Vapor-Driven Crosslinked Hydroxypropyl-β-Cyclodextrin Electrospun Nanofibrous Membranes for Ultrafast Dye Removal
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
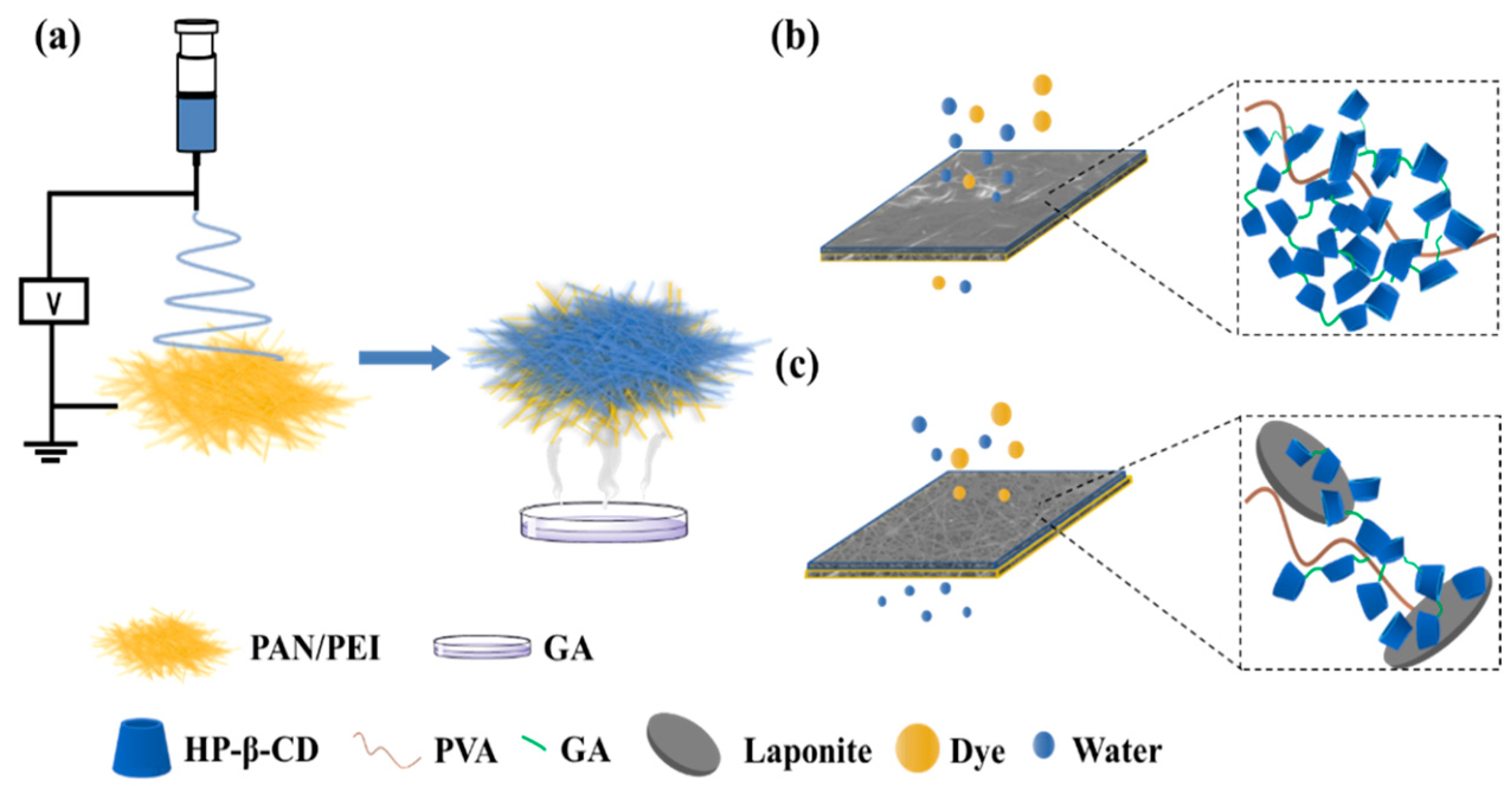
2.2. Preparation of Nanofibrous Membranes
2.3. Characterization Methods
2.4. Water Permeation Studies
2.5. Dye Filtration Experiments
3. Results
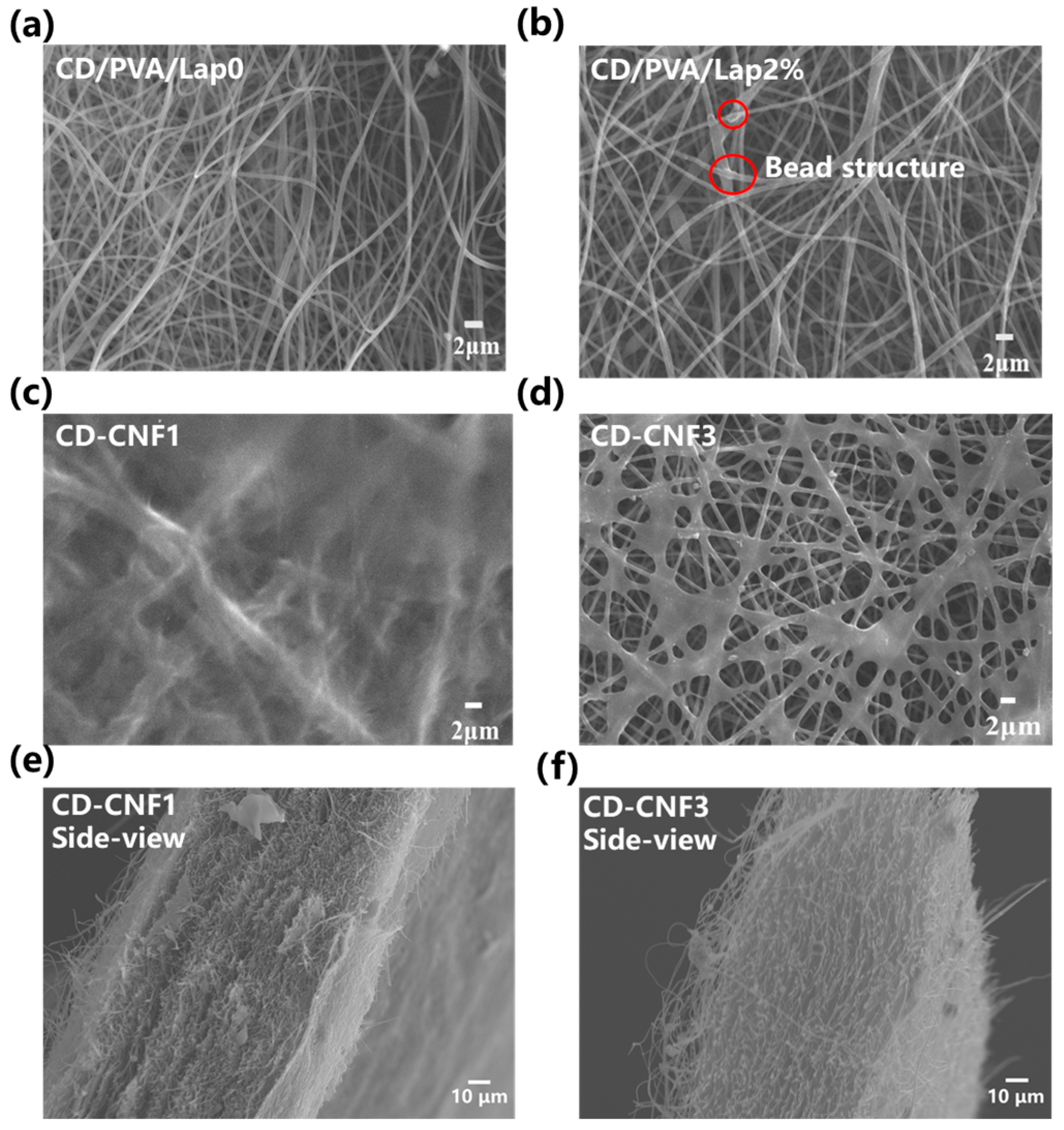
3.1. Structural and Morphological Characterizations of the CD-CNFs
3.2. Water Permeation and Dye Rejection Performance of the CD-CNFs
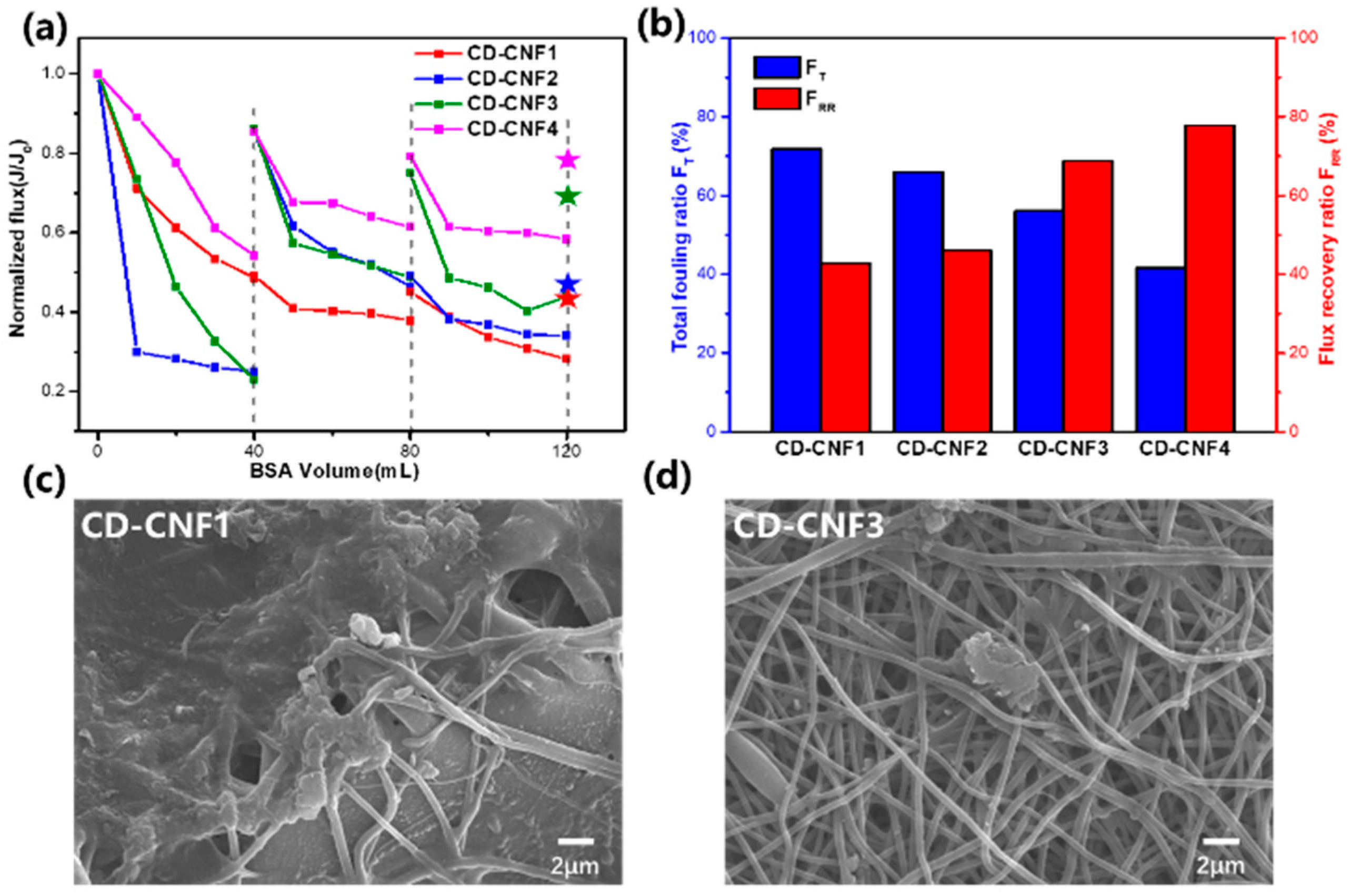
3.3. The Anti-Fouling Properties of the CD-CNFs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lin, J.; Ye, W.; Xie, M.; Seo, D.H.; Luo, J.; Wan, Y.; Van der Bruggen, B. Environmental impacts and remediation of dye-containing wastewater. Nat. Rev. Earth Environ. 2023, 4, 785–803. [Google Scholar] [CrossRef]
- Shanker, U.; Rani, M.; Jassal, V. Degradation of hazardous organic dyes in water by nanomaterials. Environ. Chem. Lett. 2017, 15, 623–642. [Google Scholar] [CrossRef]
- Tkaczyk, A.; Mitrowska, K.; Posyniak, A. Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: A review. Sci. Total Environ. 2020, 717, 137222. [Google Scholar] [CrossRef] [PubMed]
- Samuchiwal, S.; Naaz, F.; Kumar, P.; Ahammad, S.Z.; Malik, A. Life cycle assessment of sequential microbial-based anaerobic-aerobic reactor technology developed onsite for treating textile effluent. Environ. Res. 2023, 234, 116545. [Google Scholar] [CrossRef] [PubMed]
- Giannakis, S. A review of the concepts, recent advances and niche applications of the (photo) Fenton process, beyond water/wastewater treatment: Surface functionalization, biomass treatment, combatting cancer and other medical uses. Appl. Catal. B Environ. 2019, 248, 309–319. [Google Scholar] [CrossRef]
- Yan, J.; Huang, Y.; Miao, Y.E.; Tjiu, W.W.; Liu, T. Polydopamine-coated electrospun poly(vinyl alcohol/polyacrylic acid membranes as efficient dye adsorbent with good recyclability. J. Hazard. Mater. 2015, 283, 730–739. [Google Scholar] [CrossRef]
- Leudjo Taka, A.; Fosso-Kankeu, E.; Pillay, K.; Yangkou Mbianda, X. Metal nanoparticles decorated phosphorylated carbon nanotube/cyclodextrin nanosponge for trichloroethylene and Congo red dye adsorption from wastewater. J. Environ. Chem. Eng. 2020, 8, 103602. [Google Scholar] [CrossRef]
- Sarkar, P.; Modak, S.; Karan, S. Ultraselective and Highly Permeable Polyamide Nanofilms for Ionic and Molecular Nanofiltration. Adv. Funct. Mater. 2021, 31, 2007054. [Google Scholar] [CrossRef]
- Zhang, L.; Guan, H.; Zhang, N.; Jiang, B.; Sun, Y.; Yang, N. A loose NF membrane by grafting TiO2-HMDI nanoparticles on PES/β-CD substrate for dye/salt separation. Sep. Purif. Technol. 2019, 218, 8–19. [Google Scholar] [CrossRef]
- Karki, S.; Gohain, M.B.; Yadav, D.; Thakare, N.R.; Pawar, R.R.; Hazarika, S.; Ingole, P.G. Building rapid water transport channels within thin-film nanocomposite membranes based on 2D mesoporous nanosheets. Desalination 2023, 547, 116222. [Google Scholar] [CrossRef]
- Xu, W.; Chen, Y.; Xu, W.; Wu, X.; Liu, Y. Electrospinning Oriented Self-Cleaning Porous Crosslinking Polymer for Efficient Dyes Removal. Adv. Mater. Interfaces 2020, 7, 2001050. [Google Scholar] [CrossRef]
- Zhang, W.; Zheng, Z.; Lin, L.; Zhang, X.; Bae, M.; Lee, J.; Xie, J.; Diao, G.; Im, H.-J.; Piao, Y.; et al. Ultrafast Synthesis of Graphene-Embedded Cyclodextrin-Metal-Organic Framework for Supramolecular Selective Absorbency and Supercapacitor Performance. Adv. Sci. 2023, 10, 2304062. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhou, J.; Li, H. Chiral Covalent Organic Framework Packed Nanochannel Membrane for Enantioseparation. Angew. Chem. Int. Ed. 2022, 61, e202204012. [Google Scholar] [CrossRef]
- Zhang, S.; Boussouar, I.; Li, H. Selective sensing and transport in bionic nanochannel based on macrocyclic host-guest chemistry. Chin. Chem. Lett. 2021, 32, 642–648. [Google Scholar] [CrossRef]
- Jiang, Z.; Dong, R.; Evans, A.M.; Biere, N.; Ebrahim, M.A.; Li, S.; Anselmetti, D.; Dichtel, W.R.; Livingston, A.G. Aligned macrocycle pores in ultrathin films for accurate molecular sieving. Nature 2022, 609, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Alsbaiee, A.; Smith, B.J.; Xiao, L.; Ling, Y.; Helbling, D.E.; Dichtel, W.R. Rapid removal of organic micropollutants from water by a porous β-cyclodextrin polymer. Nature 2016, 529, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Puspasari, T.; Nunes, S.P.; Peinemann, K.-V. Ultrathin 2D-Layered Cyclodextrin Membranes for High- Performance Organic Solvent Nanofiltration. Adv. Funct. Mater. 2020, 30, 1906797. [Google Scholar] [CrossRef]
- Zhu, B.; Shao, R.; Li, N.; Min, C.; Liu, S.; Xu, Z.; Qian, X.; Wang, L. Progress of cyclodextrin based-membranes in water treatment: Special 3D bowl-like structure to achieve excellent separation. Chem. Eng. J. 2022, 449, 137013. [Google Scholar] [CrossRef]
- Villalobos, L.F.; Huang, T.; Peinemann, K.-V. Cyclodextrin Films with Fast Solvent Transport and Shape-Selective Permeability. Adv. Mater. 2017, 29, 1606641. [Google Scholar] [CrossRef]
- He, Y.; Miao, J.; Jiang, Z.; Tu, K.; Yang, H.; Chen, S.; Zhang, L.; Zhang, R. Improving the anti-fouling property and permeate flux of hollow fiber composite nanofiltration membrane using β-cyclodextrin. Sci. Rep. 2019, 9, 12435. [Google Scholar] [CrossRef]
- Mao, H.; Zhang, H.; Li, Y.; Xue, Y.; Pei, F.; Wang, J.; Liu, J. Tunable Solvent Permeation Properties of Thin Film Nanocomposite Membrane by Constructing Dual-Pathways Using Cyclodextrins for Organic Solvent Nanofiltration. ACS Sustain. Chem. Eng. 2015, 3, 1925–1933. [Google Scholar] [CrossRef]
- Gozali Balkanloo, P.; Mahmoudian, M.; Hosseinzadeh, M.T. A comparative study between MMT-Fe3O4/PES, MMT-HBE/PES, and MMT-acid activated/PES mixed matrix membranes. Chem. Eng. J. 2020, 396, 125188. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Y.; Mao, L.; Jiang, C.; Ang, J.; Lu, X. Doping polysulfone ultrafiltration membrane with TiO2-PDA nanohybrid for simultaneous self-cleaning and self-protection. J. Membr. Sci. 2017, 532, 20–29. [Google Scholar] [CrossRef]
- Shen, L.; Cheng, R.; Yi, M.; Hung, W.-S.; Japip, S.; Tian, L.; Zhang, X.; Jiang, S.; Li, S.; Wang, Y. Polyamide-based membranes with structural homogeneity for ultrafast molecular sieving. Nat. Commun. 2022, 13, 500. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, Y. Macrocycle crosslinked mesoporous polymers for ultrafast separation of organic dyes. Chem. Commun. 2018, 54, 7362–7365. [Google Scholar] [CrossRef]
- Xu, W.; Li, G.; Qu, H.; Ma, C.; Zhang, H.; Cheng, J.; Li, H. The Specific Removal of Perfluorooctanoic Acid Based on Pillar[5]arene-Polymer-Packed Nanochannel Membrane. ACS Nano 2023, 17, 19305–19312. [Google Scholar] [CrossRef]
- Xu, W.; Chen, Y.; Liu, Y. Directional Water Transfer Janus Nanofibrous Porous Membranes for Particulate Matter Filtration and Volatile Organic Compound Adsorption. ACS Appl. Mater. Interfaces 2021, 13, 3109–3118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chai, H.; Diao, G. Highly porous cyclodextrin functionalized nanofibrous membrane by acid etching. Colloids Surf. A Physicochem. Eng. Asp. 2019, 582, 123907. [Google Scholar] [CrossRef]
- Celebioglu, A.; Uyar, T. Electrospinning of nanofibers from non-polymeric systems: Polymer-free nanofibers from cyclodextrin derivatives. Nanoscale 2012, 4, 621–631. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, Y.; Li, X.; Sun, B.; Wang, C. Synthesis of beta-Cyclodextrin-Based Electrospun Nanofiber Membranes for Highly Efficient Adsorption and Separation of Methylene Blue. ACS Appl. Mater. Interfaces 2015, 7, 26649–26657. [Google Scholar] [CrossRef]
- Li, R.; Dou, J.; Jiang, Q.; Li, J.; Xie, Z.; Liang, J.; Ren, X. Preparation and antimicrobial activity of β-cyclodextrin derivative copolymers/cellulose acetate nanofibers. Chem. Eng. J. 2014, 248, 264–272. [Google Scholar] [CrossRef]
- Tang, Y.-J.; Shen, B.-J.; Huang, B.-Q.; Zhan, Z.-M.; Xu, Z.-L. High permselectivity thin-film composite nanofiltration membranes with 3D microstructure fabricated by incorporation of beta cyclodextrin. Sep. Purif. Technol. 2019, 227, 115718. [Google Scholar] [CrossRef]
- Shang, S.; Chiu, K.-L.; Jiang, S. Synthesis of immobilized polyvinyl alcohol/cyclodextrin eco-adsorbent and its application for the removal of ibuprofen from pharmaceutical sewage. J. Appl. Polym. Sci. 2017, 134, 44861. [Google Scholar] [CrossRef]
- Ahmad, J.; Wen, X.; Li, F.; Wang, B. Novel triangular silver nanoparticle modified membranes for enhanced antifouling performance. RSC Adv. 2019, 9, 6733–6744. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ju, J.; Tan, Y.; Hao, L.; Ma, Y.; Wu, Y.; Zhang, H.; Xia, Y.; Sui, K. Controlled synthesis of sodium alginate electrospun nanofiber membranes for multi-occasion adsorption and separation of methylene blue. Carbohydr. Polym. 2019, 205, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Yuan, L.; Wang, J.; Wu, H.; Wang, H.; Xiang, A.; Ashok, B.; Rajulu, A.V. Electrospinning of polyvinyl alcohol into crosslinked nanofibers: An approach to fabricate functional adsorbent for heavy metals. J. Hazard. Mater. 2019, 378, 120751. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.; Karki, S.; Gohain, M.B.; Ingole, P.G. Development of micropollutants removal process using thin-film nanocomposite membranes prepared by green new vapour-phase interfacial polymerization method. Chem. Eng. J. 2023, 472, 144940. [Google Scholar] [CrossRef]
- Gruppuso, M.; Iorio, F.; Turco, G.; Marsich, E.; Porrelli, D. Hyaluronic acid/lactose-modified chitosan electrospun wound dressings—Crosslinking and stability criticalities. Carbohydr. Polym. 2022, 288, 119375. [Google Scholar] [CrossRef] [PubMed]
- Prasannan, A.; Udomsin, J.; Tsai, H.-C.; Wang, C.-F.; Lai, J.-Y. Robust underwater superoleophobic membranes with bio-inspired carrageenan/laponite multilayers for the effective removal of emulsions, metal ions, and organic dyes from wastewater. Chem. Eng. J. 2020, 391, 123585. [Google Scholar] [CrossRef]
- Yang, X.; Lin, L.; Liu, Z.; Yang, J.; Cheng, Q.; Ma, W.; Xu, M.; Tang, F.; Wang, Q.; Li, X.; et al. Preparation of solvent-resistant polyimide membranes for efficient separation of dyes and salts via an “internal drive” strategy. Sep. Purif. Technol. 2024, 332, 125843. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Zhang, Y.; Chen, Y.; Liu, Y. Vapor-Driven Crosslinked Hydroxypropyl-β-Cyclodextrin Electrospun Nanofibrous Membranes for Ultrafast Dye Removal. Chemistry 2024, 6, 506-516. https://doi.org/10.3390/chemistry6040029
Xu X, Zhang Y, Chen Y, Liu Y. Vapor-Driven Crosslinked Hydroxypropyl-β-Cyclodextrin Electrospun Nanofibrous Membranes for Ultrafast Dye Removal. Chemistry. 2024; 6(4):506-516. https://doi.org/10.3390/chemistry6040029
Chicago/Turabian StyleXu, Xinmiao, Yi Zhang, Yong Chen, and Yu Liu. 2024. "Vapor-Driven Crosslinked Hydroxypropyl-β-Cyclodextrin Electrospun Nanofibrous Membranes for Ultrafast Dye Removal" Chemistry 6, no. 4: 506-516. https://doi.org/10.3390/chemistry6040029
APA StyleXu, X., Zhang, Y., Chen, Y., & Liu, Y. (2024). Vapor-Driven Crosslinked Hydroxypropyl-β-Cyclodextrin Electrospun Nanofibrous Membranes for Ultrafast Dye Removal. Chemistry, 6(4), 506-516. https://doi.org/10.3390/chemistry6040029



