Assessment of Metabolic Adaptations in Periparturient Dairy Cows Provided 3-Nitrooxypropanol and Varying Concentrate Proportions by Using the GreenFeed System for Indirect Calorimetry, Biochemical Blood Parameters and Ultrasonography of Adipose Tissues
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Sample Collection
2.3. Analyses
2.4. Calculation of Energy Metabolism Parameters by Indirect Calorimetry and Ultrasonography
2.5. Statistical Analyses
3. Results
3.1. Methane Emission and Respiratory Gas Exchange Measured with the GreenFeed SYSTEM
3.2. Energy Turnover Estimated by Indirect Calorimetric and Ultrasonic Methods
3.3. Validation of the ERtissue Outcome of the GreenFeed Indirect Calorimetry Method
3.4. Biochemical Blood Parameters
3.5. Interrelations between Metabolic RQ, Energy Metabolism and Methane Emission
4. Discussion
4.1. Limitations of the GreenFeed Technology for Its Use in Indirect Calorimetry
4.2. Validation of the Energy Partitioning Estimated by Indirect Calorimetry and Ultrasonography
4.3. Effects of 3-NOP, CFP and Parturition on Energy Metabolism Parameters
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Item | Treatments † | SEM | p-Values § | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CONHC (n = 14) | CONLC (n = 15) | NOPHC (n = 14) | NOPLC (n = 12) | 3-NOP | CFP | 3-NOP ×CFP | 3-NOP ×TIME | CFP ×TIME | 3-NOP ×CFP×TIME | ||
| Back fat thickness (cm) | |||||||||||
| d1 3 p.p. | 1.49 | 1.34 | 1.43 | 1.49 | 0.08 | 0.341 | 0.538 | 0.152 | 0.551 | 0.938 | 0.924 |
| d 28 p.p. | 1.16 | 1.01 | 1.15 | 1.21 | |||||||
| Rib fat thickness (cm) | |||||||||||
| d1 3 p.p. | 1.6 | 1.5 | 1.6 | 1.6 | 0.08 | 0.424 | 0.671 | 0.395 | 0.739 | 0.395 | 0.355 |
| d 28 p.p. | 1.2 | 1.1 | 1.2 | 1.3 | |||||||
| Absolute masses of AT depot (kg) | |||||||||||
| Mesenteric AT | |||||||||||
| d1 3 p.p. | 13.5 | 11.7 | 13.9 | 13.8 | 0.84 | 0.197 | 0.300 | 0.415 | 0.452 | 0.551 | 0.486 |
| d 28 p.p. | 7.3 | 7.0 | 7.6 | 7.4 | |||||||
| Omental AT | |||||||||||
| d 1 3 p.p. | 14.8 | 13.3 | 14.4 | 14.3 | 0.66 | 0.395 | 0.320 | 0.305 | 0.353 | 0.588 | 0.806 |
| d 28 p.p. | 10.4 | 9.3 | 10.5 | 10.6 | |||||||
| Retroperitoneal AT | |||||||||||
| d1 3 p.p. | 9.4 | 8.8 | 9.7 | 9.5 | 0.53 | 0.422 | 0.837 | 0.539 | 0.603 | 0.184 | 0.552 |
| d 28 p.p. | 6.8 | 6.6 | 6.6 | 7.3 | |||||||
| Subcutaneous AT | |||||||||||
| d1 3 p.p. | 13.6 | 12.8 | 13.9 | 13.3 | 0.73 | 0.453 | 0.499 | 0.594 | 0.559 | 0.468 | 0.336 |
| d 28 p.p. | 9.6 | 8.7 | 9.6 | 10.0 | |||||||
| Visceral AT + | |||||||||||
| d1 3 p.p. | 37.7 | 33.8 | 37.9 | 37.5 | 1.72 | 0.276 | 0.387 | 0.359 | 0.683 | 0.324 | 0.679 |
| d 28 p.p. | 24.5 | 22.9 | 24.8 | 25.3 | |||||||
References
- Knapp, J.R.; Laur, G.L.; Vadas, P.A.; Weiss, W.P.; Tricarico, J.M. Invited review: Enteric methane in dairy cattle production: Quantifying the opportunities and impact of reducing emissions. J. Dairy Sci. 2014, 97, 3231–3261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hristov, A.N.; Oh, J.; Giallongo, F.; Frederick, T.W.; Harper, M.T.; Weeks, H.L.; Branco, A.F.; Moate, P.J.; Deighton, M.H.; Williams, S.R.; et al. An inhibitor persistently decreased enteric methane emission from dairy cows with no negative effect on milk production. Proc. Natl. Acad. Sci. USA 2015, 112, 10663–10668. [Google Scholar] [CrossRef] [Green Version]
- Patra, A.; Park, T.; Kim, M.; Yu, Z. Rumen methanogens and mitigation of methane emission by anti-methanogenic compounds and substances. J. Anim. Sci. Biotechnol. 2017, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Dijkstra, J.; Bannink, A.; France, J.; Kebreab, E.; van Gastelen, S. Short communication: Antimethanogenic effects of 3-nitrooxypropanol depend on supplementation dose, dietary fiber content, and cattle type. J. Dairy Sci. 2018, 101, 9041–9047. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, C.K.; Humphries, D.J.; Kirton, P.; Kindermann, M.; Duval, S.; Steinberg, W. Effects of 3-nitrooxypropanol on methane emission, digestion, and energy and nitrogen balance of lactating dairy cows. J. Dairy Sci. 2014, 97, 3777–3789. [Google Scholar] [CrossRef] [PubMed]
- Haisan, J.; Sun, Y.; Guan, L.L.; Beauchemin, K.A.; Iwaasa, A.; Duval, S.; Barreda, D.R.; Oba, M. The effects of feeding 3-nitrooxypropanol on methane emissions and productivity of Holstein cows in mid lactation. J. Dairy Sci. 2014, 97, 3110–3119. [Google Scholar] [CrossRef] [Green Version]
- Gerber, P.J.; Steinfeld, H.; Henderson, B.; Mottet, A.; Opio, C.; Dijkman, J.; Falcucci, A.; Tempio, G. Tackling Climate Change through Livestock: A Global Assessment of Emissions and Mitigation Opportunities; 9789251079201; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2013. [Google Scholar]
- Johnson, K.A.; Johnson, D.E. Methane emissions from cattle. J. Anim. Sci. 1995, 73, 2483–2492. [Google Scholar] [CrossRef]
- Penner, G.B.; Taniguchi, M.; Guan, L.L.; Beauchemin, K.A.; Oba, M. Effect of dietary forage to concentrate ratio on volatile fatty acid absorption and the expression of genes related to volatile fatty acid absorption and metabolism in ruminal tissue. J. Dairy Sci. 2009, 92, 2767–2781. [Google Scholar] [CrossRef]
- Aschenbach, J.R.; Kristensen, N.B.; Donkin, S.S.; Hammon, H.M.; Penner, G.B. Gluconeogenesis in dairy cows: The secret of making sweet milk from sour dough. IUBMB Life 2010, 62, 869–877. [Google Scholar] [CrossRef]
- Drackley, J.K.; Overton, T.R.; Douglas, G.N. Adaptations of glucose and long-chain fatty acid metabolism in liver of dairy cows during the periparturient period. J. Dairy Sci 2001, 84, E100–E112. [Google Scholar] [CrossRef]
- Gross, J.; van Dorland, H.A.; Bruckmaier, R.M.; Schwarz, F.J. Performance and metabolic profile of dairy cows during a lactational and deliberately induced negative energy balance with subsequent realimentation. J. Dairy Sci. 2011, 94, 1820–1830. [Google Scholar] [CrossRef]
- Van Gastelen, S.; Dijkstra, J.; Binnendijk, G.; Duval, S.M.; Heck, J.M.L.; Kindermann, M.; Zandstra, T.; Bannink, A. 3-Nitrooxypropanol decreases methane emissions and increases hydrogen emissions of early lactation dairy cows, with associated changes in nutrient digestibility and energy metabolism. J. Dairy Sci. 2020, 103, 8074–8093. [Google Scholar] [CrossRef]
- Haisan, J.; Sun, Y.; Guan, L.; Beauchemin, K.A.; Iwaasa, A.; Duval, S.; Kindermann, M.; Barreda, D.R.; Oba, M. The effects of feeding 3-nitrooxypropanol at two doses on milk production, rumen fermentation, plasma metabolites, nutrient digestibility, and methane emissions in lactating Holstein cows. Anim. Prod. Sci. 2017, 57, 282–289. [Google Scholar] [CrossRef]
- Schilde, M.; von Soosten, D.; Hüther, L.; Meyer, U.; Zeyner, A.; Dänicke, S. Effects of 3-nitrooxypropanol and varying concentrate feed proportions in the ration on methane emission, rumen fermentation and performance of periparturient dairy cows. Arch. Anim. Nutr. 2021, 75, 79–104. [Google Scholar] [CrossRef]
- Jayanegara, A.; Sarwono, K.A.; Kondo, M.; Matsui, H.; Ridla, M.; Laconi, E.B. Nahrowi Use of 3-nitrooxypropanol as feed additive for mitigating enteric methane emissions from ruminants: A meta-analysis. Ital. J. Anim. Sci. 2018, 17, 650–656. [Google Scholar] [CrossRef] [Green Version]
- Melgar, A.; Welter, K.C.; Nedelkov, K.; Martins, C.M.M.R.; Harper, M.T.; Oh, J.; Räisänen, S.E.; Chen, X.; Cueva, S.F.; Duval, S.; et al. Dose-response effect of 3-nitrooxypropanol on enteric methane emissions in dairy cows. J. Dairy Sci. 2020, 103, 6145–6156. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, H.G.; Baek, Y.-C.; Lee, S.; Seo, J. The effects of dietary supplementation with 3-nitrooxypropanol on enteric methane emissions, rumen fermentation, and production performance in ruminants: A meta-analysis. J. Anim. Sci. Technol. 2020, 62, 31–42. [Google Scholar] [CrossRef] [Green Version]
- Grummer, R.R. Impact of changes in organic nutrient metabolism on feeding the transition dairy cow. J. Anim. Sci. 1995, 73, 2820–2833. [Google Scholar] [CrossRef]
- Melgar, A.; Harper, M.T.; Oh, J.; Giallongo, F.; Young, M.E.; Ott, T.L.; Duval, S.; Hristov, A.N. Effects of 3-nitrooxypropanol on rumen fermentation, lactational performance, and resumption of ovarian cyclicity in dairy cows. J. Dairy Sci. 2020, 103, 410–432. [Google Scholar] [CrossRef] [Green Version]
- Gerrits, W.J.J.; Labussiere, E. Indirect Calorimetry: Techniques, Computations and Applications; Wageningen Academic Publishers: Wageningen, The Netherlands, 2015; p. 294. [Google Scholar]
- Brouwer, E. Report of sub-committee on constants and factors. In Proceedings of the 3rd Symposium on Energy Metabolism; Blaxter, K.L., Ed.; Academic Press: London, UK, 1965; pp. 441–443. [Google Scholar]
- Hammond, K.J.; Humphries, D.J.; Crompton, L.A.; Green, C.; Reynolds, C.K. Methane emissions from cattle: Estimates from short-term measurements using a GreenFeed system compared with measurements obtained using respiration chambers or sulphur hexafluoride tracer. Anim. Feed Sci. Technol. 2015, 203, 41–52. [Google Scholar] [CrossRef]
- GfE. Empfehlung zur Energie- und Nährstoffversorgung der Milchkühe und Aufzuchtrinder; Deutsche Landwirtschafts-Gesellschaft-Verlags-GmbH: Frankfurt am Main, Germany, 2001; p. 136. [Google Scholar]
- Raschka, C.; Ruda, L.; Wenning, P.; von Stemm, C.I.; Pfarrer, C.; Huber, K.; Meyer, U.; Dänicke, S.; Rehage, J. In vivo determination of subcutaneous and abdominal adipose tissue depots in German Holstein dairy cattle. J. Anim. Sci. 2016, 94, 2821–2834. [Google Scholar] [CrossRef] [PubMed]
- Schäfers, S.; von Soosten, D.; Meyer, U.; Drong, C.; Frahm, J.; Kluess, J.; Raschka, C.; Rehage, J.; Tröscher, A.; Pelletier, W.; et al. Influence of conjugated linoleic acid and vitamin E on performance, energy metabolism, and change of fat depot mass in transitional dairy cows. J. Dairy Sci. 2017, 100, 3193–3208. [Google Scholar] [CrossRef] [Green Version]
- III Die chemische Untersuchung von Futtermitteln. In Handbuch der Landwirtschaftlichen Versuchs-und Untersuchungsmethodik (VDLUFA-Methodenbuch); VDLUFA-Verlag: Darmstadt, Germany, 2006.
- Lammers, B.P.; Buckmaster, D.R.; Heinrichs, A.J. A simple method for the analysis of particle sizes of forage and total mixed rations. J. Dairy Sci. 1996, 79, 922–928. [Google Scholar] [CrossRef]
- DLG-Verlags-GmbH. Futterwerttabellen-Wiederkäuer; DLG-Verlags-GmbH: Frankfurt am Main, Germany, 1997. [Google Scholar]
- GfE. Zur Energiebewertung beim Wiederkäuer. Proc. Soc. Nutr. Physiol. 1995, 4, 121–123. [Google Scholar]
- Huhtanen, P.; Cabezas-Garcia, E.H.; Utsumi, S.; Zimmerman, S. Comparison of methods to determine methane emissions from dairy cows in farm conditions. J. Dairy Sci. 2015, 98, 3394–3409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manafiazar, G.; Zimmerman, S.; Basarab, J.A. Repeatability and variability of short-term spot measurement of methane and carbon dioxide emissions from beef cattle using GreenFeed emissions monitoring system. Can. J. Anim. Sci. 2016, 97, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Chwalibog, A.; Jensen, K.; Thorbek, G. Oxidation of nutrients in bull calves treated with beta-adrenergic agonists. Arch. Tierernaehr 1996, 49, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Chwalibog, A.; Tauson, A.-H.; Thorbek, G. Quantitative oxidation of nutrients in growing calves. Z. Ernährungswiss 1997, 36, 313–316. [Google Scholar] [CrossRef]
- Blümmel, M.; Aiple, K.-P.; Steingaβ, H.; Becker, K. A note on the stoichiometrical relationship of short chain fatty acid production and gas formation in vitro in feedstuffs of widely differing quality. J. Anim. Physiol. Anim. Nutr. 1999, 81, 157–167. [Google Scholar] [CrossRef]
- Derno, M.; Nürnberg, G.; Schön, P.; Schwarm, A.; Röntgen, M.; Hammon, H.M.; Metges, C.C.; Bruckmaier, R.M.; Kuhla, B. Short-term feed intake is regulated by macronutrient oxidation in lactating Holstein cows. J. Dairy Sci. 2013, 96, 971–980. [Google Scholar] [CrossRef] [Green Version]
- Derno, M.; Nürnberg, G.; Kuhla, B. Characterizing the metabotype and its persistency in lactating Holstein cows: An approach toward metabolic efficiency measures. J. Dairy Sci. 2019, 102, 6559–6570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dijkstra, J.; Oenema, O.; van Groenigen, J.W.; Spek, J.W.; van Vuuren, A.M.; Bannink, A. Diet effects on urine composition of cattle and N2O emissions. Animal 2013, 7, 292–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erdmann, S.; Derno, M.; Schäff, C.T.; Börner, S.; Kautzsch, U.; Kuhla, B.; Hammon, H.M.; Tuchscherer, A.; Röntgen, M. Comparative analyses of estimated and calorimetrically determined energy balance in high-yielding dairy cows. J. Dairy Sci. 2019, 102, 4002–4013. [Google Scholar] [CrossRef] [Green Version]
- Moe, P.W.; Tyrrell, H.F.; Flatt, W.P. Energetics of body tissue mobilization. J. Dairy Sci. 1971, 54, 548–553. [Google Scholar] [CrossRef]
- Agricultural and Food Research Council (AFRC). Energy and Protein Requirements of Ruminants: An Advisory Manual Prepared by the AFRC Technical Committee on Responses to Nutrients; CAB Int.: Wallingford, UK, 1993; p. 183. [Google Scholar]
- Littell, R.C.; Henry, P.R.; Ammerman, C.B. Statistical analysis of repeated measures data using SAS procedures. J. Anim. Sci. 1998, 76, 1216–1231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Van den Borne, J.J.G.C.; Heetkamp, M.J.W.; Alferink, S.J.J.; Gerrits, W.J.J. Moving from a complete energy balance towards substrate oxidation: Use of stable isotopes. In Indirect Calorimetry Techniques, Computations and Applications; Gerrits, W.J.J., Labussiere, E., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2015; pp. 87–106. [Google Scholar]
- Caetano, M.; Wilkes, M.J.; Pitchford, W.S.; Lee, S.J.; Hynd, P.I. Energy relations in cattle can be quantified using open-circuit gas-quantification systems. Anim. Prod. Sci. 2018, 58, 1807–1813. [Google Scholar] [CrossRef]
- Gruber, L.; Knaus, W.; Susenbeth, A.; Schwarz, F.J.; Guggenberger, T.; Fischer, B.; Spiekers, H.; Steingaß, H.; Meyer, U.; Münger, A.; et al. Evaluation of the net energy for lactation system and estimation of the energy requirements of dairy cows based on a comprehensive analysis of feeding trials. Arch. Anim. Nutr. 2021, 75, 1–17. [Google Scholar] [CrossRef]
- Agnew, R.E.; Yan, T. Impact of recent research on energy feeding systems for dairy cattle. Livest. Prod. Sci. 2000, 66, 197–215. [Google Scholar] [CrossRef]
- Van Es, A.J.H. Feed evaluation for dairy cows. Livest. Prod. Sci. 1975, 2, 95–107. [Google Scholar] [CrossRef]
- Tamminga, S.; Luteijn, P.A.; Meijer, R.G.M. Changes in composition and energy content of liveweight loss in dairy cows with time after parturition. Livest Prod. Sci. 1997, 52, 31–38. [Google Scholar] [CrossRef]
- Moe, P.W. Energy metabolism of dairy cattle. J. Dairy Sci. 1981, 64, 1120–1139. [Google Scholar] [CrossRef]
- von Soosten, D.; Meyer, U.; Piechotta, M.; Flachowsky, G.; Dänicke, S. Effect of conjugated linoleic acid supplementation on body composition, body fat mobilization, protein accretion, and energy utilization in early lactation dairy cows. J. Dairy Sci. 2012, 95, 1222–1239. [Google Scholar] [CrossRef]
- Komaragiri, M.V.; Erdman, R.A. Factors affecting body tissue mobilization in early lactation dairy cows. 1. Effect of dietary protein on mobilization of body fat and protein. J. Dairy Sci. 1997, 80, 929–937. [Google Scholar] [CrossRef]
- Williams, C.B.; Jenkins, T.G. A dynamic model of metabolizable energy utilization in growing and mature cattle. I. Metabolizable energy utilization for maintenance and support metabolism. J. Anim Sci. 2003, 81, 1371–1381. [Google Scholar] [CrossRef]
- Janssen, P.H. Influence of hydrogen on rumen methane formation and fermentation balances through microbial growth kinetics and fermentation thermodynamics. Anim. Feed. Sci. Tech. 2010, 160, 1–22. [Google Scholar] [CrossRef]
- Reynolds, C.K.; Aikman, P.C.; Lupoli, B.; Humphries, D.J.; Beever, D.E. Splanchnic metabolism of dairy cows during the transition from late gestation through early lactation. J. Dairy Sci. 2003, 86, 1201–1217. [Google Scholar] [CrossRef]
- Bigner, D.R.; Goff, J.P.; Faust, M.A.; Burton, J.L.; Tyler, H.D.; Horst, R.L. Acidosis effects on insulin response during glucose tolerance tests in Jersey cows. J. Dairy Sci. 1996, 79, 2182–2188. [Google Scholar] [CrossRef]
- Kraft, W.; Dürr, U.M. Klinische Labordiagnostik in der Tiermedizin, 7th ed.; Moritz, A., Ed.; Schattauer Verlag: Stuttgart, Germany, 2013; p. 934. [Google Scholar]

 ): 70 kJ NEL/kg BW0.75 and d; p < 0.001 with confidence interval (
): 70 kJ NEL/kg BW0.75 and d; p < 0.001 with confidence interval ( ); lower limits of agreement (LoA) (
); lower limits of agreement (LoA) ( ): −149 kJ NEL/kg BW0.75 and d; upper LoA: 288 kJ NEL/kg BW0.75 and d; regression line (
): −149 kJ NEL/kg BW0.75 and d; upper LoA: 288 kJ NEL/kg BW0.75 and d; regression line ( ): y = 0.01x + 71 (RSE = 112 kJ/kg BW0.75 and d on 133 degrees of freedom, R2 = 0.0007, p = 0.756). Statistics 2B: regression line (
): y = 0.01x + 71 (RSE = 112 kJ/kg BW0.75 and d on 133 degrees of freedom, R2 = 0.0007, p = 0.756). Statistics 2B: regression line ( ; period 2 and 3): y = 0.67(0.02) x − 51(7); (RSE = 51 kJ NEL/kg BW0.75 and d on 88 degrees of freedom, R2 = 0.92, p < 0.001).
; period 2 and 3): y = 0.67(0.02) x − 51(7); (RSE = 51 kJ NEL/kg BW0.75 and d on 88 degrees of freedom, R2 = 0.92, p < 0.001).
 ): 70 kJ NEL/kg BW0.75 and d; p < 0.001 with confidence interval (
): 70 kJ NEL/kg BW0.75 and d; p < 0.001 with confidence interval ( ); lower limits of agreement (LoA) (
); lower limits of agreement (LoA) ( ): −149 kJ NEL/kg BW0.75 and d; upper LoA: 288 kJ NEL/kg BW0.75 and d; regression line (
): −149 kJ NEL/kg BW0.75 and d; upper LoA: 288 kJ NEL/kg BW0.75 and d; regression line ( ): y = 0.01x + 71 (RSE = 112 kJ/kg BW0.75 and d on 133 degrees of freedom, R2 = 0.0007, p = 0.756). Statistics 2B: regression line (
): y = 0.01x + 71 (RSE = 112 kJ/kg BW0.75 and d on 133 degrees of freedom, R2 = 0.0007, p = 0.756). Statistics 2B: regression line ( ; period 2 and 3): y = 0.67(0.02) x − 51(7); (RSE = 51 kJ NEL/kg BW0.75 and d on 88 degrees of freedom, R2 = 0.92, p < 0.001).
; period 2 and 3): y = 0.67(0.02) x − 51(7); (RSE = 51 kJ NEL/kg BW0.75 and d on 88 degrees of freedom, R2 = 0.92, p < 0.001).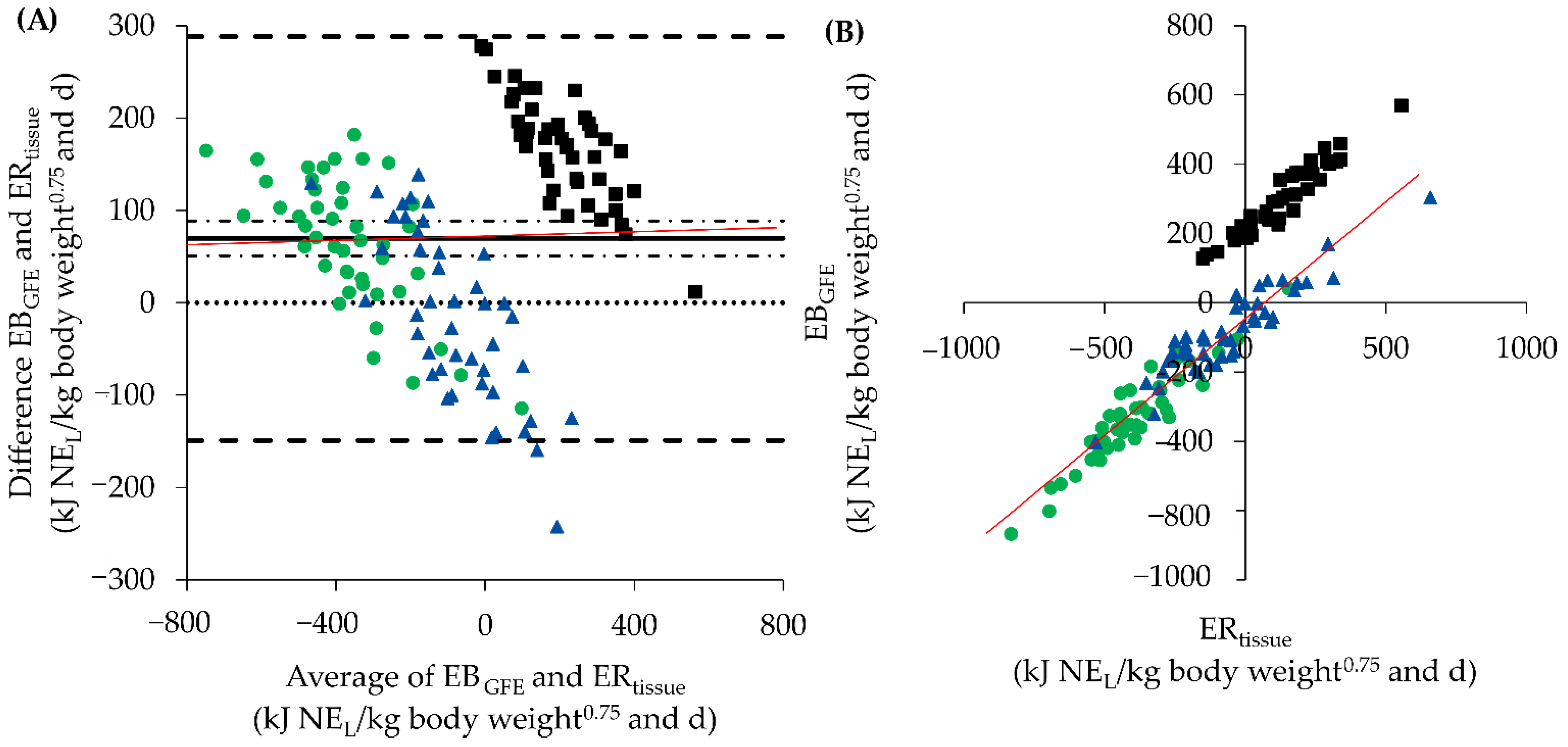



| Item | CON † | 3-NOP § | ||
|---|---|---|---|---|
| LC | HC | LC | HC | |
| DM + (g/kg) | 467 | 582 | 467 | 597 |
| Nutrient (g/kg of DM) | ||||
| Crude ash | 63 | 61 | 63 | 61 |
| Crude protein | 130 | 138 | 129 | 140 |
| Utilizable crude protein | 142 | 150 | 142 | 151 |
| Ether extract | 32 | 35 | 32 | 36 |
| aNDFom ¶ | 402 | 344 | 404 | 337 |
| Acid detergent fibreom | 226 | 191 | 227 | 187 |
| peNDF>8 mm # | 268 | 269 | 274 | 273 |
| Starch | 249 | 303 | 246 | 307 |
| Sugar | 17 | 25 | 17 | 27 |
| Energy $ (MJ/kg of DM) | ||||
| GE | 18.4 | 18.5 | 18.4 | 18.4 |
| ME | 11.0 | 11.5 | 11.0 | 11.5 |
| NEL | 6.6 | 7.0 | 6.6 | 7.1 |
| Item | Treatments † | SEM | p-Values § | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CONHC (n = 12) | CONLC (n = 13) | NOPHC (n = 11) | NOPLC (n = 9) | 3-NOP | CFP | 3-NOP ×PER | CFP ×PER | 3-NOP ×CFP ×PER | ||
| VCH4 production | ||||||||||
| Per 1 | 0490 | 0495 | 0381 | 0397 | 20 | <0.001 | <0.156 | <0.001 | <0.001 | <0.001 |
| Per 2 | 0440 | 0475 | 0314 | 0316 | ||||||
| Per 3 | 0535 | 0542 | 0341 | 0438 | ||||||
| Total VCO2 production | ||||||||||
| Per 1 | 6632 | 6378 | 6662 | 6369 | 160 | <0.064 | <0.132 | <0.001 | <0.282 | <0.001 |
| Per 2 | 6368 | 6289 | 6002 | 5823 | ||||||
| Per 3 | 7278 | 6719 | 6557 | 6553 | ||||||
| VCO2metabolic + | ||||||||||
| Per 1 | 5798 | 5537 | 6014 | 5695 | 140 | <0.653 | <0.041 | <0.001 | <0.043 | <0.003 |
| Per 2 | 5621 | 5481 | 5468 | 5285 | ||||||
| Per 3 | 6368 | 5797 | 5978 | 5809 | ||||||
| VO2 consumption | ||||||||||
| Per 1 | 6348 | 6190 | 6267 | 6205 | 138 | <0.073 | <0.436 | <0.001 | <0.342 | <0.166 |
| Per 2 | 5988 | 5933 | 5709 | 5693 | ||||||
| Per 3 | 6480 | 6167 | 5910 | 5911 | ||||||
| RQmetabolic ¶ | ||||||||||
| Per 1 | 0.91 | 0.90 | 0.96 | 0.92 | 0.007 | <0.001 | <0.001 | <0.001 | <0.014 | <0.001 |
| Per 2 | 0.94 | 0.92 | 0.95 | 0.93 | ||||||
| Per 3 | 0.98 | 0.94 | 1.01 | 0.99 | ||||||
| RQtotal # | ||||||||||
| Per 1 | 1.04 | 1.03 | 1.06 | 1.03 | 0.008 | 0.638 | 0.004 | 0.001 | 0.602 | <0.001 |
| Per 2 | 1.06 | 1.06 | 1.05 | 1.02 | ||||||
| Per 3 | 1.12 | 1.09 | 1.11 | 1.11 | ||||||
| Item | Treatments † | SEM | p-Values § | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CONHC (n = 12) | CONLC (n = 13) | NOPHC (n = 11) | NOPLC (n = 9) | 3-NOP | CFP | 3-NOP ×CFP | 3-NOP ×PER | CFP ×PER | 3-NOP ×CFP ×PER | ||
| Metabolic body weight (kg BW0.75) | |||||||||||
| Per 1 | 146 | 144 | 144 | 146 | 2.9 | 0.780 | 0.868 | 0.517 | 0.227 | <0.001 | 0.417 |
| Per 2 | 132 | 131 | 129 | 131 | |||||||
| Per 3 | 131 | 127 | 128 | 128 | |||||||
| Energy intake (kJ/kg BW0.75 and d) | |||||||||||
| Gross energy intake (GEI) | |||||||||||
| Per 1 | 1963 | 1812 | 2022 | 1738 | 64 | 0.546 | <0.001 | 0.210 | 0.146 | <0.001 | 0.092 |
| Per 2 | 2468 | 2434 | 2620 | 2335 | |||||||
| Per 3 | 3272 | 2900 | 3379 | 2961 | |||||||
| Metabolizable energy intake (MEI) | |||||||||||
| Per 1 | 1193 | 1076 | 1288 | 1031 | 40 | 0.325 | <0.001 | 0.110 | 0.379 | <0.001 | 0.084 |
| Per 2 | 1529 | 1458 | 1631 | 1402 | |||||||
| Per 3 | 2032 | 1734 | 2111 | 1769 | |||||||
| Energy expenditures (kJ/kg BW0.75 and d) | |||||||||||
| Net energy demand for pregnancy (NEP) + | |||||||||||
| Per 1 | 115 | 117 | 118 | 116 | 2.9 | 0.702 | 0.951 | 0.575 | |||
| Milk energy excretion (MEE) ¶ | |||||||||||
| Per 2 | 978 | 991 | 945 | 960 | 25 | 0.195 | 0.492 | 0.476 | 0.852 | <0.001 | 0.056 |
| Per 3 | 1010 | 931 | 948 | 935 | |||||||
| Heat production (HP) # | |||||||||||
| Per 1 | 916 | 905 | 930 | 896 | 17 | 0.028 | 0.211 | 0.738 | <0.001 | 0.750 | 0.267 |
| Per 2 | 967 | 966 | 940 | 918 | |||||||
| Per 3 | 1071 | 1044 | 1001 | 987 | |||||||
| Methane energy (CH4E) ‡ | |||||||||||
| Per 1 | 134 | 137 | 106 | 106 | 5.3 | <0.001 | 0.099 | 0.913 | <0.001 | <0.001 | <0.001 |
| Per 2 | 132 | 144 | 96 | 95 | |||||||
| Per 3 | 161 | 170 | 106 | 134 | |||||||
| Energy retention (kJ/kg BW0.75 and d) | |||||||||||
| Energy retention in body tissues and milk (ERtotal) ◊ | |||||||||||
| Per 1 | 295 | 183 | 372 | 125 | 41 | 0.084 | <0.001 | 0.137 | 0.002 | <0.001 | 0.176 |
| Per 2 | 562 | 492 | 697 | 485 | |||||||
| Per 3 | 961 | 690 | 1103 | 783 | |||||||
| Energy retention in body tissues (ERtissue) ♦ | |||||||||||
| Per 1 | 170 | 65 | 254 | 6 | 45 | 0.036 | <0.001 | 0.101 | 0.003 | 0.022 | 0.715 |
| Per 2 | −396 | −499 | −222 | −474 | |||||||
| Per 3 | −23 | −230 | 167 | −139 | |||||||
| Energy balance calculated according to GfE [24] (EBGFE) ⁑ | |||||||||||
| Per 1 | 323 | 245 | 379 | 216 | 28 | 0.104 | <0.001 | 0.077 | 0.066 | 0.059 | 0.982 |
| Per 2 | −323 | −397 | −229 | −398 | |||||||
| Per 3 | −58 | −171 | 54 | −154 | |||||||
| Energy retention in fat depots (ERfat depot) ║ | |||||||||||
| Per 2 | −185 | −175 | −169 | −181 | 24 | 0.841 | 0.974 | 0.637 | |||
| Residual energy retention (ERresidual) ∏ | |||||||||||
| Per 2 | −211 | −324 | −55 | −292 | 48 | 0.058 | 0.001 | 0.205 | |||
| Item | Treatments † | SEM | p-Values § | |||||
|---|---|---|---|---|---|---|---|---|
| CONHC (n = 14) | CONLC (n = 15) | NOPHC (n = 14) | NOPLC (n = 12) | 3-NOP | CFP | 3-NOP ×CFP | ||
| Change in fat layer thickness | ||||||||
| Back fat thickness | −0.15 | −0.15 | −0.14 | −0.12 | 0.03 | 0.709 | 0.847 | 0.786 |
| Rib fat thickness | −0.16 | −0.15 | −0.17 | −0.13 | 0.03 | 0.814 | 0.493 | 0.699 |
| Change in AT depot mass | ||||||||
| Mesenteric | −0.26 | −0.22 | −0.20 | −0.31 | 0.05 | 0.775 | 0.479 | 0.136 |
| Omental | −0.18 | −0.18 | −0.16 | −0.15 | 0.02 | 0.285 | 0.956 | 0.952 |
| Retroperitoneal | −0.12 | −0.10 | −0.13 | −0.11 | 0.02 | 0.780 | 0.367 | 0.894 |
| Subcutaneous | −0.17 | −0.18 | −0.17 | −0.14 | 0.02 | 0.410 | 0.616 | 0.399 |
| Visceral + | −0.56 | −0.50 | −0.48 | −0.57 | 0.07 | 0.998 | 0.996 | 0.330 |
| Visceral and subcutaneous | −0.73 | −0.68 | −0.65 | −0.70 | 0.08 | 0.758 | 0.994 | 0.627 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schilde, M.; von Soosten, D.; Frahm, J.; Kersten, S.; Meyer, U.; Zeyner, A.; Dänicke, S. Assessment of Metabolic Adaptations in Periparturient Dairy Cows Provided 3-Nitrooxypropanol and Varying Concentrate Proportions by Using the GreenFeed System for Indirect Calorimetry, Biochemical Blood Parameters and Ultrasonography of Adipose Tissues. Dairy 2022, 3, 100-122. https://doi.org/10.3390/dairy3010009
Schilde M, von Soosten D, Frahm J, Kersten S, Meyer U, Zeyner A, Dänicke S. Assessment of Metabolic Adaptations in Periparturient Dairy Cows Provided 3-Nitrooxypropanol and Varying Concentrate Proportions by Using the GreenFeed System for Indirect Calorimetry, Biochemical Blood Parameters and Ultrasonography of Adipose Tissues. Dairy. 2022; 3(1):100-122. https://doi.org/10.3390/dairy3010009
Chicago/Turabian StyleSchilde, Matthias, Dirk von Soosten, Jana Frahm, Susanne Kersten, Ulrich Meyer, Annette Zeyner, and Sven Dänicke. 2022. "Assessment of Metabolic Adaptations in Periparturient Dairy Cows Provided 3-Nitrooxypropanol and Varying Concentrate Proportions by Using the GreenFeed System for Indirect Calorimetry, Biochemical Blood Parameters and Ultrasonography of Adipose Tissues" Dairy 3, no. 1: 100-122. https://doi.org/10.3390/dairy3010009
APA StyleSchilde, M., von Soosten, D., Frahm, J., Kersten, S., Meyer, U., Zeyner, A., & Dänicke, S. (2022). Assessment of Metabolic Adaptations in Periparturient Dairy Cows Provided 3-Nitrooxypropanol and Varying Concentrate Proportions by Using the GreenFeed System for Indirect Calorimetry, Biochemical Blood Parameters and Ultrasonography of Adipose Tissues. Dairy, 3(1), 100-122. https://doi.org/10.3390/dairy3010009







