Abstract
Given the rising interest in functional foods for health benefits, this study aims to evaluate the antihypertensive activity of an ice cream base incorporating Lacticaseibacillus rhamnosus GG and aguamiel syrup. We assessed the probiotic viability and ACE inhibitory activity in ice cream enriched with aguamiel syrup compared to inulin. Several reports have highlighted the importance of consuming symbiotic dairy foods to modulate the intestinal microbiota and multiple pathophysiologies. Ice cream has a high worldwide consumption rate, so it is an alternative to incorporating probiotics and prebiotics. The probiotic was inoculated (109 CFU/mL) into an ice cream base enriched with aguamiel syrup and a control base with added inulin. The carbohydrate profiles in the aguamiel (used to produce the syrup) and the aguamiel syrup were obtained through HPLC. TNBS and SDS-PAGE analysis were used to determine the proteolytic action of the probiotic. Sucrose was the carbohydrate with the highest concentration in fresh aguamiel and aguamiel syrup. The probiotic remained viable for 14 days under refrigerated storage conditions, with the aguamiel syrup base showing superior protein hydrolysis (free amino groups 302.67 ± 2.29 µg/mL) and 65% ACE inhibition. Likewise, the pH remained unchanged throughout the refrigerated days. These results underscore the potential of aguamiel syrup as a prebiotic in functional dairy products.
1. Introduction
The growing consumer interest in health-promoting foods has significantly spurred research into functional foods. These foods are designed to provide benefits beyond mere nutrition, offering additional health advantages such as enhanced immune function, improved digestion, or reduced risk of chronic diseases. This trend reflects a broader shift towards proactive health management, where consumers seek foods that contribute to overall well-being and longevity. Consequently, researchers and food developers are increasingly focused on creating and optimizing functional foods that meet these evolving demands and contribute to a healthier lifestyle [1,2]. Functional foods, especially symbiotic foods combining probiotics and prebiotics, are gaining popularity because they support gut health [3] and help balance the intestinal microbiota [4]. Probiotics are live microorganisms that, when consumed in sufficient quantities, offer a range of health benefits. These beneficial bacteria enhance digestive health by promoting a balanced gut microbiota, which can alleviate issues like bloating, constipation, and diarrhea. Additionally, probiotics support the immune system by helping to regulate immune responses and enhance the body’s ability to fend off infections. As a result, incorporating probiotics into the diet can contribute to overall well-being and help maintain a healthy digestive and immune system [1]. Probiotics have been used in the treatment of different diseases. This has been supported by their effects on the intestinal microbial community, with the Lactobacillus and Streptococcus genera being the most widely used [5]. The mechanism of action of these microorganisms focuses on the inhibition of pathogens, immunomodulatory activity, and the production of vitamin and antimicrobial substances [5].
On the other hand, prebiotics, such as inulin and fructooligosaccharides (FOS), play a vital role in promoting the growth of beneficial gut bacteria. These non-digestible fibers serve as food sources for the advantageous microorganisms in the digestive tract. By selectively stimulating the growth and activity of these helpful bacteria, prebiotics contribute to a healthier gut microbiome. This enhanced microbial environment supports improved digestive function, nutrient absorption, and a stronger immune response. As a result, incorporating prebiotics into one’s diet can significantly bolster digestive health and overall well-being [2,6,7]. In this way, aguamiel has been studied for its prebiotic activity. This liquid is obtained from the Agave spp. to manufacture “pulque”, an ancient alcoholic beverage from Mexico. Aguamiel is a valuable ingredient due to its rich fermentable sugars and prebiotics content. These fermentable sugars provide an excellent energy source for beneficial gut bacteria, while the prebiotics support the growth and activity of these microorganisms. This combination makes aguamiel a promising component for functional foods, offering potential benefits such as improved digestive health and enhanced gut microbiome balance. By incorporating aguamiel into functional food products, manufacturers can leverage its natural properties to promote overall well-being and support a healthier digestive system [8,9]. Likewise, its main composition is various fermentable sugars, amino acids, proteins, and vitamins [10,11]. Aguamiel has been used to produce syrup by evaporation until 70–80 °Bx, obtaining a concentrate product with the same type of carbohydrates. Due to its nutraceutical properties, this product is popular in specific sectors in Mexico, especially in the health-conscious, organic, and functional food markets [12].
On the other hand, in recent years, new alternatives have been proposed to incorporate probiotics and prebiotics into different foods to obtain symbiotics [2,3]. Thus, the development of enriched fermented dairy foods with material such as aguamiel has been studied as a viable alternative. Due to its widespread consumption and nutrient profile, ice cream is ideal for delivering probiotics and prebiotics [13]. This is due to milk proteins, lactose, and fat. Likewise, the organoleptic properties of this food make it a product consumed worldwide [14]. Therefore, this research aimed to develop an ice cream base by adding the probiotic strain Lacticaseibacillus rhamnosus GG and enriching it with prebiotics (FOS from aguamiel syrup). Also, the probiotic strain’s survival capacity and the product’s antihypertensive capacity should be evaluated to determine its promising activity as a functional food.
2. Materials and Methods
2.1. Obtaining Aguamiel
Aguamiel was harvested from Agave salmiana plants in Zempoala, Hidalgo, and processed to prevent fermentation during transport. The aguamiel pH was detected, and samples were chosen below 5, above 7, and between 6 and 7. The collected samples were immediately filtered and refrigerated to prevent fermentation during transport. They were then divided into 100 mL portions and heated for 10 min at 90 °C. The aguamiel was stored at −4 °C until use.
2.2. Production of Aguamiel Syrup
Following traditional methods, aguamiel was heated to 80 °C and concentrated into a syrup with 70 °Bx solids content. The pH was adjusted by adding water or combining different aguamiel amounts to reach a value of 6.0, avoiding using additives; the initial aguamiel amount was 1.5 L. The entire process was carried out under continuous agitation, and the concentration of solids was constantly monitored. The syrup obtained was stored at room temperature in sterile glass containers for later analysis.
2.3. Carbohydrate Characterization
Carbohydrates in aguamiel and syrup were analyzed using HPLC (Agilent Technologies 1260 Ifinity) with a size exclusion column (Ultrahydrogel DP, 7.8 × 300 mm, Waters) and calibrated with a dextrans of different molecular weights (Sigma-Aldrich) following the Moreno-Vilet et al. [15] technique. The flow rate was 0.36 mL/min using HPLC-grade water as the mobile phase. The sample injection was 20 μL, and the analysis was performed at 61.7 °C.
2.4. Obtaining the Starter Culture
The L. rhamnosus GG from the Food Biotechnology Laboratory (Metropolitan Autonomous University campus Iztapalapa) was used. The microorganism was propagated in MRS broth (24 h at 42 °C) and used to inoculate 100 mL of 10% w/v skim milk (Dairy Gold) (37 °C for 24 h). The solution was pasteurized at 90 °C for 10 min. The starter was maintained in refrigerated conditions until its use.
2.5. Preparation of Ice Cream Base
Balthazar et al. [13] described the procedure for preparing the ice cream base with some modifications. Two ice cream bases were prepared: one with inulin and another with aguamiel syrup. Both were inoculated with L. rhamnosus GG and stored at 4 °C for 14 days. In 2 L of hot water, 200 g of skimmed milk powder were dissolved. Separately, 150 g of vegetable fat (palm kernel Aahrus) and 4 g of glyceryl monostearate were brought to melting point by mixing both compounds. Subsequently, this mixture was added to the skimmed milk, and carboxymethyl cellulose (4 g), xanthan gum (4 g), carrageenan (4 g), and inulin (40 g) were incorporated to obtain the control base and aguamiel syrup (40 g) was added to obtain the study base. Once the bases were obtained, 100 mL of the L. rhamnosus GG starter culture (109 CFU/mL) was added to each. The physical parameters, antihypertensive activity, and viability were determined each week.
2.6. Growth of L. rhamnosus GG under Refrigeration Conditions
Probiotic viability was assessed weekly using serial dilutions, and plate counts incubating at 37 °C for 72 h (anaerobic conditions) on MRS agar. The samples were divided into 3 portions and stored at 4 °C for 3 weeks. A Sorvall-Frescocentrifuge device (13,000× g at 4 °C for 15 min) was used to centrifuge 2 mL of the samples, which were used for further analysis.
The viability was determined by expressing results as CFU/mL after applying the following equation:
where C is the number of colonies counted, 10X the inverse of the dilution, and Y is the dilution factor determined by the quotient of the volume reported in the concentration (1 mL) divided by the inoculated volume (0.005 mL).
log UFC/mL = log10 (C·10X·Y)
2.7. Determination of Overrun
The inulin ice cream base and the aguamiel syrup base were placed separately in a Cuisinart brand machine for 20 min to make the ice cream, and the weight was determined. Air incorporation (overrun) was calculated using the method of Balthazar et al. [13]. Overrun was calculated by measuring the weight difference before and after freezing the ice cream mixture using the following equation:
Overrun (%) = (weight of ice cream − weight of liquid)/(weight of liquid) × 100
2.8. Proteolytic Profile Analysis
Two analyses were developed for profiling proteolysis. The free amino groups were determined using the TNBS technique, and the ice cream base peptides were separated by electrophoresis (SDS-Tris-Tricine-PAGE).
2.8.1. Analysis of Free Amino Groups from the Ice Cream Base
Determination was carried out using the 2,4,6-trinitrobenzene sulfonic acid (TNBS) technique modified by Olvera-Rosales et al. [16]. In test tubes covered with aluminum foil, 125 µL of centrifuged sample were mixed with a milliliter of 0.21 M phosphate buffer (pH 8.2). This was added to tubes, and 1 mL of 0.10% TNBS (Sigma Aldrich) was prepared with phosphate buffer (0.21 M, pH 8.2). The mixture was vortexed. All mixtures were incubated in darkness (1 h at 50 °C). To stop the reaction, 2 mL of 0.1 N hydrochloric acid was added. It was measured at 340 nm in a spectrometer using deionized water as a control. The concentration was calculated by a glycine concentration curve (0.05 to 0.25 mg/mL).
2.8.2. Tris-Tricine Polyacrylamide Gel Electrophoresis (Tris-Tricine-SDS-PAGE)
The Schägger and von Jagow methodology was used [17], applying the González-Olivares et al. [18] modifications. A 16.5% T gel was prepared (using a 19:1 acrylamide/bisacrylamide ratio and a 5% crosslinker, Bio-Rad, Hercules, CA, USA). After the staining of Coomassie Blue G-250 (Bio-Rad, Hercules, CA, USA), the gels were analyzed (Gel-Doc EZ System; Bio-Rad, Hercules, CA, USA).
2.9. ACE Inhibitory Activity
The methodology of Cushman et al. [19] was used. The following adaptations were taken. The inhibitory effect of ACE-I (EC 3.4.15.1; Sigma-Aldrich, St. Louis, MO, USA). A total of 5 mM Hippuryl-histidyl-leucine (HHL; Sigma-Aldrich, St. Louis, MO, USA) substrate solution was prepared with sodium borate buffer (0.1 M, pH 8.3, with 0.3 M sodium chloride). It was mixed with 10 µL of ACE (EC 3.4.15.1, 5.1 U/mg; Sigma-Aldrich), 100 µL of the substrate solution, and 40 µL of the sample (AbsM). The mixture was left to rest (75 min at 37 °C). Ethyl acetate was used to extract the hippuric acid formed. The extract was re-dissolved using deionized water. The spectrophotometric measure was developed at 220 nm (Power Wave XS UV-Biotek spectrometer, Kansas, MO, USA). A positive control sample (AbsC) was prepared similarly but prepared with 40 µL of borate buffer. Finally, the negative control sample (AbsB) was prepared using 50 µL of borate buffer and 100 µL of substrate solution. The following formula was used to determine the ACE inhibitory activity using the absorbance obtained:
% ACE inhibition = [(AbsC − AbsM)/(AbsC − AbsB)] × 100
2.10. Statistical Analysis
Tukey’s test followed a one-way ANOVA with a 5% significance level (p = 0.05). The experiments were performed in triplicate using NCSS statistical software (NCSS 2007, v.0, Kaysville, UT, USA, 2007).
3. Results and Discussion
3.1. Carbohydrates Present in Aguamiel and Aguamiel Syrup
HPLC analysis identified sucrose as the predominant carbohydrate in both aguamiel and syrup. According to the chromatogram, peaks corresponding to oligosaccharides (A–D) were observed until minute 6.71. Subsequently, peaks corresponding to trisaccharides (E), disaccharides (F), and monosaccharides (G) were observed. Of these compounds, both in aguamiel syrup and aguamiel, sucrose was the disaccharide with the highest concentration (Figure 1), with a peak observed at 8.42 min.
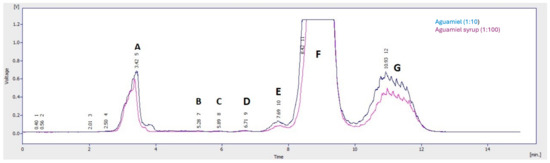
Figure 1.
Separation of carbohydrates by HPLC from aguamiel and aguamiel syrup. A–D, oligosaccharides with carbon chains longer than 4; E, trisaccharides; F, disaccharides; and G, monosaccharides.
These findings align with several researchers who have noted that sucrose is the predominant carbohydrate in aguamiel, with smaller amounts of glucose and fructose also present [10,20]. It has been observed in different varieties of agave that total sugars, fructose, and fructan concentrations are directly related to the plant age, the season, the microbiota, and the maguey’s osmoregulation to survive in extreme environments [10,20].
Likewise, it has been reported that aguamiel contains considerable concentrations of proteins, amino acids, minerals, saponins, phenolic compounds, and vitamins [21,22] in addition to sucrose. Likewise, the presence of fructooligosaccharides (FOS) is a strong indicator of its potential as a prebiotic by promoting the growth of microorganisms of the genus Lactobacillus and Bifidobacterium [22,23]. The concentrations for each fraction obtained by HPLC were calculated to determine the proportion of carbohydrates in the aguamiel and syrup (Table 1). There was no discernible difference in the carbohydrate profiles between the aguamiel and syrup samples.

Table 1.
Concentrations of carbohydrates present in aguamiel and aguamiel syrup separated by HPLC.
The sucrose concentration was higher in both samples, consistent with what was observed in the HPLC determination. However, the total concentration of carbohydrates was higher in the syrup due to the concentration process, increasing by 10 times concerning the concentration of each component in the fresh aguamiel sample. As previously noted, the carbohydrate profile typically varies depending on the agave species or the year’s season. Studies have reported that aguamiel collected from A. atrovirens and A. salmiana tends to exhibit higher concentrations of glucose when harvested during the winter and spring seasons. However, in the present investigation, the aguamiel was collected during the summer, which would explain why sucrose is the most abundant carbohydrate in the sample. This is consistent with what has been reported in previous studies, which highlight the influence of the year’s season on the variability of carbohydrate concentrations in aguamiel [10,24].
3.2. Overrun, pH Changes, and Viability during Fermentation
The aguamiel syrup base had lower overrun and pH, but maintained probiotic viability, indicating stability under refrigerated conditions. Incorporating aguamiel syrup into the ice cream base reduced the percentage to less than 10% after 14 days of refrigeration (Table 2). Overrun is a crucial physical parameter in ice cream since it indicates quality by interfering with the product’s texture, softness, and stability [1]. However, this parameter is influenced by multiple factors: the correct homogenization of the mixture, the incorporation of foaming additives and milk proteins, and the fermentation process. The above would respond to the lower percentage of overrun in the base of the study.

Table 2.
Overrun percentage, pH, and microbial viability based on inulin (I) and aguamiel syrup (AS) during refrigerated storage.
Furthermore, it has been noted that incorporating inulin into ice cream significantly increases the overrun, as this prebiotic is responsible for air incorporation [1,25]. In this context, the pattern observed in the base with inulin was similar to that described in other studies where the incorporation of inulin positively affected the overrun percentage. However, it has also been observed that the overrun percentage in probiotic ice creams is lower than in non-probiotic ice creams. Furthermore, inulin does not appear to alter the overrun percentage compared to ice creams made with whole sheep’s milk [14].
The differences in pH changes between both bases could be attributed to the viability of the probiotics. Studies have shown that probiotics maintain their metabolic activity even when frozen. This activity is reportedly linked to the hydrolysis of milk protein polypeptide chains [8]. Our study found that viability remained consistent in both ice cream bases (Table 2).
In general, it has been observed that microbial viability in probiotic ice creams decreases as the refrigeration and freezing time increases, especially when they are in the form of free cells and not encapsulated [6]. However, our study determined that probiotic viability remained stable throughout storage. This, in turn, is related to the reported pH values, which did not show a significant decrease in both bases. Lactic acid production during fermentation is due to lactose conversion derived from decreased pH. However, releasing amino groups during fermentation could explain why, in the study base, the pH decreased more slowly [26]. In the development of probiotic ice creams, it is understood that the decrease in microorganisms occurs primarily during the initial processing hours due to ice crystal formation, thermal shock, and air incorporation during refrigerated storage [27]. However, despite these factors, it has been observed that probiotic microorganisms can remain viable for more than 90 days at −18 °C [28]. Similarly, it has been observed that the viability of probiotic ice cream is influenced by factors such as the specific strain used, acidity levels in the product, and cytoprotection [27,28].
3.3. Proteolytic Profile
3.3.1. TNBS Determination
Proteolytic activity increased in both bases, with higher amino group release in the aguamiel syrup base, suggesting enhanced protein hydrolysis (Figure 2). In both ice cream bases, the probiotic probably utilized the peptides initially present in the ice cream base (time 0). Subsequently, starting from this initial concentration of amino groups, an increase was observed up to 7 days of fermentation. Specifically, in the control base, the concentration increased from 122.65 ± 0.9 to 140.26 ± 1.3 µg/mL, while in the base with the added aguamiel syrup, it increased from 159.63 ± 4.8 to 209.63 ± 1.99 µg/mL. Towards the end of fermentation (14 days), these concentrations increased again. The above indicates the capability of L. rhamnosus GG to release amino groups, which is dependent on the strain’s proteolytic system. Regarding L. rhamnosus, its auxotrophies necessitate peptides containing cysteine, serine, arginine, proline, and glutamine [29].
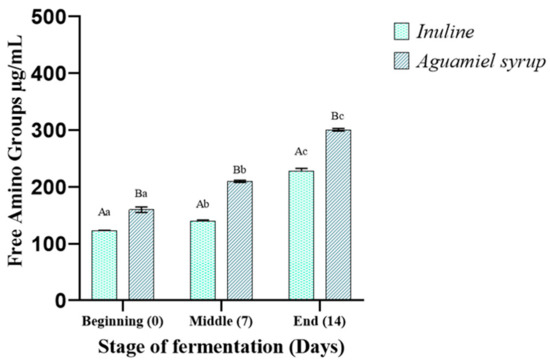
Figure 2.
Concentration of amino groups released during the refrigeration of a probiotic ice cream base with added inulin and a base with added aguamiel syrup. The results represent the average of three determinations ± the standard deviation. Lowercase letters (a–c) indicate the comparisons of means between different fermentation times within the same fermentation system. Uppercase letters (A, B) indicate the comparisons of means between different fermentation systems at the same fermentation time.
The higher concentrations of amino groups in the aguamiel syrup base indicates stronger proteolytic activity compared to the inulin base. After 14 days of storage, the concentrations of amino groups reached a maximum of 228.32 ± 4.0 µg/mL in the base with inulin and 302.67 ± 2.29 µg/mL in the base with aguamiel syrup. In general terms, it has been observed that Lactobacillus species exhibit higher initial proteolytic activity compared to other species of lactic acid bacteria [30]. Protein hydrolysis is primarily activated by protease (PrtR), which initiates the breakdown of proteins to meet their auxotrophic requirements. After obtaining the necessary amino acids for growth, the probiotic release both peptides and amino acids [26].
The initial free amino acids in the aguamiel syrup influenced different concentrations observed in both systems, which was higher in the base supplemented with aguamiel syrup (159.63 ± 4.8) compared to the base supplemented with inulin (122.65 ± 0.9) at the initial time. The L. rhamnosus aminopeptidases are regulated by a system of operons, which are activated by various amino acids and act as system promoters [31]. Likewise, a constant release and increased amount of amino groups at the end of fermentation in milk enriched with aguamiel has been reported [9].
3.3.2. Separation of Peptides by Tris-Tricine-SDS-PAGE
Electropherograms showed a higher accumulation of small peptides in the aguamiel syrup base, correlating with increased proteolytic activity. Results revealed the presence of small peptides (<10 kDa) in both systems (Figure 3). Therefore, this accumulation was more pronounced in the ice cream base supplemented with aguamiel syrup. Results are related to free amino groups concentration, leading to a higher concentration in the ice cream base enriched with aguamiel syrup. As previously mentioned, it has been observed that adding this material to different dairy matrices improves the L. rhamnosus proteolysis.
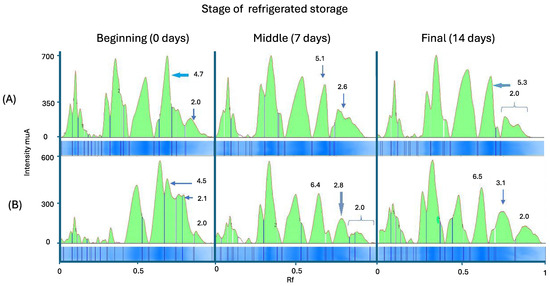
Figure 3.
Electropherograms of the peptide separation by SDS-PAGE in the ice cream bases with added L. rhamnosus GG during 14 days of refrigerated storage enriched with (A) inulin and (B) aguamiel syrup.
Some authors have indicated that low-molecular-weight peptide accumulation is related to the fermentation duration and the enrichment with an optimal carbon source with prebiotic capacity. In this sense, incorporating compounds such as aguamiel and oat β-glucans increases proteolytic activity [8,9]. The proteolytic system of LAB is unique to each strain and is influenced by the disposable amino acids and the microorganism’s ability to adapt [30]. In this context, the majority of the proteolytic activity of L. rhamnosus is performed by the cell wall-bound proteinase PrtR. Subsequently, intracellular peptidases and aminopeptidases continue by cleaving peptide fractions and excreting unnecessary ones [32].
On the other hand, a decrease in peptides higher than 10 kDa and in proteins was observed. This trend is more evident in the ice cream base system with added aguamiel syrup, with a higher concentration at day 14 of storage. Results are related to those observed in TNBS analysis, in which a higher concentration of amino groups was found in both systems towards the end of fermentation (14 days). However, this increase was more evident in the fermentation system of the base under study. In fact, new peaks were observed throughout all days of storage and appeared sequentially. This pattern indicates the importance of lactic bacteria metabolism during storage, even at refrigeration temperatures. The lowest molecular weight protein in milk is about 14.4 kDa, a weight that corresponds to α-lactalbumin. All fractions found below this molecular weight represent fractions derived from hydrolysis, which is carried out through the probiotic microorganism.
Low-molecular-weight peptide concentrations were higher in the base with added aguamiel syrup than in the base with inulin (Figure 3). Specifically, the study noted an increase in peptides smaller than 2 kDa and a reduction in the levels of caseins and whey proteins. It has been observed that LABs prefer caseins over whey proteins to generate high- and medium-molecular-weight peptides. However, these peptides are derived into smaller peptides when they are hydrolyzed by the action of endopeptidases and excreted into the medium once the microorganism has covered its auxotrophies [33].
Various studies report obtaining peptides less than 10 kDa from the hydrolysis of milk proteins with significant biological activity [34,35]. Similarly, the presence of small peptides is a focal point in many studies investigating the production of bioactive peptides. It has been reported that there is a fundamental relationship between peptide size and biological activity. In this way, low-molecular-weight peptides have been observed to result in greater antihypertensive or antidiabetic activity [30,35,36].
3.4. Antihypertensive Analysis: ACE Inhibition
Results showed that distinct variations in the antihypertensive efficacy were noticeable between the two fermentation systems. For the ice cream base enriched with the probiotic inulin, at the onset of fermentation (Figure 4), an inhibition percentage of 47.5% was noted. Afterward, this percentage rose to 54.8% after 7 days of fermentation, followed by a slight decrease, resulting in an inhibition percentage of 43.5% after 14 days of storage. In this context, studies have indicated that L. rhamnosus GG initially requires aromatic amino acids to fulfill its metabolic requirements during fermentation [37]. This might be associated with increased ACE inhibition after 7 days of storage. Similarly, studies have suggested that a longer fermentation time may reduce antihypertensive activity, resulting in a lower percentage of ACE inhibition.
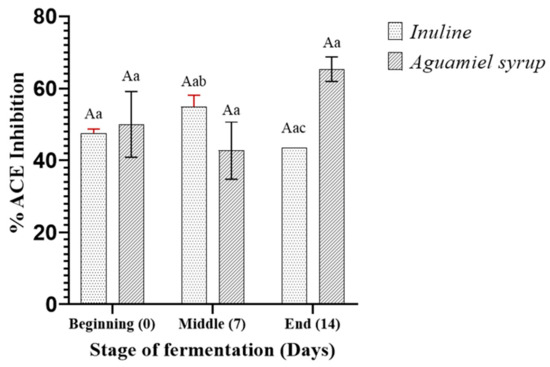
Figure 4.
Inhibition of ACE activity by peptide fractions derived from protein fractionation during refrigerated storage of ice cream base with L. rhamnosus GG and enriched with inulin and aguamiel syrup. Lowercase letters (a–c) indicate the comparisons of means between different refrigerated storage times within the same systems. Uppercase letters (A, B) indicate the comparisons of means between different systems at the same refrigerated storage time.
In the case of the ice cream base with added aguamiel syrup, ACE inhibition decreased from 50% to 42.5% after 7 days of storage. Subsequently, this percentage increased to 65% after 14 days. Despite a slight increase in inhibitory activity, it is understood that once L. rhamnosus is present, it excretes any excess into the medium, leading to their accumulation. Due to no significant changes in bacterial growth during the storage time in both ice cream bases, the inhibition percentage was probably not substantial either. Likewise, it did not exhibit a higher rate of inhibition, which could be related to the results observed in its growth, which did display an increased rate. Yet, during electrophoresis analysis, the base supplemented with inulin showed a lower proteolytic capacity for producing low-molecular-weight peptides compared to the base supplemented with aguamiel syrup. This could suggest that inulin does not promote small peptides released during fermentation, potentially resulting in lower ACE inhibitory activity.
Based on the study of ACE, it has been pointed out that there is a close relationship between the structure of peptides and their antihypertensive capacity [38,39]. The substrate and inhibitors preferred by ACE are those with structures that include hydrophobic amino acids as well as aromatic amino acids [39]. In this regard, its antihypertensive activity has been linked to the sequence found at the C-terminal site [16]. In this way, investigations have reported that peptides with amino acids such as tryptophan, phenylalanine, or isoleucine in their structure couple at the ACE active site, and consequently, the antihypertensive activity increases [40]. Similar to how incorporating aguamiel syrup into the ice cream base enhances its proteolytic capacity, it is also expected to improve antihypertensive activity by increasing the percentage of ACE inhibition. It should be noted that the above will depend largely on the specific conditions of the aguamiel syrup. As previously mentioned, the season of the year in which the collection is carried out, the concentration of carbohydrates, the age of the plant, and the native microbiota are factors to consider in the characterization and quality of the aguamiel syrup that could ultimately influence the bioactive capacity of the products enriched with this material [10,24].
Currently, no dairy products with aguamiel syrup are on the market. However, these results suggest the potential for developing new products incorporating aguamiel syrup derivatives and conferring important beneficial properties.
4. Conclusions
When incorporated into an ice cream base, aguamiel syrup significantly boosts the viability and proteolytic activity of Lactobacillus rhamnosus GG. This enhancement of bacterial activity is crucial as it produces bioactive compounds that can exert antihypertensive effects. By improving this probiotic strain’s survival and functional performance, aguamiel syrup helps elevate the ice cream’s overall health benefits, making it a tasty treat and a portion of functional food with potential cardiovascular benefits.
The antihypertensive activity observed in this study could be attributed to the peptides found after the refrigeration period of the ice cream base enriched with inulin and aguamiel syrup. Additionally, low-molecular-weight peptides (<10 kDa) would indicate the probability of finding amino acid structures with therapeutic potential, especially in the ice cream base, in which peptides of 2 kDa were found. This is why ice cream, a globally consumed product, presents an opportunity for deriving bioactive peptides through the metabolism of lactic acid bacteria, as demonstrated in this study. These results suggest that aguamiel syrup can be used to develop new functional dairy products with health benefits. Incorporating aguamiel syrup into dairy products offers a promising avenue for enhancing their bioactive potential. Likewise, adding new prebiotic ingredients, such as those in aguamiel syrup, opens a path to a new field of study in the evaluation of emerging prebiotics, which not only provides the benefits of this type of ingredients but also allows the improvement of the metabolic aspects of probiotic bacteria, enabling the release of the secondary metabolites of therapeutic interest such as antihypertensive peptides. Nevertheless, further studies are crucial to evaluate the enhancement of techno-functional properties, such as overrun, in ice cream bases enriched with symbiotic additions like these. Additionally, it is necessary to expand the study of fermented dairy products that incorporate aguamiel derivatives since no commercial proposal exists for any of these. The results of this research lay the foundations for an emerging alternative in using matrices such as aguamiel syrup that, when added to everyday consumption products, helps to enhance their bioactive capacity.
Author Contributions
Conceptualization, L.G.G.-O. and A.E.C.-G.; experimentation and technical support, L.B.O.-R., E.H.-R. and E.P.-E.; writing original draft preparation, L.B.O.-R. and L.G.G.-O.; funding acquisition and experimental support, E.C.-L., A.E.C.-G. and J.J.-O.; review and editing, L.G.G.-O., J.J.-O. and L.B.O.-R.; developing of figures and tables, L.B.O.-R. and L.G.G.-O.; project administration, L.G.G.-O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
Authors thank CONAHCyT for the stipend received. This paper is included in the compromises made in the Project 317510 from the announcement CONAHCYT FOP07-2021-03 “Valorización del aguamiel producido en comunidades del Estado de Hidalgo: producción sustentable de jarabe rico en oligofructanos destinado a sectores económicos medio y medio alto”.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Villalva, F.J.; Cravero Bruneri, A.P.; Vinderola, G.; Gonçalvez De Oliveira, E.; Paz, N.F.; Ramón, A.N. Formulation of a Peach Ice Cream as Potential Symbiotic Food. Food Sci. Technol. 2017, 37, 456–461. [Google Scholar] [CrossRef]
- Kowalczyk, M.; Znamirowska-Piotrowska, A.; Buniowska-Olejnik, M.; Pawlos, M. Sheep Milk Symbiotic Ice Cream: Effect of Inulin and Apple Fiber on the Survival of Five Probiotic Bacterial Strains during Simulated In Vitro Digestion Conditions. Nutrients 2022, 14, 4454. [Google Scholar] [CrossRef] [PubMed]
- Aritonang, S.N.; Roza, E.; Rossi, E. The Effect of Whippy Cream Adding on the Quality of Frozen Soyghurt as Symbiotic Ice Cream. IOP Conf. Ser. Earth Environ. Sci. 2019, 287, 012029. [Google Scholar] [CrossRef]
- De Oliveira, A.P.D.; De Oliveira Almeida, T.J.; Santos, T.M.B.; Dias, F.S. Symbiotic Goat Milk Ice Cream with Umbu Fortified with Autochthonous Goat Cheese Lactic Acid Bacteria. LWT 2021, 141, 110888. [Google Scholar] [CrossRef]
- Olvera-Rosales, L.-B.; Cruz-Guerrero, A.-E.; Ramírez-Moreno, E.; Quintero-Lira, A.; Contreras-López, E.; Jaimez-Ordaz, J.; Castañeda-Ovando, A.; Añorve-Morga, J.; Calderón-Ramos, Z.-G.; Arias-Rico, J.; et al. Impact of the Gut Microbiota Balance on the Health–Disease Relationship: The Importance of Consuming Probiotics and Prebiotics. Foods 2021, 10, 1261. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, H.S.; Salama, H.H.; EL-Sayed, S.M. Production of Synbiotic Ice Cream. Int. J. Chemtech Res. 2014, 7, 138–147. [Google Scholar]
- Cheng, J.; Ma, Y.; Li, X.; Yan, T.; Cui, J. Effects of Milk Protein-Polysaccharide Interactions on the Stability of Ice Cream Mix Model Systems. Food Hydrocoll. 2015, 45, 327–336. [Google Scholar] [CrossRef]
- Jaimez-Ordaz, J.; Martínez-Ramírez, X.; Cruz-Guerrero, A.E.; Contreras-López, E.; Ayala-Niño, A.; Castro-Rosas, J.; González-Olivares, L.G. Survival and Proteolytic Capacity of Probiotics in a Fermented Milk Enriched with Agave Juice and Stored in Refrigeration. Food Sci. Technol. 2019, 39, 188–194. [Google Scholar] [CrossRef]
- Ramírez-Godínez, J.; Gutiérrez-Rodríguez, J.F.; Contreras-López, E.; Rodríguez-Serrano, G.M.; Castañeda-Ovando, A.; Jaimez-Ordaz, J.; González-Olivares, L.G. Agave Juice Improves Survival and Proteolytic Activity of Lactobacillus rhamnosus GG during Ripening of Semi-Ripened Mexican Cheese. Food Sci. Technol. 2022, 42, e30820. [Google Scholar] [CrossRef]
- Isabel Enríquez-Salazar, M.; Veana, F.; Aguilar, C.N.; De La Garza-Rodríguez, I.M.; López, M.G.; Rutiaga-Quiñones, O.M.; Morlett-Chávez, J.A.; Rodríguez-Herrera, R. Microbial Diversity and Biochemical Profile of Aguamiel Collected from Agave salmiana and A. atrovirens during Different Seasons of Year. Food Sci. Biotechnol. 2017, 26, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Basurto, R.I.; Pourcelly, G.; Doco, T.; Williams, P.; Dornier, M.; Belleville, M.-P. Analysis of the Main Components of the Aguamiel Produced by the Maguey-Pulquero (Agave mapisaga) throughout the Harvest Period. J. Agric. Food Chem. 2008, 56, 3682–3687. [Google Scholar] [CrossRef]
- Castañeda-Ovando, A.; Moreno-Vilet, L.; Jaimez-Ordaz, J.; Ramírez-Godínez, J.; Pérez-Escalante, E.; Cruz-Guerrero, A.E.; Contreras-López, E.; Alatorre-Santamaría, S.A.; Guzmán-Rodríguez, F.J.; González-Olivares, L.G. Aguamiel Syrup as a Technological Diversification Product: Composition, Bioactivity and Present Panorama. Future Foods 2023, 8, 100249. [Google Scholar] [CrossRef]
- Cruz, A.G.; Antunes, A.E.C.; Sousa, A.L.O.P.; Faria, J.A.F.; Saad, S.M.I. Ice-Cream as a Probiotic Food Carrier. Food Res. Int. 2009, 42, 1233–1239. [Google Scholar] [CrossRef]
- Balthazar, C.F.; Silva, H.L.A.; Esmerino, E.A.; Rocha, R.S.; Moraes, J.; Carmo, M.A.V.; Azevedo, L.; Camps, I.; Abud, Y.K.D.; Sant’Anna, C.; et al. The Addition of Inulin and Lactobacillus casei 01 in Sheep Milk Ice Cream. Food Chem. 2018, 246, 464–472. [Google Scholar] [CrossRef]
- Moreno-Vilet, L.; Bostyn, S.; Flores-Montaño, J.-L.; Camacho-Ruiz, R.-M. Size-Exclusion Chromatography (HPLC-SEC) Technique Optimization by Simplex Method to Estimate Molecular Weight Distribution of Agave Fructans. Food Chem. 2017, 237, 833–840. [Google Scholar] [CrossRef]
- Olvera-Rosales, L.B.; Cruz-Guerrero, A.E.; García-Garibay, J.M.; Gómez-Ruíz, L.C.; Contreras-López, E.; Guzmán-Rodríguez, F.; González-Olivares, L.G. Bioactive Peptides of Whey: Obtaining, Activity, Mechanism of Action, and Further Applications. Crit. Rev. Food Sci. Nutr. 2023, 63, 10351–10381. [Google Scholar] [CrossRef]
- Schägger, H.; Von Jagow, G. Tricine-Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis for the Separation of Proteins in the Range from 1 to 100 kDa. Anal. Biochem. 1987, 166, 368–379. [Google Scholar] [CrossRef]
- González-Olivares, L.G.; Añorve-Morga, J.; Castañeda-Ovando, A.; Contreras-López, E.; Jaimez-Ordaz, J. Peptide Separation of Commercial Fermented Milk during Refrigerated Storage. Food Sci. Technol. 2014, 34, 674–679. [Google Scholar] [CrossRef]
- Cushman, D.W.; Wang, F.L.; Fung, W.C.; Harvey, C.M.; DeForrest, J.M. Differentiation of Angiotensin–Converting Enzyme (ACE) Inhibitors by Their SelectiveInhibition of ACE in Physiologically Important Target Organs. Am. J. Hypertens. 1989, 2, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Peralta-García, I.; González-Muñoz, F.; Elena, R.-A.M.; Sánchez-Flores, A.; López Munguía, A. Evolution of Fructans in Aguamiel (Agave Sap) During the Plant Production Lifetime. Front. Nutr. 2020, 7, 566950. [Google Scholar] [CrossRef]
- Romero-López, M.R.; Osorio-Díaz, P.; Flores-Morales, A.; Robledo, N.; Mora-Escobedo, R. Chemical Composition, Antioxidant Capacity and Prebiotic Effect of Aguamiel (Agave atrovirens) during in Vitro Fermentation. Rev. Mex. Ing. Química 2015, 14, 281–292. [Google Scholar]
- Villarreal-Morales, S.L.; Muñiz-Márquez, D.B.; Michel-Michel, M.; González-Montemayor, Á.-M.; Escobedo-García, S.; Salas-Tovar, J.A.; Flores-Gallegos, A.C.; Rodríguez-Herrera, R. Aguamiel a Fresh Beverage from Agave spp. Sap with Functional Properties. In Natural Beverages; Elsevier: Amsterdam, The Netherlands, 2019; pp. 179–208. ISBN 978-0-12-816689-5. [Google Scholar]
- Kolida, S.; Tuohy, K.; Gibson, G.R. Prebiotic Effects of Inulin and Oligofructose. Br. J. Nutr. 2002, 87, S193–S197. [Google Scholar] [CrossRef] [PubMed]
- González-Cruz, L.; Jaramillo-Flores, M.E.; Bernardino-Nicanor, A.; Mora-Escobedo, R. Influence of the Harvest Age on Fructan Content and Fructosyltransferase Activity in Agave atrovirens Karw Pine. Int. J. Plant Physiol. Biochem. 2012, 4, 110–119. [Google Scholar] [CrossRef]
- Akalın, A.S.; Erişir, D. Effects of Inulin and Oligofructose on the Rheological Characteristics and Probiotic Culture Survival in Low-Fat Probiotic Ice Cream. J. Food Sci. 2008, 73, M184–M188. [Google Scholar] [CrossRef] [PubMed]
- Olvera-Rosales, L.B.; Pérez-Escalante, E.; Castañeda-Ovando, A.; Contreras-López, E.; Cruz-Guerrero, A.E.; Regal-López, P.; Cardelle-Cobas, A.; González-Olivares, L.G. ACE-Inhibitory Activity of Whey Proteins Fractions Derived of Fermentation by Lacticaseibacillus rhamnosus GG and Streptococcus thermophilus SY-102. Foods 2023, 12, 2416. [Google Scholar] [CrossRef]
- Silva, P.D.L.D.; Bezerra, M.D.F.; Santos, K.M.O.D.; Correia, R.T.P. Potentially Probiotic Ice Cream from Goat’s Milk: Characterization and Cell Viability during Processing, Storage and Simulated Gastrointestinal Conditions. LWT-Food Sci. Technol. 2015, 62, 452–457. [Google Scholar] [CrossRef]
- Dos Santos Cruxen, C.E.; Hoffmann, J.F.; Zandoná, G.P.; Fiorentini, Â.M.; Rombaldi, C.V.; Chaves, F.C. Probiotic Butiá (Butia odorata) Ice Cream: Development, Characterization, Stability of Bioactive Compounds, and Viability of Bifidobacterium lactis during Storage. LWT 2017, 75, 379–385. [Google Scholar] [CrossRef]
- Sun, J.; Chen, H.; Qiao, Y.; Liu, G.; Leng, C.; Zhang, Y.; Lv, X.; Feng, Z. The Nutrient Requirements of Lactobacillus rhamnosus GG and Their Application to Fermented Milk. J. Dairy. Sci. 2019, 102, 5971–5978. [Google Scholar] [CrossRef] [PubMed]
- Sebastián-Nicolas, J.L.; Contreras-López, E.; Ramírez-Godínez, J.; Cruz-Guerrero, A.E.; Rodríguez-Serrano, G.M.; Añorve-Morga, J.; Jaimez-Ordaz, J.; Castañeda-Ovando, A.; Pérez-Escalante, E.; Ayala-Niño, A.; et al. Milk Fermentation by Lacticaseibacillus rhamnosus GG and Streptococcus thermophilus SY-102: Proteolytic Profile and ACE-Inhibitory Activity. Fermentation 2021, 7, 215. [Google Scholar] [CrossRef]
- Laakso, K.; Koskenniemi, K.; Koponen, J.; Kankainen, M.; Surakka, A.; Salusjärvi, T.; Auvinen, P.; Savijoki, K.; Nyman, T.A.; Kalkkinen, N.; et al. Growth Phase-associated Changes in the Proteome and Transcriptome of Lactobacillus rhamnosus GG in Industrial-type Whey Medium. Microb. Biotechnol. 2011, 4, 746–766. [Google Scholar] [CrossRef]
- Hafeez, Z.; Cakir-Kiefer, C.; Lecomte, X.; Miclo, L.; Dary-Mourot, A. The X-Prolyl Dipeptidyl-Peptidase PepX of Streptococcus thermophilus Initially Described as Intracellular Is Also Responsible for Peptidase Extracellular Activity. J. Dairy. Sci. 2019, 102, 113–123. [Google Scholar] [CrossRef]
- González-Olivares, L.G.; Jiménez-Guzmán, J.; Cruz-Guerrero, A.; Rodríguez-Serrano, G.; Gómez-Ruiz, L.; García-Garibay, M. Bioactive Peptides Released by Lactic Acid Bacteria in Commercial Fermented Milks. Rev. Mex. Ing. Química 2011, 10, 179–188. [Google Scholar]
- Quirós, A.; Ramos, M.; Muguerza, B.; Delgado, M.A.; Miguel, M.; Aleixandre, A.; Recio, I. Identification of Novel Antihypertensive Peptides in Milk Fermented with Enterococcus faecalis. Int. Dairy J. 2007, 17, 33–41. [Google Scholar] [CrossRef]
- Ali, E.; Nielsen, S.D.; Abd-El Aal, S.; El-Leboudy, A.; Saleh, E.; LaPointe, G. Use of Mass Spectrometry to Profile Peptides in Whey Protein Isolate Medium Fermented by Lactobacillus helveticus LH-2 and Lactobacillus Acidophilus La-5. Front. Nutr. 2019, 6, 152. [Google Scholar] [CrossRef] [PubMed]
- Olvera-Rosales, L.B.; Cruz-Guerrero, A.E.; Jaimez-Ordaz, J.; Pérez-Escalante, E.; Quintero-Lira, A.; Ramírez-Moreno, E.; Contreras-López, E.; González-Olivares, L.G. Differences in the Proteolytic System of Lactic Acid Bacteria Affect the Release of DPP-IV Inhibitory Peptides from Whey Proteins. Dairy 2023, 4, 515–526. [Google Scholar] [CrossRef]
- Innocente, N.; Biasutti, M.; Rita, F.; Brichese, R.; Comi, G.; Iacumin, L. Effect of Indigenous Lactobacillus rhamnosus Isolated from Bovine Milk on Microbiological Characteristics and Aromatic Profile of Traditional Yogurt. LWT-Food Sci. Technol. 2016, 66, 158–164. [Google Scholar] [CrossRef]
- Murray, B.; FitzGerald, R. Angiotensin Converting Enzyme Inhibitory Peptides Derived from Food Proteins: Biochemistry, Bioactivity and Production. CPD 2007, 13, 773–791. [Google Scholar] [CrossRef]
- Yu, Y.; Yu, W.; Jin, Y. Peptidomic Analysis of Milk Fermented by Lactobacillus delbrueckii Subsp. bulgaricus and Streptococcus thermophilus. Food Hydrocoll. Health 2021, 1, 100033. [Google Scholar] [CrossRef]
- Li, G.; Le, G.; Shi, Y.; Shrestha, S. Angiotensin I–Converting Enzyme Inhibitory Peptides Derived from Food Proteins and Their Physiological and Pharmacological Effects. Nutr. Res. 2004, 24, 469–486. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).