An Efficient and Eco-Friendly Procedure for Electrophilic Thiocyanation of Anilines and 1-(Substituted benzylidene)-2-phenyl Hydrazines
Abstract
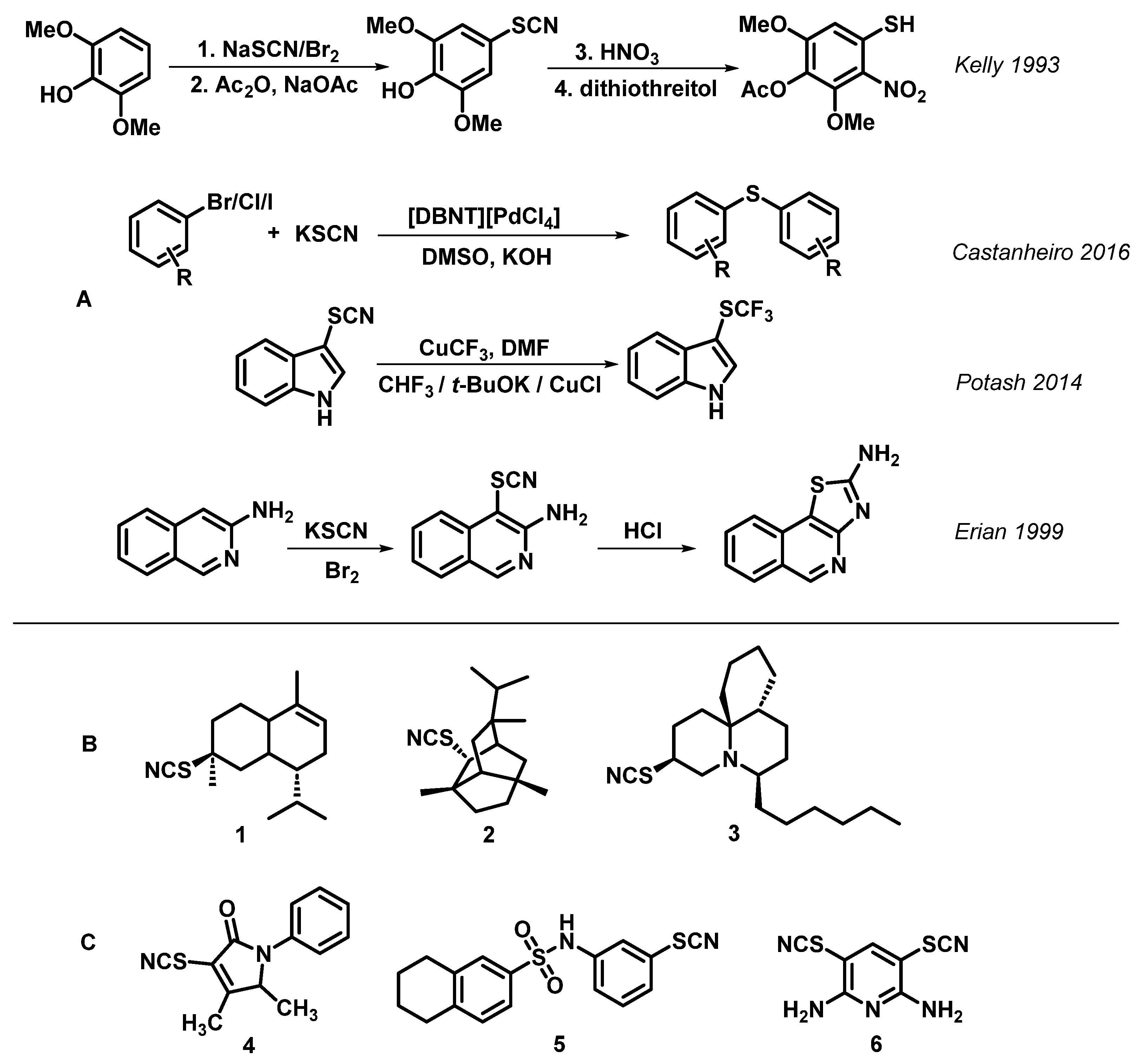
:1. Introduction
2. Experimental Section
2.1. Materials and Methods
- (i)
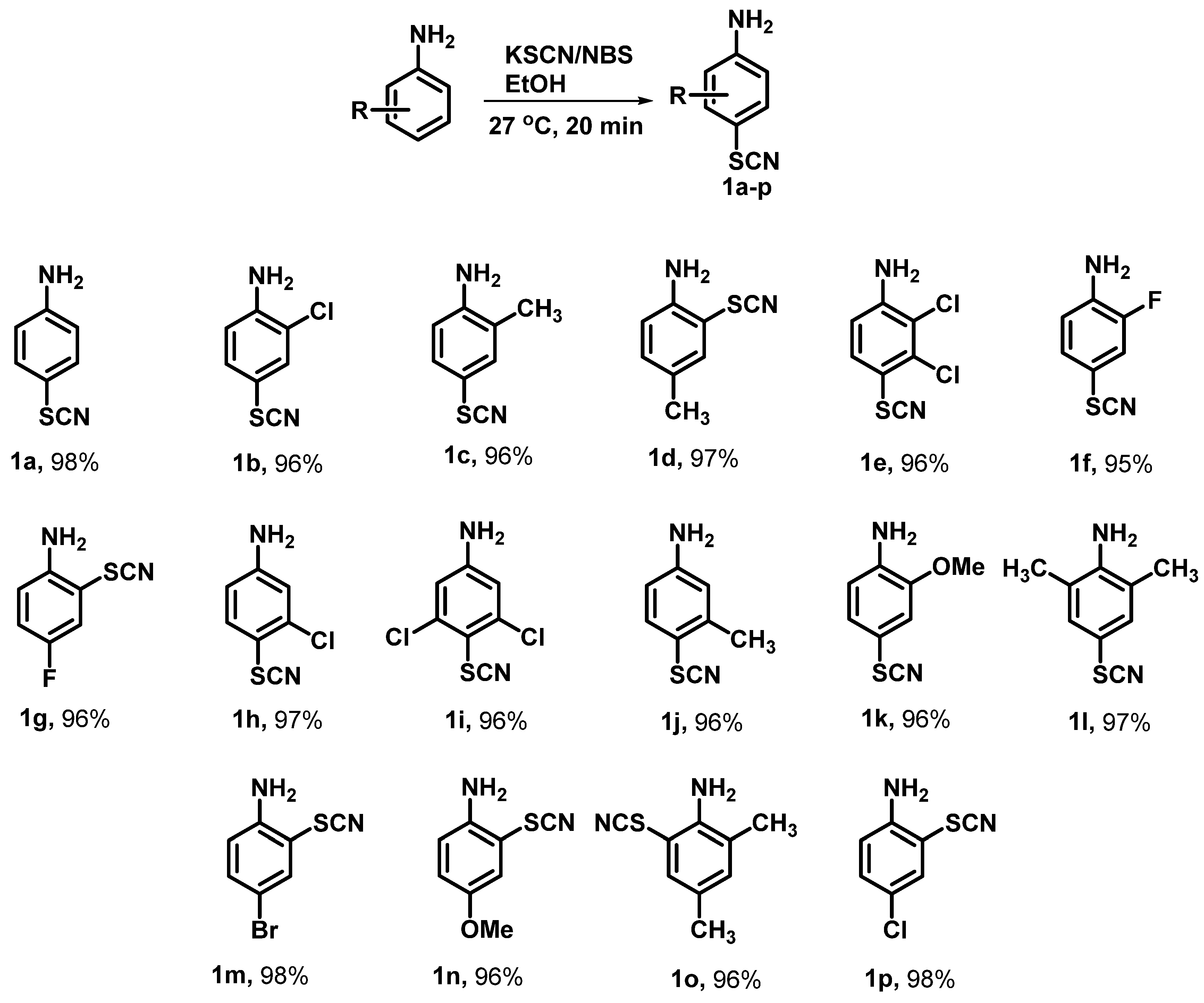
- General procedure for the synthesis of thiocyanatoaniline analogues
- (ii)
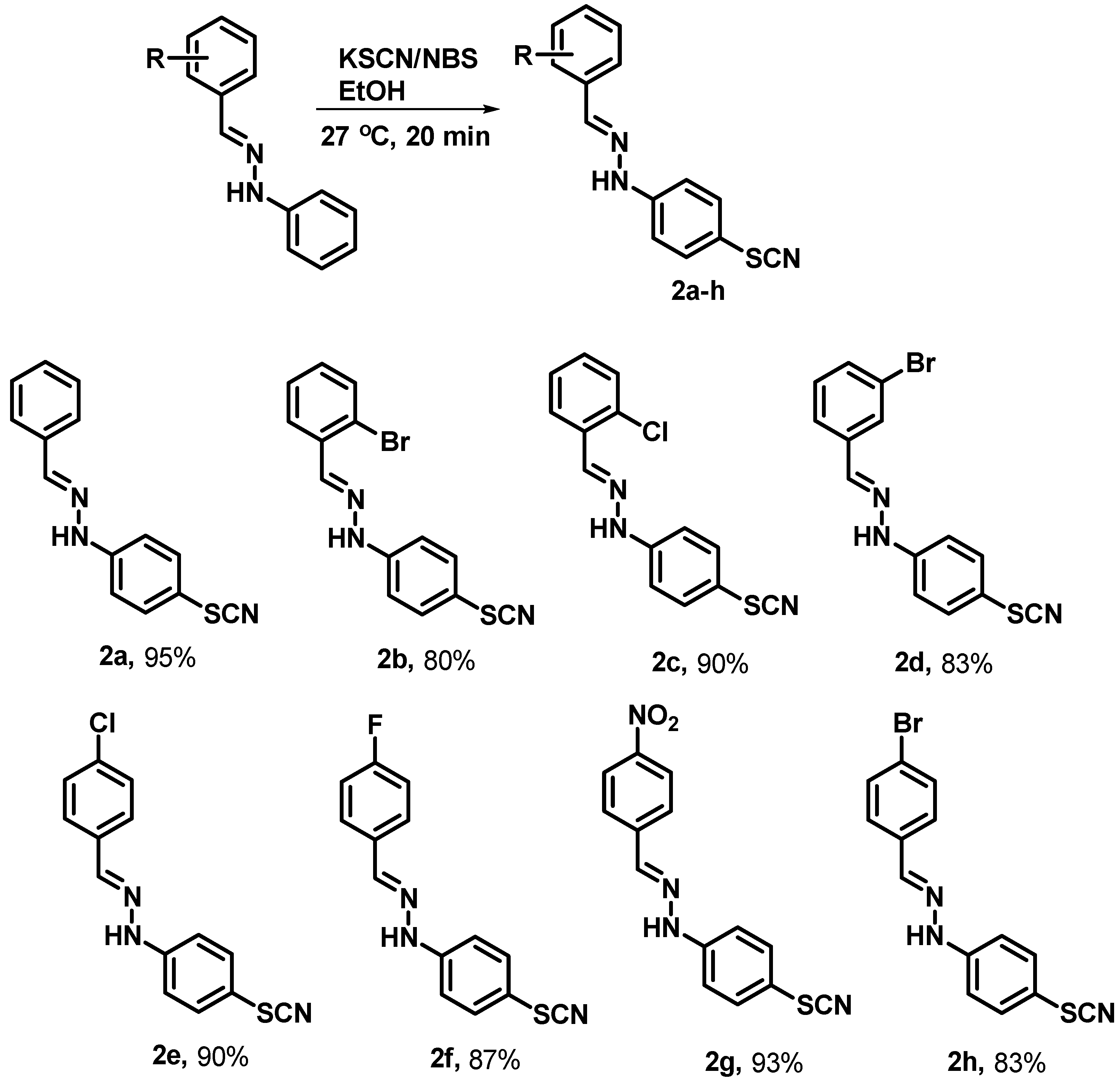
- General procedure for the synthesis of substituted (E)-1-benzylidene-2-(4-thiocyanatophenyl) hydrazine analogues.
2.2. X-ray Diffraction Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, H.; Shi, X.; Liu, X.; Zhao, L. Recent progress of direct thiocyanation reactions. Org. Biomol. Chem. 2022, 20, 6508–6527. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Vuagnat, M.; Chen, M.Y.; Pannecoucke, X.; Jubault, P.; Besset, T. Design and use of electrophilic thiocyanating and selenocyanating reagents: A new trend for the construction of SCN-and SeCN-containing ompounds. Chem. Eur. J. 2021, 27, 6145–6160. [Google Scholar] [CrossRef] [PubMed]
- Kelly, T.R.; Kim, M.H.; Curtis, A.D. Structure correction and synthesis of the naturally occurring benzothiazinone BMY 40662. J. Org. Chem. 1993, 58, 5855–5857. [Google Scholar] [CrossRef]
- Johnson, T.B.; Douglass, I.B. The action of chlorine on thiocyanates. J. Am. Chem. Soc. 1939, 61, 2548–2550. [Google Scholar] [CrossRef]
- Castanheiro, T.; Suffert, J.; Donnard, M.; Gulea, M. Recent advances in the chemistry of organic thiocyanates. Chem. Soc. Rev. 2016, 45, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Potash, S.; Rozen, S. A new synthesis of trifluoromethyl sulfides utilizing thiocyanates and fluoroform. J. Fluor. Chem. 2014, 168, 172–176. [Google Scholar] [CrossRef]
- Bayarmagnai, B.; Matheis, C.; Jouvin, K.; Goossen, L.J. Synthesis of difluoromethyl thioethers from difluoromethyl trimethylsilane and organothiocyanates generated in situ. Angew. Chem. Int. Ed. Engl. 2015, 4, 5753–5756. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Wang, H.; Gao, R.; Sun, D.; Bi, X. Microwave-assisted synthesis of asymmetric disulfides. RSC Adv. 2014, 4, 28794–28797. [Google Scholar] [CrossRef]
- Renard, P.Y.; Schwebel, H.; Vayron, P.; Josien, L.; Valleix, A.; Mioskowski, C. Easy access to phosphonothioates. Chem. Eur. J. 2002, 8, 2910–2916. [Google Scholar] [CrossRef]
- Erian, A.W.; Sherif, S.M. The chemistry of thiocyanic esters. Tetrahedron 1999, 55, 7957–8027. [Google Scholar] [CrossRef]
- Patai, S. Chemistry of Cyanates and Their Thio Derivatives; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 1977; ISBN 978-0471994251. [Google Scholar]
- He, H.Y.; Faulkner, D.J.; Shumsky, J.S.; Hong, K.; Clardy, J. A sesquiterpene thiocyanate and three sesquiterpene isothiocyanates from the sponge Trachyopsis aplysinoides. J. Org. Chem. 1989, 54, 2511–2514. [Google Scholar] [CrossRef]
- Burreson, B.J.; Scheuer, P.J.; Finer, J.; Clardy, J. 9-Isocyanopupukeanane, a marine invertebrate allomone with a new sesquiterpene skeleton. J. Am. Chem. Soc. 1975, 97, 4763–4764. [Google Scholar] [CrossRef]
- Patil, A.D.; Freyer, A.J.; Reichwein, R.; Carte, B.; Killmer, L.B.; Faucette, L.; Johnson, R.K.; Faulkner, D.J. Fasicularin, a novel tricyclic alkaloid from the ascidian Nephteis fasicularis with selective activity against a DNA repair-deficient organism. Tetrahedron Lett. 1997, 38, 363–364. [Google Scholar] [CrossRef]
- Jiménez, C.; Crews, P. Novel marine sponge derived amino acids 13. Additional psammaplin derivatives from Psammaplysilla purpurea. Tetrahedron 1991, 47, 2097–2102. [Google Scholar] [CrossRef]
- Prawat, H.; Mahidol, C.; Kaweetripob, W.; Prachyawarakorn, V.; Tuntiwachwuttikul, P.; Ruchirawat, S. Sesquiterpene isocyanides, isothiocyanates, thiocyanates, and formamides from the Thai sponge Halichondria sp. Tetrahedron 2016, 72, 4222–4229. [Google Scholar] [CrossRef]
- Capon, R.J.; Skene, C.; Liu, E.H.T.; Lacey, E.; Gill, J.H.; Heiland, K.; Friedel, T. The isolation and synthesis of novel nematocidal dithiocyanates from an Australian marine sponge, Oceanapia sp. J. Org. Chem. 2001, 66, 7765–7769. [Google Scholar] [CrossRef] [PubMed]
- Szajnman, S.H.; Yan, W.; Bailey, B.N.; Docampo, R.; Elhalem, E.; Rodriguez, J.B. Design and synthesis of aryloxyethyl thiocyanate derivatives as potent inhibitors of Trypanosoma cruzi proliferation. J. Med. Chem. 2000, 43, 1826–1840. [Google Scholar] [CrossRef] [PubMed]
- Abreu, M.E.; Rzeszotarski, W.; Kyle, D.J.; Hiner, R. Preparation of Oxopyrazolinyl Thiocyanate and bis(Oxopyrazolinyl) Disulfides as Corticotropin-Releasing Factor Antagonist. U.S. Patent 5063245 A, 5 November 1991. [Google Scholar]
- Wössner, N.; Alhalabi, Z.; González, J.; Swyter, S.; Gan, J.; Schmidtkunz, K.; Zhang, L.; Vaquero, A.; Ovaa, H.; Einsle, O.; et al. Sirtuin 1 inhibiting thiocyanates (S1th)-a new class of isotype selective inhibitors of NAD+ dependent lysine deacetylases. Front. Oncol. 2020, 10, 657–671. [Google Scholar] [CrossRef] [PubMed]
- Laali, K.K.; Greves, W.J.; Zwarycz, A.T.; Correa Smits, S.J.; Troendle, F.J.; Borosky, G.L.; Akhtar, S.; Manna, A.; Paulus, A.; Chanan-Khan, A.; et al. Synthesis, computational docking study, and biological evaluation of a library of heterocyclic curcuminoids with remarkable antitumor activity. ChemMedChem 2018, 13, 1895–1908. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.C.; Li, S.G.; Lalonde, T.J.; Yang, K.S.; Yu, G.; Qiao, Y.; Xu, S.; Liu, W.R. Drug repurposing for the SARS-CoV-2 papain-like protease. ChemMedChem 2022, 17, e202100455. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, M.; Sarkar, S. A clay-mediated eco-friendly thiocyanation of indoles and carbazoles. Tetrahedron Lett. 2003, 44, 8131–8133. [Google Scholar] [CrossRef]
- Iranpoor, N.; Firouzabadi, H.; Azadi, R. A new diphenylphosphinite ionic liquid (IL-OPPh2) as reagent and solvent for highly selective bromination, thiocyanation or isothiocyanation of alcohols and trimethylsilyl and tetrahydropyranyl ethers. Tetrahedron Lett. 2006, 47, 5531–5534. [Google Scholar] [CrossRef]
- Kumar, A.; Ahamd, P.; Maurya, R.A. Direct α-thiocyanation of carbonyl and β-dicarbonyl compounds using potassium peroxydisulfate–copper (II). Tetrahedron Lett. 2007, 48, 1399–1401. [Google Scholar] [CrossRef]
- Gitkis, A.; Becker, J.Y. Anodic thiocyanation of mono- and disubstituted aromatic compounds. Electrochim. Acta. 2010, 55, 5854–5859. [Google Scholar] [CrossRef]
- Yadav, J.S.; Reddy, B.V.S.; Krishna, B.B.M. IBX: A novel and versatile oxidant for electrophilic thiocyanation of indoles, pyrrole and arylamines. Synthesis 2008, 23, 3779–3782. [Google Scholar] [CrossRef]
- Das, B.; Kumar, A.S. Efficient thiocyanation of indoles using para-toluene sulfonic acid. Synth. Commun. 2010, 40, 337–341. [Google Scholar] [CrossRef]
- Ichake, S.S.; Rajawinslin, R.R.; Kavala, V.; Villuri, B.K.; Yang, H.-T.; Kuo, C.-W.; Yao, C.-F. N-bromosuccinide-mediated thiocyanation of cyclohexene-fused isooxazoline N-oxides. Asian J. Org. Chem. 2016, 5, 343–352. [Google Scholar] [CrossRef]
- Akhlaghinia, B.; Pourali, A.R.; Rahmani, M. Efficient and novel method for thiocyanation of aromatic compounds using trichloroisocyanuric acid/ammonium thiocyanate/wet SiO2. Synth. Commun. 2012, 42, 1184–1191. [Google Scholar] [CrossRef]
- Nair, V.; George, T.G.; Nair, L.G.; Panicker, S.B. A direct synthesis of aryl thiocyanates using cerium(IV) ammonium nitrate. Tetrahedron Lett. 1999, 40, 1195–1196. [Google Scholar] [CrossRef]
- Yadav, J.S.; Reddy, B.V.S.; Shubashree, S.; Sadashiv, K. Iodine/MeOH: A novel and efficient reagent system for thiocyanation of aromatics and heteroaromatics. Tetrahedron Lett. 2004, 45, 2951–2954. [Google Scholar] [CrossRef]
- Jadhav, V.K.; Pal, R.R.; Wadgaonkar, P.P.; Salunkhe, M.M. A facile synthesis of aryl thiocyanates using sodium perborate. Synth. Commun. 2001, 31, 3041–3045. [Google Scholar] [CrossRef]
- Wu, G.; Liu, Q.; Shen, Y.; Wu, W.; Wu, L. Regioselective thiocyanation of aromatic and heteroaromatic compounds using ammonium thiocyanate and oxone. Tetrahedron Lett. 2005, 46, 5831–5834. [Google Scholar] [CrossRef]
- Pan, X.Q.; Lei, M.Y.; Zou, J.P.; Zhang, W. Mn(OAc)3-promoted regioselective free radical thiocyanation of indoles and anilines. Tetrahedron Lett. 2009, 50, 347–349. [Google Scholar] [CrossRef]
- Iranpoor, N.; Firouzabadi, H.; Khalili, D.; Shahin, R. A new application for diethyl azodicarboxylate: Efficient and regioselective thiocyanation of aromatics amines. Tetrahedron Lett. 2010, 51, 3508–3510. [Google Scholar] [CrossRef]
- Yadav, J.S.; Reddy, B.V.S.; Krishna, A.D.; Reddy, C.S.; Narsaiah, A.V. Ferric(III) chloride-promoted electrophilic thiocyanation of aromatic and heteroaromatic compounds. Synthesis 2005, 2, 961–964. [Google Scholar] [CrossRef]
- Murthy, Y.L.N.; Govindh, B.; Diwakar, B.S.; Nagalakshmi, K.; Venu, R. Microwave-assisted neat reaction technology for regioselective thiocyanation of substituted anilines and indoles in solid media. J. Iran. Chem. Soc. 2011, 8, 292–297. [Google Scholar] [CrossRef]
- Memarian, H.R.; Mohammadpoor-Baltork, I.; Nikoofar, K. DDQ-promoted thiocyanation of aromatic and heteroaromatic compounds. Can. J. Chem. 2007, 85, 930–937. [Google Scholar] [CrossRef]
- Wu, J.; Wu, G.; Wu, L. Thiocyanation of aromatic and heteroaromatic compounds using ammonium thiocyanate and I2O5. Synth. Commun. 2008, 38, 2367–2373. [Google Scholar] [CrossRef]
- Mahajan, U.S.; Akamanchi, K.G. Facile method for thiocyanation of activated arenes using iodic acid in combination with ammonium thiocyanate. Synth. Commun. 2009, 39, 2674–2682. [Google Scholar] [CrossRef]
- Khazaei, A.; Zolfigol, M.A.; Mokhlesi, M.; Panah, F.D.; Sajjadifar, S. Simple and highly efficient catalytic thiocyanation of aromatic compounds in aqueous media. Helv. Chim. Acta 2012, 95, 106–114. [Google Scholar] [CrossRef]
- Prakash, O.; Kaur, H.; Pundeer, R.; Dhillon, R.S.; Singh, S.P. An improved iodine(III) mediated method for thiocyanation of 2-arylindan-1,3-diones, phenols, and anilines. Synth. Commun. 2003, 33, 4037–4042. [Google Scholar] [CrossRef]
- Toste, F.D.; Stefano, V.D.; Still, I.W. A versatile procedure for the preparation of aryl thiocyanates using N-thiocyanatosuccinimide (NTS). Synth. Commun. 1995, 25, 1277–1286. [Google Scholar] [CrossRef]
- Bruker. SMART, SAINT-Plus, SADABS; Bruker Axs Inc.: Madison, WI, USA, 1998. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Nardelli, M. Ring asymmetry parameters from out-of-plane atomic displacements. Acta Cryst. 1983, 39, 1141–1412. [Google Scholar] [CrossRef]
- Farrugia, L.J.J. ORTEP-3 for Windows-a version of ORTEP-III with a Graphical User Interface (GUI). Appl. Cryst. 1997, 30, 565. [Google Scholar] [CrossRef]
- Spek, A.L. Structure validation in chemical crystallography. Acta Cryst. 2009, 65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Watkin, D.J.; Prout, C.K.; Pearce, L.J. CAMERON; Chemical Crystallography Laboratory, University of Oxford: Oxford, UK, 1996. [Google Scholar]
- Brandenburg, K.; Putz, H. Crystal Impact; GbR: Bonn, Germany, 2005. [Google Scholar]


 | |||||
|---|---|---|---|---|---|
| Entry | Solvent | KSCN (Eq) | NBS (Eq) | Time h/min | Yield a (%) |
| 1 | DMSO | 2 | 1 | 2 h | 60 |
| 2 | DCE | 2 | 1 | 1.5 h | 50 |
| 3 | DCM | 2 | 1 | 1.5 h | 70 |
| 4 | DMF | 2 | 1 | 2 h | 50 |
| 5 | THF | 2 | 1 | 1 h | 60 |
| 6 | Dioxane | 2 | 1 | 1.5 h | 40 |
| 7 | Acetonitrile | 2 | 1 | 1 h | 80 |
| 8 | MeOH | 2 | 1 | 45 min | 85 |
| 9 | EtOH | 2 | 0.25 | 1 h | 70 |
| 10 | EtOH | 2 | 0.50 | 1 h | 85 |
| 11 | EtOH | 2 | 0.75 | 1 h | 90 |
| 12 | EtOH | 2 | 1 | 20 min | 98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mallikarjunaswamy, A.M.M.; Kuruvalli, G.; Syed, K.; Reddy, V.D.; Nair, V.A. An Efficient and Eco-Friendly Procedure for Electrophilic Thiocyanation of Anilines and 1-(Substituted benzylidene)-2-phenyl Hydrazines. Chemistry 2024, 6, 476-488. https://doi.org/10.3390/chemistry6030027
Mallikarjunaswamy AMM, Kuruvalli G, Syed K, Reddy VD, Nair VA. An Efficient and Eco-Friendly Procedure for Electrophilic Thiocyanation of Anilines and 1-(Substituted benzylidene)-2-phenyl Hydrazines. Chemistry. 2024; 6(3):476-488. https://doi.org/10.3390/chemistry6030027
Chicago/Turabian StyleMallikarjunaswamy, A. M. M., Gouthami Kuruvalli, Khajamohiddin Syed, Vaddi Damodara Reddy, and Vipin A. Nair. 2024. "An Efficient and Eco-Friendly Procedure for Electrophilic Thiocyanation of Anilines and 1-(Substituted benzylidene)-2-phenyl Hydrazines" Chemistry 6, no. 3: 476-488. https://doi.org/10.3390/chemistry6030027
APA StyleMallikarjunaswamy, A. M. M., Kuruvalli, G., Syed, K., Reddy, V. D., & Nair, V. A. (2024). An Efficient and Eco-Friendly Procedure for Electrophilic Thiocyanation of Anilines and 1-(Substituted benzylidene)-2-phenyl Hydrazines. Chemistry, 6(3), 476-488. https://doi.org/10.3390/chemistry6030027








