MES-FES Interface Enhances Quadriceps Muscle Response in Sitting Position in Incomplete Spinal Cord Injury: Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Report
2.2. Assessments
2.2.1. Neuromuscular Assessment—Surface Electromyography (EMG)
2.2.2. Strength Assessment
2.2.3. Range of Motion Assessment
2.2.4. Spasticity Assessment
2.2.5. Quality-of-Life Assessment
2.2.6. Assessment of Current Motivation
2.3. Interventions
MES-FES Interface
2.4. Feasibility of the Intervention
- Intervention duration;
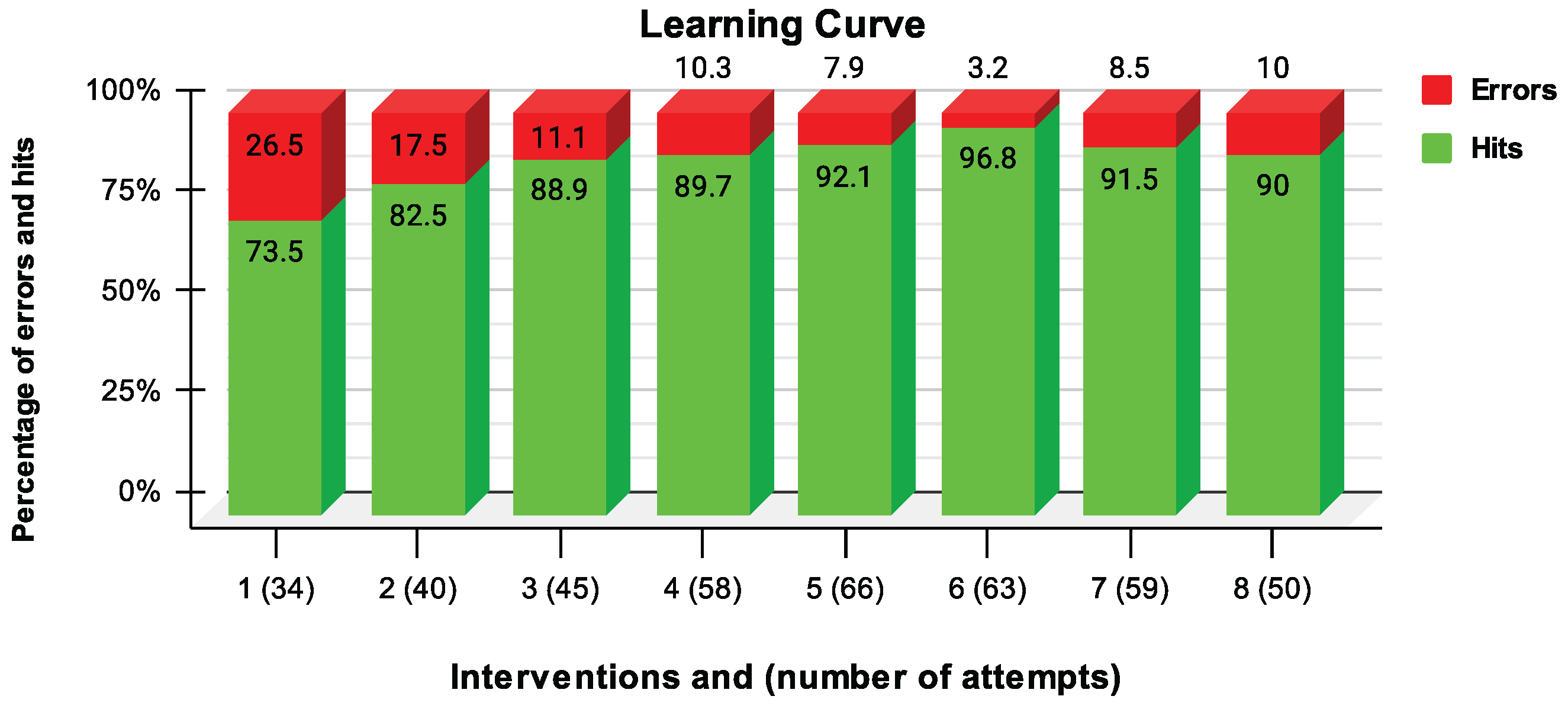
- Learning curve of the MES-FES interface by the participant: Number of hits and errors as a percentage of FES activation.
2.5. Data Analysis
3. Results and Discussion
3.1. Neuromuscular Assessment
3.2. Strength Assessment
3.3. Assessment of Range of Motion
3.4. Assessment of Spasticity
3.5. Quality-of-Life Assessment
3.6. Current Motivation Assessment
3.7. Interface MES-FES
3.8. Intervention Viability
3.8.1. Duration of MES-FES Interface Protocol Steps
3.8.2. Learning Curve of the MES-FES Interface by User
3.9. Study Limitations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SCI | Spinal cord injury |
| BCI | Brain–computer interface |
| EEG | Electroencephalography |
| MES | Myoelectric signal |
| NES | Neuroelectrical signal |
| FES | Functional electrical stimulation |
| EMG | Electromyography |
| RMS | Root mean squared |
| MDF | Median frequency |
| MAS | Modified Ashworth Scale |
| ERD | Event-related desynchronization |
| ERS | Event-related synchronization |
| MF | Magnetic field |
| SMF | Static magnetic field |
| MI | Motor imagery |
| CSP | Common spatial pattern |
| LDA | Linear discriminant analysis |
| VRPN | Virtual reality peripheral network |
| ASIA | American Spinal Cord Injury Association |
| AIS | ASIA Impairment Scale |
| ROM | Range of motion |
| BB | Biceps brachii |
References
- Willis, M.D.; Robertson, N.P. Technology-assisted recovery of walking after spinal cord injury. J. Neurol. 2019, 266, 1046–1048. [Google Scholar] [PubMed]
- Cerezetti, C.R.N.; Nunes, G.R.; Cordeiro, D.R.C.L.; Tedesco, S. Lesão medular traumática e estratégias de enfrentamento: Revisão crítica. O Mundo Saúde 2012, 36, 318–326. [Google Scholar] [CrossRef]
- Atkins, K.D.; Bickel, C.S. Effects of functional electrical stimulation on muscle health after spinal cord injury. Curr. Opin. Pharmacol. 2021, 60, 226–231. [Google Scholar] [CrossRef] [PubMed]
- de Pinho Borella, M.; Sacchelli, T. Os efeitos da prática de atividades motoras sobre a neuroplasticidade. Rev. Neurociênc. 2009, 17, 161–169. [Google Scholar] [CrossRef][Green Version]
- Johnston, M.V. Plasticity in the developing brain: Implications for rehabilitation. Dev. Disabil. Res. Rev. 2009, 15, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Balbinot, G.; Li, G.; Wiest, M.J.; Pakosh, M.; Furlan, J.C.; Kalsi-Ryan, S.; Zariffa, J. Properties of the surface electromyogram following traumatic spinal cord injury: A scoping review. J. Neuroeng. Rehabil. 2021, 18, 105. [Google Scholar] [CrossRef] [PubMed]
- Krueger, E.; Magri, L.; Botelho, A.; Bach, F.; Rebellato, C.; Fracaro, L.; Fragoso, F.; Villanova, J., Jr.; Brofman, P.; Popović-Maneski, L. Effects of low-intensity electrical stimulation and adipose derived stem cells transplantation on the time-domain analysis-based electromyographic signals in dogs with SCI. Neurosci. Lett. 2019, 696, 38–45. [Google Scholar] [CrossRef]
- Fukuda, T.Y.; Echeimberg, J.O.; Pompeu, J.E.; Lucareli, P.R.G.; Garbelotti, S.; Gimenes, R.O.; Apolinário, A. Root mean square value of the electromyographic signal in the isometric torque of the quadriceps, hamstrings and brachial biceps muscles in female subjects. J. Appl. Res. 2010, 10, 32–39. [Google Scholar]
- Feniman, S.P.; Cardoso, J.R.; Villegas, I.L.P.; Faganello, L.; Bela, D.; Santos, S.M.S.; Lavado, E.L. Desenvolvimento e validação de um questionário de qualidade de vida em indivíduos com lesão da medula espinal. CEP 2016, 86038, 440. [Google Scholar]
- Likert, R. A technique for the measurement of attitudes. Arch. Psychol. 1932, 22 140, 55. [Google Scholar]
- Solnik, S.; Rider, P.; Steinweg, K. Teager–Kaiser energy operator signal conditioning improves EMG onset detection. Eur. J. Appl. Physiol. 2010, 110, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Nogueira-Neto, G.; Scheeren, E.; Krueger, E.; Nohama, P.; Button, V.L.S.N. The Influence of Window Length Analysis on the Time and Frequency Domain of Mechanomyographic and Electromyographic Signals of Submaximal Fatiguing Contractions. Open J. Biophys. 2013, 3, 178–190. [Google Scholar] [CrossRef]
- IEC 60601-2-10:2012; Medical Electrical Equipment—Part 2-10: Particular Requirements for the Basic Safety and Essential Performance of nerve and Muscle Stimulators. International Electrotechnical Commission: Geneva, Switzerland, 2012.
- Krueger, E.; Scheeren, E.; Nogueira-Neto, G.; Nohama, P. Mechanomyography energy decreases during muscular fatigue in paraplegics. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society—EMBC 2014, Chicago, IL, USA, 26–30 August 2014; Volume 2014, pp. 5824–5827. [Google Scholar] [CrossRef]
- Rabischong, E. Surface action potentials related to torque output in paraplegics’ electrically stimulated quadriceps muscle. Med. Eng. Phys. 1996, 18, 538–547. [Google Scholar] [CrossRef]
- Schiefer, M.A.; Triolo, R.J.; Tyler, D.J. A model of selective activation of the femoral nerve with a flat interface nerve electrode for a lower extremity neuroprosthesis. IEEE Trans. Neural Syst. Rehabil. Eng. 2008, 16, 195–204. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, X.; Tang, X.; Chen, X.; Gao, X. Examining and monitoring paretic muscle changes during stroke rehabilitation using surface electromyography: A pilot study. Math. Biosci. Eng. 2020, 17, 216–234. [Google Scholar] [CrossRef]
- Bélanger, M.; Stein, R.B.; Wheeler, G.D.; Gordon, T.; Leduc, B. Electrical stimulation: Can it increase muscle strength and reverse osteopenia in spinal cord injured individuals? Arch. Phys. Med. Rehabil. 2000, 81, 1090–1098. [Google Scholar] [CrossRef]
- Granat, M.; Ferguson, A.; Andrews, B.; Delargy, M. The role of functional electrical stimulation in the rehabilitation of patients with incomplete spinal cord injury-observed benefits during gait studies. Spinal Cord 1993, 31, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Karamian, B.A.; Siegel, N.; Nourie, B.; Serruya, M.D.; Heary, R.F.; Harrop, J.S.; Vaccaro, A.R. The role of electrical stimulation for rehabilitation and regeneration after spinal cord injury. J. Orthop. Traumatol. 2022, 23, 2. [Google Scholar] [CrossRef]
- Bekhet, A.H.; Bochkezanian, V.; Saab, I.M.; Gorgey, A.S. The effects of electrical stimulation parameters in managing spasticity after spinal cord injury: A systematic review. Am. J. Phys. Med. Rehabil. 2019, 98, 484–499. [Google Scholar] [CrossRef]
- Martino Cinnera, A.; Bonnì, S.; Pellicciari, M.C.; Giorgi, F.; Caltagirone, C.; Koch, G. Health-related quality of life (HRQoL) after stroke: Positive relationship between lower extremity and balance recovery. Top. Stroke Rehabil. 2020, 27, 534–540. [Google Scholar] [CrossRef]
- Meadows, C.C.; Gable, P.A.; Lohse, K.R.; Miller, M.W. Motivation and motor cortical activity can independently affect motor performance. Neuroscience 2016, 339, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Fumagali, G.L.C.; Krueger, E. Efeito modulador da interface EMG-FES na abertura de mão em indivíduos após AVC. Rev. Neurociênc. 2021, 29, 1–23. [Google Scholar] [CrossRef]
- Zhuang, M.; Wu, Q.; Wan, F.; Hu, Y. State-of-the-art non-invasive brain–computer interface for neural rehabilitation: A review. J. Neurorestoratology 2020, 8, 12–25. [Google Scholar] [CrossRef]
- Marquez-Chin, C.; Marquis, A.; Popovic, M.R. EEG-triggered functional electrical stimulation therapy for restoring upper limb function in chronic stroke with severe hemiplegia. Case Rep. Neurol. Med. 2016, 2016, 9146213. [Google Scholar] [CrossRef]
- Dutta, A.; Kobetic, R.; Triolo, R.J. Ambulation after incomplete spinal cord injury with EMG-triggered functional electrical stimulation. IEEE Trans. Biomed. Eng. 2008, 55, 791–794. [Google Scholar] [CrossRef]



| Descriptor | RF (%) | RF (%) | VL (%) | VL (%) |
|---|---|---|---|---|
| Pre | Post8 | Pre | Post8 | |
| EMGRMS | 6.8 | 8.8 | 8.2 | 11.5 |
| EMGMDF | 146.6 | 123.8 | 108.5 | 102.6 |
| Assessment | Pre | Post8 |
|---|---|---|
| Quadriceps femoris strength | 11.5 kgf | 12.9 kgf |
| Knee extension ROM | 57.7° | 68.3° |
| Spasticity of knee flexors | 2 | 1+ |
| Domain | Pre | Post8 |
|---|---|---|
| General Health Status (28–140) | 45 | 38 |
| Social Relationship (11–55) | 19 | 18 |
| Functional Independence (14–70) | 17 | 16 |
| Accessibility (4–20) | 4 | 4 |
| Emotional Aspects (17–85) | 28 | 25 |
| Total (74–370) | 113 | 101 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, D.B.; Sartori, L.G.; Méndez, M.V.G.; Souza, R.B.d.; Campos, D.P.; Júnior, P.B.; Junior, J.J.A.M.; Krueger, E. MES-FES Interface Enhances Quadriceps Muscle Response in Sitting Position in Incomplete Spinal Cord Injury: Pilot Study. Prosthesis 2024, 6, 643-656. https://doi.org/10.3390/prosthesis6030045
Ribeiro DB, Sartori LG, Méndez MVG, Souza RBd, Campos DP, Júnior PB, Junior JJAM, Krueger E. MES-FES Interface Enhances Quadriceps Muscle Response in Sitting Position in Incomplete Spinal Cord Injury: Pilot Study. Prosthesis. 2024; 6(3):643-656. https://doi.org/10.3390/prosthesis6030045
Chicago/Turabian StyleRibeiro, Denise Bolonhezi, Larissa Gomes Sartori, María Verónica González Méndez, Roger Burgo de Souza, Daniel Prado Campos, Paulo Broniera Júnior, José J. A. Mendes Junior, and Eddy Krueger. 2024. "MES-FES Interface Enhances Quadriceps Muscle Response in Sitting Position in Incomplete Spinal Cord Injury: Pilot Study" Prosthesis 6, no. 3: 643-656. https://doi.org/10.3390/prosthesis6030045
APA StyleRibeiro, D. B., Sartori, L. G., Méndez, M. V. G., Souza, R. B. d., Campos, D. P., Júnior, P. B., Junior, J. J. A. M., & Krueger, E. (2024). MES-FES Interface Enhances Quadriceps Muscle Response in Sitting Position in Incomplete Spinal Cord Injury: Pilot Study. Prosthesis, 6(3), 643-656. https://doi.org/10.3390/prosthesis6030045





