Effect of Drying Aids on the Quality Properties of Kefir Powder
Abstract
1. Introduction
2. Materials and Methods
2.1. Kefir Preparation and Carriers Used
2.2. Drying Process
2.3. Physicochemical Properties
2.4. Powder Properties
2.5. Adsorption Isotherms
2.6. Particle Size Distribution
2.7. Microbiological Analyses
2.8. Morphology Analysis
2.9. Statistical Analysis
3. Results and Discussion
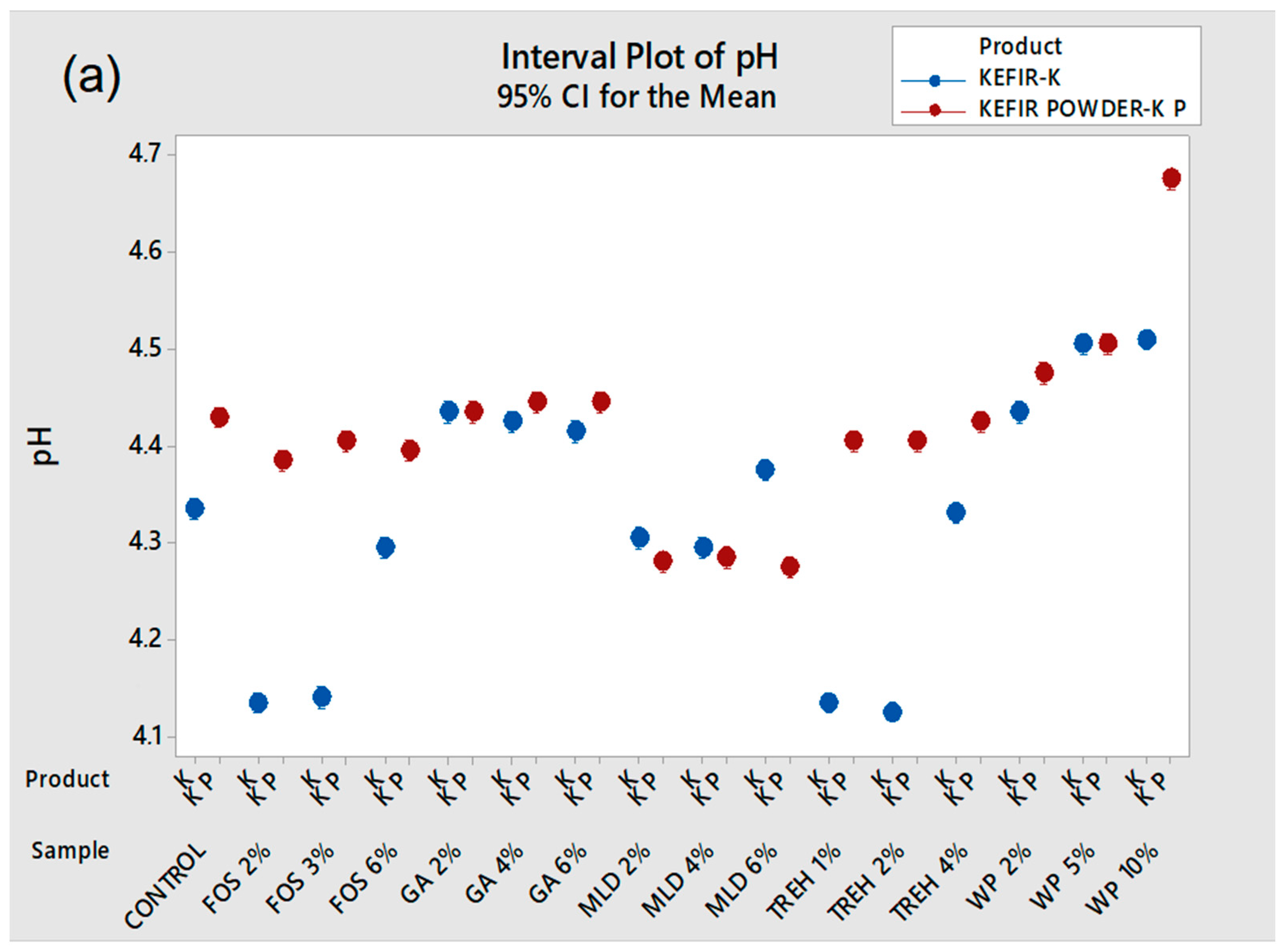
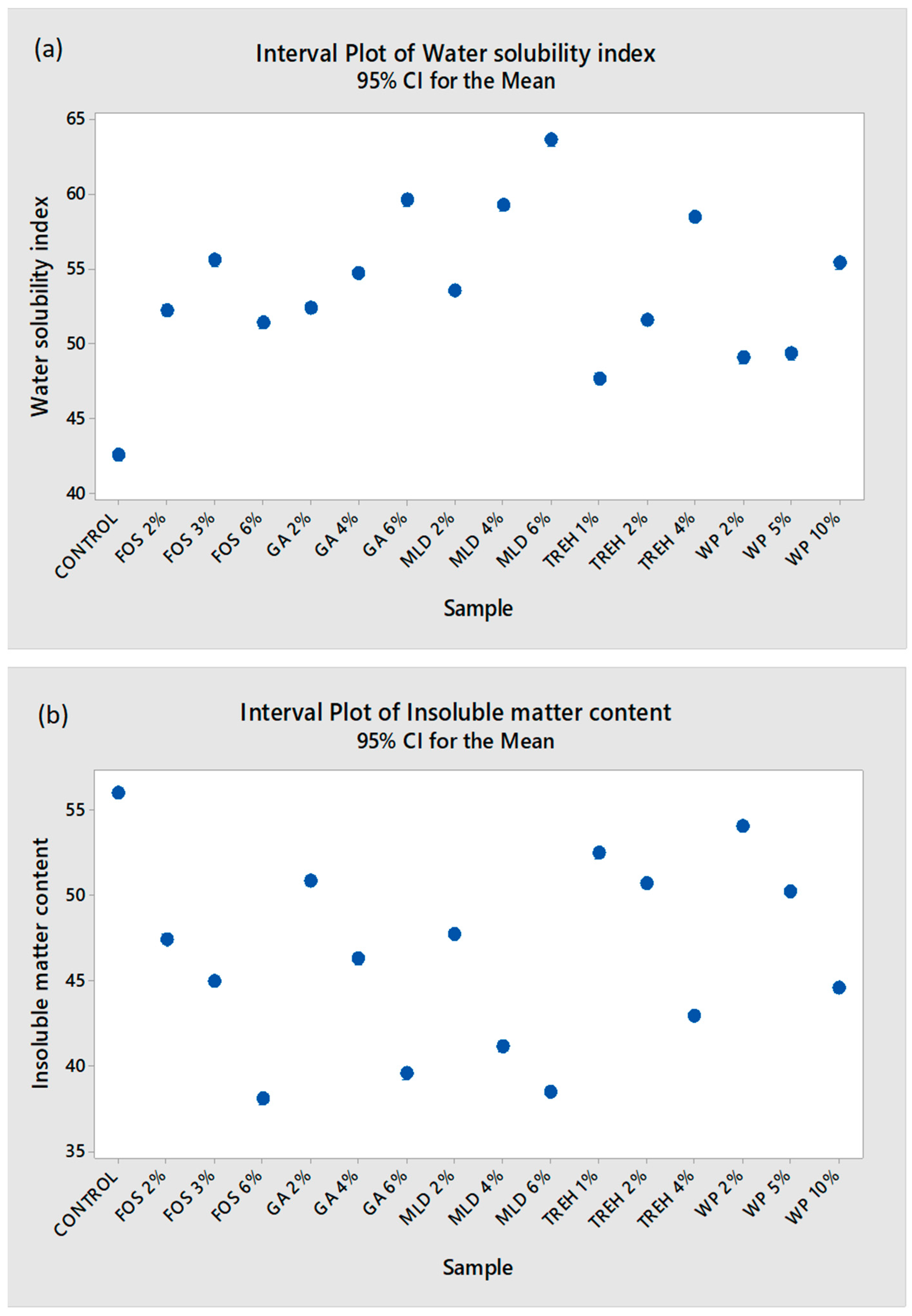
3.1. Physicochemical Properties of Kefir Powder and the Effect of Drying Aids
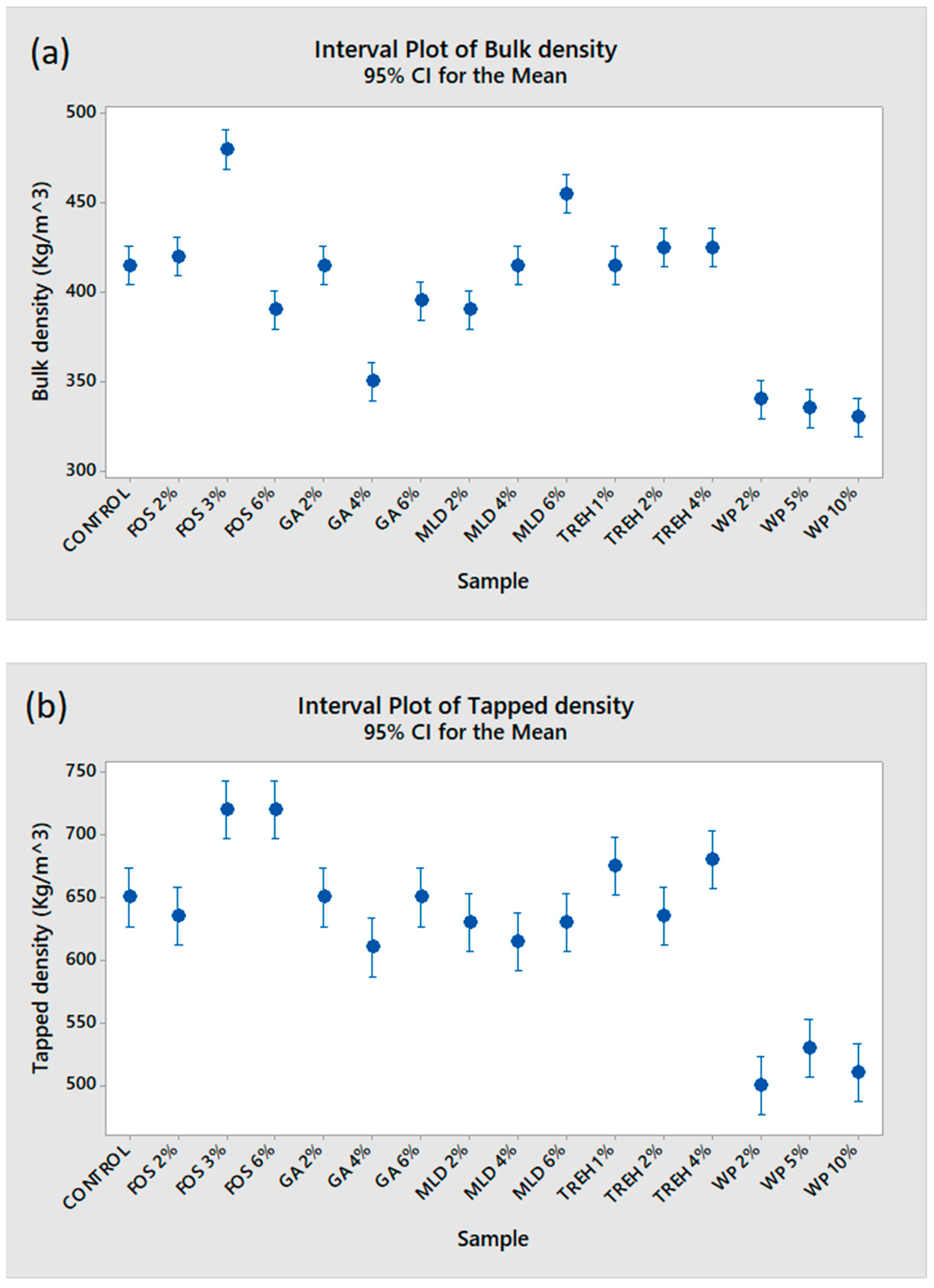
3.2. Physical Properties of Kefir Powder and the Effect of the Different Drying Aids
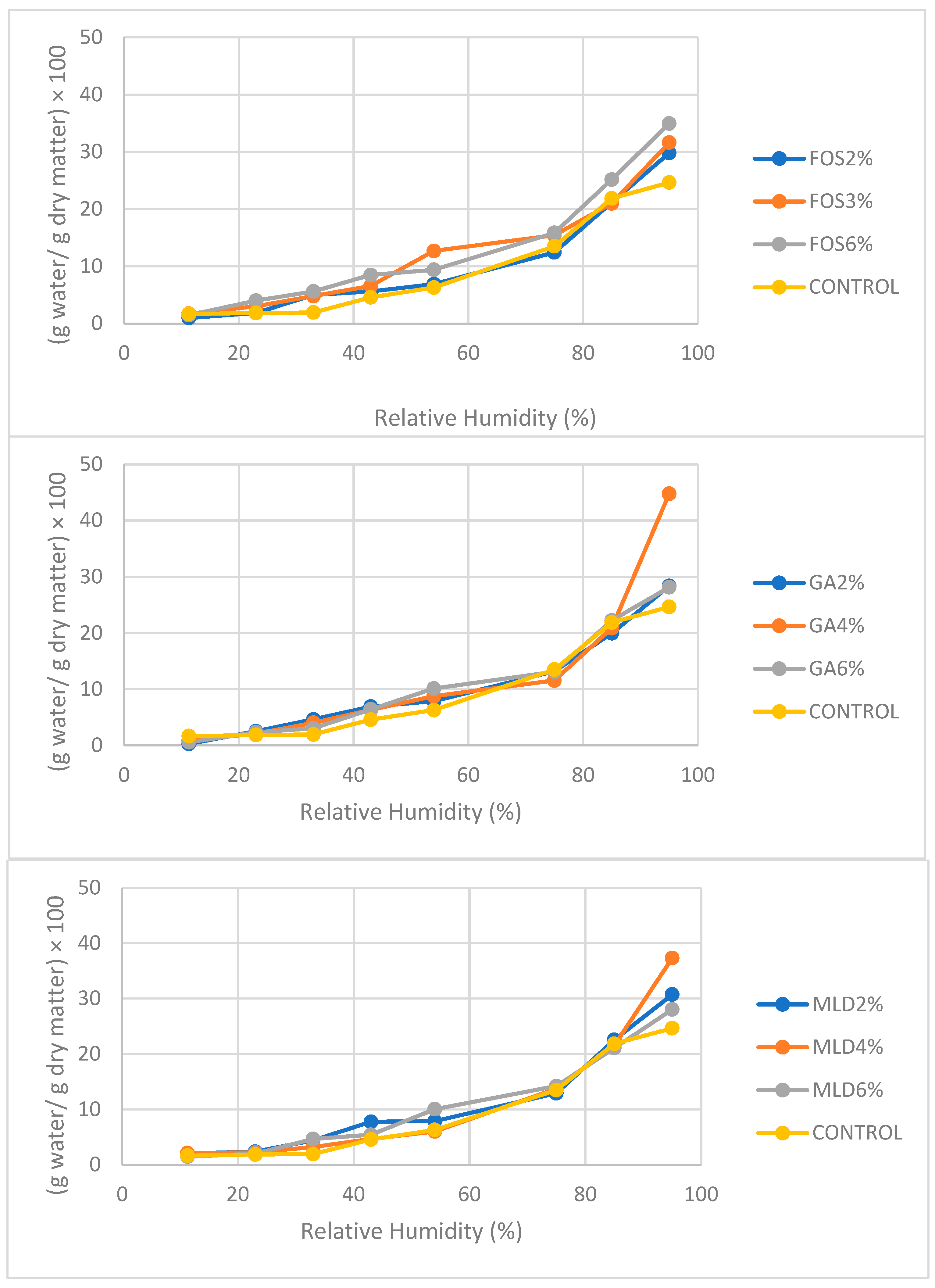
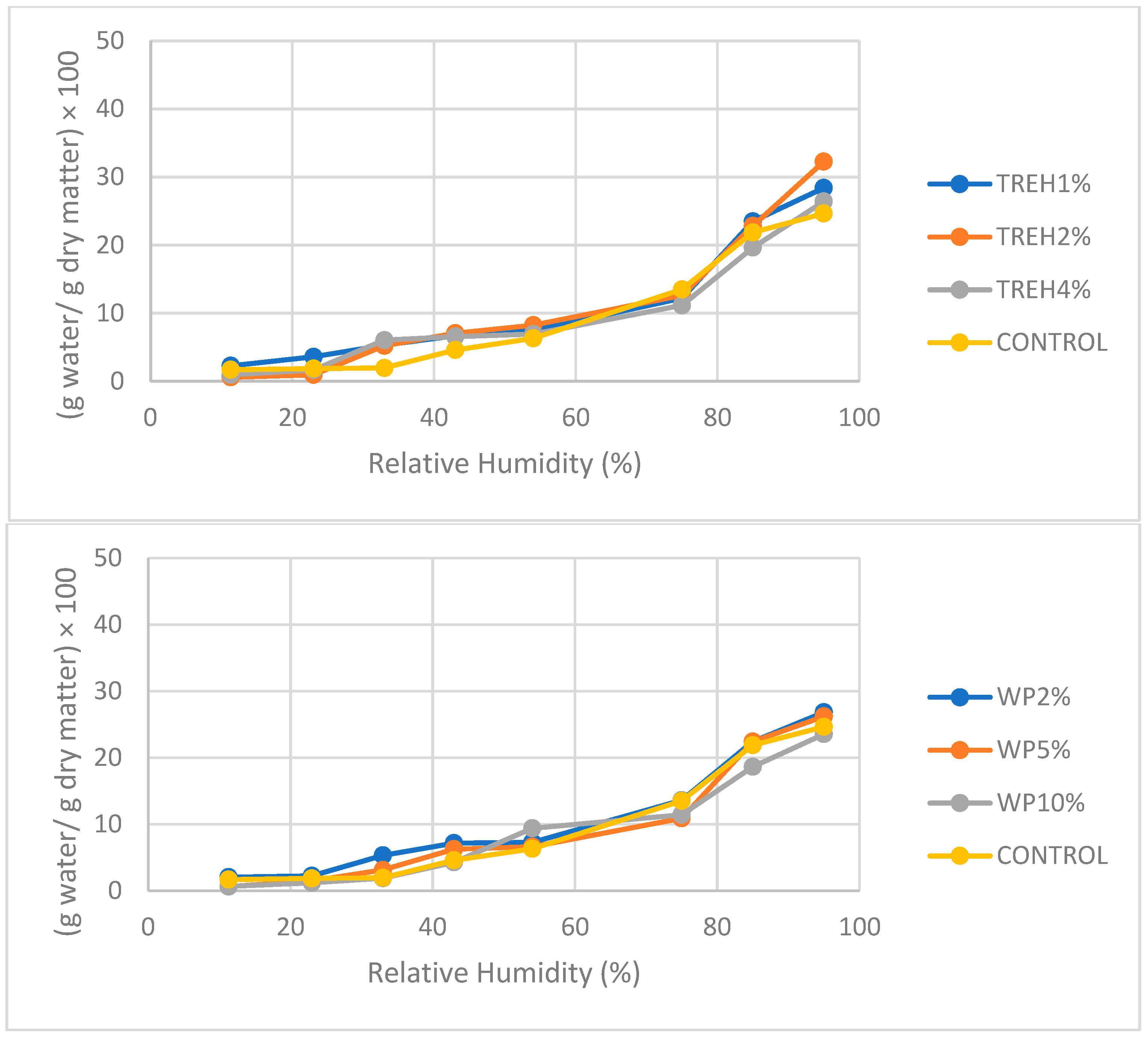
3.3. Adsorption Isotherms of Powder Kefir Samples
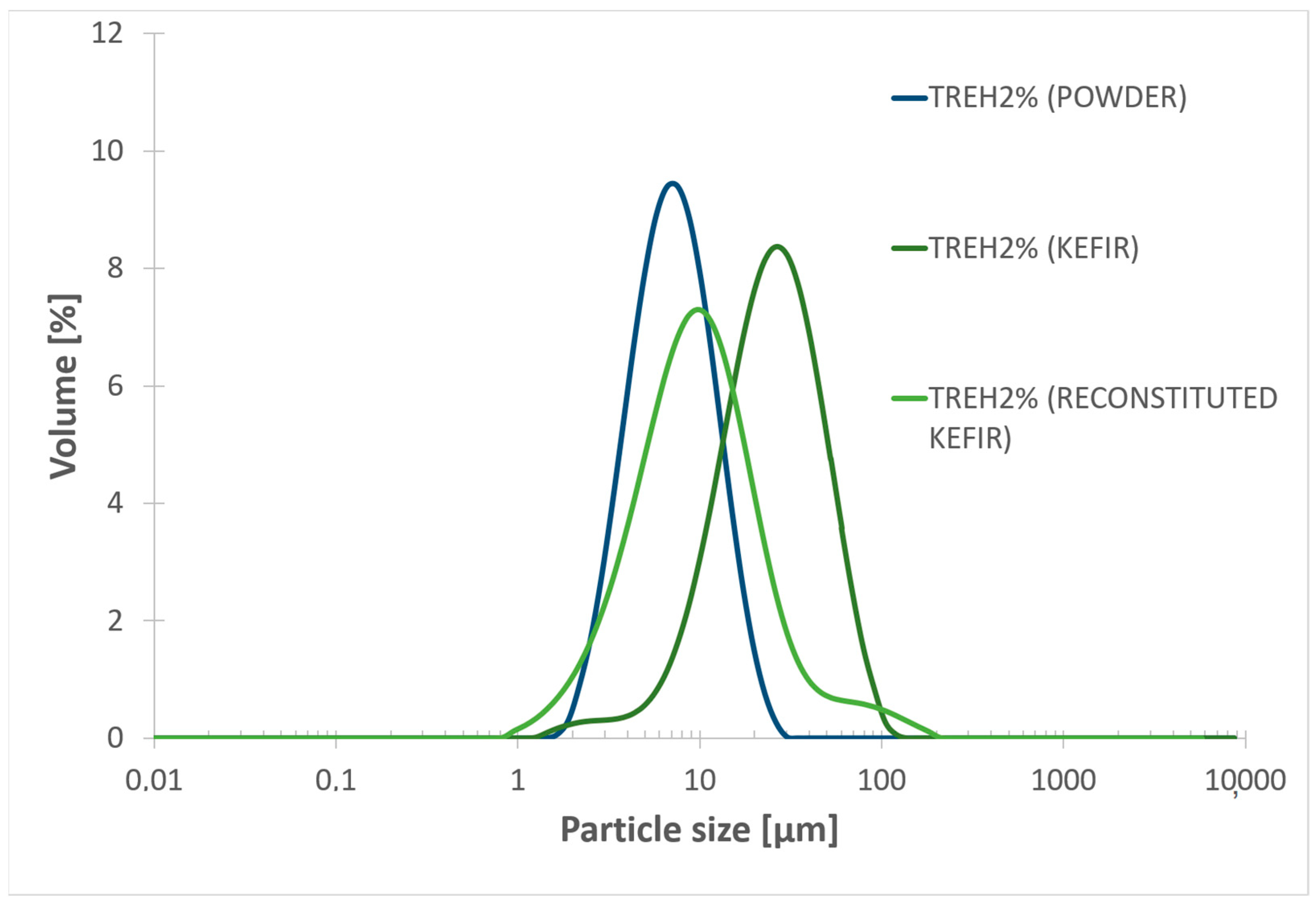
3.4. Particle Size Distribution
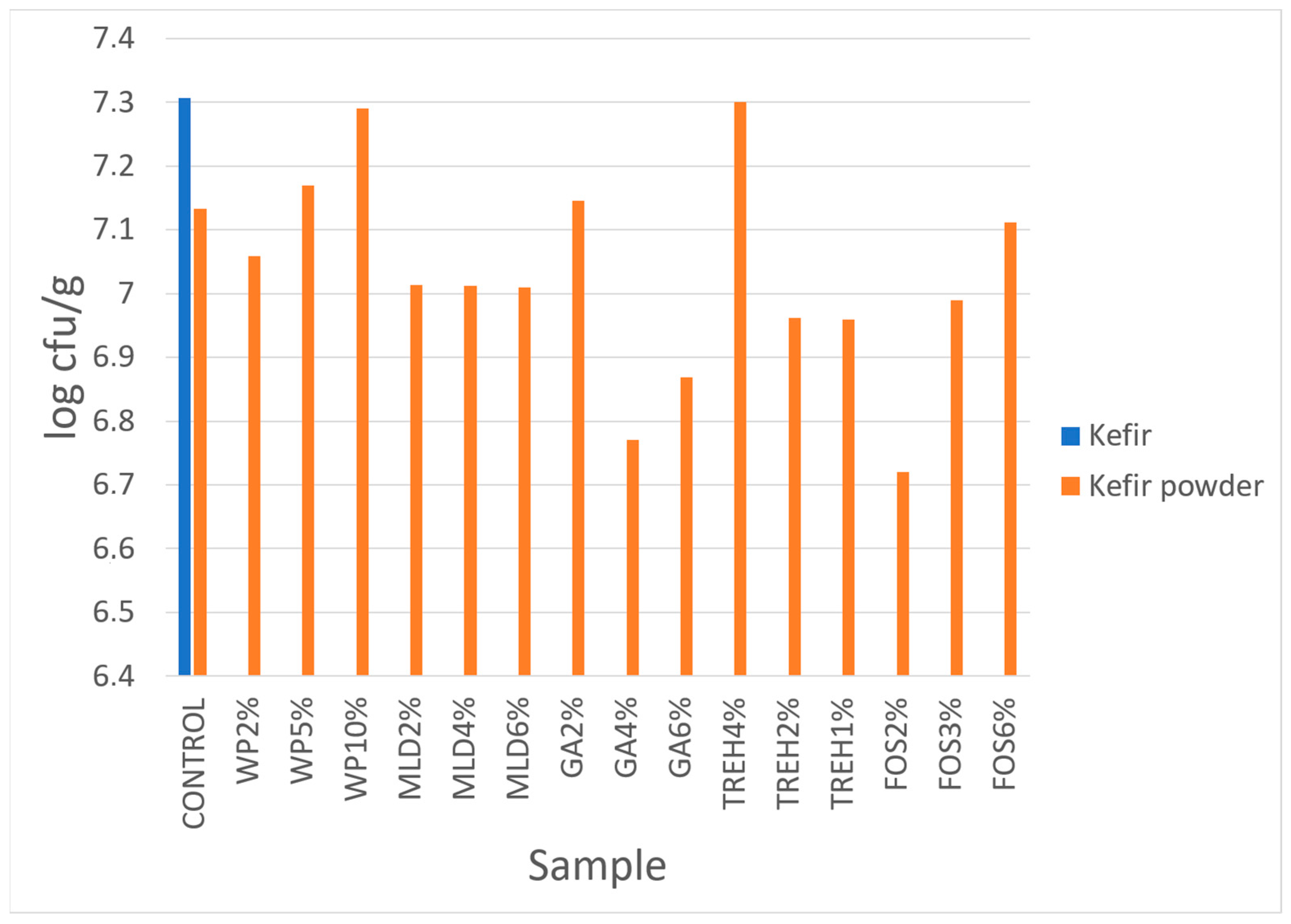
3.5. Microbiological Properties
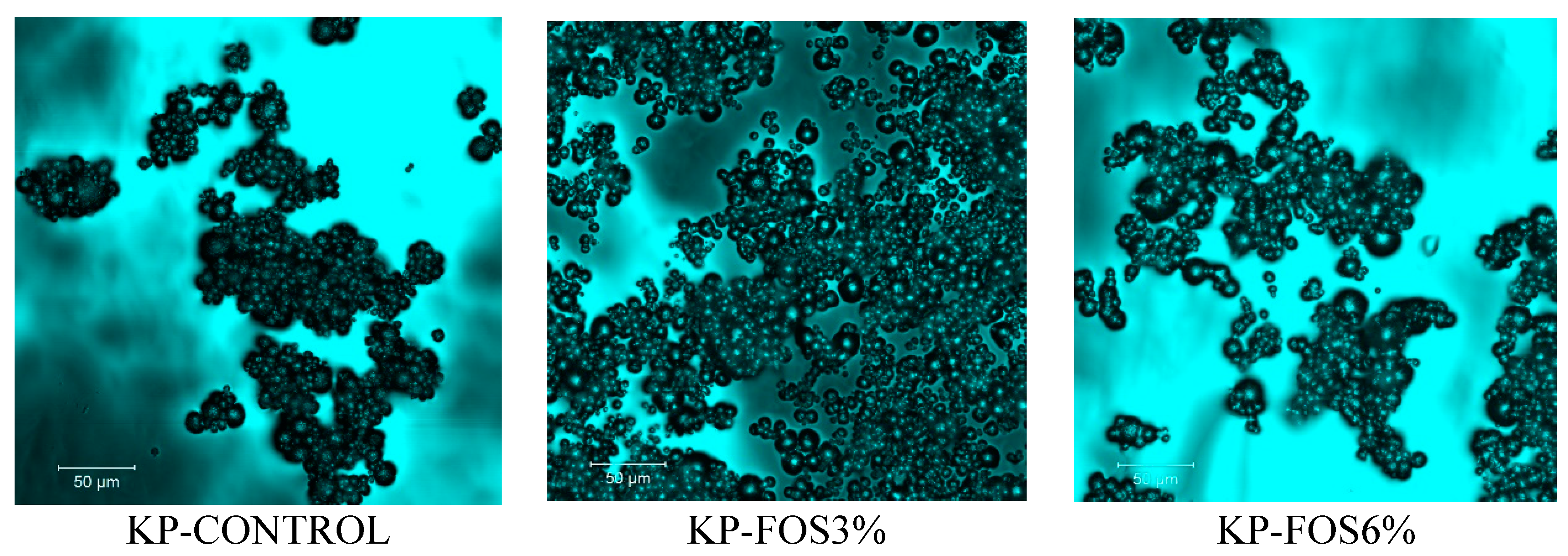
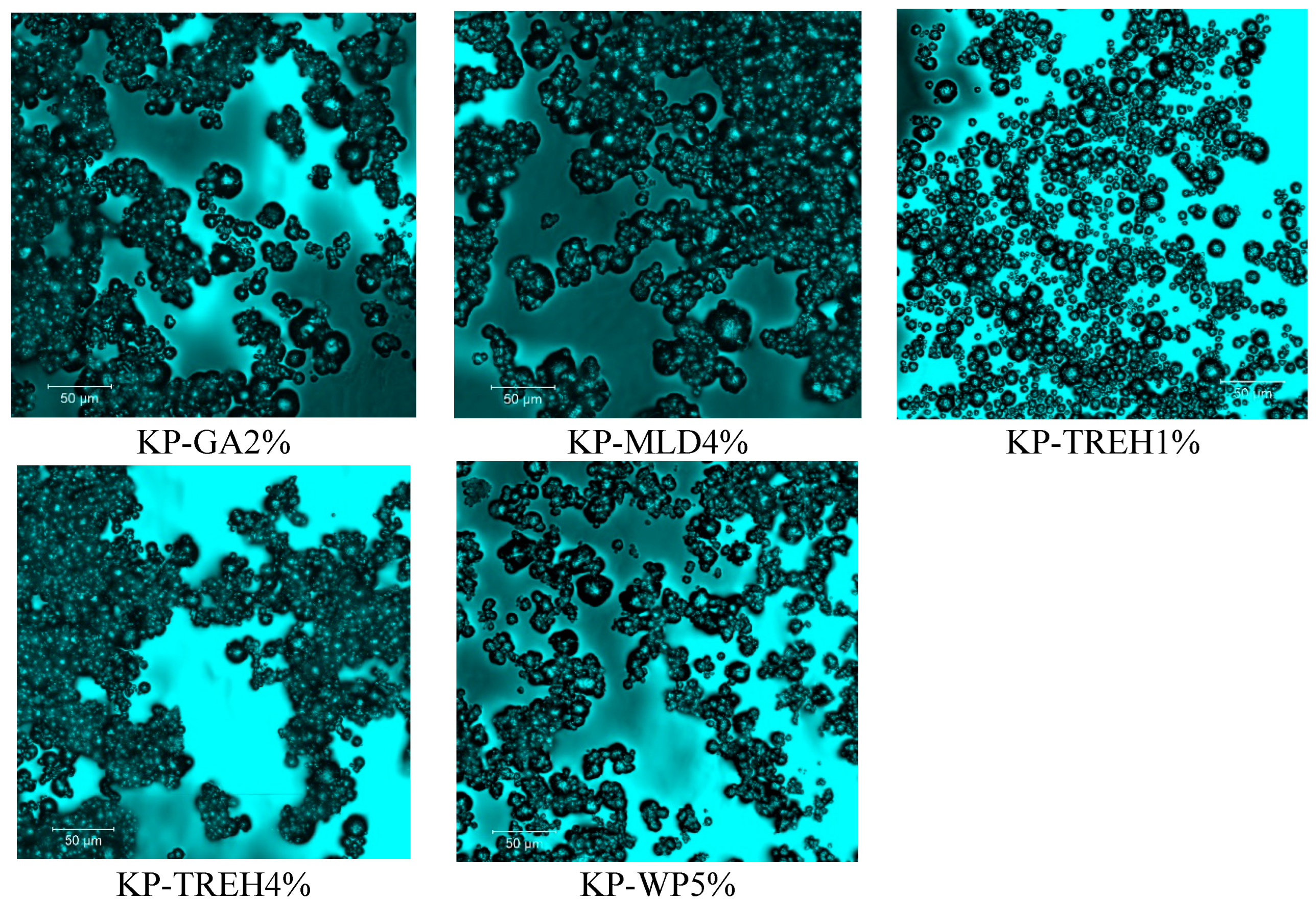
3.6. Structure
4. Conclusions
Author Contributions
Funding

Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Otles, S.; Cadingi, O. Kefir: A probiotic dairy—composition, nutritional and therapeutic aspects. Pak. J. Nutr. 2003, 2, 54–59. [Google Scholar]
- Rodrigues, K.L.; Caputo, L.R.G.; Carvalho, J.C.T.; Evangelista, J.; Schneedorf, J.M. Antimicrobial and Healing Activity of Kefir and Kefiran Extract. Int. J. Antimicrob. Agents 2005, 25, 404–408. [Google Scholar] [CrossRef]
- Farnworth, E.R.; Mainville, I. Kefir—A Fermented Milk Product. In Handbook of Fermented Functional Foods, 2nd ed.; Farnworth, E.R., Ed.; Taylor & Francis Group, LLC.: New York, NY, USA, 2008; pp. 89–127. [Google Scholar]
- Arslan, S. A Review: Chemical, Microbiological and Nutritional Characteristics of Kefir. CyTA J. Food 2015, 13, 340–345. [Google Scholar] [CrossRef]
- Filkova, I.; Huang, L.X.; Mujumbar, A.S. Industrial Spray Drying Systems. In Handbook of Industrial Drying, 3rd ed.; Mujumbar, A.S., Ed.; Taylor & Francis Group, LLC.: New York, NY, USA, 2006; Chapter 10; pp. 215–254. [Google Scholar]
- Santos, D.; Maurício, A.C.; Sencadas, V.; Santos, J.D.; Fernandes, M.H.; Gomes, P.S. Spray Drying: An Overview. In Biomaterials —Physics and Chemistry—New Edition; IntechOpen: London, UK, 2017; ISBN 978-1-78923-065-9. [Google Scholar]
- Yonekura, L.; Sun, H.; Soukoulis, C.; Fisk, I. Microencapsulation of Lactobacillus Acidophilus NCIMB 701748 in Matrices Containing Soluble Fibre by Spray Drying: Technological Characterization, Storage Stability and Survival after in Vitro Digestion. J. Funct. Foods 2014, 6, 205–214. [Google Scholar] [CrossRef]
- Anandharamakrishnan, C.; Padma Ishwarya, S. Spray Drying Techniques for Food Ingredient Encapsulation; John Wiley & Sons: Hoboken, NJ, USA, 2015; ISBN 978-1-118-86419-7. [Google Scholar]
- Nurwantoro, N.; Susanti, S.; Rizqiati, H. The Effect of Different Type Drying Methods on Chemical Characteristics and Microbiology of Goat Milk Powder Kefir. J. Appl. Food Technol. 2020, 7, 19–24. [Google Scholar] [CrossRef]
- Brasiel, P.G.D.A.; Costa de Almeida, T.; Mateus, K.; Fernandes de Carvalho, A.; Potente Dutra Luquetti, S.C.; Gouveia Peluzio, M.D.C. Maintenance of Probiotic Characteristics of Dry Kefir: Is It Possible? J. Culin. Sci. Technol. 2022, 20, 463–472. [Google Scholar] [CrossRef]
- Atalar, I.; Dervisoglu, M. Optimization of Spray Drying Process Parameters for Kefir Powder Using Response Surface Methodology. LWT Food Sci. Technol. 2015, 60, 751–757. [Google Scholar] [CrossRef]
- Teijeiro, M.; Pérez, P.F.; De Antoni, G.L.; Golowczyc, M.A. Suitability of Kefir Powder Production Using Spray Drying. Food Res. Int. 2018, 112, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Favilla, A.L.C.; dos Santos Junior, E.R.; Rodrigues, M.C.N.L.; dos Santos Baião, D.; Paschoalin, V.M.F.; Miguel, M.A.L.; da Silva Carneiro, C.; Pierucci, A.P.T.R. Microbial and Physicochemical Properties of Spray Dried Kefir Microcapsules during Storage. LWT 2022, 154, 112710. [Google Scholar] [CrossRef]
- Nale, Z.; Tontul, I.; Aşçi Arslan, A.; Sahin Nadeem, H.; Kucukcetin, A. Microbial Viability, Physicochemical and Sensory Properties of Kefir Microcapsules Prepared Using Maltodextrin/Arabic Gum Mixes. Int. J. Dairy Technol. 2018, 71, 61–72. [Google Scholar] [CrossRef]
- Setiyawan, A.I.; Auberta, C.; Nurwantoro, N.; Febrisiantosa, A. Studies in Physical, Chemical and Microbiological Characteristics of Spray Dried Kefir with Skim Milk Filler. In Proceedings of the International Seminar on Livestock Production and Veterinary Technology, Bogor, Indonesia, 6–7 September 2021; p. 14. [Google Scholar]
- Tontul, I.; Ergin, F.; Eroğlu, E.; Küçükçetin, A.; Topuz, A. The Impact of Refractance Window Drying Conditions on the Physical and Microbiological Properties of Kefir Powder. Food Biosci. 2021, 43, 101317. [Google Scholar] [CrossRef]
- Rizqiati, H.; Nurwantoro, N.; Susanti, S.; Prayoga, M.I.Y. The Effects of Dextrin Concentration as Filler on Physical, Chemical, and Microbiology Properties of Powdered Goat Milk Kefir. J. Indones. Trop. Anim. Agric. 2021, 46, 145–153. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists: Official Methods of Analysis of AOAC International, 21st ed.; AOAC: Washington, DC, USA, 2019. [Google Scholar]
- Pugliese, A.; Cabassi, G.; Chiavaro, E.; Paciulli, M.; Carini, E.; Mucchetti, G. Physical Characterization of Whole and Skim Dried Milk Powders. J. Food Sci. Technol. 2017, 54, 3433–3442. [Google Scholar] [CrossRef] [PubMed]
- Padma, M.; Rao, P.V.K.J.; Edukondalu, L.; Aparna, K.; Babu, G.R. The Effects of Spray Drying Conditions on Water Absorption Index, Water Solubility Index, Solubility and Water Activity (Aw) of Rice Milk Powder. Int. J. Environ. Clim. Chang. 2022, 9, 517–529. [Google Scholar] [CrossRef]
- Skanderby, M.; Westergaard, V.; Partridge, A.; Muir, D.D. Dried Milk Products. In Dairy Powders and Concentrated Products; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009; pp. 180–234. ISBN 978-1-4443-2272-9. [Google Scholar]
- Exarhopoulos, S.; Goulas, A.; Dimitreli, G. Biodegradable Films from Kefiran-Based Cryogel Systems. Macromol 2022, 2, 324–345. [Google Scholar] [CrossRef]
- Tamime, A.Y.; Robinson, R.K. Tamime and Robinson’s Yoghurt, 3rd ed.; Woodhead Publishing Limited and CRC Press: New York, NY, USA, 2007. [Google Scholar]
- Greek Codex Alimentarius. Official Journal of the Hellenic Republic (Article 80A); National Printing Office: Athens, Greece, 2018. [Google Scholar]
- Walstra, P.; Wouters, J.T.M.; Geurts, T.J. Dairy Science and Technology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2005; ISBN 978-0-429-11614-8. [Google Scholar]
- Toniolo, S.P.; Afkhami, S.; D’Agostino, M.R.; Lichty, B.D.; Cranston, E.D.; Xing, Z.; Thompson, M.R. Spray Dried VSV-Vectored Vaccine Is Thermally Stable and Immunologically Active in Vivo. Sci. Rep. 2020, 10, 13349. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Cánovas, G.V.; Ortega-Rivas, E.; Juliano, P.; Yan, H. Drying. Food Powders: Physical Properties, Processing, and Functionality; Barbosa-Cánovas, G.V., Ortega-Rivas, E., Juliano, P., Yan, H., Eds.; Springer: Boston, MA, USA, 2005; pp. 271–304. ISBN 978-0-387-27613-7. [Google Scholar]
- Koç, B.; Sakin-Yılmazer, M.; Kaymak-Ertekin, F.; Balkır, P. Physical Properties of Yoghurt Powder Produced by Spray Drying. J. Food Sci. Technol. 2014, 51, 1377–1383. [Google Scholar] [CrossRef] [PubMed]
- Roos, Y.H. Water Activity and Physical State Effects on Amorphous Food Stability. J. Food Process. Preserv. 1993, 16, 433–447. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, W. Water Adsorption and Glass Transition of Spray-Dried Soy Sauce Powders Using Maltodextrins as Carrier. Food Bioprocess Technol. 2013, 6, 2791–2799. [Google Scholar] [CrossRef]
- Chen, X.; Mujumdar, A. Drying Technologies in Food Processing; Blackwell: Chennai, India, 2008. [Google Scholar]
- Codex Alimentarius. Standard for Fermented Milks CXS 243-2003. Adopted in 2003. Revised in 2008, 2010, 2018. Amended in 2022. World Health Organization, Food and Agriculture Organization: Rome, Italy, 2008. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B243-2003%252FCXS_243e.pdf (accessed on 3 October 2023).
- Alamilla-Beltrán, L.; Chanona-Pérez, J.J.; Jiménez-Aparicio, A.R.; Gutiérrez-López, G.F. Description of Morphological Changes of Particles along Spray Drying. J. Food Eng. 2005, 67, 179–184. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Exarhopoulos, S.; Karipoglou, D.; Groztidou, O.; Georgiou, D.; Kalogianni, E.P.; Goulas, A.; Dimitreli, G. Effect of Drying Aids on the Quality Properties of Kefir Powder. Dairy 2025, 6, 9. https://doi.org/10.3390/dairy6010009
Exarhopoulos S, Karipoglou D, Groztidou O, Georgiou D, Kalogianni EP, Goulas A, Dimitreli G. Effect of Drying Aids on the Quality Properties of Kefir Powder. Dairy. 2025; 6(1):9. https://doi.org/10.3390/dairy6010009
Chicago/Turabian StyleExarhopoulos, Stylianos, Dimitris Karipoglou, Olga Groztidou, Despoina Georgiou, Eleni P. Kalogianni, Athanasios Goulas, and Georgia Dimitreli. 2025. "Effect of Drying Aids on the Quality Properties of Kefir Powder" Dairy 6, no. 1: 9. https://doi.org/10.3390/dairy6010009
APA StyleExarhopoulos, S., Karipoglou, D., Groztidou, O., Georgiou, D., Kalogianni, E. P., Goulas, A., & Dimitreli, G. (2025). Effect of Drying Aids on the Quality Properties of Kefir Powder. Dairy, 6(1), 9. https://doi.org/10.3390/dairy6010009






