Dual Mobility Hip Arthroplasty: Innovative Technological Advances
Abstract
1. Introduction
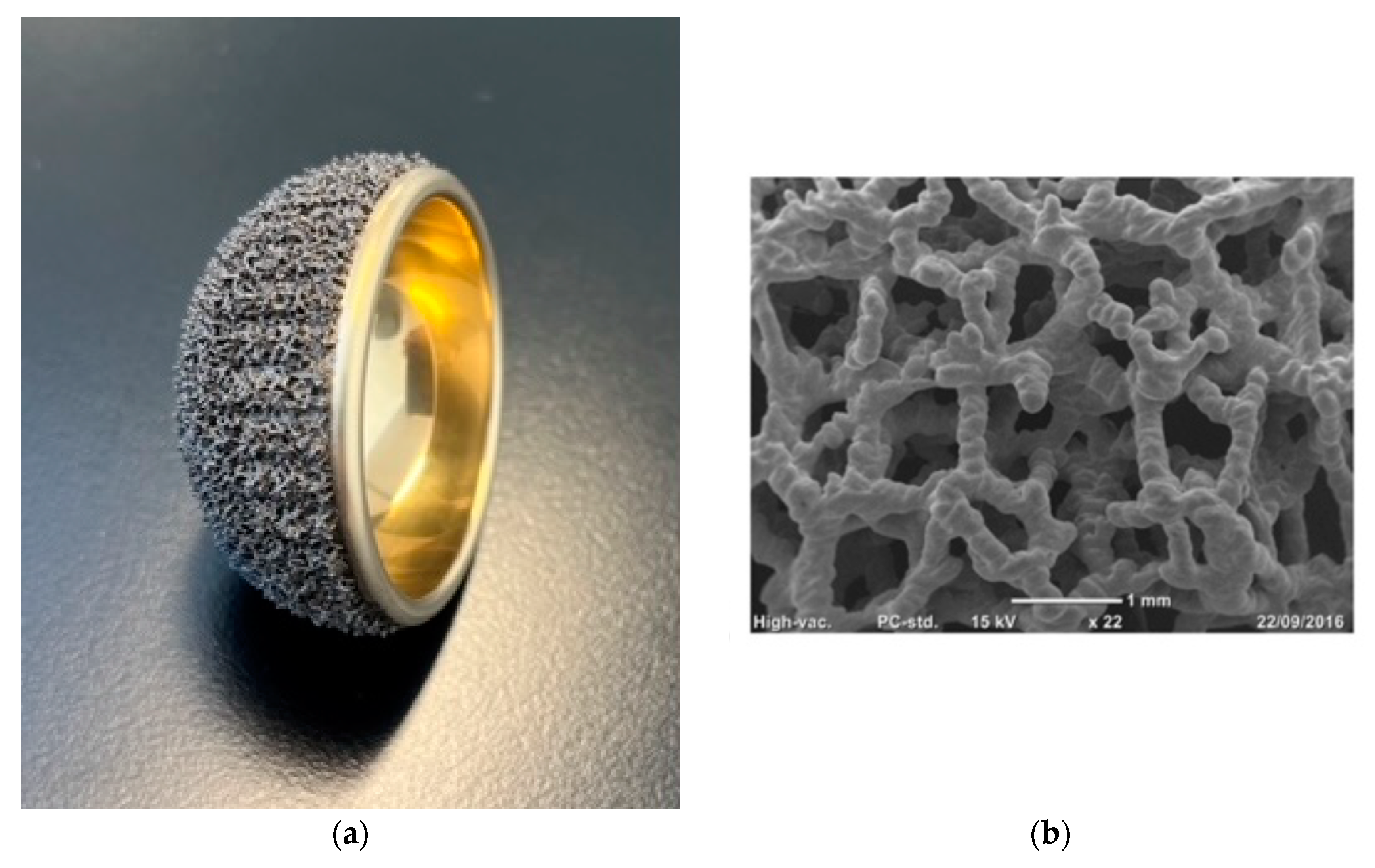
Characteristics and Rationale of the Acorn Traser System
2. Materials and Methods
2.1. Patients and Radiographic Evaluation
2.2. Statistical Analysis
3. Results
3.1. Patient Population
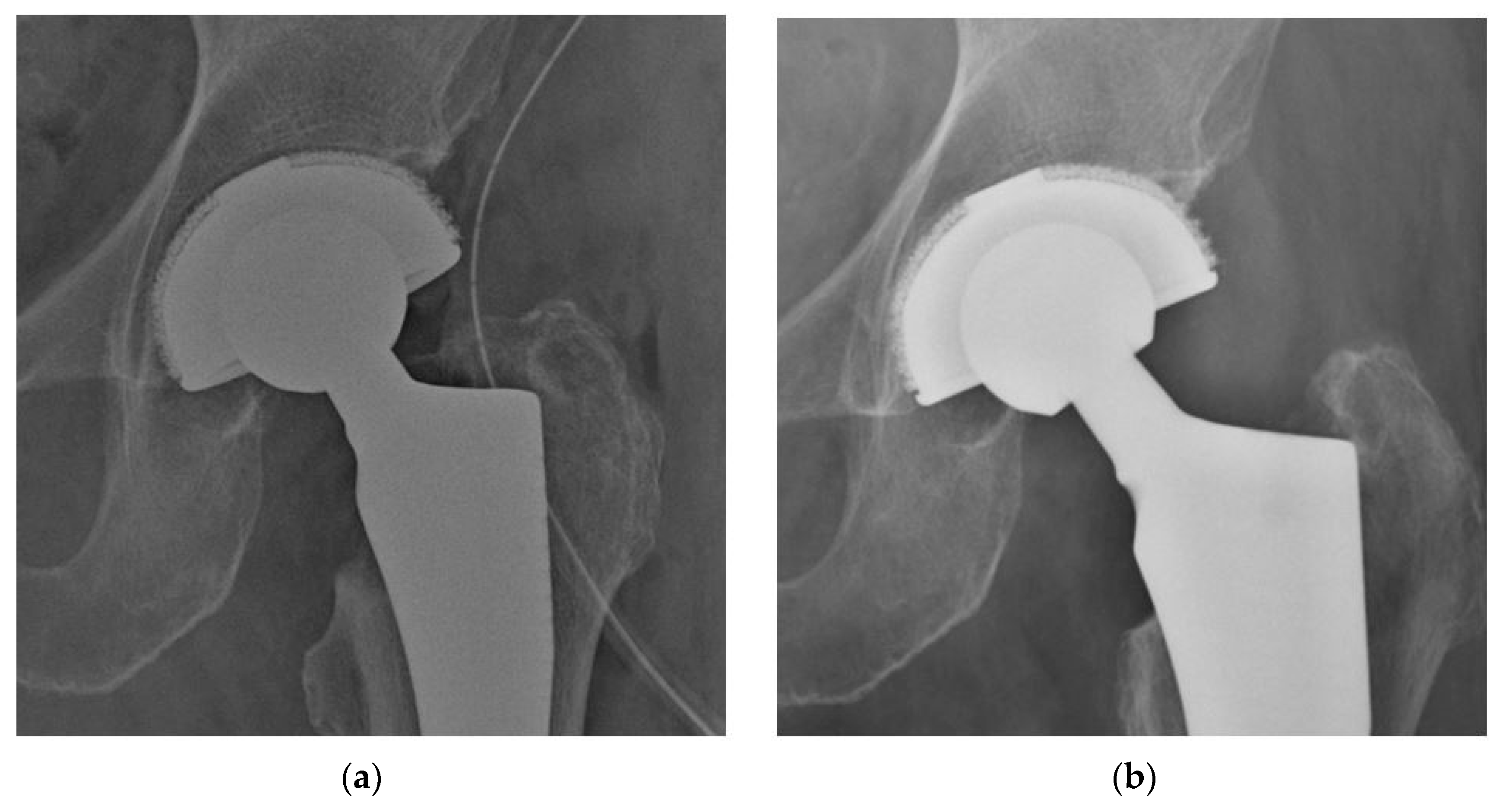
3.2. Radiographic Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Christiansen, T. A New Hip Prosthesis with Trunnion-Bearing. Acta Chir. Scand. 1969, 135, 43–46. [Google Scholar] [PubMed]
- Charnley, J.; Halley, D. Rate of Wear in Total Hip Replacement. Clin. Orthop. Relat. Res. 1975, 112, 170–179. [Google Scholar] [CrossRef]
- McKee, G.K.; Watson-Farrar, J. Replacement of Arthritic Hips by the McKee-Farrar Prosthesis. J. Bone Jt. Surg. Br. 1966, 48, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Eskildsen, S.M.; Olsson, E.C.; Del Gaizo, D.J. Canted Seating of the Stryker Modular Dual Mobility Liner within a Trident Hemispherical Acetabular Shell. Arthroplast. Today 2016, 2, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Massin, P.; Orain, V.; Philippot, R.; Farizon, F.; Fessy, M.H. Fixation Failures of Dual Mobility Cups: A Mid-Term Study of 2601 Hip Replacements. Clin. Orthop. Relat. Res. 2012, 470, 1932–1940. [Google Scholar] [CrossRef]
- Hanawa, T. Biocompatibility of Titanium from the Viewpoint of Its Surface. Sci. Technol. Adv. Mater. 2022, 23, 457–472. [Google Scholar] [CrossRef] [PubMed]
- Hanawa, T. Overview of Metals and Applications. In Metals for Medical Devices; Niinomi, M., Ed.; Woodhead Publishing: Cambridge, UK, 2019; pp. 3–30. [Google Scholar]
- Wu, D.; Bhalekar, R.M.; Marsh, J.S.; Langton, D.J.; Stewart, A.J. Periarticular Metal Hypersensitivity Complications of Hip Bearings Containing Cobalt–Chromium. EFORT Open Rev. 2022, 7, 758–771. [Google Scholar] [CrossRef] [PubMed]
- Bogdanova-Bennett, A.; Sagi, A.; Asopa, V.; Field, R.E.; Sochart, D.H. Nickel Hypersensitivity and Skin Patch Testing in Total Hip Replacement Surgery: A Systematic Review. EFORT Open Rev. 2021, 6, 825–838. [Google Scholar] [CrossRef] [PubMed]
- Tigani, D.; Banci, L.; Stallone, S.; Melucci, G.; Pieratelli, G.; Castiello, E. Evolution and New Generation of Dual Mobility Cups. Orthopedics 2023, 46, e273–e280. [Google Scholar] [CrossRef]
- Matsen Ko, L.J.; Pollag, K.E.; Yoo, J.Y.; Sharkey, P.F. Serum Metal Ion Levels Following Total Hip Arthroplasty With Modular Dual Mobility Components. J. Arthroplasty 2016, 31, 186–189. [Google Scholar] [CrossRef]
- Nam, D.; Salih, R.; Nahhas, C.R.; Barrack, R.L.; Nunley, R.M. Is a Modular Dual Mobility Acetabulum a Viable Option for the Young, Active Total Hip Arthroplasty Patient? Bone Jt. J. 2019, 101, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Marie-Hardy, L.; O’Laughlin, P.; Bonnin, M.; Ait Si Selmi, T. Are Dual Mobility Cups Associated with Increased Metal Ions in the Blood? Clinical Study of Nickel and Chromium Levels with 29 Months’ Follow-Up. Orthop. Traumatol. Surg. Res. 2018, 104, 1179–1182. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, A.; Nocon, A.; De Martino, I.; Mayman, D.J.; Sculco, T.P.; Sculco, P.K. Serum Metal Ions in Contemporary Monoblock and Modular Dual Mobility Articulations. Arthroplast. Today 2021, 12, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.; Skipor, A.; Patterson, L.; Hallab, N.; Paprosky, W.; Black, J.; Galante, J. Metal Release in Patients Who Have Had a Primary Total Hip Arthroplasty. A Prospective, Controlled, Longitudinal Study. J. Bone Jt. Surg. 1998, 80, 1447–1458. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, B.P.; Dubin, J.; Westrich, G.H. Modular Dual-Mobility Liner Malseating: A Radiographic Analysis. Arthroplast. Today 2020, 6, 699–703. [Google Scholar] [CrossRef] [PubMed]
- Solarino, G.; Simone, F.; Panella, A.; Carlet, A.; Riefoli, F.; Moretti, B. Preliminary Results of Total Hip Arthroplasty in Subjects at Risk for Dislocation Using a Novel Modular Cementless Dual-Mobility Cup. A Single-Center Prospective Study. Prosthesis 2021, 3, 53–60. [Google Scholar] [CrossRef]
- Moghnie, A.; Tigani, D.; Consoli, A.; Castiello, E.; Ganci, M.; Amendola, L. Modular Dual Mobility Articulation in Primary and Revision Hip Arthroplasty: Lights and Shadows. J. Orthop. Surg. Res. 2023, 18, 278. [Google Scholar] [CrossRef]
- Niki, Y.; Matsumoto, H.; Otani, T.; Yatabe, T.; Kondo, M.; Yoshimine, F.; Toyama, Y. Screening for Symptomatic Metal Sensitivity: A Prospective Study of 92 Patients Undergoing Total Knee Arthroplasty. Biomaterials 2005, 26, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Dall’Ava, L.; Hothi, H.; Henckel, J.; Di Laura, A.; Tirabosco, R.; Eskelinen, A.; Skinner, J.; Hart, A. Osseointegration of Retrieved 3D-Printed, off-the-Shelf Acetabular Implants. Bone Jt. Res. 2021, 10, 388–400. [Google Scholar] [CrossRef]
- Ponader, S.; von Wilmowsky, C.; Widenmayer, M.; Lutz, R.; Heinl, P.; Körner, C.; Singer, R.F.; Nkenke, E.; Neukam, F.W.; Schlegel, K.A. In Vivo Performance of Selective Electron Beam-melted Ti-6Al-4V Structures. J. Biomed. Mater. Res. Part A 2010, 92, 56–62. [Google Scholar] [CrossRef]
- Taniguchi, N.; Fujibayashi, S.; Takemoto, M.; Sasaki, K.; Otsuki, B.; Nakamura, T.; Matsushita, T.; Kokubo, T.; Matsuda, S. Effect of Pore Size on Bone Ingrowth into Porous Titanium Implants Fabricated by Additive Manufacturing: An in Vivo Experiment. Mater. Sci. Eng. C 2016, 59, 690–701. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Issa, K.; Kapadia, B.; Pivec, R.; Khanuja, H.; Mont, M. Highly-Porous Metal Option for Primary Cementless Acetabular Fixation. What Is the Evidence? Hip Int. 2013, 23, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Massari, L.; Bistolfi, A.; Grillo, P.; Borré, A.; Gigliofiorito, G.; Pari, C.; Francescotto, A.; Tosco, P.; Deledda, D.; Ravera, L.; et al. Periacetabular Bone Densitometry after Total Hip Arthroplasty with Highly Porous Titanium Cups: A 2-Year Follow-up Prospective Study. Hip Int. 2017, 27, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Castagnini, F.; Bordini, B.; Stea, S.; Calderoni, P.; Masetti, C.; Busanelli, L. Highly Porous Titanium Cup in Cementless Total Hip Arthroplasty: Registry Results at Eight Years. Int. Orthop. 2019, 43, 1815–1821. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.S.; McAuley, J.P.; Young, A.M.; Engh, C.A. Radiographic Signs of Osseointegration in Porous-Coated Acetabular Components. Clin. Orthop. Relat. Res. 2006, 444, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Nunn, D.; Freeman, M.A.R.; Hill, P.F.; Evans, S.J.W. The Measurement of Migration of the Acetabular Component of Hip Prostheses. J. Bone Jt. Surg. Br. 1989, 71, 629–631. [Google Scholar] [CrossRef] [PubMed]
- Cordero-ampuero, J.; Garcia-Cimbrelo, E.; Munuera, L. Fixation of Cementless Acetabular Cups. A Radiographic 4-8-Year Study of 102 Porous-Coated Components. Acta Orthop. Scand. 1994, 65, 263–266. [Google Scholar] [CrossRef]
- Hodgkinson, J.P.; Shelley, P.; Wroblewski, B.M. The Correlation between the Roentgenographic Appearance and Operative Findings at the Bone-Cement Junction of the Socket in Charnley Low Friction Arthroplasties. Clin. Orthop. Relat. Res. 1988, 228, 105–109. [Google Scholar] [CrossRef]
- Hartmann, A.; Hannemann, F.; Lützner, J.; Seidler, A.; Drexler, H.; Günther, K.P.; Schmitt, J. Metal Ion Concentrations in Body Fluids after Implantation of Hip Replacements with Metal-on-Metal Bearing-Systematic Review of Clinical and Epidemiological Studies. PLoS ONE 2013, 8, e70359. [Google Scholar] [CrossRef]
- Van Der Merwe, J.M. Metal Hypersensitivity in Joint Arthroplasty. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 2021, 5, e20. [Google Scholar] [CrossRef]
- Mesinkovska, N.A.; Tellez, A.; Molina, L.; Honari, G.; Sood, A.; Barsoum, W.; Taylor, J.S. The Effect of Patch Testing on Surgical Practices and Outcomes in Orthopedic Patients with Metal Implants. Arch. Dermatol. 2012, 148, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Skjöldebrand, C.; Tipper, J.L.; Hatto, P.; Bryant, M.; Hall, R.M.; Persson, C. Current Status and Future Potential of Wear-Resistant Coatings and Articulating Surfaces for Hip and Knee Implants. Mater. Today Bio 2022, 15, 100270. [Google Scholar] [CrossRef] [PubMed]
- Ragone, V.; Canciani, E.; Arosio, M.; Olimpo, M.; Piras, L.A.; von Degerfeld, M.M.; Augusti, D.; D’Ambrosi, R.; Dellavia, C. In Vivo Osseointegration of a Randomized Trabecular Titanium Structure Obtained by an Additive Manufacturing Technique. J. Mater. Sci. Mater. Med. 2020, 31, 1–11. [Google Scholar] [CrossRef] [PubMed]

| Patients Baseline Variables | |
|---|---|
| Patients (Male/Female) | 50 (21/29) |
| Hips (Left/Right) | 50 (24/26) |
| Mean Age at THA 1 (years) | 75 ± 9.6 (40–86) |
| BMI * (Kg/m2) | 22.8 ± 4.1 (18–29) |
| Cause of THA (trauma/degenerative) | 27/23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tigani, D.; Solito, L.; Stallone, S.; Leonida, C.M.; Dieterich, T.; Taverniti, F.; Banci, L.; Melucci, G. Dual Mobility Hip Arthroplasty: Innovative Technological Advances. Prosthesis 2024, 6, 393-400. https://doi.org/10.3390/prosthesis6020029
Tigani D, Solito L, Stallone S, Leonida CM, Dieterich T, Taverniti F, Banci L, Melucci G. Dual Mobility Hip Arthroplasty: Innovative Technological Advances. Prosthesis. 2024; 6(2):393-400. https://doi.org/10.3390/prosthesis6020029
Chicago/Turabian StyleTigani, Domenico, Ludovica Solito, Stefano Stallone, Corrado Maria Leonida, Tommaso Dieterich, Francesco Taverniti, Lorenzo Banci, and Giuseppe Melucci. 2024. "Dual Mobility Hip Arthroplasty: Innovative Technological Advances" Prosthesis 6, no. 2: 393-400. https://doi.org/10.3390/prosthesis6020029
APA StyleTigani, D., Solito, L., Stallone, S., Leonida, C. M., Dieterich, T., Taverniti, F., Banci, L., & Melucci, G. (2024). Dual Mobility Hip Arthroplasty: Innovative Technological Advances. Prosthesis, 6(2), 393-400. https://doi.org/10.3390/prosthesis6020029








