Enhancing the Wetting Properties of Activated Biochar by Oxidation with Hydrogen Peroxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Modification of Materials
2.2. Characterization of Synthesized Materials
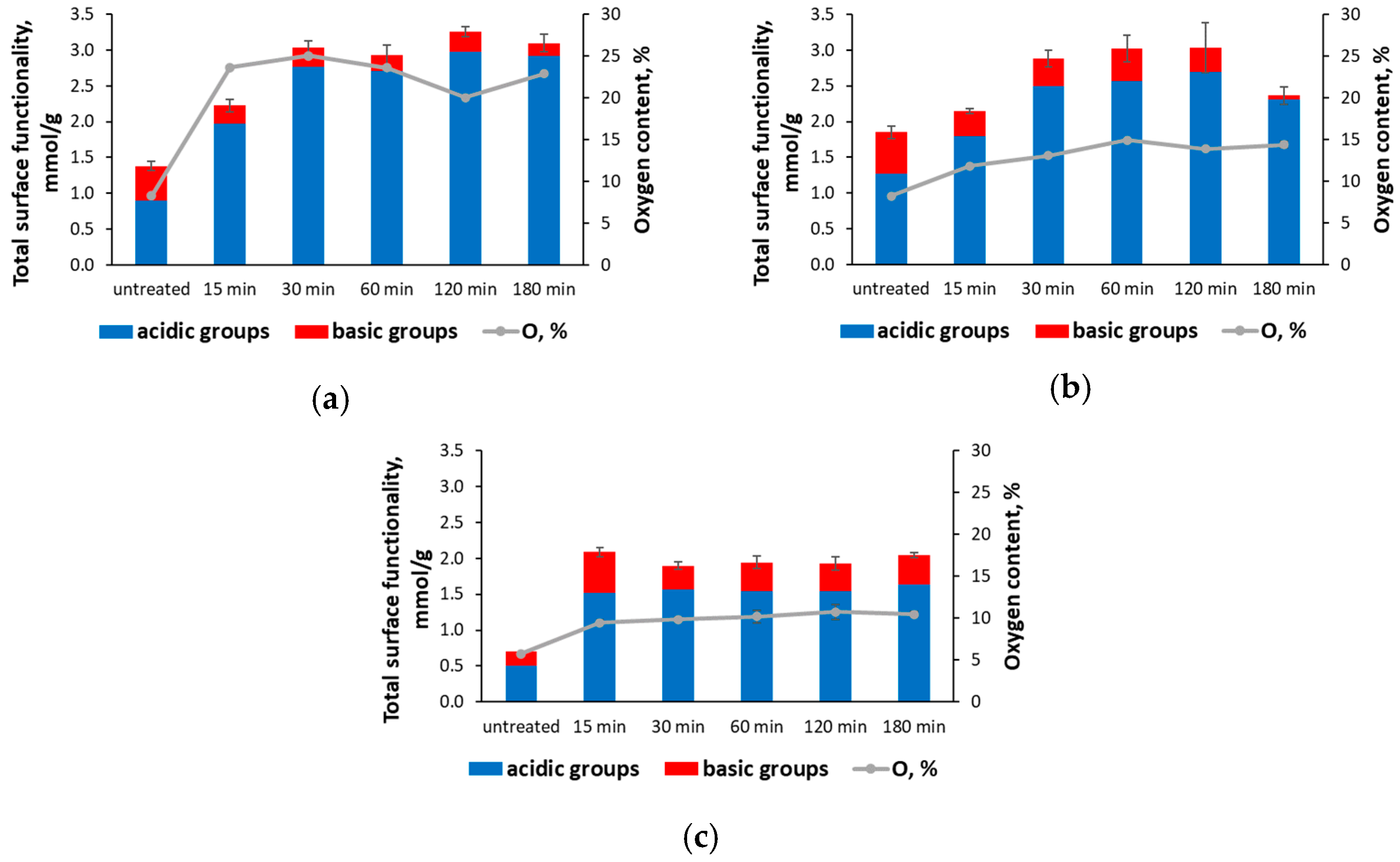
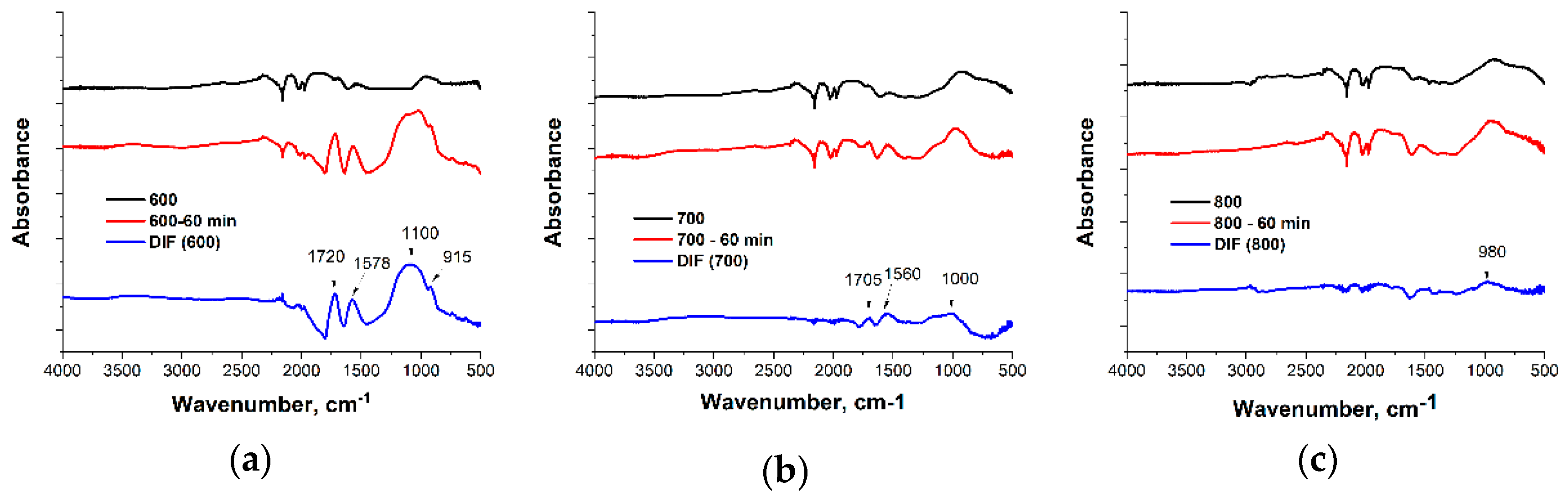
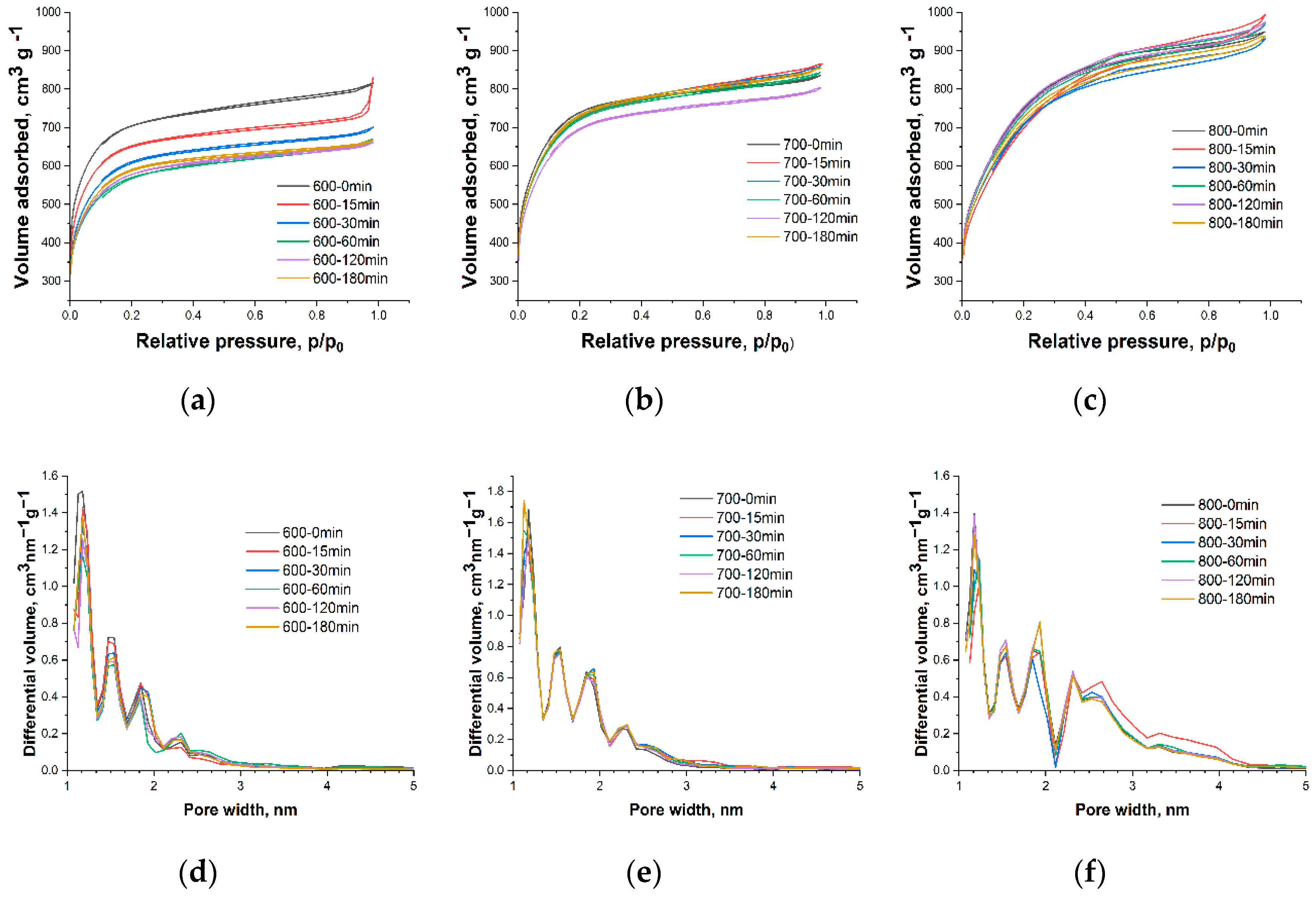
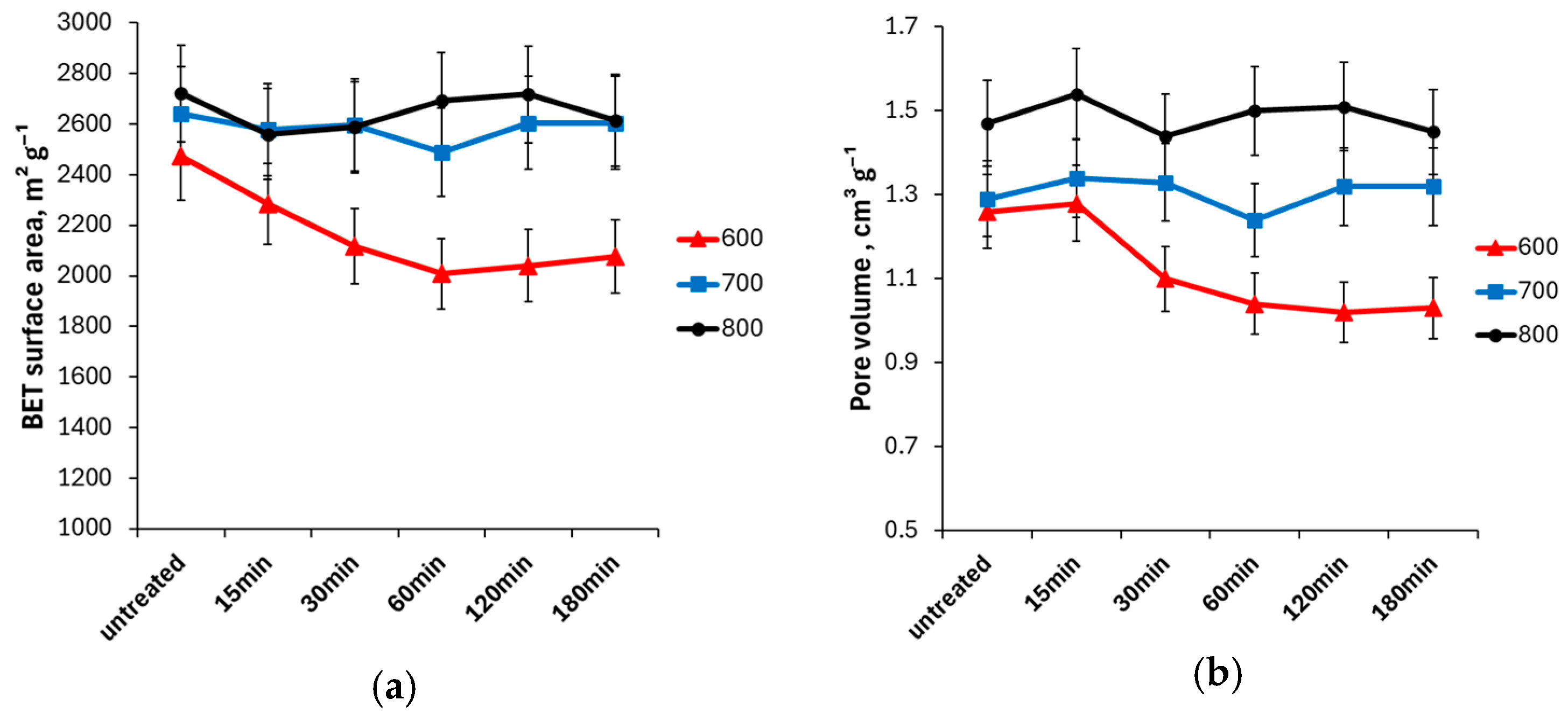
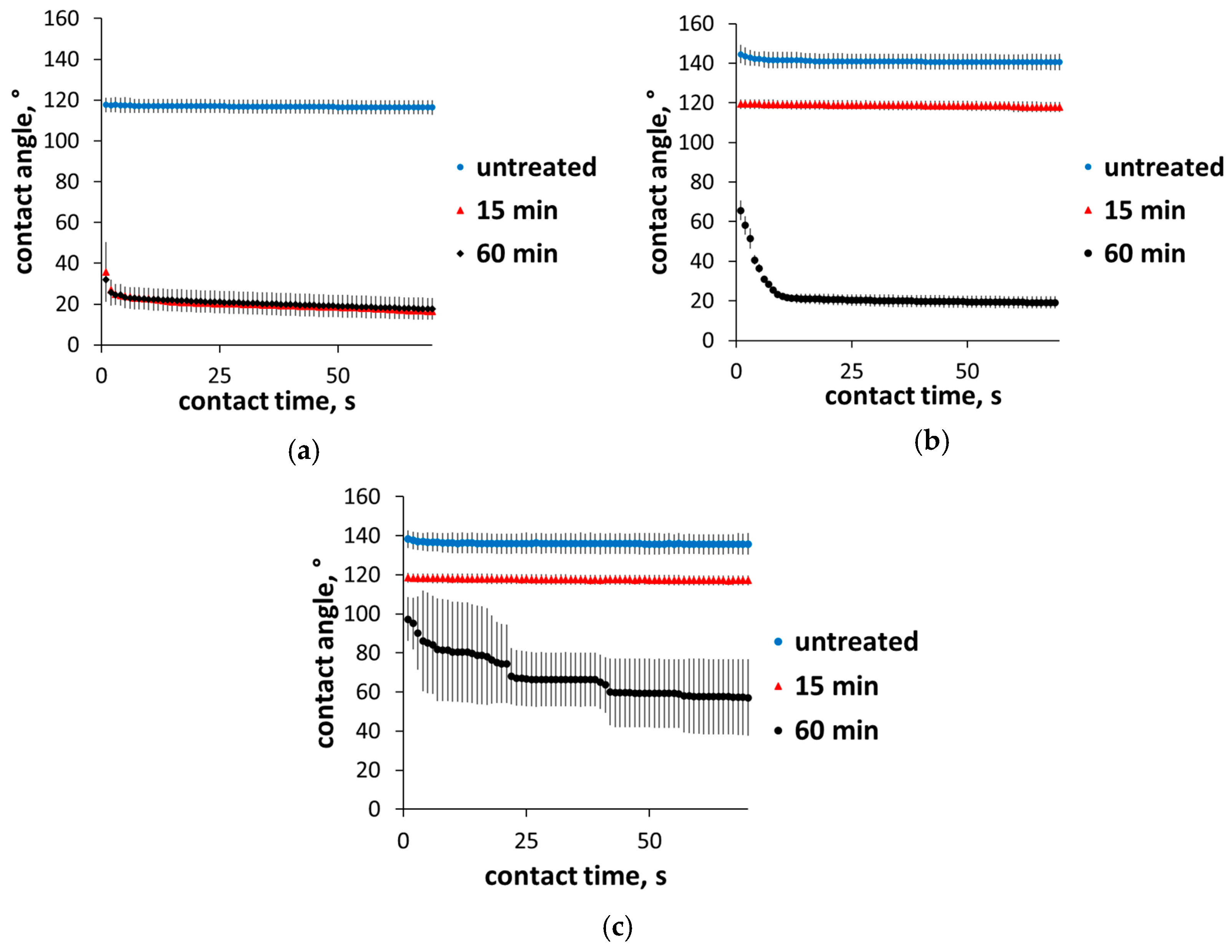
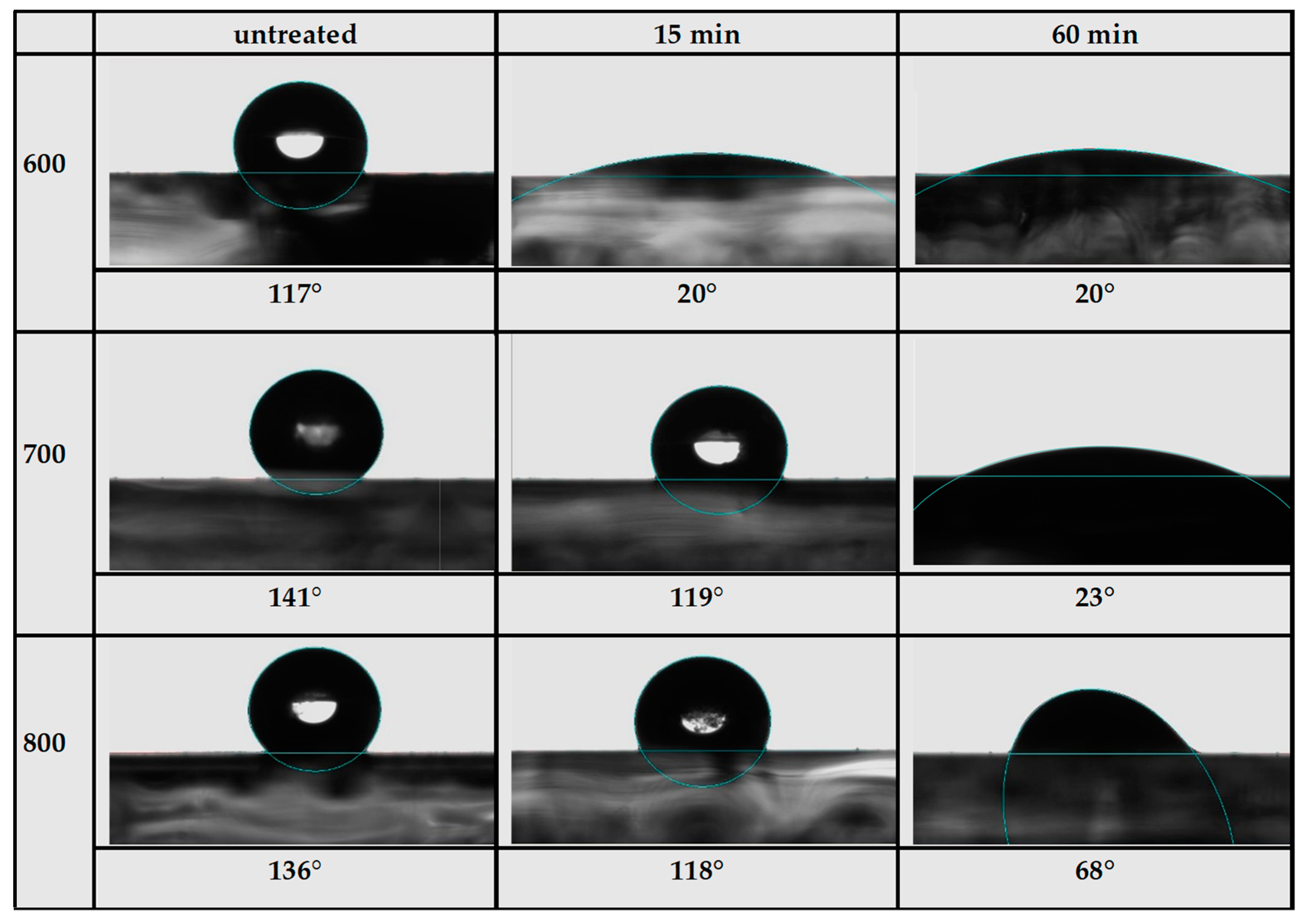
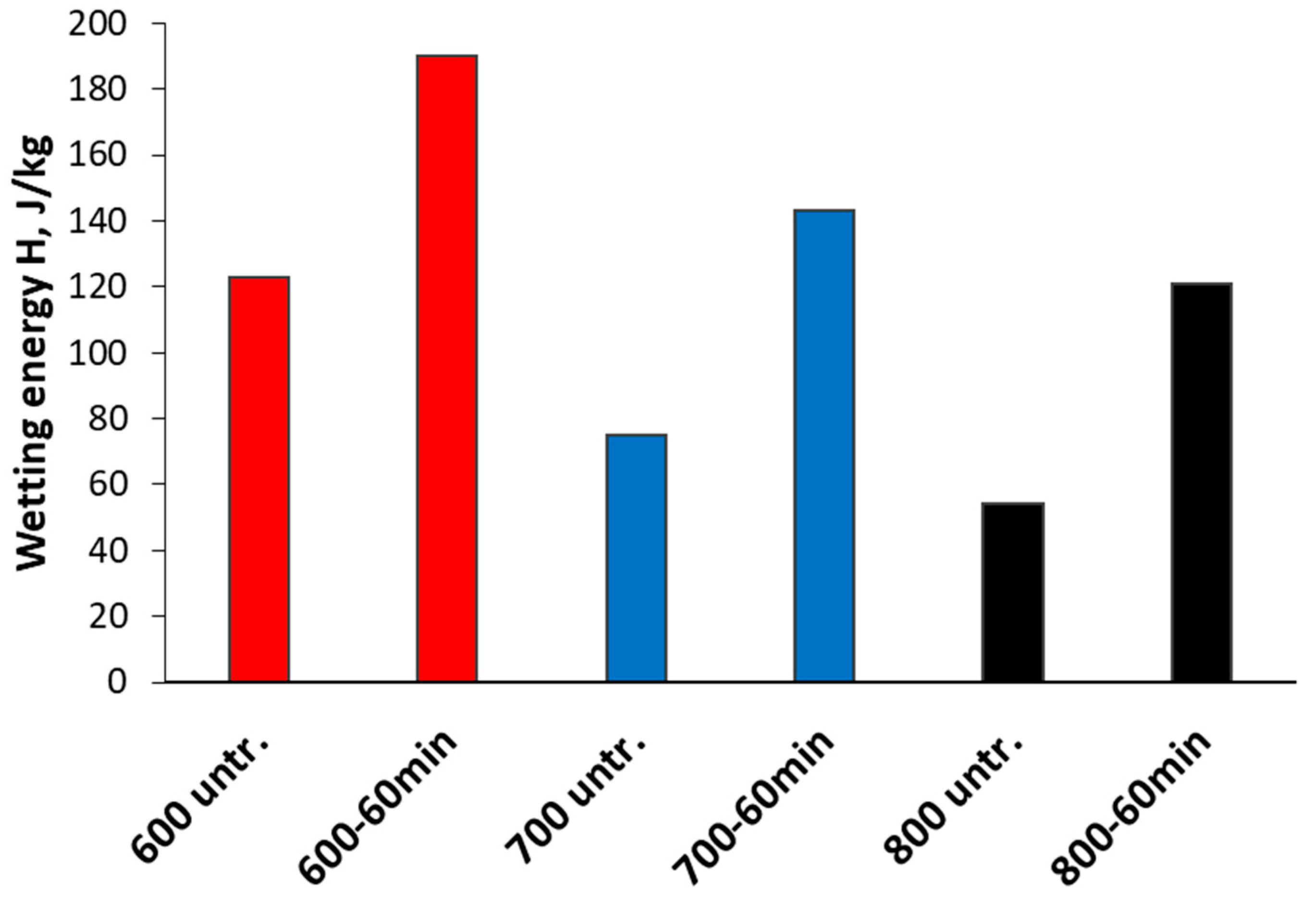
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Duta, L.; Popescu, A.C.; Zgura, I.; Mihailescu, I.N. Wettability of Nanostructured Surfaces. In Wetting Wettability; Intechopen: London, UK, 2015; p. 60808. [Google Scholar] [CrossRef]
- Feng, J.; Guo, Z. Wettability of graphene: From influencing factors and reversible conversions to potential applications. Nanoscale Horiz 2019, 4, 339–364. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, F.; Periolatto, M.; Ferrero, F.; Periolatto, M. Modification of Surface Energy and Wetting of Textile Fibers. In Wetting Wettability; Intechopen: London, UK, 2015; p. 60812. [Google Scholar] [CrossRef]
- Yuan, Y.; Lee, T.R. Contact angle and wetting properties. Springer Ser. Surf. Sci. 2013, 51, 3–34. [Google Scholar] [CrossRef]
- Daniel, D.; Vuckovac, M.; Backholm, M.; Latikka, M.; Karyappa, R.; Koh, X.Q.; Timonen, J.V.I.; Tomczak, N.; Ras, R.H.A. Probing surface wetting across multiple force, length and time scales. Commun. Phys. 2023, 6, 152. [Google Scholar] [CrossRef]
- Wang, X.Y.; Xia, X.H.; Wang, H.X.; Yang, Y.X.; Yang, S.L.; Zhang, A.Y.; Yuan, R.; Zhu, H.; Wang, B.; Zhang, Y.B.; et al. Special-wettability-mediating electrode interfaces for new energy devices: Opportunities and challenges. Nano Energy 2024, 120, 109185. [Google Scholar] [CrossRef]
- Krainer, S.; Hirn, U. Contact angle measurement on porous substrates: Effect of liquid absorption and drop size. Colloids Surf A Physicochem Eng Asp. 2021, 619, 126503. [Google Scholar] [CrossRef]
- Butkus, M.A.; Grasso, D. The nature of surface complexation: A continuum approach. Environ. Geology 2001, 40, 446–453. [Google Scholar] [CrossRef]
- Qiu, C.; Jiang, L.; Gao, Y.; Sheng, L. Effects of oxygen-containing functional groups on carbon materials in supercapacitors: A review. Mater Des. 2023, 230, 111952. [Google Scholar] [CrossRef]
- Gray, M.; Johnson, M.G.; Dragila, M.I.; Kleber, M. Water uptake in biochars: The roles of porosity and hydrophobicity. Biomass Bioenergy 2014, 61, 196–205. [Google Scholar] [CrossRef]
- Kinney, T.J.; Masiello, C.A.; Dugan, B.; Hockaday, W.C.; Dean, M.R.; Zygourakis, K.; Barnes, R.T. Hydrologic properties of biochars produced at different temperatures. Biomass Bioenergy 2012, 41, 34–43. [Google Scholar] [CrossRef]
- Belyaeva, L.A.; Schneider, G.F. Wettability of graphene. Surf Sci Rep. 2020, 75, 100482. [Google Scholar] [CrossRef]
- Sun, Z.; Dai, L.; Lai, P.; Shen, F.; Shen, F.; Zhu, W. Air oxidation in surface engineering of biochar-based materials: A critical review. Carbon Res. 2022, 1, 32. [Google Scholar] [CrossRef]
- Jaramillo, J.; Álvarez, P.M.; Gómez-Serrano, V. Oxidation of activated carbon by dry and wet methods: Surface chemistry and textural modifications. Fuel Process. Technol. 2010, 91, 1768–1775. [Google Scholar] [CrossRef]
- Pavese, M.; Musso, S.; Bianco, S.; Giorcelli, M.; Pugno, N. An analysis of carbon nanotube structure wettability before and after oxidationtreatment. J. Phys. Condens. Matter 2008, 20, 474206. [Google Scholar] [CrossRef]
- Huff, M.D.; Lee, J.W. Biochar-surface oxygenation with hydrogen peroxide. J Environ Manage 2016, 165, 17–21. [Google Scholar] [CrossRef]
- Chemerys, V.; Baltrėnaitė-Gedienė, E.; Baltrėnas, P.; Dobele, G. Influence of H2O2 Modification on the Adsorptive Properties of Birch-Derived Biochar. Pol J Environ Stud. 2019, 29, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Zhurinsh, A.; Zandersons, J. Lump charcoal production in Latvia: State of the art and prospects. In Pyrolysis and Gasification of Biomass and Waste; Bridgwater, A.V., Ed.; CPL Press: Newbury, UK, 2003; pp. 307–314. [Google Scholar]
- Volperts, A.; Plavniece, A.; Dobele, G.; Zhurinsh, A.; Kruusenberg, I.; Kaare, K.; Locs, J.; Tamasauskaite-Tamasiunaite, L.; Norkus, E. Biomass based activated carbons for fuel cells. Renew Energy 2019, 141, 40–45. [Google Scholar] [CrossRef]
- Volperts, A.; Plavniece, A.; Kaare, K.; Dobele, G.; Zhurinsh, A.; Kruusenberg, I. Influence of Chemical Activation Temperatures on Nitrogen-Doped Carbon Material Structure, Pore Size Distribution and Oxygen Reduction Reaction Activity. Catalysts 2021, 11, 1460. [Google Scholar] [CrossRef]
- Boehm, H.P. Chemical Identification of Surface Groups. Adv. Catal. 1966, 16, 179–274. [Google Scholar] [CrossRef]
- Schönherr, J.; Buchheim, J.R.; Scholz, P.; Adelhelm, P. Boehm Titration Revisited (Part I): Practical Aspects for Achieving a High Precision in Quantifying Oxygen-Containing Surface Groups on Carbon Materials. C 2018, 4, 21. [Google Scholar] [CrossRef]
- Sun, J.; Niu, J.; Liu, M.; Ji, J.; Dou, M.; Wang, F. Biomass-derived nitrogen-doped porous carbons with tailored hierarchical porosity and high specific surface area for high energy and power density supercapacitors. Appl Surf Sci. 2018, 427, 807–813. [Google Scholar] [CrossRef]
- Tratnik, B.; Van de Velde, N.; Jerman, I.; Kapun, G.; Tchernychova, E.; Tomšič, M.; Jamnik, A.; Genorio, B.; Vizintin, A.; Dominko, R. Correlating Structural Properties with Electrochemical Behavior of Non-graphitizable Carbons in Na-Ion Batteries. ACS Appl Energy Mater 2022, 5, 10667–10679. [Google Scholar] [CrossRef] [PubMed]
- Leng, L.J.; Xiong, Q.; Yang, L.H.; Li, H.; Zhou, Y.Y.; Zhang, W.J.; Jiang, S.J.; Li, H.L.; Huang, H.J. An overview on engineering the surface area and porosity of biochar. Sci. Total Environ. 2021, 763, 144204. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wang, Q.; Lu, Y.; Li, B.; Chen, L.; Hu, Y.S. High-temperature treatment induced carbon anode with ultrahigh Na storage capacity at low-voltage plateau. Sci. Bull. 2018, 63, 1125–1129. [Google Scholar] [CrossRef] [PubMed]
- Ibarra, J.V.; Muñoz, E.; Moliner, R. FTIR study of the evolution of coal structure during the coalification process. Org Geochem. 1996, 24, 725–735. [Google Scholar] [CrossRef]
- Bellamy, L.J. The Infra-red Spectra of Complex Molecules; Springer Dordrecht: Dordrecht, The Netherlands, 1975. [Google Scholar] [CrossRef]
| Activation Temperature, °C | Oxidation Time | N, % | C, % | H, % | O, % |
|---|---|---|---|---|---|
| 600 | 0 min | 0.547 ± 0.005 | 90.24 ± 0.26 | 0.95 ± 0.03 | 8.26 ± 0.23 |
| 15 min | 0.28 ± 0.03 | 75.31 ± 0.24 | 0.78 ± 0.05 | 23.63 ± 0.16 | |
| 30 min | 0.30 ± 0.09 | 73.61 ± 0.14 | 1.040 ± 0.016 | 25.05 ± 0.04 | |
| 60 min | 0.243 ± 0.004 | 75.36 ± 0.19 | 0.81 ± 0.04 | 23.59 ± 0.24 | |
| 120 min | 0.34 ± 0.06 | 80.1 ± 1.9 | 0.84 ± 0.12 | 20 ± 2 | |
| 180 min | 0.257 ± 0.016 | 76.11 ± 0.33 | 0.75 ± 0.17 | 22.88 ± 0.14 | |
| 700 | 0 min | 0.53 ± 0.07 | 90.973 ± 0.015 | 0.270 ± 0.012 | 8.23 ± 0.06 |
| 15 min | 0.54 ± 0.14 | 87.18 ± 0.07 | 0.473 ± 0.016 | 11.81 ± 0.08 | |
| 30 min | 0.41 ± 0.05 | 85.7 ± 0.2 | 0.823 ± 0.012 | 13.1 ± 0.2 | |
| 60 min | 0.55 ± 0.10 | 83.6 ± 0.2 | 0.93 ± 0.07 | 14.95 ± 0.18 | |
| 120 min | 0.60 ± 0.06 | 84.59 ± 0.14 | 0.93 ± 0.03 | 13.88 ± 0.17 | |
| 180 min | 0.54 ± 0.18 | 84.19 ± 0.19 | 0.912 ± 0.010 | 14.3 ± 0.4 | |
| 800 | 0 min | 0.6 ± 0.3 | 92.80 ± 0.02 | 0.88 ± 0.12 | 5.74 ± 0.11 |
| 15 min | 0.6 ± 0.3 | 89.4 ± 0.2 | 0.51 ± 0.15 | 9.5 ± 0.4 | |
| 30 min | 0.63 ± 0.08 | 89.00 ± 0.09 | 0.55 ± 0.15 | 9.8 ± 0.3 | |
| 60 min | 0.57 ± 0.12 | 88.9 ± 0.8 | 0.36 ± 0.10 | 10.2 ± 0.7 | |
| 120 min | 0.47 ± 0.19 | 88.3 ± 0.8 | 0.45 ± 0.11 | 10.8 ± 0.9 | |
| 180 min | 0.57 ± 0.12 | 88.540 ± 0.004 | 0.4 ± 0.2 | 10.5 ± 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liepins, K.; Volperts, A.; Dobele, G.; Plavniece, A.; Bikovens, O.; Sansonetti, E.; Zhurinsh, A. Enhancing the Wetting Properties of Activated Biochar by Oxidation with Hydrogen Peroxide. Chemistry 2024, 6, 911-921. https://doi.org/10.3390/chemistry6050053
Liepins K, Volperts A, Dobele G, Plavniece A, Bikovens O, Sansonetti E, Zhurinsh A. Enhancing the Wetting Properties of Activated Biochar by Oxidation with Hydrogen Peroxide. Chemistry. 2024; 6(5):911-921. https://doi.org/10.3390/chemistry6050053
Chicago/Turabian StyleLiepins, Kalvis, Aleksandrs Volperts, Galina Dobele, Ance Plavniece, Oskars Bikovens, Errj Sansonetti, and Aivars Zhurinsh. 2024. "Enhancing the Wetting Properties of Activated Biochar by Oxidation with Hydrogen Peroxide" Chemistry 6, no. 5: 911-921. https://doi.org/10.3390/chemistry6050053






