Lactic Acid Bacteria: Food Safety and Human Health Applications
Abstract
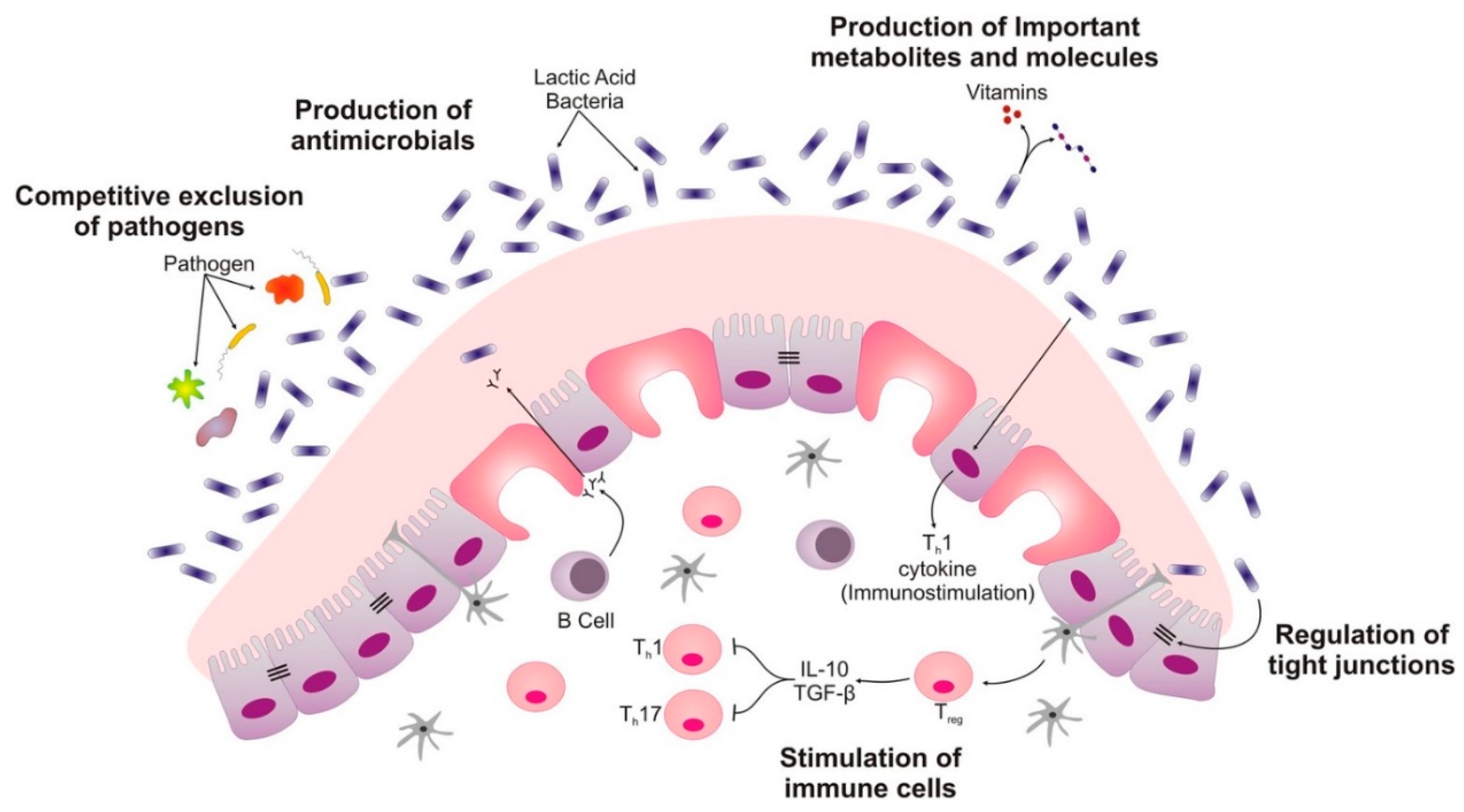
:1. Introduction
2. Lactic Acid Bacteria
2.1. Taxonomic Classification of Lactic Acid Bacteria
2.2. Niche or Habitat of Lactic Acid Bacteria
2.3. Lactic Acid Bacteria in Bio-Preservation
2.4. Lactic Acid Bacteria in Fermented Foods
2.5. Milk Fermentation with Lactic Acid Bacteria
2.6. Lactic Acid Bacteria as an Essential Strain in Dairy Starter Cultures
2.7. Lactobacillus delbrueckii subsp. bulgaricus
3. History of Probiotics
3.1. Origin of Probiotics
3.2. Mechanism of Probiotics
- (i)
- Probiotics can modulate the host’s defenses which include the innate as well as the acquired immune system. This mode of action is most critical for prevention and therapy for infectious diseases but also for the treatment of chronic inflammation of the gastrointestinal tract.
- (ii)
- Probiotics could also directly impact other microorganisms, commensal, and/or pathogenic ones in general. This property could be of immense benefit and vital in prevention and therapy for infections and the overall restoration of the microbial equilibrium in the gut.
- (iii)
- Additionally, probiotic effects may be linked to actions affecting microbial products such as toxins and host products, e.g., bile salts and food ingredients. This property may result in the inactivation of toxins and aids in detoxification in the gastrointestinal gut. It is also worth noting that the kind of effects depicted by certain strains of probiotics largely depends on the strain’s metabolic properties, the molecules presented on their surfaces or on their secreted components.
- Probiotics compete against pathogenic bacteria to bind to intestinal epithelial cells [86].
- Probiotics enhance the intestinal epithelial barrier function by increasing mucin production, preventing pathogens from causing injury to the epithelium and reducing cell permeability. In addition, probiotics also enhance the mucosal barrier function by inducing the expression of antimicrobial peptides such as defensins [86].
- They inhibit pathogenic growth through the secretion of antimicrobial peptides such as bacteriocins and reuterin. For example, lactic acid bacteria inhibit pathogen growth by creating an acidic environment through the production of organic acids [86].
- Probiotics also stimulate the production of serum Immunoglobin A (IgA) and secrete IgA which plays a vital role in intestinal humoral immunity [86].
- They enhance phagocytosis, increase the activity of natural killer cells, promote cell-mediated immunity, and stimulate various other non-specific immune responses against pathogens [86].
- Probiotics down-regulate pro-inflammatory cytokine production, prevent apoptosis, and suppress the proliferation of T cells thus preventing various inflammatory conditions [86].
- They produce hydrogen peroxide which suppresses pathogens associated with bacterial vaginosis [88].
3.3. Probiotics and Human Health
3.4. Health Benefits of Probiotics in Some Disease Conditions
3.4.1. Lactose Intolerance
3.4.2. Diabetes and Obesity
3.4.3. Acute Diarrheal Disease
3.4.4. Inflammatory Bowel Diseases and Irritable Bowel Syndrome
3.4.5. Cancer
3.4.6. Cardiovascular Diseases
3.4.7. Urogenital Infections
3.4.8. Allergy
3.4.9. Gut–Brain Axis
3.5. Antiviral Activity of Lactic Acid Bacteria
3.5.1. Mechanisms of Probiotic Action on Viruses
- Probiotic bacteria is irreversibly attached to the virus, therefore limiting the virus’ binding effect to the host cell receptor.
- Probiotic adhesive property is capable of obstructing viral attachment on the epithelial surface through steric hindrance.
- Virus replication is inhibited by mucin attachments produced by probiotics through the process of mucosal regeneration.
- Antimicrobial metabolites produced by probiotics act against pathogens.
- Synthesis of dehydrogenase by probiotics may possess and contribute to antiviral processes.
- Epithelial cells generally promote the modulation of immune responses.
- Macrophages and dendritic cells are induced, thus stimulating the immune response.
- Viral cells are destroyed by the joint action of cluster of differentiation 8 (CD8) T cells and T lymphocytes that differentiate into cytotoxic T lymphocytes (CTLs).
- Further differentiation of CD4 and T lymphocytes into helper T cells (Th1 and Th2) occurs.
- Activated phagocytes eliminate viruses through induction of the Th1 cells.
- B-cells are proliferated by stimulation of Th2, which migrates to secondary lymphatic organs resident in mucosa-associated lymphoid tissue (MALT). Differentiation of B cells into Ig producing plasma cells occurs afterward.
- Antibodies activated during this immune response completely eliminate the virus.
3.5.2. Strain-Specific Antiviral Properties of Lactic Acid Bacteria
3.5.3. Antiviral Properties of Bacteriocins
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bintsis, T. Lactic acid bacteriaas starter cultures: An update in their metabolism and genetics. Aims Microbiol. 2018, 4, 665–684. [Google Scholar] [CrossRef] [PubMed]
- Hati, S.; Mandal, S.; Prajapat, J.B. Novel Starters for Value Added Fermented Dairy Products. Curr. Res. Nutr. Food Sci. J. 2013, 1, 83–91. [Google Scholar] [CrossRef]
- Perez, R.H.; Zendo, T.; Sonomoto, K. Novel bacteriocins from lactic acid bacteria (LAB), various structures and applications. Microb. Cell Factories 2014, 13 (Suppl. 1), S3. [Google Scholar] [CrossRef] [Green Version]
- Quinto, E.J.; Jimenez, P.; Caro, I.; Tejero, J.; Mateo, J.; Girbes, T. Probiotic Lactic Acid Bacteria: A Review. Food Nutr. Sci. 2014, 5, 1765–1775. [Google Scholar] [CrossRef] [Green Version]
- Sadishkumar, V.; Jeevaratnam, K. In vitro probiotic evaluation of potential antioxidant lactic acid bacteria isolated from Idli batter fermented with Piper betle leaves. Int. J. Food Sci. Technol. 2017, 52, 329–340. [Google Scholar] [CrossRef]
- Hayek, S.A.; Ibrahim, S.A. Current limitations and challenges with lactic acid bacteria: A review. Food Nutr. Sci. 2013, 4, 73–87. [Google Scholar] [CrossRef] [Green Version]
- Hayek, S.A.; Gyawali, R.; Aljaloud, S.O.; Krastanov, A.; Ibrahim, S.A. Cultivation media for lactic acid bacteria used in dairy products. J. Dairy Res. 2019, 86, 490–502. [Google Scholar] [CrossRef] [Green Version]
- Orla-Jensen, S. The Lactic Acid Bacteria; Andr Fred Host and Sons imp: Copenhagen, Denmark, 1919. [Google Scholar]
- Parvez, S.; Malik, K.A.; Ah Kang, S.; Kim, H.Y. Probiotics and their fermented food products are beneficial for health. J. Appl. Microbiol. 2006, 100, 1171–1185. [Google Scholar] [CrossRef]
- Mokoena, M.P. Lactic Acid Bacteria and Their Bacteriocins: Classification, Biosynthesis and Applications against Uropathogens: A Mini-Review. Molecules 2017, 22, 1255. [Google Scholar] [CrossRef]
- Salminen, S.; Bouley, C.; Boutron-Ruault, M.C.; Cummings, J.H.; Franck, A.; Gibson, G.R.; Isolauri, E.; Moreau, M.C.; Roberfroid, M.; Rowland, I. Functional food science and gastrointestinal physiology and function. Br. J. Nutr. 1998, 80 (Suppl. 1), S147–S171. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Kaur, J.; Lee, S.; Park, Y.S. Tracking of Intentionally Inoculated Lactic Acid Bacteria Strains in Yogurt and Probiotic Powder. Microorganisms 2020, 8, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, K.; Wang, Y.; Li, D.; Cai, Y.; Pang, H. Characterization, identification and application of lactic acid bacteria isolated from forage paddy rice silage. PLoS ONE 2015, 10, e0121967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.; Harris, H.M.; Mattarelli, P.; Watanabe, K. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Pang, H.; Zhang, H.; Cai, Y. Biodiversity of lactic acid bacteria. In Lactic Acid Bacteria; Zhang, H., Cai, Y., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 103–203. [Google Scholar] [CrossRef]
- Hooper, L.V.; Macpherson, A.J. Immune Adaptations That Maintain Homeostasis with the Intestinal Microbiota. Nat. Rev. Immunol. 2010, 10, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Lebeer, S.; Vanderleyden, J.; De Keersmaecker, S.C. Genes and Molecules of Lactobacilli Supporting Probiotic Action. Microbiol. Mol. Biol. Rev. 2008, 72, 728–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz, L.; Margolles, A.; Sánchez, B. Bile resistance mechanisms in Lactobacillus and Bifidobacterium. Front. Microbiol. 2013, 4, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Corfield, A.P.; Myerscough, N.; Longman, R.; Sylvester, P.; Arul, S.; Pignatelli, M. Mucins and Mucosal Protection in the Gastrointestinal Tract: New Prospects for Mucins in the Pathology of Gastrointestinal Disease. Gut 2000, 47, 589–594. [Google Scholar] [CrossRef] [Green Version]
- Moal VL, L.; Servin, A.L. The Front Line on Enteric Host Defence against Unwelcome Intrusion of Harmful Microorganisms: Mucins, Antimicrobial Peptides and Microbiota. Clin. Microbiol. Rev. 2006, 19, 315–337. [Google Scholar] [CrossRef] [Green Version]
- Servin, A.L. Antagonistic Activities of Lactobacilli and Bifidobacteria against Microbial Pathogens. FEMS Microbiol. Rev. 2004, 28, 405–440. [Google Scholar] [CrossRef] [Green Version]
- Sieuwerts, S. Microbial Interactions in the Yoghurt Consortium: Current Status and Product Implications. SOJ Microbiol Infect. Dis. 2016, 4, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Asioli, D.; Aschemann-Witzel, J.; Caputo, V.; Vecchio, R.; Annunziata, A.; Næs, T.; Varela, P. Making sense of the “clean label” trends: A review of consumer food choice behavior and discussion of industry implications. Food Res. Int. 2017, 99, 58–71. [Google Scholar] [CrossRef]
- Yang, S.-C.; Lin, C.-H.; Sung, C.T.; Fang, J.Y. Antibacterial Activities of Bacteriocins: Application in Foods and Pharmaceuticals. Front. Microbiol. 2014, 5, 1–10. [Google Scholar]
- Cotter, P.D.; Ross, R.P.; Hill, C. Bacteriocins—A Viable Alternative to Antibiotics? Nat. Rev. Microbiol. 2013, 11, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Quinto, E.J.; Caro, I.; Villalobos-Delgado, L.H.; Mateo, J.; De-Mateo-Silleras, B.; Redondo-Del-Río, M.P. Food safety through natural antimicrobials. Antibiotics 2019, 8, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernbom, N.; Licht, T.R.; Brogren, C.H.; Jelle, B.; Johansen, A.H.; Badiola, I.; Vogensen, F.K.; Norrung, B. Effects of Lactococcus lactis on Composition of Intestinal Microbiota: Role of Nisin. Appl. Environ. Microbiol. 2006, 72, 239–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagpal, R.; Kumar, A.; Kumar, M.; Behare, P.V.; Jain, S.; Yadav, H. Probiotics, their health benefits and applications for developing healthier foods: A review. FEMS Microbiol. Lett. 2012, 334, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Papadimitriou, K.; Zoumpopoulou, G.; Foligné, B.; Alexandraki, V.; Kazou, M.; Pot, B.; Tsakalidou, E. Discovering probiotic microorganisms: In vitro, in vivo, genetic and omics approaches. Front. Microbiol. 2015, 6, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Ponce, A.G.; Moreira, M.R.; Del Valle, C.E.; Roura, S.I. Preliminary characterization of bacteriocin-like substances from lactic acid bacteria isolated from organic leafy vegetables. LWT Food Sci. Technol. (Campinas.) 2008, 41, 432–441. [Google Scholar] [CrossRef]
- Seifu, E.; Buys, E.M.; Donkin, E.F. Significance of the lactoperoxidase system in the dairy industry and its potential applications: A review. Trends Food Sci. Technol. 2005, 16, 137–154. [Google Scholar] [CrossRef]
- Ibrahim, O.O. Classification of Antimicrobial Peptides Bacteriocins, and the Nature of some Bacteriocins with Potential Applications in Food Safety and Bio-Pharmaceuticals. EC Microbiol. 2019, 15, 591–608. [Google Scholar]
- Ramu, R.; Shirahatti, P.S.; Devi, A.T.; Prasad, A.; Kumuda, J.; Lochana, M.S.; Zameer, F.; Dhananjaya, B.L.; Nagendra-Prasad, M.N. Bacteriocins and Their Applications in Food Preservation. Crit. Rev. Food Sci. Nutr. 2015, 1–42. [Google Scholar] [CrossRef]
- Gupta, R.; Jeevaratnam, K. Lactic Acid Bacteria: Probiotic Characteristic, Selection Criteria, and its Role in Human Health (A Review). J. Emerg. Technol. Innov. Res. (JETIR) 2018, 5, 411–424. [Google Scholar]
- FAO/WHO Codex Alimentarius Commission; Joint FAO/WHO Food Standards Programme; World Health Organization. Codex Alimentarius: Food Hygiene, Basic Texts; Food & Agriculture Organization, Wiley Press: London, UK, 2003. [Google Scholar]
- Ghosh, T.; Beniwal, A.; Semwal, A.; Navani, N.K. Mechanistic insights into probiotic properties of lactic acid bacteria associated with ethnic fermented dairy products. Front. Microbiol. 2019, 10, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balamurugan, R.; Chandragunasekaran, A.S.; Chellappan, G.; Rajaram, K.; Ramamoorthi, G.; Ramakrishna, B.S. Probiotic potential of lactic acid bacteria present in homemade curd in southern India. Indian J. Med. Res. 2014, 140, 345–355. [Google Scholar] [PubMed]
- Luo, F.; Feng, S.; Sun, Q.; Xiang, W.; Zhao, J.; Zhang, J.; Yang, Z. Screening for bacteriocin-producing lactic acid bacteria from kurut, a traditional naturally-fermented yak milk from Qinghai–Tibet plateau. Food Control 2011, 22, 50–53. [Google Scholar] [CrossRef]
- Seo, M.K.; Park, E.J.; Ko, S.Y.; Choi, E.W.; Kim, S. Therapeutic effects of kefir grain Lactobacillus-derived extracellular vesicles in mice with 2, 4, 6-trinitrobenzene sulfonic acid-induced inflammatory bowel disease. J. Dairy Sci. 2018, 101, 8662–8671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Ahmed, Z.; Feng, W.; Li, C.; Song, S. Physicochemical properties of exopolysaccharide produced by Lactobacillus kefiranofaciens ZW3 isolated from Tibet kefir. Int. J. Biol. Macromol. 2008, 43, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Bonczar, G.; Walczycka, M.B.; Domagała, J.; Maciejowski, K.; Najgebauer-Lejko, D.; Sady, M.; Wszołek, M. Effect of dairy animal species and of the type of starter cultures on the cholesterol content of manufactured fermented milks. Small Rumin. Res. 2016, 136, 22–26. [Google Scholar] [CrossRef]
- Li, S.; Zhao, Y.; Zhang, L.; Zhang, X.; Huang, L.; Li, D.; Niu, C.; Yang, Z.; Wang, Q. Antioxidant activity of Lactobacillus plantarum strains isolated from traditional Chinese fermented foods. Food Chem. 2012, 135, 1914–1919. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, S.; Yuan, Y.; Yue, T.; Li, J. Comparison of lactobacilli isolated from Chinese suan-tsai and koumiss for their probiotic and functional properties. J. Funct. Foods 2015, 12, 294–302. [Google Scholar] [CrossRef]
- Akabanda, F.; Owusu-Kwarteng, J.; Tano-Debrah, K.; Glover, R.L.; Nielsen, D.S.; Jespersen, L. Taxonomic and molecular characterization of lactic acid bacteria and yeasts in nunu, a Ghanaian fermented milk product. Food Microbiol. 2013, 34, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.S.; Ray, R.C.; Zdolec, N. Lactobacillus plantarum with Functional Properties: An Approach to Increase Safety and Shelf-Life of Fermented Foods. BioMed Res. Int. 2018, 9361614, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Kwak, M.K.; Liu, R.; Kang, S.O. Antimicrobial activity of cyclic dipeptides produced by Lactobacillus plantarum LBP-K10 against multidrug-resistant bacteria, pathogenic fungi, and influenza A virus. Food Control 2017, 85, 223–234. [Google Scholar] [CrossRef]
- Parente, E.; Cogan, T.M. Starter cultures: General aspects. Cheese 2004, 1, 123–148. [Google Scholar] [CrossRef]
- Settanni, L.; Gaglio, R.; Guarcello, R.; Francesca, N.; Carpino, S.; Sannino, C.; Todaro, M. Selected lactic acid bacteria as a hurdle to the microbial spoilage of cheese: Application on a traditional raw ewes’ milk cheese. Int. Dairy J. 2013, 32, 126–132. [Google Scholar] [CrossRef] [Green Version]
- Panesar, P.S. Fermented Dairy Products: Starter Cultures and Potential Nutritional Benefits. Food Nutr. Sci. 2011, 2, 47–51. [Google Scholar] [CrossRef] [Green Version]
- Daly, D.F.M.; McSweeney, P.L.H.; Sheehan, J.J. Split defect and secondary fermentation in Swiss-type cheeses—A review. Dairy Sci. Technol. 2010, 90, 3–26. [Google Scholar] [CrossRef]
- Abou-Donia, S.A. Recent developments in Zabady and Egyptian Labneh research: A review. Egypt. J. Dairy Sci. 2004, 32, 1–15. [Google Scholar]
- Obodai, M.; Dodd, C.E.R. Characterization of dominant microbiota of a Ghanaian fermented milk product, nyarmie, by culture- and nonculture-based methods. J. Appl. Microbiol. 2006, 100, 1355–1363. [Google Scholar] [CrossRef]
- Gyawali, R.; Nwamaioha, N.; Fiagbor, R.; Zimmerman, T.; Newman, R.H.; Ibrahim, S.A. The role of prebiotics in disease prevention and health promotion. In Dietary Interventions in Gastrointestinal Diseases; Academic Press: Cambridge, MA, USA, 2019; pp. 151–167. [Google Scholar]
- Deng, K.; Fang, W.; Zheng, B.; Miao, S.; Huo, G. Phenotypic, fermentation characterization, and resistance mechanism analysis of bacteriophage-resistant mutants of Lactobacillus delbrueckii ssp. bulgaricus isolated from traditional Chinese dairy products. J. Dairy Sci. 2018, 101, 1901–1914. [Google Scholar] [CrossRef] [Green Version]
- Todorov, S.D.; Ho, P.; Vaz-Velho, M.; Dicks, L.M. Characterization of bacteriocins produced by two strains of Lactobacillus plantarum isolated from Beloura and Chourico, traditional pork products from Portugal. Meat Sci. 2010, 84, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Biscola, V.; Todorov, S.D.; Capuano, V.S.C.; Abriouel, H.; Gálvez, A.; Franco, B.D. Isolation and characterization of a nisin-like bacteriocin produced by a Lactococcus lactis strain isolated from charqui, a Brazilian fermented, salted and dried meat product. Meat Sci. 2013, 93, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Cerning, J. Production of exopolysaccharides by lactic acid bacteria and dairy propionibacteria. Le Lait 1995, 75, 463–472. [Google Scholar] [CrossRef]
- Rogosa, M.; Hansen, P.A. Nomenclatural considerations of certain species of Lactobacillus Beijerinck: Request for an opinion. Int. J. Syst. Bacteriol. 1971, 21, 177–186. [Google Scholar] [CrossRef] [Green Version]
- Weiss, N.; Schillinger, U.; Kandler, O. Lactobacillus lactis, Lactobacillus leichmanii, and Lactobacillus bulgaricus, subjective synonyms of Lactobacillus delbrueckii subsp. lactis comb. nov. and Lactobacillus delbrueckii subsp. bulgaricus comb. nov. Syst. Appl. Microbiol. 1983, 4, 552–557. [Google Scholar] [CrossRef]
- Sieuwerts, S. Analysis of Molecular Interactions between Yoghurt Bacteria by an Integrated Genomics Approach. Ph.D. Thesis, Wageningen Universiteit, Wageningen, The Netherlands, 2009. [Google Scholar]
- Nicolas, P.; Bessieres, P.; Ehrlich, D.S.; Maguin, E.; van de Guchte, M. Extensive horizontal transfer of core genome genes between two Lactobacillus species found in the gastrointestinal tract. BMC Evol. Biol. 2007, 7, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcus, J.; van Sinderen, C.D.; O’Toole, P.W. The genus Lactobacillus a genomic basis for understanding its diversity. FEMS Microbiol. Lett. 2006, 269, 22–28. [Google Scholar]
- Klaenhammer, T.; Altermann, E.; Pfeiler, E.; Buck, B.L.; Goh, Y.J.; O’Flaherty, S.; Barrangou, R.; Duong, T. Functional genomics of probiotic lactobacilli. J. Clin. Gastroenterol. 2008, 42, 160–161. [Google Scholar] [CrossRef]
- Oyeniran, A.; Ibrahim, S.A.; Gyawali, R.; Tahergorabi, R.; Zimmerman, T.; Krastanov, A. A modified reinforced clostridial medium for the isolation and enumeration of Lactobacillus delbrueckii ssp. bulgaricus in a mixed culture. J. Dairy Sci. 2020, 103, 5030–5042. [Google Scholar] [CrossRef]
- Nwamaioha, N.O.; Ibrahim, S.A. A selective medium for the enumeration and differentiation of Lactobacillus delbrueckii ssp. bulgaricus. J. Dairy Sci. 2018, 101, 4953–4961. [Google Scholar] [CrossRef]
- Petry, S.; Furlan, S.; Crepeau, M.J.; Cerning, J.; Desmazeaud, M. Factors affecting exocellular polysaccharide production by Lactobacillus delbrueckii subsp. bulgaricus grown in a chemically defined medium. Appl. Environ. Microbiol. 2000, 8, 3427–3431. [Google Scholar]
- Hassan, A.N.; Frank, J.F.; Marth, E.H.; Steele, J.L. Starter Cultures and Their Use; Applied Dairy Microbiology; Marcel Dekker, Inc.: New York, NY, USA, 2001. [Google Scholar]
- Adolfsson, O.; Meydani, S.N.; Russell, R.M. Yogurt and gut function. Am. J. Clin. Nutr. 2004, 80, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Hols, P.; Hancy, F.; Fontaine, L.; Grossiord, B.; Prozzi, D.; Leblond-Bourget, N.; Decaris, B.; Bolotin, A.; Delorme, C.; Dusko Ehrlich, S.; et al. New insights in the molecular biology and physiology of Streptococcus thermophilus revealed by comparative genomics. FEMS Microbiol. Rev. 2005, 29, 435–463. [Google Scholar]
- Oberman, H.; Libudzist, Z. Fermented Milks. Fermented Milks, Microbiology of Fermented Foods; Elsiever Applied Science Publication: London, UK, 1985; Volume 1, pp. 167–186. [Google Scholar]
- Hughes, D.B.; Hoover, D.G. Bifidobacteria: Their potential for use in American dairy products. Food Technol. 1991, 45, 74–83. [Google Scholar]
- O’Sullivan, M.G.; Thornton, G.; O’Sullivan, G.C.; Collins, J.K. Probiotic bacteria: Myth or reality? Trends Food Sci. Technol. 1992, 3, 309–314. [Google Scholar] [CrossRef]
- Lourens-Hattingh, A.; Viljoen, B.C. Review: Yogurt as probiotic carrier food. Int. Dairy J. 2001, 11, 1–17. [Google Scholar] [CrossRef]
- Siezen, R.J.; Wilson, G. Probiotics genomics. Microb. Biotechnol. 2010, 3, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Soud, N.H.A.; Said, R.N.; Mosallam, D.S.; Barakat NA, M.; Sabry, M.A. Bifidobacterium lactis in treatment of children with acute diarrhea. A randomized double blind controlled trial. Open Access Maced. J. Med. Sci. 2015, 3, 403–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Upadhyay, N.; Moudgal, V. Probiotics: A Review. Jcom 2012, 19, 76–84. [Google Scholar]
- Chmielewska, A.; Szajewska, H. Systematic review of randomised controlled trials: Probiotics for functional constipation. World J. Gastroenterol. 2010, 16, 69–75. [Google Scholar] [PubMed]
- Song, D.; Ibrahim, S.A.; Hayek, S. Recent applications of probiotics in food and agricultural science. In Probiotics 10; Rigobelo, E.C., Ed.; InTech: Manhattan, NY, USA, 2012; Chapter 1; pp. 1–34. [Google Scholar]
- Hossain, Z. Bacteria: Streptococcus. Encycl. Food Saf. 2014, 1, 535–545. [Google Scholar]
- Hadji-Sfaxi, I.; El-Ghaish, S.; Ahmadova, A.; Batdorj, B.; Le Blay-Laliberté, G.; Barbier, G.; Haertlé, T.; Chobert, J.M. Antimicrobial activity and safety of use of Enterococcus faecium PC4.1 isolated from Mongol yogurt. Food Contr. 2011, 22, 2020–2027. [Google Scholar] [CrossRef]
- Pieniz, S.; Andreazza, R.; Pereira, J.Q.; de Oliveira Camargo, F.A.; Brandelli, A. Production of selenium-enriched biomass by Enterococcus durans. Biol Trace. Elem. Res. 2013, 155, 447–454. [Google Scholar] [CrossRef]
- Fijan, S. Microorganisms with claimed probiotic properties: An overview of recent literature. Int. J. Environ. Res. Public Health 2014, 11, 4745–4767. [Google Scholar] [CrossRef] [PubMed]
- Khalighi, A.; Behdani, R.; Kouhestani, S. Probiotics: A comprehensive review of their classification, mode of action and role in human nutrition. Probiotics Prebiotics Hum. Nutr. Health 2016, 10, 63646. [Google Scholar]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of action of probiotics. Adv. Nutr. 2019, 10 (Suppl. 1), S49–S66. [Google Scholar] [CrossRef] [Green Version]
- Shah, N.P. Probiotics and prebiotics. Agro Food Ind. Hi-Tech 2014, 15, 13–16. [Google Scholar]
- Bermudez-Brito, M.; Plaza-Díaz, J.; Munoz-Quezada, S.; Gomez-Llorente, C.; Gil, A. Probiotic mechanisms of action. Ann. Nutr. Metab. 2012, 61, 160–174. [Google Scholar] [CrossRef]
- Oelschlaeger, T.A. Mechanisms of probiotic actions—A review. Int. J. Med. Microbiol. 2010, 300, 57–62. [Google Scholar] [CrossRef]
- O’Hanlon, D.E.; Moench, T.R.; Cone, R.A. In vaginal fluid, bacteria associated with bacterial vaginosis can be suppressed with lactic acid but not hydrogen peroxide. BMC Infect. Dis. 2011, 11, 200. [Google Scholar] [CrossRef] [Green Version]
- Masood, M.I.; Qadir, M.I.; Jafr Hussain Shirazi, J.H.; Khan, I.U. Beneficial effects of lactic acid bacteria on human beings (Review). Crit. Rev. Microbiol. 2011, 37, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Ouwehand, A.C.; Lahtinen, S.; Nurminen, P. Lactobacillus rhamnosus HN001 and Bifidobacterium lactis HN019. In Handbook of Probiotics and Prebiotics; Lee, Y.K., Salminen, S., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 473–477. [Google Scholar]
- Salminen, S.J.; Gueimonde, M.; Isolauri, E. Probiotics that modify disease risk. J. Nutr. 2005, 135, 1294–1298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nomoto, K. Prevention of infections by probiotics. J. Biosci. Bioeng. 2005, 100, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Isolauri, E.; Sutus, Y.; Kankaanpää, P.; Arvilommi, H.; Salminen, S. Probiotics: Effects on immunity. Am. J. Clin. Nutr. 2001, 73, 444–450. [Google Scholar] [CrossRef] [Green Version]
- Maldonado Galdeano, C.; de Moreno de LeBlanc, A.; Carmuega, E.; Weill, R.; Perdigón, G. Mechanisms involved in the immunostimulation by probiotic fermented milk. J. Dairy Res. 2009, 76, 446–454. [Google Scholar] [CrossRef]
- Soccol, C.R.; Vandenberghe, L.C.S.; Spier, M.R.; Medeiros, A.B.P.; Yamaguishi, C.T.; Lindner, J.D.D.; Pandey, A.; Thomaz-Soccol, V. The Potential of Probiotics. Food Technol. Biotechnol. 2010, 48, 413–434. [Google Scholar]
- Shu, Q.H.; Gill, S. A dietary probiotic (Bifidobacterium lactis HN019) reduces the severity of Escherichia coli O157: H7 infection in mice. Med. Microbiol. Immunol. 2001, 189, 147–152. [Google Scholar] [CrossRef]
- FAO/WHO. Guidelines for the Evaluation of Probiotics in Food. In Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food, London Ontario, Canada, April 30 and May 1, 2002; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy; World Health Organization (WHO): Geneva, Switzerland, 2002. [Google Scholar]
- Nagpal, R.; Yadav, H.; Puniya, A.K.; Singh, K.; Jain, S.; Marotta, F. Potential of probiotics and prebiotics for symbiotic functional dairy foods. Int. J. Probiotics Prebiotics 2007, 2, 75–84. [Google Scholar]
- Wedajo, B. Lactic Acid Bacteria: Benefits, Selection Criteria and Probiotic Potential in Fermented Food. J. Probiotics Health 2015, 3, 2. [Google Scholar] [CrossRef]
- Kumar, M.; Behare, P.V.; Mohania, D.; Arora, S.; Kaur, A.; Nagpal, R. Health-promoting probiotic functional foods: Potential and prospects. Agro Food Ind. Hi-Tech 2009, 20, 29–33. [Google Scholar]
- Ezendam, J.; Van Loveren, H. Probiotics: Immunomodulation and Evaluation of Safety and Efficacy. Nutr. Rev. 2008, 64, 1–14. [Google Scholar] [CrossRef]
- Park, S.; Kang, J.; Choi, S.; Park, H.; Hwang, E.; Kang, Y.; Kim, A.; Holzapfel, W.; Ji, Y. Cholesterol-lowering effect of Lactobacillus rhamnosus BFE5264 and its influence on the gut microbiome and propionate level in a murine model. PLoS ONE 2018, 13, e0203150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahouli, I.; Tomaro-Duchesneau, C.; Prakash, S. Probiotics in colorectal cancer (CRC) with emphasis on mechanisms of action and current perspectives. J. Med. Microbiol. 2013, 62, 1107–1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gill, H.; Prasad, J. Bioactive Components of milk: Probiotics, immunomodulation, and health benefits. Adv. Exp. Med. Biol. 2008, 606, 423–454. [Google Scholar]
- Russell, D.A.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Metabolic activities and probiotic potential of bifidobacteria. Int. J. Food Microbiol. 2011, 149, 88–105. [Google Scholar] [CrossRef]
- Guerra, P.V.; Lima, L.N.; Souza, T.C.; Mazochi, V.; Penna, F.J.; Silva, A.M.; Nicoli, J.R.; Guimarães, E.V. Pediatric functional constipation treatment with Bifidobacterium-containing yogurt: A crossover, double-blind, controlled trial. World J. Gastroenterol. 2011, 17, 3916–3921. [Google Scholar] [CrossRef] [PubMed]
- Mazlyn, M.M.; Nagarajah, L.H.-L.; Fatimah, A.; Norimah, A.K.; Goh, K.-L. Effects of a probiotic fermented milk on functional constipation a randomized, double-blind, placebo-controlled study. J. Gastroenterol. Hepatol. 2013, 28, 1141–1147. [Google Scholar] [CrossRef]
- Hsieh, P.S.; Tsai, Y.C.; Chen, Y.C.; Teh, S.F.; Ou, C.M.; King VA, E. Eradication of Helicobacter pylori infection by the probiotic strains Lactobacillus johnsonii MH-68 and L. salivarius ssp. salicinius AP-32. Helicobacter 2012, 17, 466–477. [Google Scholar] [CrossRef]
- Gonzalez-Ochoa, G.; Flores-Mendoza, L.K.; Icedo-Garcia, R.; Gomez-Flores, R.; Tamez-Guerra, P. Modulation of rotavirus severe gastroenteritis by the combination of probiotics and prebiotics. Arch. Microbiol. 2017, 199, 953–961. [Google Scholar] [CrossRef] [Green Version]
- Siener, R.; Bangen, U.; Sidhu, H.; Hönow, R.; Von Unruh, G.; Hesse, A. The role of Oxalobacter formigenes colonization in calcium oxalate stone disease. Kidney Int. 2013, 83, 1144–1149. [Google Scholar] [CrossRef] [Green Version]
- Andreasen, A.S.; Larsen, N.; Pedersen-Skovsgaard, T.; Berg, R.M.; Møller, K.; Svendsen, K.D.; Jakobsen, M.; Pedersen, B.K. Effects of Lactobacillus acidophilus NCFM on insulin sensitivity and the systemic inflammatory response in human subjects. Br. J. Nutr. 2010, 104, 1831–1838. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, M.; Darimont, C.; Drapeau, V.; Emady-Azar, S.; Lepage, M.; Rezzonico, E.; Ngom-Bru, C.; Berger, B.; Philippe, L.; Ammon-Zuffrey, C.; et al. Effect of Lactobacillus rhamnosus CGMCC1.3724 supplementation on weight loss and maintenance in obese men and women. Br. J. Nutr. 2013, 111, 1507–1519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daliri, E.B.-M.; Lee, B.H. New perspectives on probiotics in health and disease. Food Sci. Hum. Wellness 2015, 4, 56–65. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.H.; Liong, M.T.; Choi, S.B. Probiotics in health and disease. In Beneficial Microbes in Fermented and Functional Foods; Rai, V.R., Bai, J.A., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 167–183. [Google Scholar]
- Ibrahim, S.A.; Gyawali, R. Lactose intolerance. In Milk and Dairy Products in Human Nutrition: Production, Composition and Health; Park, Y.W., Haenlein, G.F.W., Eds.; John & Wiley and Sons Ltd.: Hoboken, NJ, USA, 2013; pp. 246–260. [Google Scholar]
- Barling, P.M. Lactose tolerance and intolerance in Malaysians. IeJSME 2012, 6 (Suppl. 1), S12–S23. [Google Scholar]
- Vonk, R.J.; Reckman, G.A.R.; Harmsen, H.J.M.; Priebe, M.G. Probiotics and Lactose Intolerance. IntechChapter 2012, 7, 149–160. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, S.; O’Sullivan, D. Use of chemical mutagenesis for the isolation of food grade beta-galactosidase over producing mutants of bifidobacteria, lactobacilli and Streptococcus thermophilus. J. Dairy Sci. 2000, 83, 923–930. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Alazzeh, A.Y.; Awaisheh, S.S.; Song, D.; Shahbazi, A.; AbuGhazaleh, A. Enhancement of α- and β-Galactosidase activity in Lactobacillus reuteri by different metal ions. Biol. Trace Elem. Res. 2010, 136, 106–116. [Google Scholar] [CrossRef]
- Goh, Y.J.; Klaenhammer, T.R. A functional glycogen biosynthesis pathway in Lactobacillus acidophilus: Expression and analysis of the glg operon. Mol. Microbiol. 2013, 89, 1187–1200. [Google Scholar] [CrossRef] [Green Version]
- Foxx-Orenstein, A.E.; Chey, W.D. Manipulation of the gut microbiota as a novel treatment strategy for gastrointestinal disorders. Am. J. Gastroenterol. 2012, 1 (Suppl. 1), 41. [Google Scholar] [CrossRef]
- Karlton Senaye, B.D.; Tahergorabi, R.; Giddings, V.L.; Ibrahim, S.A. Effect of gums on viability and βgalactosidase activity of Lactobacillus spp. in milk drink during refrigerated storage. Int. J. Food Sci. Technol. 2014, 50, 32–40. [Google Scholar] [CrossRef]
- Gyawali, R.; Oyeniran, A.; Zimmerman, T.; Aljaloud, S.O.; Krastanov, A.; Ibrahim, S.A. A comparative study of extraction techniques for maximum recovery of β-galactosidase from the yogurt bacterium Lactobacillus delbrueckii ssp. bulgaricus. J. Dairy Res. 2020, 87, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Backhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Delzenne, N.M.; Neyrinck, A.M.; Backhed, F.; Cani, P.D. Targeting gut microbiota in obesity: Effects of prebiotics and probiotics. Nat. Rev. Endocrinol. 2011, 7, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Le Barz, M.; Anhê, F.F.; Varin, T.V.; Desjardins, Y.; Levy, E.; Roy, D.; Marette, A. Probiotics as complementary treatment for metabolic disorders. Diabetes Metab. J. 2015, 39, 291–303. [Google Scholar] [CrossRef]
- Kobyliak, N.; Conte, C.; Cammarota, G.; Haley, A.P.; Styriak, I.; Gaspar, L.; Kruzliak, P. Probiotics in prevention and treatment of obesity: A critical view. Nutr. Metab. 2016, 13, 14. [Google Scholar] [CrossRef] [Green Version]
- Barrett, H.L.; Callaway, L.K.; Nitert, M.D. Probiotics: A potential role in the prevention of gestational diabetes? Acta Diabetol. 2012, 49, 1–13. [Google Scholar] [CrossRef]
- Karimi, G.; Sabran, M.R.; Jamaluddin, R.; Parvaneh, K.; Mohtarrudin, N.; Ahmad, Z.; Khodavandi, A. The anti-obesity effects of Lactobacillus casei strain Shirota versus Orlistat on high fat diet-induced obese rats. Food Nutr. Res. 2015, 59, 29273. [Google Scholar] [CrossRef] [Green Version]
- McFarland, L.V.; Elmer, G.W.; McFarland, M. Meta-analysis of probiotics for the prevention and treatment of acute pediatric diarrhea. Int. J. Probiotics Prebiotics 2006, 1, 63–76. [Google Scholar]
- Allen, S.J.; Martinez, E.G.; Gregorio, G.V.; Dans, L.F. Probiotics for treating acute infectious diarrhea. Cochrane Database Syst. Rev. 2010, 11, CD003048. [Google Scholar]
- Urbańska, M.; Gieruszczak-Białek, D.; Szajewska, H. Systematic review with meta-analysis: Lactobacillus reuteri DSM 17938 for diarrhoeal diseases in children. Aliment. Pharmacol. Ther. 2016, 43, 1025–1034. [Google Scholar] [CrossRef] [Green Version]
- Szajewska, H.; Skorka, A.; Ruszczyński, M.; Gieruszczak-Białek, D. Meta-analysis: L actobacillus GG for treating acute gastroenteritis in children–updated analysis of randomised controlled trials. Aliment. Pharmacol. Ther. 2013, 38, 467–476. [Google Scholar] [CrossRef]
- Tiwari, G.; Tiwari, R.; Pandey, S.; Pandey, P. Promising future of probiotics for human health: Current scenario. Chron. Young Sci. 2012, 3, 17–28. [Google Scholar] [CrossRef]
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef]
- Mendoza, L. Potential effect of probiotics in the treatment of breast cancer. Oncol. Rev. 2019, 13, 422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reid, G.; Jass, J.; Sebulsky, M.T.; McCormick, J.K. Potential uses of probiotics in clinical practice. Clin. Microbiol. Rev. 2003, 16, 658–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oxman, T.; Shapira, M.; Klein, R.; Avazov, N.; Rabinowitz, B. Oral administration of Lactobacillus induces cardioprotection. J. Altern. Complement Med. 2001, 7, 345–354. [Google Scholar] [CrossRef] [PubMed]
- De Roos, N.M.; Katan, M.B. Effects of probiotic bacteria on diarrhea, lipid metabolism, and carcinogenesis: A review of papers published between 1988 and 1998. Am. J. Clin. Nutr. 2000, 71, 405–411. [Google Scholar] [CrossRef]
- Antony, S.; de Leon, M.P. Probiotics and Its Relationship with the Cardiovascular System. Probiotics Curr. Knowl. Future Prospect. 2018, 52–68. [Google Scholar] [CrossRef] [Green Version]
- Thushara, R.M.; Gangadaran, S.; Solati, Z.; Moghadasian, M.H. Cardiovascular benefits of probiotics: A review of experimental and clinical studies. Food Funct. 2016, 7, 632–642. [Google Scholar] [CrossRef]
- Waigankar, S.; Patel, S. Role of probiotics in urogenital healthcare. J. Mid-Life Health 2011, 2, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Hanson, L.; VandeVusse, L.; Jerme, M.; Abad, C.L.; Safdar, N. Probiotics for treatment and prevention of urogenital infections in women: A systematic review. J. Midwifery Women’s Health 2016, 61, 339–355. [Google Scholar] [CrossRef]
- Kerry, R.G.; Patra, J.K.; Gouda, S.; Park, Y.; Shin, H.S.; Das, G. Benefaction of probiotics for human health: A review. J. Food Drug Anal. 2018, 26, 927–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, S.; Lee, S.J.; Park, D.J.; Oh, S.; Lim, K.T. The anti-allergic activity of Lactobacillus plantarum L67 and its application to yogurt. J. Dairy Sci. 2016, 99, 9372–9382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kukkonen, K.; Savilahti, E.; Haahtela, T.; Juntunen-Backman, K.; Korpela, R.; Poussa, T.; Kuitunen, M. Probiotics and prebiotic galacto-oligosaccharides in the prevention of allergic diseases: A randomized, double-blind, placebo-controlled trial. J. Allergy Clin. Immunol. 2007, 119, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Neau, E.; Delannoy, J.; Marion, C.; Cottart, C.H.; Labellie, C.; Holowacz, S.; Waligora-Dupriet, A.J. Three novel candidate probiotic strains with prophylactic properties in a murine model of cow’s milk allergy. Appl. Environ. Microbiol. 2016, 82, 1722–1733. [Google Scholar] [CrossRef] [Green Version]
- Umbrello, G.; Esposito, S. Microbiota and neurologic diseases: Potential effects of probiotics. J. Transl. Med. 2016, 14, 298. [Google Scholar] [CrossRef] [Green Version]
- Daliri EB, M.; Oh, D.H.; Lee, B.H. Psychobiotics; a promise for neurodevelopmental therapy. J Probiotics Health 2016, 4, 1e4. [Google Scholar]
- Harata, G.; He, F.; Hiruta, N.; Kawase, M.; Kubota, A.; Hiramatsu, M.; Yausi, H. Intranasal administration of Lactobacillus rhamnosus GG protects mice from H1N1 influenza virus infection by regulating respiratory immune responses. Lett. Appl. Microbiol. 2010, 50, 597–602. [Google Scholar] [CrossRef]
- Kobayashi, N.; Saito, T.; Uematsu, T.; Kishi, K.; Toba, M.; Kohda, N.; Suzuki, T. Oral administration of heat-killed Lactobacillus pentosus strain b240 augments protection against influenza virus infection in mice. Int. Immunopharmacol. 2011, 11, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Makino, S.; Ikegami, S.; Itoh, H.; Yamada, H. Effects of oral administration of yogurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1 and its exopolysaccharides against influenza virus infection in mice. Int. Immunopharmacol. 2011, 11, 2246–2250. [Google Scholar] [CrossRef] [PubMed]
- Al Kassaa, I.; Hober, D.; Hamze, M.; Chihib, N.E.; Drider, D. Antiviral potential of lactic acid bacteria and their bacteriocins. Probiotics Antimicrob. Proteins 2014, 6, 177–185. [Google Scholar] [CrossRef]
- Botic, T.; Klingberg, T.D.; Weingartl, H.; Cencič, A. A novel eukaryotic cell culture model to study antiviral activity of potential probiotic bacteria. Int. J. Food Microbiol. 2007, 115, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chai, W.; Burwinkel, M.; Twardziok, S.; Wrede, P.; Palissa, C.; Esch, B.; Schmid, M.F.G. Inhibitory influence of Enterococcus faecium on the propagation of swine influenza a virus in vitro. PLoS ONE 2013, 8, e53043. [Google Scholar] [CrossRef] [Green Version]
- Kawashima, T.; Hayashi, K.; Kosaka, A.; Kawashima, M.; Igarashi, T.; Tsutsui, H.; Tsuji, N.M.; Nishimura, I.; Hayashi, T.; Obata, A. Lactobacillus plantarum strain YU from fermented foods Probiotics & Antimicro. Prot. activates Th1 and protective immune responses. Int. Immunopharmacol. 2011, 11, 2017–2024. [Google Scholar]
- Roselli, M.; Pieper, R.; Rogel-Gaillard, C.; De Vries, H.; Bailey, M.; Smidt, H.; Lauridsen, C. Immunomodulating effects of probiotics for microbiota modulation, gut health and disease resistance in pigs. Anim. Feed Sci. Technol. 2017, 233, 104–119. [Google Scholar] [CrossRef] [Green Version]
- Salminen, M.K.; Rautelin, H.; Tynkkynen, S.; Poussa, T.; Saxelin, M.; Valtonen, V.; Järvinen, A. Lactobacillus bacteremia, clinical significance, and patient outcome, with special focus on probiotic L-Rhamnosus GG. Clin. Infect. Dis. 2004, 38, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Van Niel, C.W.; Feudtner, C.; Garrison, M.M.; Christakis, D.A. Lactobacillus therapy for acute infectious diarrhea in children: A meta-analysis. Pediatrics 2002, 109, 678–684. [Google Scholar] [CrossRef] [Green Version]
- Lehtoranta, L. Probiotics and Virus Infections: The Effects of Lactobacillus Rhamnosus GG on Respiratory and Gastrointestinal Virus Infections. Ph.D. Thesis, Institute of Biomedicine, University of Helsinki, Helsinki, Finland, 2012. [Google Scholar]
- Boge, T.; Rémigy, M.; Vaudaine, S.; Tanguy, J.; Bourdet-Sicard, R.; Van Der Werf, S. A probiotic fermented dairy drink improves antibody response to influenza vaccination in the elderly in two randomized controlled trials. Vaccine 2009, 27, 5677–5684. [Google Scholar] [CrossRef]
- Rather, I.A.; Choi, K.H.; Bajpai, V.K.; Park, Y.H. Antiviral mode of action of Lactobacillus plantarum YML009 on influenza virus. Bang J. Pharm. 2014, 9, 475–482. [Google Scholar] [CrossRef] [Green Version]
- Villena, J.; Oliveira, M.L.S.; Ferreira, P.C.; Salva, S.; Alvarez, S. Lactic acid bacteria in the prevention of pneumococcal respiratory infection: Future opportunities and challenges. Int. Immunopharmacol. 2011, 11, 1633–1645. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, K.; Fujitani, N.; Nakagawa, H.; Miyazaki, T. Prevention of respiratory syncytial virus infection with probiotic lactic acid bacterium Lactobacillus gasseri SBT2055. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cavicchioli, V.Q.; de Carvalho, O.V.; de Paiva, J.C.; Todorov, S.D.; Júnior, A.S.; Nero, L.A. Inhibition of herpes simplex virus 1 (HSV-1) and poliovirus (PV-1) by bacteriocins from Lactococcus lactis subsp. lactis and Enterococcus durans strains isolated from goat milk. Int. J. Antimicrob. Agents 2018, 51, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Leyer, G.J.; Li, S.; Mubasher, M.E.; Reifer, C.; Ouwehand, A.C. Probiotic effects on cold and influenza-like symptom incidence and duration in children. Pediatrics 2009, 124, e172–e179. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.Y.; Lee, D.K.; Ha, N.J.; Shin, H.S. Antiviral effects of Lactobacillus ruminis SPM0211 and Bifidobacterium longum SPM1205 and SPM1206 on rotavirus-infected Caco-2 cells and a neonatal mouse model. J. Microbiol. 2015, 53, 796–803. [Google Scholar] [CrossRef]
- Khani, S.; Motamedifar, M.; Golmoghaddam, H.; Hosseini, H.M.; Hashemizadeh, Z. In vitro study of the effect of a probiotic bacterium Lactobacillus rhamnosus against herpes simplex virus type 1. Braz. J. Infect. Dis. 2012, 16, 129–135. [Google Scholar]
- Kechaou, N.; Chain, F.; Gratadoux, J.J.; Blugeon, S.; Bertho, N.; Chevalier, C.; Langella, P. Identification of one novel candidate probiotic Lactobacillus plantarum strain active against influenza virus infection in mice by a large-scale screening. Appl. Environ. Microbiol. 2013, 79, 1491–1499. [Google Scholar] [CrossRef] [Green Version]
- Chai, W.; Burwinkel, M.; Wang, Z.; Palissa, C.; Esch, B.; Twardziok, S.; Schmidt, M.F. Antiviral effects of a probiotic Enterococcus faecium strain against transmissible gastroenteritis coronavirus. Arch. Virol. 2013, 158, 799–807. [Google Scholar] [CrossRef] [Green Version]
- Grandy, G.; Medina, M.; Soria, R.; Terán, C.G.; Araya, M. Probiotics in the treatment of acute rotavirus diarrhea. A randomized, double-blind, controlled trial using two different probiotic preparations in Bolivian children. BMC Infect. Dis. 2010, 10, 253. [Google Scholar] [CrossRef] [Green Version]
- Wachsman, M.B.; Castilla, V.; de Ruiz Holgado, A.P.; de Torres, R.A.; Sesma, F.; Coto, C.E. Enterocin CRL35 inhibits late stages of HSV-1 and HSV-2 replication in vitro. Antivir. Res. 2003, 58, 17–24. [Google Scholar] [CrossRef]
- Imran, S. Bacteriocin: A Potential Antiviral and Antimicrobial Agent; an Alternative to Antibiotics. EC Microbiol. 2019, 15, 263–266. [Google Scholar]
- Quintana, V.M.; Torres, N.I.; Wachsman, M.B.; Sinko, P.J.; Castilla, V.; Chikindas, M. Antiherpes simplex virus type 2 activity of the antimicrobial peptide subtilosin. J. Appl. Microbiol. 2014, 117, 1253–1259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serkedjieva, J.; Danova, S.; Ivanova, I. Anti-influenza virus activity of a bacteriocin produced by Lactobacillus delbrueckii. Appl. Biochem. Biotechnol. 2000, 88, 285–298. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Dicks, L.M.; Popov, I.V.; Karaseva, A.; Ermakov, A.M.; Suvorov, A.; Chikindas, M.L. Probiotics at war against viruses: What is missing from the picture? Front. Microbiol. 2020, 11, 1–21. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Gyawali, R.; Fidan, H. Self-Defense: A Practical Approach to Combatting COVID-19. Acta Sci. Nutr. Health 2020, 4, 33–37. [Google Scholar] [CrossRef]



| Antimicrobial Compounds/Metabolites | Characteristic Property | Species | References |
|---|---|---|---|
| Organic acids, hydrogen peroxide | Promotes significant inhibitory, antagonistic effect and an important target for pathogens (Gram-positives and Gram-negatives) and food spoilage microorganisms | Lactobacillus species | Nagpal et al., 2012 [28]; Papadimitriou et al., 2015 [29]; Ponce et al., 2008 [30] |
| Lactoperoxidase system | Thiocyanate and hydrogen peroxide have a broad-spectrum antibacterial action on pathogens | Lactobacillus species | Seifu et al., 2005 [31] |
| Bacteriocins | Characteristic Property | Species/Compound | References |
| Class I Bacteriocins (Lantibiotics) | Antimicrobial peptides synthesized ribosomally and have an inhibitory effect on pathogens. Widely used in food preservation operations. Lantibiotics are post-translationally modified and are low molecular weight peptides (<5 kDa). Consists of superior amino acids i.e., lanthionine and β-methyllanthionine |
| Yang et al., 2014 [24]; Mokoena 2017 [10], Perez et al., 2014 [3] |
| Class II Bacteriocins (Non Lantibiotics) | Heat stable and small peptides with a high molecular weight (5–10 kDa). They are non-lanthionine molecules with or without post-translational modifications | Yang et al., 2014 [24]; Mokoena 2017 [10], Perez et al., 2014 [3] | |
| Class IIa Bacteriocins (Non Lantibiotics) | Functional peptides are synthesized from several genes as a requirement | Yang et al., 2014 [24]; Mokoena 2017 [10], Perez et al., 2014 [3] | |
| Bacteriocins | Characteristic Property | Species/Compound | References |
| Class IIb Bacteriocins (Non Lantibiotics) | Two different peptides, mostly linear coupled with or without post translational modifications at the C-terminal are required |
| Yang et al., 2014 [24]; Mokoena 2017 [10], Perez et al., 2014 [3] |
| Class IIc Bacteriocins (Non Lantibiotics) | Bacteriocins have a circular structure with both the N- and C-terminals linked by covalent bonds | Yang et al., 2014 [24]; Mokoena 2017 [10], Perez et al., 2014 [3] | |
| Class III Bacteriocins (Non-lantibiotics) Class IIIa Bacteriocins (Bacteriolytic) Class IIIb Bacteriocins (Non-lytic) | Large heat-labile peptides with molecular weight > 30 kDa. They are sub-classified under Class IIIa and Class IIIb. Class IIIa they are mainly bacteriolysins. Lysostaphin, is an antimicrobial peptide produced by staphylococci that targets Gram-positives and destroys them. Class IIIb (Helveticin) is a non-lytic protein produced from Gram-positive bacteria Lactobacillus helveticus | Ibrahim, 2019 [32]; Ramu et al., (2015) [33]. | |
| Class IV Bacteriocins | Bacteriocins classified as complex with compositions of lipids and carbohydrates moieties | Ibrahim, 2019 [32]; Ramu et al., (2015) [33]. |
| Traditional Fermented Foods | Microbiota | Associated Action | References |
|---|---|---|---|
| Dahi | Lactobacillus acidophilus | Production of antibacterial substances | Balamurugan et al., 2014 [37] |
| Kefir | Lactobacillus kefir, Lactobacillus kefiranofaciens, and Lactobacillus kefirgranum | Production of bacteriocin enhances antibacterial activity Epithelial cells of the intestine have reduced inflammation Serum cholesterol level is reduced Produce an EPS known as kefiran. | Luo et al., 2011 [38]; Seo et al., 2018 [39]; Wang et al., 2008 [40]; Bonczar et al., 2016 [41] |
| Tofu | Lactobacillus plantarum | Antioxidant activity | Li et al., 2012 [42] |
| Koumiss | Lactobacillus sp. | Excellent antimicrobial properties against pathogens | Guo et al., 2015 [43] |
| Swiss Cheese | Lactobacillus helveticus R389 | Enhancement of the immune system by increasing IgA and CD4 positive cells. | Ghosh et al., 2019 [36] |
| Nunu | Lactobacillus plantarum, Lactobacillus fermentum, and Saccharomyces cerevisiae | Produces EPS, and β-galactosidase Produces bacteriocins known as plantaricins promoting antibacterial activity against pathogens | Akabanda et al., 2013 [44]; Behera et al., 2018 [45] |
| Korean kimchi | Lactobacillus plantarum | Antimicrobial activity against pathogens | Kwak et al., 2017 [46] |
| Fermented Dairy Foods | Starter Cultures | References |
|---|---|---|
| Hard cheese without eyes | Lactococcus lactis lactis, Lactococcus lactis ssp. cremoris | Settani et al., 2013 [48] |
| Kefir | Lactobacillus kefir, Lactobacillus kefiranofaciens, | Luo et al., 2011 [38] |
| Yogurt | Lb. acidophilus, S. thermophilus, Lb. delbrueckii ssp. bulgaricus | Panesar, 2011 [49], Hati et al., 2013 [2] |
| Swiss cheese | Lactobacillus delbrueckii ssp. lactis, Lb. helveticus, Lb. casei | Daly et al., 2010 [50] |
| Zabady | Lb. delbrueckii ssp. bulgaricus, S. thermophilus | Abou-Donia, 2004 [51] |
| Bulgarian butter milk | L. delbrueckii subsp. bulgaricus | Panesar, 2011 [49] |
| Nyarmie | Lactobacillus sp., Lactococcus lactis | Obodai & Dodd, 2006 [52] |
| Starter bacteria | Functionality | Benefits | References |
|---|---|---|---|
| Lactobacillus plantarum, Lactococcus lactis | Production of Bacteriocins | Bio-preservation | Todorov et al., 2010 [55]; Biscola et al., 2013 [56] |
| Lactobacillus sp. (EPS efficient) | Formation of stabilizers and production of exopolysaccharides | Enhanced viscosity and body development (polysaccharide materials) | Cerning, 1995 [57] |
| Vitamins producing lactic acid (Strepotococci and propionibacteria) | Vitamin content in fermented dairy products are improved | Enhances the overall health of the bacteria, Promotes vitamin malnutrition | Hati et al., 2013 [2] |
| Leuconostoc spp. | Acid production | Promotes flavor development, Formation of gels | Bintsis, 2018 [1] |
| Lactococcus lactis ssp. cremoris | Proteolyis and lipolysis | Ensures accelerated ripening and maturation of cheese | Hati et al., 2013 [2] |
| Probiotic Strain | Health Benefits | Mechanism of Action | References |
|---|---|---|---|
| Lactic acid bacteria | Prevention and treatment of colon cancer | Ensures biodegradation of susceptible and potential carcinogens Boost the immune response system of the host and inhibits pro-cancerous enzymatic activity of colonic microorganisms | Kahouli et al., 2013 [103] |
| Bifidobacterium bifidum | Inhibition of enteric pathogens | Prevents and reduces diarrhea Inhibits invasive pathogens by secretion of acids and increases antibacterial action of the intestinal microflora | Gill & Prasad, 2008 [104]; Russell et al., 2011 [105] |
| Bifidobacterium lactis, L. bulgaricus, L. plantarum, L. acidophilus | Irritable bowel syndrome and constipation prevention and treatment | Alleviates symptoms of irritable bowel syndrome. Modulates and alters gastrointestinal microflora to offset abnormal conditions. | Guerra et al., 2011 [106]; Mena et al., 2013 [107] |
| Lactobacillus, Bifidobacterium | Treatment of Helicobacter pylori infection | Epithelial and mucosal cells are competitively colonized. Production of bacteriocins and organic acids to impede action of the bacteria. | Hsieh et al., 2012 [108] |
| Bifidobacterium breve | Rotaviral gastroenteritis treatment | Promotes and boosts the production of anti-rotavirus IgA or anti-influenza virus | Gonzalez-Ochoa et al., (2017) [109] |
| Oxalobacter formigenes Lactobacillus and Bifidobacterium species | Treatment of kidney or Urogenital infections | Metabolic and mopping up action on toxic compounds. | Roswitha et al., 2013 [110] |
| Lactobacillus acidophilus NCFM | Diabetes and obesity | Minimizes risks associated with type 2 diabetes mellitus and enhances host metabolic system ensuring weight management | Andreasen et al., 2010 [111] Sanchez et al., 2013 [112] |
| Lactic Acid Bacteria Strain | Origin of Strain | Virus Evaluated | Mode of Action | References |
|---|---|---|---|---|
| L. fermentum CECT5716 | Human breast milk | Influenza virus | Enhances the response of antibodies | Boge et al., 2009 [163] |
| Lactobacillus delbrueckii ssp. Bulgaricus OLL1073R-1 (1073R-1) | Fermented food (Yogurt) | Influenza virus | Promotes antagonistic antibodies | Nagai et al., 2011 [154] |
| L. plantarum YML009 | Fermented food (Kimchi) | H1N1 Influenza virus | Activation of Th1 immune response | Rather et al., 2014 [164] |
| L. rhamnosus CRL1505 | Commercial probiotic strains | Respiratory syncytial virus (RSV) | Production of IFN-γ and Ils | Villena et al., 2011 [165] |
| Lactobacillus gasseri SBT2055 (LG2055) | Human feces | RSV | Proinflammatory activity | Eguchi et al., 2019 [166] |
| Enterococcus durans | Goat milk | Herpes Simplex Virus (HSV-1) and Human papillomavirus (PV-1) | Decreases viral cell replication | Cavicchioli et al., 2018 [167] |
| L. acidophilus strain NCFM | Newborn feces | Reduce influenza like symptoms | Immunomodulation | Leyer et al., 2009 [168] |
| Lactobacillus ruminis SPM0211 | Isolated from a young Korean girl | Rotavirus (ROV) | Immunomodulation and promotion of interferons (IFNs) | Kang et al., 2015 [169] |
| L. rhamnosus | Gut flora | HSV-1 | Stimulation of macrophages and elimination of HSV-1 | Khani et al., 2012 [170] |
| L. plantarum CNRZ 1997 | - | H1N1 strain A | Proinflammatory response | Kechaou et al., 2013 [171] |
| E. faecium NCIMB 10415 | - | Transmissible gastroenteritis virus) TGEV | Promotion of nitric oxide (NO) production and secretion of Interleukins (IL-6 and IL-8) | Chai et al., 2013 [172] |
| L. acidophilus | - | ROVs | Reduction in duration of diarrhea | Grandy et al., 2010 [173] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayivi, R.D.; Gyawali, R.; Krastanov, A.; Aljaloud, S.O.; Worku, M.; Tahergorabi, R.; Silva, R.C.d.; Ibrahim, S.A. Lactic Acid Bacteria: Food Safety and Human Health Applications. Dairy 2020, 1, 202-232. https://doi.org/10.3390/dairy1030015
Ayivi RD, Gyawali R, Krastanov A, Aljaloud SO, Worku M, Tahergorabi R, Silva RCd, Ibrahim SA. Lactic Acid Bacteria: Food Safety and Human Health Applications. Dairy. 2020; 1(3):202-232. https://doi.org/10.3390/dairy1030015
Chicago/Turabian StyleAyivi, Raphael D., Rabin Gyawali, Albert Krastanov, Sulaiman O. Aljaloud, Mulumebet Worku, Reza Tahergorabi, Roberta Claro da Silva, and Salam A. Ibrahim. 2020. "Lactic Acid Bacteria: Food Safety and Human Health Applications" Dairy 1, no. 3: 202-232. https://doi.org/10.3390/dairy1030015
APA StyleAyivi, R. D., Gyawali, R., Krastanov, A., Aljaloud, S. O., Worku, M., Tahergorabi, R., Silva, R. C. d., & Ibrahim, S. A. (2020). Lactic Acid Bacteria: Food Safety and Human Health Applications. Dairy, 1(3), 202-232. https://doi.org/10.3390/dairy1030015









