Abstract
Diradicals have attracted the attention of chemists due to their unique electronic structures and properties originating from unpaired electrons. One of the fundamental motifs of diradicals is quinodimethane; p- and o-quinodimethanes are singlet Kekulé hydrocarbons, while m-quinodimethane is a triplet non-Kekulé hydrocarbon. Most of the hydrocarbon diradicals studied to date have been limited to p- and o-quinodimethane-based non-fused-ring and fused-ring open-shell singlet diradicals and m-quinodimethane-based non-fused-ring triplet diradicals. In this account, studies on m-quinodimethane-based fused-ring diradicals, including an open-shell singlet Kekulé hydrocarbon, an open-shell singlet zwitterion, non-Kekulé hydrocarbon-based triplet diradical and diradical cation, and a triplet Kekulé hydrocarbon, are summarized. They are designed, successfully synthesized, and isolated as crystals, and their fundamental electronic structures and properties have been elucidated by optical, electrochemical, and magnetic measurements, together with DFT calculations. A series of studies showed that controlling the interaction between the two unpaired electrons of m-quinodimethane through an appropriate molecular design would produce polycyclic diradicals with various open-shell singlet diradical characters and the energy differences between singlet and triplet states.
1. Introduction
Diradicals have attracted the attention of chemists as magnetic, electrochemical, and optical materials due to their unique electronic structure and physical properties derived from two unpaired electrons [1]. When the two unpaired electrons of a diradical interact, the electron spins become antiparallel due to the bonding interaction, resulting in a singlet ground state, or the electron spins become parallel due to the ferromagnetic interaction, resulting in a triplet ground state. Establishing a method to control these interactions is essential to create diradicals with different ground spin multiplicities, unique electronic states, and physical properties.
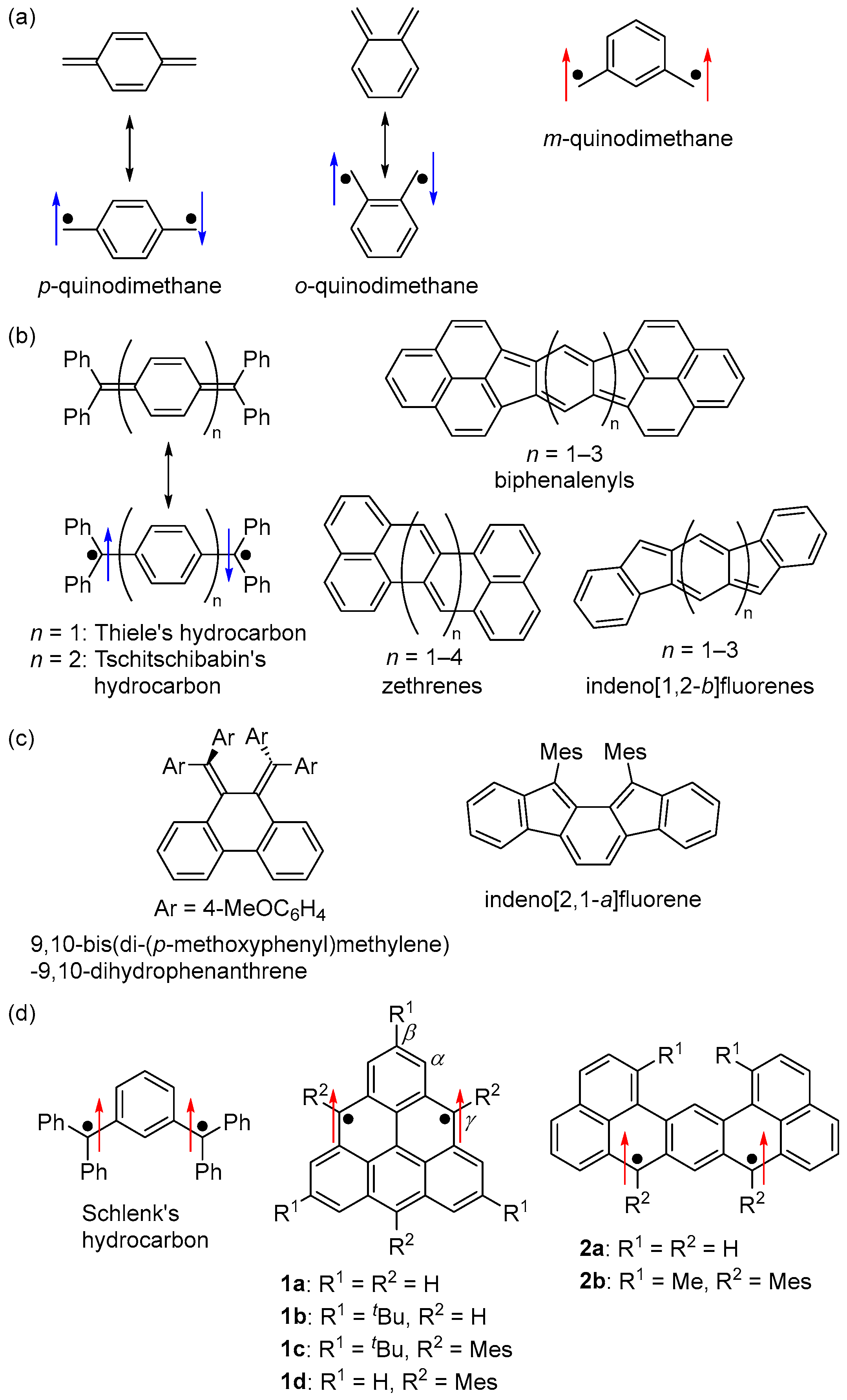
One of the fundamental diradical skeletons is quinodimethane (Figure 1a). p- and o-quinodimethanes are open-shell singlet Kekulé hydrocarbons, while m-quinodimethane is a triplet non-Kekulé hydrocarbon. Most open-shell singlet hydrocarbon diradicals are p-quinodimethane-based Kekulé hydrocarbons, described as the resonance of the closed-shell and diradical structures (Figure 1b) [2,3,4,5,6,7,8,9]. A variety of studies have been conducted on hydrocarbon diradicals, spanning from non-fused-ring hydrocarbons, including Thiele’s hydrocarbons [10] and Tschitschibabin’s hydrocarbons [11,12], to fused-ring hydrocarbons, including biphenalenyls [13,14,15,16,17], zethrenes [18,19,20,21,22,23,24], and indeno [1,2-b]fluorenes [25,26,27,28]. The scope of study also extends to o-quinodimethane-based open-shell singlet Kekulé hydrocarbons (Figure 1c), such as 9,10-bis(di-(p-methoxyphenyl)methylene)-9,10-dihydrophenanthrene [29] and indeno[2,1-a]fluorene derivatives [30], serving as notable examples of non-fused-ring and fused-ring hydrocarbons, respectively.
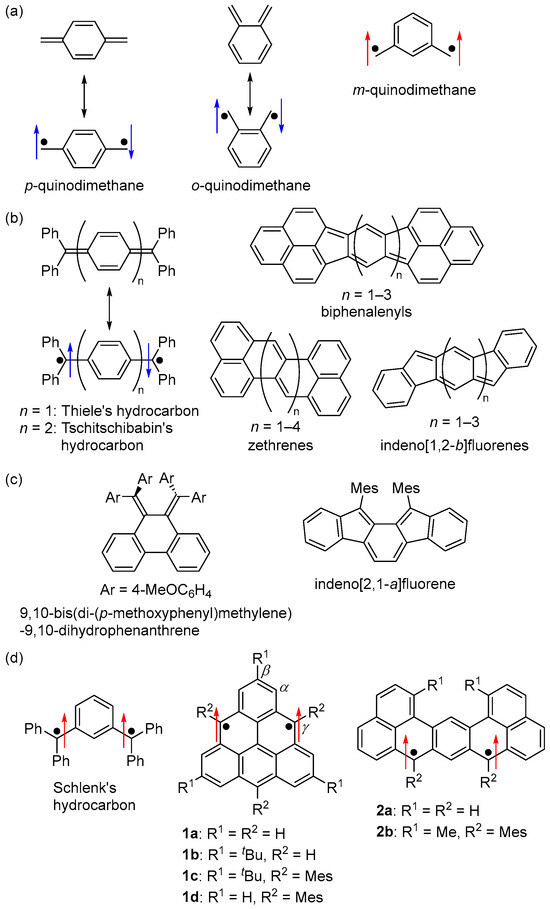
Figure 1.
Structures of (a) quinodimethanes, (b) p-quinodimethane-based and (c) o-quinodimethane-based open-shell singlet hydrocarbons, and (d) m-quinodimethane-based triplet hydrocarbons. Arrows indicate electron spin (blue and red colors are for singlet and triplet diradicals, respectively). Mes = 2,4,6-Me3C6H2.
On the other hand, m-quinodimethane-based triplet non-fused-ring non-Kekulé hydrocarbons have long been studied. Schlenk’s hydrocarbon [31,32], synthesized in 1915, is a typical example (Figure 1d). Rajca et al. synthesized and isolated a kinetically stabilized Schlenk’s hydrocarbon derivative [33,34]. Fused-ring m-quinodimethane-based triplet hydrocarbons, such as triangulene (1a) [35,36,37] and dibenzo[de,jk]pentacene (2a) [21,38], have also been studied (Figure 1d). However, due to their high reactivity and instability, these diradicals have not been isolated, nor have their physical properties been clarified in detail.
Based on these backgrounds, we focused on m-quinodimethane, considering that by controlling the magnitude of the interaction between the two unpaired electrons through appropriate molecular design, not only the energy difference between the singlet and triplet states (ΔEST) but also the ground spin multiplicity of diradicals could be controlled. Such spin multiplicity control is not applicable to p- and o-quinodimethane, which have singlet ground states. We designed and synthesized the following m-quinodimethane-based polycyclic diradicals: (1) an open-shell singlet Kekulé hydrocarbon, (2) an open-shell singlet zwitterion, (3) a non-Kekulé hydrocarbon-based triplet diradical and diradical cation, and (4) a triplet Kekulé hydrocarbon [39].
2. Open-Shell Singlet Kekulé Hydrocarbon
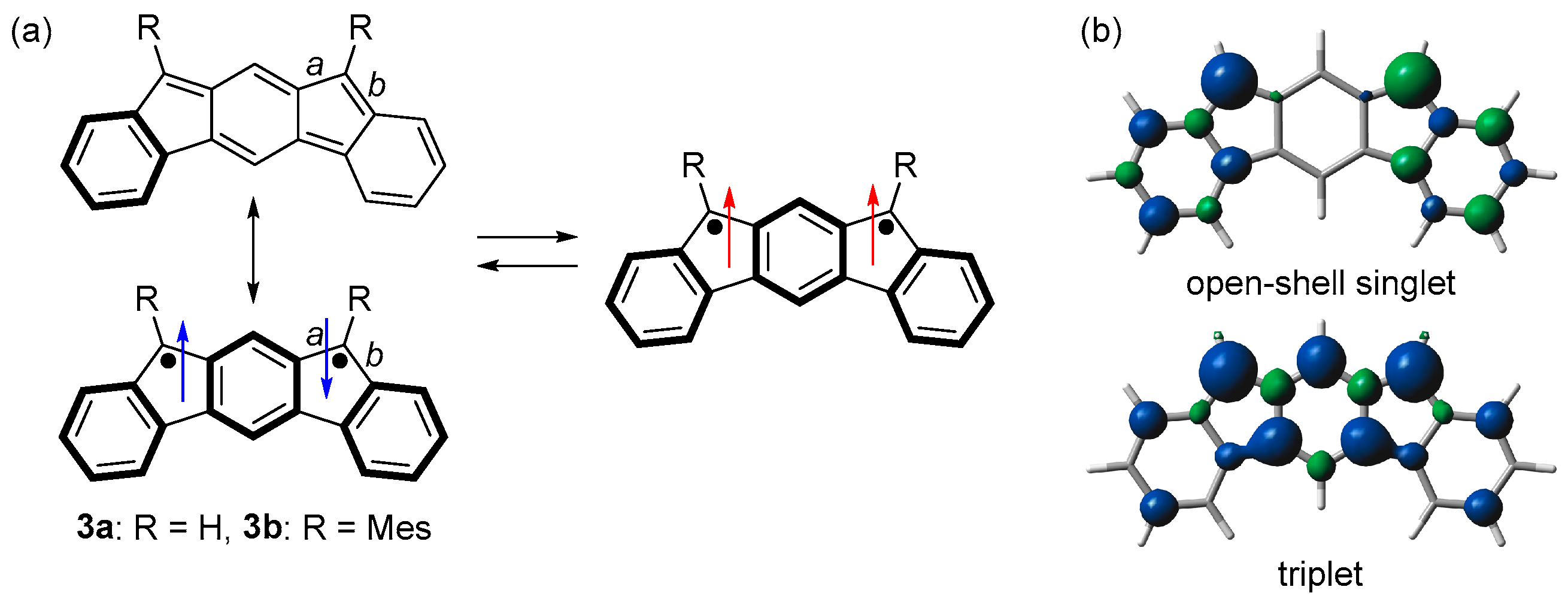
When we started this study in 2010, there was no study of m-quinodimethane-based open-shell singlet Kekulé hydrocarbons. We then designed indeno[2,1-b]fluorene (3a), an isomer of indenofluorenes (Figure 2a) [40]. Although no Kekulé structure can be drawn to m-quinodimethane, a Kekulé structure could be drawn to 3a. The singlet state of 3a is stabilized because of the bonding interaction between the two unpaired electrons through the outer benzene ring. Considering the resonance structures of 3a, the Kekulé structure is stabilized by the bond formation and one Clar’s aromatic sextet (benzene rings shown in the bold line), while the singlet diradical structure is stabilized by three Clar’s aromatic sextets. Therefore, 3a would comprise the contribution of both structures and have an open-shell singlet diradical character.
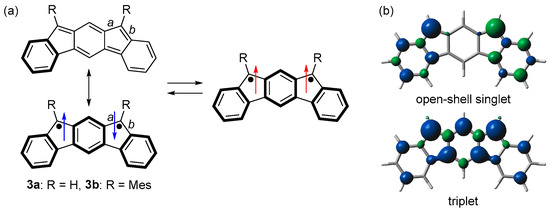
Figure 2.
(a) Resonance structures of 3. Arrows indicate electron spin (blue and red colors are for singlet and triplet diradicals, respectively). (b) Spin densities of open-shell singlet and triplet states of 3a. Blue and green surfaces represent α and β spin densities, respectively.
DFT calculations predicted that 3a has a singlet ground state with an open-shell singlet diradical character of 0.71 (LC-UBLYP/6-311G(d,p)//UB3LYP/6-311G(d,p)) and ΔEST of −11.3 kJ mol−1 (UB3LYP/6-311G(d,p)). The spin density distributions were predicted to be similar to those of the two benzyl radicals in the singlet state and m-quinodimethane in the triplet state (Figure 2b). Unlike p- and o-quinodimethane-based open-shell singlet Kekulé hydrocarbons, the spin density distributions of 3a in the singlet and triplet states are quite different because the triplet state is stabilized by m-quinodimethane moiety.
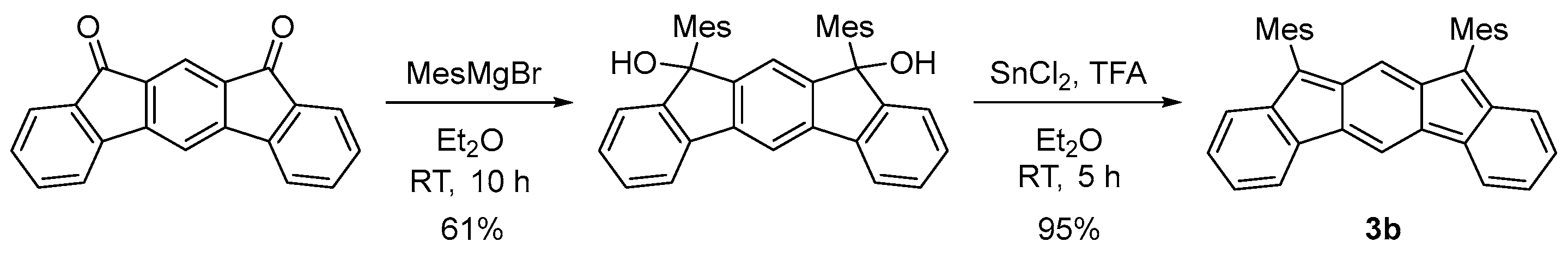
We designed and synthesized a derivative 3b by introducing mesityl groups for kinetic stabilization (Scheme 1 and Figure 2a). 3b was found to be stable enough to be purified by column chromatography. Single crystals of 3b were obtained by recrystallization from acetonitrile solution in a degassed sealed tube (Figure 3a). Bonds a (1.437 Å) and b (1.431 Å) are longer than the C(sp2)–C(sp2) bond of benzene (1.39 Å), indicating the contribution of the diradical structure in addition to the Kekulé structure (Figure 2a).
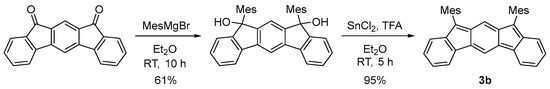
Scheme 1.
Synthesis of 3b.
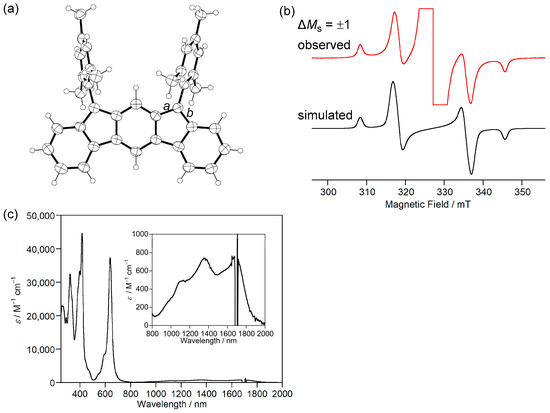
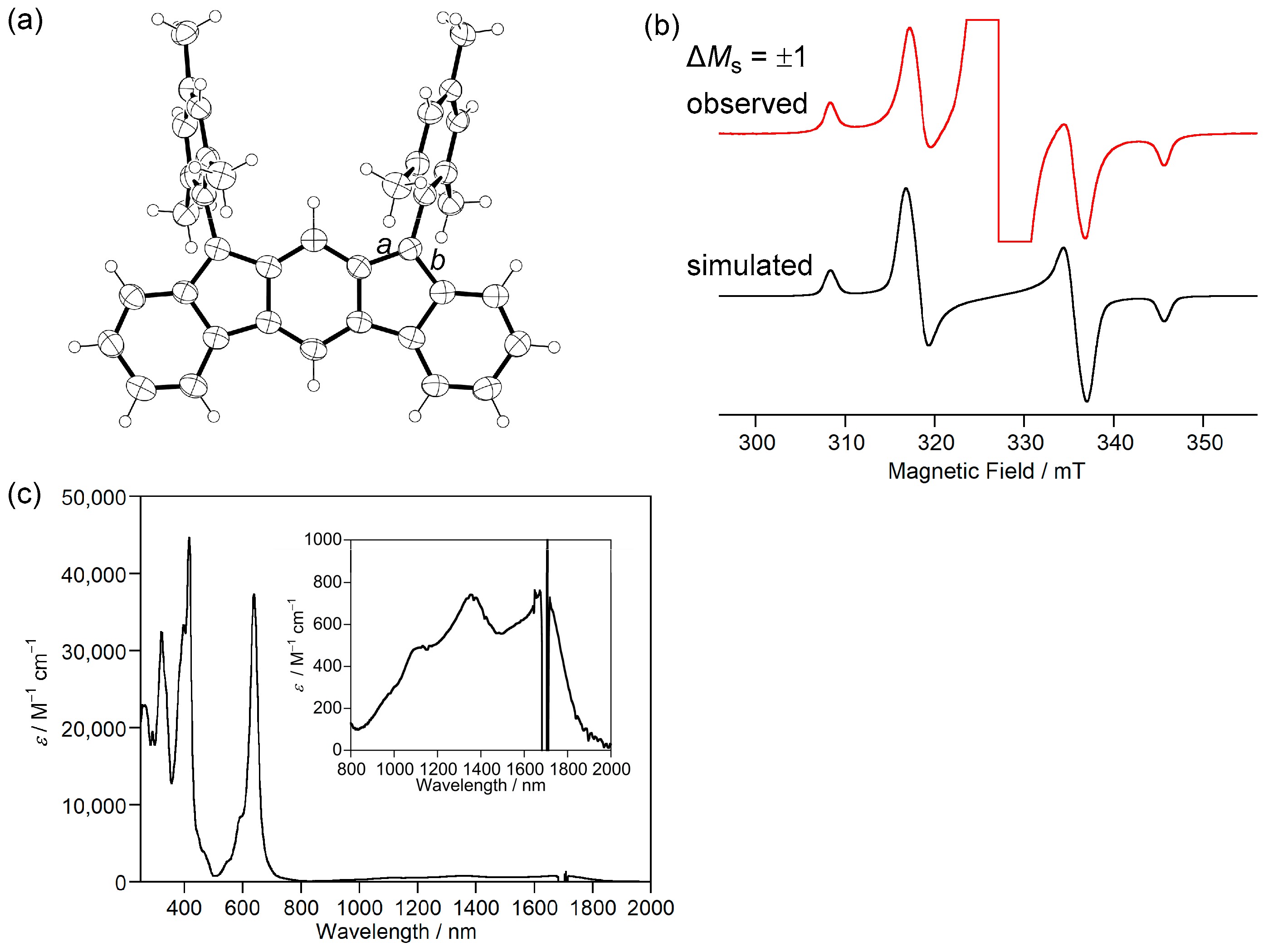
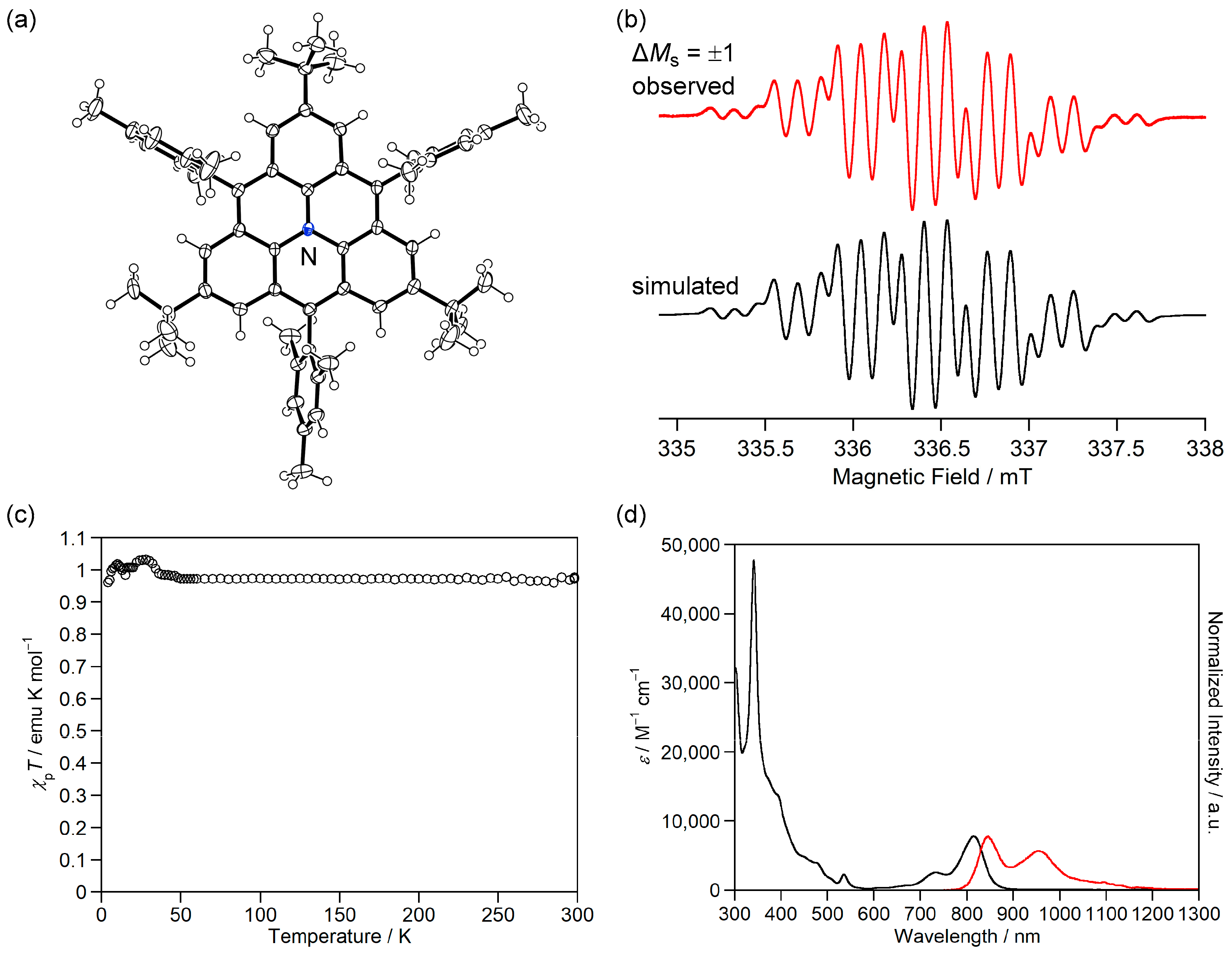
Figure 3.
(a) ORTEP drawing of 3b. Displacement ellipsoids are drawn at the 50% probability level. (b) ESR spectrum of 3b. (c) UV-Vis-NIR absorption spectra of 3b in CH2Cl2. Reprinted with permission from Ref. [40], Wiley-VCH, 2013.
ESR measurements showed a signal with a |D| value of 0.017 cm−1 originating from the thermally excited triplet state of 3b (Figure 3b). Due to the small energy difference between HOMO and LUMO, 3b exhibits absorption in the near-infrared (NIR) region of ca. 1700 nm in CH2Cl2 (Figure 3c), although 3b has only 20 π electrons in its main core structure. 3b is the first example of a m-quinodimethane-based open-shell singlet Kekulé hydrocarbon. This study introduced new aspects of open-shell singlet Kekulé hydrocarbon and led to the study of fluoreno[2,3-b]fluorene [41] and dianthraceno[2,3-a:3′,2′-h]-s-indacene [42].
3. Open-Shell Singlet Zwitterion
In contrast to the numerous studies of open-shell singlet Kekulé hydrocarbons, few studies were conducted on the open-shell singlet zwitterions, which were described as the resonance of the zwitterionic and diradical structures. This means that many aspects of their electronic structures and physical properties are unresolved [43,44].
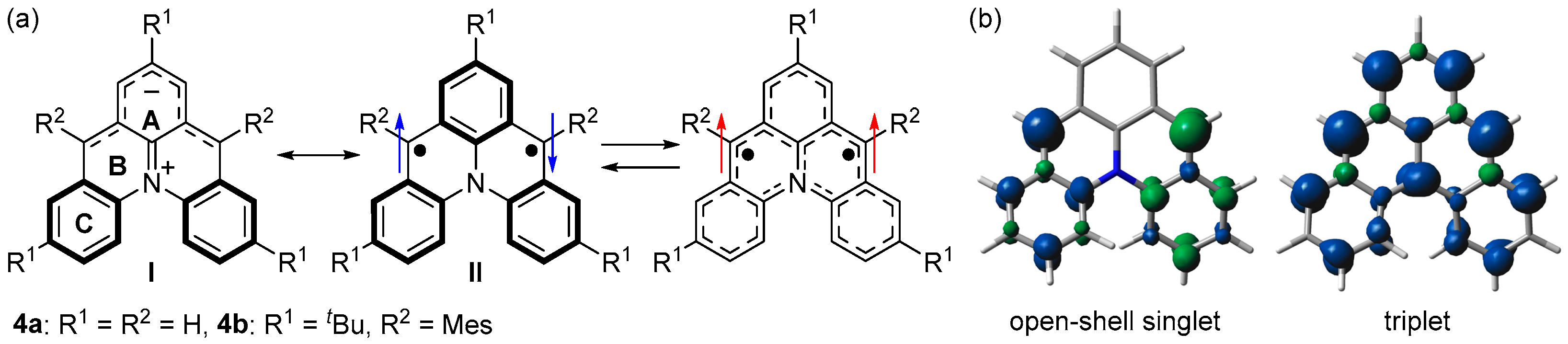
We designed azoniadibenzo[a,j]phenalenide (4a), in which m-quinodimethane is bridged by diphenylamine (Figure 4a) [45]. DFT calculations predicted that 4a has a singlet ground state with an open-shell singlet diradical character of 0.57 (LC-UBLYP/6-311G(d,p)//UB3LYP/6-311G(d,p)) and ΔEST of −24.0 kJ mol−1 (UB3LYP/6-311G(d,p)). The spin density distribution was predicted to be similar to that of 3a; spins delocalize over two benzyl radicals in the singlet state and m-quinodimethane moiety in the triplet state (Figure 4b).
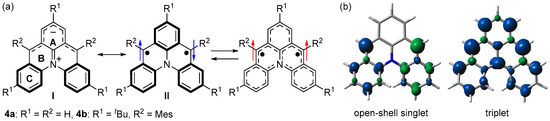
Figure 4.
(a) Resonance structures of 4. Arrows indicate electron spin (blue and red colors are for singlet and triplet diradicals, respectively). (b) Spin densities of open-shell singlet and triplet states of 4a. Blue and green surfaces represent α and β spin densities, respectively.
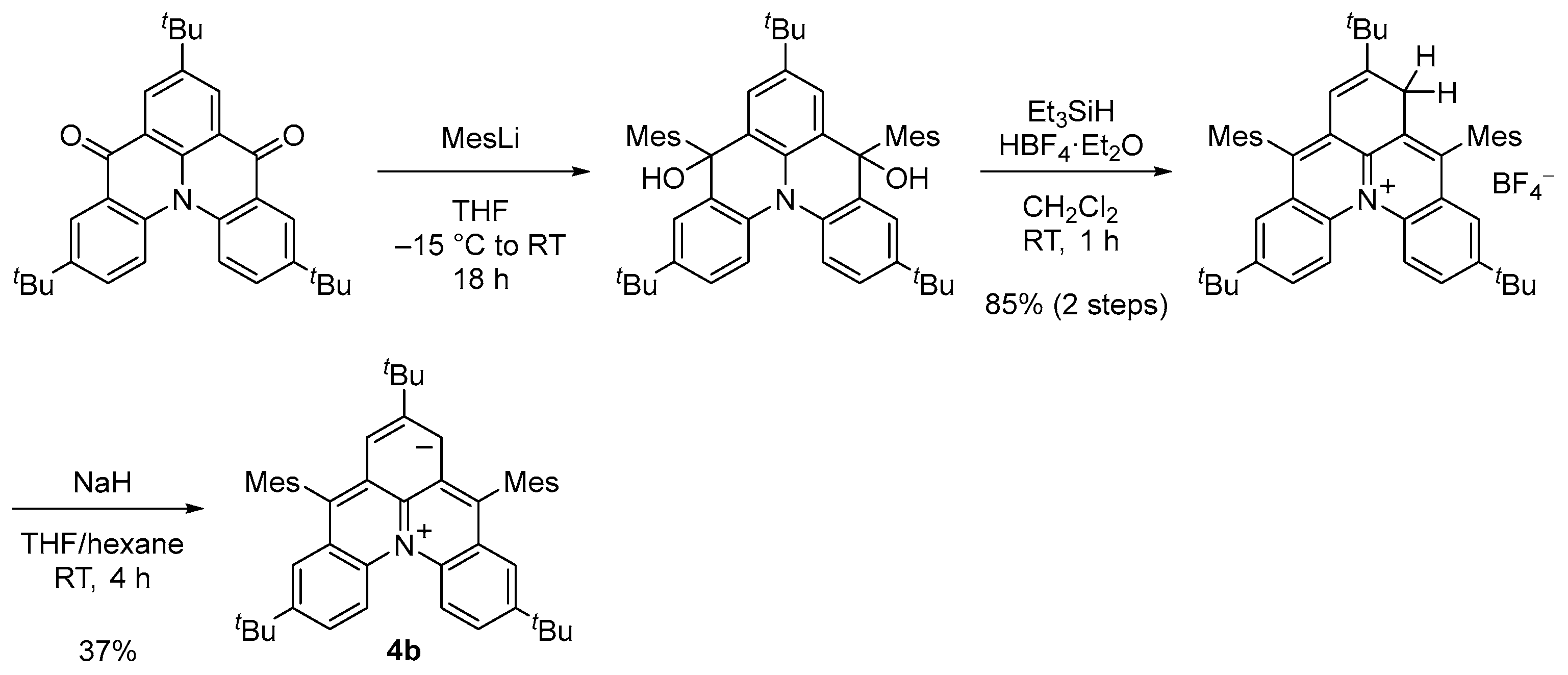
We designed and synthesized a derivative 4b with tert-butyl and mesityl groups for kinetic stabilization (Scheme 2, Figure 4a). Single crystals of 4b were obtained by recrystallization from tetrahydrofuran–hexane solution in a degassed sealed tube (Figure 5a). Unlike the open-shell singlet Kekulé hydrocarbons such as 3b, there was no significant difference in the bond length between the singlet ground state and the thermally excited triplet state of 4b (Figure 5a), and thus, its open-shell singlet diradical character could not be clarified based on the bond length.
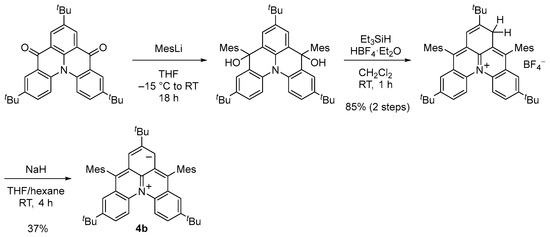
Scheme 2.
Synthesis of 4b.
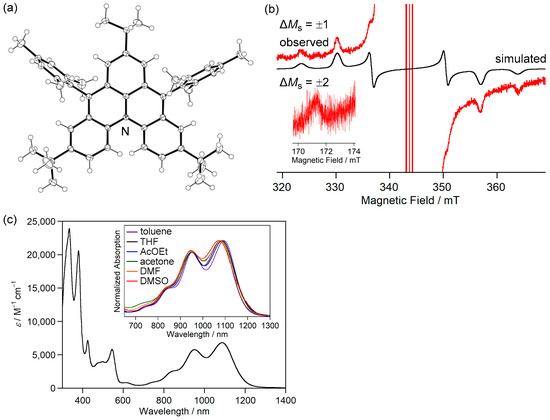
Figure 5.
(a) ORTEP drawing of 4b. Displacement ellipsoids are drawn at the 50% probability level. (b) ESR spectra of 4b at 470 K. (c) UV-Vis-NIR spectra of 4b in THF. Reprinted with permission from Ref. [45], Wiley-VCH, 2019.
To clarify the electronic structure, we focused on aromaticity. HOMA (Harmonic Oscillator Model of Aromaticity) values [46,47], Nucleus-Independent Chemical Shift (NICS) values [48,49,50], and Anisotropy of the Induced Current Density (ACID) plot [51,52] showed that rings A and C are aromatic, while ring B is non-aromatic. The chemical shifts of the 1H NMR spectra revealed that the negative charge is delocalized over the upper part of 4b, indicating the contribution of the zwitterionic structure I (Figure 4a).
ESR measurements were conducted to experimentally clarify the open-shell singlet diradical character of 4b. An ESR signal with a |D| value of 0.019 cm−1 originating from the thermally excited triplet state of 4b was observed (Figure 5b). The ΔEST was determined to be −36.4 kJ mol−1 by variable-temperature ESR spectra. These results showed that 4b is described as the resonance of the zwitterionic structure I and diradical structure II (Figure 4a).
4b showed an absorption band in the NIR region of 1086 nm in tetrahydrofuran, indicating a small energy difference between HOMO and LUMO (Figure 5c). Despite the contribution of the zwitterionic structure, the absorption wavelength did not significantly depend on the polarity of solvents, probably because of the small dipole moment. Therefore, open-shell singlet zwitterions with large dipole moments are required to respond to various external stimuli. A careful molecular design is required because the zwitterionic structure significantly stabilizes the singlet state, as observed in the study of bis-periazulene (cyclohepta[def]fluorene) [53].
4. Non-Kekulé Hydrocarbon-Based Triplet Diradical and Diradical Cation
The first attempted synthesis of triangulene (1a), a fused-ring triplet non-Kekulé hydrocarbon, was conducted by Clar et al. in 1953, but 1a was highly reactive and polymerized at low temperatures [35,36]. A kinetically stabilized derivative 1b was designed and generated by Nakasuji et al. in 2001, and its triplet ground state was experimentally confirmed [37]. However, 1b was not isolated due to its high reactivity. Recently, many unstable triplet diradicals have been generated on metal surfaces and observed using STM and AFM. 1a and 2a were reported by Gross et al. in 2017 [54] and Wang et al. in 2020 [55], respectively. However, due to the influence of metals, the fundamental optical, electrochemical, and magnetic properties of these radicals have not been elucidated.
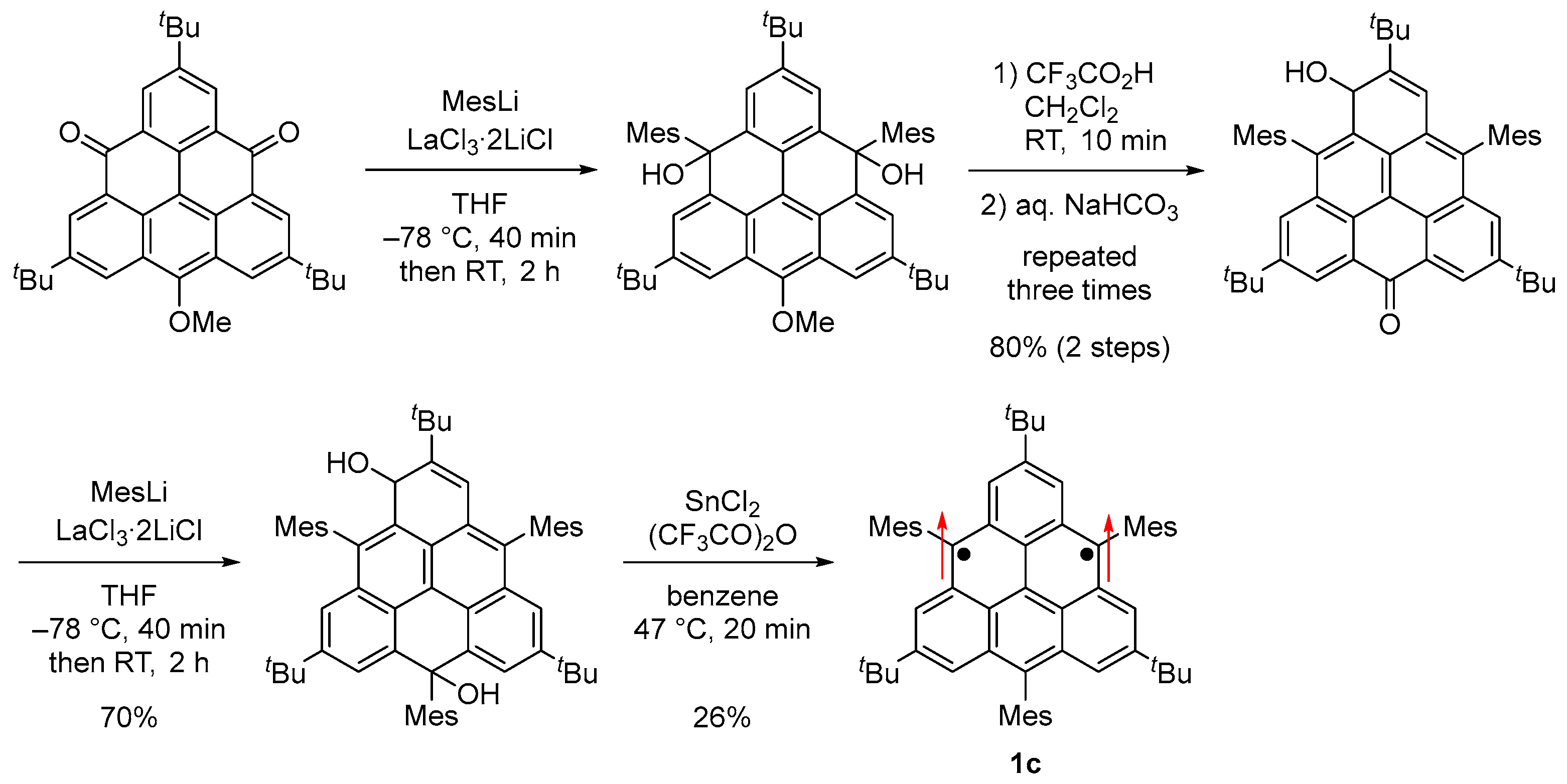
Because 1b [37] polymerized at γ carbons with a large spin density, we designed and synthesized 1c by introducing mesityl groups at the γ carbons for kinetic stabilization (Scheme 3, Figure 1d and Figure 6a) [56]. 1c was stable in solution at room temperature in a degassed sealed tube and in the solid state in a glove box under an argon atmosphere but gradually decomposed when the solution was exposed to air.
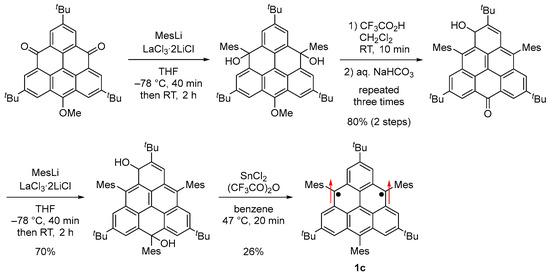
Scheme 3.
Synthesis of 1c. Arrows indicate electron spin.
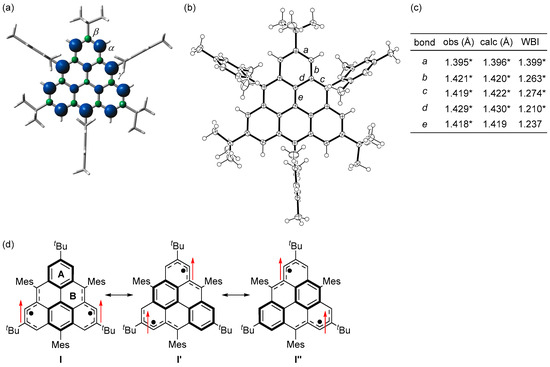
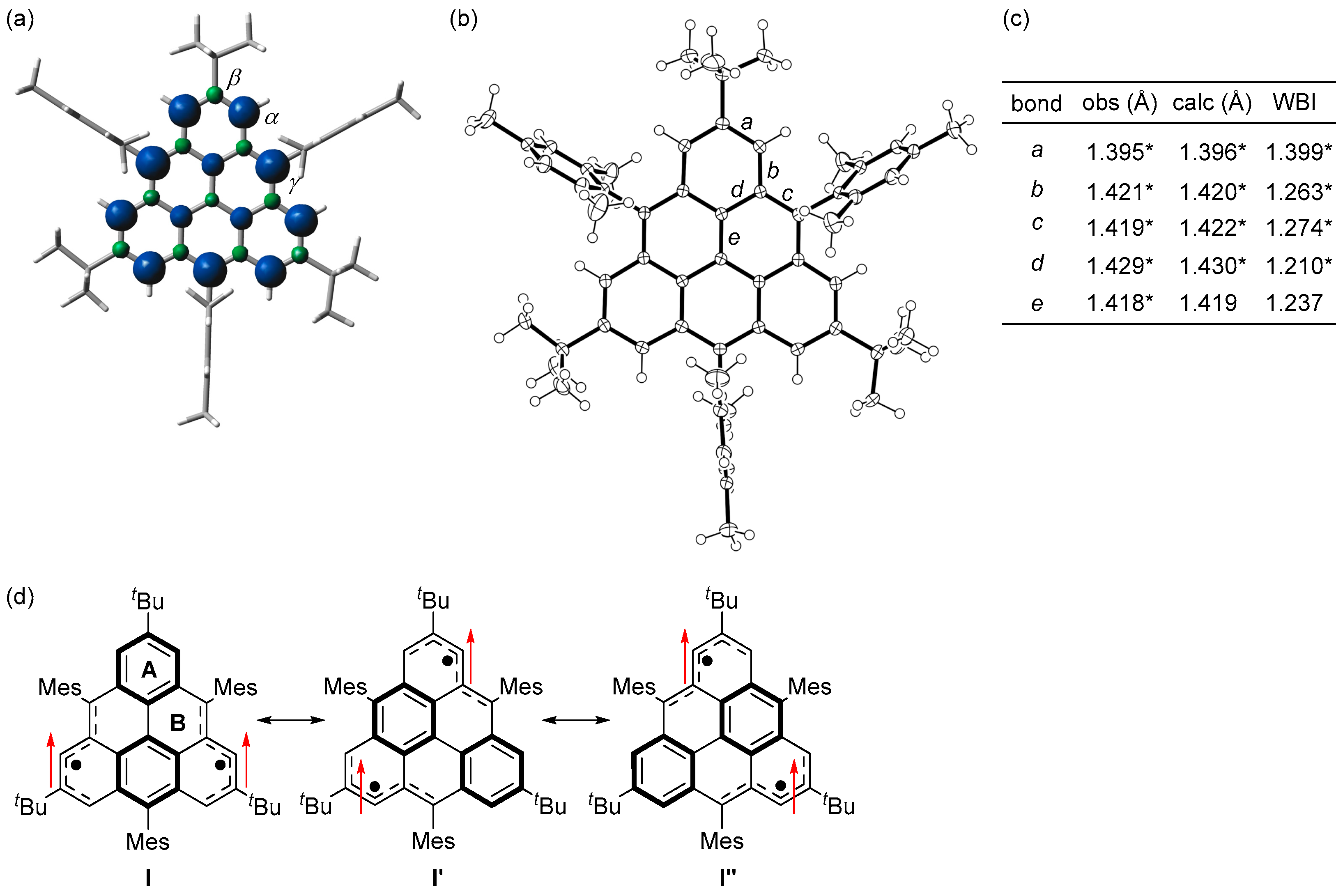
Figure 6.
(a) Spin densities of the triplet state of 1c. Blue and green surfaces represent α and β spin densities, respectively. (b) ORTEP drawing of 1c. Displacement ellipsoids are drawn at the 50% probability level. (c) Observed and calculated bond lengths and calculated Wiberg bond indices (WBI) of 1c. * Mean value. (d) Resonance structures of 1c. Arrows indicate electron spin. Reprinted with permission from Ref. [56], American Chemical Society, 2021.
Single crystals of 1c obtained by recrystallization from a CH2Cl2–hexane solution in a degassed sealed tube had an almost D3h symmetric main core structure with no significant bond length alternation based on the bond lengths and Wiberg bond indices [57] (Figure 6c). The HOMA and NICS(1)zz values and the ACID plot indicate that rings A and B are aromatic. Therefore, the canonical structure I with two Clar’s aromatic sextets contributes significantly, and I′ and I″ also contribute equally due to the molecular symmetry (Figure 6d).
ESR spectra of the toluene solution of 1c at room temperature showed an ESR signal split by six α-protons (Figure 7a). The experimental spin density of α-carbon atoms (0.293) agreed well with the calculated spin density (+0.279, UBLYP/6-311G(d)//UB3LYP/6-311G(d)) (Figure 6a). These results indicate that the two unpaired electrons of 1c are mainly delocalized over the periphery of the main core structure, with 54% (9% per carbon atom) in the six α carbons and 33% (11% per carbon atom) in the three γ carbons.
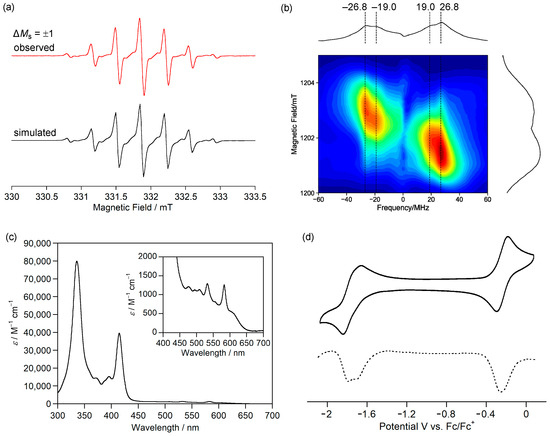
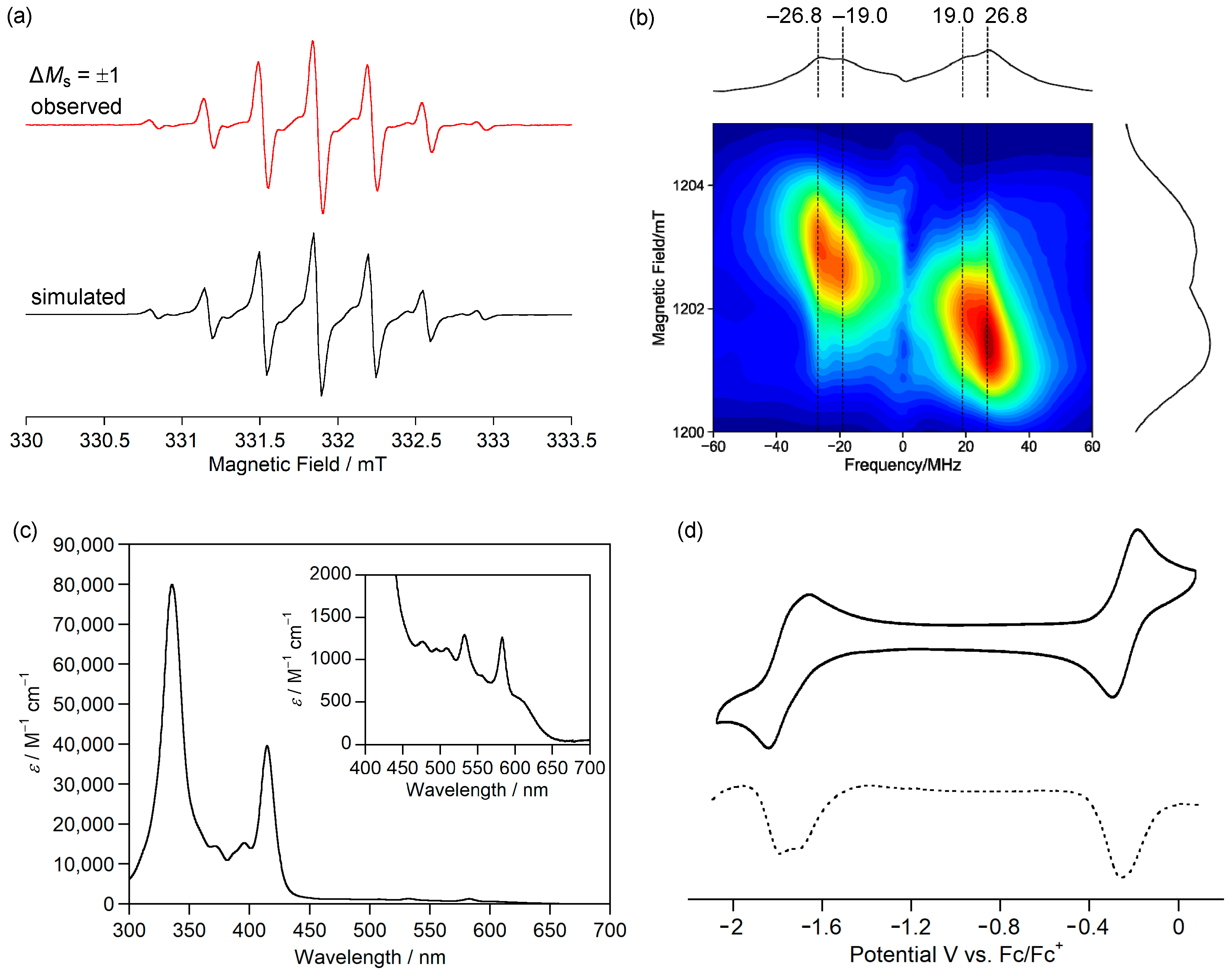
Figure 7.
(a) X-band cw ESR spectra of 1c in toluene at room temperature (red, experiment; black, simulation). (b) Q-band pulse ESR-based two-dimensional electron spin transient nutation spectra of 1c in frozen toluene solution at 50 K. (c) UV-Vis absorption spectrum of 1c in CH2Cl2. (d) Cyclic voltammogram (solid line) and differential pulse voltammogram (dashed line) of 1c in CH2Cl2. Reprinted with permission from Ref. [56], American Chemical Society, 2021.
The ESR spectrum of the frozen toluene solution of 1c did not show the characteristic fine structure of the triplet species and showed a very weak forbidden transition of ΔMs = ±2. Although the reason for these phenomena is unclear, the triangulene derivative 1d, which was reported by Juríček et al., also did not show the fine structure of the transition of ΔMs = ±1 and the forbidden transition at ΔMs = ±2 [58]. The triplet state of 1c was confirmed by a two-dimensional electron spin transient nutation (2D-ESTN) spectrum of a frozen toluene solution of 1c (Figure 7b) [59,60]; the signals at the nutation frequencies of 26.8 and 19.0 MHz were attributed to the triplet state of 1c and a small amount of monoradical impurity, respectively, because the ratio was 21/2:1.
Magnetic susceptibility measurements showed that χpT of 1c was 0.88 emu K mol−1, which is larger than the theoretical value (0.75 emu K mol−1) of the singlet diradical. The χpT value was constant at 10–300 K, indicating that 1c has a triplet ground state with a large ΔEST, as expected from the calculated ΔEST of +53.5 kJ mol−1 (UB3LYP/6-311G(d,p)).
The fundamental optical and electrochemical properties of 1c were elucidated by measuring the UV-Vis absorption spectrum and cyclic voltammogram. 1c exhibited weak absorption bands at 450–600 nm in CH2Cl2 (Figure 7c), which were assigned to transitions from α-HOMO to α-LUMO and β-HOMO to β-LUMO. 1c in dichloromethane showed a reversible two-electron oxidation wave to dication and reversible two-stage one-electron reduction waves to radical anion and dianion (Figure 7d). The small differences between the first and second oxidation potentials and between the first and second reduction potentials are consistent with the degenerated α-HOMOs (–4.50 eV) and β-LUMOs (–2.55 eV). 1c is the first hydrocarbon with a triplet ground state isolated in crystalline forms.
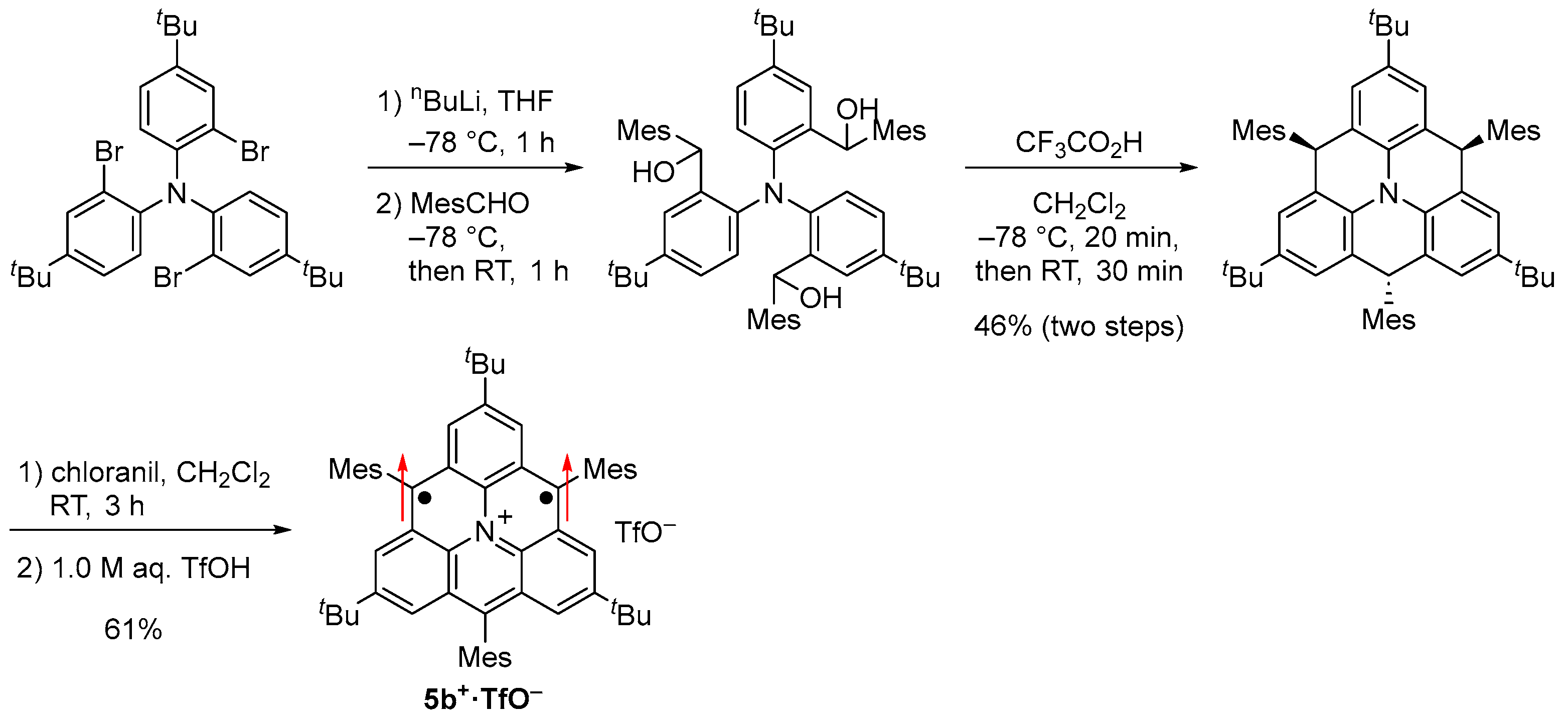
We also designed and synthesized a nitrogen-doped triangulene cation derivative 5b+·TfO− with tert-butyl groups for kinetic protection, which is isoelectronic to 1c (Figure 8 and Scheme 4) [61]. During our study, Wang et al. generated 5a+ on Au surface and elucidated its triplet ground state [62], but its optical, electrochemical, and detailed magnetic properties remain to be elucidated.
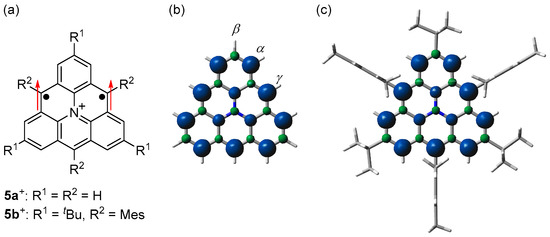
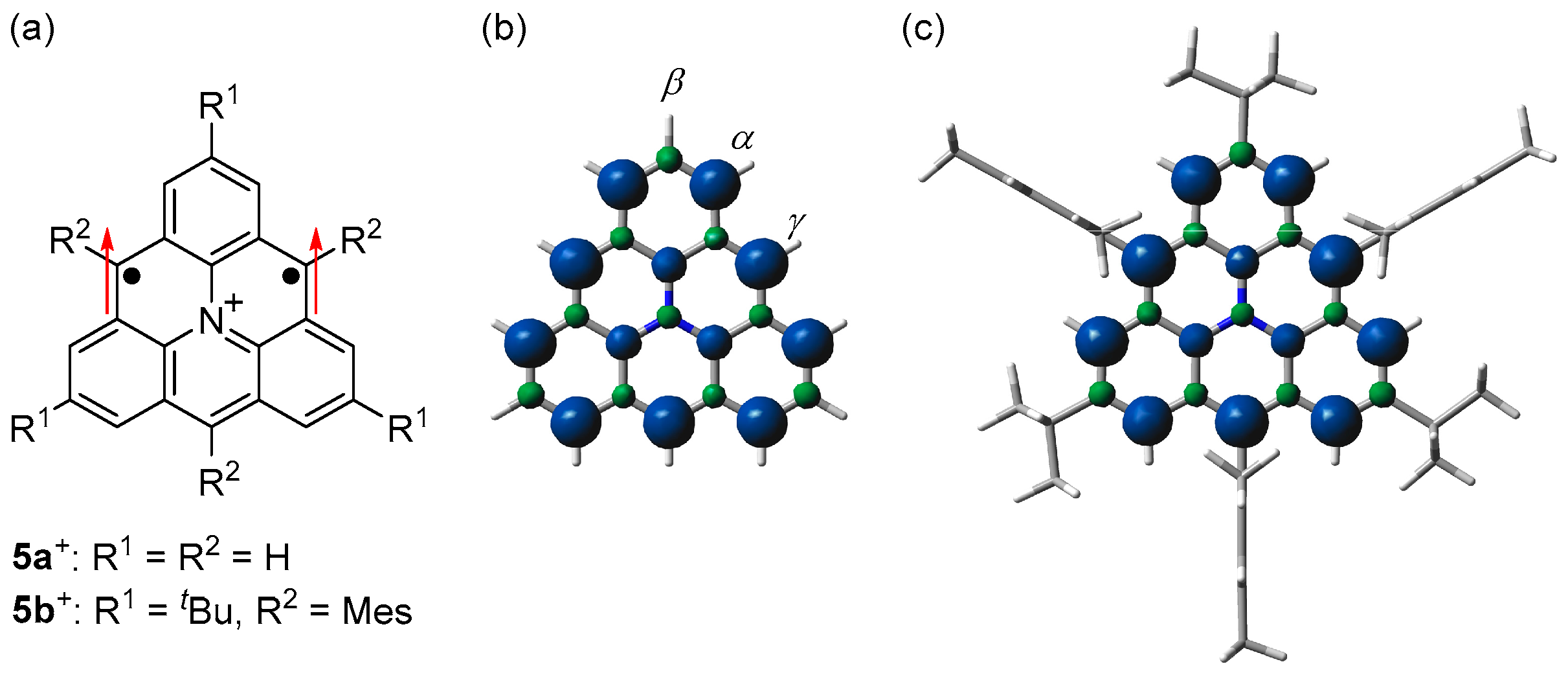
Figure 8.
(a) Structures of 5+. Arrows indicate electron spin. Spin densities of triplet states of (b) 5a+ and (c) 5b+. Blue and green surfaces represent α and β spin densities, respectively. Reprinted with permission from Ref. [61], Wiley-VCH, 2023.
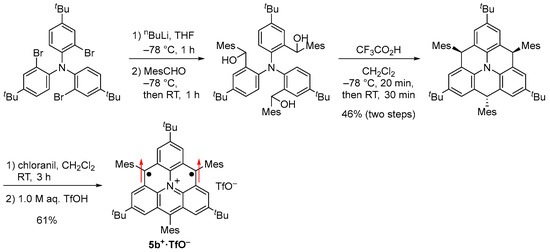
Scheme 4.
Synthesis of 5b+·TfO−.
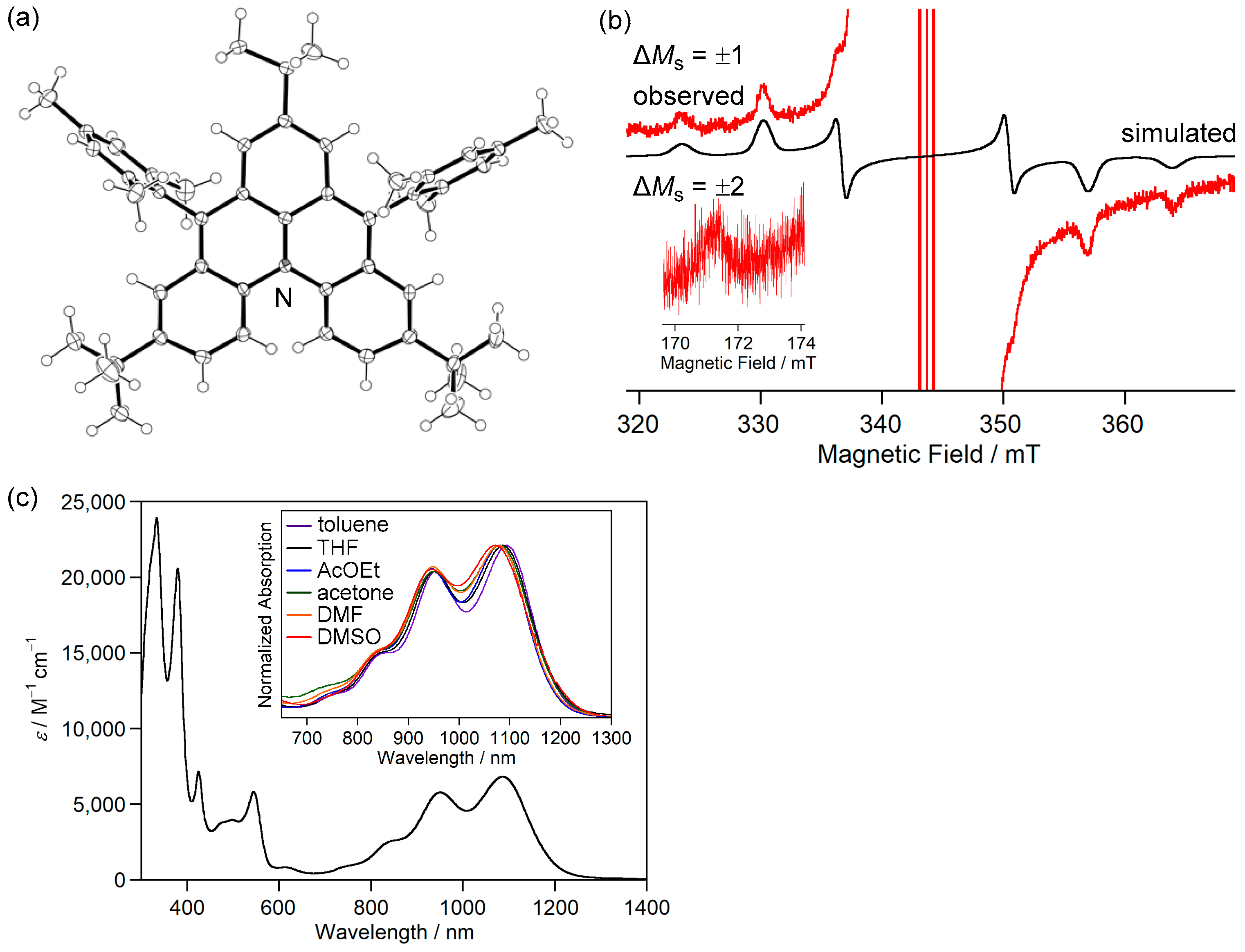
5b+·TfO− was stable in a glove box under argon atmosphere in the solid state and even in solution under air. Single crystals of 5b+·TfO− obtained by recrystallization from a dichloromethane–hexane solution in a degassed sealed tube had an almost D3h symmetric main core structure with similar lengths of C–C bonds to 1c (Figure 9a). ESR spectra of the benzene solution of 5b+·TfO− at room temperature showed a signal split by six α-protons and a nitrogen atom (Figure 9b). The experimental spin density of α-carbon atoms (0.300) agreed well with the calculated spin density (+0.281, Figure 8c) and that of 1c. 5b+·TfO−, which also showed a weak forbidden transition of ΔMs = ±2, and its triplet state was confirmed by the 2D-ESTN spectrum.
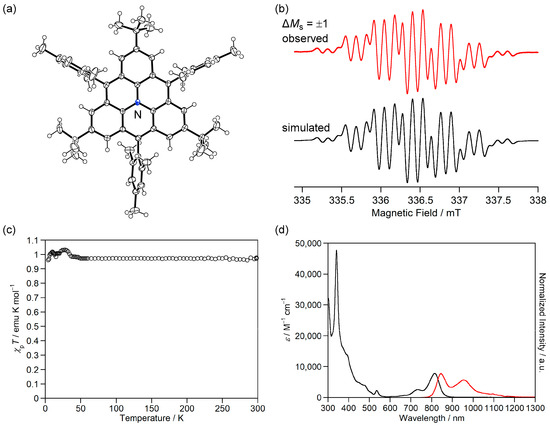
Figure 9.
(a) ORTEP drawing of 5b+·TfO−. Displacement ellipsoids are drawn at the 50% probability level. TfO− is omitted for clarity. (b) ESR spectrum of 5b+·TfO− in benzene at room temperature. (c) χpT–T curves of 5b+·TfO−. (d) UV-Vis-NIR absorption (black) and fluorescence (red, excited at 730 nm) spectra of 5b+·TfO− in CH2Cl2. Reprinted with permission from Ref. [61], Wiley-VCH, 2023.
Magnetic susceptibility measurements showed that χpT of 5b+·TfO− was 0.97 emu K mol−1, which is very close to the theoretical value (1.00 emu K mol−1) of the triplet diradical (Figure 9c). The χpT value was constant at 50–300 K, indicating that 5b+·TfO− has a triplet ground state with a large ΔEST, as expected from the calculated ΔEST of +51.6 kJ mol−1 (UB3LYP/6-311G(d,p)).
Although 5b+·TfO− has magnetic properties similar to 1c, its optical properties are significantly different. 5b+·TfO− showed an NIR absorption band of 815 nm in CH2Cl2 (Figure 9d), which was assigned to the transition from degenerate α-HOMOs to α-LUMO, which is longer and stronger than the transitions from α-HOMO to α-LUMO and β-HOMO to β-LUMO of 1c (Figure 7c). Because 1c is an alternant hydrocarbon, its T0 to T1 transition is symmetry-forbidden, while the nitrogen cation of 5b+ breaks the alternancy symmetry and allows the T0 to T1 transition. In addition, the nitrogen cation lowers the energy level of α-LUMO and reduces the energy difference between α-HOMO and α-LUMO, which causes the longer-wavelength shift of the T0 to T1 transition. Furthermore, 5b+·TfO− exhibited an NIR fluorescence at 846 nm in CH2Cl2 (Figure 9d), which is the first example of an NIR-light emissive triplet diradical [63,64].
5. Triplet Kekulé Hydrocarbon
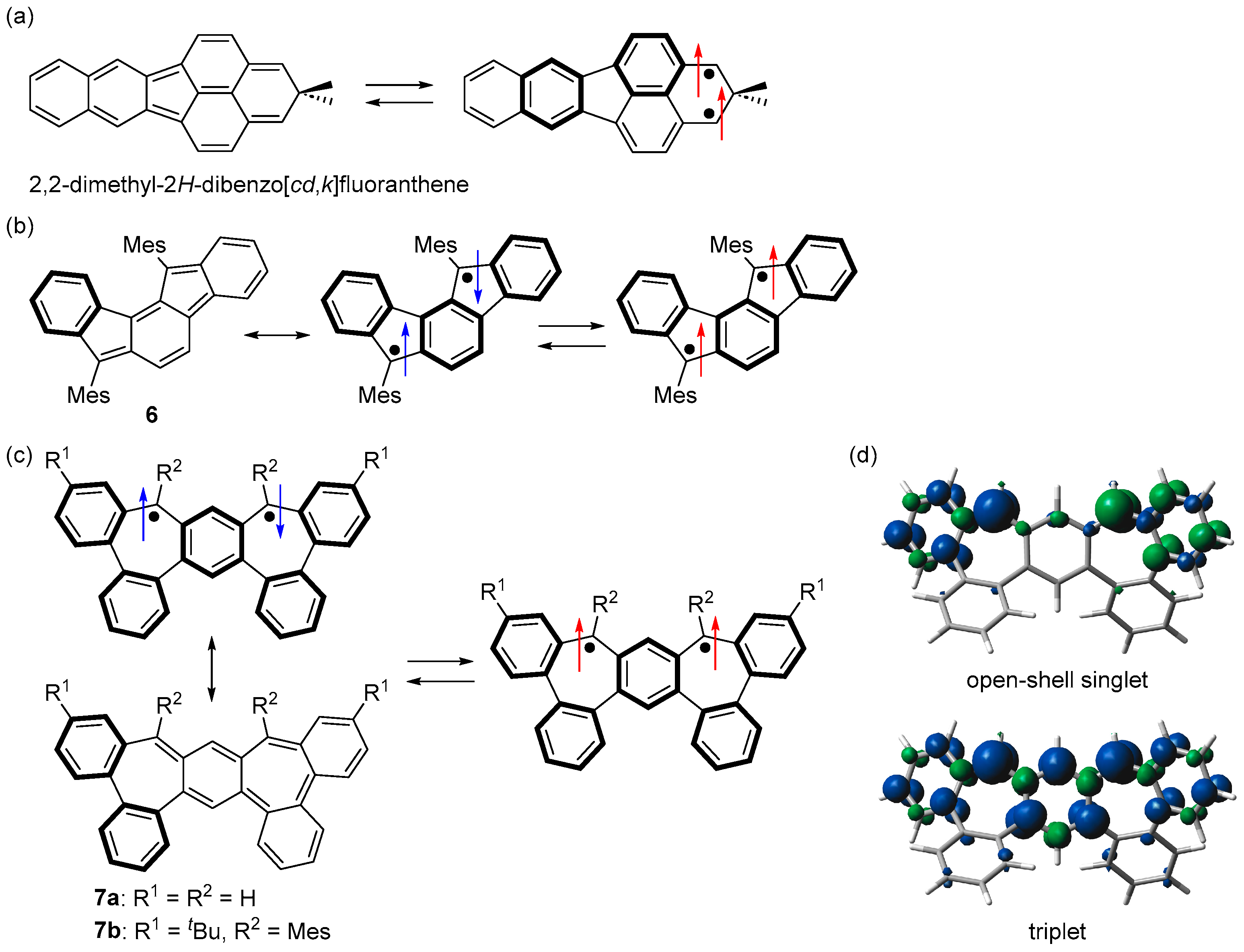
In contrast to the many studies of triplet non-Kekulé hydrocarbons, including Schlenk’s hydrocarbon and its derivatives, few triplet Kekulé hydrocarbons have been studied. 2,2-Dimethyl-2H-dibenzo[cd,k]fluoranthene was generated by McMasters et al. in cryogenic matrices, and its triplet ground state was experimentally confirmed (Figure 10a) [65]. An indeno[1,2-a]fluorene derivative 6 was reported by Haley et al. as a m-quinodimethane-based plausible triplet Kekulé hydrocarbon (Figure 10b) [66]. Although the triplet ESR signal of 6 was successfully observed (|D| = 0.021 cm−1), 6 has not been isolated, and its ground state has not been fully elucidated.
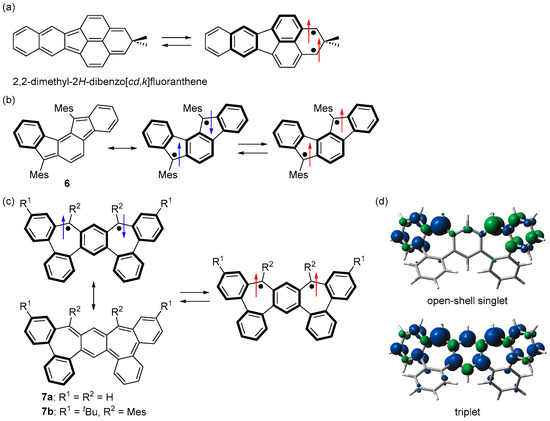
Figure 10.
Structures of (a) 2,2-dimethyl-2H-dibenzo[cd,k]fluoranthene, (b) 6, and (c) 7. Arrows indicate electron spin (blue and red colors are for singlet and triplet diradicals, respectively). (d) Spin densities of open-shell singlet and triplet states of 7a. Blue and green surfaces represent α and β spin densities, respectively.
In the structures of 3a and 6, where both sides of m-quinodimethane are bridged with benzene, the difference in the number of Clar’s aromatic sextet between the Kekulé and diradical structures is 2. We thought that the bonding interaction between the two unpaired electrons could be further reduced by increasing this difference and designed bisdibenzo[3,4:5,6]cyclohepta[1,2-a:2,1-d]benzene (7a), where both sides of m-quinodimethane are bridged with biphenyl (Figure 10c) [67]. Because the number of Clar’s aromatic sextet is 2 in the Kekulé structure and 5 in the singlet diradical structure, the difference is 3, the bonding interaction between the two unpaired electrons is very weak, and the open-shell singlet diradical character of the singlet state of 7a was estimated to be 0.99 (LC-UBLYP/6-311G(d,p)//UB3LYP/6-311G(d,p)). On the other hand, the triplet state of 7a was stabilized because its spin density was similar to m-quinodimethane (Figure 10d). DFT calculations predicted that 7a would have a triplet ground state with a ΔEST of +28.7 kJ mol−1 (UB3LYP/6-311G(d,p)).
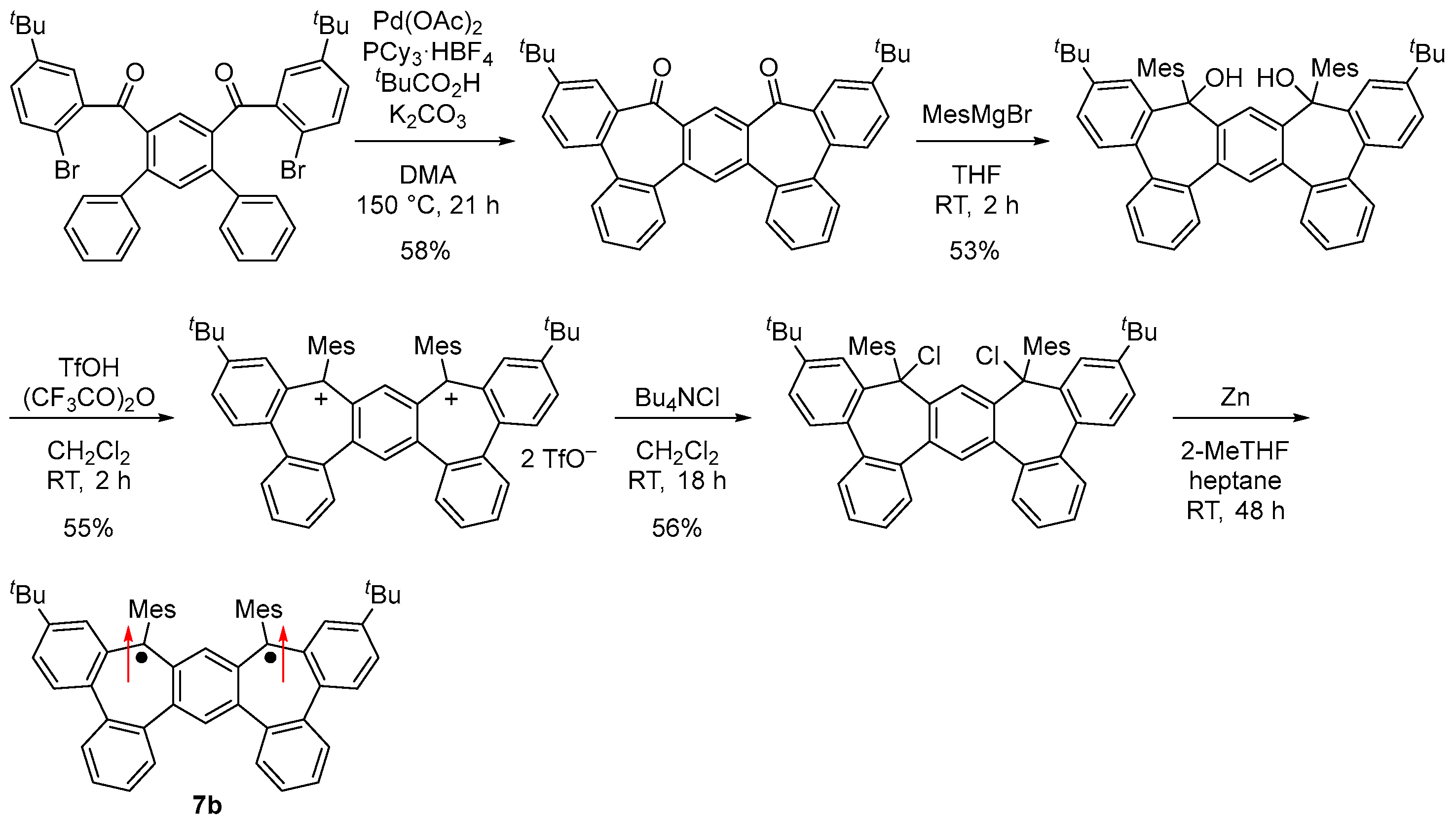
A kinetically stabilized derivative 7b was designed and synthesized by introducing tert-butyl and mesityl groups for kinetic stabilization (Figure 10c and Scheme 5). Single crystals of 7b obtained by recrystallization from 2-methyltetrahydrofuran–heptane solution in a degassed sealed tube had an almost C2 symmetric main core structure due to steric repulsion at the m-terphenyl moiety.
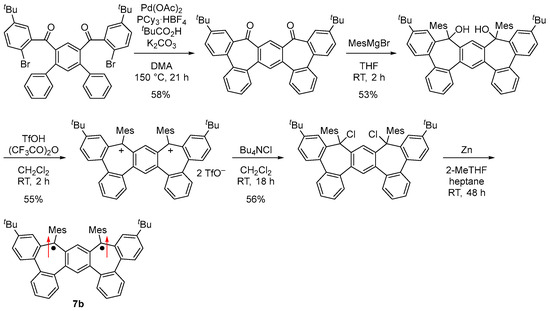
Scheme 5.
Synthesis of 7b. Arrows indicate electron spin.
The Wiberg bond indices of bonds a and b were close to 1, indicating that these bonds have a single bond character. The HOMA and NICS(1)zz values and the ACID plot of the triplet state of 7a indicate that ring A is weakly aromatic, ring B is nonaromatic, and rings C and D are aromatic. Therefore, canonical structure I with four Clar’s aromatic sextets and m-quinodimethane moieties contributes significantly, and canonical structure II with three Clar’s aromatic sextets contributes slightly (Figure 11b).
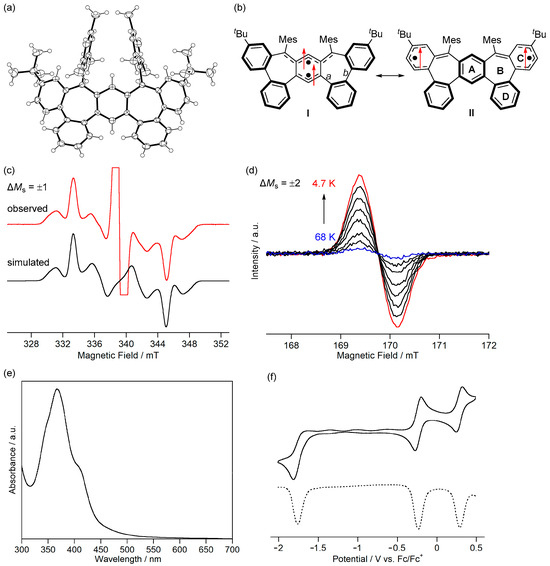
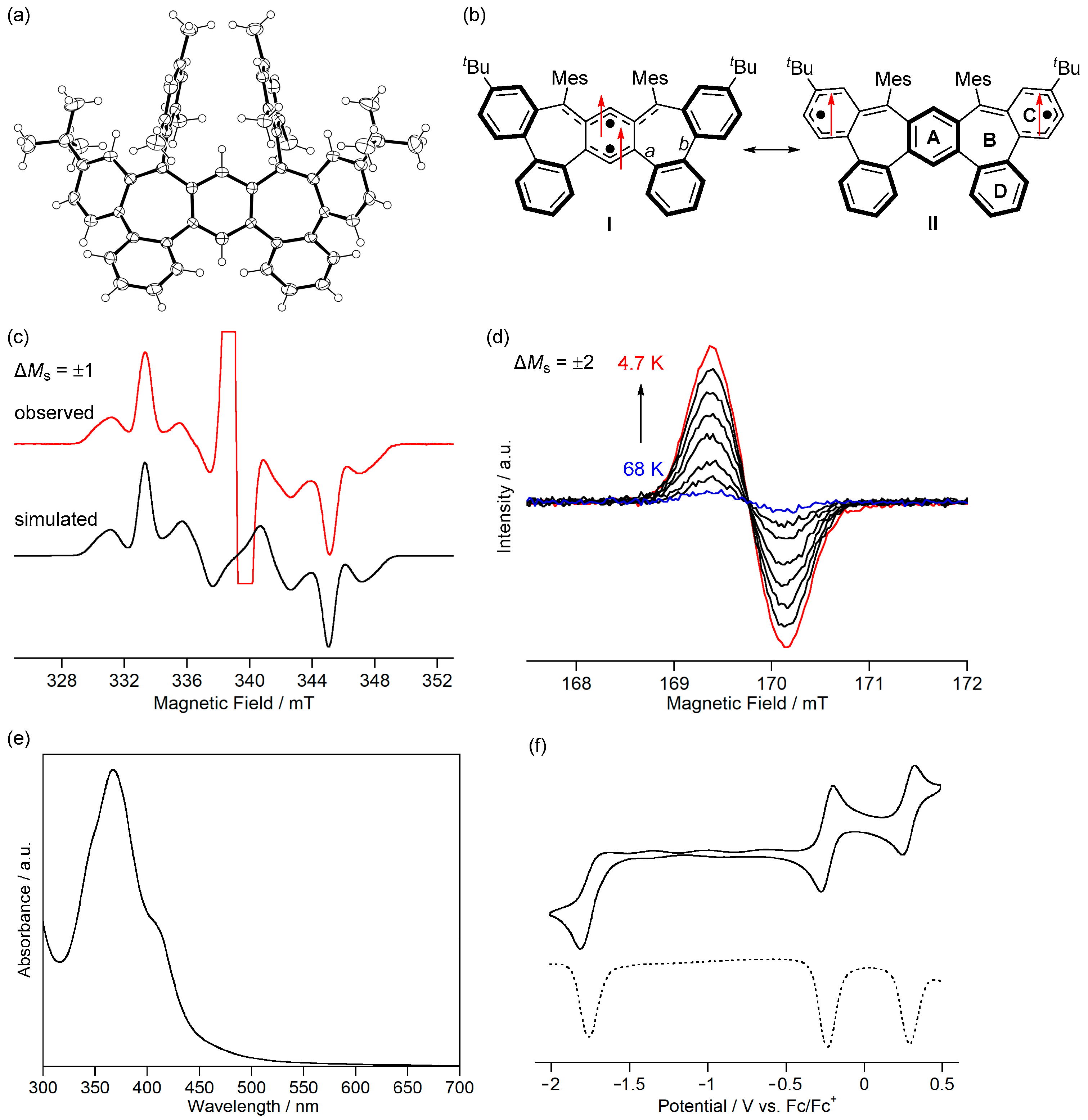
Figure 11.
(a) ORTEP drawing of 7b. Displacement ellipsoids are drawn at the 50% probability level. (b) Resonance structures of 7b. Arrows indicate electron spin. ESR spectra of 7b in toluene (c) at 69 K and (d) from 68 to 4.7 K. (e) UV-Vis absorption spectrum of 7b in CH2Cl2. (f) Cyclic voltammogram (solid line) and differential pulse voltammogram (dashed line) of 7b in CH2Cl2. Reprinted with permission from Ref. [67], Wiley-VCH, 2022.
The ESR spectrum of a frozen toluene solution of 7b showed a signal of the allowed transition with ΔMs = ±1 (Figure 11c). By superimposing the ESR spectra of the two triplet patterns, we found that two conformational isomers existed. The major conformer had zero-field splitting parameters of |D| = 0.0074 cm−1 and |E| = 0.0012 cm−1 and the minor conformer had those of |D| = 0.0083 cm−1 and |E| = 0.0009 cm−1. Because the |D| values are smaller than the triplet states of 3b (0.017 cm−1) and 6 (0.021 cm−1), the two unpaired electrons of 7b would delocalize over ring C (Figure 11b). The signal intensity of the forbidden transition of ΔMs = ±2 increased when the temperature was decreased (Figure 11d). The linear relationship of the triplet signal intensity versus T−1 experimentally confirmed the triplet ground state of 7b.
The fundamental optical and electrochemical properties of 7b were elucidated by measuring UV-Vis absorption spectra and cyclic voltammograms. 7b exhibited weak absorption bands below 600 nm in CH2Cl2, assigned to the transition from α-HOMO to α-LUMO of the triplet state (523 nm, TD-B3LYP/6-311G(d,p)//B3LYP/6-311G(d,p)) (Figure 11e). In addition, 7b did not show the NIR absorption expected for the open-shell singlet state (956 nm). Therefore, the population of thermally excited singlet states of 7b at room temperature is negligible, as expected from the calculated ΔEST (+26.3 kJ mol−1, UB3LYP/6-311G(d,p)). 7b was unstable in air; when the solution was exposed to air, the absorption of 7b disappeared within 15 min. The cyclic voltammogram of 7b showed reversible oxidation waves to radical cation at −0.24 V and to dication at +0.29 V vs. Fc/Fc+ and an irreversible reduction wave to radical anion at −1.82 V (peak potential) (Figure 11f). The small difference between the oxidation potentials would be due to the small energy difference between the α-HOMO (−4.47 eV) and α-HOMO−1 (−4.66 eV). 7b is the first triplet Kekulé hydrocarbon isolated. The difference in the electronic structures and properties of triplet non-Kekulé hydrocarbons between triplet Kekulé hydrocarbons will be elucidated.
6. Conclusions
In this account, studies of m-quinodimethane-based polycyclic diradicals, including ideno[2,1-b]fluorene (3a), an open-shell singlet Kekulé hydrocarbon, azoniadibenzo[a,j]phenalenide (4a), an open-shell singlet zwitterion, triangulene (1a) and nitrogen-doped triangulene cation 5a+, non-Kekulé hydrocarbon-based triplet diradical and diradical cation, and bisdibenzo[3,4:5,6]cyclohepta[1,2-a:2,1-d]benzene (7a), a triplet Kekulé hydrocarbon, are summarized. Kinetically stabilized derivatives of these diradicals were successfully synthesized and isolated, revealing their detailed electronic structures. These diradicals exhibited unique physical properties, such as near-infrared (NIR) absorption/emission, amphoteric redox properties, and a significantly large ΔEST.
Given the strong correlation between the energy difference between the HOMO and LUMO (ΔEHL, RB3LYP/6-311G(d,p)), the open-shell singlet diradical character of the singlet state, calculated using the open-shell singlet structure (y, LC-UBLYP/6-311G(d,p)//UB3LYP/6-311G(d,p)), and the energy difference between the singlet and triplet states (ΔEST, UB3LYP/6-311G(d,p)) of these m-quinodimethane-based polycyclic diradicals (Table 1), controlling the interaction between the two unpaired electrons of m-quinodimethane through appropriate molecular design would produce polycyclic diradicals with various y and ΔEST. These studies could pave the way for the development of functional organic materials based on polycyclic diradicals, a promising avenue for future research and applications.

Table 1.
ΔEHL, y, and ΔEHL of m-quinodimethane-based polycyclic diradicals.
Funding
This work was partially supported by JSPS KAKENHI, grant number JP23H01951, and JST CREST, grant number JPMJCR20R3.
Acknowledgments
The author is grateful to Yoshito Tobe and Ryo Shintani (Osaka University) for valuable discussion, the late Masayoshi Nakano and Ryohei Kishi (Osaka University) for quantum chemical calculations, Daisuke Shiomi, Kazunobu Sato, and Takeji Takui (Osaka Metropolitan University) for magnetic measurements, Ichiro Hisaki, Mikiji Miyata (Osaka University), and Hiroyasu Sato (Rigaku Corporation) for X-ray crystallographic analysis, Hikaru Sotome, Hiroshi Miyasaka (Osaka University), Masahito Murai, and Shigehiro Yamaguchi (Nagoya University) for optical measurements.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Abe, M. Diradicals. Chem. Rev. 2013, 113, 7011–7088. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Ye, Q.; Chi, C.; Wu, J. Low band gap polycyclic hydrocarbons: From closed-shell near infrared dyes and semiconductors to open-shell radicals. Chem. Soc. Rev. 2012, 41, 7857–7889. [Google Scholar] [CrossRef]
- Zeng, Z.; Shi, X.; Chi, C.; López Navarrete, J.T.; Casado, J.; Wu, J. Pro-aromatic and anti-aromatic π-conjugated molecules: An irresistible wish to be diradicals. Chem. Soc. Rev. 2015, 44, 6578–6596. [Google Scholar] [CrossRef] [PubMed]
- Kubo, T. Recent Progress in Quinoidal Singlet Biradical Molecules. Chem. Lett. 2015, 44, 111–122. [Google Scholar] [CrossRef]
- Konishi, A.; Kubo, T. Benzenoid Quinodimethanes. Top. Curr. Chem. 2017, 375, 83. [Google Scholar] [CrossRef] [PubMed]
- Tobe, Y. Quinodimethanes Incorporated in Non-Benzenoid Aromatic or Antiaromatic Frameworks. Top. Curr. Chem. 2018, 376, 12. [Google Scholar] [CrossRef]
- Dressler, J.J.; Haley, M.M. Learning how to fine-tune diradical properties by structure refinement. J. Phys. Org. Chem. 2020, 33, e4114. [Google Scholar] [CrossRef]
- Konishi, A.; Yasuda, M. Breathing New Life into Nonalternant Hydrocarbon Chemistry: Syntheses and Properties of Polycyclic Hydrocarbons Containing Azulene, Pentalene, and Heptalene Frameworks. Chem. Lett. 2021, 50, 195–212. [Google Scholar] [CrossRef]
- Shu, C.; Yang, Z.; Rajca, A. From Stable Radicals to Thermally Robust High-Spin Diradicals and Triradicals. Chem. Rev. 2023, 123, 11954–12003. [Google Scholar] [CrossRef]
- Thiele, J.; Balhorn, H. Ueber einen chinoïden Kohlenwasserstoff. Berichte Dtsch. Chem. Ges. 1904, 37, 1463–1470. [Google Scholar] [CrossRef]
- Tschitschibabin, A.E. Über einige phenylierte Derivate des p,p-Ditolyls. Berichte Dtsch. Chem. Ges. 1907, 40, 1810–1819. [Google Scholar] [CrossRef]
- Montgomery, L.K.; Huffman, J.C.; Jurczak, E.A.; Grendze, M.P. The molecular structures of Thiele’s and Chichibabin’s hydrocarbons. J. Am. Chem. Soc. 1986, 108, 6004–6011. [Google Scholar] [CrossRef] [PubMed]
- Kubo, T.; Shimizu, A.; Sakamoto, M.; Uruichi, M.; Yakushi, K.; Nakano, M.; Shiomi, D.; Sato, K.; Takui, T.; Morita, Y.; et al. Synthesis, Intermolecular Interaction, and Semiconductive Behavior of a Delocalized Singlet Biradical Hydrocarbon. Angew. Chem. Int. Ed. 2005, 44, 6564–6568. [Google Scholar] [CrossRef] [PubMed]
- Kubo, T.; Shimizu, A.; Uruichi, M.; Yakushi, K.; Nakano, M.; Shiomi, D.; Sato, K.; Takui, T.; Morita, Y.; Nakasuji, K. Singlet Biradical Character of Phenalenyl-Based Kekulé Hydrocarbon with Naphthoquinoid Structure. Org. Lett. 2007, 9, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, A.; Uruichi, M.; Yakushi, K.; Matsuzaki, H.; Okamoto, H.; Nakano, M.; Hirao, Y.; Matsumoto, K.; Kurata, H.; Kubo, T. Resonance Balance Shift in Stacks of Delocalized Singlet Biradicals. Angew. Chem. Int. Ed. 2009, 48, 5482–5486. [Google Scholar] [CrossRef]
- Shimizu, A.; Kubo, T.; Uruichi, M.; Yakushi, K.; Nakano, M.; Shiomi, D.; Sato, K.; Takui, T.; Hirao, Y.; Matsumoto, K.; et al. Alternating Covalent Bonding Interactions in a One-Dimensional Chain of a Phenalenyl-Based Singlet Biradical Molecule Having Kekulé Structures. J. Am. Chem. Soc. 2010, 132, 14421–14428. [Google Scholar] [CrossRef]
- Shimizu, A.; Hirao, Y.; Matsumoto, K.; Kurata, H.; Kubo, T.; Uruichi, M.; Yakushi, K. Aromaticity and π-bond covalency: Prominent intermolecular covalent bonding interaction of a Kekulé hydrocarbon with very significant singlet biradical character. Chem. Commun. 2012, 48, 5629–5631. [Google Scholar] [CrossRef]
- Clar, E.; Lang, K.F.; Schulz-Kiesow, H. Aromatische Kohlenwasserstoffe, LXX. Mitteil.1): Zethren (1.12; 6.7-Dibenztetracen). Chem. Berichte 1955, 88, 1520–1527. [Google Scholar] [CrossRef]
- Wu, T.-C.; Chen, C.-H.; Hibi, D.; Shimizu, A.; Tobe, Y.; Wu, Y.-T. Synthesis, Structure, and Photophysical Properties of Dibenzo[de,mn]naphthacenes. Angew. Chem. Int. Ed. 2010, 49, 7059–7062. [Google Scholar] [CrossRef]
- Umeda, R.; Hibi, D.; Miki, K.; Tobe, Y. Tetradehydrodinaphtho[10]annulene: A Hitherto Unknown Dehydroannulene and a Viable Precursor to Stable Zethrene Derivatives. Org. Lett. 2009, 11, 4104–4106. [Google Scholar] [CrossRef]
- Clar, E.; Macpherson, I.A. The significance of Kekulé structures for the stability of aromatic systems—II. Tetrahedron 1962, 18, 1411–1416. [Google Scholar] [CrossRef]
- Li, Y.; Heng, W.-K.; Lee, B.S.; Aratani, N.; Zafra, J.L.; Bao, N.; Lee, R.; Sung, Y.M.; Sun, Z.; Huang, K.-W.; et al. Kinetically Blocked Stable Heptazethrene and Octazethrene: Closed-Shell or Open-Shell in the Ground State? J. Am. Chem. Soc. 2012, 134, 14913–14922. [Google Scholar] [CrossRef]
- Hu, P.; Lee, S.; Herng, T.S.; Aratani, N.; Gonçalves, T.P.; Qi, Q.; Shi, X.; Yamada, H.; Huang, K.-W.; Ding, J.; et al. Toward Tetraradicaloid: The Effect of Fusion Mode on Radical Character and Chemical Reactivity. J. Am. Chem. Soc. 2016, 138, 1065–1077. [Google Scholar] [CrossRef]
- Huang, R.; Phan, H.; Herng, T.S.; Hu, P.; Zeng, W.; Dong, S.; Das, S.; Shen, Y.; Ding, J.; Casanova, D.; et al. Higher Order π-Conjugated Polycyclic Hydrocarbons with Open-Shell Singlet Ground State: Nonazethrene versus Nonacene. J. Am. Chem. Soc. 2016, 138, 10323–10330. [Google Scholar] [CrossRef]
- Chase, D.T.; Rose, B.D.; McClintock, S.P.; Zakharov, L.N.; Haley, M.M. Indeno[1,2-b]fluorenes: Fully Conjugated Antiaromatic Analogues of Acenes. Angew. Chem. Int. Ed. 2011, 50, 1127–1130. [Google Scholar] [CrossRef]
- Chase, D.T.; Fix, A.G.; Kang, S.J.; Rose, B.D.; Weber, C.D.; Zhong, Y.; Zakharov, L.N.; Lonergan, M.C.; Nuckolls, C.; Haley, M.M. 6,12-Diarylindeno[1,2-b]fluorenes: Syntheses, Photophysics, and Ambipolar OFETs. J. Am. Chem. Soc. 2012, 134, 10349–10352. [Google Scholar] [CrossRef] [PubMed]
- Barker, J.E.; Frederickson, C.K.; Jones, M.H.; Zakharov, L.N.; Haley, M.M. Synthesis and Properties of Quinoidal Fluorenofluorenes. Org. Lett. 2017, 19, 5312–5315. [Google Scholar] [CrossRef] [PubMed]
- Rudebusch, G.E.; Zafra, J.L.; Jorner, K.; Fukuda, K.; Marshall, J.L.; Arrechea-Marcos, I.; Espejo, G.L.; Ponce Ortiz, R.; Gómez-García, C.J.; Zakharov, L.N.; et al. Diindeno-fusion of an anthracene as a design strategy for stable organic biradicals. Nat. Chem. 2016, 8, 753–759. [Google Scholar] [CrossRef]
- Iwashita, S.; Ohta, E.; Higuchi, H.; Kawai, H.; Fujiwara, K.; Ono, K.; Takenaka, M.; Suzuki, T. First stable 7,7,8,8-tetraaryl-o-quinodimethane: Isolation, X-ray structure, electrochromic response of 9,10-bis(dianisylmethylene)-9,10-dihydrophenanthrene. Chem. Commun. 2004, 2076–2077. [Google Scholar] [CrossRef]
- Shimizu, A.; Tobe, Y. Indeno[2,1-a]fluorene: An Air-Stable ortho-Quinodimethane Derivative. Angew. Chem. Int. Ed. 2011, 50, 6906–6910. [Google Scholar] [CrossRef]
- Schlenk, W.; Brauns, M. Zur Frage der Metachinoide. Berichte Dtsch. Chem. Ges. 1915, 48, 661–669. [Google Scholar] [CrossRef]
- Kothe, G.; Denkel, K.; Sümmermann, W. Schlenk’s Biradical.—A Molecule in the Triplet Ground State. Angew. Chem. Int. Ed. Engl. 1970, 9, 906–907. [Google Scholar] [CrossRef]
- Rajca, A.; Utamapanya, S.; Xu, J. Control of magnetic interactions in polyarylmethyl triplet diradicals using steric hindrance. J. Am. Chem. Soc. 1991, 113, 9235–9241. [Google Scholar] [CrossRef]
- Rajca, A.; Utamapanya, S. π-Conjugated systems with unique electronic structure: A case of planarized 1,3-connected polyarylmethyl carbodianion and stable triplet hydrocarbon diradical. J. Org. Chem. 1992, 57, 1760–1767. [Google Scholar] [CrossRef]
- Clar, E.; Stewart, D.G. Aromatic Hydrocarbons. LXV. Triangulene Derivatives1. J. Am. Chem. Soc. 1953, 75, 2667–2673. [Google Scholar] [CrossRef]
- Clar, E.; Stewart, D.G. Aromatic Hydrocarbons. LXVIII. Triangulene Derivatives. Part II1. J. Am. Chem. Soc. 1954, 76, 3504–3507. [Google Scholar] [CrossRef]
- Inoue, J.; Fukui, K.; Kubo, T.; Nakazawa, S.; Sato, K.; Shiomi, D.; Morita, Y.; Yamamoto, K.; Takui, T.; Nakasuji, K. The First Detection of a Clar’s Hydrocarbon, 2,6,10-Tri-tert-Butyltriangulene: A Ground-State Triplet of Non-Kekulé Polynuclear Benzenoid Hydrocarbon. J. Am. Chem. Soc. 2001, 123, 12702–12703. [Google Scholar] [CrossRef]
- Li, Y.; Huang, K.; Sun, Z.; Webster, R.; Zeng, Z.; Zeng, W.; Chi, C.; Furukawa, K.; Wu, J. A kinetically blocked 1,14:11,12-dibenzopentacene: A persistent triplet diradical of a non-Kekulé polycyclic benzenoid hydrocarbon. Chem. Sci. 2014, 5, 1908–1914. [Google Scholar] [CrossRef]
- Shimizu, A.; Arikawa, S.; Morikoshi, T.; Shintani, R. m-Quinodimethane-based fused-ring triplet hydrocarbons. Pure Appl. Chem. 2023, 95, 401–412. [Google Scholar] [CrossRef]
- Shimizu, A.; Kishi, R.; Nakano, M.; Shiomi, D.; Sato, K.; Takui, T.; Hisaki, I.; Miyata, M.; Tobe, Y. Indeno[2,1-b]fluorene: A 20-π-Electron Hydrocarbon with Very Low-Energy Light Absorption. Angew. Chem. Int. Ed. 2013, 52, 6076–6079. [Google Scholar] [CrossRef]
- Miyoshi, H.; Miki, M.; Hirano, S.; Shimizu, A.; Kishi, R.; Fukuda, K.; Shiomi, D.; Sato, K.; Takui, T.; Hisaki, I.; et al. Fluoreno[2,3-b]fluorene vs Indeno[2,1-b]fluorene: Unusual Relationship between the Number of π Electrons and Excitation Energy in m-Quinodimethane-Type Singlet Diradicaloids. J. Org. Chem. 2017, 82, 1380–1388. [Google Scholar] [CrossRef]
- Jiang, Q.; Wang, L.; Wei, H.; Peng, Y.; Xu, G.; Li, Z.; Liu, P.; Hu, Z.; Niu, W.; Chen, Y.; et al. A Kinetically Stabilized Dianthraceno[2,3-a:3′,2′-h]-s-Indacene: Stable Kekule Diradical Polycyclic Hydrocarbon with Triplet Ground State, Angew. Chem. Int. Ed. 2024, e202422994. [Google Scholar] [CrossRef]
- Abe, M.; Adam, W.; Nau, W.M. Photochemical Generation and Methanol Trapping of Localized 1,3 and 1,4 Singlet Diradicals Derived from a Spiroepoxy-Substituted Cyclopentane-1,3-diyl. J. Am. Chem. Soc. 1998, 120, 11304–11310. [Google Scholar] [CrossRef]
- Niecke, E.; Fuchs, A.; Baumeister, F.; Nieger, M.; Schoeller, W.W. A P2C2 Four-Membered Ring with Unusual Bonding—Synthesis, Structure, and Ring Opening of a 1,3-Diphosphacyclobutane-2,4-diyl. Angew. Chem. Int. Ed. Engl. 1995, 34, 555–557. [Google Scholar] [CrossRef]
- Arikawa, S.; Shimizu, A.; Shintani, R. Azoniadibenzo[a,j]phenalenide: A Polycyclic Zwitterion with Singlet Biradical Character. Angew. Chem. Int. Ed. 2019, 58, 6415–6419. [Google Scholar] [CrossRef] [PubMed]
- Kruszewski, J.; Krygowski, T.M. Definition of aromaticity basing on the harmonic oscillator model. Tetrahedron Lett. 1972, 13, 3839–3842. [Google Scholar] [CrossRef]
- Krygowski, T.M. Crystallographic studies of inter- and intramolecular interactions reflected in aromatic character of .pi.-electron systems. J. Chem. Inf. Model. 1993, 33, 70–78. [Google Scholar]
- Schleyer, P.V.R.; Maerker, C.; Dransfeld, A.; Jiao, H.; van Eikema Hommes, N.J.R. Nucleus-Independent Chemical Shifts: A Simple and Efficient Aromaticity Probe. J. Am. Chem. Soc. 1996, 118, 6317–6318. [Google Scholar] [CrossRef]
- Chen, Z.; Wannere, C.S.; Corminboeuf, C.; Puchta, R.; Schleyer, P.V.R. Nucleus-Independent Chemical Shifts (NICS) as an Aromaticity Criterion. Chem. Rev. 2005, 105, 3842–3888. [Google Scholar] [CrossRef]
- Schleyer, P.V.R.; Manoharan, M.; Wang, Z.-X.; Kiran, B.; Jiao, H.; Puchta, R.; van Eikema Hommes, N.J.R. Dissected Nucleus-Independent Chemical Shift Analysis of π-Aromaticity and Antiaromaticity. Org. Lett. 2001, 16, 2465–2468. [Google Scholar] [CrossRef]
- Herges, R.; Geuenich, D. Delocalization of Electrons in Molecules. J. Phys. Chem. A 2001, 105, 3214–3220. [Google Scholar] [CrossRef]
- Geuenich, D.; Hess, K.; Köhler, F.; Herges, R. Anisotropy of the Induced Current Density (ACID), a General Method to Quantify and Visualize Electronic Delocalization. Chem. Rev. 2005, 105, 3758–3772. [Google Scholar] [CrossRef] [PubMed]
- Horii, K.; Kishi, R.; Nakano, M.; Shiomi, D.; Sato, K.; Takui, T.; Konishi, A.; Yasuda, M. Bis-periazulene (Cyclohepta[def]fluorene) as a Nonalternant Isomer of Pyrene: Synthesis and Characterization of Its Triaryl Derivatives. J. Am. Chem. Soc. 2022, 144, 3370–3375. [Google Scholar] [CrossRef]
- Pavliček, N.; Mistry, A.; Majzik, Z.; Moll, N.; Meyer, G.; Fox, D.J.; Gross, L. Synthesis and characterization of triangulene. Nat. Nanotechnol. 2017, 12, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Li, C.; Du, Q.; Tao, K.; Wang, S.; Yu, P. Atomically Precise Synthesis and Characterization of Heptauthrene with Triplet Ground State. Nano Lett. 2020, 20, 6859–6864. [Google Scholar] [CrossRef]
- Arikawa, S.; Shimizu, A.; Shiomi, D.; Sato, K.; Shintani, R. Synthesis and Isolation of a Kinetically Stabilized Crystalline Triangulene. J. Am. Chem. Soc. 2021, 143, 19599–19605. [Google Scholar] [CrossRef]
- Wiberg, K.B. Application of the pople-santry-segal CNDO method to the cyclopropylcarbinyl and cyclobutyl cation and to bicyclobutane. Tetrahedron 1968, 24, 1083–1096. [Google Scholar] [CrossRef]
- Valenta, L.; Mayländer, M.; Kappeler, P.; Blacque, O.; Šolomek, T.; Richert, S.; Juríček, M. Trimesityltriangulene: A persistent derivative of Clar’s hydrocarbon. Chem. Commun. 2022, 58, 3019–3022. [Google Scholar] [CrossRef]
- Astashkin, A.V.; Schweiger, A. Electron-spin transient nutation: A new approach to simplify the interpretation of ESR spectra. Chem. Phys. Lett. 1990, 174, 595–602. [Google Scholar] [CrossRef]
- Sato, K.; Yano, M.; Furuichi, M.; Shiomi, D.; Takui, T.; Abe, K.; Itoh, K.; Higuchi, A.; Katsuma, K.; Shirota, Y. Polycationic High-Spin States of One- and Two-Dimensional (Diarylamino)benzenes, Prototypical Model Units for Purely Organic Ferromagnetic Metals as Studied by Pulsed ESR/Electron Spin Transient Nutation Spectroscopy. J. Am. Chem. Soc. 1997, 119, 6607–6613. [Google Scholar] [CrossRef]
- Arikawa, S.; Shimizu, A.; Shiomi, D.; Sato, K.; Takui, T.; Sotome, H.; Miyasaka, H.; Murai, M.; Yamaguchi, S.; Shintani, R. A Kinetically Stabilized Nitrogen-Doped Triangulene Cation: Stable and NIR Fluorescent Diradical Cation with Triplet Ground State. Angew. Chem. Int. Ed. 2023, 62, e202302714. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Berdonces-Layunta, A.; Friedrich, N.; Vilas-Varela, M.; Calupitan, J.P.; Pascual, J.I.; Pena, D.; Casanova, D.; Corso, M.; de Oteyza, D.G. Aza-Triangulene: On-Surface Synthesis and Electronic and Magnetic Properties. J. Am. Chem. Soc. 2022, 144, 4522–4529. [Google Scholar] [CrossRef] [PubMed]
- Namai, H.; Ikeda, H.; Hoshi, Y.; Kato, N.; Morishita, Y.; Mizuno, K. Thermoluminescence and a New Organic Light-Emitting Diode (OLED) Based on Triplet−Triplet Fluorescence of the Trimethylenemethane (TMM) Biradical. J. Am. Chem. Soc. 2007, 129, 9032–9036. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Chong, Y.; Tang, S.; Fang, Y.; Zhao, Y.; Jiang, J.; Wang, X. A stable triplet diradical emitter. Chem. Sci. 2021, 12, 15151–15156. [Google Scholar] [CrossRef]
- McMasters, D.R.; Wirz, J. Spectroscopy and Reactivity of Kekulé Hydrocarbons with Very Small Singlet−Triplet Gaps. J. Am. Chem. Soc. 2001, 123, 238–246. [Google Scholar] [CrossRef]
- Dressler, J.J.; Zhou, Z.; Marshall, J.L.; Kishi, R.; Takamuku, S.; Wei, Z.; Spisak, S.N.; Nakano, M.; Petrukhina, M.A.; Haley, M.M. Synthesis of the Unknown Indeno [1,2-a]fluorene Regioisomer: Crystallographic Characterization of Its Dianion. Angew. Chem. Int. Ed. 2017, 56, 15363–15367. [Google Scholar] [CrossRef]
- Shimizu, A.; Morikoshi, T.; Sugisaki, K.; Shiomi, D.; Sato, K.; Takui, T.; Shintani, R. Synthesis and Isolation of a Kekulé Hydrocarbon with a Triplet Ground State. Angew. Chem. Int. Ed. 2022, 61, e202205729. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).