The Outcomes for Different Biological Heart Valve Prostheses in Surgical Aortic Valve Replacement before and after the Introduction of Transcatheter Aortic Valve Implantation
Abstract
1. Introduction
- (1)
- What is the change in the referral pattern for isolated SAVR (i-SAVR) and SAVR with additional procedures (c-SAVR) over time?
- (2)
- What are the changes in the complexity of the surgical procedures for c-SAVR over time?
- (3)
- What are the early and long-term outcomes for both surgical groups?
- (4)
- Is there a difference in the outcomes between the different types of BHV prostheses that were used in the current population?
2. Materials and Methods
3. Results
3.1. Temporal Trends in the Referral, Preoperative, and Operative Parameters
3.2. Temporal Trends in Short-Term Postoperative Outcomes, Need for Resources, and Long-Term Survival
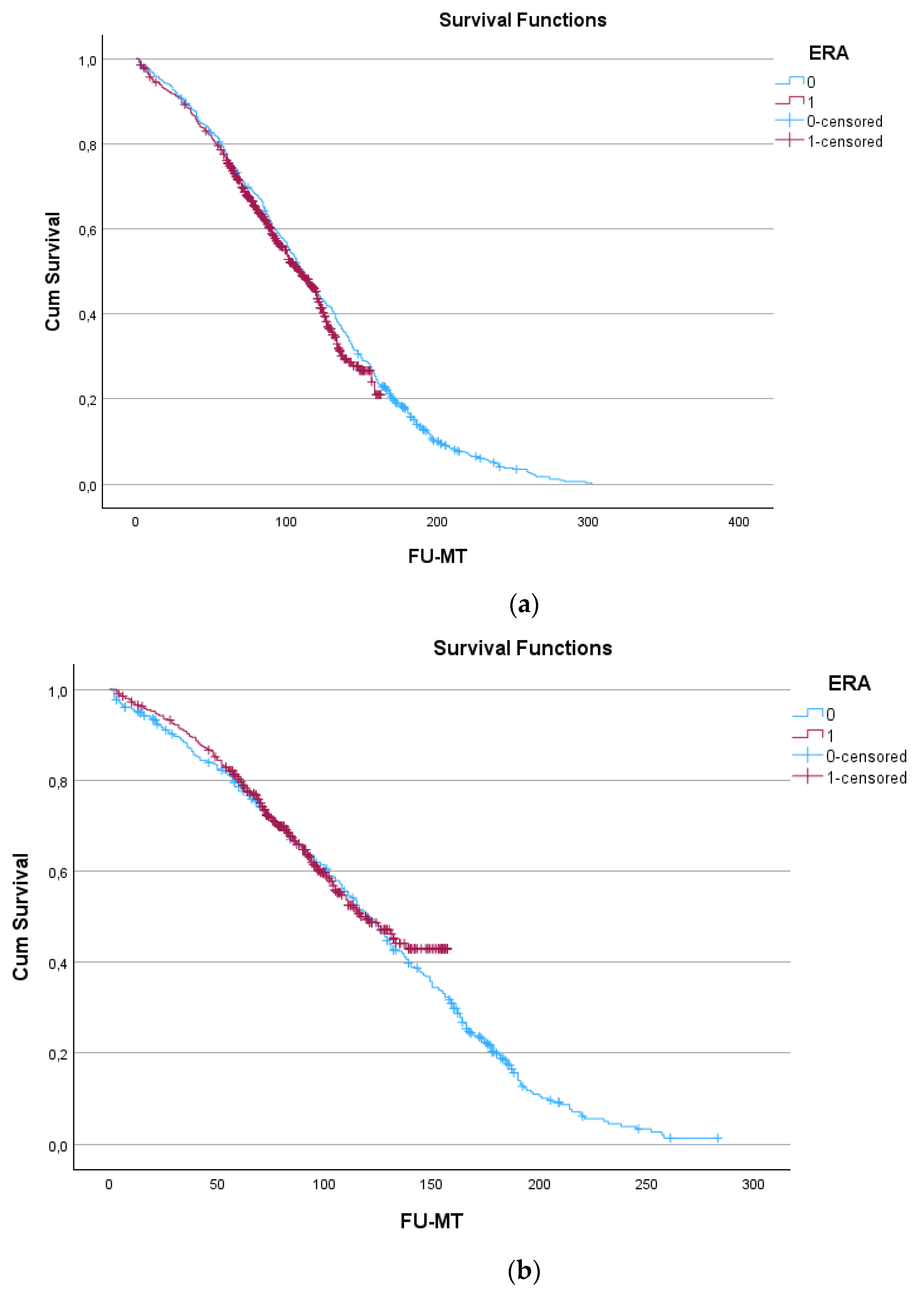
4. Discussion
4.1. Numbers of Referrals for Surgery
4.2. Age and Comorbid Conditions
4.3. Complexity of the Procedure and the Use of Biological Valve Prostheses
4.4. Short-Term Mortality, Adverse Events, and the Need for Resources
4.5. Long-Term Survival and Long-Term Events
4.6. The Carpentier-Edwards Valve and Other Potential Alternatives
5. Future Prospects
6. Conclusions
7. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eveborn, G.W.; Schirmer, H.; Heggelund, G.; Lunde, P.; Rasmussen, K. The evolving epidemiology of valvular aortic stenosis. The Tromsø Study. Heart 2013, 99, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.Y.; Chow, V.; Brieger, D.; Yan, T.D.; Kritharides, L.; Ng, A.C. Outcomes of 16,436 patients requiring isolated aortic valve surgery: A statewide cohort study. Int. J. Cardiol. 2021, 326, 55–61. [Google Scholar] [CrossRef]
- Amanullah, M.R.; Pio, S.M.; Ng, A.C.; Sin, K.Y.; Marsan, N.A.; Ding, Z.P.; Leon, M.B.; Généreux, P.; Delgado, V.; Ewe, S.H.; et al. Prognostic Implications of Associated Cardiac Abnormalities Detected on Echocardiography in Patients With Moderate Aortic Stenosis. JACC Cardiovasc. Imaging 2021, 14, 1724–1737. [Google Scholar] [CrossRef] [PubMed]
- Généreux, P.; Pibarot, P.; Redfors, B.; Mack, M.J.; Makkar, R.R.; A Jaber, W.; Svensson, L.G.; Kapadia, S.; Tuzcu, E.M.; Thourani, V.H.; et al. Staging classification of aortic stenosis based on the extent of cardiac damage. Eur. Heart J. 2017, 38, 3351–3358. [Google Scholar] [CrossRef]
- Strange, G.; Stewart, S.; Celermajer, D.; Prior, D.; Scalia, G.M.; Marwick, T.; Ilton, M.; Joseph, M.; Codde, J.; Playford, D.; et al. National Echocardiography Database of Australia contributing sites. Poor Long-Term Survival in Patients With Moderate Aortic Stenosis. J. Am. Coll. Cardiol. 2019, 74, 1851–1863. [Google Scholar] [CrossRef]
- Généreux, P.; Pibarot, P.; Redfors, B.; Bax, J.J.; Zhao, Y.; Makkar, R.R.; Kapadia, S.; Thourani, V.H.; Mack, M.J.; Nazif, T.M.; et al. Evolution and Prognostic Impact of Cardiac Damage After Aortic Valve Replacement. J. Am. Coll. Cardiol. 2022, 80, 783–800. [Google Scholar] [CrossRef] [PubMed]
- Mistiaen, W.; Van Cauwelaert, P.; Muylaert, P.; Wuyts, F.; Harrisson, F.; Bortier, H. Risk factors and survival after aortic valve replacement in octogenarians. J. Heart Valve Dis. 2004, 13, 538–544. [Google Scholar] [PubMed]
- Jiritano, F.; Serraino, G.F.; Sorrentino, S.; Napolitano, D.; Costa, D.; Ielapi, N.; Bracale, U.M.; Mastroroberto, P.; Andreucci, M.; Serra, R. Risk of Bleeding in Elderly Patients Undergoing Transcatheter Aortic Valve Implantation or Surgical Aortic Valve Replacement. Prosthesis 2024, 6, 175–185. [Google Scholar] [CrossRef]
- Mistiaen, W.; Deblier, I.; Dossche, K.; Vanermen, A. Clinical Outcomes after Surgical Aortic Valve Replacement in 681 Octogenarians: A Single-Center Real-World Experience Comparing the Old Patients with the Very Old Patients. Geriatrics 2024, 9, 44. [Google Scholar] [CrossRef]
- Mistiaen, W.P.; Van Cauwelaert, P.; Muylaert, P. One Thousand Carpentier-Edwards Pericardial Valves in the Aortic Position: What has Changed in the Past 20 years, and What are the Effects on Hospital Complications? J. Heart Valve Dis. 2007, 16, 417–420. [Google Scholar]
- Mylotte, D.; Osnabrugge, R.L.; Windecker, S.; Lefèvre, T.; de Jaegere, P.; Jeger, R.; Wenaweser, P.; Maisano, F.; Moat, N.; Søndergaard, L.; et al. Transcatheter Aortic Valve Replacement in Europe Adoption Trends and Factors Influencing Device Utilization. J. Am. Coll. Cardiol. 2013, 62, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Si, S.; Hillis, G.S.; Sanfilippo, F.M.; Smith, J.; Tran, L.; Reid, C.M.; Briffa, T. Surgical aortic valve replacement in Australia, 2002–2015: Temporal changes in clinical practice, patient profiles and outcomes. ANZ J. Surg. 2019, 89, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Neyt, M.; Van Brabandt, H.; Van De Sande, S.; Devriese, S. Transcatheter Aortakunstklep Implantatie (TAVI): Een Health Technology Assessment Actualisatie; Health Technology Assessment (HTA); KCE Reports 163A. D/2011/10.273/46; Federaal Kenniscentrum voor de Gezondheidszorg (KCE): Brussel, Belgium, 2011. [Google Scholar]
- Mistiaen, W.P.; Deblier, I.; Dossche, K.; Vanermen, A. Is it worthwhile to perform surgical aortic valve replacement in elderly patients with symptomatic aortic valve disease and malignancy: A short and long-term study in 2500 patients. Eur. Heart J. 2022, 43 (Suppl. S2), ehac544.1605. [Google Scholar] [CrossRef]
- Masraf, H.; Sef, D.; Chin, S.L.; Hunduma, G.; Trkulja, V.; Miskolczi, S.; Velissaris, T.; Luthra, S. Long-Term Survival among Octogenarians Undergoing Aortic Valve Replacement with or without Simultaneous Coronary Artery Bypass Grafting: A 22-Year Tertiary Single-Center Experience. J. Clin. Med. 2023, 12, 4841. [Google Scholar] [CrossRef] [PubMed]
- De Backer, O.; Luk, N.H.V.; Olsen, N.T.; Olsen, P.S.; Søndergaar, L. Choice of Treatment for Aortic Valve Stenosis in the Era of Transcatheter Aortic Valve Replacement in Eastern Denmark (2005 to 2015). JACC Cardiovasc. Interv. 2016, 9, 1152–1158. [Google Scholar] [CrossRef] [PubMed]
- Brennan, J.M.; Holmes, D.R.; Sherwood, M.W.; Edwards, F.H.; Carroll, J.D.; Grover, F.L.; Tuzcu, E.M.; Thourani, V.; Brindis, R.G.; Shahian, D.M.; et al. The Association of Transcatheter Aortic Valve Replacement Availability and Hospital Aortic Valve Replacement Volume and Mortality in the United States. Ann. Thorac. Surg. 2014, 98, 2016–2022. [Google Scholar] [CrossRef] [PubMed]
- Culler, S.D.; Cohen, D.J.; Brown, P.P.; Kugelmass, A.D.; Reynolds, M.R.; Ambrose, K.; Schlosser, M.L.; Simon, A.W.; Katz, M.R. Trends in Aortic Valve Replacement Procedures Between 2009 and 2015: Has Transcatheter Aortic Valve Replacement Made a Difference? Ann. Thorac. Surg. 2018, 105, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.E.; McAlexander, W.W.; Sasse, M.F.; Leesar, M.A.; Melby, S.J.; Singh, S.P.; Jernigan, L.B.; Booker, O.J.; Alli, O.O. Impact of Transcatheter Aortic Valve Replacement on Surgical Volumes and Outcomes in a Tertiary Academic Cardiac Surgical Practice. J. Am. Coll. Surg. 2016, 222, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.; Dagenais, F.; Voisine, P.; Dumont, E.; Charbonneau, E.; Baillot, R.; Kalavrouziotis, D.; Mohammadi, S. Surgical aortic valve replacement outcomes in the transcatheter era. J. Thorac. Cardiovasc. Surg. 2015, 150, 1582–1588. [Google Scholar] [CrossRef]
- Mullan, C.W.; Mori, M.; Oichert, M.D.; Bin Mahmood, S.U.; Yousef, S.; Geirsson, A. United States national trends in comorbidity and outcomes of adult cardiac surgery patients. J. Cardiac Surg. 2020, 35, 2248–2253. [Google Scholar] [CrossRef]
- Sharma, T.; Krishnan, A.M.; Lahoud, R.; Polomsky, M.; Dauerman, H.L. National Trends in TAVR and SAVR for Patients With Severe Isolated Aortic Stenosis. J. Am. Coll. Cardiol. 2022, 80, 2054–2056. [Google Scholar] [CrossRef] [PubMed]
- Akintoye, E.; Ando, T.; Sandio, A.; Adegbala, O.; Salih, M.; Zubairu, J.; Oseni, A.; Sistla, P.; Alqasrawi, M.; Egbe, A.; et al. Aortic Valve Replacement for Severe Aortic Stenosis Before and During the Era of Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2020, 126, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; O’Brien, S.M.; Wu, C.; Sikora, J.A.H.; Griffith, B.P.; Gammie, J.S. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years: Changes in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database. J. Thorac. Cardiovasc. Surg. 2009, 137, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Guimaron, S.; De Brux, J.; Verhoye, J.; Guihaire, J. Surgical aortic valve replacement in the modern era: Insights from the French Registry EPICARD. J. Card. Surg. 2021, 36, 4573–4581. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-García, R.; Perez-Farinos, N.; De Miguel-Díez, J.; Hernándezbarrera, V.; Méndez-Bailón, M.; Jimenez-Trujillo, I.; De Miguel-Yanes, J.M.; López-De-Andrés, A. National Trends in Utilization and In-Hospital Outcomes of Surgical Aortic Valve Replacements in Spain, 2001–2015. Braz. J. Cardiovasc. Surg. 2020, 35, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Siregar, S.; de Heer, F.; Groenwold, R.H.; Versteegh, M.I.; Bekkers, J.A.; Brinkman, E.S.; Bots, M.L.; van der Graaf, Y.; van Herwerden, L.A. Trends and outcomes of valve surgery: 16-year results of Netherlands Cardiac Surgery National Database. Eur. J. Cardiothorac. Surg. 2014, 46, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Tam, D.Y.; Rocha, R.V.; Wijeysundera, H.C.; Austin, P.C.; Dvir, D.; Fremes, S.E. Surgical valve selection in the era of transcatheter aortic valve replacement in the Society of Thoracic Surgeons Database. J. Thorac. Cardiovasc. Surg. 2019, 159, 416–427.e8. [Google Scholar] [CrossRef] [PubMed]
- Formica, F.; Mariani, S.; D’alessandro, S.; Singh, G.; Di Mauro, M.; Cerrito, M.G.; Messina, L.A.; Scianna, S.; Papesso, F.; Sangalli, F. Does additional coronary artery bypass grafting to aortic valve replacement in elderly patients affect the early and long-term outcome? Heart Vessels 2020, 35, 487–501. [Google Scholar] [CrossRef] [PubMed]
- Czarnecki, A.; Qiu, F.; Koh, M.; Alter, D.A.; Austin, P.C.; Fremes, S.E.; Tu, J.V.; Wijeysundera, H.C.; Yan, A.T.; Ko, D.T. Trends in the incidence and outcomes of patients with aortic stenosis hospitalization. Am. Heart J. 2019, 199, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Mistiaen, W. Referral of Patients for Surgical Aortic Valve Replacement before and after Introduction of the Transcatheter Aortic Valve Implantation-Changing Patterns of Preoperative Characteristics and Volume and Postoperative Outcome. J. Cardiovasc. Dev. Dis. 2023, 22, 223. [Google Scholar] [CrossRef]
- Kundi, H.; Strom, J.B.; Valsdottir, L.R.; Elmariah, S.; Popma, J.J.; Shen, C.; Yeh, R.W. Trends in Isolated Surgical Aortic Valve Replacement According to Hospital-Based Transcatheter Aortic Valve Replacement Volumes. JACC Cardiovasc. Interv. 2018, 11, 2148–2156. [Google Scholar] [CrossRef] [PubMed]
- Khounlaboud, M.; Donal, E.; Auffret, V.; Anselmi, A.; Ingels, A.; Flécher, E.; Verhoye, J.-P.; Daubert, C.; Le Breton, H.; Mabo, P.; et al. Comparison of Preoperative and Postoperative Characteristics in Octogenarians Having Isolated Surgical Aortic Valve Replacement Before Versus After Introduction of Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2015, 116, 933–937. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Bin Mahmood, S.U.; Geirsson, A.; Yun, J.J.; Cleman, M.W.; Forrest, J.K.; Mangi, A.A. Trends in volume and risk profiles of patients undergoing isolated surgical and transcatheter aortic valve replacement. Catheter. Cardiovasc. Interv. 2019, 93, E337–E342. [Google Scholar] [CrossRef] [PubMed]
- Gandjian, M.; Verma, A.; Tran, Z.; Sanaiha, Y.; Downey, P.; Shemin, R.J.; Benharash, P. Influence of center surgical aortic valve volume on outcomes of transcatheter aortic valve replacement. JTCVS Open 2022, 11, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.R.; Chew, D.P.; Horsfall, M.J.; Chuang, A.M.-Y.; Sinhal, A.R.; Joseph, M.X.; A Baker, R.; Bennetts, J.S.; Selvanayagam, J.B.; Lehman, S.J. Multidisciplinary transcatheter aortic valve replacement heart team programme improves mortality in aortic stenosis. Open Heart 2019, 6, e000983. [Google Scholar] [CrossRef] [PubMed]
- Gaede, L.; Blumenstein, J.; Kim, W.-K.; Liebetrau, C.; Dörr, O.; Nef, H.; Hamm, C.; Elsässer, A.; Möllmann, H. Trends in aortic valve replacement in Germany in 2015: Transcatheter versus isolated surgical aortic valve repair. Clin. Res. Cardiol. 2017, 106, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.; Willner, N.; Eltchaninoff, H.; Burwash, I.G.; Michel, M.; Durand, E.; Gilard, M.; Dindorf, C.; Iung, B.; Cribier, A.; et al. Trends in aortic valve replacement for aortic stenosis: A French nationwide study. Eur. Heart J. 2022, 43, 666–679. [Google Scholar] [CrossRef] [PubMed]
- Reinöhl, J.; Kaier, K.; Reinecke, H.; Schmoor, C.; Frankenstein, L.; Vach, W.; Cribier, A.; Beyersdorf, F.; Bode, C.; Zehender, M. Effect of Availability of Transcatheter Aortic-Valve Replacement on Clinical Practice. N. Engl. J. Med. 2015, 373, 2438–2447. [Google Scholar] [CrossRef]
- Attias, D.; Maillet, J.-M.; Copie, X.; Bonnet, N.; Mesnildrey, P.; Benvenuti, C.; Benacerraf, M.; Scheublé, A.; Digne, F.; Stratiev, V.; et al. Prevalence, clinical characteristics and outcomes of high-risk patients treated for severe aortic stenosis prior to and after transcatheter aortic valve implantation availability. Eur. J. Cardiothorac. Surg. 2015, 47, e212. [Google Scholar] [CrossRef][Green Version]
- Englum, B.R.; Ganapathi, A.M.; Schechter, M.A.; Harrison, J.K.; Glower, D.D.; Hughes, G.C. Changes in Risk Profile and Outcomes of Patients Undergoing Surgical Aortic Valve Replacement from the Pre- to Post-Transcatheter Aortic Valve Replacement Eras. Ann. Thorac. Surg. 2015, 101, 110–117. [Google Scholar] [CrossRef][Green Version]
- Heinze, G.; Christ, T.; Leonards, C.O.; Dohmen, P.M.; Konertz, W. Risk and Outcome of Aortic Valve Surgery in the Transcatheter Valve Era: The Gender Aspect. Ann. Thorac. Cardiovasc. Surg. 2015, 21, 446–451. [Google Scholar] [CrossRef][Green Version]
- Wang, T.K.M.; Sathanathan, J.; Ramanathan, T.; Webster, M.; Ruygrok, P. Isolated Aortic Valve Replacement in Octogenarians Before and After the Introduction of Trans-catheter Aortic Valve Implantation. Heart Lung Circ. 2014, 23, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Dimagli, A.; Sinha, S.; Caputo, M.; Angelini, G.D.; Benedetto, U. Trend in morbidity and mortality in surgical aortic valve replacement: A retrospective, observational, single-centre study. Interact. Cardiovasc. Thorac. Surg. 2020, 31, 796–802. [Google Scholar] [CrossRef]
- Chahine, J.; Jedeon, Z.; Fiocchi, J.; Shaffer, A.; Knoper, R.; John, R.; Yannopoulos, D.; Raveendran, G.; Gurevich, S. A retrospective study on the trends in surgical aortic valve replacement outcomes in the post-transcatheter aortic valve replacement era. Health Sci. Rep. 2022, 5, e660. [Google Scholar] [CrossRef]
- Nguyen, T.C.; Terwelp, M.D.; Thourani, V.H.; Zhao, Y.; Ganim, N.; Hoffmann, C.; Justo, M.; Estrera, A.L.; Smalling, R.W.; Balan, P.; et al. Clinical trends in surgical, minimally invasive and transcatheter aortic valve replacement†. Eur. J. Cardiothorac. Surg. 2017, 51, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Silashi, M.; Conrad, L.; Treede, H.; Reiter, B.; Schaefer, U.; Blankenberg, S.; Reichenspurner, H. Trends in Surgical Aortic Valve Replacement in More Than 3000 Consecutive Cases in the Era of Transcatheter Aortic Valve Implantations. Thorac. Cardiovasc. Surg. 2016, 64, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Malaisrie, S.C.; Tuday, E.; Lapin, B.; Wang, E.; Lee, R.; McGee, E.C.; Davidson, C.; McCarthy, P.M. Transcatheter aortic valve implantation decreases the rate of unoperated aortic stenosis. Eur. J. Cardiothorac. Surg. 2011, 40, 43–48. [Google Scholar] [CrossRef][Green Version]
- D’Onofrio, A.; Alfieri, O.R.; Cioni, M.; Alamanni, F.; Fusari, M.; Tarzia, V.; Rizzoli, G.; Gerosa, G. The impact of transcatheter aortic valve implantation on patients’ profiles and outcomes of aortic valve surgery programmes: A multi-institutional appraisal. Interact. Cardiovasc. Thorac. Surg. 2013, 16, 608–611. [Google Scholar] [CrossRef]
- Goel, S.S.; Ige, M.; Tuzcu, E.M.; Ellis, S.G.; Stewart, W.J.; Svensson, L.G.; Lytle, B.W.; Kapadia, S.R. Severe aortic stenosis and coronary artery disease–implications for management in the transcatheter aortic valve replacement era: A comprehensive review. J. Am. Coll. Cardiol. 2013, 62, 1–10. [Google Scholar] [CrossRef]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, J.; Iung, B.; Lancellotti, P.; Lansac, E.; Munoz, D.R.; et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur. Heart J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef]
- Weserling, M.; Hamm, C.W.; Kim, W.K. Percutaneous Coronary Intervention in Transcatheter Aortic Valve Implantation Patients: Overview and Practical Management. Front. Cardiovasc. Med. 2021, 8, 653768. [Google Scholar] [CrossRef]
- Durko, A.P.; Osnabrugge, R.L.; Van Mieghem, N.M.; Milojevic, M.; Mylotte, D.; Nkomo, V.T.; Kappetein, A.P. Annual number of candidates for transcatheter aortic valve implantation per country: Current estimates and future projections. Eur. Heart J. 2018, 39, 2635–2642. [Google Scholar] [CrossRef]
- Maximus, S.; Milliken, J.C.; Danielsen, B.; Shemin, R.; Khan, J.; Carey, J.S. Implementation of transcatheter aortic valve replacement in California: Influence on aortic valve surgery. J. Thorac. Cardiovasc. Surg. 2018, 155, 1447–1456. [Google Scholar] [CrossRef]
- Thourani, V.H.; Suri, R.M.; Gunter, R.L.; Sheng, S.; O’brien, S.M.; Ailawadi, G.; Szeto, W.Y.; Dewey, T.M.; Guyton, R.A.; Bavaria, J.E.; et al. Contemporary Real-World Outcomes of Surgical Aortic Valve Replacement in 141,905 Low-Risk, Intermediate-Risk, and High-Risk Patients. Ann. Thorac. Surg. 2015, 99, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Lacquaniti, A.; Ceresa, F.; Campo, S.; Smeriglio, A.; Trombetta, D.; Patanè, F.; Monardo, P. Surgical Aortic Valve Replacement and Renal Dysfunction: From Acute Kidney Injury to Chronic Disease. J. Clin. Med. 2024, 13, 2933. [Google Scholar] [CrossRef]
- Berretta, P.; Meuris, B.; Kappert, U.; Andreas, M.; Fiore, A.; Solinas, M.; Misfeld, M.; Carrel, T.P.; Villa, E.; Savini, C.; et al. Sutureless Versus Rapid Deployment Aortic Valve Replacement: Results From a Multicenter Registry. Ann. Thorac. Surg. 2022, 114, 758–765. [Google Scholar] [CrossRef]
- Dokollari, A.; Ramlawi, B.; Torregrossa, G.; Sá, M.P.; Sicouri, S.; Prifti, E.; Gelsomino, S.; Bonacchi, M. Benefits and Pitfalls of the Perceval Sutureless Bioprosthesis. Front. Cardiovasc. Med. 2022, 8, 789392. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, S.J.; Salmasi, M.Y.; Zientara, A.; Roussin, I.; Quarto, C.; Asimakopoulos, G. Perceval sutureless bioprosthesis versus Perimount sutured bioprosthesis for aortic valve replacement in patients with aortic stenosis: A retrospective, propensity-matched study. J. Cardiothorac. Surg. 2024, 19, 95. [Google Scholar] [CrossRef]
- Pelce, E.; Porto, A.; Gariboldi, V.; Ben Lagha, A.; Amanatiou, C.; Collart, F.; Theron, A. Five-year outcomes of rapid-deployment aortic valve replacement with the Edwards Intuity valve. J. Card Surg. 2021, 36, 2826–2833. [Google Scholar] [CrossRef] [PubMed]
- Klop, I.D.G.; Kougioumtzoglou, A.M.; Kloppenburg, G.T.L.; van Putte, B.P.; Sprangers, M.A.G.; Klein, P.; Nieuwkerk, P.T. Short-term outcome of the intuity rapid deployment prosthesis: A systematic review and meta-analysis. Interact Cardiovasc. Thorac. Surg. 2020, 31, 427–436. [Google Scholar] [CrossRef]
- Bavaria, J.E.; Griffith, B.; Heimansohn, D.A.; Rozanski, J.; Johnston, D.R.; Bartus, K.; Girardi, L.N.; Beaver, T.; Takayama, H.; Mumtaz, M.A.; et al. COMMENCE Trial Investigators. Five-year Outcomes of the COMMENCE Trial Investigating Aortic Valve Replacement With RESILIA Tissue. Ann. Thorac. Surg. 2023, 115, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Sef, D.; Thet, M.S.; Klokocovnik, T.; Luthra, S. Early and mid-term outcomes after aortic valve replacement using a novel tissue bioprosthesis: A systematic review. Eur. J. Cardiothorac. Surg. 2024, 65, ezae045. [Google Scholar] [CrossRef] [PubMed]
- Lansac, E.; Youssefi, P.; de Heer, F.; Bavaria, J.; De Kerchove, L.; El-Hamamsy, I.; Elkhoury, G.; Enriquez-Sarano, M.; Jondeau, L.d.G.; Kluin, J.; et al. Aortic Valve Repair Research Network Investigators from the Heart Valve Society, Collaborators. Aortic Valve Surgery in Nonelderly Patients: Insights Gained From AVIATOR. Semin. Thorac. Cardiovasc. Surg. 2019, 31, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Persson, M.; Glaser, N.; Nilsson, J.; Friberg, Ö.; Franco-Cereceda, A.; Sartipy, U. Comparison of Long-term Performance of Bioprosthetic Aortic Valves in Sweden From 2003 to 2018. JAMA Netw. Open 2022, 5, e220962. [Google Scholar] [CrossRef] [PubMed]
- Sansone, F.; Dato, G.M.A.; Zingarelli, E.; Ferrero, E.; Prot, S.; Ceresa, F.; Patanè, F.; Casabona, R. Long-term follow-up of stentless prosthesis. J. Cardiol. 2014, 63, 365–372. [Google Scholar] [CrossRef]
- Porterie, J.; Kalavrouziotis, D.; Dumont, E.; Paradis, J.M.; De Larochellière, R.; Rodés-Cabau, J.; Mohammadi, S. Clinical impact of the heart team on the outcomes of surgical aortic valve replacement among octogenarians. J. Thorac. Cardiovasc. Surg. 2023, 165, 1010–1019.e5. [Google Scholar] [CrossRef]

| Factor | c-SAVR Pre (%) | c-SAVR Post (%) | p | i-SAVR Pre (%) | i-SAVR Post (%) | p |
|---|---|---|---|---|---|---|
| Age > 80 | 175/810 (21.6) | 285/803 (35.5) | <0.001 | 85/446 (19.1) | 130/421 (30.9) | <0.001 |
| Prior PCI | 67/810 (8.3) | 143/803 (17.8) | <0.001 | 15/446 (3.4) | 41/421 (9.7) | <0.001 |
| Diabetes | 149/810 (18.4) | 215/803 (26.8) | <0.001 | 65/446 (14.6) | 99/421 (23.5) | <0.001 |
| Chronic kidney disease | 110/805 (13.7) | 167/800 (20.9) | <0.001 | 53/445 (11.9) | 78/421 (18.5) | 0.007 |
| Male gender | 475/810 (58.6) | 539/803 (67.1) | <0.001 | 189/446 (42.4) | 217/421 (51.5) | 0.007 |
| Atrial fibrillation | 168/807 (20.8) | 233/803 (29.0) | <0.001 | 96/445 (21.6) | 106/421 (25.2) | 0.210 |
| LMCA involvement | 105/810 (13.0) | 153/803 (19.1) | <0.001 | 1/446 (0.2) | 1/142 (0.2) | 0.967 |
| Emergent SAVR | 36/810 (4.4) | 63/803 (7.8) | 0.004 | 14/446 (3.1) | 18/420 (4.3) | 0.371 |
| Treated malignancy | 101/807 (12.5) | 140/800 (17.5) | 0.005 | 71/445 (16.0) | 73/420 (17.4) | 0.574 |
| Arterial hypertension | 574/809 (71.0) | 614/800 (76.8) | 0.008 | 294/433 (66.4) | 302/420 (71.9) | 0.078 |
| Endocarditis | 10/808 (1.2) | 24/803 (3.0) | 0.014 | 10/446 (2.2) | 16/421 (3.8) | 0.179 |
| Conduction defects (all types) | 232/803 (28.9) | 277/803 (34.5) | 0.016 | 125/445 (28.1) | 227/421 (33.0) | 0.115 |
| AV block degree 1 or 2 | 44/779 (5.6) | 63/767 (8.2) | 0.047 | 21/432 (4.9) | 27/403 (6.7) | 0.254 |
| Cerebrovascular accident | 76/806 (9.4) | 106/802 (13.2) | 0.017 | 33/446 (7.4) | 38/421 (9.0) | 0.383 |
| Peripheral artery disease | 126/552 (22.8) | 209/779 (26.2) | 0.163 | 26/322 (8.1) | 64/421 (15.2) | 0.003 |
| Factor | c-SAVR Pre (%) | c-SAVR Post (%) | p | i-SAVR Pre (%) | i-SAVR Post (%) | p |
|---|---|---|---|---|---|---|
| CABG | 785/810 (96.9) | 735/803 (91.5) | <0.001 | NA | NA | NA |
| Mitral valve repair | 24/810 (3.0) | 74/803 (9.2) | <0.001 | NA | NA | NA |
| >2 procedures | 68/810 (8.4) | 125/803 (15.6) | <0.001 | NA | NA | NA |
| Other procedures | 30/615 (4.9) | 74/800 (9.3) | 0.002 | NA | NA | NA |
| CPB time > 120 min | 275/607 (45.3) | 469/725 (64.7) | <0.001 | 25/372 (6.7) | 27/369 (7.3) | 0.751 |
| Use of smallest valve size | 25/810 (3.1) | 14/801 (1.7) | 0.080 | 16/446 (3.6) | 8/420 (1.9) | 0.132 |
| Predictor | Odds Ratio | 95%CI | p |
|---|---|---|---|
| Urgent SAVR | 2.96 | 1.77–4.95 | <0.001 |
| Chronic obstructive pulmonary disease | 2.32 | 1.43–3.77 | <0.001 |
| Chronic renal disease | 2.13 | 1.31–3.46 | 0.002 |
| Congestive heart failure | 1.97 | 1.17–3.33 | 0.011 |
| Age > 80 years | 1.82 | 1.13–2.94 | 0.014 |
| Factor | c-SAVR Pre (%) | c-SAVR Post (%) | p | i-SAVR Pre (%) | i-SAVR Post (%) | p |
|---|---|---|---|---|---|---|
| Adverse event | ||||||
| Mortality | 42/809 (5.2) | 57/803 (7.1) | 0.111 | 15/446 (3.4) | 18/420 (4.3) | 0.479 |
| Acute renal injury | 92/809 (11.4) | 250/801 (31.2) | <0.001 | 32/446 (7.0) | 88/420 (21.0) | <0.001 |
| Conduction defect | 103/806 (12.8) | 190/802 (23.7) | <0.001 | 45/446 (10.1) | 77/421 (18.3) | <0.001 |
| Bleeding | 39/806 (4.8) | 80/802 (10.0) | <0.001 | 10/446 (2.2) | 21/421 (5.0) | 0.030 |
| Acute myocardial infarction | 7/809 (0.9) | 14/802 (1.7) | 0.119 | 2/446 (0.4) | 2/421 (0.5) | 0.954 |
| Atrial fibrillation | 317/807 (39.3) | 328/802 (40.9) | 0.508 | 165/446 (37.0) | 66/421 (39.4) | 0.461 |
| Thromboembolism | 31/806 (3.8) | 33/802 (4.1) | 0.783 | 11/446 (2.5) | 10/421 (2.4) | 0.931 |
| Need for resources | ||||||
| Renal replacement therapy | 15/803 (1.9) | 61/801 (7.6) | <0.001 | 5.455 (1.1) | 14/419 (3.3) | 0.026 |
| Permanent PM implantation | 9/805 (1.1) | 32/803 (4.0) | <0.001 | 14/446 (3.1) | 14/421 (3.3) | 0.887 |
| Thrombocyte concentrate | 15/187 (8.0) | 128/791 (16.2) | 0.005 | 6/92 (6.5) | 28/412 (6.8) | 0.924 |
| Reintervention | 18/810 (2.2) | 36/803 (4.5) | 0.012 | 12/446 (2.7) | 15/421 (3.6) | 0.460 |
| Plasma | 49/187 (26.2) | 272/791 (34.4) | 0.032 | 15/91 (16.5) | 81/412 (19.7) | 0.485 |
| >4 units packed cells | 54/187 (28.9) | 199/792 (25.1) | 0.292 | 15/92 (16.3) | 45/413 (10.9) | 0.147 |
| Survival (Years) | c-SAVR Pre | n | c-SAVR Post | n | i-SAVR Pre | n | i-SAVR Post | n |
|---|---|---|---|---|---|---|---|---|
| 1 year | 96.0 ± 0.7% | 739 | 94.5 ± 0.8% | 699 | 95.1 ± 1.0% | 407 | 96.5 ± 0.9% | 385 |
| 5 years | 76.6 ± 1.5% | 593 | 76.1 ± 1.6% | 561 | 78.6 ± 2.0% | 326 | 80.2 ± 2.0% | 302 |
| 10 years | 44.6 ± 1.8% | 340 | 43.6 ± 2.3% | 129 | 43.6 ± 2.3% | 129 | 49.4 ± 3.2% | 74 |
| Early Era | Later Era | |||||
|---|---|---|---|---|---|---|
| Predictor | Odds Ratio | 95%CI | p | Odds Ratio | 95%CI | p |
| Age over 80 years | 1.99 | 1.66–2.38 | <0.001 | 2.75 | 2.28–3.31 | <0.001 |
| COPD | 1.35 | 1.15–1.78 | <0.001 | 1.51 | 1.23–1.86 | <0.001 |
| CKD | 1.53 | 1.42–1.89 | <0.001 | 1.52 | 1.23–1.89 | <0.001 |
| Diabetes mellitus | - | - | - | 1.67 | 1.37–2.05 | <0.001 |
| Atrial fibrillation | - | - | - | 1.56 | 1.28–1.91 | <0.001 |
| AV block 1 or 2 | - | - | - | 1.62 | 1.18–2.22 | 0.003 |
| Malignancy | 1.30 | 1.06–1.60 | 0.013 | 1.40 | 1.11–1.75 | 0.004 |
| Peripheral art disease | 1.23 | 1.05–1.53 | 0.010 | 1.32 | 1.08–1.62 | 0.006 |
| CPB time > 120 min | 1.27 | 1.07–1.57 | 0.007 | 1.27 | 1.06–1.53 | 0.012 |
| Urgent/emergent SAVR | 1.27 | 1.03–1.57 | 0.007 | 1.54 | 1.07–2.23 | 0.021 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deblier, I.; Dossche, K.; Vanermen, A.; Mistiaen, W. The Outcomes for Different Biological Heart Valve Prostheses in Surgical Aortic Valve Replacement before and after the Introduction of Transcatheter Aortic Valve Implantation. Prosthesis 2024, 6, 708-725. https://doi.org/10.3390/prosthesis6030050
Deblier I, Dossche K, Vanermen A, Mistiaen W. The Outcomes for Different Biological Heart Valve Prostheses in Surgical Aortic Valve Replacement before and after the Introduction of Transcatheter Aortic Valve Implantation. Prosthesis. 2024; 6(3):708-725. https://doi.org/10.3390/prosthesis6030050
Chicago/Turabian StyleDeblier, Ivo, Karl Dossche, Anthony Vanermen, and Wilhelm Mistiaen. 2024. "The Outcomes for Different Biological Heart Valve Prostheses in Surgical Aortic Valve Replacement before and after the Introduction of Transcatheter Aortic Valve Implantation" Prosthesis 6, no. 3: 708-725. https://doi.org/10.3390/prosthesis6030050
APA StyleDeblier, I., Dossche, K., Vanermen, A., & Mistiaen, W. (2024). The Outcomes for Different Biological Heart Valve Prostheses in Surgical Aortic Valve Replacement before and after the Introduction of Transcatheter Aortic Valve Implantation. Prosthesis, 6(3), 708-725. https://doi.org/10.3390/prosthesis6030050







