A Study on Milk and Caciocavallo Cheese from Podolica Breed in Basilicata, Italy
Abstract
1. Introduction
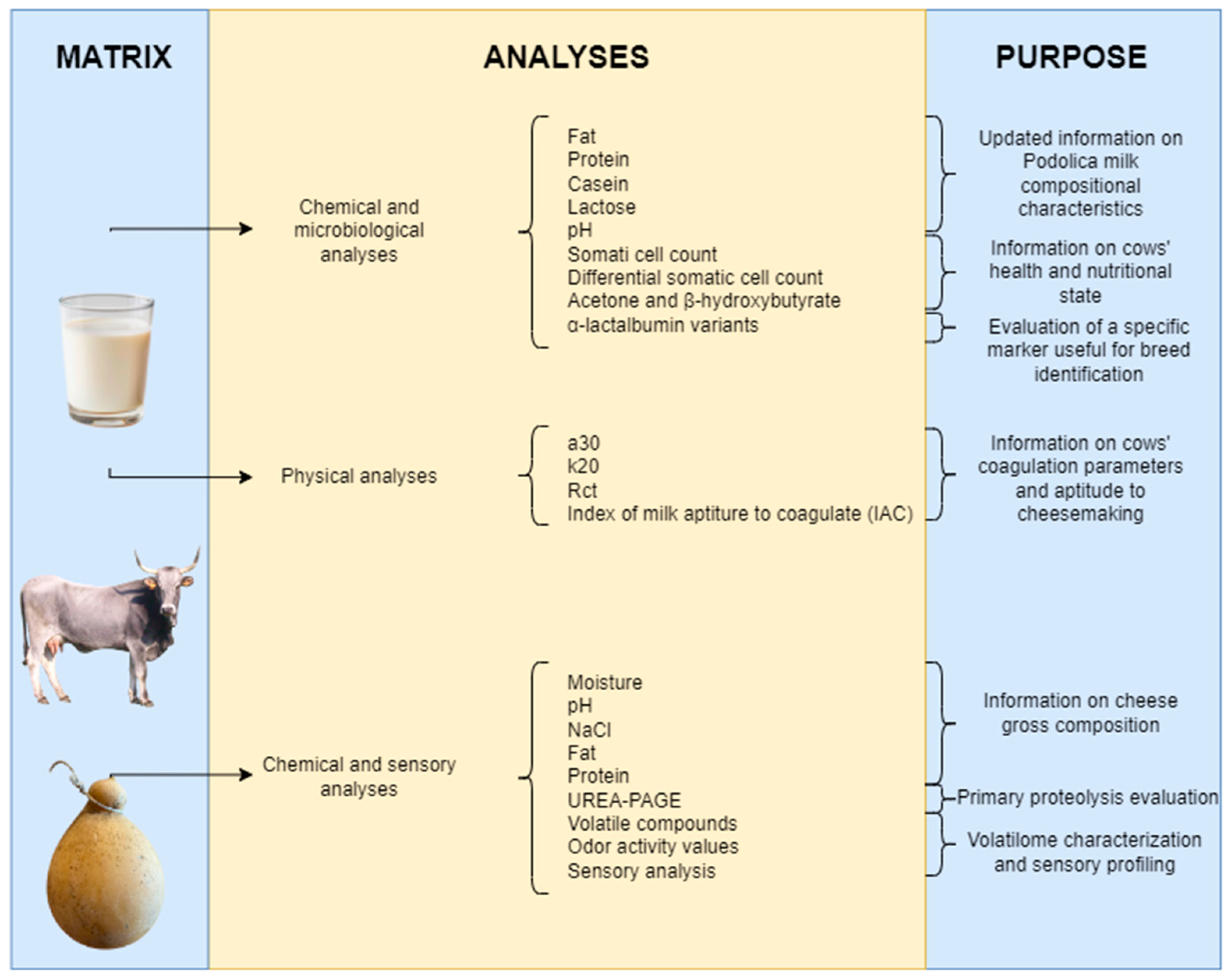
2. Materials and Methods
2.1. Milk and Cheese Samples
2.2. Chemical-Physical Analyses
2.3. Sensory Analyses
2.4. Statistical Analysis
3. Results and Discussion
3.1. Milk
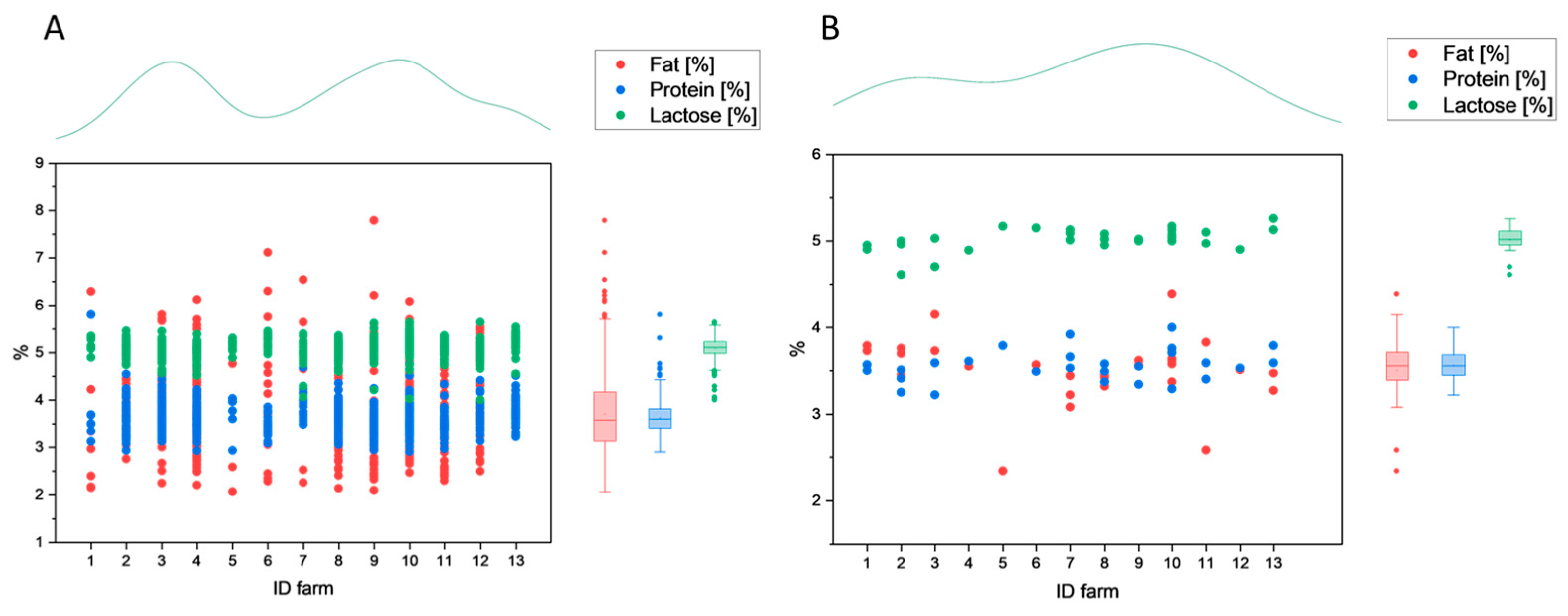
3.1.1. Compositional Parameters
3.1.2. Health Parameters
3.1.3. Technological Parameters
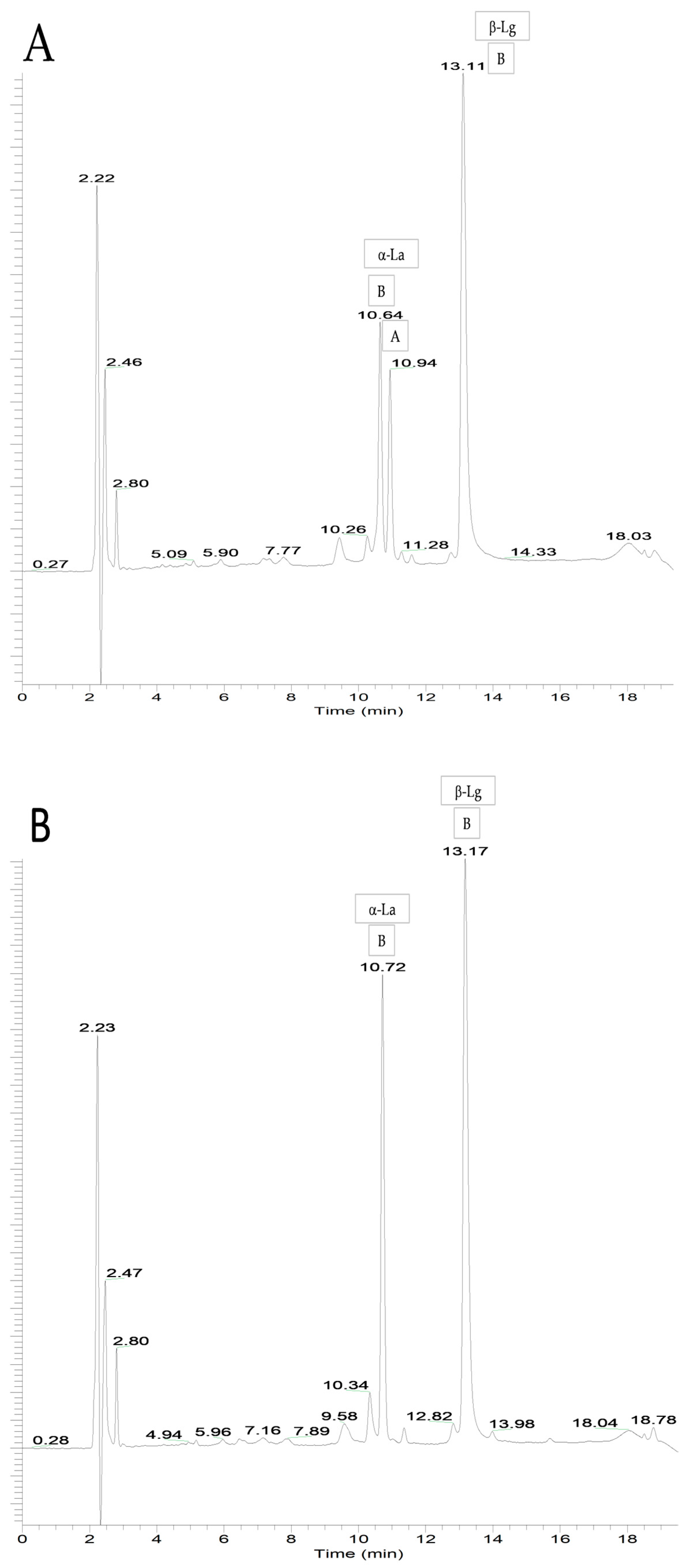
3.1.4. Whey Protein Profile
3.2. Caciocavallo Podolico
3.2.1. Gross Composition
3.2.2. Urea-PAGE
3.2.3. VOC Analysis
3.2.4. Sensory Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guerci, M.; Trydeman, M.K.; Bava, L.; Zucali, M.; Schönbach, P.; Kristensen, T. Parameters affecting the environmental impact of a range of dairy farming systems in Denmark, Germany and Italy. J. Clean. Prod. 2013, 54, 133–141. [Google Scholar] [CrossRef]
- Lovarelli, D.; Bacenetti, J.; Guarino, M. A review on dairy cattle farming: Is precision livestock farming the compromise for an environmental, economic and social sustainable production? J. Clean. Prod. 2020, 262, 121409. [Google Scholar] [CrossRef]
- Brito, L.; Bedere, N.; Douhard, F.; Oliveira, H.; Arnal, M.; Peñagaricano, F.; Schinckel, A.; Baes, C.; Miglior, F. Review: Genetic selection of high-yielding dairy cattle toward sustainable farming systems in a rapidly changing world. Animal 2021, 15, 100292. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, P.; Lancioni, H.; Ceccobelli, S.; Colli, L.; Cardinali, I.; Karsli, T.; Capodiferro, M.R.; Sahin, E.; Ferretti, L.; Marsan, P.A.; et al. Mitochondrial DNA Variants of Podolian Cattle Breeds Testify for a Dual Maternal Origin. PLoS ONE 2018, 13, e0192567. [Google Scholar] [CrossRef]
- Braghieri, A.; Pacelli, C.; Bragaglio, A.; Sabia, E.; Napolitano, F. The Hidden Costs of Livestock Environmental Sustainability: The Case of Podolian Cattle. In The Sustainability of Agro-Food and Natural Resource Systems in the Mediterranean Basin; Vastola, A., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 47–56. ISBN 978-3-319-16356-7. [Google Scholar]
- ANABIC. Consistency of Podolica Breed Updated on 31 December 2022. Available online: http://www.anabic.it/index1.htm (accessed on 2 August 2023).
- Cosentino, C.; D’Adamo, C.; Naturali, S.; Pecora, G.; Paolino, R.; Musto, M.; Adduci, F.; Freschi, P. Podolian cattle: Reproductive activity, milk and future prospects. Ital. J. Agron. 2018, 13, 200–207. [Google Scholar] [CrossRef]
- Uzun, P.; Serrapica, F.; Masucci, F.; Assunta, B.C.M.; Yildiz, H.; Grasso, F.; Di Francia, A. Diversity of traditional Caciocavallo cheeses produced in Italy. Int. J. Dairy Technol. 2020, 73, 234–243. [Google Scholar] [CrossRef]
- Perna, A.; Marsico, D.; Santarsiere, C.; Freschi, P.; Gambacorta, E. Quantitative and Qualitative Aspects of Milk in the Podolian Breed in Extensive Rearing. In Proceedings of the 4th World Italian Beef Cattle Congress, Gubbio, Italy, 29 April–1 May 2005; pp. 457–460. [Google Scholar]
- Pistoia, A.; Casarosa, L.; Poli, P.; Mani, D.; Ferruzzi, G. Caratteristiche Qualitative Del Latte e Del Formaggio Caciocavallo Nella Razza Bovina Podolica. Large Anim. Rev. 2015, 21, 63–66. [Google Scholar]
- Pieragostini, E.; Scaloni, A.; Rullo, R.; Di Luccia, A. Identical marker alleles in Podolic cattle (Bos taurus) and Indian zebu (Bos indicus). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2000, 127, 1–9. [Google Scholar] [CrossRef]
- Farrell, H.; Jimenez-Flores, R.; Bleck, G.; Brown, E.; Butler, J.; Creamer, L.; Hicks, C.; Hollar, C.; Ng-Kwai-Hang, K.; Swaisgood, H. Nomenclature of the Proteins of Cows’ Milk—Sixth Revision. J. Dairy Sci. 2004, 87, 1641–1674. [Google Scholar] [CrossRef]
- Licitra, G.; Carpino, S. The Microfloras and Sensory Profiles of Selected Protected Designation of Origin Italian Cheeses. Microbiol. Spectr. 2014, 2, 151–165. [Google Scholar] [CrossRef]
- Quinto, M.; Sevi, A.; Di Caterina, R.; Albenzio, M.; Muscio, A.; Rotunno, T. Quality of Milkand Caciocavallo Cheesefrom Farms Rearing Podolicaand Italian Friesian Cows. Ital. J. Food Sci. 2003, 15, 485–498. [Google Scholar]
- Cammarota, G.; Laurino, C.; Pellicano, M.P.; Pellicano, M.P. Sensory Evaluation of Different “Caciocavallo” Typical Cheeses. La Rivista di scienza deLL’aLimentazione 2014, 43, 31–39. [Google Scholar]
- Pazzola, M.; Balia, F.; Dettori, M.L.; Mura, M.C.; Carcangiu, V.; Vacca, G.M. Effects of different storage conditions, the farm and the stage of lactation on renneting parameters of goat milk investigated using the Formagraph method. J. Dairy Res. 2011, 78, 343–348. [Google Scholar] [CrossRef]
- Beux, S.; Cassandro, M.; Nogueira, A.; Waszczynskyj, N. Effect of THI on milk coagulation properties of Holstein-Friesian dairy cattle. Rev. Bras. Zootec. 2017, 46, 429–432. [Google Scholar] [CrossRef]
- Andrews, A.T. Proteinases in normal bovine milk and their action on caseins. J. Dairy Res. 1983, 50, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Faccia, M.; Trani, A.; Loizzo, P.; Gagliardi, R.; La Gatta, B.; Di Luccia, A. Detection of αs1-I casein in mozzarella Fiordilatte: A possible tool to reveal the use of stored curd in cheesemaking. Food Control 2014, 42, 101–108. [Google Scholar] [CrossRef]
- Natrella, G.; Gambacorta, G.; Faccia, M. Application of Commercial Biopreservation Starter in Combination with MAP for Shelf-Life Extension of Burrata Cheese. Foods 2023, 12, 1867. [Google Scholar] [CrossRef]
- Natrella, G.; Gambacorta, G.; Faccia, M. Influence of the stretching temperature on the volatile compounds and odour intensity of high moisture mozzarella: A model study. Int. Dairy J. 2021, 130, 105282. [Google Scholar] [CrossRef]
- Trani, A.; Gambacorta, G.; Loizzo, P.; Cassone, A.; Faccia, M. Short communication: Chemical and sensory characteristics of Canestrato di Moliterno cheese manufactured in spring. J. Dairy Sci. 2016, 99, 6080–6085. [Google Scholar] [CrossRef]
- Michele, F.; Luisa, A.; Marianna, M.; Amalia, C.; Alessandro, D.N.M. The effect of incorporating calcium lactate in the saline solution on improving the shelf life of Fiordilatte cheese. Int. J. Dairy Technol. 2013, 66, 373–381. [Google Scholar] [CrossRef]
- Faccia, M.; Natrella, G.; Gambacorta, G.; Trani, A. Cheese ripening in nonconventional conditions: A multiparameter study applied to Protected Geographical Indication Canestrato di Moliterno cheese. J. Dairy Sci. 2022, 105, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Kindstedt, P.; Duthie, A.; Nilson, K. Estimation of Casein from Total Protein in Commingled Milk. J. Dairy Sci. 1983, 66, 2459–2463. [Google Scholar] [CrossRef]
- Molik, E.; Bonczar, G.; Misztal, T.; Ebrowska, A.; Zib, D. The Effect of the Photoperiod and Exogenous Melatonin on the Protein Content in Sheep Milk. In Milk Protein 2012, 12, 325–340. [Google Scholar] [CrossRef]
- Bijl, E.; van Valenberg, H.; Huppertz, T.; van Hooijdonk, A. Protein, casein, and micellar salts in milk: Current content and historical perspectives. J. Dairy Sci. 2013, 96, 5455–5464. [Google Scholar] [CrossRef]
- Ikonen, T.; Morri, S.; Tyrisevä, A.-M.; Ruottinen, O.; Ojala, M. Genetic and Phenotypic Correlations between Milk Coagulation Properties, Milk Production Traits, Somatic Cell Count, Casein Content, and pH of Milk. J. Dairy Sci. 2004, 87, 458–467. [Google Scholar] [CrossRef]
- Sharma, N.; Singh, N.K.; Bhadwal, M.S. Relationship of Somatic Cell Count and Mastitis: An Overview. Asian-Australas. J. Anim. Sci. 2011, 24, 429–438. [Google Scholar] [CrossRef]
- Li, N.; Richoux, R.; Boutinaud, M.; Martin, P.; Gagnaire, V. Role of somatic cells on dairy processes and products: A review. Dairy Sci. Technol. 2014, 94, 517–538. [Google Scholar] [CrossRef] [PubMed]
- Bobbo, T.; Cipolat-Gotet, C.; Bittante, G.; Cecchinato, A. The nonlinear effect of somatic cell count on milk composition, coagulation properties, curd firmness modeling, cheese yield, and curd nutrient recovery. J. Dairy Sci. 2016, 99, 5104–5119. [Google Scholar] [CrossRef]
- Schwarz, D.; Diesterbeck, U.; König, S.; Brügemann, K.; Schlez, K.; Zschöck, M.; Wolter, W.; Czerny, C.-P. Flow cytometric differential cell counts in milk for the evaluation of inflammatory reactions in clinically healthy and subclinically infected bovine mammary glands. J. Dairy Sci. 2011, 94, 5033–5044. [Google Scholar] [CrossRef]
- Damm, M.; Holm, C.; Blaabjerg, M.; Bro, M.N.; Schwarz, D. Differential somatic cell count—A novel method for routine mastitis screening in the frame of Dairy Herd Improvement testing programs. J. Dairy Sci. 2017, 100, 4926–4940. [Google Scholar] [CrossRef]
- Zecconi, A.; Vairani, D.; Cipolla, M.; Rizzi, N.; Zanini, L. Assessment of subclinical mastitis diagnostic accuracy by differential cell count in individual cow milk. Ital. J. Anim. Sci. 2019, 18, 460–465. [Google Scholar] [CrossRef]
- Jonker, J.; Kohn, R.; Erdman, R. Using Milk Urea Nitrogen to Predict Nitrogen Excretion and Utilization Efficiency in Lactating Dairy Cows. J. Dairy Sci. 1998, 81, 2681–2692. [Google Scholar] [CrossRef] [PubMed]
- Roy, B.; Brahma, B.; Ghosh, S.; Pankaj, P.; Mandal, G. Evaluation of Milk Urea Concentration as Useful Indicator for Dairy Herd Management: A Review. Asian J. Anim. Vet.-Adv. 2010, 6, 1–19. [Google Scholar] [CrossRef]
- Sanjayaranj, I.; Lopez-Villalobos, N.; Blair, H.T.; Janssen, P.W.M.; Holroyd, S.E.; MacGibbon, A.K.H. A Study of Milk Composition and Coagulation Properties of Holstein-Friesian, Jersey, and Their Cross Milked Once or Twice a Day. Dairy 2023, 4, 167–179. [Google Scholar] [CrossRef]
- Niero, G.; Franzoi, M.; Manuelian, C.L.; Visentin, G.; Penasa, M.; De Marchi, M. Protein profile of cow milk from multibreed herds and its relationship with milk coagulation properties. Ital. J. Anim. Sci. 2021, 20, 2232–2242. [Google Scholar] [CrossRef]
- Penasa, M.; Toffanin, V.; Cologna, N.; Cassandro, M.; De Marchi, M. Effects of dairy factory, milk casein content and titratable acidity on coagulation properties in Trentingrana dairy industry. J. Dairy Res. 2016, 83, 242–248. [Google Scholar] [CrossRef]
- Malacarne, M.; Fieni, S.; Tosi, F.; Franceschi, P.; Formaggioni, P.; Summer, A. Seasonal variations of the rennet-coagulation properties of herd milks in Parmigiano-Reggiano cheese manufacture: Comparison between Italian Friesian and Italian Brown cattle breeds. Ital. J. Anim. Sci. 2005, 4, 215–217. [Google Scholar] [CrossRef]
- De Marchi, M.; Zotto, R.D.; Cassandro, M.; Bittante, G. Milk Coagulation Ability of Five Dairy Cattle Breeds. J. Dairy Sci. 2007, 90, 3986–3992. [Google Scholar] [CrossRef]
- Franzoi, M.; Manuelian, C.; Penasa, M.; De Marchi, M. Effects of somatic cell score on milk yield and mid-infrared predicted composition and technological traits of Brown Swiss, Holstein Friesian, and Simmental cattle breeds. J. Dairy Sci. 2020, 103, 791–804. [Google Scholar] [CrossRef]
- Aschaffenburg, R. Reviews of the Progress of Dairy Science. J. Dairy Sci. 1968, 35, 447–460. [Google Scholar]
- Stobnicka-Kupiec, A.; Gołofit-Szymczak, M.; Górny, R. Microbial contamination level and microbial diversity of occupational environment in commercial and traditional dairy plants. Ann. Agric. Environ. Med. 2019, 26, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Gobbetti, M.; Morea, M.; Baruzzi, F.; Corbo, M.; Matarante, A.; Considine, T.; Di Cagno, R.; Guinee, T.; Fox, P. Microbiological, compositional, biochemical and textural characterisation of Caciocavallo Pugliese cheese during ripening. Int. Dairy J. 2002, 12, 511–523. [Google Scholar] [CrossRef]
- Delgado, F.J.; González-Crespo, J.; Cava, R.; Ramírez, R. Formation of the aroma of a raw goat milk cheese during maturation analysed by SPME–GC–MS. Food Chem. 2011, 129, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Kilcawley, K.N.; Faulkner, H.; Clarke, H.J.; O’sullivan, M.G.; Kerry, J.P. Factors Influencing the Flavour of Bovine Milk and Cheese from Grass Based versus Non-Grass Based Milk Production Systems. Foods 2018, 7, 37. [Google Scholar] [CrossRef]
- Faccia, M. The Flavor of Dairy Products from Grass-Fed Cows. Foods 2020, 9, 1188. [Google Scholar] [CrossRef] [PubMed]



| Individual Milk | Bulk Milk | Literature Range * | |
|---|---|---|---|
| RCT | 23.42 ± 5.5 a | 22.47 ± 2.52 a | 21.32–21.82 |
| k20 | 5.17 ± 1.55 a | 5.23 ± 0.77 a | 5.28–6.49 |
| a30 | 38.94 ± 11.07 a | 37.48 ± 5.18 a | 14.15–17.76 |
| IAC | 100.84 ± 3.65 a | 102.48 ± 3.00 a | 100.32–100.97 |
| Ripening Time (Days) | pH | Moisture % | NaCl % | Protein % | Fat % | Protein to Fat Ratio |
|---|---|---|---|---|---|---|
| Podolica | ||||||
| 20 | 5.07 b | 40.8 f | 3.0 a | 23.9 a | 28.1 a | 0.85 a |
| 45 | 5.30 d | 32.8 c | 3.4 b | 27.5 b | 30.9 b | 0.89 b |
| 60 | 5.19 c | 36.5 d | 3.5 b | 25.1 a | 29.7 b | 0.85 a |
| 60 | 5.32 d | 35.3 d | 3.6 bc | 26.5 b | 29.5 b | 0.90 b |
| 90 | 5.15 c | 38.7 e | 3.5 b | 25.4 ab | 28.3 ab | 0.90 b |
| 150 | 5.40 e | 27.8 a | 3.8 cd | 29.7 c | 34.0 c | 0.87 ab |
| 180 | 5.30 d | 28.2 a | 3.8 cd | 29.5 c | 32.8 c | 0.90 b |
| 180 | 4.93 a | 32.3 bc | 3.7 c | 27.9 b | 30.4 b | 0.92 c |
| 340 | 5.28 d | 29.4 ab | 4.0 d | 29.0 c | 32.5 bc | 0.89 b |
| Specialized breeds | ||||||
| 90 | 5.33 d | 41.1 f | 3.1 a | 24.6 a | 26.5 a | 0.93 c |
| 90 | 5.23 cd | 37.5 e | 2.9 a | 27.5 b | 28.8 ab | 0.95 c |
| 120 | 4.97 a | 42.0 f | 3.2 ab | 24.6 a | 26.0 a | 0.95 c |
| 120 | 5.16 c | 33.7 c | 3.2 a | 27.8 b | 29.7 c | 0.94 c |
| 120 | 5.10 b | 39.8 ef | 3.5 c | 25.1 ab | 27.9 ab | 0.90 bc |
| 135 | 5.06 b | 31.1 b | 3.5 c | 29.3 c | 29.9 b | 0.98 d |
| 150 | 5.09 b | 31.3 b | 3.2 ab | 29.6 c | 30.8 b | 0.96 c |
| 165 | 5.16 c | 35.6 d | 3.3 b | 27.2 b | 28.2 a | 0.96 cd |
| 180 | 5.29 d | 34.1 c | 3.4 b | 28.3 bc | 29.4 b | 0.96 cd |
| Moisture | Ripening Time | Proteolysis | Breed | |
|---|---|---|---|---|
| Groups | ||||
| Acids | ** | * | ** (Podolica) | |
| Alcohols | ** | |||
| Esters | ** | ** | ** (Podolica) | |
| Aldehydes | ||||
| Ketones | ** (specialized breeds) | |||
| Single compounds | ||||
| Acetic acid | ** | * | ||
| Acetoin | ** | ** | ||
| 1-butanol | ** | |||
| 2-butanol | ** | ** | ||
| 1-butanol, 2-methyl | ** | |||
| 2-butanone | ** | ** | ||
| Butyl butanoate | ** | ** (Podolica) | ||
| Butyl hexanoate | * | |||
| Ethanol | ** | |||
| Ethylacetate | ** | * | ||
| Ethyldecanoate | ** | |||
| Ethylpentanoate | ** | |||
| Ethylpropionate | ** | |||
| 2-hexanone | ** | |||
| 2-heptanol | ** | |||
| 2-heptanone | ** | ** | ** | ** (specialized breeds) |
| Hexanal | ** | |||
| Hexanoic acid | ** | |||
| Isobutyric acid | ** | |||
| D-limonene | * | |||
| Methylbutyl butanoate | * | |||
| 2-nonanone | ** | |||
| 8-nonen-2-one | ** | |||
| 2-octanone | * | |||
| 1-pentanol | ** | |||
| 2-pentanol | ** | |||
| 3-pentanol,2-metil | ** | |||
| Phenylethyl alcohol | * | |||
| Propyl butanoate | * | ** (Podolica) | ||
| Propyl hexanoate | ** | |||
| 2-undecanone | * | |||
| Compound | Odor Threshold in Water (ppb) | OAV in Cheese (Average Value) Podolico | OAV in Cheese (Average Value) Specialized Breeds | Descriptors |
|---|---|---|---|---|
| Butanoic acid | 1 | 780 a | 720 a | sweat, dirty socks |
| Hexanoic acid | 5 | 93 a | 81 b | pungent, goaty |
| Acetic acid | 10 | 38 a | 41 a | vinegar |
| Ethyl butyrate | 7,5 | 18 a | 22 a | fruity, pineapple |
| Ethyl hexanoate | 6 | 13 a | 16 a | fruity, apple peel |
| Limonene | 38 | 3 a | 2 a | lemon-like |
| Propyl butyrate | 11 | 3 a | 2 a | fruity, pear |
| Butyl butyrate | 5 | 1 a | 2 a | fruity, pineapple |
| Propionic acid | 6 | 2 b | 3 a | pungent, sweat, dairy |
| 1-hexanol | 6 | 1 a | 1 a | fresh grass |
| 2-nonanone | 80 | 1 a | 1 a | green, earthy, musty |
| Descriptor | Podolica | Min–Max | Specialize Breeds | Min–Max | Sig |
|---|---|---|---|---|---|
| TEXTURE | |||||
| Adhesive | 2 | 1–2 | 1 | 0–1 | * |
| Crumbly | 2 | 1–3 | 2 | 1–3 | |
| Eyes | 1 | 0–1 | 1 | 0–1 | |
| Hard | 3 | 2–4 | 3 | 3–4 | |
| Soluble | 2 | 0–3 | 2 | 1–3 | |
| AROMA | |||||
| Butter | 1 | 0–1 | 1 | 0–1 | |
| Boiled cabbage | 2 | 1–3 | 3 | 2–3 | |
| Cowy/barn | 4 | 3–5 | 3 | 1–3 | * |
| Smoky | 2 | 2–3 | 1 | 0–1 | * |
| Solvent | 2 | 2–3 | 1 | 0–1 | * |
| TASTE | |||||
| Salty | 3 | 3–4 | 2 | 1-3 | * |
| Bitter | 0 | 0 | 0 | 0–1 | |
| Pungent | 2 | 0–3 | 2 | 0–2 | |
| Acid | 1 | 0–2 | 1 | 0–2 | |
| Umami | 2 | 1–3 | 2 | 0–3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Natrella, G.; De Palo, P.; Maggiolino, A.; Faccia, M. A Study on Milk and Caciocavallo Cheese from Podolica Breed in Basilicata, Italy. Dairy 2023, 4, 482-496. https://doi.org/10.3390/dairy4030032
Natrella G, De Palo P, Maggiolino A, Faccia M. A Study on Milk and Caciocavallo Cheese from Podolica Breed in Basilicata, Italy. Dairy. 2023; 4(3):482-496. https://doi.org/10.3390/dairy4030032
Chicago/Turabian StyleNatrella, Giuseppe, Pasquale De Palo, Aristide Maggiolino, and Michele Faccia. 2023. "A Study on Milk and Caciocavallo Cheese from Podolica Breed in Basilicata, Italy" Dairy 4, no. 3: 482-496. https://doi.org/10.3390/dairy4030032
APA StyleNatrella, G., De Palo, P., Maggiolino, A., & Faccia, M. (2023). A Study on Milk and Caciocavallo Cheese from Podolica Breed in Basilicata, Italy. Dairy, 4(3), 482-496. https://doi.org/10.3390/dairy4030032









