Visible-Light Photochromic Properties of an Inorganic-Organic Phosphomolybdic Acid/Polythiophene Hybrid Thin Film
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation
2.2. Characterization
2.3. Experiments
3. Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Qin, B.; Chen, H.Y.; Liang, H.; Fu, L.; Liu, X.; Qiu, X.; Liu, S.; Song, R.; Tang, Z. Reversible photoswitchable fluorescence in thin films of inorganic nanoparticle and polyoxometalate assemblies. J. Am. Chem. Soc. 2010, 132, 2886–2888. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.X.; Bi, L.H.; Fu, Y.; Wang, N.; Liu, S.; Tang, Z. Multistate electrically controlled photoluminescence switching. Chem. Sci. 2013, 4, 4371–4377. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, X.; Ma, P.; Singh, V.; Zhang, C.; Niu, J.; Wang, J. Photochromic behavior of a new polyoxomolybdate/alkylamine composite in solid state. J. Mater. Sci. 2018, 53, 3078–3086. [Google Scholar] [CrossRef]
- Graca, V.C.; Sousa, C.M.; Coelho, P. Towards grey coloring photochromic materials using vinylidene-naphthofurans. Dye. Pigment. 2020, 176. [Google Scholar] [CrossRef]
- Bao, H.F.; Wang, X.Y.; Yang, G.Q.; Li, H.; Zhang, F.; Feng, W. UV-light and visible-light photochromism of inorganic–organic multilayer films based on polyoxometalate and poly(acrylamide). Colloid Polym. Sci. 2014, 292, 2883–2889. [Google Scholar] [CrossRef]
- Jing, X.F.; Zou, D.L.; Meng, Q.Q.; Zhang, W.; Zhang, F.; Feng, W.; Han, X. Fabrication and visible-light photochromism of novel hybrid inorganic–organic film based on polyoxometalates and ethyl cellulose. Inorg. Chem. Commun. 2014, 46, 149–154. [Google Scholar] [CrossRef]
- Zhang, J.; Zou, Q.; Tian, H. Photochromic materials: More than meets the eye. Adv. Mater. 2013, 25, 378–399. [Google Scholar] [CrossRef] [PubMed]
- Toshihiro, Y. Photo- and electrochromism of polyoxometalates and related materials. Chem. Rev. 1998, 98, 307–326. [Google Scholar]
- Chen, J.; MeiAi, L.; Feng, W.; Xiong, D.Q.; Liu, Y.; Cai, W.M. Preparation and photochromism of nanocomposite thin film based on polyoxometalate and polyethyleneglycol. Mater. Lett. 2007, 61, 5247–5249. [Google Scholar] [CrossRef]
- Zeng, O.R.; Guo, S.Y.; Sun, Y.B.; Li, Z.; Feng, W. Protonation Induced Enhanced Optical-Light Photochromic Properties of an lnorganic-Organic Phosphomolybdic Acid/Polyaniline Hybrid Thin Film. Nanomaterials 2020, 10, 1839. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Sun, Y.; Liu, J.L.; Wang, X.; Liu, S.L.; Feng, W. Enhanced photochromism of heteropolyacid/polyvinylpyrolidone composite film by TiO2 doping. J. Appl. Polym. Sci. 2015, 132, 41583. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, X.; Lu, Y.; Xuan, L.; Xia, S.; Feng, W.; Han, X. Preparation and visible-light photochromism of phosphomolybdic acid/polyvinylpyrrolidone hybrid film. Chem. Res. Chin. Univ. 2014, 30, 703–708. [Google Scholar] [CrossRef]
- Wei, Y.; Han, B.; Dong, Z.; Feng, W. Phosphomolybdic acid-modified highly organized TiO2 nanotube arrays with rapid photochromic performance. J. Mater. Sci. Technol. 2019, 35, 1951–1958. [Google Scholar] [CrossRef]
- Yue, T.; Han, B.; Wang, X.; Bai, L.; Feng, W. Instantaneous visible-light photochromic performance of composite powders based on PMoA and ZnO nanotubes. Chem. Lett. 2019, 48, 851–854. [Google Scholar] [CrossRef]
- Liu Qi Hu Ch Wang, X. Hydrothermal synthesis of oxygen-deficiency tungsten oxide quantum dots with excellent photochromic reversibility. Appl. Surf. Sci. 2019, 480, 404–409. [Google Scholar]
- Li, D.; Wei, J.; Dong, S.; Li, H.; Xia, Y.; Jiao, X.; Wang, T.; Chen, D. Novel PVP/HTA hybrids for multifunctional rewritable paper. ACS Appl. Mater. Interfaces 2018, 10, 1701–1706. [Google Scholar] [CrossRef] [PubMed]
- Qaid, S.M.; Alharbi, F.H.; Bedja, I.; Nazeeruddin, M.K.; Aldwayyan, A.S. Reducing Amplified Spontaneous Emission Threshold in CsPbBr3 Quantum Dot Films by Controlling TiO2 Compact Layer. Nanomaterials 2020, 10, 1605. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Li, J.; Deng, Q.; Gao, Y. Preparation, characterization, photochromic properties, and mechanism of PMoA/ZnO/PVP composite film. Molecules 2023, 28, 7605. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Gupta, A.K.; Devi, M.; Gonsalves, K.E.; Pradeep, C.P. Engineering Multifunctionality in Hybrid Polyoxometalates: Aromatic Sulfonium Octamolybdates as Excellent Photochromic Materials and Self-Separating Catalysts for Epoxidation. Inorg. Chem. 2017, 56, 10325–10336. [Google Scholar] [CrossRef] [PubMed]


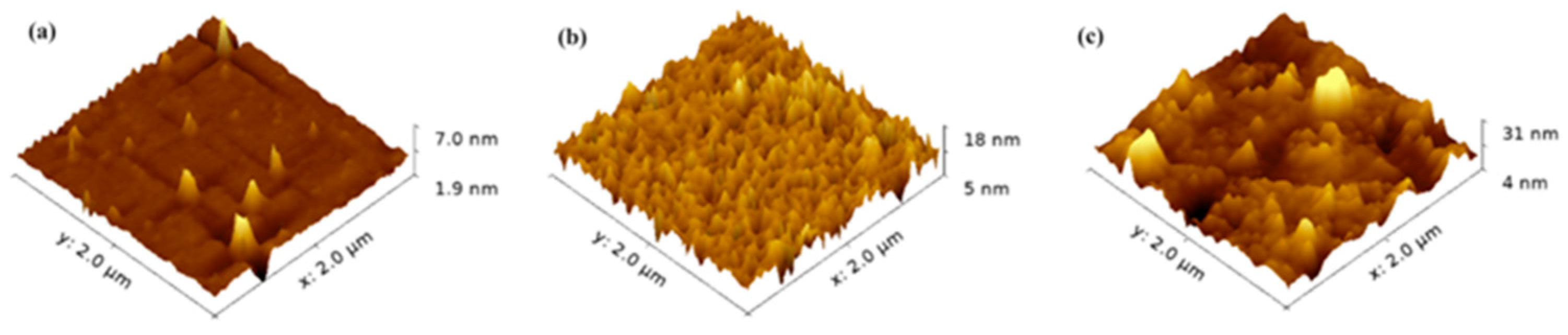
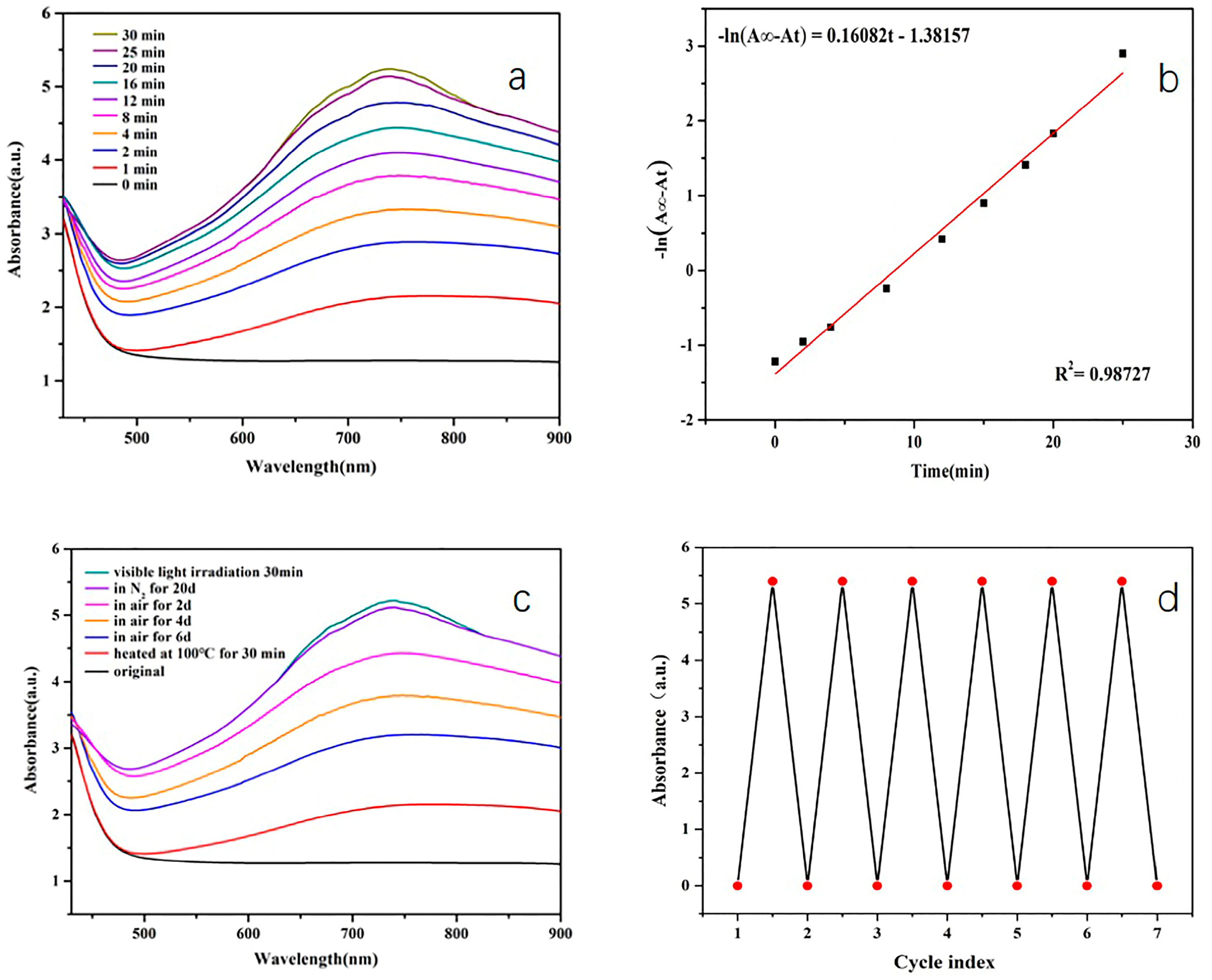

| Photochromic Material | The Thickness of Samples | The Maximum Absorbance | Reference |
|---|---|---|---|
| PMoA/PANI Hybridizing Thin Film | 1.8 μm | 3.46 | [10] |
| ZnO/PMoA | - | 0.21 | [14] |
| WO3-x QDs | - | 2.75 | [15] |
| PVP/HTA hybrids | - | 0.78 | [16] |
| CsPbBr3 Quantum Dot Films | - | 0.78 | [17] |
| PMoA/ZnO/PVP composite film | - | 0.32 | [18] |
| Aromatic Sulfonium Octamolybdates | solid-state | 3.2 | [19] |
| PMoA/PTh composite film | 2.0 μm | 5.27 | This work |
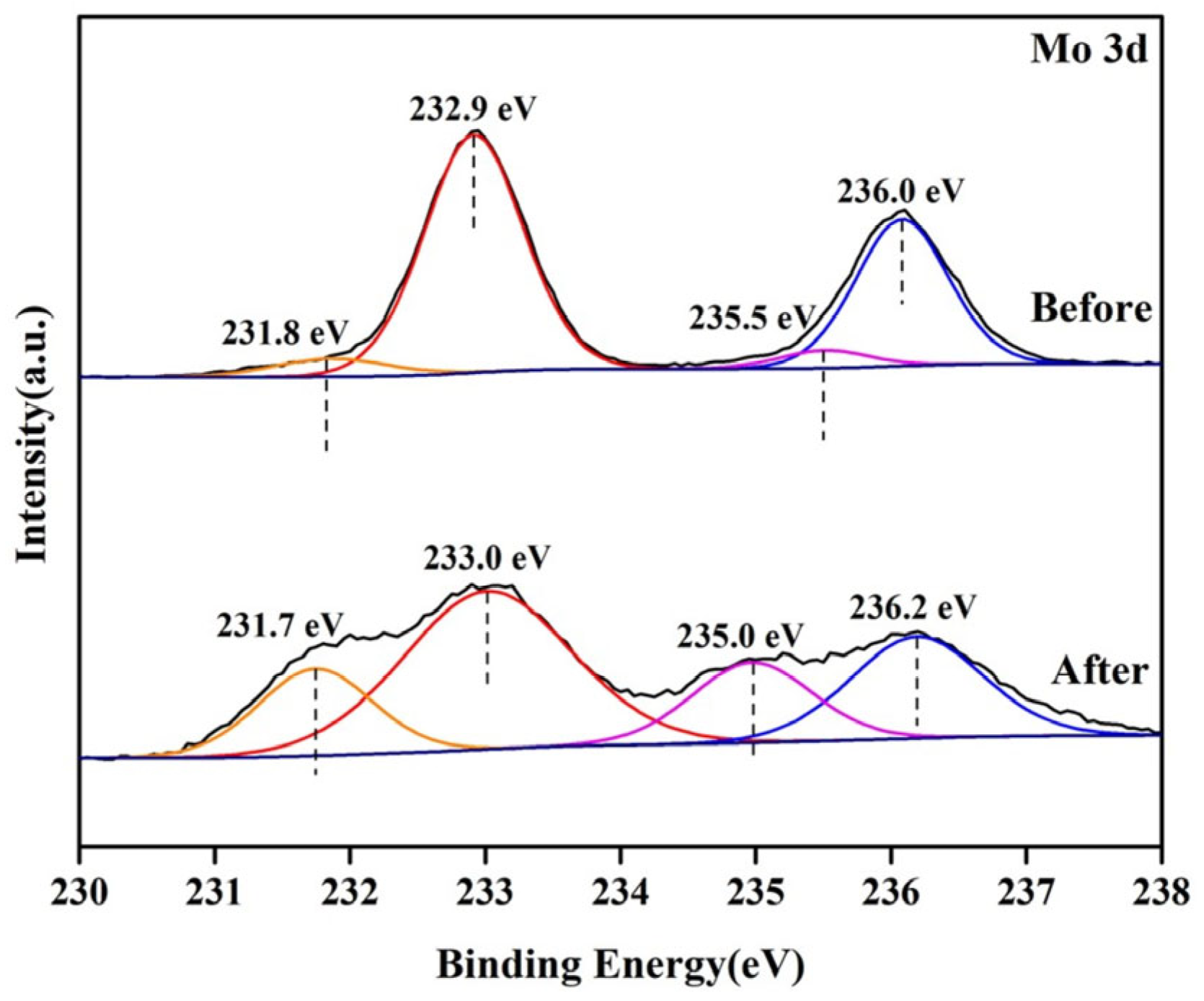
| Sample | Mo5+ | Mo6+ | Mo5+/Mo Ratios | ||
|---|---|---|---|---|---|
| 3d3/2 | 3d5/2 | 3d3/2 | 3d5/2 | ||
| Before | 231.8 | 235.5 | 232.9 | 236.0 | 0.09 |
| After | 231.7 | 235.0 | 233.0 | 236.2 | 0.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, W.; Zhao, H.; Feng, W.; Zhao, H. Visible-Light Photochromic Properties of an Inorganic-Organic Phosphomolybdic Acid/Polythiophene Hybrid Thin Film. Chemistry 2024, 6, 469-475. https://doi.org/10.3390/chemistry6030026
Zhao W, Zhao H, Feng W, Zhao H. Visible-Light Photochromic Properties of an Inorganic-Organic Phosphomolybdic Acid/Polythiophene Hybrid Thin Film. Chemistry. 2024; 6(3):469-475. https://doi.org/10.3390/chemistry6030026
Chicago/Turabian StyleZhao, Wanqing, Hongmei Zhao, Wei Feng, and Honggang Zhao. 2024. "Visible-Light Photochromic Properties of an Inorganic-Organic Phosphomolybdic Acid/Polythiophene Hybrid Thin Film" Chemistry 6, no. 3: 469-475. https://doi.org/10.3390/chemistry6030026
APA StyleZhao, W., Zhao, H., Feng, W., & Zhao, H. (2024). Visible-Light Photochromic Properties of an Inorganic-Organic Phosphomolybdic Acid/Polythiophene Hybrid Thin Film. Chemistry, 6(3), 469-475. https://doi.org/10.3390/chemistry6030026






