; El-Maghraby, R.M.; Albo, J.; Fateen, S.E.K.; Abdelghany, A. Modeling and Numerical Investigation of the Performance of Gas Diffusion Electrodes for the Electrochemical Reduction of Carbon Dioxide to Methanol. Ind. Eng. Chem. Res. 2020, 59, 20929–20942. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, J.; Guo, C.; Zhao, S. Investigation of Electrochemical Machining for Gradual Change Special-Shaped Deep Spiral Hole Based on COMSOL. Int. J. Adv. Manuf. Technol. 2020, 108, 2717–2725. [Google Scholar] [CrossRef]
- Cordova-Huaman, A.V.; Jauja-Ccana, V.R.; La Rosa-Toro, A. Low-Cost Smartphone-Controlled Potentiostat Based on Arduino for Teaching Electrochemistry Fundamentals and Applications. Heliyon 2021, 7, e06259. [Google Scholar] [CrossRef]
- Ahmad, N.J.; Yakob, N.; Bunyamin, M.A.H.; Winarno, N.; Akmal, W.H. The Effect of Interactive Computer Animation and Simulation on Students’ Achievement and Motivation in Learning Electrochemistry. J. Pendidik. IPA Indones. 2021, 10, 311–324. [Google Scholar] [CrossRef]


| Provider List of Electrochemical Instruments and Accessories | Website of the Provider |
|---|---|
| Metrohm | www.metrohm.com, accessed on 16 May 2024 |
| Bio-Logic Sciences instruments | www.biologic.net, accessed on 16 May 2024 |
| PalmSens | www.palmsens.com, accessed on 16 May 2024 |
| Solartron | www.solartronmetrology.com, accessed on 16 May 2024 |
| Hanna Instruments | www.hannainst.com, accessed on 16 May 2024 |
| Gamry instruments | www.gamry.com, accessed on 16 May 2024 |
| Sciospec | www.sciospec.com, accessed on 16 May 2024 |
| Princeton Applied Research | www.ameteksi.com, accessed on 16 May 2024 |
| PINE RESEARCH | www.pineresearch.com, accessed on 16 May 2024 |
| Texas Instruments | www.ti.com, accessed on 16 May 2024 |
| Kanopytech | www.kanopytech.com, accessed on 16 May 2024 |
| Ivium Technologies | www.ivium.com, accessed on 16 May 2024 |
| Corrtest Instruments | www.corrtest.com.cn, accessed on 16 May 2024 |
| Xiamen Tob New Energy Technology | www.tobmachine.com, accessed on 16 May 2024 |
| Phadke Instruments | www.phadkeinstruments.com, accessed on 16 May 2024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yammine, P.; El-Nakat, H.; Kassab, R.; Mansour, A.; El Khoury, B.; Koumeir, D.; Matar, Z.; Chmayssem, A. Recent Advances in Applied Electrochemistry: A Review. Chemistry 2024, 6, 407-434. https://doi.org/10.3390/chemistry6030024
Yammine P, El-Nakat H, Kassab R, Mansour A, El Khoury B, Koumeir D, Matar Z, Chmayssem A. Recent Advances in Applied Electrochemistry: A Review. Chemistry. 2024; 6(3):407-434. https://doi.org/10.3390/chemistry6030024
Chicago/Turabian StyleYammine, Paolo, Hanna El-Nakat, Rima Kassab, Agapy Mansour, Bilal El Khoury, Diala Koumeir, Zeinab Matar, and Ayman Chmayssem. 2024. "Recent Advances in Applied Electrochemistry: A Review" Chemistry 6, no. 3: 407-434. https://doi.org/10.3390/chemistry6030024
APA StyleYammine, P., El-Nakat, H., Kassab, R., Mansour, A., El Khoury, B., Koumeir, D., Matar, Z., & Chmayssem, A. (2024). Recent Advances in Applied Electrochemistry: A Review. Chemistry, 6(3), 407-434. https://doi.org/10.3390/chemistry6030024







