Lactone-Terminated Self-Assembled Monolayers for Mimicking Nanoscale Polyester Surfaces
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the SAMs
2.3. Characterization of the SAMs
2.4. Spin-Coating Method
2.5. Synthesis Procedure
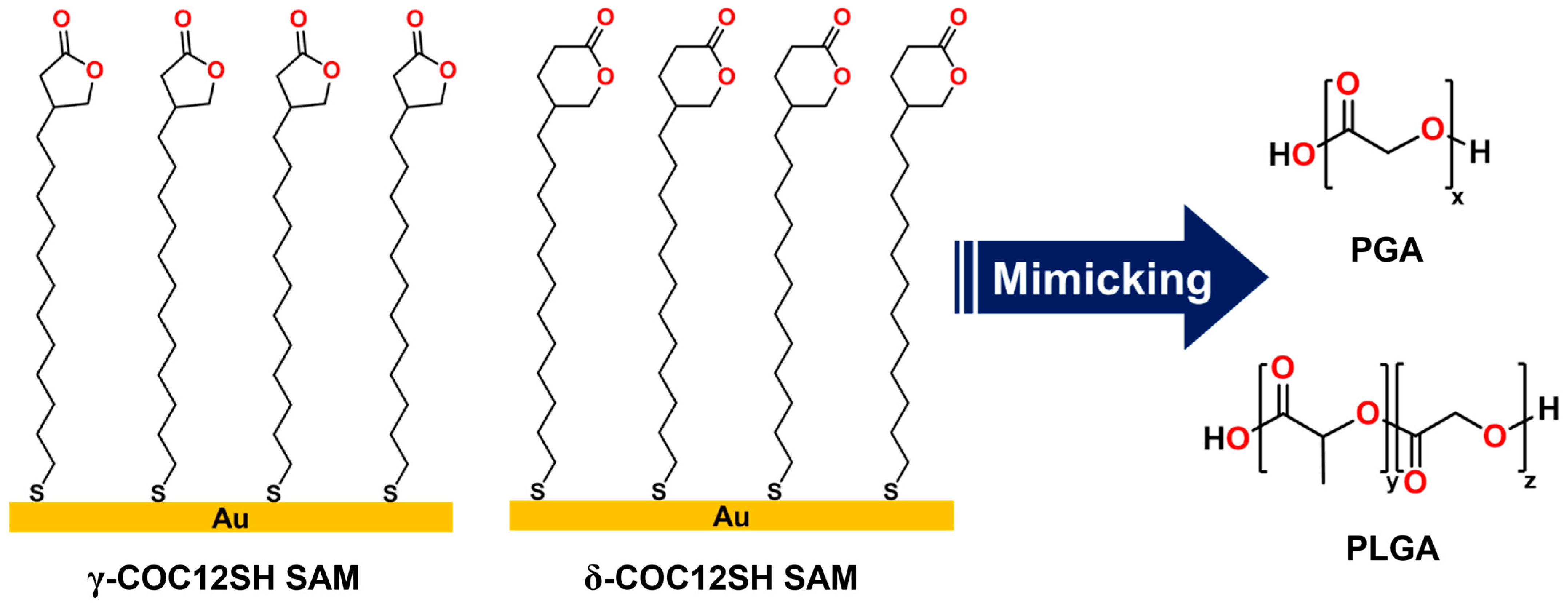
3. Results and Discussion
3.1. Ellipsometric Measurements of the SAMs
3.2. XPS Analysis of the SAMs
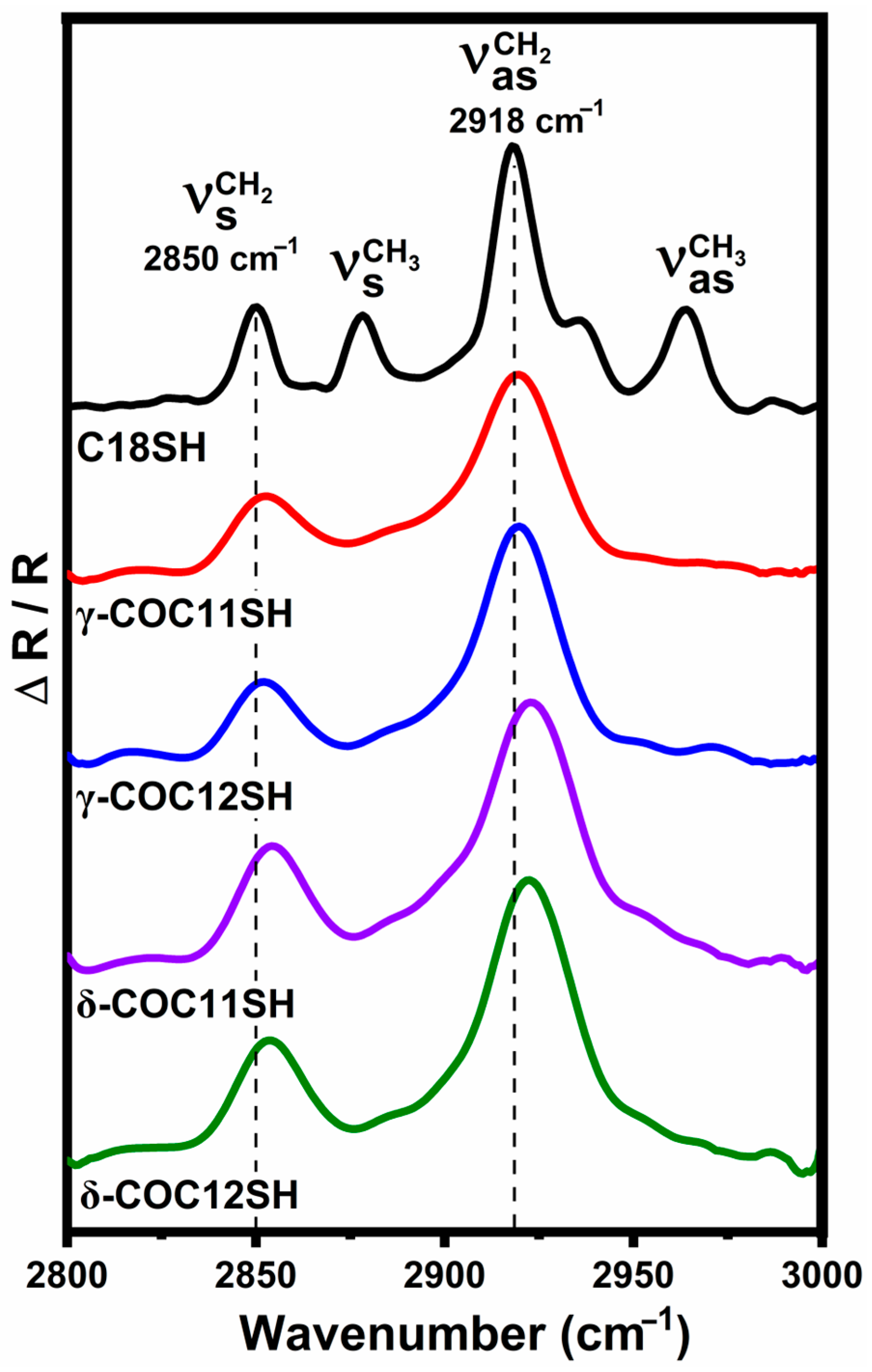
3.3. PM-IRRAS Analysis of the SAMs
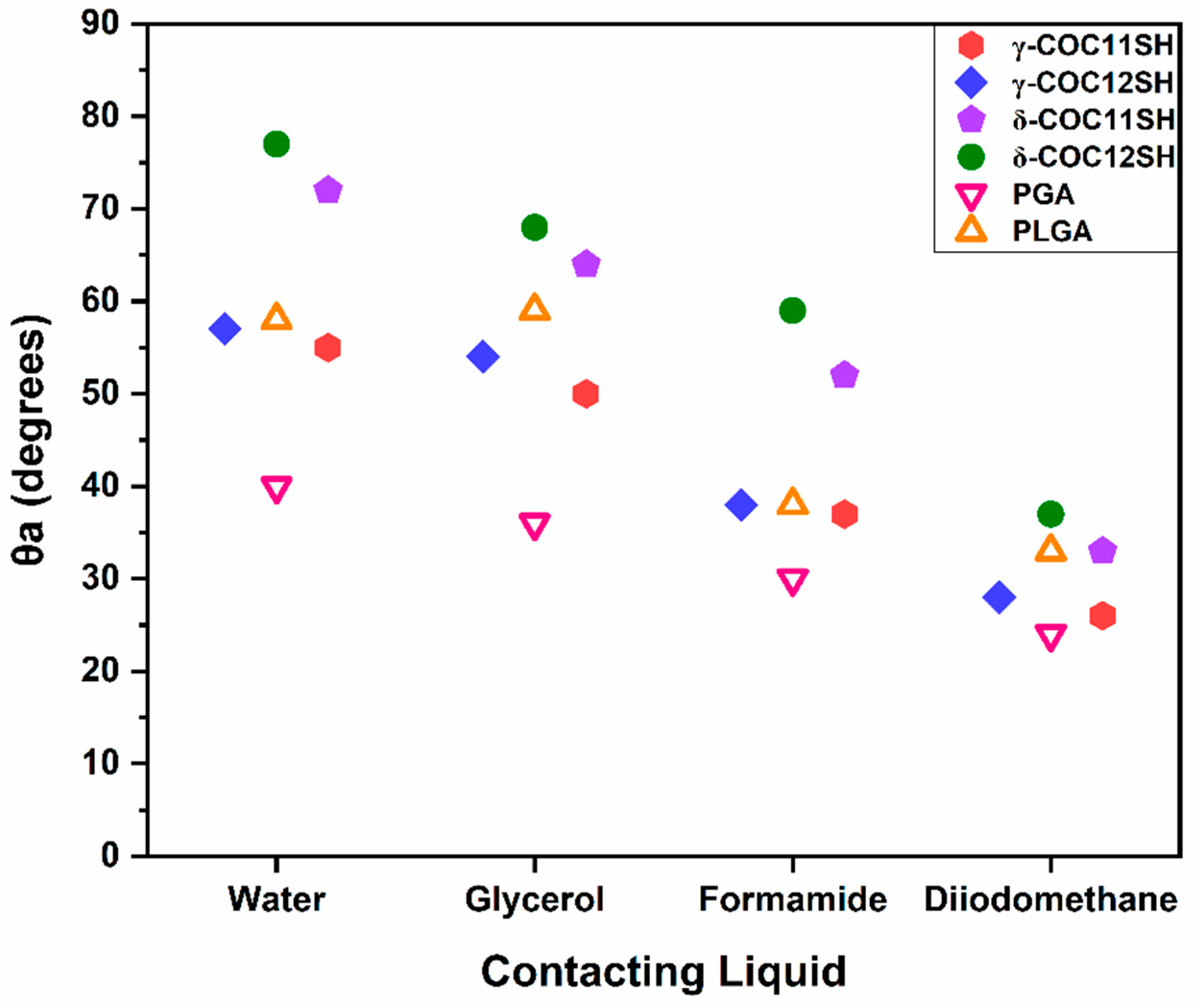
3.4. Wettability of the SAMs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beckman, I.P.; Lozano, C.; Freeman, E.; Riveros, G. Fiber Selection for Reinforced Additive Manufacturing. Polymers 2021, 13, 2231. [Google Scholar] [CrossRef]
- Jem, K.J.; Tan, B. The Development and Challenges of Poly(lactic acid) and Poly(glycolic acid). Adv. Ind. Eng. Polym. Res. 2020, 3, 60–70. [Google Scholar] [CrossRef]
- Budak, K.; Sogut, O.; Aydemir Sezer, U. A Review on Synthesis and Biomedical Applications of Polyglycolic Acid. J. Polym. Res. 2020, 27, 208. [Google Scholar] [CrossRef]
- Rocha, C.V.; Gonçalves, V.; da Silva, M.C.; Bañobre-López, M.; Gallo, J. PLGA-Based Composites for Various Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 2034. [Google Scholar] [CrossRef]
- Ong, C.S.; Lay, H.T.; Tamilselvam, N.R.; Chew, J.W. Cross-Linked Polycarbonate Microfiltration Membranes with Improved Solvent Resistance. Langmuir 2021, 37, 4025–4032. [Google Scholar] [CrossRef]
- Ogieglo, W.; Ghanem, B.; Ma, X.; Pinnau, I.; Wessling, M. How Much Do Ultrathin Polymers with Intrinsic Microporosity Swell in Liquids? J. Phys. Chem. B 2016, 120, 10403–10410. [Google Scholar] [CrossRef]
- Gugliuzza, A. Solvent Swollen Polymer. In Encyclopedia of Membranes; Drioli, E., Giorno, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1801–1802. [Google Scholar] [CrossRef]
- Zaleski, R.; Krasucka, P.; Skrzypiec, K.; Goworek, J. Macro- and Nanoscopic Studies of Porous Polymer Swelling. Macromolecules 2017, 50, 5080–5089. [Google Scholar] [CrossRef]
- Wang, J.; Klok, H.-A. Swelling-Induced Chain Stretching Enhances Hydrolytic Degrafting of Hydrophobic Polymer Brushes in Organic Media. Angew. Chem. Int. Ed. 2019, 58, 9989–9993. [Google Scholar] [CrossRef] [PubMed]
- Tajalli, P.; Hernandez Rivera, J.M.; Omidiyan, M.; Tran, H.-V.; Lee, T.R. Carbonate-Terminated Self-Assembled Monolayers for Mimicking Nanoscale Polycarbonate Surfaces. ACS Appl. Nano Mater. 2023, 6, 2472–2477. [Google Scholar] [CrossRef]
- Cooper, O.; Phan, H.-P.; Wang, B.; Lowe, S.; Day, C.J.; Nguyen, N.-T.; Tiralongo, J. Functional Microarray Platform with Self-Assembled Monolayers on 3C-Silicon Carbide. Langmuir 2020, 36, 13181–13192. [Google Scholar] [CrossRef]
- Kahsar, K.R.; Schwartz, D.K.; Medlin, J.W. Control of Metal Catalyst Selectivity through Specific Noncovalent Molecular Interactions. J. Am. Chem. Soc. 2014, 136, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Coan, P.D.; Farberow, C.A.; Griffin, M.B.; Medlin, J.W. Organic Modifiers Promote Furfuryl Alcohol Ring Hydrogenation via Surface Hydrogen-Bonding Interactions. ACS Catal. 2021, 11, 3730–3739. [Google Scholar] [CrossRef]
- Sui, W.; Zhao, W.; Zhang, X.; Peng, S.; Zeng, Z.; Xue, Q. Comparative Anti-Corrosion Properties of Alkylthiols SAMs and Mercapto Functional Silica Sol–Gel Coatings on Copper Surface in Sodium Chloride Solution. J. Sol-Gel Sci. Technol. 2016, 80, 567–578. [Google Scholar] [CrossRef]
- Telegdi, J. Formation of Self-Assembled Anticorrosion Films on Different Metals. Materials 2020, 13, 5089. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Tran, H.-V.; Lee, T.R. Self-Assembled Monolayer Coatings on Gold and Silica Surfaces for Antifouling Applications: A Review. Coatings 2022, 12, 1462. [Google Scholar] [CrossRef]
- St. Hill, L.R.; Tran, H.-V.; Chinwangso, P.; Lee, H.J.; Marquez, M.D.; Craft, J.W.; Lee, T.R. Antifouling Studies of Unsymmetrical Oligo(Ethylene Glycol) Spiroalkanedithiol Self-Assembled Monolayers. Micro 2021, 1, 151–163. [Google Scholar] [CrossRef]
- Barriet, D.; Chinwangso, P.; Lee, T.R. Can Cyclopropyl-Terminated Self-Assembled Monolayers on Gold Be Used to Mimic the Surface of Polyethylene? ACS Appl. Mater. Interfaces 2010, 2, 1254–1265. [Google Scholar] [CrossRef]
- Yu, T.; Marquez, M.D.; Zenasni, O.; Lee, T.R. Mimicking Polymer Surfaces Using Cyclohexyl- and Perfluorocyclohexyl-Terminated Self-Assembled Monolayers. ACS Appl. Nano Mater. 2019, 2, 5809–5816. [Google Scholar] [CrossRef]
- Rodriguez, D.; Marquez, M.D.; Zenasni, O.; Han, L.T.; Baldelli, S.; Lee, T.R. Surface Dipoles Induce Uniform Orientation in Contacting Polar Liquids. Chem. Mater. 2020, 32, 7832–7841. [Google Scholar] [CrossRef]
- Bain, C.D.; Troughton, E.B.; Tao, Y.T.; Evall, J.; Whitesides, G.M.; Nuzzo, R.G. Formation of Monolayer Films by the Spontaneous Assembly of Organic Thiols from Solution onto Gold. J. Am. Chem. Soc. 1989, 111, 321–335. [Google Scholar] [CrossRef]
- Choi, Y.; Park, C.S.; Tran, H.-V.; Li, C.-H.; Crudden, C.M.; Lee, T.R. Functionalized N-Heterocyclic Carbene Monolayers on Gold for Surface-Initiated Polymerizations. ACS Appl. Mater. Interfaces 2022, 14, 44969–44980. [Google Scholar] [CrossRef]
- Zharnikov, M. High-Resolution X-ray Photoelectron Spectroscopy in Studies of Self-Assembled Organic Monolayers. J. Electron Spectrosc. Relat. Phenom. 2010, 178–179, 380–393. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X.; Fang, D. A Review on C 1s XPS-Spectra for Some Kinds of Carbon Materials. Fuller. Nanotub. Carbon Nanostruct. 2020, 28, 1048–1058. [Google Scholar] [CrossRef]
- Aitchison, H.; Lu, H.; Hogan, S.W.L.; Früchtl, H.; Cebula, I.; Zharnikov, M.; Buck, M. Self-Assembled Monolayers of Oligophenylenecarboxylic Acids on Silver Formed at the Liquid–Solid Interface. Langmuir 2016, 32, 9397–9409. [Google Scholar] [CrossRef]
- Ishida, T.; Hara, M.; Kojima, I.; Tsuneda, S.; Nishida, N.; Sasabe, H.; Knoll, W. High Resolution X-ray Photoelectron Spectroscopy Measurements of Octadecanethiol Self-Assembled Monolayers on Au(111). Langmuir 1998, 14, 2092–2096. [Google Scholar] [CrossRef]
- Porter, M.D.; Bright, T.B.; Allara, D.L.; Chidsey, C.E.D. Spontaneously Organized Molecular Assemblies. 4. Structural Characterization of n-Alkyl Thiol Monolayers on Gold by Optical Ellipsometry, Infrared Spectroscopy, and Electrochemistry. J. Am. Chem. Soc. 1987, 109, 3559–3568. [Google Scholar] [CrossRef]
- MacPhail, R.A.; Strauss, H.L.; Snyder, R.G.; Elliger, C.A. Carbon-Hydrogen Stretching Modes and the Structure of n-Alkyl Chains. 2. Long, All-Trans Chains. J. Phys. Chem. 1984, 88, 334–341. [Google Scholar] [CrossRef]
- Snyder, R.G.; Strauss, H.L.; Elliger, C.A. Carbon-Hydrogen Stretching Modes and the Structure of n-Alkyl Chains. 1. Long, Disordered Chains. J. Phys. Chem. 1982, 86, 5145–5150. [Google Scholar] [CrossRef]
- Smallwood, I. Handbook of Organic Solvent Properties; Butterworth-Heinemann: Woburn, MA, USA, 2014. [Google Scholar]
- Wohlfarth, C. Surface Tension of Pure Liquids and Binary Liquid Mixtures, 2008th ed.; Lechner, M.D., Ed.; Landolt-Börnstein: Numerical Data and Functional Relationships in Science and Technology—New Series; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar] [CrossRef]
- Lee, S.; Park, J.-S.; Lee, T.R. The Wettability of Fluoropolymer Surfaces: Influence of Surface Dipoles. Langmuir 2008, 24, 4817–4826. [Google Scholar] [CrossRef]
- Beitollahpoor, M.; Farzam, M.; Pesika, N.S. Determination of the Sliding Angle of Water Drops on Surfaces from Friction Force Measurements. Langmuir 2022, 38, 2132–2136. [Google Scholar] [CrossRef]
- Beitollahpoor, M.; Farzam, M.; Pesika, N.S. Friction Force-Based Measurements for Simultaneous Determination of the Wetting Properties and Stability of Superhydrophobic Surfaces. J. Colloid Interface Sci. 2023, 648, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Soon Park, C.; Zenasni, O.; D Marquez, M.; Justin Moore, H.; Randall Lee, T. Hydrophilic Surfaces via the Self-Assembly of Nitrile-Terminated Alkanethiols on Gold. AIMS Mater. Sci. 2018, 5, 171–189. [Google Scholar] [CrossRef]
- Lee, H.J.; Jamison, A.C.; Lee, T.R. Surface Dipoles: A Growing Body of Evidence Supports Their Impact and Importance. Acc. Chem. Res. 2015, 48, 3007–3015. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Y.; Cao, Y.; Li, G.; Liao, Y. Influence of Surface Roughness on Contact Angle Hysteresis and Spreading Work. Colloid Polym. Sci. 2020, 298, 1107–1112. [Google Scholar] [CrossRef]
- Farzam, M.; Beitollahpoor, M.; Solomon, S.E.; Ashbaugh, H.S.; Pesika, N.S. Advances in the Fabrication and Characterization of Superhydrophobic Surfaces Inspired by the Lotus Leaf. Biomimetics 2022, 7, 196. [Google Scholar] [CrossRef]
- Aktas, C.; Polat, O.; Beitollahpoor, M.; Farzam, M.; Pesika, N.S.; Sahiner, N. Force-Based Characterization of the Wetting Properties of LDPE Surfaces Treated with CF4 and H2 Plasmas. Polymers 2023, 15, 2132. [Google Scholar] [CrossRef]

| Adsorbate | Thickness (Å) |
|---|---|
| C18SH | 20 ± 1 |
| γ-COC11SH | 15 ± 1 |
| γ-COC12SH | 16 ± 1 |
| δ-COC11SH | 16 ± 1 |
| δ-COC12SH | 17 ± 1 |
| Adsorbate | S 2p3/2 (eV) | C 1s (CH2) (eV) | C 1s (C–O) (eV) | C 1s (C=O) (eV) | O 1s (C–O) (eV) | O 1s (C=O) (eV) | Relative Packing Density (%) |
|---|---|---|---|---|---|---|---|
| C18SH | 162 | 284.9 | – | – | – | – | 100 |
| γ-COC11SH | 161.9 | 284.8 | 286.8 | 289.2 | 533.3 | 532.5 | 91 ± 4 |
| γ-COC12SH | 161.9 | 284.9 | 286.9 | 289.3 | 533.3 | 532.4 | 90 ± 2 |
| δ-COC11SH | 161.9 | 284.9 | 286.8 | 289.3 | 533.2 | 532.5 | 87 ± 2 |
| δ-COC12SH | 161.9 | 284.7 | 286.6 | 289.2 | 533.2 | 532.3 | 85 ± 3 |
| Adsorbate | (cm−1) | (cm−1) | (cm−1) | |
|---|---|---|---|---|
| C18SH | 2850 | 2878 | 2918 | 2964 |
| γ-COC11SH | 2853 | – | 2920 | – |
| γ-COC12SH | 2853 | – | 2920 | – |
| δ-COC11SH | 2854 | – | 2923 | – |
| δ-COC12SH | 2854 | – | 2923 | – |
| Adsorbate | Polar Protic Solvents | Polar Aprotic Solvents | Non-Polar Solvents | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| W | Gly | FA | MF | DMSO | DMF | MeCN | DIM | DC | SQ | HD | |
| γ-COC11SH | 55 (6) | 50 (4) | 37 (8) | <10 (–) | <10 (–) | <10 (–) | <10 (–) | 26 (6) | <10 (–) | <10 (–) | <10 (–) |
| γ-COC12SH | 57 (8) | 54 (8) | 38 (5) | <10 (–) | <10 (–) | <10 (–) | <10 (–) | 28 (6) | <10 (–) | <10 (–) | <10 (–) |
| δ-COC11SH | 72 (12) | 64 (8) | 52 (8) | <10 (–) | <10 (–) | <10 (–) | <10 (–) | 33 (5) | <10 (–) | <10 (–) | <10 (–) |
| δ-COC12SH | 77 (13) | 68 (11) | 59 (10) | <10 (–) | <10 (–) | <10 (–) | <10 (–) | 37 (10) | <10 (–) | <10 (–) | <10 (–) |
| PGA | 40 (9) | 36 (6) | 30 (7) | <10 (–) | <10 (–) | <10 (–) | <10 (–) | 24 (4) | <10 (–) | <10 (–) | <10 (–) |
| PLGA | 58 (12) | 59 (6) | 38 (12) | 14 (–) | 16 (5) | 13 (–) | <10 (–) | 33 (9) | <10 (–) | <10 (–) | <10 (–) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tajalli, P.; Hernandez Rivera, J.M.; Omidiyan, M.; Lee, J.M.; Tran, H.-V.; Lee, T.R. Lactone-Terminated Self-Assembled Monolayers for Mimicking Nanoscale Polyester Surfaces. Chemistry 2024, 6, 666-676. https://doi.org/10.3390/chemistry6040039
Tajalli P, Hernandez Rivera JM, Omidiyan M, Lee JM, Tran H-V, Lee TR. Lactone-Terminated Self-Assembled Monolayers for Mimicking Nanoscale Polyester Surfaces. Chemistry. 2024; 6(4):666-676. https://doi.org/10.3390/chemistry6040039
Chicago/Turabian StyleTajalli, Pooria, Jennifer M. Hernandez Rivera, Mina Omidiyan, Jong Moon Lee, Hung-Vu Tran, and T. Randall Lee. 2024. "Lactone-Terminated Self-Assembled Monolayers for Mimicking Nanoscale Polyester Surfaces" Chemistry 6, no. 4: 666-676. https://doi.org/10.3390/chemistry6040039
APA StyleTajalli, P., Hernandez Rivera, J. M., Omidiyan, M., Lee, J. M., Tran, H.-V., & Lee, T. R. (2024). Lactone-Terminated Self-Assembled Monolayers for Mimicking Nanoscale Polyester Surfaces. Chemistry, 6(4), 666-676. https://doi.org/10.3390/chemistry6040039










