Abstract
4-Acylpyrazolones are important ligands in analytical chemistry and technologies used for the separation and concentration of various metals. We have proposed a novel method for obtaining a material that consists of covalently immobilized functionalized ionic liquid on the surface of a mineral carrier featuring a coordination-active fragment of 4-acylpyrazolone. For its synthesis, we have introduced a strategy based on the quaternization of surface azolyl groups from 3-(1H-imidazol-1-yl)propyl silica with an alkylating reagent containing a 4-acylpyrazolone motif-4-(6-bromohexanoyl)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one. This method of covalent immobilization preserves the 1,3-dioxo fragment, which ensures the effective binding of metal ions. The success of this functionalization has been confirmed by IR and 13C NMR spectroscopy data, as well as by thermogravimetric analysis. The overall functional capacity was found to be 0.3 mmol/g. The potential of the synthesized organomineral material to concentrate five rare earth elements (REEs) representing the cerium (Eu(III), Sm(III)) and yttrium groups (Gd(III), Dy(III), Er(III)) has been demonstrated. It was shown that during extraction from multicomponent systems, both under static and dynamic preconcentration conditions, there is a competitive influence of analytes, and their separation can be evaluated under dynamic conditions based on dynamic output curves and calculated distribution coefficients. It was shown that for systems where Kd > 1.8, quantitative separation can be performed in a dynamic mode of sorption under selected conditions.
1. Introduction
In a little more than 30 years, ionic liquids transformed from very exotic compounds to an indispensable attribute in different fields of chemical science. They also became an important tool in chemical technology. Currently, almost all fields of chemistry use ionic liquids: they act as unique reaction media [1,2,3], extracting agents [4,5,6,7], catalyst supports [8,9] and have many other applications [10,11,12]. Dozens of monographs and hundreds of reviews are dedicated to various chemical aspects of ionic liquids [13,14].
Of specific interest are functionalized ionic liquids, which contain chelating moieties, among others. Such compounds find application in many methods for the separation and concentration of both inorganic ions [15,16,17] and organic compounds [18]. Despite the structural diversity of these compounds, this field has many “blind spots”, leaving a large space for modern researchers to provide new information about their properties and application.
For example, the technique for the development of new ionic liquids [19] consisting of a conjugation of the onium moiety and a functional analytic group with knowingly efficient chelating [20] or other characteristics was not embodied in all types of efficient ligands. In addition, the so-called immobilized ionic liquids [21,22], which are, in fact, solid-phase materials with covalently immobilized onium fragments, including those containing further functional analytic groups, constitute a different area of knowledge. Materials of this type are of interest as chromatographic phases [23], sorbents for solid-phase extraction [24], substrates for heterogeneous catalysts [25], etc. Acylpyrazolone derivatives are promising for use as additional chelating agents [26,27,28,29,30]. The representatives of this compound class are in-demand ligands, the properties of which are often compared with cyclic beta-diketones, forming stable intracomplex compounds with metal ions in quite acidic media. Such complexation enables us to decrease the effect of competitive hydrolytic and polymerization processes and provides high distribution coefficients for these elements upon liquid–liquid extraction and solid-phase extraction, as well as resistance to light and heat, and it enables the extraction of more than 50 elements, including REEs. These properties distinguish this type of compound from other analytical reagents [27,28,29,30,31]. The increased demand for the efficient processing, separation, and concentration of REEs from secondary raw materials is due to their growing consumption in hi-tech industrial and household products [32,33]. The problem of the efficient recovery of REEs [34] from samples with a complex composition, followed by their separation, gives impetus to the synthesis of new supramolecular ligands [35] and ionic ligands [36,37,38] and their adoption as extracting agents in liquid–liquid extraction, as well as to the design of polymers [39] and organomineral materials [40] containing a grafted [41] and impregnated functional layer for solid-phase extraction [42].
Despite the existing variety of reagents used for extraction in liquid–liquid extraction conditions and materials for solid-phase extraction, the class of widely used ligands such as 4-acylpyrazolones remains largely unexplored in terms of its immobilization on inorganic carriers. The high coordination activity of 4-acylpyrazolones in solution with respect to rare earth elements (REEs) suggests the potential effectiveness of the resulting solid-phase materials. From this perspective, the presented work has a fundamental interest in the search for new materials that could be suitable for the extraction and separation of REEs. For silica, several approaches to obtain 4-acylpyrazolone-modified solid-phase materials for separation and preconcentration are known. The simplest approach includes impregnation of the silica surface, which is achieved by evaporation of a solution of 4-pyrazolone 1, 2 in an organic solvent in the presence of silica [42,43,44].
A general drawback of the materials obtained by the impregnation of ligands onto different supports is the absence of covalent bonding to the surface, which results in the leaching of the impregnated ligand and a poor reproducibility of the properties of such sorbents. This drawback is absent in sorption materials where a ligand is bound to the support surface in a covalent manner. However, the implementation of the covalent immobilization of 4-acylpyrazolone 3 by the above-mentioned methods affects one of the carbonyl groups, resulting in a change in chelating properties [45,46] (Scheme 1).
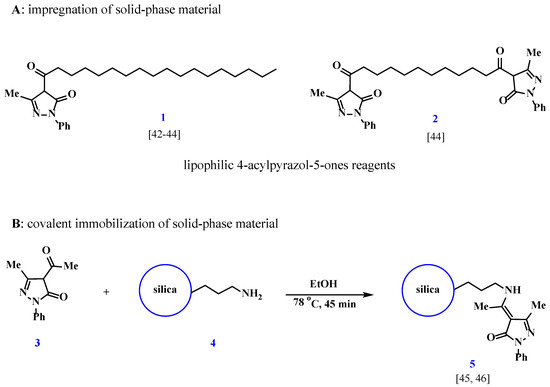
Scheme 1.
Variations in synthesis of materials based on 4-acylpyrazol-5-ones [42,43,44,45,46].
In the present work, a novel synthesis approach is proposed, and some characteristics of a material are described. The material consists of covalently immobilized functionalized ionic liquid on the surface of a mineral carrier, featuring a coordination-active fragment of 1,3-dioxo compounds. We suggest a strategy for obtaining silica with covalently immobilized 4-acylpyrazolone fragments based on the use of alkylating agents that form stable quaternary salts upon interaction with surface azole groups of modified silica. The synthesized organomineral material with the immobilized ionic liquid, containing an acylpyrazolone moieties as a further chelating agent, was characterized using the data from IR, 13C NMR spectroscopy and thermal gravimetric analyses. To demonstrate the potential application of the obtained material and to characterize it as a sorbent, we studied the solid-phase extraction of five rare earth elements (REEs) representing the cerium (Eu(III), Sm(III)) and yttrium groups (Gd(III), Dy(III), Er(III)). It was shown that Gd(III), Dy(III), Er(III), Eu(III) and Sm(III) can be separated and concentrated under dynamic conditions using the synthesized sorbent.
2. Experimental Section
2.1. Materials and Methods
Infrared diffuse reflectance spectra were recorded on a Shimadzu Prestige-21 FTIR spectrometer within 4000–400 cm−1 at a 0.5 cm−1 resolution. 13C NMR spectra were measured using a 400 MHz Bruker WB Avance III spectrometer operating at 9.39 Tesla equipped with a Bruker H-F/X 4 mm pencil CP/MAS probehead. 13C chemical shifts were referenced to the external solid TSP ((trimethylsilyl)propionic acid sodium salt) standard. The cross-polarization technique from 1H with spinning sideband suppression (CP TOSS) and contact pulse durations of 2–4 ms at a MAS rate of 10 kHz was used. The thermal stabilities of the modified silica samples were studied on an STA 409 PC Luxx synchronous thermal analyzer (Netzsch, Selb, Germany) in a temperature range from 30 to 1000 °C at a heating rate of 10 °C/min in an air atmosphere in Al2O3 ceramic crucibles.
The concentrations of all metal ions were determined by ICP-AES using an iCAP-6000 Series atomic emission spectrometer (Thermo Scientific, Waltham, MA, USA) under the optimum instrument parameters, providing the maximum assay sensitivity. The main emission lines of elements free of spectral overlaps from the matrix components of a sample were chosen based on the analysis of their entire spectra.
A standard 5∙10−2 mol/L stock solution of Gd(III), Dy(III), Er(III), Eu(III) and Sm(III) was prepared by dissolving the corresponding metal oxide in 15% HCl.
The experiment included the equilibration of the sorbent (0.05 g) with acetate buffer (50 mL) (pH 3, 3.5, 4, 4.5 and 5). The equilibration experiment was performed in triplicate, and the concentration of Gd(III), Dy(III), Er(III), Eu(III) and Sm(III) in the sample in each extraction experiment was determined three times to minimize experimental and statistical errors. Aliquots were sampled from the aqueous phase prior to and after equilibration.
The absorbances of the solutions were measured on a Leki SS2107 spectrophotometer (Mediora Oy, Helsinki, Finland) using a standard cell with an optical path length of 10 mm. Solutions were stirred using a KS 4000i control incubator shaker (IKA, Staufen im Breisgau, Germany). The residual concentration of Gd(III), Dy(III), Er(III), Eu(III) and Sm(III) after extraction was determined using a spectrophotometric procedure with arsenazo III [47].
A standard stock solution of 5∙10−2 mol/L of Gd(III), Dy(III), Er(III), Eu(III) and Sm(III) was prepared by dissolving the oxides of the metals in 15% HCl.
The sorbent was prepared using Kieselgel 60 (0.040–0.063 mm) (Macherey-Nagel, Düren, Germany).
6-Bromohexanoyl chloride was synthesized from 6-bromohexanoic acid [48]. 6-Bromohexanoic acid was prepared by oxidation with Jones reagent 6-bromohexanol-1 [49]. 1-(3-(trimethoxysilyl)propyl)-1H-imidazole was synthesized according to the protocol we proposed [50].
2.2. Synthesis Protocol
2.2.1. 4-(6-Bromohexanoyl)-5-Methyl-2-Phenyl-2,4-Dihydro-3H-Pyrazol-3-One
To a solution of 2.26 g (0.013 mol) of 1-phenyl-3-methylpyrazolone-5, 20 mL of 1,4-dioxane was added, as was 1.93 g (0.026 mol) of Ca(OH)2. The reaction mixture was stirred for 30 min, after which a solution of 2 mL (0.013 mol) of 6-bromohexanoyl chloride in 5 mL of 1,4-dioxane was added and the solvent was kept at boiling for 2 h. Then, the reaction mixture was poured into a beaker with 70 mL of 2M HCl, and the resulting precipitate was filtered through a Buchner funnel and washed with water. Recrystallization from EtOH gave 3.56 g of beige crystals. Yield 74%; m.p. 102–103 °C. IR (KBr), ν/cm−1: 3145 (OH), 3086, 3005 (Csp2-H), 2949, 2933, 2860, 2850 (Csp3-H), 1625 (C=O). 1H NMR (399.78 MHz, CDCl3, δ, ppm) enol: 1.54–1.60 (m, 2H, CH2), 1.74–1.81 (m, 2H, CH2), 1.88–1.95 (m, 2H, CH2), 2.47 (s, 3H, CH3), 2.74 (t., J = 7.3 Hz, 2H, CH2), 3.43 (t., J = 6.8 Hz, 2H, CH2), 7.25–7.29 (m, 1H, CH), 7.41–7.47 (m, 2H, CH), 7.79–7.82 (m, 2H, CH2). 13C NMR (100.5 MHz, CDCl3, δ, ppm) enol: 15.8 (CH3), 23.5 (CH2), 27.9 (CH2), 32.5 (CH2), 33.4 (CH2), 38.8 (CH2), 103.7 (C), 120.7 (CH), 126.6 (CH), 129.1 (CH), 137.2 (C), 147.3 (C), 160.5 (C), 197.0 (C=O).
2.2.2. Modified Silica
Into a flask equipped with a magnetic stirring element and a reflux condenser we added 10 g of 3-(1H-imidazol-1-yl)propyl silica (11) (capacity for imidazole groups 0.7 mmol/g), 50 mL of MeCN and 0.5 g of 4-(6-bromohexanoyl)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one (8). The reaction mixture was kept at the solvent’s boiling point and under vigorous stirring for 12 h. Then, the silica was filtered, washed on a Schott filter with hot ethanol and acetone and dried in a vacuum at a residual pressure of 1 mm Hg to a constant mass.
2.3. Adsorption Experiments
Modified silica (12) (50 mg) and an aqueous solution of Gd(III), Dy(III), Er(III), Eu (III) or Sm(III) (50 mL), with different pH and concentrations, were mixed at 298 K. The contact time was 30 min, and the value was chosen based on the dependence of the distribution coefficients of Gd(III), Dy(III), Er(III), Eu(III) and Sm(III) on time (Figure 1).
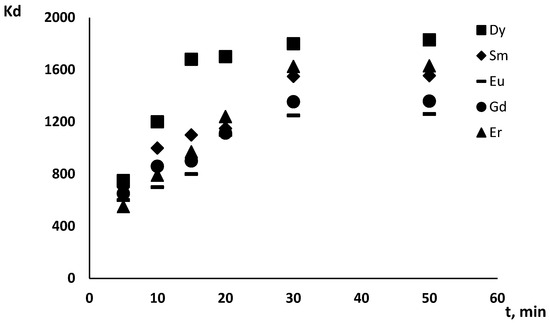
Figure 1.
The dependence of Gd(III), Dy(III), Er(III), Eu(III) and Sm(III)’s (C = 3.75 × 10−5 mol/L) distribution coefficients on the phase contact time in a system based on 50 mg of silica 12 at pH 5.
After extraction, the solid phase was separated by filtration. The concentration of the residual metal was determined by ICP-AES using an iCAP-6000 Series atomic emission spectrometer (Thermo Scientific, Waltham, MA, USA) or Leki SS2107 spectrophotometer (Mediora Oy, Helsinki, Finland). The extraction efficiency was estimated by calculating the distribution coefficient (under static conditions) using Equation (1).
where
A0—the maximum sorption capacity;
Ae—the equilibrium sorption capacity;
V—the volume of the solution;
m—the sorbent weight, g.
2.4. Dynamic Sorption Experiment
To perform the dynamic experiment, a microcolumn (d = 6 mm) was packed with modified silica 12 (m sorbent = 0.2 g). A solution was pumped in using the peristaltic pump, allowing one to vary the volumetric flow rate to 1.25 mL/min. The solution was pumped in aliquots (V = 5 mL) through the packed column, the effluent was collected, and the residual concentration of Gd(III), Dy(III), Er(III), Eu(III) and Sm(III) was determined by spectrophotometry and ICP-MS.
3. Results
3.1. Synthesis and Characterizations
We propose a material assembly strategy which is based on the pre-functionalization of a solid-phase matrix with fragments containing tertiary nitrogen atoms. The subsequent alkylation of these reaction centers should provide the formation of strong conjugates (Scheme 2). In this work, we used silica gel as the matrix, and alkyl fragments served as spacers; however, this strategy can be extended to a wide range of materials.
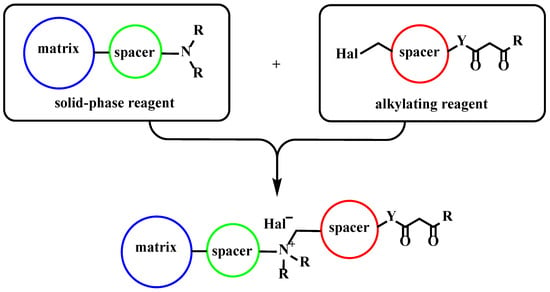
Scheme 2.
Strategy for material preparation (matrix—mineral, organomineral, or organic solid-phase material; spacer—alkyl, aromatic, or heteroaromatic fragment).
For the covalent immobilization of the 4-acylpyrazolone moiety, we propose using 4-(6-bromohexanoyl)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one (8) as the alkylating agent, which was synthesized according to the classical route [51] by the acylation of 1-phenyl-3-methylpyrazol-5-one (6) with 6-bromohexanoyl chloride (7) in the presence of calcium hydroxide upon reflux in 1,4-dioxane for 2 h (Scheme 3).
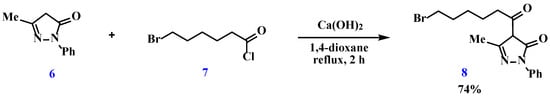
Scheme 3.
Preparation of 4-(6-bromohexanoyl)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one.
The solid-phase matrix was a 3-(1H-imidazol-1-yl)propyl-modified silica (11) obtained by the reaction of 1-(3-(trimethoxysilyl)propyl)-1H-imidazole (10) with silica upon heating in toluene (Scheme 4).
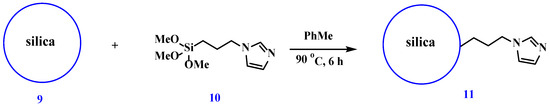
Scheme 4.
Preparation of 3-(1H-imidazol-1-yl)propyl silica.
The synthesized alkylating derivative (8) was covalently immobilized by reflux in acetonitrile for 12 h (Scheme 5).
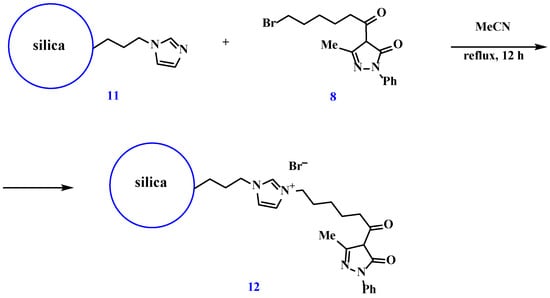
Scheme 5.
Covalent immobilization of 4-(6-bromohexanoyl)-5-methyl-2-phenyl-3H-pyrazol-3-one.
The covalent immobilization of 4-(6-bromohexanoyl)-5-methyl-2-phenyl-3H-pyrazol-3-one and the formation of material (12) were confirmed by IR and NMR spectroscopy.
The recorded diffuse reflectance IR spectra display a broad absorption band in the region of 3000–3700 cm−1(Figure 2), which results from the overlapping of the stretching vibration bands of the Si−OH hydroxyl group and the stretching H–O–H vibrations of adsorbed water molecules bound through both intra- and intermolecular hydrogen bonds. The spectra also display absorption bands corresponding to grafted functional groups: there are absorption bands corresponding to the stretching vibrations of C=O and C=N bonds and to the aromatic Csp2–Csp2 bond at 1625, 1593 and 1516 cm−1; there were also absorption bands of the symmetric and asymmetric vibrations of Csp3−H bonds against the broad absorption band of the hydroxyl group in a region of 2895–2945 cm−1.
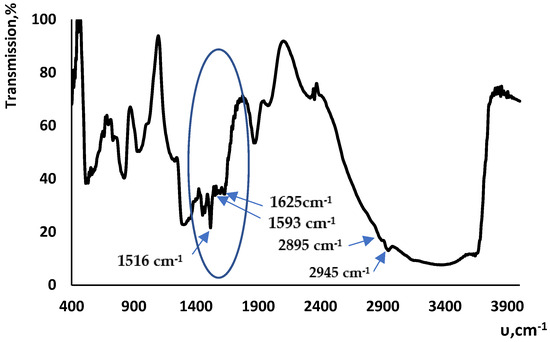
Figure 2.
IR spectrum of the modified silica (12).
Figure 3 shows the 13C NMR spectrum of the obtained silica. Two groups of signals are seen. The first group includes signals at δ 118.5, 119.4, 126.8, 136.1 and 137.8 corresponding to carbon nuclei in nitrogen-containing and benzene rings and signals at δ 152.8 and 165.1 corresponding to C=O groups. The second group includes signals at δ 49.4, 30.1, 24.5 and 14.3 corresponding to different -CH2- fragments and a signal at 9.3 corresponding to the -CH3 group.
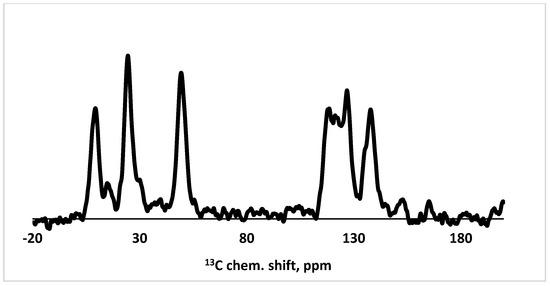
Figure 3.
13C NMR spectrum of the synthesized silica (12).
The synthesized organomineral materials (12) were characterized using the data of their thermal gravimetric analysis. The thermogram displays three main steps of weight loss (Figure 4). Upon heating from 20 to 120 °C, the adsorbed solvent and water are removed, which is accompanied by an endothermic effect in the DSC curves and a relative weight loss of 2.38%. The subsequent steps of weight loss in the temperature range from 200 to 700 °C are manifested as peaks in DSC curves, demonstrating the exothermicity of the occurring processes, which suggests thermal destruction and the subsequent total burnout of the immobilized organic layer. The total weight loss in the second and third sections of the TG curves was 12%. This weight loss value was used to calculate the total functional capacity of the silica, which was 0.3 mmol/g.
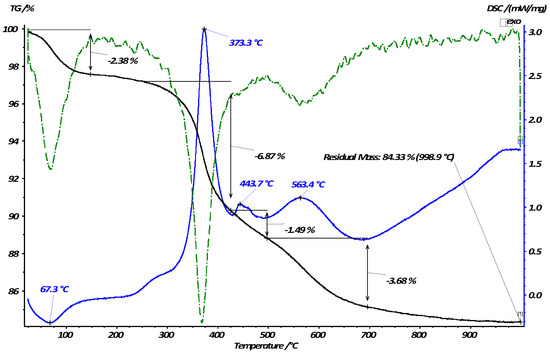
Figure 4.
Thermogravimetric curves of the modified silica (12) sample.
3.2. Distribution Coefficient
The efficiency of extraction for systems containing the modified silica 12 and Gd(III), Dy(III), Er(III), Eu(III) and Sm(III) was studied in a pH range of 3.5–5, which is limited, on the one hand, by the deactivation of the functional group through its protonation and, on the other hand, by hydrolytic processes with metal ions. The contribution of the nonmodified silica matrix, i.e., nonspecific sorption, to the extractive power was estimated by parallel experiments under similar conditions. The ratio between the volume of the liquid and solid phases upon solid-phase extraction under steady-state conditions was maintained at a value of 1:800; the initial concentrations of the rare earth elements were selected based on the functional capacity value of 0.3 mmol/g and maintaining an equimolar ratio between functional groups and metal ions (m (sorbent) = 50 mg, C of Gd(III), Dy(III), Er(III), Eu(III) and Sm(III) = 3.75 × 10–5 mol/L). The highest efficiency of extraction upon individual recovery for all studied metals was observed at pH 5 and their distribution coefficients are quite close in value, which suggests low-probability selectivity upon sorption from multicomponent systems under static conditions (Table 1). The distribution coefficients on the nonmodified surface for the same initial concentrations of Gd(III), Dy(III), Er(III), Eu(III) and Sm(III) and for each pH value of the aqueous phase varied around the zero value, which suggests a virtual absence of the contribution of nonspecific sorption due to hydroxocomplex formation.

Table 1.
Distribution coefficients of Gd(III), Dy(III), Er(III), Eu(III) and Sm(III) in their static individual preconcentrations (p = 0.95; n = 4).
The driving force of extraction when complexing sorbents under static conditions is the stability constant of a produced complex compound. As for liquid–liquid extraction, the distribution in the system can be changed by the addition of additional complexons, or synergetic agents, to the aqueous phase in order to obtain an adduct, which makes the energy of solvation lower to increase the efficiency of solid-phase extraction. We used 1,10-phenanthroline as the synergetic agent, which was added to the aqueous phase in an equimolar ratio with respect to the rare earth ions and in a two-fold molar excess. The calculated distribution coefficients throughout the chosen pH range of the aqueous phase upon the addition of the two-fold molar excess of 1,10-phenathroline with respect to the analytes are slightly higher (Table 2) than those obtained for similar systems free of this synergetic agent; at the same time, the pattern of dependence, as compared to that in the absence of the synergetic agent, and the closeness of the distribution coefficients’ values remained unchanged, which also suggests that the chosen group of REEs cannot be separated.

Table 2.
Distribution coefficients of Gd(III), Dy(III), Er(III), Eu(III) and Sm(III) in static preconcentrations with 1,10-phenathroline (p = 0.95; n = 4).
3.3. Dynamic Sorption
The selectivity of extraction upon recovery on complexing sorbents can be controlled by adding further complexons and by changing the conditional stability constant or changing the mode of extraction itself, static or dynamic, which allows one to take into account the complexation of the adduct on the silica’s surface.
Dynamic breakthrough curves are one of the most important tools used to study the kinetic aspects of ion exchange. We obtained dynamic elution curves as plots of the ratio of the determined Gd(III), Dy(III), Er(III), Eu(III) and Sm(III) preconcentrations in the effluent to their initial value versus the passed solution’s volume.
The method of frontal dynamic curves under dynamic conditions was used to obtain the extraction curves of Gd(III), Dy(III), Er(III), Eu(III) and Sm(III) for both their individual presence (C0(Gd(III)) = C0(Dy(III)) = C0(Er(III)) = C0(Eu(III)) = C0(Sm(III)) = 2.5 × 10−5 mol/L, V = 50 mL, and the rate of the solution’s passage was 1.25 mL/min) and presence in a solution containing all REE ions in an equimolar ratio.
The resulting plots have a typical S shape, which suggests relatively high kinetic parameters of mass transfer in the given system. The obtained curves were described by the equations used for linear frontal chromatography [52]. The distribution coefficients (Kd) were calculated from the obtained dynamic curves. For each i-th fraction of the passed solution (corresponding to one point in the dynamic elution curve), the total amount of the sorbed Me(III), where Me(III) is Gd(III), Dy(III), Er(III), Eu(III), or Sm(III), was calculated using the following equation:
where C0(Me(III)) is the initial concentration of Gd(III), Dy(III), Er(III), Eu(III), or Sm(III); Ci(Me(III)) is the concentration of Gd(III), Dy(III), Er(III), Eu(III), or Sm(III) in the i-th fraction; and Vi fraction is the volume of the fraction.
ni (Me(III)) = (C0(Me(III)) − Ci(Me(III)) × Vi fraction
The distribution coefficient was estimated at the point where Ci(Me(III)) = 0.5 C0(Me(III)):
where Vr is the retention volume corresponding to Ci(Me(III)) = 0.5 C0(Me(III)).
Kd = Vr/m (sorbent)
Based on the distribution coefficients calculated from the frontal dynamic curves under conditions of the individual extraction of REEs, we can arrange the ions in terms of their affinity to the modified surface as follows: Er(III) > Dy(III)> Sm(III) ~ Eu(III) ~ Gd(III) (Table 3), which shows that, under dynamic conditions, REE ions can be divided into two groups.

Table 3.
Dynamic distribution coefficients of Gd(III), Dy(III), Er(III), Eu(III) and Sm(III) in their individual preconcentrations (p = 0.95; n = 4).
Taking into account the obtained data for individual systems, a five-component system containing Gd(III), Dy(III), Er(III), Eu(III) and Sm(III) in equimolar amounts was simulated and frontal dynamic curves were plotted (Figure 5). While the calculated distribution coefficients suggest a mutual competitive effect of REE ions, it remains possible to separate the REE ions into groups: Er(III) ~ Dy(III) > Sm(III) ~ Eu(III) ~ Gd(III) (Table 4).
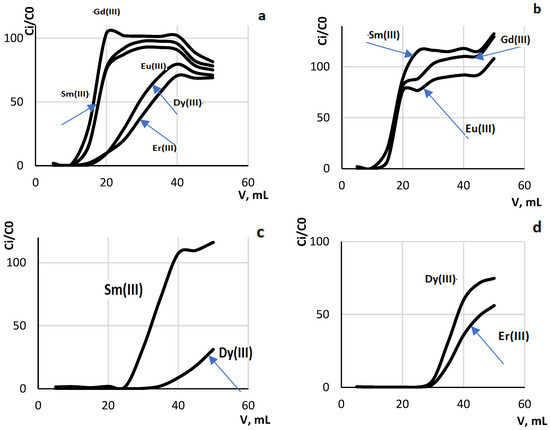
Figure 5.
Dynamic breakthrough curves of multicomponent system with V = 50 mL and rate of solution of passage 1.25 mL/min, m(silica (12) = 0.2 g ((a)– (C0(Gd(III)) = C0(Dy(III)) = C0(Er(III)) = C0(Eu(III)) = C0(Sm(III)) = 2.5 × 10−5 mol/L; (b) (C0(Gd(III)) = C0(Eu(III)) = C0(Sm(III)) = 2.5 × 10−5 mol/L; (c) Dy(III)) = C0(Sm(III)) = 2.5 × 10−5 mol/L; (d) C0(Dy(III)) = C0(Er(III)) = 2.5 × 10−5 mol/L).

Table 4.
Dynamic distribution coefficients of Gd(III), Dy(III), Er(III), Eu(III) and Sm(III) in five-component system’s preconcentration (p = 0.95; n = 4).
Relying on the obtained values of the distribution coefficients for the five-component system, which allow us to distinguish two groups of ions, we simulated three additional systems (preserving the equimolar ratio between the components): two systems contained ions with close distribution coefficients, Er(III) + Dy(III) and Sm(III) + Eu(III) + Gd(III), and one system contained ions with different distribution coefficients, Dy(III) + Sm(III).
As follows from the given values of the distribution coefficients Table 5, within systems with close Kd values, quantitative separation between the Er(III) + Dy(III) and Sm(III) + Eu(III) + Gd(III) systems cannot be achieved using a cartridge packed with the modified silica and a solution flow rate of 1.25 mL/min. For the system where Kd in > 1.8 Dy(III) + Sm(III), quantitative separation can be performed using a dynamic mode of sorption under selected conditions.

Table 5.
Dynamic distribution coefficients of Gd(III), Dy(III), Er(III), Eu(III) and Sm(III) in preconcentration of three-component and two-component systems (p = 0.95; n = 4) ((a)—three-component system C0(Gd(III)) = C0(Eu(III)) = C0(Sm(III)) = 2.5 × 10−5 mol/L; (b)—two-component system Dy(III)) = C0(Sm(III)) = 2.5 × 10−5 mol/L; (c)—two-component system C0(Dy(III)) = C0(Er(III)) = 2.5 × 10−5 mol/L).
4. Conclusions
We suggest a strategy for obtaining silica with covalently immobilized 4-acylpyrazolone fragments based on the use of alkylating agents that form stable quaternary salts upon interaction with the surface azole groups of modified silica. The synthesized organomineral material with an immobilized ionic liquid containing acylpyrazolone moieties as a further chelating agent was characterized using the data from IR, 13C NMR spectroscopy and thermal gravimetric analyses. This material is proposed for the extraction, separation and preconcentration of Gd(III), Dy(III), Er(III), Eu(III) and Sm(III) from multicomponent systems. The contribution of competitive sorption upon their extraction from multicomponent systems under dynamic conditions is shown. Using the method of frontal dynamic elution curves, inflow curves were obtained and distribution coefficients were calculated. It was shown that both group extraction and individual separation in systems containing Gd(III), Dy(III), Er(III), Eu(III) and Sm(III) in equimolar amounts can be performed.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/chemistry6050066/s1, Figure S1: IR spectrum of unmodified silica gel.
Author Contributions
Conceptualization, D.N.K. and V.V.K.; methodology, D.N.K. and V.V.K.; software, D.N.K. and V.V.K.; validation, D.N.K., I.A.L. and V.V.K.; formal analysis, D.N.K., I.A.L. and V.V.K.; investigation, D.N.K., I.A.L. and V.V.K.; resources, D.N.K., I.A.L. and V.V.K.; data curation, D.N.K., I.A.L. and V.V.K.; writing—original draft preparation, D.N.K. and V.V.K.; writing—review and editing, D.N.K. and V.V.K.; visualization, D.N.K. and V.V.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Higher Education of the Russian Federation, project no. FZEN-2023-0006.
Data Availability Statement
The data presented in this study are available in this article and Supplementary Materials.
Acknowledgments
This research was performed using the equipment of the Center for Joint Use, the “Ecological and Analytical Center” (KubSU).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Vygodskii, Y.S.; Lozinskaya, E.I.; Shaplov, A.S. Ionic Liquids as Novel Reaction Media for the Synthesis of Condensation Polymers. Macromol. Rapid Commun. 2002, 12, 678–680. [Google Scholar] [CrossRef]
- Yoshimitsu, H.; Kanazawa, A.; Kanaoka, S.; Aoshima, S. Cationic polymerization of vinyl ethers with alkyl or ionic side groups in ionic liquids. J. Polym. Sci. Part A Polym. Chem. 2016, 12, 1774–1784. [Google Scholar] [CrossRef]
- Pletnev, I.V.; Smirnova, S.V.; Sharov, A.V.; Zolotov, Y.A. New generation extraction solvents: From ionic liquids and aqueous biphasic systems to deep eutectic solvents. Russ. Chem. Rev. 2021, 90, 1109–1141. [Google Scholar] [CrossRef]
- Pletnev, I.V.; Smirnova, S.V.; Shvedene, N.V. New Directions in Using Ionic Liquids in Analytical Chemistry. 1: Liquid–Liquid Extraction. J. Anal. Chem. 2019, 74, 625–658. [Google Scholar] [CrossRef]
- Huddleston, J.G.; Willauer, H.D.; Swatloski, R.P.; Visser, A.E.; Rogers, R.D. Room temperature ionic liquids as novel media for ‘clean’ liquid-liquid extraction. Chem. Commun. 1998, 16, 1765–1766. [Google Scholar] [CrossRef]
- Visser, A.E.; Swatloski, R.P.; Reichert, W.M.; Griffin, S.T.; Rogers, R.D. Traditional extractants in nontraditional solvents: Groups 1 and 2 extraction by crown ethers in room-temperature ionic liquids. Ind. Eng. Chem. Res. 2000, 39, 3596–3604. [Google Scholar] [CrossRef]
- Smirnova, S.V.; Pletnev, I.V. New Ionic Liquids for Extraction Preconcentration. J. Anal. Chem. 2019, 74, 1–11. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, J.; Wang, H.; Wang, K.; Tian, Y.; Xue, X.; Qiao, Y.; Ji, X.; Zhang, S. Ionic liquids as a new cornerstone to support hydrogen energy. Green Chem. 2023, 25, 4981–4994. [Google Scholar] [CrossRef]
- Romanovsky, B.V.; Tarkhanova, I.G. Supported ionic liquids in catalysis. Russ. Chem. Rev. 2017, 86, 444–458. [Google Scholar] [CrossRef]
- Shashkov, M.V.; Sidelnikov, V.N.; Parmon, V.N. Ionic Liquids—New Gas Chromatographic Phases with Unique Properties. A Review. Dokl. Phys. Chem. 2023, 508, 1–16. [Google Scholar] [CrossRef]
- Wolny, A.; Chrobok, A. Silica-Based Supported Ionic Liquid-like Phases as Heterogeneous Catalysts. Molecules 2022, 27, 5900. [Google Scholar] [CrossRef] [PubMed]
- Yavir, K.; Konieczna, K.; Marcinkowski, L.; Kloskowski, A. Ionic liquids in the microextraction techniques: The influence of ILs structure and properties. Trends Anal. Chem. 2020, 130, 115994. [Google Scholar] [CrossRef]
- Lebedeva, O.; Kultin, D.; Kustov, L. Advanced research and prospects on polymer ionic liquids: Trends, potential and application. Green Chem. 2023, 25, 9001–9019. [Google Scholar] [CrossRef]
- Qader, I.B.; Prasad, K. Recent Developments on Ionic Liquids and Deep Eutectic Solvents for Drug Delivery Applications. Pharm. Res. 2022, 39, 2367–2377. [Google Scholar] [CrossRef]
- Harjani, J.R.; Friščić, T.; MacGillivray, L.R.; Singer, R.D. Removal of metal ions from aqueous solutions using chelating task-specific ionic liquids. Dalton Trans. 2008, 34, 4595–4601. [Google Scholar] [CrossRef]
- Sosa, F.H.B.; Carvalho, P.J.; Coutinho, J.A.P. Preconcentration of Superbase Ionic Liquid from Aqueous Solution by Membrane Filtration. Ind. Eng. Chem. Res. 2022, 61, 14626–14636. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Huynh, T.T.T.; Nguyen, N.H.; Nguyen, T.H.; Tran, P.H. Recent advances in the application of ionic liquid-modified silica gel in solid-phase extraction. J. Mol. Liq. 2022, 368, 120623. [Google Scholar] [CrossRef]
- Khezeli, T.; Ghaedi, M.; Daneshfar, A.; Bahrani, S.; Asfaram, A.; Soylak, M. Chapter 6—Ionic liquids in separation and preconcentration of organic and inorganic species. In New Generation Green Solvents for Separation and Preconcentration of Organic and Inorganic Species; Elsevier: Amsterdam, The Netherlands, 2020; pp. 267–318. [Google Scholar] [CrossRef]
- Zazybin, A.G.; Rafikova, K.Y.; Zolotareva, V.; Dembitsky, D.; Sasaki, V.M. Metal-containing ionic liquids: Current paradigm and applications. Russ. Chem. Rev. 2017, 86, 1254–1270. [Google Scholar] [CrossRef]
- Shirzaei, F.; Shaterian, H.R. Preparation of novel functionalized ionic liquid: Green, stable, and reusable catalyst for the synthesis of new 2-(phenylsulfonyl)-1H-benzo[a]pyrano[2,3-c]phenazin-3-amine derivatives. J. Mol. Liq. 2022, 345, 117893. [Google Scholar] [CrossRef]
- Farzam, S.F.; Shemirani, F.; Karimi, S. Synthesis of imidazolium ionic liquid immobilized on magnetic mesoporous silica: A sorbent material in a green micro-solid phase extraction of multiclass pesticides in water. Talanta 2024, 272, 125744. [Google Scholar] [CrossRef]
- Jahanbakhshi, A.; Eskandaria, S.; Farahi, M. A novel ionic liquid immobilized on FSM-16: A novel recoverable nanocatalyst for the synthesis of biscoumarin derivatives. New J. Chem. 2024, 48, 1381–1389. [Google Scholar] [CrossRef]
- Shang, D.; Liu, X.; Bai, L.; Zeng, S.; Xu, Q.; Gao, H.; Zhang, X. Ionic liquids in gas separation processing. Curr. Opin. Green Sustain. Chem. 2017, 5, 74–81. [Google Scholar] [CrossRef]
- Muhammad, S.; Javed, M.N.; Gill, K.A.; Ali, F.I.; Henderson, W.; Bari, A.; Musharraf, S.G.; Baig, J.A.; Hashmi, I.A. Selective extraction of heavy metals (Fe, Co, Ni) from their aqueous mixtures by Task-Specific salicylate functionalized imidazolium based ionic liquid. J. Clean. Prod. 2022, 344, 131119. [Google Scholar] [CrossRef]
- Taib, L.A.; Keshavarz, M. Non-covalent immobilized sultone based protic ionic liquids on nano silica sulfuric acid as novel heterogeneous catalysts for the efficient synthesis of tetrazolopyrimidines. J. Porous Mater. 2023, 30, 565–578. [Google Scholar] [CrossRef]
- Marchetti, F.; Pettinari, C.; Pettinari, R. Acylpyrazolone ligands: Synthesis, structures, metal coordination chemistry and applications. Coord. Chem. Rev. 2005, 249, 2909–2945. [Google Scholar] [CrossRef]
- Marchetti, F.; Pettinari, C.; Pettinari, R. Recent advances in acylpyrazolone metal complexes and their potential applications. Coord. Chem. Rev. 2015, 303, 1–31. [Google Scholar] [CrossRef]
- Mies, T.; White, A.J.P.; Rzepa, H.S.; Barluzzi, L.; Devgan, M.; Layfield, R.A.; Barrett, A.G.M. Syntheses and Characterization of Main Group, Transition Metal, Lanthanide, and Actinide Complexes of Bidentate Acylpyrazolone Ligands. Inorg. Chem. 2023, 62, 13253–13276. [Google Scholar] [CrossRef]
- Polikovskiy, T.; Korshunov, V.; Gontcharenko, V.; Kiskin, M.; Belousov, Y.; Pettinari, C.; Taydakov, I. Dynamics of the Ligand Excited States Relaxation in Novel β-Diketonates of Non-Luminescent Trivalent Metal Ions. Int. J. Mol. Sci. 2023, 24, 8131. [Google Scholar] [CrossRef]
- Di Nicola, C.; Marchetti, F.; Pettinari, R.; Tombesi, A.; Pettinari, C.; Grappasonni, I.; Dyson, P.J.; Scuri, S. Tethering (Arene)Ru(II) Acylpyrazolones Decorated with Long Aliphatic Chains to Polystyrene Surfaces Provides Potent Antibacterial Plastics. Materials 2020, 13, 526. [Google Scholar] [CrossRef]
- Umetani, S.; Kawase, Y.; Le, Q.T.H.; Matsuia, M. Acylpyrazolone derivatives of high selectivity for lanthanide metal ions: Effect of the distance between the two donating oxygens. J. Chem. Soc. Dalton Trans. 2000, 16, 2787–2791. [Google Scholar] [CrossRef]
- Liu, T.; Chen, J. Extraction and separation of heavy rare earth elements: A review. Sep. Purif. Technol. 2021, 276, 119263. [Google Scholar] [CrossRef]
- Sharifian, S.; Wang, N.-H.L. Resin-based approaches for selective extraction and purification of rare earth elements: A comprehensive review. J. Environ. Chem. Eng. 2024, 12, 112402. [Google Scholar] [CrossRef]
- Fisher, A.; Kara, D. Determination of rare earth elements in natural water samples—A review of sample separation, preconcentration and direct methodologies. Anal. Chim. Acta 2016, 935, 1–29. [Google Scholar] [CrossRef]
- Dam, H.H.; Reinhoudt, D.N.; Verboom, W. Multicoordinate ligands for actinide/lanthanide separations. Chem. Soc. Rev. 2007, 36, 367–377. [Google Scholar] [CrossRef]
- Atanassova, M.; Kurteva, V.; Lubenov, L.; Billard, I. Solvent extraction and separation of light lanthanoids with mixtures of two chelating extractants: Benzene vs. ionic liquid. Sep. Sci. Technol. 2016, 51, 290–299. [Google Scholar] [CrossRef]
- Zeng, Z.; Gao, Y.; Liu, C.; Sun, X. A novel functionalized ionic liquid [DOC4mim][DEHG] for impurity removal of aluminum in rare earth leaching solution. Sep. Sci. Technol. 2022, 296, 121388. [Google Scholar] [CrossRef]
- Mishra, B.B.; Devi, N.; Sarangi, K. Solvent extraction and separation of samarium from transition and rare-earth metals using phosphonium ionic liquid Cyphos IL 104. Monatsh. Chem. 2021, 152, 767–775. [Google Scholar] [CrossRef]
- Ouardi, Y.E.; Virolainen, S.; Mouele, E.S.M.; Laatikainen, M.; Repo, E.; Laatikainen, K. The recent progress of ion exchange for the separation of rare earths from secondary resources—A review. Hydrometallurgy 2023, 218, 106047. [Google Scholar] [CrossRef]
- Wilfong, W.C.; Ji, T.; Duan, Y.; Shi, F.; Wang, Q.; Gray, M.L. Critical review of functionalized silica sorbent strategies for selective extraction of rare earth elements from acid mine drainage. J. Hazard. Mater. 2022, 424, 127625. [Google Scholar] [CrossRef]
- Ehrlich, G.V.; Lisichkin, G.V. Sorption in the Chemistry of Rare Earth Elements. Russ. J. Gen. Chem. 2017, 87, 1220–1245. [Google Scholar] [CrossRef]
- Ouargli, R.; Hamacha, R.; Benharrats, N.; Boos, A.; Bengueddach, A. β-Diketone functionalized SBA-15 and SBA-16 for rapid liquid–solid extraction of copper. J. Porous Mater. 2015, 22, 511–520. [Google Scholar] [CrossRef]
- Boos, A.; Brunette, J.-P.; Leroy, M.J.F. Surfactant-templated silica doped with 1-phenyl-3-methyl-4-stearoylpyrazol-5-one (HPMSP) as a new sorbent. J. Mater. Chem. 2002, 12, 886–889. [Google Scholar] [CrossRef]
- Bou-Maroun, E.; Goetz-Grandmont, G.J.; Boos, A. Solid-Liquid Extraction of Lanthanum(III), Europium(III), and Lutetium(III) by Acyl-Hydroxypyrazoles Entrapped in Mesostructured Silica. Sep. Sci. Technol. 2007, 42, 1913–1930. [Google Scholar] [CrossRef]
- Suhail, F.; Batool, M.; Din, M.I.; Khan, M.A.; Chotana, G.A.; Zubair, I.; Shah, A.T. Facile synthesis of hetaryl-modified MCM-41 and targeted removal of Pb(II) ions for water purification. J. Porous Mater. 2020, 27, 1491–1504. [Google Scholar] [CrossRef]
- Amarasekara, A.S.; Owereh, O.S.; Aghara, S.K. Synthesis of 4-acylpyrazolone Schiff base ligand grafted silica and selectivity in adsorption of lanthanides from aqueous solutions. J. Rare Earths 2009, 27, 870–874. [Google Scholar] [CrossRef]
- Savvin, S.B. Analytical use of arsenazo III: Determination of thorium, zirconium, uranium and rare earth elements. Talanta 1961, 8, 673–685. [Google Scholar] [CrossRef]
- Gu, R.; Lehn, J.-M. Constitutional Dynamic Selection at Low Reynolds Number in a Triple Dynamic System: Covalent Dynamic Adaptation Driven by Double Supramolecular Self-Assembly. J. Am. Chem. Soc. 2021, 143, 14136–14146. [Google Scholar] [CrossRef]
- Rama Rao, A.V.; Pulla Reddy, S.; Rajarathnam Reddy, E. Short and efficient syntheses of coriolic acid. J. Org. Chem. 1986, 51, 4158–4159. [Google Scholar] [CrossRef]
- El’kov, N.A.; Konshina, D.N.; Konshin, V.V. A Practical Synthesis of 1-[3-(Trimethoxysilyl)propyl]-1H-imidazole. Russ. J. Gen. Chem. 2023, 93, 909–911. [Google Scholar] [CrossRef]
- Jensen, B.S. The synthesis of 1-phenyl-3-methyl-4-acyl-pyrazolones-5. Acta Chem. Scand. 1959, 13, 1668–1670. [Google Scholar] [CrossRef]
- Golshan-Shirazi, S.; Guiochon, G. Modeling of preparative liquid chromatography. J. Chromatogr. A 1994, 658, 149–171. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).