Abstract
Cordyceps militaris, well known for its therapeutic potential in managing type-2 diabetes through the inhibition of α-amylase and α-glucosidase enzymes, was the central focus of this research, which investigated the influence of various cultivation substrates on its enzymatic inhibitory properties and bioactive compound content. Previous studies have primarily focused on the general pharmacological benefits of C. militaris but have not thoroughly explored how different substrates affect its bioactive profile and enzyme inhibitory activities. This study aimed to evaluate the impact of substrate selection on the enzyme inhibition activities and the levels of bioactive compounds such as cordycepin and adenosine in C. militaris, demonstrating that substrate selection markedly affects both these enzymes’ inhibition activities and bioactive compound levels. Particularly, C. militaris fruiting bodies grown on Brihaspa atrostigmella showed the highest concentrations of cordycepin (2.932 mg/g) and adenosine (1.062 mg/g). This substrate also exhibited the most potent α-glucosidase inhibition with an IC50 value of 336.4 ± 16.0 µg/mL and the most effective α-amylase inhibition with an IC50 value of 504.6 ± 4.2 µg/mL. Conversely, C. militaris cultivated on the solid residues of Gryllus bimaculatus displayed the strongest xanthine oxidase (XOD) inhibition, with the lowest IC50 value of 415.7 ± 11.2 µg/mL. These findings highlight the critical role of substrate choice in enhancing the medicinal properties of C. militaris, suggesting that optimized cultivation can enhance the bioactive properties for more effective natural therapies for diabetes and other metabolic disorders. This study not only extends the understanding of C. militaris’ pharmacological potential but also illustrates its applicability in developing customized treatment options.
1. Introduction
The Cordyceps genus is esteemed for its considerable therapeutic and nutritional value and plays a significant role in both Asian traditional medicine and Western healthcare practices [1]. Among its species, C. militaris is distinguished for its potent pharmacological effects, such as immunomodulation and anticancer properties [2,3,4]. The use of Cordyceps for treating diseases like cancer, immune deficiencies, and age-related illnesses is supported by extensive research demonstrating its antioxidant, anti-inflammatory, and immunomodulatory properties [5,6,7]. These characteristics emphasize the mushroom’s capacity to regulate bodily functions and effectively counteract disease states [8]. C. militaris parasitizes various insects to produce fruiting bodies, which are rich in essential phytochemicals including cordycepin, polysaccharides, and phenolic compounds that enhance its health-promoting attributes [9]. With a growing demand for natural health products, the Cordyceps extract market has expanded significantly, valued at over USD 473 million in 2018, with predictions for continued growth [10]. This burgeoning demand has driven innovations in the artificial cultivation of C. militaris, tailored to enhance yield and the production of bioactive compounds. Current cultivation methods utilize diverse substrates like cereal grains and insect bodies, which not only imitate the fungus’s natural growing conditions but also boost specific bioactive constituents known for their notable antitumor and immunomodulatory effects [4].
Despite these advantages, Cordyceps cultivation faces challenges, especially regarding sustainability and environmental impacts [11]. The leftover biomass from Cordyceps cultivation presents substantial waste management challenges, necessitating innovative solutions such as column chromatographic extraction techniques to salvage valuable compounds from the residual substrates [12]. The choice of cultivation hosts is crucial, as it not only affects the yield and quality of the bioactive compounds but also the sustainability of production practices. Each host offers a unique physiological environment that influences the metabolic pathways of C. militaris, affecting the synthesis of essential compounds such as cordycepin and polysaccharides [13]. For example, research has shown that silkworm larvae yield higher cordycepin levels than other hosts, highlighting the importance of careful host selection to enhance the fungus’s medicinal properties [14,15]. Adapting a broad range of hosts from natural arthropods to synthetic media helps reduce ecological impacts and provides economic opportunities for large-scale, sustainable production [16,17,18]. The genetic diversity among C. militaris strains also plays a critical role, influencing compatibility with various hosts and the efficacy of bioactive compound synthesis [19]. Advances in molecular biology have deepened our understanding of how C. militaris adjusts its metabolic pathways to maximize resource utilization, thus optimizing cultivation efficiency [20].
This study leverages this extensive background to explore the impact of various edible insects as substrates on the production of cordycepin and other bioactive components. By utilizing five different insects approved for consumption in Vietnam—Bombyx mori Pupae (silkworm pupae), Brihaspa atrostigmella (chit worm), Halyomorpha halys (brown stink bug), Oxya chinensis (grasshoppers), and Gryllus bimaculatus (cricket)—we aim to elucidate the influence of substrate choice on the medicinal properties of C. militaris. Brown rice was supplied to all media as a basal substrate to provide essential nutrients necessary for the initial growth and development of C. militaris [21,22,23]. Each insect blend served as a supplemental substrate to enhance the production of bioactive compounds such as adenosine, cordycepin, phenols, and flavonoids [24]. This dual-substrate approach was designed to investigate the synergistic effects on fungal growth and its pharmacological properties. The inclusion of insect blends is particularly significant as they provide specific nutrients and bioactive compounds, such as proteins, fats, vitamins, and minerals, which are essential for optimizing the metabolic processes in C. militaris [25,26,27]. These nutrients potentially improve the biological activities of the fungus, leading to enhanced production of bioactive compounds with strong antioxidant and enzyme inhibitory activities. This approach not only offers insights into optimizing cultivation practices but also highlights the potential for developing natural therapies for metabolic disorders, thus contributing to the broader pharmacological applications of C. militaris. This investigation includes an analysis of the antioxidant and xanthine oxidase inhibition activities, as well as α-amylase and α-glucosidase inhibition activities of both the fruiting bodies and solid-based residues. Through this research, we hope to contribute to the pharmacological understanding of Cordyceps and provide insights into sustainable cultivation practices that could enhance the therapeutic efficacy of this valuable medicinal fungus. Thus, we have embarked on a detailed exploration of the effects of these five edible insects on cordycepin production, aiming to optimize the health benefits derived from C. militaris cultivated on these substrates.
2. Materials and Methods
2.1. Materials
2.1.1. Fungal Strain and Edible Insects
The fungal strain C. militaris VCCM 34117 was provided by the Vietnam Academy of Science and Technology’s (VAST) Culture Collection of Microorganisms (VCCM), Hanoi Vietnam. We maintained the stock culture on potato dextrose agar (PDA) slant plates at 4 °C and refreshed the subcultures bi-monthly. We obtained edible insects, including B. mori Pupae (silkworm pupae), B. atrostigmella (chit worm), H. halys (brown stink bug), O. chinensis (grasshoppers), and G. bimaculatus (cricket) from the Institute of Ecology and Biological Resources, Vietnam Academy of Science and Technology, Hanoi Vietnam (Figure 1).

Figure 1.
Edible insects used in the study.
2.1.2. Cultivation of C. militaris VCCM 34117
C. militaris VCCM 34,117 was initially cultivated on PDA plates using an active slant for 14 days at 22 °C. A sterilized cork borer extracted 9 mm mycelial agar discs, which were then transferred to 500 mL Erlenmeyer flasks containing 150 mL of seed culture medium (glucose, 30 g/L; peptone, 5 g/L; yeast extract, 2 g/L; MgSO4·7H2O, 0.5 g/L; KH2PO4, 0.5 g/L; pH 6.0). The flasks were incubated at 22 °C for 4 days on a rotary shaker and the resulting cultures were used as liquid spawn.
All edible insects were dried at 60 °C and processed into a blend for solid-state fermentation. Plastic containers were filled with 30 g of brown rice (Oryza sativa), 10 g of each insect blend, and 40 mL of medium (glucose, 20 g/L; potato extract, 4 g/L; peptone, 5 g/L; yeast extract, 2 g/L; vitamin B complex, 0.5 g/L; MgSO4·7H2O, 0.5 g/L; KH2PO4, 0.5 g/L; pH 6.0), and then autoclaved for 30 min at 121 °C. After cooling to room temperature, the containers were inoculated with 1 mL of seed culture and maintained under dark conditions and 70% relative humidity for 10 days at 22 °C to promote vegetative growth. To induce fruiting body formation, the samples were exposed to a light intensity of 1000 lx for 12 h alternating with 12 h of darkness, maintaining a relative humidity of 85% at 22 °C. After 65 days, the samples were collected. Consequently, five types of C. militaris corresponding to five hosts were obtained: C. militaris cultivated on Halyomorpha halys (Figure 2a), C. militaris cultivated on Gryllus bimaculatus (Figure 2b), C. militaris cultivated on Oxya chinensis (Figure 2c), C. militaris cultivated on Bombyx mori pupae (Figure 2d), and C. militaris cultivated on Brihaspa atrostigmella (Figure 2e). We acknowledge the methodology adopted for the extraction of C. militaris, which was meticulously detailed and executed in collaboration under project code UDSPTM.01/22-23.

Figure 2.
Fruiting bodies and solid-based residues of C. militaris cultivated on various insects: (a) Halyomorpha halys, (b) Gryllus bimaculatus, (c) Oxya chinensis, (d) Bombyx mori Pupae, and (e) Brihaspa atrostigmella.
Fruiting bodies and solid-based residues were meticulously separated, then subjected to freeze-drying and fine grinding. The resultant materials were filtered under vacuum using a Whatman No. 1 filter (Whatman plc, Maidstone, UK), after which the filtrate was concentrated through rotary evaporation. The concentrated samples were preserved in methanol at −20 °C for subsequent analysis. To ensure reproducibility and reliability, all experimental procedures were performed in triplicate. For clarity, abbreviations used throughout this study are detailed in Table 1.

Table 1.
Nutritional characteristics and contributions of selected edible insects to Cordyceps militaris cultivation.
Following sample preparation, including drying and grinding, the freeze-dried Cordyceps militaris samples underwent an extraction procedure. The ground samples were extracted using a methanol–water solution (80:20, v/v) at a ratio of 1:20 (w/v). The mixture was sonicated for 30 min at 25 °C, followed by centrifugation at 5000 rpm for 15 min. The supernatant was collected, and the residue was re-extracted twice under the same conditions. The combined extracts were filtered through Whatman No. 1 filter paper and concentrated under reduced pressure using a rotary evaporator at 40 °C until dry. The dried extracts were stored at −20 °C for subsequent analysis.
2.2. Reagents
The reagents utilized for all experiments were sourced from Sigma-Aldrich Pte Ltd., Singapore, and included 1,1-Diphenyl-2-picrylhydrazyl (DPPH), 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), dimethyl sulfoxide (DMSO), monopotassium phosphate, dipotassium phosphate, sodium hydroxide, hydrochloric acid, xanthine, microbial xanthine oxidase, and allopurinol. Additionally, acetate buffer (250 mM, pH 5.0), phosphate buffer (50 mM, pH 7.4), sodium phosphate buffer (0.02 M, pH 6.9 with 6 mM NaCl), wheat starch, α-amylase, α-glucosidase enzymes, acarbose, Folin–Ciocalteu reagent, sodium carbonate, aluminum chloride, sodium nitrite, sodium hydroxide, and catechin were also used. All reagents were of analytical grade.
2.3. Adenosine and Cordycepin Content in C. militaris
The quantification of adenosine and cordycepin was conducted using an HPLC system, following the method described by Li et al. [21]. Initially, 5 mL of solid-based residue (SBR) extract (in MeOH) was filtered through a hydrophilic filter (0.2 μm) prior to injection into the HPLC system (Thermo Ulti-Mate 3000, column Hypersil Gold 250 × 4.6 mm − 5 µm, Thermo Fisher Scientific Inc., Waltham, MA, USA). The separation was achieved using two solvent systems: (A) water with 10 mM ammonium acetate and 0.1% acetic acid, and (B) 90% MeOH with 10 mM ammonium acetate and 0.1% acetic acid, in a gradient program. The program commenced with a 20 min phase where the concentration of solvent B increased from 5% to 95%, then rose to 100% over the next 5 min, and finally reduced back to 5% in the concluding 5 min. The HPLC parameters included a detection wavelength of 260 nm, a flow rate of 1.6 mL/min, a column temperature of 40 °C, and a sample temperature of 15 °C.
2.4. Total Phenolic Content and Evaluation of Total Flavonoid Content
The phenolic content in all samples was determined using the Folin–Ciocalteu method [22]. Results were expressed in milligrams of gallic acid equivalent (GAE) per gram of dry weight (DW) after establishing a calibration curve. For flavonoid quantification, the aluminum chloride (AlCl3) colorimetric method was utilized, and the total flavonoid content was measured using a calibration curve, with the results expressed in milligrams of rutin equivalent per gram dry weight (DW) [23].
2.5. Antioxidant and Xanthine Oxidase Inhibition (XOD) Activities
The antioxidant activity was determined using DPPH (2,2-diphenyl-1-picrylhydrazyl) radical scavenging and ABTS (2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid)) radical cation decolorization assays [24]. Modifications were made to the established spectrophotometric techniques to measure the inhibitory effect on xanthine oxidase (XO) for all samples [25].
2.6. α-Amylase Inhibition (AAI) Assay and α-Glucosidase Inhibition (AGI) Assay
The inhibitory effect of all samples on α-amylase was evaluated using a starch-iodine method, with spectrophotometric measurements based on a previously reported method, incorporating minor modifications [25]. Acarbose, a commercial diabetes inhibitor, was employed as a positive reference. Solutions of α-amylase and soluble starch were prepared and utilized on the day of the experiment. Additionally, the anti-α-glucosidase activity of all samples was assessed using a previously described method [25], also with some modifications.
2.7. Statistical Analysis
Results are expressed as mean ± standard deviation (SD). Statistical significance was assessed using Duncan’s test with a significance level of 5%. Additionally, differences due to the solvent system were analyzed by one-way analysis of variance (ANOVA) using Minitab Statistical Software, Minitab® 21.2, based in Philadelphia, PA, USA.
3. Results
3.1. Analysis of Adenosine and Cordycepin Concentrations in C. militaris Cultivars
The analysis of adenosine and cordycepin content across different C. militaris cultivars cultivated on various insects provides significant insights into the influence of substrates on the biosynthesis of these compounds (Table 2, Figure S1–S13 and Table S1 (Supplementary Materials)).

Table 2.
Comparative analysis of bioactive compounds in C. militaris.
The concentration of adenosine ranges from 0.053 mg/g in SBMP to 1.062 mg/g in FBA, demonstrating a substantial variability dependent on the host material. Notably, the FBA sample, which involves cultivation on B. atrostigmella, shows the highest concentration of adenosine, indicating a potential optimization of growth conditions or inherent metabolic capabilities of the fungus when grown on this particular host. On the other hand, the lowest concentrations of adenosine are found in C. militaris solid-based residues from B. mori Pupae and B. atrostigmella (SBMP and SBA), suggesting that the nutrient composition or structure of these residues might be less conducive to adenosine production. The cordycepin content displays a similar trend of variability, with concentrations ranging from 0.207 mg/g in SOC to 2.932 mg/g in FBA. This wide range highlights the significant impact of host choice on cordycepin accumulation, with FBA again showing the highest levels. The marked increase in cordycepin in samples associated with B. atrostigmella (both fruiting bodies and residues) compared to other substrates suggests that specific components or the physical nature of the host substrate might enhance the biosynthesis of cordycepin. The statistical grouping indicated by the post hoc test underscores these observations, as samples from similar host types do not show significant differences in compound concentrations, emphasizing consistent metabolic responses to similar cultivation conditions.
3.2. Variations in Phenolic and Flavonoid Contents across Different C. militaris Hosts
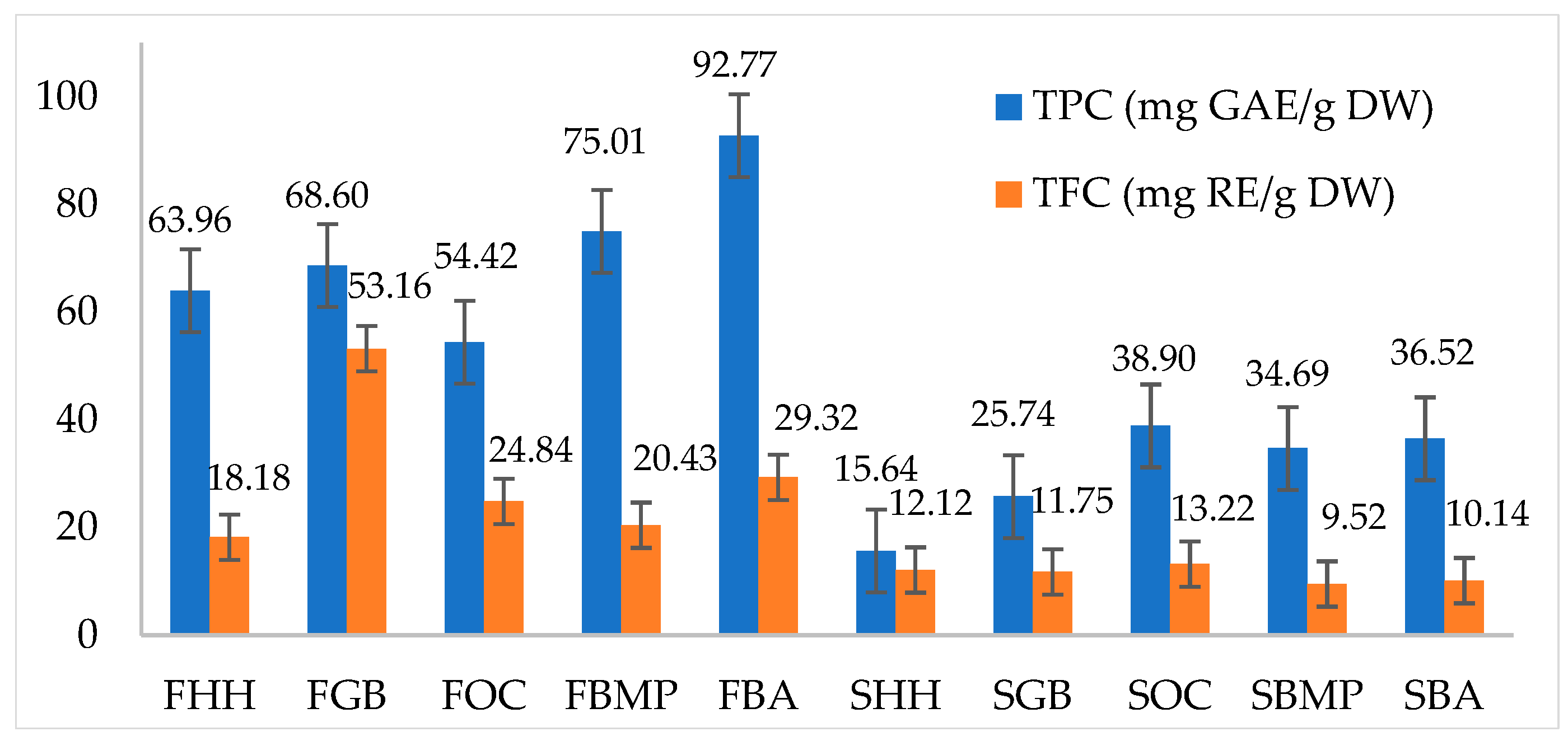
The quantitative analysis of Total Phenolic Content (TPC) and Total Flavonoid Content (TFC) in different hosts of C. militaris is presented in Figure 3. The results demonstrate a substantial variation in the concentrations of these bioactive compounds, critical for their antioxidative activities, which contribute to the medicinal properties of the fungus. The highest phenolic content was observed in the FBA, reaching 92.77 mg GAE/g DW, which also showed a relatively high flavonoid content at 29.32 mg RE/g DW. Conversely, the SHH exhibited the lowest phenolic content at 15.63 mg GAE/g DW and a correspondingly low flavonoid content of 12.12 mg RE/g DW. Notably, the FGB stood out with a particularly high flavonoid content of 53.16 mg RE/g DW, despite having a moderate phenolic content of 68.59 mg GAE/g DW. These data suggest that specific host substrates may differentially influence the biosynthesis pathways of phenolic and flavonoid compounds in C. militaris.
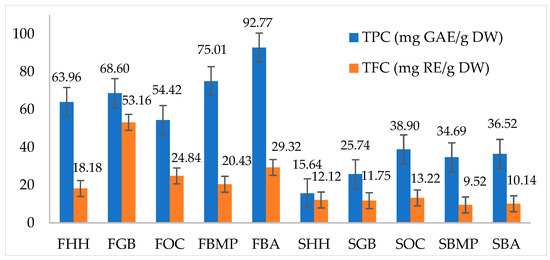
Figure 3.
Total phenolic and flavonoid contents of C. militaris cultivated on various hosts.
3.3. Antioxidant and Xanthine Oxidase Inhibitory Activities of Cordyceps militaris Cultivations
The evaluation of enzyme inhibition capabilities of C. militaris, cultivated on various substrates, underscores their potential in therapeutic applications, particularly in oxidative stress and glycemic control management. The data presented reveal a spectrum of inhibitory effects against DPPH, ABTS, and xanthine oxidase (XOD) enzymes, with each substrate yielding distinct results (Table 3).

Table 3.
Inhibitory concentrations of C. militaris on DPPH, ABTS, and XOD.
The IC50 values, which indicate the concentration needed to inhibit 50% of the radical or enzymatic activity, serve as a benchmark for comparing the potency of different samples. Lower IC50 values indicate higher potency. For the DPPH assay, FBA (C. militaris fruiting bodies cultivated on Brihaspa atrostigmella) shows the most potent activity with an IC50 of 88.34 ± 6.3 µg/mL, suggesting strong antioxidant capabilities. This is closely followed by FBMP and FGB, indicating that these strains or cultivation conditions also foster significant antioxidant production.
In the ABTS assay, FBA again shows a strong result with an IC50 of 247.3 ± 17.7 µg/mL, underscoring its potential in scavenging different types of free radicals. This potent activity in both DPPH and ABTS assays highlights FBA’s robustness in neutralizing oxidative molecules, which is beneficial for preventing oxidative stress-related diseases.
The XOD inhibition assay results are crucial for applications in treating hyperuricemia and gout, where the inhibition of xanthine oxidase is beneficial. The FGB sample exhibits the most potent inhibition with an IC50 of 415.7 ± 11.2 µg/mL. This result is significant because it suggests that the substrate or conditions under which FGB is cultivated are particularly effective at enhancing the production of bioactive compounds that inhibit xanthine oxidase.
3.4. Analysis of Enzymatic Inhibition by C. militaris Cultivations in Type 2 Diabetes Management
The results of α-amylase and α-glucosidase inhibition assays reveal a notable variation in the inhibitory capacities of different C. militaris cultivations, which can potentially be harnessed for therapeutic applications, particularly in the management of type-2 diabetes. These assays are essential for understanding how different substrates used in the cultivation of C. militaris can influence the production of bioactive compounds with enzyme inhibition properties. For α-amylase inhibition, the IC50 values indicate that C. militaris fruiting bodies and solid-based residues cultivated on various substrates exhibit a broad range of enzyme inhibition activities (Table 4). The most notable results were observed in Cordyceps cultivated on B. atrostigmella and B. mori Pupae, showing significantly high IC50 values (3924.3 ± 11.3 µg/mL and 3867.4 ± 15.2 µg/mL, respectively), indicating lower enzyme inhibition efficiency. In contrast, the cultivation on O. chinensis fruiting bodies showed more potent inhibition (504.6 ± 4.2 µg/mL).

Table 4.
Inhibitory activities of C. militaris hosts against α-Amylase and α-Glucosidase.
Similarly, in the α-glucosidase inhibition assay, the variations are equally significant. The strongest inhibition was observed again in Cordyceps cultivated on B. atrostigmella fruiting bodies (3018.7 ± 11.6 µg/mL), suggesting that the substrate may enrich compounds favorable for inhibiting this specific enzyme. Conversely, C. militaris fruiting bodies cultivated on B. atrostigmella (FBA) showed the lowest IC50 value (336.4 ± 16.0 µg/mL), highlighting its potential as an effective natural inhibitor for α-glucosidase. These findings suggest a direct correlation between the type of substrate used for cultivation and the enzymatic inhibition properties of the resulting C. militaris, pointing to the possibility of tailoring cultivation processes to enhance specific bioactive properties.
4. Discussion
In the field of mycological research, substrate selection has been repeatedly emphasized as a critical factor influencing the biosynthesis of bioactive compounds in fungi, especially in species like C. militaris, known for its medicinal properties [26,27,28]. This study corroborates previous findings by demonstrating that different cultivation substrates significantly affect both the enzyme inhibition capabilities and the levels of bioactive compounds such as cordycepin and adenosine in C. militaris. Particularly, C. militaris fruiting bodies cultivated on B. atrostigmella showcased the highest concentrations of cordycepin at 2.932 mg/g and adenosine at 1.062 mg/g, alongside exhibiting potent α-glucosidase and α-amylase inhibition. This aligns with the findings of Li et al. (2019) who noted that the physical and chemical properties of substrates influence fungal metabolism by affecting conditions such as oxygenation and moisture content [29]. Additionally, the work by Yu et al. (2023) highlights similar variations in bioactive compound production across different strains of Cordyceps, further supporting our observations regarding the impact of substrate choice [30]. In contrast, C. militaris grown on solid media without adding edible insects was reported to produce very low amounts of cordycepin and adenosine [31]. Therefore, it is important to use edible insects for the commercial production of C. militaris and its bioactive metabolites. This study not only extends the understanding of C. militaris’ pharmacological potential but also illustrates the critical role of optimized substrate selection in enhancing the therapeutic efficacy of fungal bioactive compounds, thus offering valuable insights for the development of more effective natural therapies for diabetes and other metabolic disorders.
The inclusion of various insects as supplemental substrates in the cultivation of C. militaris leverages their rich nutritional profiles to enhance the production of bioactive compounds [32]. Each insect provides a unique blend of proteins, fats, vitamins, and minerals that significantly influence the metabolic pathways of C. militaris, potentially leading to increased production of adenosine, cordycepin, phenols, and flavonoids [24]. This dual-substrate approach explores synergistic effects on fungal growth and pharmacological properties, optimizing cultivation practices for enhanced medicinal benefits. For example, B. mori Pupae are rich in protein, essential amino acids, fats, vitamins, and minerals [14]. B. atrostigmella, although less documented, provides proteins, fats, and antioxidant bioactive compounds [27]. H. halys offers high protein content, healthy fats, and essential vitamins and minerals [33]. O. chinensis contains high protein levels, unsaturated fats, and vital vitamins and minerals [33]. G. bimaculatus, rich in protein and fats, also provides essential vitamins and minerals [34]. These profiles support the enhanced production of bioactive compounds in C. militaris, contributing to its medicinal potential.
Our results show that C. militaris cultivated from different insects exhibits varying pharmacological properties [35,36]. For instance, the solid-based residues of B. mori Pupae and G. bimaculatus showed significantly high IC50 values, indicating lower enzyme inhibition efficiency. In contrast, the fruiting bodies cultivated on B. atrostigmella (FBA) exhibited the lowest IC50 value of 336.4 ± 16.0 μg/mL, reflecting the highest enzyme inhibitory activity. This suggests that the substrate provided by B. atrostigmella significantly enhances the production of bioactive compounds with potent enzyme inhibitory properties. The rich nutrient profile of B. atrostigmella likely provides an optimal environment for the biosynthesis of these compounds, maximizing the medicinal potential of C. militaris. The lower contents of adenosine and cordycepin in solid-based residues compared to fruiting bodies could be attributed to the differing metabolic pathways activated during fungal growth on solid residues versus fruiting bodies [9,37]. The solid-based residues may lack certain nutrients or conditions that are crucial for the optimal synthesis of these bioactive compounds [38].
The phenolic and flavonoid contents also varied significantly across different substrates. The high phenolic and flavonoid content in FBA suggests that the cultivation conditions on this insect might optimize the synthesis of these compounds, possibly due to a rich nutrient profile or specific environmental stress factors that induce higher metabolic activity. Phenols and flavonoids, known for their antioxidant properties, are indicative of the potential health benefits of C. militaris [39]. Specifically, phenolic compounds such as gallic acid, catechin, and epicatechin contribute to its potent antioxidant activities, while flavonoids like quercetin, kaempferol, and rutin enhance its therapeutic profile [38]. In contrast, the lower values recorded for SHHs (solid-based residues) indicate suboptimal conditions for the synthesis of these bioactive molecules. The unique case of FGB, where flavonoid production is significantly enhanced relative to phenolic content, points to specific metabolic pathways being preferentially activated in response to the unique properties of the insects used. This preferential activation might be influenced by the specific nutrient availability and environmental factors provided by the host substrate, leading to a differential expression of metabolic enzymes involved in the biosynthesis of these compounds. Understanding the variability in phenolic and flavonoid contents based on substrate can provide deeper insights into optimizing cultivation conditions to enhance the medicinal properties of C. militaris. Future studies should focus on identifying and quantifying individual phenolic and flavonoid compounds using advanced analytical techniques such as high-performance liquid chromatography (HPLC) and mass spectrometry (MS) to further elucidate the specific bioactive profiles of C. militaris grown on different substrates. Such detailed characterization will aid in developing targeted cultivation strategies that maximize the therapeutic potential of C. militaris. Additionally, exploring the molecular mechanisms underlying the differential biosynthesis of these compounds in response to various substrates will provide valuable information for optimizing fungal cultivation for pharmaceutical applications.
These findings not only advance our understanding of fungal metabolism but also highlight the potential of manipulating cultivation conditions to enhance the yield of valuable bioactive compounds. This approach could significantly impact commercial cultivation strategies, offering a route to maximize the health-promoting properties of C. militaris products. Comparison with previous studies reveals that our results are consistent with the established knowledge regarding the influence of substrates on bioactive compound production. For example, previous studies have demonstrated that substrates rich in complex carbohydrates and proteins, such as B. atrostigmella, provide a rich source of precursors for the synthesis of nucleoside analogues and other secondary metabolites, leading to higher yields of these compounds [30,40]. Furthermore, the physical structure and composition of the substrate can affect the availability of oxygen and nutrients, which influences the fungal metabolic pathways and enzyme activities involved in the degradation of substrate materials into simpler forms that the fungus can readily assimilate [41].
Research into the genetic expression profiles of C. militaris when cultivated on different substrates reveals the upregulation of genes involved in secondary metabolism, which are crucial for the synthesis of bioactive compounds [20]. For instance, the enhanced production of cordycepin on specific substrates correlates with the increased activity of enzymes such as cordycepin phosphoramidate, which plays a direct role in the biosynthesis pathway of this compound [42]. Moreover, environmental stressors associated with different substrates, such as varying pH levels or nutrient deficiencies, can induce stress responses in fungi, leading to the activation of survival pathways that include the upregulation of secondary metabolite production as a protective mechanism [43]. Substrates that can induce mild oxidative stress, like those containing specific types of phenolic compounds, can enhance the fungal production of antioxidants as a countermeasure, which in turn increases the overall yield of bioactive molecules with antioxidant properties [44]. This adaptive response to substrate-induced stress is a key factor in the elevated levels of bioactive compounds observed in certain cultivations, as seen in our experiments with O. chinensis substrates which led to potent α-glucosidase and α-amylase inhibition activities [42,45]. The interaction between C. militaris and its cultivation substrate embodies a complex interplay of nutrient availability, genetic regulation, and environmental stress, all of which converge to influence the fungal metabolic profile. This holistic understanding allows for the strategic manipulation of cultivation conditions to maximize the production of desired bioactive compounds, potentially revolutionizing the use of C. militaris in pharmaceutical and therapeutic applications. Future research should aim to delve deeper into the molecular mechanisms at play, utilizing advanced genomic, proteomic, and metabolomic technologies to unravel the intricate dynamics that govern the interaction between fungal metabolism and substrate characteristics [44].
The implications of C. militaris cultivation on various substrates for type-2 diabetes management are profound, offering a novel approach to enhancing the therapeutic efficacy of natural interventions. The enzymatic inhibition properties of Cordyceps, particularly its effects on α-amylase and α-glucosidase, pivotal enzymes in carbohydrate metabolism, suggest its potential as a complementary treatment to control blood glucose levels [42]. Our research demonstrates that substrate selection significantly affects the fungus’s ability to produce bioactive compounds like cordycepin and adenosine, which are critical in modulating enzymatic activity. Specifically, the potent inhibition observed with substrates such as B. atrostigmella, which yielded the highest levels of these compounds, indicates a targeted approach to enhancing these bioactivities. These findings align with recent studies highlighting the importance of natural products in diabetes management, where compounds exhibiting inhibitory action against carbohydrate-hydrolyzing enzymes can significantly reduce postprandial blood glucose spikes, a key factor in managing diabetes symptoms and complications [45,46]. Moreover, the variability in enzyme inhibition and bioactive compound production across different substrates points to the possibility of customizing C. militaris cultivation techniques to produce specific compounds that can be harnessed for therapeutic purposes [47]. Additionally, the pharmacological benefits of Cordyceps extend beyond simple enzyme inhibition. The anti-inflammatory and antioxidant properties of its bioactive components contribute to ameliorating chronic inflammation and oxidative stress, which are integral to the pathophysiology of diabetes [48]. By modulating these underlying processes, C. militaris can provide a multifaceted approach to diabetes care, addressing both glycemic control and the broader systemic disturbances that accompany the disease [49]. The holistic effect of Cordyceps on metabolic health, as evidenced through our research, underscores its potential integration into diabetes treatment protocols, offering a complementary strategy alongside conventional pharmaceuticals. This approach is particularly valuable given the increasing interest in and the necessity for treatments that have fewer side effects and are derived from natural sources. To this end, further clinical studies and trials are needed to quantify the exact impact of these findings in clinical settings, thereby confirming the efficacy of C. militaris as a supportive treatment for diabetes and potentially other metabolic disorders.
The findings of this study represent a significant advancement in our understanding of how substrate selection can optimize the production of bioactive compounds in C. militaris. The inclusion of various insects as substrates provides a rich source of nutrients that enhance the medicinal properties of this fungus. By bridging the disciplines of mycology, nutritional chemistry, and pharmacology, this research offers new insights into sustainable and effective cultivation practices that can maximize the therapeutic potential of C. militaris. These findings not only contribute to the broader field of medicinal chemistry but also highlight the practical applications of this research in developing natural therapies for metabolic disorders such as type-2 diabetes.
5. Conclusions
This study elucidates the significant role of cultivation substrates in modulating the enzymatic inhibitory efficacy and bioactive compound synthesis of C. militaris, which has potential applications in managing type-2 diabetes and other metabolic disorders. Our findings reveal that substrate selection crucially influences the levels of pivotal compounds such as cordycepin and adenosine, with the highest concentrations observed in Cordyceps cultivated on B. atrostigmella. This substrate not only yielded the greatest amounts of bioactive compounds but also exhibited the strongest inhibitory activity against α-glucosidase and α-amylase, highlighting the substrate’s composition as a key factor in enhancing medicinal properties. Conversely, Cordyceps grown on G. bimaculatus solid residues showed remarkable inhibition of xanthine oxidase, suggesting that different substrates can be optimized to target specific therapeutic outcomes. These insights advance our understanding of C. militaris’s pharmacological potential and suggest a framework for future biotechnological applications aimed at developing effective natural therapies. This research advocates for tailored cultivation strategies to maximize the health benefits of C. militaris, promising enhanced therapeutic options for chronic disease management.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/chemistry6040030/s1, Figure S1 (Calibration Curve for Adenosine Quantification); Figure S2 (Calibration Curve for Cordycepin Quantification); Figure S3 (HPLC Chromatogram of Adenosine and Cordycepin in C. militaris solid-based residues from Halyomorpha halys); Figure S4 (HPLC Chromatogram of Adenosine and Cordycepin in C. militaris solid-based residues from Oxya chinensis); Figure S5 (HPLC Chromatogram of Adenosine and Cordycepin in C. militaris solid-based residues from Gryllus bimaculatus); Figure S6 (HPLC Chromatogram of Adenosine and Cordycepin in C. militaris solid-based residues from Bombyx mori Pupae); Figure S7 (HPLC Chromatogram of Adenosine and Cordycepin in C. militaris solid-based residues from Brihaspa atrostigmella); Figure S8 (HPLC Chromatogram of Adenosine and Cordycepin in C. militaris Fruiting Bodies Cultivated on Halyomorpha halys); Figure S9 (HPLC Chromatogram of Adenosine and Cordycepin in C. militaris Fruiting Bodies Cultivated on Oxya chinensis); Figure S10 (HPLC Chromatogram of Adenosine and Cordycepin in C. militaris Fruiting Bodies Cultivated on Gryllus bimaculatus); Figure S11 (HPLC Chromatogram of Adenosine and Cordycepin in C. militaris Fruiting Bodies Cultivated on Bombyx mori Pupae); Figure S12 (HPLC Chromatogram of Adenosine and Cordycepin in C. militaris Fruiting Bodies Cultivated on Brihaspa atrostigmella); Figure S13 (UV-Vis Spectroscopy and HPLC Chromatogram of C. militaris), and Table S1 (Retention Times and Peak Areas of Adenosine and Cordycepin in C. militaris).
Author Contributions
Conceptualization, N.Q.T. and T.N.M.; methodology, T.N.M., V.T.H.N., Q.N.T., H.N.B.V., N.M.N.V. and H.N.A.; validation, V.T.H.N., N.T.D., H.N.A. and Q.N.T.; formal analysis, T.N.M.; investigation, Q.N.T. and H.N.A.; resources, Q.N.T.; data curation, H.N.B.V., N.M.N.V. and T.N.M.; writing—original draft preparation, T.N.M. and H.N.B.V.; writing—review and editing, T.N.M., N.M.N.V., N.T.D. and N.Q.T.; supervision, N.Q.T.; funding acquisition, T.N.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Vietnam Academy of Science and Technology under grant number THTEXS.02/21-24.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
The fungal strain C. militaris VCCM 34117, provided by the VAST Culture Collection of Microorganisms (VCCM), Vietnam Academy of Science and Technology (VAST), was utilized in this study. Additionally, the Center for High Technology Research and Development, also under VAST, is acknowledged for its partial support in providing the necessary equipment for this research.
Conflicts of Interest
Author Truong Ngoc Minh was employed by the company Vicomi Tam An Investment and Commercial Company Limited. The authors declare no conflict of interest.
References
- Shrestha, S.; Shrestha, B.; Park, J.H.; Lee, D.Y.; Cho, J.G.; Baek, N.I. Chemical constituents of Yarsagumba (Ophiocordyceps sinensis Berk.), a valued traditional Himalayan medicine. Nep. J. Sci. Technol. 2012, 13, 43–58. [Google Scholar] [CrossRef]
- Jeong, M.H.; Lee, C.M.; Lee, S.W.; Seo, S.Y.; Seo, M.J.; Kang, B.W.; Jo, W.S. Cordycepin-enriched Cordyceps militaris induces immunomodulation and tumor growth delay in mouse-derived breast cancer. Oncol. Rep. 2013, 30, 1996–2002. [Google Scholar] [CrossRef]
- Liu, J.Y.; Feng, C.P.; Li, X.; Chang, M.C.; Meng, J.L.; Xu, L.J. Immunomodulatory and antioxidative activity of Cordyceps militaris polysaccharides in mice. Int. J. Biol. Macromol. 2016, 86, 594–598. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.T.; Huang, K.S.; Shaw, J.F.; Chen, J.R.; Kuo, W.S.; Shen, G.; Yang, C.H. Trends in the immunomodulatory effects of Cordyceps militaris: Total extracts, polysaccharides and cordycepin. Front. Pharmacol. 2020, 11, 575704. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, D. Superfoods: The Food and Medicine of the Future; North Atlantic Books: Berkeley, CA, USA, 2009. [Google Scholar]
- Hanser, M.; Hudson, S.L. Alternative Therapies: Growing Options in Nursing Practice; National Center of Continuing Education, Inc.: Lakeway, TX, USA, 2006. [Google Scholar]
- Munch, A.H.A.B.G. Neuroprotective Mechanisms: Oxidative Stress as a Target for Neuroprotective Therapies in Alzheimer’s and Parkinson’s. Handb. Neurochem. Mol. Neurobiol. Degener. Dis. Nerv. Syst. 2007, 84, 77–102. [Google Scholar]
- Bains, A.; Chawla, P.; Kaur, S.; Najda, A.; Fogarasi, M.; Fogarasi, S. Bioactives from mushroom: Health attributes and food industry applications. Materials 2021, 14, 7640. [Google Scholar] [CrossRef] [PubMed]
- Jedrejko, K.; Kała, K.; Sułkowska-Ziaja, K.; Krakowska, A.; Zieba, P.; Marzec, K.; Sekara, A.; Muszynska, B. Cordyceps militaris—Fruiting Bodies, Mycelium, and Supplements: Valuable Component of Daily Diet. Antioxidants 2022, 11, 1861. [Google Scholar] [CrossRef]
- Niego, A.G.; Rapior, S.; Thongklang, N.; Raspé, O.; Jaidee, W.; Lumyong, S.; Hyde, K.D. Macrofungi as a nutraceutical source: Promising bioactive compounds and market value. J. Fungi. 2021, 7, 397. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.T.; Wasser, S.P. Current and future research trends in agricultural and biomedical applications of medicinal mushrooms and mushroom products. Int. J. Med. Mushrooms 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Phoungthong, K.; Aiphuk, W.; Maneerat, T.; Suwunwong, T.; Choto, P.; Chomnunti, P. Utilization of corncob biochar in cultivation media for cordycepin production and biomass of C. militaris. Sustainability 2022, 14, 9362. [Google Scholar]
- Zeng, Z.; Mou, D.; Luo, L.; Zhong, W.; Duan, L.; Zou, X. Different cultivation environments affect the yield, bacterial community and metabolites of Cordyceps cicadae. Front. Microbiol. 2021, 12, 669785. [Google Scholar] [CrossRef]
- Aramwit, P.; Bang, N.; Ratanavaraporn, J.; Nakpheng, T.; Srichana, T. An anticancer cordycepin produced by Cordyceps militaris growing on the dead larva of Bombyx mori silkworm. J. Agric. Sci. 2014, 6, 41. [Google Scholar]
- Li, Y.T.; Yao, H.T.; Huang, Z.L.; Gong, L.C.; Herman, R.A.; Wu, F.A.; Wang, J. Silkworm Pupae globulin promotes Cordyceps militaris fermentation: Regulation of metabolic pathways enhances cordycepin synthesis and extends the synthesis phase. Food Biosci. 2024, 59, 103971. [Google Scholar] [CrossRef]
- Kaewkam, A.; Sornchai, P.; Chanprame, S.; Iamtham, S. Utilization of Spirulina maxima to enhance yield and cordycepin content in Cordyceps militaris artificial cultivation. Antioxidants 2021, 11, 1861. [Google Scholar]
- Li, X.; Liu, Q.; Li, W.; Li, Q.; Qian, Z.; Liu, X.; Dong, C. A breakthrough in the artificial cultivation of Chinese cordyceps on a large-scale and its impact on science, the economy, and industry. Crit. Rev. Biotechnol. 2019, 39, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Guo, S.; Wang, W.; Liu, X. Cordyceps industry in China. Mycology 2015, 6, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.H.; Cheng, Z.; Yang, X.L.; Li, S.; Ding, Z.Q.; Zhou, T.S.; Chen, J.K. Genetic diversity and structure of Cordyceps sinensis populations from extensive geographical regions in China as revealed by inter-simple sequence repeat markers. J. Microbiol. 2008, 46, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Thananusak, R.; Laoteng, K.; Raethong, N.; Koffas, M.; Vongsangnak, W. Dissecting metabolic regulation in mycelial growth and fruiting body developmental stages of Cordyceps militaris through integrative transcriptome analysis. Biotechnol. Bioprocess Eng. 2023, 28, 406–418. [Google Scholar] [CrossRef]
- Li, S.P.; Li, P.; Dong, T.T.; Tsim, K.W. Determination of Nucleosides in Natural Cordyceps sinensis and Cultured Cordyceps mycelia by Capillary Electrophoresis. Electrophoresis 2001, 22, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Blainski, A.; Lopes, G.C.; De Mello, J.C.P. Application and Analysis of the Folin Ciocalteu Method for the Determination of theTotal Phenolic Content from Limonium brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef] [PubMed]
- Matić, P.; Sabljić, M.; Jakobek, L. Validation of Spectrophotometric Methods for the Determination of Total Polyphenol and Total Flavonoid Content. J. AOAC Int. 2017, 100, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Fukuta, M.; Xuan, T.D.; Deba, F.; Tawata, S.; Khanh, T.D.; Chung, I.M. Comparative Efficacies In Vitro of Antibacterial, Fungicidal, Antioxidant, and Herbicidal Activities of Momilatones A and B. J. Plant Interact. 2007, 2, 245–251. [Google Scholar] [CrossRef]
- Minh, T.N.; Van, T.M.; Andriana, Y.; Hau, D.V.; Duyen, D.H.; Guzman-Gelani, C.D. Antioxidant, Xanthine oxidase, α-Amylaseand α-Glucosidase Inhibitory Activities of Bioactive Compounds from Rumex crispus L. Root. Molecules 2019, 24, 3899. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.C.; Liang, C.H.; Wu, C.Y. Various grain substrates for the production of fruiting bodies and bioactive compounds of the medicinal caterpillar mushroom, Cordyceps militaris (Ascomycetes). Int. J. Med. Mushrooms 2014, 16, 6. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.X.; Xue, D.; Lu, Z.H.; Huang, H.L. Effects of substrates on the production of fruiting bodies and the bioactive components by different Cordyceps militaris strains (ascomycetes). Int. J. Med. Mushrooms 2020, 22, 1. [Google Scholar] [CrossRef] [PubMed]
- Cleaver, P.D.; Loomis-Powers, M.; Patel, D. Analysis of quality and techniques for hybridization of medicinal fungus Cordyceps sinensis (Berk.) Sacc. (Ascomycetes). Int. J. Med. Mushrooms 2004, 6, 2. [Google Scholar]
- Li, Y.; Yang, H.; Yang, H.; Wang, J.; Chen, H. Assessment of drying methods on the physiochemical property and antioxidant activity of Cordyceps militaris. J. Food Meas. Charact. 2019, 13, 513–520. [Google Scholar] [CrossRef]
- Yu, J.; Sun, M.; Wang, X.; Qi, D.; Han, C. Poly-pathways metabolomics for high-yielding cordycepin of Cordyceps militaris. Biomed. Chromatogr. 2023, 37, e5551. [Google Scholar] [CrossRef] [PubMed]
- Turk, A.; Abdelhamid, M.A.; Yeon, S.W.; Ryu, S.H.; Lee, S.; Ko, S.M.; Kim, B.S.; Pack, S.P.; Hwang, B.Y.; Lee, M.K. Cordyceps mushroom with increased cordycepin content by the cultivation on edible insects. Front. Microbiol. 2022, 13, 1017576. [Google Scholar] [CrossRef] [PubMed]
- Krishna, K.V.; Balasubramanian, B.; Park, S.; Bhattacharya, S.; Kadanthottu Sebastian, J.; Liu, W.C.; Malaviya, A. Conservation of Endangered Cordyceps sinensis Through Artificial Cultivation Strategies of Cordyceps militaris, an Alternate. Mol. Biotechnol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Nephale, L.E.; Moyo, N.A.; Rapatsa, M.M. Dietary Full-fat Stinkbug (Encosternum delegorguei) Meal Effects on Growth Performance, Blood Chemistry, Liver and Intestinal Histology of Juvenile Mozambique tilapia (Oreochromis mossambicus). Cogent Food Agric. 2023, 9, 2253717. [Google Scholar] [CrossRef]
- Murugu, D.K.; Onyango, A.N.; Ndiritu, A.K.; Osuga, I.M.; Xavier, C.; Nakimbugwe, D.; Tanga, C.M. From Farm to Fork: Crickets as Alternative Source Of Protein, Minerals, and Vitamins. Front. Nutr. 2021, 8, 704002. [Google Scholar] [CrossRef] [PubMed]
- Woolley, V.C.; Teakle, G.R.; Prince, G.; de Moor, C.H.; Chandler, D. Cordycepin, a metabolite of Cordyceps militaris, reduces immune-related gene expression in insects. J. Invertebr. Pathol. 2020, 177, 107480. [Google Scholar] [CrossRef] [PubMed]
- Phull, A.R.; Ahmed, M.; Park, H.J. Cordyceps militaris as a biofunctional food source: Pharmacological potential, anti-inflammatory actions and related molecular mechanisms. Microorganisms 2022, 10, 405. [Google Scholar] [CrossRef] [PubMed]
- Minh, T.N.; Anh, L.V.; Trung, N.Q.; Minh, B.Q.; Xuan, T.D. Efficacy of Green Extracting Solvents on Antioxidant, Xanthine Oxidase, and Plant Inhibitory Potentials of Solid-Based Residues (SBRs) of Cordyceps militaris. Stresses 2023, 3, 11–21. [Google Scholar] [CrossRef]
- Osemwegie, O.O.; Adetunji, C.O.; Ayeni, E.A.; Adejobi, O.I.; Arise, R.O.; Nwonuma, C.O.; Oghenekaro, A.O. Exopolysaccharides from bacteria and fungi: Current status and perspectives in Africa. Heliyon 2020, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Wen, T.C.; Kang, C.; Meng, Z.B.; Qi, Y.B.; Hyde, K.D.; Kang, J.C. Enhanced production of cordycepin by solid state fermentation of Cordyceps militaris using additives. Chiang Mai J. Sci. 2016, 43, 972–984. [Google Scholar]
- Sripilai, K.; Chaicharoenaudomrung, N.; Phonchai, R.; Chueaphromsri, P.; Kunhorm, P.; Noisa, P. Development of an animal-free nitrogen source for the liquid surface culture of Cordyceps militaris. Lett. Appl. Microbiol. 2023, 76, ovad053. [Google Scholar] [CrossRef] [PubMed]
- Zu, Z.; Wang, S.; Zhao, Y.; Fan, W.; Li, T. Integrated enzymes activity and transcriptome reveal the effect of exogenous melatonin on the strain degeneration of Cordyceps militaris. Front. Microbiol. 2023, 14, 1112035. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Wang, Q.; Wang, Y.; Li, L.; Zhu, L.; Kuang, X.; Dai, H. Design, synthesis, antibacterial/antitumor activity and in vitro stability of novel cordycepin derivatives with unsaturated fatty acid chain. Eur. J. Pharm. Sci. 2023, 187, 106466. [Google Scholar] [CrossRef]
- Hoang, C.Q.; Duong, G.H.; Tran, M.H.; Vu, T.X.; Tran, T.B.; Pham, H.T. Molecular mechanisms underlying phenotypic degeneration Cordyceps militaris: Insights from transcriptome reanalysis and osmotic stress studies. Sci. Rep. 2024, 14, 2231. [Google Scholar] [CrossRef] [PubMed]
- Soraksa, N.; Heebkaew, N.; Promjantuek, W.; Kunhorm, P.; Kaokean, P.; Chaicharoenaudomung, N.; Noisa, P. Cordycepin, a bioactive compound from Cordyceps spp., moderates Alzheimer’s disease-associated pathology via anti-oxidative stress and autophagy activation. J. Asian Nat. Prod. Res. 2024, 26, 583–603. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Liu, J.; Zhou, Z.; Li, Z.; Zhou, Y.; Xu, S.; Zou, X. Positive effects of Cordyceps cateniannulata colonization in tobacco: Growth promotion and resistance to abiotic stress. Front. Microbiol. 2023, 14, 1131184. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.S.; Huang, R.Y.; Wei, Y.J.; Tsai, G.J.; Huang, C.H. Influence of C. militaris-fermented grain substrate extracts on alleviating food allergy in mice. Heliyon 2023, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.; Valado, A. Algae-Derived Natural Products in Diabetes and Its Complications—Current Advances and Future Prospects. Life 2023, 13, 1831. [Google Scholar] [CrossRef] [PubMed]
- Soltani, M.; Malek, R.A.; Elmarzugi, N.A.; Mahomoodally, M.F.; Uy, D.; Leng, O.M.; El-Enshasy, H.A. Cordycepin: A biotherapeutic molecule from medicinal mushroom. In Biology of Macrofungi; Springer: Berlin/Heidelberg, Germany, 2018; pp. 319–349. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.J. Cordyceps as potential therapeutic agents for atherosclerosis. J. Integr. Med. 2024, 22, 102–114. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).