Embryo Morphokinetic Activity Evident in Short Videos of In Vitro Bovine Embryos
Abstract
:1. Introduction
2. Materials and Methods
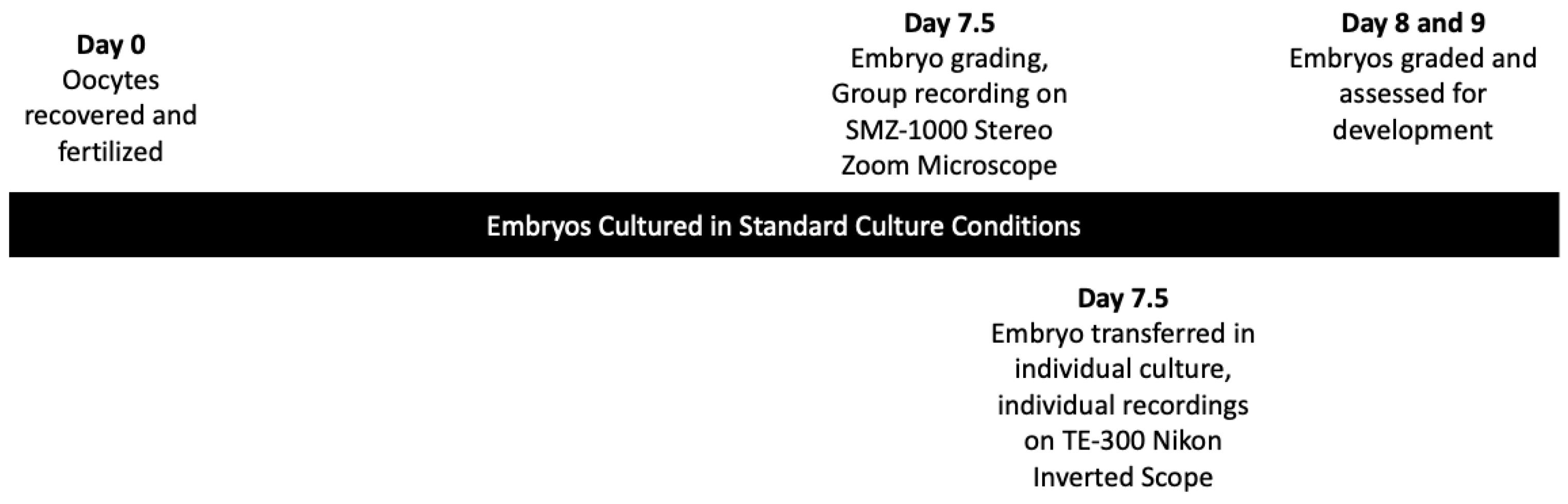
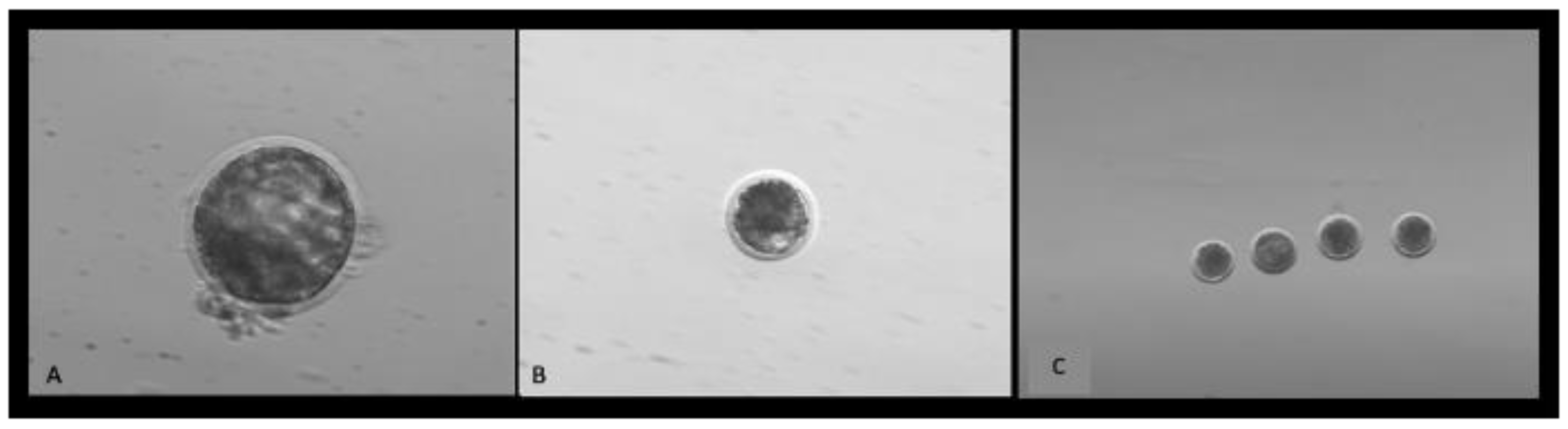
2.1. Laboratory Study to Collect Video Data of Bovine Embryos
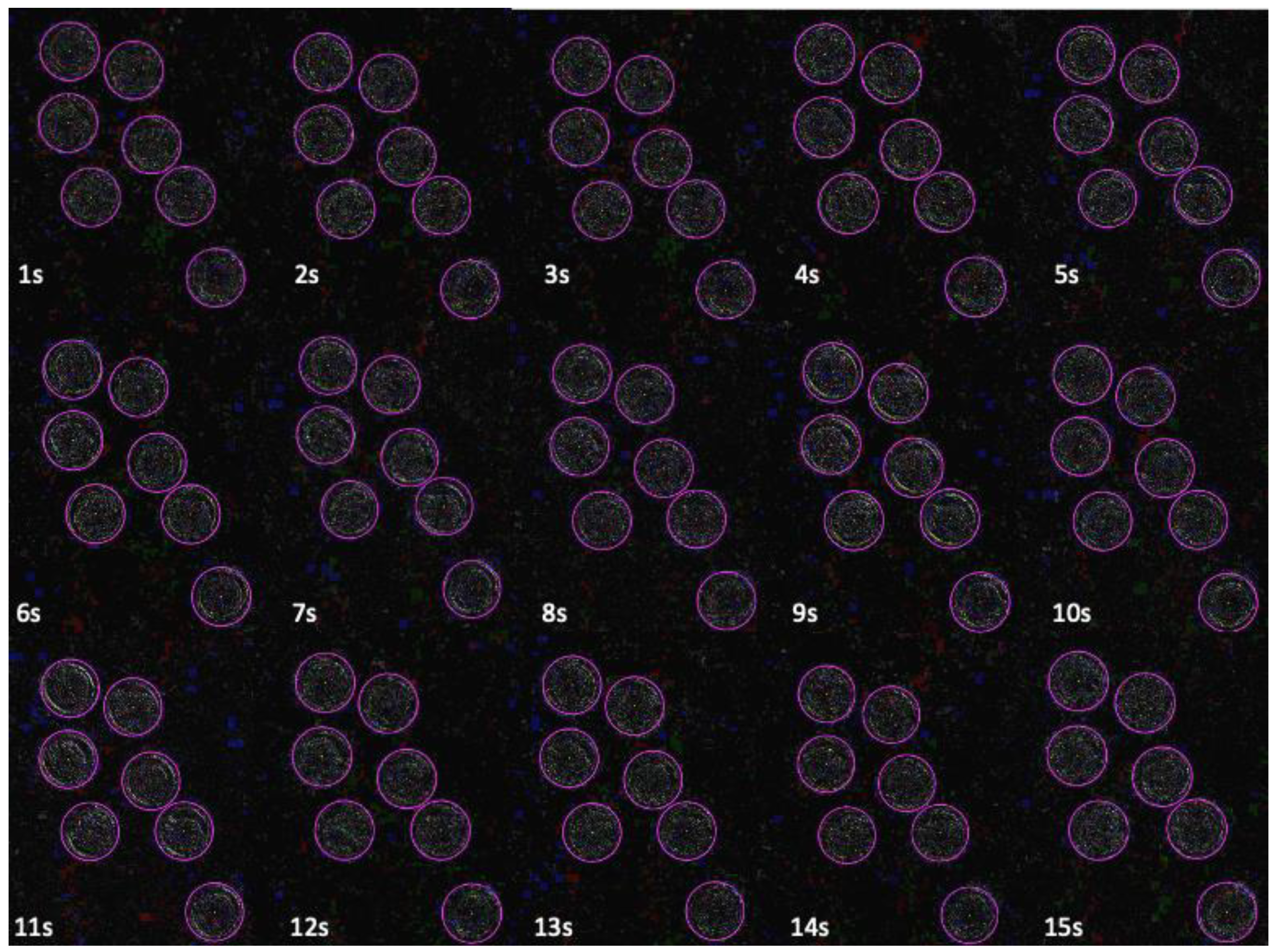
2.2. Graphic Imaging Techniques to Evaluate Videos of Bovine Embryos
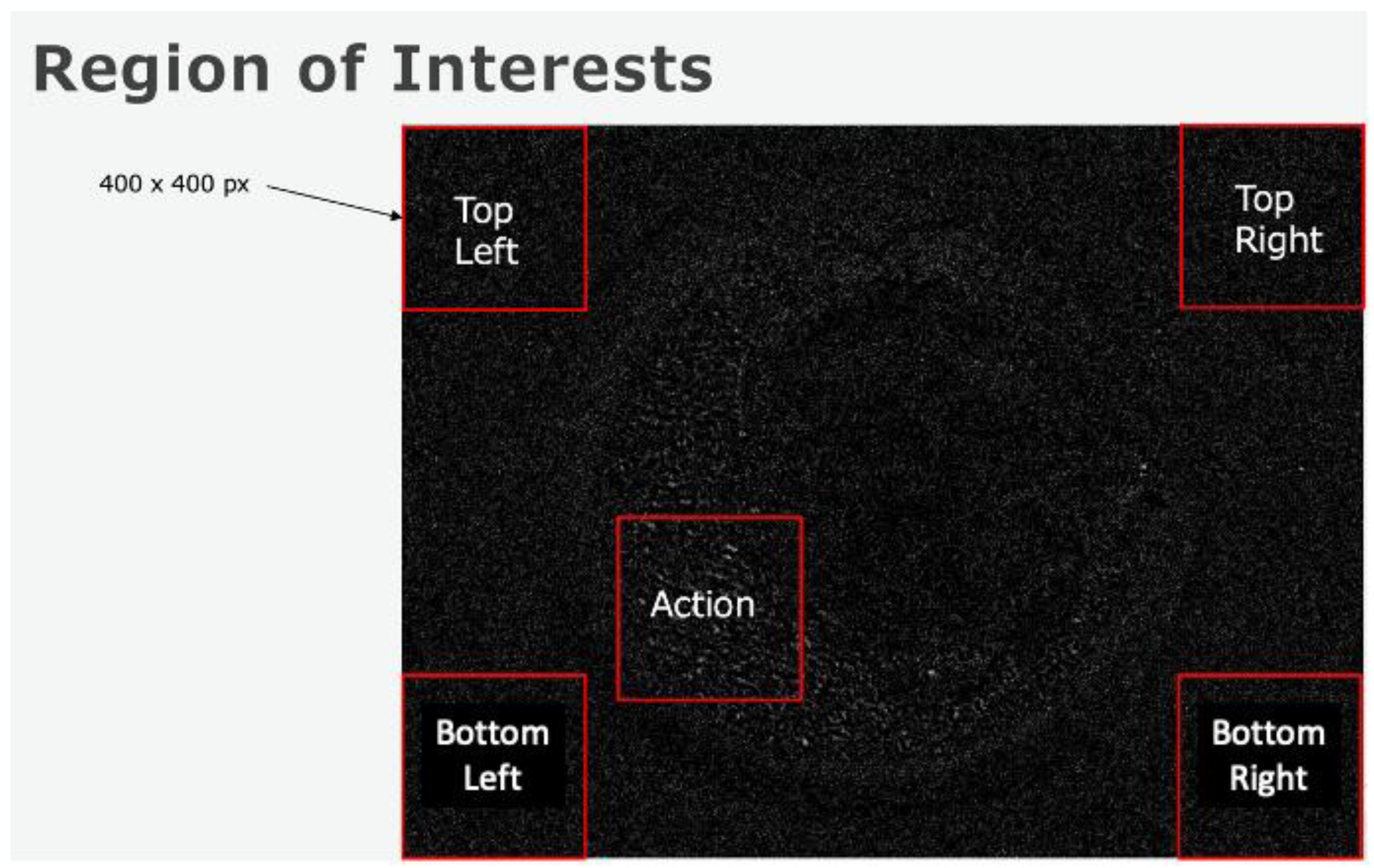
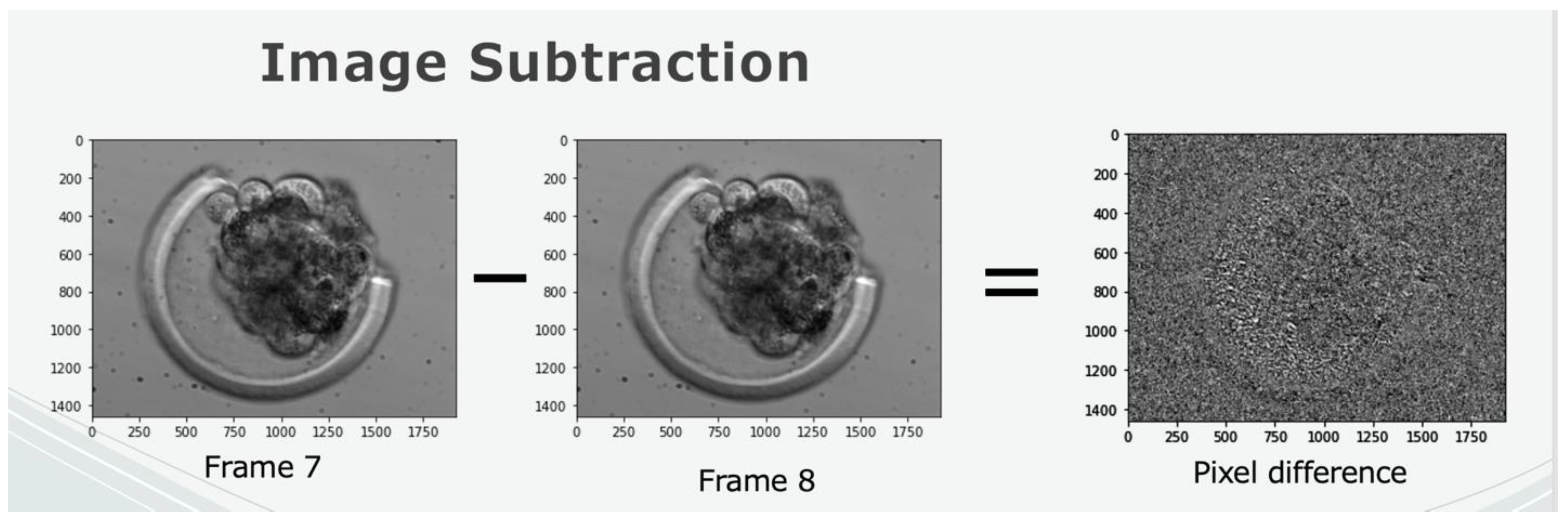
2.3. Implementation of Quality Control Methods and Evaluation of Noise
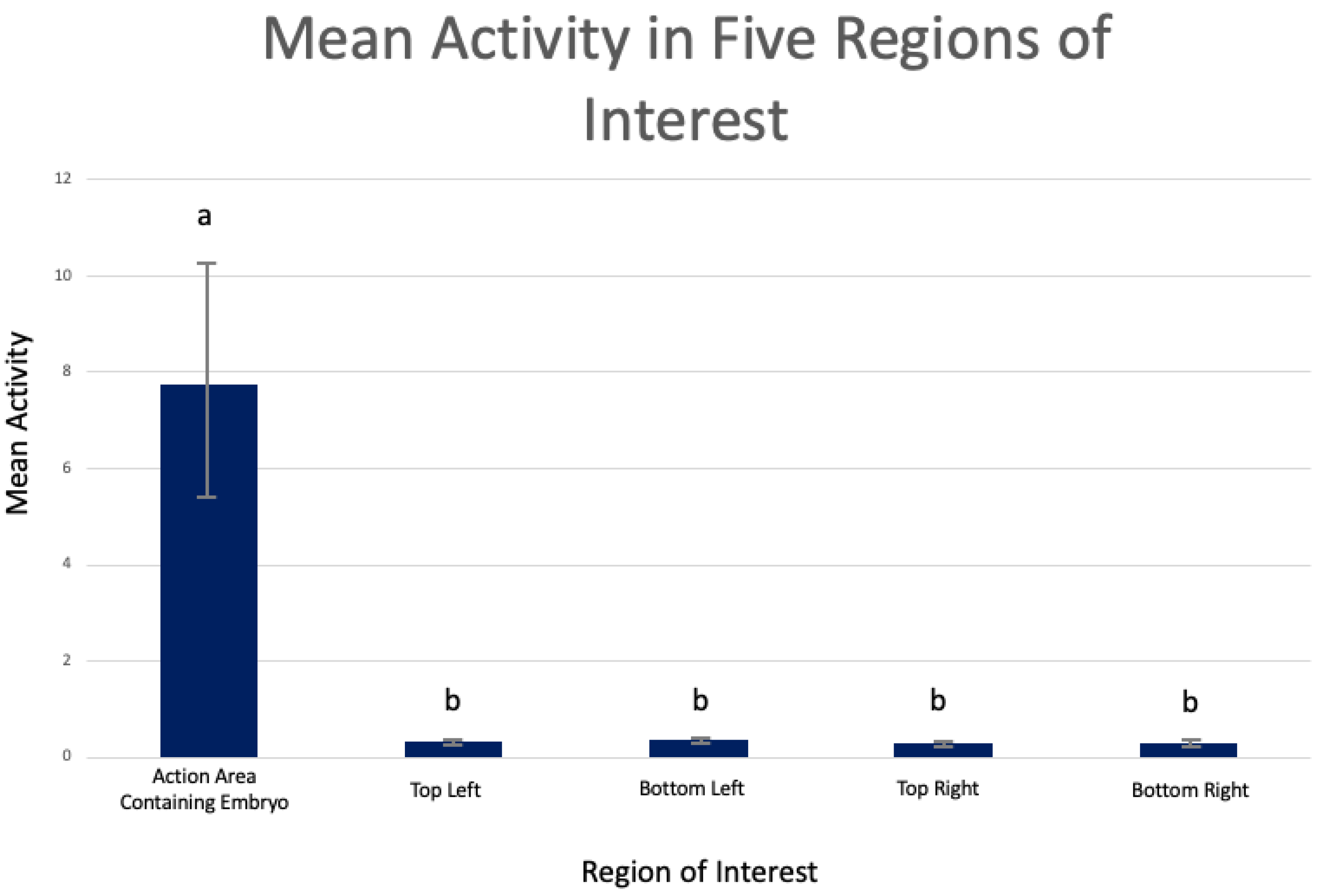
2.4. Identification of Metrics Correlating to Embryo Viability
3. Statistical Analysis
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Henchion, M.; Hayes, M.; Mullen, A.M.; Fenelon, M.; Tiwari, B. Future Protein Supply and Demand: Strategies and Factors Influencing a Sustainable Equilibrium. Foods 2017, 6, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capper, J. The environmental impact of beef production in the United States: 1977 compared with 2007. J. Anim. Sci. 2011, 89, 4249–4261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capper, J.; Cady, R.; Bauman, D. The environmental impact of dairy production: 1944 compared with 2007. J. Anim. Sci. 2009, 87, 2160–2167. [Google Scholar] [CrossRef] [PubMed]
- Brito, L.; Bedere, N.; Douhard, F.; Oliveria, H.; Arnal, M.; Penagaricano, F.; Schnickel, A.; Baes, C.; Miglior, F. Review: Genetic selection of high-yielding dairy cattle toward sustainable farming systems in a rapidly changing world. Animals 2021, 15, 1. [Google Scholar] [CrossRef]
- Dillon, P.; Buckley, F.; O’Connor, P.; Hegarty, D.; Rath, M. A comparison of different dairy cow breeds on a seasonal grass-based system of milk production: 1. Milk production, live weight, body condition score and DM intake. Livest. Prod. Sci. 2003, 83, 21–33. [Google Scholar] [CrossRef]
- Dillon, P.; Snijders, S.; Buckley, F.; Harris, B.; O’Connor, P.; Mee, J. A comparison of different dairy cow breeds on a seasonal grass-based system of milk production: 2. Reproduction and survival. Livest. Prod. Sci. 2003, 83, 35–42. [Google Scholar] [CrossRef]
- Walsh, S.; Buckley, F.; Berry, D.; Rath, M.; Pierce, K.; Byrne, N.; Dillon, P. Effects of breed, feeding system, and parity on udder health and milking characteristics. J. Dairy Sci. 2007, 90, 5767–5779. [Google Scholar] [CrossRef]
- Walsh, S.; Buckley, F.; Pierce, K.; Byrne, N.; Patton, J.; Dillon, P. Effects of breed and feeding system on milk production, body weight, body condition score, reproductive performance, and postpartum ovarian function. J. Dairy Sci. 2008, 91, 4401–4413. [Google Scholar] [CrossRef] [Green Version]
- Azzarello, A.; Hoest, T.; Hay-Schmidt, A.; Mikkelsen, A.L. Live birth potential of good morphology and vitrified blastocysts presenting abnormal cell divisions. Reprod. Biol. 2017, 17, 144–150. [Google Scholar] [CrossRef]
- Bedere, N.; Disenhaus, C.; Ducrocq, V.; Leurent-Colette, S.; Delaby, L. Ability of dairy cows to be inseminated according to breed and genetic merit for production traits under contrasting pasture-based feeding systems. Animal 2017, 11, 826–835. [Google Scholar] [CrossRef]
- Bedere, N.; Disenhaus, C.; Ducrocq, V.; Leurent-Colette, S.; Delaby, L. Ability of dairy cows to ensure pregnancy according to breed and genetic merit for production traits under contrasted pasture-based systems. J. Dairy Sci. 2017, 100, 2812–2827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potts, B.; Shaughness, M.; Erdman, R.A. The decline in digestive efficiency of US dairy cows from 1970 to 2014. J. Dairy Sci. 2017, 100, 5400–5410. [Google Scholar] [CrossRef] [Green Version]
- Wells, C. EmGenisys Pitch Deck. In Luminate NextCorps Accelerator; EmGenisys: Rochester, NY, USA, 2022. [Google Scholar]
- Demetrio, D.; Looney, C.; Rees, H.; Werhman, M. 2020 Statistical Information Committee Report (2019 Data). 2020. Annual Report of the AETA Statistical Information Committee. Available online: https://www.aeta.org/docs/2019_Stats.pdf (accessed on 22 September 2022).
- Bo, G.; Mapletoft, R. Evaluation and classification of bovine embryos. Anim. Reprod. 2013, 10, 344–348. [Google Scholar]
- Wessels, C.; Penrose, L.; Prien, S. Current State of Art Embryo Selection Techniques. Investig. Obstet. Gynecol. Res. 2017, 1, 1–6. [Google Scholar] [CrossRef]
- Cruz, M.; Blanca, G.; Garrido, N.; Pedersen, K.; Martínez, M. Embryo Quality, Blastocyst and Ongoing Pregnancy Rates in Oocyte Donation Patients Whose Embryos Were Monitored by Time-lapse Imaging. J. Assist. Reprod. Genet. 2011, 28, 569–573. [Google Scholar] [CrossRef] [Green Version]
- Del Gallego, R.; Remohi, J.; Meseguer, M. Time-lapse imaging: State of the art. Biol. Reprod. 2019, 101, 1146–1154. [Google Scholar] [CrossRef]
- Zaninovic, N.; Irani, M.; Meseguer, M. Assessment of embryo morphology and developmental dynamics by time-lapse microscopy: Is there a relation to implantation and ploidy? Fertil. Steril. 2017, 108, 722–729. [Google Scholar] [CrossRef] [Green Version]
- Athayde Wirka, K.; Chen, A.A.; Conaghan, J.; Ivani, K.; Gvakharia, M.; Behr, B.; Suraj, V.; Tan, L.; Shen, S. Atypical Embryo Phenotypes Identified by Time-Lapse Microscopy: High Prevalence and Association with Embryo Development. Fertil. Steril. 2014, 101, 1637–1648. [Google Scholar] [CrossRef] [PubMed]
- Goodman, L.R.; Goldberg, J.; Falcone, T.; Austin, C.; Desai, N. Does the Addition of Time-Lapse Morphokinetics in the Selection of Embryos for Transfer Improve Pregnancy Rates? A Randomized Controlled Trial. Fertil. Steril. 2016, 105, 275–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhan, Q.; Ye, Z.; Clarke, R.; Rosenwaks, Z.; Zaninovic, N. Direct Unequal Cleavages: Embryo Developmental Competence, Genetic Constitution and Clinical Outcome. PLoS ONE 2016, 11, e0166398. [Google Scholar] [CrossRef] [Green Version]
- Desai, N.; Ploskonka, S.; Goodman, L.; Attaran, M.; Goldberg, J.M.; Austin, C.; Falcone, T. Delayed blastulation, multinucleation, and expansion grade are independently associated with live-birth rates in frozen blastocyst transfer cycles. Fertil. Steril. 2016, 106, 1370–1378. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, J.; Rubio, I.; Muñoz, E.; Pellicer, A.; Meseguer, M. Study of nucleation status in the second cell cycle of human embryo and its impact on implantation rate. Fertil. Steril. 2016, 106, 291–299. [Google Scholar] [CrossRef] [Green Version]
- Rubio, I.; Kuhlmann, R.; Agerholm, I.; Kirk, J.; Herrero, J.; Escribá, M.-J.; Bellver, J.; Meseguer, M. Limited Implantation Success of Direct-Cleaved Human Zygotes: A Time-Lapse Study. Fertil. Steril. 2012, 98, 1458–1463. [Google Scholar] [CrossRef] [PubMed]
- Ebner, T.; Höggerl, A.; Oppelt, P.; Radler, E.; Enzelsberger, S.H.; Mayer, R.B.; Petek, E.; Shebl, O. Time-lapse imaging provides further evidence that planar arrangement of blastomeres is highly abnormal. Arch. Gynecol. Obstet. 2017, 296, 1199–1205. [Google Scholar] [CrossRef]
- Desch, L.; Bruno, C.; Luu, M.; Barberet, J.; Choux, C.; Lamotte, M.; Schmutz, E.; Sagot, P.; Fauque, P. Embryo multinucleation at the two-cell stage is an independent predictor of intracytoplasmic sperm injection outcomes. Fertil. Steril. 2017, 107, 97–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.T.; Shi, J.X.; Gong, F.; Zhang, S.P.; Lu, C.F.; Tan, K.; Leng, L.Z.; Hao, M.; He, H.; Gu, Y.F.; et al. Cleavage Pattern Predicts Developmental Potential of Day 3 Human Embryos Produced by IVF. Reprod. Biomed. Online 2015, 30, 625–634. [Google Scholar] [CrossRef] [Green Version]
- Barrie, A.; Homburg, R.; McDowell, G.; Brown, J.; Kingsland, C.; Troup, S. Preliminary Investigation of the Prevalence and Implantation Potential of Abnormal Embryonic Phenotypes Assessed Using Time-Lapse Imaging. Reprod. Biomed. 2017, 34, 455–462. [Google Scholar] [CrossRef] [Green Version]
- Wong, C.C.; Loewke, K.E.; Bossert, N.L.; Behr, B.; De Jonge, C.J.; Baer, T.M.; Pera, R.A.R. Non-invasive imaging of human embryos before embryonic genome activation predicts development to the blastocyst stage. Nat. Biotechnol. 2010, 28, 1115–1121. [Google Scholar] [CrossRef]
- Aparicio-Ruiz, B.; Basile, N.; Pérez Albalá, S.; Bronet, F.; Remohí, J.; Meseguer, M. Automatic time-lapse instrument is superior to single-point morphology observation for selecting viable embryos: Retrospective study in oocyte donation. Fertil. Steril. 2016, 106, 1379–1385.e10. [Google Scholar] [CrossRef] [Green Version]
- Yeung, S.; Downing, N.L.; Fei-Fei, L.; Milstein, A. Bedside computer vision—Moving artificial intelligence from driver assistance to patient safety. N. Engl. J. Med. 2018, 378, 1271–1273. [Google Scholar] [CrossRef]
- Wells, C.; Killingsworth, R.; Real-time embryo morphokinetic activity can be measured in short observatory periods to provide objective evaluation of embryo health. A Closer Look. Am. Embryo Transf. Assoc. Newsl. Available online: https://www.aeta.org/newsletter/?p=2623 (accessed on 20 September 2022).
- Sayed, S.; Reigstad, M.M.; Petersen, B.M.; Schwennicke, A.; Wegner Hausken, J.; Storeng, R. Time-lapse imaging derived morphokinetic variables reveal association with implantation and live birth following in vitro fertilization: A retrospective study using data from transferred human embryos. PLoS ONE 2010, 15, e0242377. [Google Scholar] [CrossRef] [PubMed]
- Rienzi, L.; Capalbo, A.; Stoppa, M.; Romano, S.; Maggiulli, R.; Albricci, L.; Sarica, C.; Farcomeni, A.; Vajta, G.; Ubaldi, F.M. No evidence of association between blastocyst aneuploidy and morphokinetic assessment in a selected population of poor-prognosis patients: A longitudinal cohort study. Reprod. Biomed. Online 2015, 30, 57–66. [Google Scholar] [CrossRef] [Green Version]
- Sundvall, L.; Ingerslev, H.J.; Breth Knudsen, U.; Kirkegaard, K. Inter- and intra-observer variability of time-lapse annotations. Hum. Reprod. 2013, 28, 3215–3221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Storr, A.; Venetis, C.A.; Cooke, S.; Kilani, S.; Ledger, W. Inter-observer and intra-observer agreement between embryologists during selection of a single Day 5 embryo for transfer: A multicenter study. Hum. Reprod. 2017, 32, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Adolfsson, E.; Andershed, A.N. Morphology vs morphokinetics: A retrospective comparison of inter-observer and intra-observer agreement between embryologists on blastocysts with known implantation outcome. JBRA Assist. Reprod. 2018, 22, 228–237. [Google Scholar] [CrossRef]
- Boucret, L.; Tramon, L.; Saulnier, P.; Ferré-L’Hôtellier, V.; Bouet, P.-E.; May-Panloup, P. Change in the Strategy of Embryo Selection with Time-Lapse System Implementation—Impact on Clinical Pregnancy Rates. J. Clin. Med. 2021, 10, 4111. [Google Scholar] [CrossRef]
- Yang, S.-H.; Wu, C.-H.; Chen, Y.-C.; Yang, C.-K.; Wu, T.-H.; Chen, P.-C.; Tsai, H.-D. Effect of Morphokinetics and Morphological Dynamics of Cleavage Stage on Embryo Developmental Potential: A Time-Lapse Study. Taiwan J. Obstet. Gynecol. 2018, 57, 76–82. [Google Scholar] [CrossRef]
- Desai, N.; Goldberg, J.M.; Austin, C.; Falcone, T. Are Cleavage Anomalies, Multinucleation, or Specific Cell Cycle Kinetics Observed with Time-Lapse Imaging Predictive of Embryo Developmental Capacity or Ploidy? Fertil. Steril. 2018, 109, 665–674. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Chapple, V.; Roberts, P.; Matson, P. Prevalence, Consequence, and Significance of Reverse Cleavage by Human Embryos Viewed with the Use of the Embryoscope Time-Lapse Video System. Fertil. Steril. 2014, 102, 295–1300. [Google Scholar] [CrossRef]
- Conaghan, J.; Chen, A.A.; Willman, S.P.; Ivani, K.; Chenette, P.E.; Boostanfar, R.; Baker, V.L.; Adamson, G.D.; Abusief, M.E.; Gvakharia, M.; et al. Improving Embryo Selection Using a Computer-Automated Time-Lapse Image Analysis Test plus Day 3 Morphology: Results from a Prospective Multicenter Trial. Fertil. Steril. 2013, 100, 412–419. [Google Scholar] [CrossRef]
- Motato, Y.; de los Santos, M.J.; Escriba, M.J.; Ruiz, B.A.; Remohí, J.; Meseguer, M. Morphokinetic Analysis and Embryonic Prediction for Blastocyst Formation through an Integrated Time-Lapse System. Fertil. Steril. 2016, 105, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Dal Canto, M.; Coticchio, G.; Mignini Renzini, M.; De Ponti, E.; Novara, P.V.; Brambillasca, F.; Comi, R.; Fadini, R. Cleavage Kinetics Analysis of Human Embryos Predicts Development to Blastocyst and Implantation. Reprod. Biomed. 2012, 25, 474–480. [Google Scholar] [CrossRef]
- Cruz, M.; Garrido, N.; Herrero, J.; Pérez-Cano, I.; Muñoz, M.; Meseguer, M. Timing of Cell Division in Human Cleavage-Stage Embryos Is Linked with Blastocyst Formation and Quality. Reprod. Biomed. Online 2012, 25, 371–381. [Google Scholar] [CrossRef]
- Carrasco, B.; Arroyo, G.; Gil, Y.; Gómez, M.J.; Rodríguez, I.; Barri, P.N.; Veiga, A.; Boada, M. Selecting Embryos with the Highest Implantation Potential Using Data Mining and Decision Tree Based on Classical Embryo Morphology and Morphokinetics. J. Assist. Reprod. Genet. 2017, 34, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Leese, H.J. Quiet please, do not disturb: A hypothesis of embryo metabolism and viability. Bioessay 2002, 24, 845–849. [Google Scholar] [CrossRef]
- Leese, H.J.; Sturmey, R.; Baumann, C.G.; McEvoy, T.G. Embryo viability and metabolism: Obeying the quiet rules. Hum. Reprod. 2007, 22, 3047–3050. [Google Scholar] [CrossRef] [PubMed]
- Brison, D.; Leese, H. Energy metabolism in late preimplantation rat embryos. J. Reprod. Fert. 1991, 93, 245–251. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wells, C.; Wiik, A.; Hanks, J.; Zavareh, A.; Killingsworth, R. Embryo Morphokinetic Activity Evident in Short Videos of In Vitro Bovine Embryos. Dairy 2022, 3, 849-861. https://doi.org/10.3390/dairy3040058
Wells C, Wiik A, Hanks J, Zavareh A, Killingsworth R. Embryo Morphokinetic Activity Evident in Short Videos of In Vitro Bovine Embryos. Dairy. 2022; 3(4):849-861. https://doi.org/10.3390/dairy3040058
Chicago/Turabian StyleWells, Cara, Anders Wiik, John Hanks, Amir Zavareh, and Russell Killingsworth. 2022. "Embryo Morphokinetic Activity Evident in Short Videos of In Vitro Bovine Embryos" Dairy 3, no. 4: 849-861. https://doi.org/10.3390/dairy3040058
APA StyleWells, C., Wiik, A., Hanks, J., Zavareh, A., & Killingsworth, R. (2022). Embryo Morphokinetic Activity Evident in Short Videos of In Vitro Bovine Embryos. Dairy, 3(4), 849-861. https://doi.org/10.3390/dairy3040058





