Bacterial Endotoxins and Their Role in Periparturient Diseases of Dairy Cows: Mucosal Vaccine Perspectives
Abstract
:1. Introduction
2. High-Grain Diets, Rumen Microbiota, and Periparturient Disease
2.1. Structure and Function of Endotoxins
2.1.1. Structure of Lipopolysaccharide
2.1.2. Relationship between Lipid A Structure and Activity of LPS
2.1.3. Structure of Lipoteichoic Acid
3. Sources of Endotoxins
3.1. Gastrointestinal Tract
3.2. Mammary Gland
3.3. Uterus
4. Translocation of Endotoxin
4.1. Possible Mechanisms of Paracellular Transport
4.2. Possible Mechanisms of Transcellular Transport
5. Periparturient Diseases and Their Connection to Endotoxin
5.1. Acute and Subacute Ruminal Acidosis
5.2. Fatty Liver
5.3. Mastitis
5.4. Retained Placenta
5.5. Metritis and Endometritis
5.6. Laminitis
5.7. Displaced Abomasum
5.8. Milk Fever
5.9. Downer Cow Syndrome
6. Mucosal Immunity and Vaccination against Bacterial Antigens
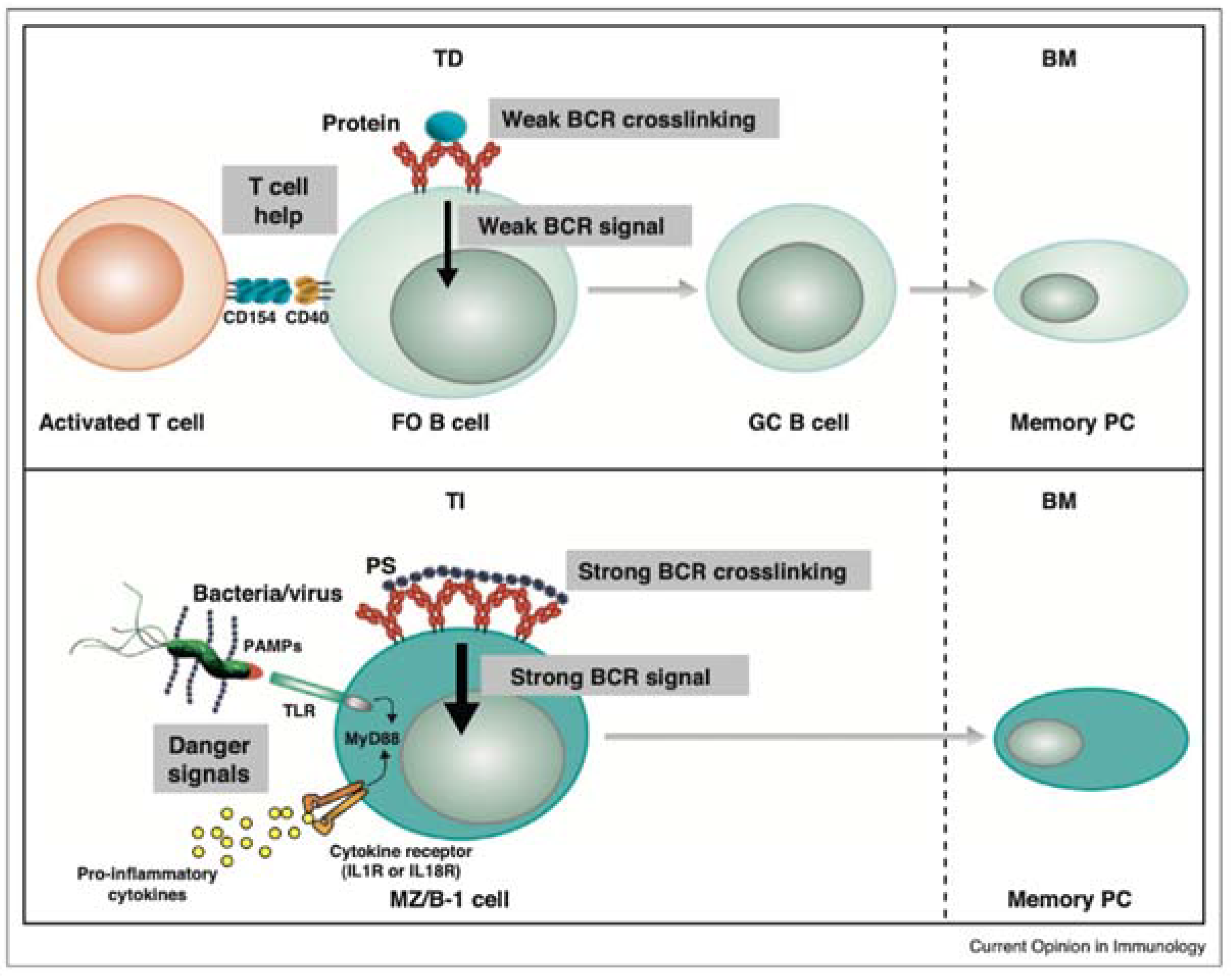
6.1. Why Mucosal Vaccination Against LPS and LTA?
6.2. Secretory Immunoglobulin A (sIgA)
6.3. Induction of the Mucosal Immune Responses
6.4. Connectivity and Compartmentalization of the Mucosal Immune System
6.5. Optimal Route of Immunization
6.6. Endotoxin and Immunological Memory
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ingvartsen, K.L.; Moyes, K. Nutrition, immune function and health of dairy cattle. Animal 2013, 1, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roche, J.R.; Bell, A.W.; Overton, T.R.; Loor, J.J. Nutritional management of the transition cow in the 21st century—A paradigm shift in thinking. Anim. Prod. Sci. 2013, 53, 1000–1023. [Google Scholar] [CrossRef]
- Mallard, B.A.; Dekkers, J.C.; Ireland, M.J.; Leslie, K.E.; Sharif, S.; Lacey Vankampen, C.; Wagter, L.; Wilkie, B.N. Alteration in immune responsiveness during the peripartum period and its ramification on dairy cow and calf health. J. Dairy Sci. 1998, 81, 585–595. [Google Scholar] [CrossRef]
- Dosogne, H.; Meyer, E.; Sturk, A.; van Loon, J.; Massart-Leen, A.M.; Burvenich, C. Effect of enrofloxacin treatment on plasma endotoxin during bovine Escherichia coli mastitis. Inflamm. Res. 2002, 51, 201–205. [Google Scholar] [CrossRef]
- Emmanuel, D.G.V.; Madsen, K.L.; Churchill, T.A.; Dunn, S.M.; Ametaj, B.N. Acidosis and lipopolysaccharide from Escherichia coli B:055 cause hyperpermeability of rumen and colon tissues. J. Dairy Sci. 2007, 90, 5552–5557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mateus, L.; Lopes da Costa, L.; Diniz, P.; Ziecik, A.J. Relationship between endotoxin and prostaglandin (PGE2 and PGFM) concentrations and ovarian function in dairy cows with puerperal endometritis. Anim. Reprod. Sci. 2003, 76, 143–154. [Google Scholar] [CrossRef]
- Ametaj, B.N.; Zebeli, Q.; Iqbal, S. Nutrition, microbiota, and endotoxin-related diseases in dairy cows. R. Bras. Zootec. 2010, 39, 433–444. [Google Scholar] [CrossRef] [Green Version]
- Nagaraja, T.G.; Titgemeyer, E.C. Ruminal acidosis in beef cattle: The current microbiological and nutritional outlook. J. Dairy Sci. 2007, 90, E17–E38. [Google Scholar] [CrossRef] [Green Version]
- Garrett, E.F.; Nordlund, K.V.; Goodger, W.J.; Oetzel, G.R. A cross-sectional field study investigating the effect of periparturient dietary management on ruminal pH in early lactation dairy cows. J. Dairy Sci. 1997, 80, 169. [Google Scholar]
- Fernando, S.C.; Purvis, H.T., 2nd; Najar, F.Z.; Sukharnikov, L.O.; Krehbiel, C.R.; Nagaraja, T.G.; Roe, B.A.; Desilva, U. Rumen microbial population dynamics during adaptation to a high-grain diet. Appl. Environ. Microbiol. 2010, 76, 7482–7490. [Google Scholar] [CrossRef] [Green Version]
- Draing, C.; Sigel, S.; Deininger, S.; Traub, S.; Munke, R.; Mayer, C.; Hareng, L.; Hartung, T.; von Aulock, S.; Hermann, C. Cytokine induction by Gram-positive bacteria. Immunobiology 2008, 213, 285–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azizi, A.; Kumar, A.; Diaz-Mitoma, F.; Mestecky, J. Enhancing oral vaccine potency by targeting intestinal M cells. PLoS Pathog. 2010, 6, 1001147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iqbal, S.; Zebeli, Q.; Mansmann, D.A.; Dunn, S.M.; Ametaj, B.N. Oral administration of LPS and lipoteichoic acid prepartum modulated reactants of innate and humoral immunity in periparturient dairy cows. Innate Immun. 2014, 20, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Ametaj, B.N.; Sivaraman, S.; Dunn, S.M.; Zebeli, Q. Repeated oral administration of lipopolysaccharide from Escherichia coli 0111: B4 modulated humoral immune responses in periparturient dairy cows. Innate Immun. 2012, 18, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Zebeli, Q.; Mansmann, D.A.; Dunn, S.M.; Ametaj, B.N. Oronasal administration of lipopolysaccharide prepartum modulated plasma metabolite patterns in periparturient dairy cows. Open J. Anim. Sci. 2013, 3, 184–192. [Google Scholar] [CrossRef] [Green Version]
- Zebeli, Q.; Mansmann, D.; Sivaraman, S.; Dunn, S.M.; Ametaj, B.N. Oral challenge with increasing doses of LPS modulated the patterns of plasma metabolites and minerals in periparturient dairy cows. Innate Immun. 2013, 19, 298–314. [Google Scholar] [CrossRef]
- Van Soest, P.J. Nutritional Ecology of the Ruminant, 2nd ed.; Cornell University Press: Ithaca, NY, USA, 1994. [Google Scholar]
- Jing, L.; Zhang, R.; Liu, Y.; Zhu, W.; Mao, S. Intravenous lipopolysaccharide challenge alters ruminal bacterial microbiota and disrupts ruminal metabolism in dairy cattle. Br. J. Nutr. 2014, 112, 170–182. [Google Scholar] [CrossRef] [Green Version]
- Mao, S.Y.; Zhang, R.Y.; Wang, D.S.; Zhu, W.Y. Impact of subacute ruminal acidosis (SARA) adaptation on rumen microbiota in dairy cattle using pyrosequencing. Anaerobe 2013, 24, 12–19. [Google Scholar] [CrossRef]
- Van Amersfoort, E.S.; Van Berkel, T.J.; Kuiper, J. Receptors, mediators, and mechanisms involved in bacterial sepsis and septic shock. Clin. Microbiol. Rev. 2003, 16, 379–414. [Google Scholar] [CrossRef] [Green Version]
- Nankar, S.A.; Pande, A.H. Physicochemical properties of bacterial pro-inflammatory lipids influence their interaction with apolipoprotein-derived peptides. Biochim. Biophys. Acta 2013, 4, 853–862. [Google Scholar] [CrossRef]
- Kulp, A.; Kuehn, M.J. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu. Rev. Microbiol. 2010, 64, 163–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erridge, C.; Bennett-Guerrero, E.; Poxton, I.R. Structure and function of lipopolysaccharides. Microbes Infect. 2002, 4, 837–851. [Google Scholar] [CrossRef]
- Netea, M.G.; van Deuren, M.; Kullberg, B.J.; Cavaillon, J.M.; Van der Meer, J.W. Does the shape of lipid A determine the interaction of LPS with Toll-like receptors? Trends Immunol. 2002, 23, 135–139. [Google Scholar] [CrossRef]
- Percy, M.G.; Grundling, A. Lipoteichoic acid synthesis and function in gram-positive bacteria. Annu. Rev. Microbiol. 2014, 68, 81–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morath, S.; von Aulock, S.; Hartung, T. Structure/function relationships of lipoteichoic acids. J. Endotoxin Res. 2005, 11, 348–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khafipour, E.; Li, S.; Plaizier, J.C.; Krause, D.O. Rumen microbiome composition determined using two nutritional models of subacute ruminal acidosis. Appl. Environ. Microbiol. 2009, 75, 7115–7124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, J.M.; Duckworth, C.A.; Watson, A.J.; Frey, M.R.; Miguel, J.C.; Burkitt, M.D.; Sutton, R.; Hughes, K.R.; Hall, L.J.; Caamano, J.H.; et al. A mouse model of pathological small intestinal epithelial cell apoptosis and shedding induced by systemic administration of lipopolysaccharide. Dis. Model. Mech. 2013, 6, 1388–1399. [Google Scholar] [CrossRef] [Green Version]
- Hakogi, E.; Tamura, H.; Tanaka, S.; Kohata, A.; Shimada, Y.; Tabuchi, K. Endotoxin levels in milk and plasma of mastitis-affected cows measured with a chromogenic limulus test. Vet. Microbiol. 1989, 20, 267–274. [Google Scholar] [CrossRef]
- Jorgensen, H.B.; Buitenhuis, B.; Rontved, C.M.; Jiang, L.; Ingvartsen, K.L.; Sorensen, P. Transcriptional profiling of the bovine hepatic response to experimentally induced E. coli mastitis. Physiol. Genom. 2012, 44, 595–606. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, K.; Oyama, S.; Numata, A.; Rahman, M.M.; Kumura, H. Lipopolysaccharide disrupts the milk-blood barrier by modulating claudins in mammary alveolar tight junctions. PLoS ONE 2013, 8, e62187. [Google Scholar] [CrossRef] [Green Version]
- Leitner, G.; Yadlin, B.; Glickman, A.; Chaffer, M.; Saran, A. Systemic and local immune response of cows to intramammary infection with Staphylococcus aureus. Res. Vet. Sci. 2000, 69, 181–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peter, A.T.; Bosu, W.T.; Gilbert, R.O. Absorption of Escherichia coli endotoxin (lipopolysaccharide) from the uteri of postpartum dairy cows. Theriogenology 1990, 33, 1011–1014. [Google Scholar] [CrossRef]
- Mani, V.; Weber, T.E.; Baumgard, L.H.; Gabler, N.K. Growth and Development Symposium: Endotoxin, inflammation, and intestinal function in livestock. J. Anim. Sci. 2012, 90, 1452–1465. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.S.; Wang, J.; Yannie, P.J.; Ghosh, S. Intestinal Barrier Dysfunction, LPS Translocation, and Disease Development. J. Endocr. Soc. 2020, 4, bvz039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emmanuel, D.G.; Dunn, S.M.; Ametaj, B.N. Feeding high proportions of barley grain stimulates an inflammatory response in dairy cows. J. Dairy Sci. 2008, 91, 606–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, L.A.; Li, J.S.; Li, Y.S.; Zhu, N.T.; Liu, F.N.; Tan, L. Intestinal barrier damage caused by trauma and lipopolysaccharide. World J. Gastroenterol. 2004, 10, 2373–2378. [Google Scholar] [CrossRef]
- Han, X.; Fink, M.P.; Yang, R.; Delude, R.L. Increased iNOS activity is essential for intestinal epithelial tight junction dysfunction in endotoxemic mice. Shock 2004, 21, 261–270. [Google Scholar] [CrossRef]
- Potoka, D.A.; Upperman, J.S.; Nadler, E.P.; Wong, C.T.; Zhou, X.; Zhang, X.R.; Ford, H.R. NF-kappaB inhibition enhances peroxynitrite-induced enterocyte apoptosis. J. Surg. Res. 2002, 106, 7–14. [Google Scholar] [CrossRef]
- Potoka, D.A.; Nadler, E.P.; Upperman, J.S.; Ford, H.R. Role of nitric oxide and peroxynitrite in gut barrier failure. World J. Surg. 2002, 26, 806–811. [Google Scholar] [CrossRef]
- Sugi, K.; Musch, M.W.; Field, M.; Chang, E.B. Inhibition of Na+,K+-ATPase by interferon gamma down-regulates intestinal epithelial transport and barrier function. Gastroenterology 2001, 120, 1393–1403. [Google Scholar] [CrossRef]
- Neal, M.D.; Leaphart, C.; Levy, R.; Prince, J.; Billiar, T.R.; Watkins, S.; Li, J.; Cetin, S.; Ford, H.; Schreiber, A.; et al. Enterocyte TLR4 mediates phagocytosis and translocation of bacteria across the intestinal barrier. J. Immunol. 2006, 176, 3070–3079. [Google Scholar] [CrossRef] [PubMed]
- Abreu, M.T.; Arnold, E.T.; Thomas, L.S.; Gonsky, R.; Zhou, Y.; Hu, B.; Arditi, M. TLR4 and MD-2 expression is regulated by immune-mediated signals in human intestinal epithelial cells. J. Biol. Chem. 2002, 277, 20431–20437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cario, E.; Brown, D.; McKee, M.; Lynch-Devaney, K.; Gerken, G.; Podolsky, D.K. Commensal-associated molecular patterns induce selective toll-like receptor-trafficking from apical membrane to cytoplasmic compartments in polarized intestinal epithelium. Am. J. Pathol. 2002, 160, 165–173. [Google Scholar] [CrossRef]
- Guo, S.; Al-Sadi, R.; Said, H.M.; Ma, T.Y. Lipopolysaccharide causes an increase in intestinal tight junction permeability in vitro and in vivo by inducing enterocyte membrane expression and localization of TLR-4 and CD14. Am. J. Pathol. 2013, 182, 375–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beatty, W.L.; Meresse, S.; Gounon, P.; Davoust, J.; Mounier, J.; Sansonetti, P.J.; Gorvel, J.P. Trafficking of Shigella lipopolysaccharide in polarized intestinal epithelial cells. J. Cell Biol. 1999, 145, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, S.; Witta, J.; Zhong, J.; de Villiers, W.; Eckhardt, E. Chylomicrons promote intestinal absorption of lipopolysaccharides. J. Lipid Res. 2009, 50, 90–97. [Google Scholar] [CrossRef] [Green Version]
- Grunfeld, C.; Feingold, K.R. Endotoxin in the gut and chylomicrons: Translocation or transportation? J. Lipid Res. 2009, 50, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Moreira, A.P.; Texeira, T.F.; Ferreira, A.B.; Peluzio Mdo, C.; Alfenas Rde, C. Influence of a high-fat diet on gut microbiota, intestinal permeability and metabolic endotoxaemia. Br. J. Nutr. 2012, 108, 801–809. [Google Scholar] [CrossRef]
- Meissner, H.H.; Henning, P.H.; Horn, C.H.; Leeuw, K.-J.; Hagg, F.M.; Fouché, G. Ruminal acidosis: A review with detailed reference to the controlling agent Megasphaera elsdenii NCIMB 41125. S. Afr. J. Anim. Sci. 2010, 40, 79–100. [Google Scholar] [CrossRef] [Green Version]
- Krause, K.M.; Oetzel, G.R. Understanding and preventing subacute ruminal acidosis in dairy herds: A review. Anim. Feed Sci. Technol. 2006, 126, 215–236. [Google Scholar] [CrossRef]
- Maulfair, D.D.; McIntyre, K.K.; Heinrichs, A.J. Subacute ruminal acidosis and total mixed ration preference in lactating dairy cows. J. Dairy Sci. 2013, 96, 6610–6620. [Google Scholar] [CrossRef] [PubMed]
- Emmanuel, D.G.V.; Shanthipoosan, S.; Ametaj, B.N. High grain diets perturb rumen and plasma metabolites and induce inflammatory responses in early lactation dairy cows. Ital. J. Anim. Sci. 2010, 6, 424–426. [Google Scholar] [CrossRef]
- Goff, J.P. Major advances in our understanding of nutritional influences on bovine health. J. Dairy Sci. 2006, 89, 1292–1301. [Google Scholar] [CrossRef] [Green Version]
- Grummer, R.R. Nutritional and management strategies for the prevention of fatty liver in dairy cattle. Vet. J. 2008, 176, 10–20. [Google Scholar] [CrossRef]
- Ametaj, B.N.; Bradford, B.J.; Bobe, G.; Nafikov, R.A.; Lu, Y.; Young, J.W.; Beitz, D.C. Strong relationships between mediators of the acute phase response and fatty liver in dairy cows. Can. J. Anim. Sci. 2005, 85, 165–175. [Google Scholar] [CrossRef]
- Bradford, B.J.; Mamedova, L.K.; Minton, J.E.; Drouillard, J.S.; Johnson, B.J. Daily injection of tumor necrosis factor-increases hepatic triglycerides and alters transcript abundance of metabolic genes in lactating dairy cattle. J. Nutr. 2009, 139, 1451–1456. [Google Scholar] [CrossRef] [Green Version]
- Rossol, M.; Heine, H.; Meusch, U.; Quandt, D.; Klein, C.; Sweet, M.J.; Hauschildt, S. LPS-induced cytokine production in human monocytes and macrophages. Crit. Rev. Immunol. 2011, 31, 379–446. [Google Scholar] [CrossRef]
- Wellnitz, O.; Arnold, E.T.; Bruckmaier, R.M. Lipopolysaccharide and lipoteichoic acid induce different immune responses in the bovine mammary gland. J. Dairy Sci. 2011, 94, 5405–5412. [Google Scholar] [CrossRef] [Green Version]
- Endo, H.; Niioka, M.; Kobayashi, N.; Tanaka, M.; Watanabe, T. Butyrate-producing probiotics reduce nonalcoholic fatty liver disease progression in rats: New insight into the probiotics for the gut-liver axis. PLoS ONE 2013, 8, e63388. [Google Scholar] [CrossRef] [Green Version]
- Ritze, Y.; Bárdos, G.; Claus, A.; Ehrmann, V.; Bergheim, I.; Schwiertz, A.; Bischoff, S.C. Lactobacillus rhamnosus GG Protects against Non-Alcoholic Fatty Liver Disease in Mice. PLoS ONE 2014, 9, e80169. [Google Scholar] [CrossRef] [Green Version]
- Aitken, S.L.; Corl, C.M.; Sordillo, L.M. Immunopathology of mastitis: Insights into disease recognition and resolution. J. Mammary Gland Biol. Neoplasia 2011, 16, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Hamadani, H.; Khan, A.A.; Banday, M.T.; Ashraf, I.; Handoo, N.; Shah, A.B.; Hamadani, A. Bovine mastitis—A disease of serious concern for dairy farmers. Int. J. Livest. Res. 2013, 3, 42–55. [Google Scholar] [CrossRef] [Green Version]
- Rainard, P.; Riollet, C. Innate immunity of the bovine mammary gland. Vet. Res. 2006, 37, 369–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiwari, J.G.; Babra, C.; Tiwari, H.K.; Williams, V.; Wet, S.D.; Gibson, J.; Paxman, A.; Morgan, E.; Costantino, P.; Sunagar, R.; et al. Trends in therapeutic and prevention strategies for management of bovine mastitis: An overview. J. Vaccines Vaccin. 2013, 4, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Goldammer, T.; Zerbe, H.; Molenaar, A.; Schuberth, H.J.; Brunner, R.M.; Kata, S.R.; Seyfert, H.M. Mastitis increases mammary mRNA abundance of beta-defensin 5, toll-like-receptor 2 (TLR2), and TLR4 but not TLR9 in cattle. Clin. Diagn. Lab. Immunol. 2004, 11, 174–185. [Google Scholar] [CrossRef] [Green Version]
- Ibeagha-Awemu, E.M.; Lee, J.W.; Ibeagha, A.E.; Bannerman, D.D.; Paape, M.J.; Zhao, X. Bacterial lipopolysaccharide induces increased expression of toll-like receptor (TLR) 4 and downstream TLR signaling molecules in bovine mammary epithelial cells. Vet. Res. 2008, 39, 11. [Google Scholar] [CrossRef] [Green Version]
- Bannerman, D.D.; Paape, M.J.; Lee, J.W.; Zhao, X.; Hope, J.C.; Rainard, P. Escherichia coli and Staphylococcus aureus elicit differential innate immune responses following intramammary infection. Clin. Diagn. Lab. Immunol. 2004, 11, 463–472. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Shen, L.; Jiang, J.; Xu, Q.; Luo, Z.; Luo, Q.; Yu, S.; Yao, X.; Ren, Z.; Hu, Y.; et al. Metabolomic Profiles of Bovine Mammary Epithelial Cells Stimulated by Lipopolysaccharide. Sci. Rep. 2019, 9, 19131. [Google Scholar] [CrossRef] [Green Version]
- Moyes, K.M.; Larsen, T.; Sørensen, P.; Ingvartsen, K.L. Changes in various metabolic parameters in blood and milk during experimental Escherichia coli mastitis for primiparous Holstein dairy cows during early lactation. J. Anim. Sci. Biotechnol. 2014, 5, 47. [Google Scholar] [CrossRef] [Green Version]
- Paape, M.J.; Bannerman, D.D.; Zhao, X.; Lee, J.W. The bovine neutrophil: Structure and function in blood and milk. Vet. Res. 2003, 34, 597–627. [Google Scholar]
- Zhao, X.; Lacasse, P. Mammary tissue damage during bovine mastitis: Causes and control. J. Anim. Sci. 2008, 86, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Lauzon, K.; Zhao, X.; Bouetard, A.; Delbecchi, L.; Paquette, B.; Lacasse, P. Antioxidants to prevent bovine neutrophil-induced mammary epithelial cell damage. J. Dairy Sci. 2005, 88, 4295–4303. [Google Scholar] [CrossRef] [Green Version]
- Mehrzad, J.; Desrosiers, C.; Lauzon, K.; Robitaille, G.; Zhao, X.; Lacasse, P. Proteases involved in mammary tissue damage during endotoxin-induced mastitis in dairy cows. J. Dairy Sci. 2005, 88, 211–222. [Google Scholar] [CrossRef] [Green Version]
- Lavon, Y.; Leitner, G.; Moallem, U.; Klipper, E.; Voet, H.; Jacoby, S.; Glick, G.; Meidan, R.; Wolfenson, D. Immediate and carryover effects of Gram-negative and Gram-positive toxin-induced mastitis on follicular function in dairy cows. Theriogenology 2011, 76, 942–953. [Google Scholar] [CrossRef]
- Jacobsen, S.; Toelboell, T.; Andersen, P.H. Dose dependency and individual variability in selected clinical, haematological and blood biochemical responses after systemic lipopolysaccharide challenge in cattle. Vet. Res. 2005, 36, 167–178. [Google Scholar] [CrossRef] [Green Version]
- Hoeben, D.; Burvenich, C.; Trevisi, E.; Bertoni, G.; Hamann, J.; Bruckmaier, R.M.; Blum, J.W. Role of endotoxin and TNF-alpha in the pathogenesis of experimentally induced coliform mastitis in periparturient cows. J. Dairy Res. 2000, 67, 503–514. [Google Scholar] [CrossRef]
- Minuti, A.; Zhou, Z.; Graugnard, D.E.; Rodriguez-Zas, S.L.; Palladino, A.R.; Cardoso, F.C.; Trevisi, E.; Loor, J.J. Acute mammary and liver transcriptome responses after an intramammary Escherichia coli lipopolysaccharide challenge in postpartal dairy cows. Physiol. Rep. 2015, 4, e12388. [Google Scholar] [CrossRef] [Green Version]
- Khovidhunkit, W.; Kim, M.S.; Memon, R.A.; Shigenaga, J.K.; Moser, A.H.; Feingold, K.R.; Grunfeld, C. Effects of infection and inflammation on lipid and lipoprotein metabolism: Mechanisms and consequences to the host. J. Lipid Res. 2004, 45, 1169–1196. [Google Scholar] [CrossRef] [Green Version]
- Perkins, K.H.; VandeHaar, M.J.; Burton, J.L.; Liesman, J.S.; Erskine, R.J.; Elsasser, T.H. Clinical responses to intramammary endotoxin infusion in dairy cows subjected to feed restriction. J. Dairy Sci. 2002, 85, 1724–1731. [Google Scholar] [CrossRef]
- Doll, K.; Sickinger, M.; Seeger, T. New aspects in the pathogenesis of abomasal displacement. Vet. J. 2009, 181, 90–96. [Google Scholar] [CrossRef]
- Zebeli, Q.; Sivaraman, S.; Dunn, S.M.; Ametaj, B.N. Intermittent parenteral administration of endotoxin triggers metabolic and immunological alterations typically associated with displaced abomasum and retained placenta in periparturient dairy cows. J. Dairy Sci. 2011, 94, 4968–4983. [Google Scholar] [CrossRef] [PubMed]
- Vlaminck, K.; van Meirhaeghe, H.; van den Hende, C.; Oyaert, W.; Muylle, E. Effect of endotoxins on abomasal emptying in cattle. Dtsch. Tierarztl. Wochenschr. 1985, 92, 392–395. [Google Scholar] [PubMed]
- Kaze, C.; Mevissen, M.; Hirsbrunner, G.; Steiner, A. Effect of endotoxins on contractility of smooth muscle preparations from the bovine abomasal antrum. Dtsch. Tierarztl. Wochenschr. 2004, 111, 28–35. [Google Scholar] [PubMed]
- Ziv, G. Treatment of peracute and acute mastitis. Vet. Clin. N. Am. Food Anim. Pract. 1992, 8, 1–15. [Google Scholar] [CrossRef]
- Ohtsuka, H.; Kudo, K.; Mori, K.; Nagai, F.; Hatsugaya, A.; Tajima, M.; Tamura, K.; Hoshi, F.; Koiwa, M.; Kawamura, S. Acute phase response in naturally occurring coliform mastitis. J. Vet. Med. Sci. 2001, 63, 675–678. [Google Scholar] [CrossRef] [Green Version]
- Petzl, W.; Günther, J.; Pfister, T.; Sauter-Louis, C.; Goetze, L.; von Aulock, S.; Hafner-Marx, A.; Schuberth, H.J.; Seyfert, H.M.; Zerbe, H. Lipopolysaccharide pretreatment of the udder protects against experimental Escherichia coli mastitis. Innate Immun. 2012, 18, 467–477. [Google Scholar] [CrossRef]
- LeBlanc, S.J. Postpartum uterine disease and dairy herd reproductive performance: A review. Vet. J. 2008, 176, 102–114. [Google Scholar] [CrossRef]
- Kimura, K.; Goff, J.P.; Kehrli, M.E., Jr.; Reinhardt, T.A. Decreased neutrophil function as a cause of retained placenta in dairy cattle. J. Dairy Sci. 2002, 85, 544–550. [Google Scholar] [CrossRef]
- Dohmen, M.J.; Joop, K.; Sturk, A.; Bols, P.E.; Lohuis, J.A. Relationship between intra-uterine bacterial contamination, endotoxin levels and the development of endometritis in postpartum cows with dystocia or retained placenta. Theriogenology 2000, 54, 1019–1032. [Google Scholar] [CrossRef]
- Diez-Fraile, A.; Meyer, E.; Duchateau, L.; Burvenich, C. L-selectin and beta2-integrin expression on circulating bovine polymorphonuclear leukocytes during endotoxin mastitis. J. Dairy Sci. 2003, 86, 2334–2342. [Google Scholar] [CrossRef]
- Boro, P.; Kumaresan, A.; Singh, A.K.; Gupta, D.; Kumar, S.; Manimaran, A.; Mohanty, A.K.; Mohanty, T.K.; Pathak, R.; Attupuram, N.M.; et al. Expression of short chain fatty acid receptors and pro-inflammatory cytokines in utero-placental tissues is altered in cows developing retention of fetal membranes. Placenta 2014, 35, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Azawi, O.I. Postpartum uterine infection in cattle. Anim. Reprod. Sci. 2008, 105, 187–208. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, I.M.; Cronin, J.; Goetze, L.; Donofrio, G.; Schuberth, H.J. Defining postpartum uterine disease and the mechanisms of infection and immunity in the female reproductive tract in cattle. Biol. Reprod. 2009, 81, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, R.O.; Shin, S.T.; Guard, C.L.; Erb, H.N.; Frajblat, M. Prevalence of endometritis and its effects on reproductive performance of dairy cows. Theriogenology 2005, 64, 1879–1888. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.J.; Sibley, K.; Miller, A.N.; Lane, E.A.; Fishwick, J.; Nash, D.M.; Herath, S.; England, G.C.; Dobson, H.; Sheldon, I.M. The effect of Escherichia coli lipopolysaccharide and tumour necrosis factor alpha on ovarian function. Am. J. Reprod. Immunol. 2008, 60, 462–473. [Google Scholar] [CrossRef] [Green Version]
- Herath, S.; Lilly, S.T.; Fischer, D.P.; Williams, E.J.; Dobson, H.; Bryant, C.E.; Sheldon, I.M. Bacterial lipopolysaccharide induces an endocrine switch from prostaglandin F2 to prostaglandin E2 in bovine endometrium. Endocrinology 2009, 150, 1912–1920. [Google Scholar] [CrossRef]
- Purba, F.Y.; Ueda, J.; Nii, T.; Yoshimura, Y.; Isobe, N. Effects of intrauterine infusion of bacterial lipopolysaccharides on the mammary gland inflammatory response in goats. Vet. Immunol. Immunopathol. 2020, 219, 109972. [Google Scholar] [CrossRef]
- Machado, V.S.; Bicalho, M.L.; Meira Junior, E.B.; Rossi, R.; Ribeiro, B.L.; Lima, S.; Santos, T.; Kussler, A.; Foditsch, C.; Ganda, E.K.; et al. Subcutaneous immunization with inactivated bacterial components and purified protein of Escherichia coli, Fusobacterium necrophorum and Trueperella pyogenes prevents puerperal metritis in Holstein dairy cows. PLoS ONE 2014, 9, e91734. [Google Scholar] [CrossRef]
- Nocek, J.E. Bovine acidosis: Implications on laminitis. J. Dairy Sci. 1997, 80, 1005–1028. [Google Scholar] [CrossRef]
- Danscher, A.M.; Enemark, J.M.; Telezhenko, E.; Capion, N.; Ekstrøm, C.T.; Thoefner, M.B. Oligofructose overload induces lameness in cattle. J. Dairy Sci. 2009, 92, 607–616. [Google Scholar] [CrossRef]
- Shearer, J.K.; van Amstel, S.R. Pathogenesis and treatment of sole ulcers and white line disease. Vet. Clin. N. Am. Food Anim. Pract. 2017, 33, 283–300. [Google Scholar] [CrossRef] [PubMed]
- Boosman, R.; Mutsaers, C.W.; Klarenbeek, A. The role of endotoxin in the pathogenesis of acute bovine laminitis. Vet. Q. 1991, 13, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.S.; Murray, R.D.; Ward, W.R. Gross and histopathological study of endotoxin-induced hoof lesions in cattle. J. Comp. Pathol. 1994, 110, 103–115. [Google Scholar] [CrossRef]
- Thoefner, M.B.; Pollitt, C.C.; Van Eps, A.W.; Milinovich, G.J.; Trott, D.J.; Wattle, O.; Andersen, P.H. Acute bovine laminitis: A new induction model using alimentary oligofructose overload. J. Dairy Sci. 2004, 87, 2932–2940. [Google Scholar] [CrossRef] [Green Version]
- Bailey, S.R.; Adair, H.S.; Reinemeyer, C.R.; Morgan, S.J.; Brooks, A.C.; Longhofer, S.L.; Elliott, J. Plasma concentrations of endotoxin and platelet activation in the developmental stage of oligofructose-induced laminitis. Vet. Immunol. Immunopathol. 2009, 129, 167–173. [Google Scholar] [CrossRef]
- Brooks, A.C.; Menzies-Gow, N.J.; Wheeler-Jones, C.; Bailey, S.R.; Cunning-ham, F.M.; Elliott, J. Endotoxin-induced activation of equine platelets: Evidence for direct activation of p38 MAPK pathways and vasoactive mediator production. Inflamm. Res. 2007, 56, 154–161. [Google Scholar] [CrossRef]
- Brandt, E.; Petersen, F.; Ludwig, A.; Ehlert, J.E.; Bock, L.; Flad, H.D. The beta-thromboglobulins and platelet factor 4: Blood platelet-derived CXC chemokines with divergent roles in early neutrophil regulation. J. Leukoc. Biol. 2000, 67, 471–478. [Google Scholar] [CrossRef]
- Reisinger, N.; Schaumberger, S.; Nagl, V.; Hessenberger, S.; Schatzmayr, G. Concentration Dependent Influence of Lipopolysaccharides on Separation of Hoof Explants and Supernatant Lactic Acid Concentration in an Ex Vivo/In Vitro Laminitis Model. PLoS ONE 2015, 10, e0143754. [Google Scholar] [CrossRef]
- Eades, S.C. Overview of current laminitis research. Vet. Clin. N. Am. Equine Pract. 2010, 26, 51–63. [Google Scholar] [CrossRef]
- Kelton, D.F.; Lissemore, K.D.; Martin, R.E. Recommendations for recording and calculating the incidence of selected clinical diseases of dairy cattle. J. Dairy Sci. 1998, 81, 2502–2509. [Google Scholar] [CrossRef]
- Caixeta, L.S.; Herman, J.A.; Johnson, G.W.; McArt, J.A.A. Herd-Level monitoring and prevention of displaced abomasum in dairy cattle. Vet. Clin. N. Am. Food Anim. Pract. 2018, 34, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Whitlock, R.H. Diseases of the abomasum associated with current feeding practices. J. Am. Vet. Med. Assoc. 1969, 154, 1203–1205. [Google Scholar]
- Markusfeld, O. The association of displaced abomasum withvarious periparturient factors in dairy cows. A retrospective study. Prev. Vet. Med. 1986, 4, 173–183. [Google Scholar] [CrossRef]
- Constable, P.D.; Miller, G.Y.; Hoffsis, G.F.; Hull, B.L.; Rings, D.M. Risk factors for abomasal volvulus and left abomasal displace-ment in cattle. Am. J. Vet. Res. 1992, 53, 1184–1192. [Google Scholar] [PubMed]
- Coppock, C.E. Displaced abomasum in dairy cattle: Etiological factors. J. Dairy Sci. 1974, 57, 926–933. [Google Scholar] [CrossRef]
- Detilleux, J.C.; Grohn, Y.T.; Eicker, S.W.; Quaas, R.L. Effects of left displaced abomasum on test day milk yields of Holstein cows. J. Dairy Sci. 1997, 80, 121–126. [Google Scholar] [CrossRef]
- Cameron, R.E.; Dyk, P.B.; Herdt, T.H.; Kaneene, J.B.; Miller, R.; Bucholtz, H.F.; Liesman, J.S.; Vandehaar, M.J.; Emery, R.S. Dry cow diet, management, and energy balance as risk factors for displaced abomasum in high producing dairy herds. J. Dairy Sci. 1998, 81, 132–139. [Google Scholar] [CrossRef]
- Shaver, R.D. Nutritional risk factors in the etiology of left displaced abomasum in dairy cows: A review. J. Dairy Sci. 1997, 80, 2449–2453. [Google Scholar] [CrossRef]
- Stengarde, L.; Holtenius, K.; Traven, M.; Hultgren, J.; Niskanen, R.; Emanuelson, U. Blood profiles in dairy cows with displaced abomasum. J. Dairy Sci. 2010, 93, 4691–4699. [Google Scholar] [CrossRef] [Green Version]
- LeBlanc, S.J.; Leslie, K.E.; Duffield, T.F. Metabolic predictors of displaced abomasum in dairy cattle. J. Dairy Sci. 2005, 88, 159–170. [Google Scholar] [CrossRef] [Green Version]
- Suthar, V.S.; Canelas-Raposo, J.; Deniz, A.; Heuwieser, W. Prevalence of subclinical ketosis and relationships with postpartum diseases in European dairy cows. J. Dairy Sci. 2013, 96, 2925–2938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zadnik, T. A comparative study of the hemato-biochemical parameters between clinically healthy cows and cows with displacement of the abomasum. Acta Vet. Beogr. 2003, 53, 297–310. [Google Scholar]
- Tsuneyoshi, I.; Kanmura, Y.; Yoshimura, N. Lipoteichoic acid from Staphylococcus aureus depresses contractile function of human arteries in vitro due to the induction of nitric oxide synthase. Anesth. Analg. 1996, 82, 948–953. [Google Scholar] [PubMed]
- Gray, C.P.; St George, T.D.; Jonsson, N.N. Milk fever in dairy cattle: A novel hypothesis for an immune mediated aetiology. Cattle Pract. 2007, 15, 277–282. [Google Scholar]
- DeGaris, P.J.; Lean, I.J. Milk fever in dairy cows: A review of pathophysiology and control principles. Vet. J. 2008, 176, 58–69. [Google Scholar] [CrossRef]
- Waldron, M.R.; Nonnecke, B.J.; Nishida, T.; Horst, R.L.; Overton, T.R. Effect of lipopolysaccharide infusion on serum macromineral and vitamin D concentrations in dairy cows. J. Dairy Sci. 2003, 86, 3440–3446. [Google Scholar] [CrossRef]
- Holowaychuk, M.K.; Hansen, B.D.; DeFrancesco, T.C.; Marks, S.L. Ionized hypocalcemia in critically ill dogs. J. Vet. Intern. Med. 2009, 23, 509–513. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Karl, I.E. Calcium: A regulator of the inflammatory response in endotoxemia and sepsis. New Horiz. 1996, 4, 58–71. [Google Scholar]
- Malcolm, D.S.; Zaloga, G.P.; Holaday, J.W. Calcium administration increases the mortality of endotoxic shock in rats. Crit. Care Med. 1989, 17, 900–903. [Google Scholar] [CrossRef]
- Zaloga, G.P.; Malcolm, D.; Chernow, B.; Holaday, J.W. Endotoxin-induced hypocalcemia results in defective calcium mobilization in rats. Circ. Shock 1988, 24, 143–148. [Google Scholar]
- Collage, R.D.; Howell, G.M.; Zhang, X.; Stripay, J.L.; Lee, J.S.; Angus, D.C.; Rosengart, M.R. Calcium supplementation during sepsis exacerbates organ failure and mortality via calcium/calmodulin-dependent protein kinase kinase signaling. Crit. Care Med. 2013, 41, e352–e360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chmielinska, J.J.; Tejero-Taldo, M.I.; Mak, I.T.; Weglicki, W.B. Intestinal and cardiac inflammatory response shows enhanced endotoxin receptor (CD14) expression in magnesium deficiency. Mol. Cell. Biochem. 2005, 278, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Zebeli, Q.; Beitz, D.C.; Bradford, B.J.; Dunn, S.M.; Ametaj, B.N. Peripartal alterations of calcitonin gene-related peptide and minerals in dairy cows affected by milk fever. Vet. Clin. Pathol. 2013, 42, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Dervishi, E.; Ametaj, B.N. Milk fever in dairy cows is preceded by activation of innate immunity and alterations in carbohydrate metabolism prior to disease occurrence. Res. Vet. Sci. 2018, 117, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Ametaj, B.N.; Hosseini, A.; Odhiambo, J.F.; Zebeli, Q.; Deng, Q.; Sharma, S.; Iqbal, S.; Dunn, S.M.; Lam, T.H.; Farooq, U. Application of Acute Phase Proteins for Monitoring Inflammatory States in Cattle; Veas, F., Ed.; InTech Europe: Rijeka, Croatia, 2011; pp. 299–354. ISBN 978-953-307-873-1. [Google Scholar]
- Schulte, W.; Bernhagen, J.; Bucala, R. Cytokines in sepsis: Potent immunoregulators and potential therapeutic targets-an updated view. Mediat. Inflamm. 2013, 2013, 165974. [Google Scholar] [CrossRef]
- Correa, M.T.; Erb, H.N.; Scarlett, J.M. Risk factors for downer cow syndrome. J. Dairy Sci. 1993, 76, 3460–3463. [Google Scholar] [CrossRef]
- Eckel, E.F.; Ametaj, B.N. Invited review: Role of bacterial endotoxins in the etiopathogenesis of periparturient diseases of transition dairy cows. J. Dairy Sci. 2016, 99, 5967–5990. [Google Scholar] [CrossRef]
- Kalaitzakis, E.; Panousis, N.; Roubies, N.; Giadinis, N.; Kaldrymidou, E.; Georgiadis, M.; Karatzias, K. Clinicopathological evaluation of downer dairy cows with fatty liver. Can. Vet. J. 2010, 51, 615–622. [Google Scholar]
- Byrne, C.M.; Erol, I.; Call, J.E.; Kaspar, C.W.; Buege, D.R.; Hiemke, C.J.; Fedorka-Cray, P.J.; Benson, A.K.; Wallace, F.M.; Luchansky, J.B. Characterization of Escherichia coli O157:H7 from downer and healthy dairy cattle in the upper Midwest region of the United States. Appl. Environ. Microbiol. 2003, 69, 4683–4688. [Google Scholar] [CrossRef] [Green Version]
- Neutra, M.R.; Kozlowski, P.A. Mucosal vaccines: The promise and the challenge. Nat. Rev. Immunol. 2006, 6, 148–158. [Google Scholar] [CrossRef]
- De Magistris, M.T. Mucosal delivery of vaccine antigens and its advantages in pediatrics. Adv. Drug Deliv. Rev. 2006, 58, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Dlugonska, H.; Grzybowski, M. Mucosal vaccination—An old but still vital strategy. Ann. Parasitol. 2012, 58, 1–8. [Google Scholar] [PubMed]
- Gerdts, V.; Mutwiri, G.K.; Tikoo, S.K.; Babiuk, L.A. Mucosal delivery of vaccines in domestic animals. Vet. Res. 2006, 37, 487–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clements, J.D.; Freytag, L.C. Parenteral Vaccination Can Be an Effective Means of Inducing Protective Mucosal Responses. Clin. Vaccine Immunol. 2016, 23, 438–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simecka, J.W. Mucosal immunity of the gastrointestinal tract and oral tolerance. Adv. Drug Deliv. Rev. 1998, 34, 235–259. [Google Scholar] [CrossRef]
- Holmgren, J.; Czerkinsky, C. Mucosal immunity and vaccines. Nat. Med. 2005, 11, S45–S53. [Google Scholar] [CrossRef]
- Wershil, B.K.; Furuta, G.T. 4. Gastrointestinal mucosal immunity. J. Allergy Clin. Immunol. 2008, 121, S380–S383. [Google Scholar] [CrossRef]
- Apter, F.M.; Michetti, P.; Winner, L.S., 3rd; Mack, J.A.; Mekalanos, J.J.; Neutra, M.R. Analysis of the roles of antilipopolysaccharide and anti-cholera toxin immunoglobulin A (IgA) antibodies in protection against Vibrio cholerae and cholera toxin by use of monoclonal IgA antibodies in vivo. Infect. Immun. 1993, 61, 5279–5285. [Google Scholar] [CrossRef] [Green Version]
- Hanihara-Tatsuzawa, F.; Miura, H.; Kobayashi, S.; Isagawa, T.; Okuma, A.; Manabe, I.; MaruYama, T. Control of Toll-like receptor–mediated T cell–independent type 1 antibody responses by the inducible nuclear protein IκB-ζ. J. Biol. Chem. 2014, 289, 30925–30936. [Google Scholar] [CrossRef] [Green Version]
- Weill, F.S.; Cela, E.M.; Paz, M.L.; Ferrari, A.; Leoni, J.; Gonzalez Maglio, D.H. Lipoteichoic acid from Lactobacillus rhamnosus GG as an oral photoprotective agent against UV-induced carcinogenesis. Br. J. Nutr. 2013, 109, 457–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandtzaeg, P. Induction of secretory immunity and memory at mucosal surfaces. Vaccine 2007, 25, 5467–5484. [Google Scholar] [CrossRef] [PubMed]
- Brandtzaeg, P. Secretory immunity with special reference to the oral cavity. J. Oral Microbiol. 2013, 5, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Ogra, P.L.; Faden, H.; Welliver, R.C. Vaccination strategies for mucosal immune responses. Clin. Microbiol. Rev. 2001, 14, 430–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hopkins, S.; Kraehenbuhl, J.P.; Schodel, F.; Potts, A.; Peterson, D.; de Grandi, P.; Nardelli-Haefliger, D. A recombinant Salmonella typhimurium vaccine induces local immunity by four different routes of immunization. Infect. Immun. 1995, 63, 3279–3286. [Google Scholar] [CrossRef] [Green Version]
- Deauvieau, F.; Dussurgey, S.; Rossignol, D.; de Montfort, A.; Burdin, N.; Guy, B. Memory B and T cell responses induced by serotype 4 Streptococcus pneumoniae vaccines: Longitudinal analysis comparing responses elicited by free polysaccharide, conjugate and carrier. Vaccine 2009, 28, 576–582. [Google Scholar] [CrossRef]
- Alugupalli, K.R.; Leong, J.M.; Woodland, R.T.; Muramatsu, M.; Honjo, T.; Gerstein, R.M. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity 2004, 21, 379–390. [Google Scholar] [CrossRef] [Green Version]
- Defrance, T.; Taillardet, M.; Genestier, L. T cell-independent B cell memory. Curr. Opin. Immunol. 2011, 23, 330–336. [Google Scholar] [CrossRef]
- Obukhanych, T.V.; Nussenzweig, M.C. T-independent type II immune responses generate memory B cells. J. Exp. Med. 2006, 203, 305–310. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.M.; Rahman, M.A.; Mohasin, M.; Riyadh, M.A.; Leung, D.T.; Alam, M.M.; Chowdhury, F.; Khan, A.I.; Weil, A.A.; Aktar, A.; et al. Memory B cell responses to Vibrio cholerae O1 lipopolysaccharide are associated with protection against infection from household contacts of patients with cholera in Bangladesh. Clin. Vaccine Immunol. 2012, 19, 842–848. [Google Scholar] [CrossRef] [Green Version]
- Simon, J.K.; Wahid, R.; Maciel, M., Jr.; Picking, W.L.; Kotloff, K.L.; Levine, M.M.; Sztein, M.B. Antigen-specific B memory cell responses to lipopolysaccharide (LPS) and invasion plasmid antigen (Ipa) B elicited in volunteers vaccinated with live-attenuated Shigella flexneri 2a vaccine candidates. Vaccine 2009, 27, 565–572. [Google Scholar] [CrossRef] [Green Version]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eckel, E.F.; Ametaj, B.N. Bacterial Endotoxins and Their Role in Periparturient Diseases of Dairy Cows: Mucosal Vaccine Perspectives. Dairy 2020, 1, 61-90. https://doi.org/10.3390/dairy1010006
Eckel EF, Ametaj BN. Bacterial Endotoxins and Their Role in Periparturient Diseases of Dairy Cows: Mucosal Vaccine Perspectives. Dairy. 2020; 1(1):61-90. https://doi.org/10.3390/dairy1010006
Chicago/Turabian StyleEckel, Emily F., and Burim N. Ametaj. 2020. "Bacterial Endotoxins and Their Role in Periparturient Diseases of Dairy Cows: Mucosal Vaccine Perspectives" Dairy 1, no. 1: 61-90. https://doi.org/10.3390/dairy1010006
APA StyleEckel, E. F., & Ametaj, B. N. (2020). Bacterial Endotoxins and Their Role in Periparturient Diseases of Dairy Cows: Mucosal Vaccine Perspectives. Dairy, 1(1), 61-90. https://doi.org/10.3390/dairy1010006





