Biomechanical Factors in the Prognosis of Implants: A Clinical Study
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
- Measurement of peri-implant health: History of periodontal disease, bleeding on probing, increased probing depth, orthopantomography, and standardized intraoral periapical radiographs, among other parameters, have been employed to evaluate the levels of supporting bone around the implants [25,26,27,28].Additionally, it should be considered that emerging adjuvant preventive treatments such as Ozone [29] and photobiomodulation [30] can have a significant influence on the oral environment and they could also have an impact on the long-term prognosis of dental implants. Therefore, future studies are needed to mix biomechanical considerations with biological variables and preventive treatments to obtain a complete overview.
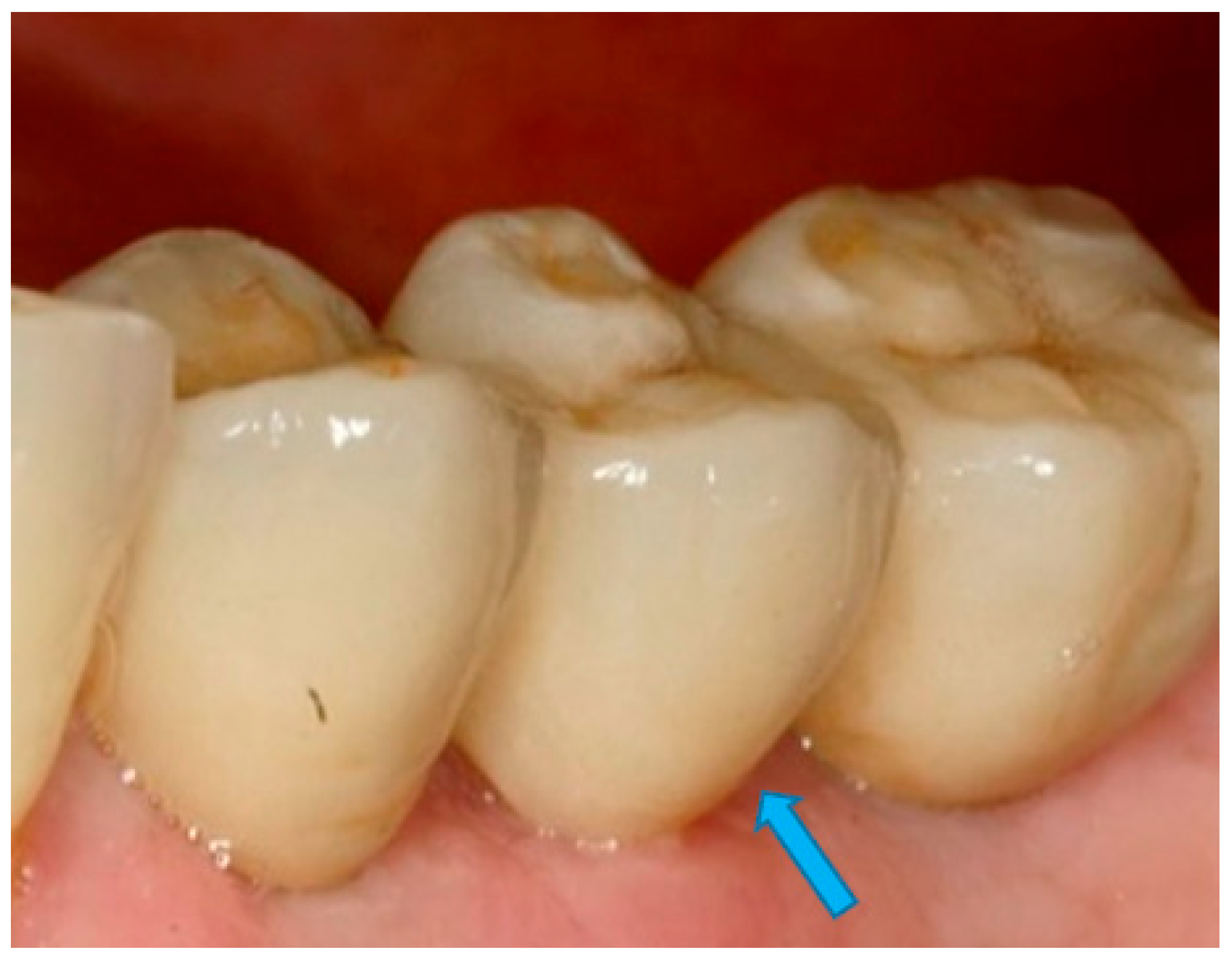
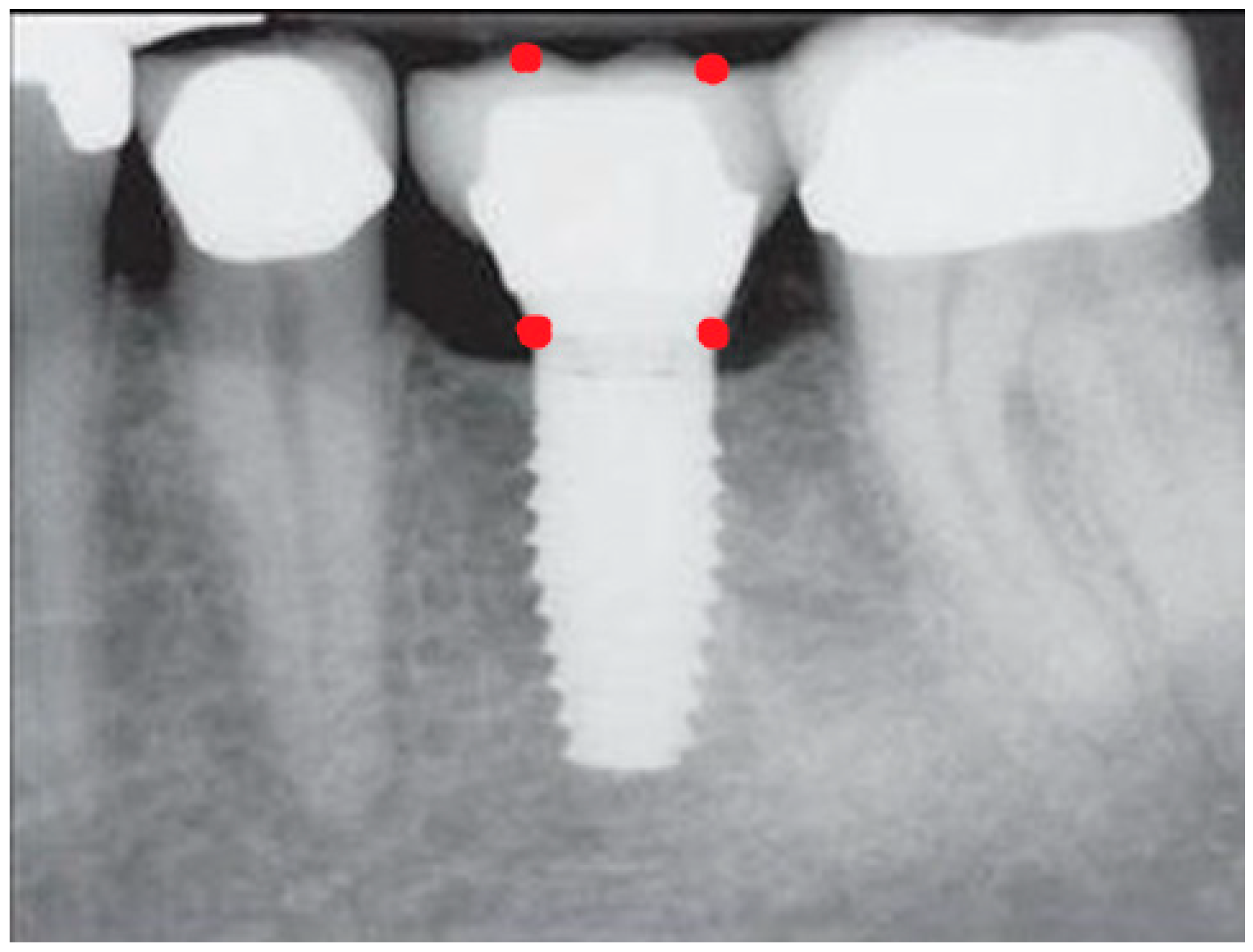
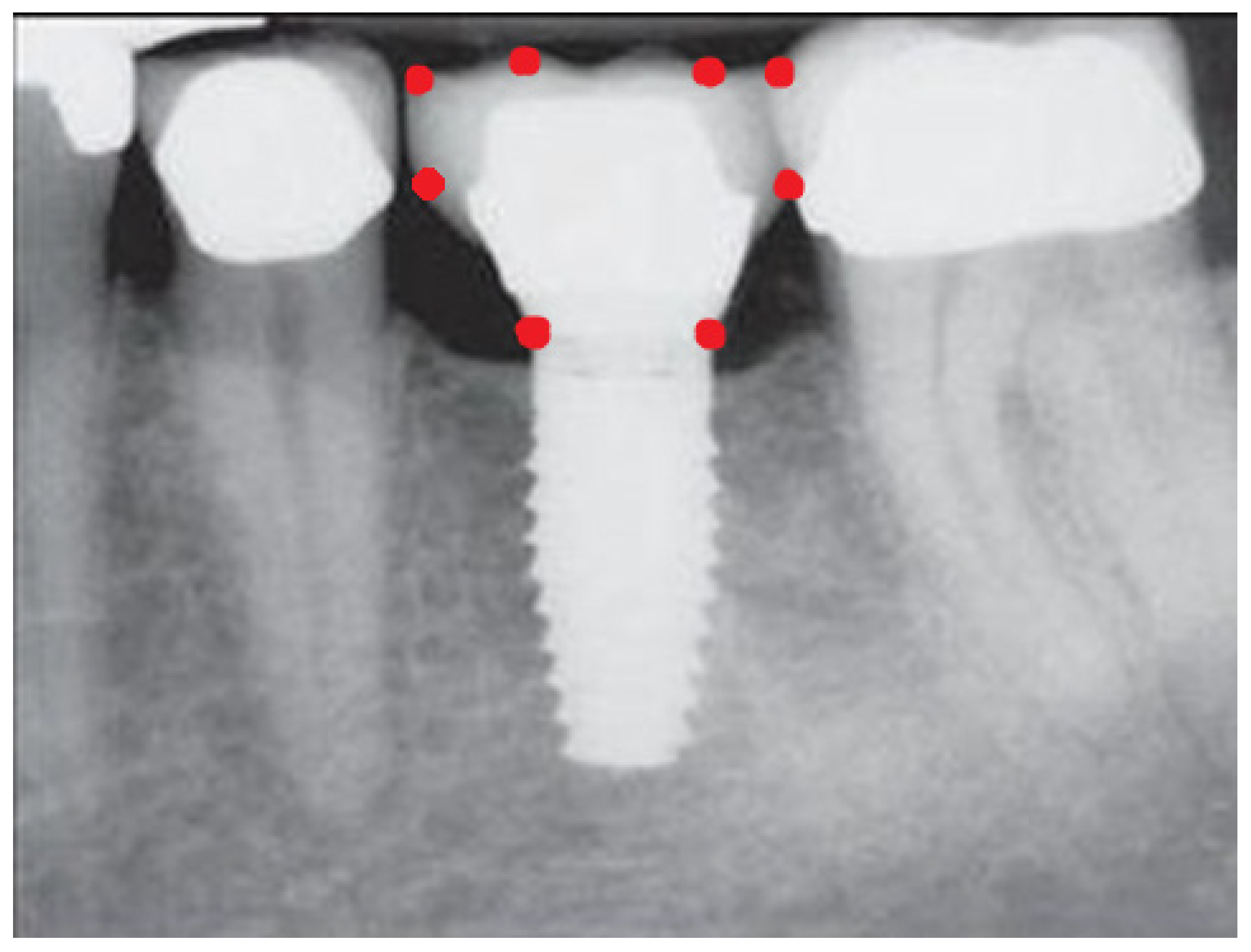
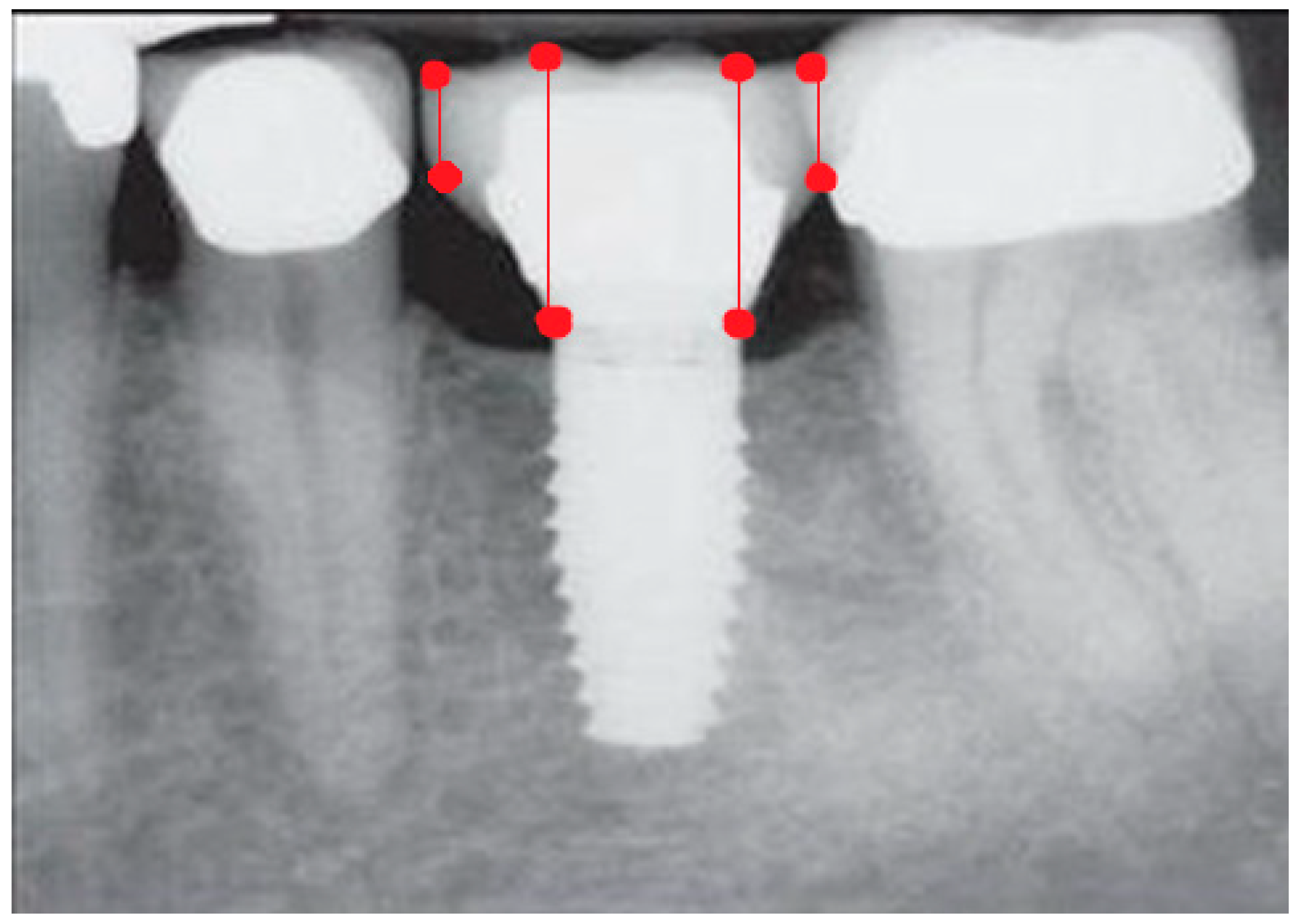
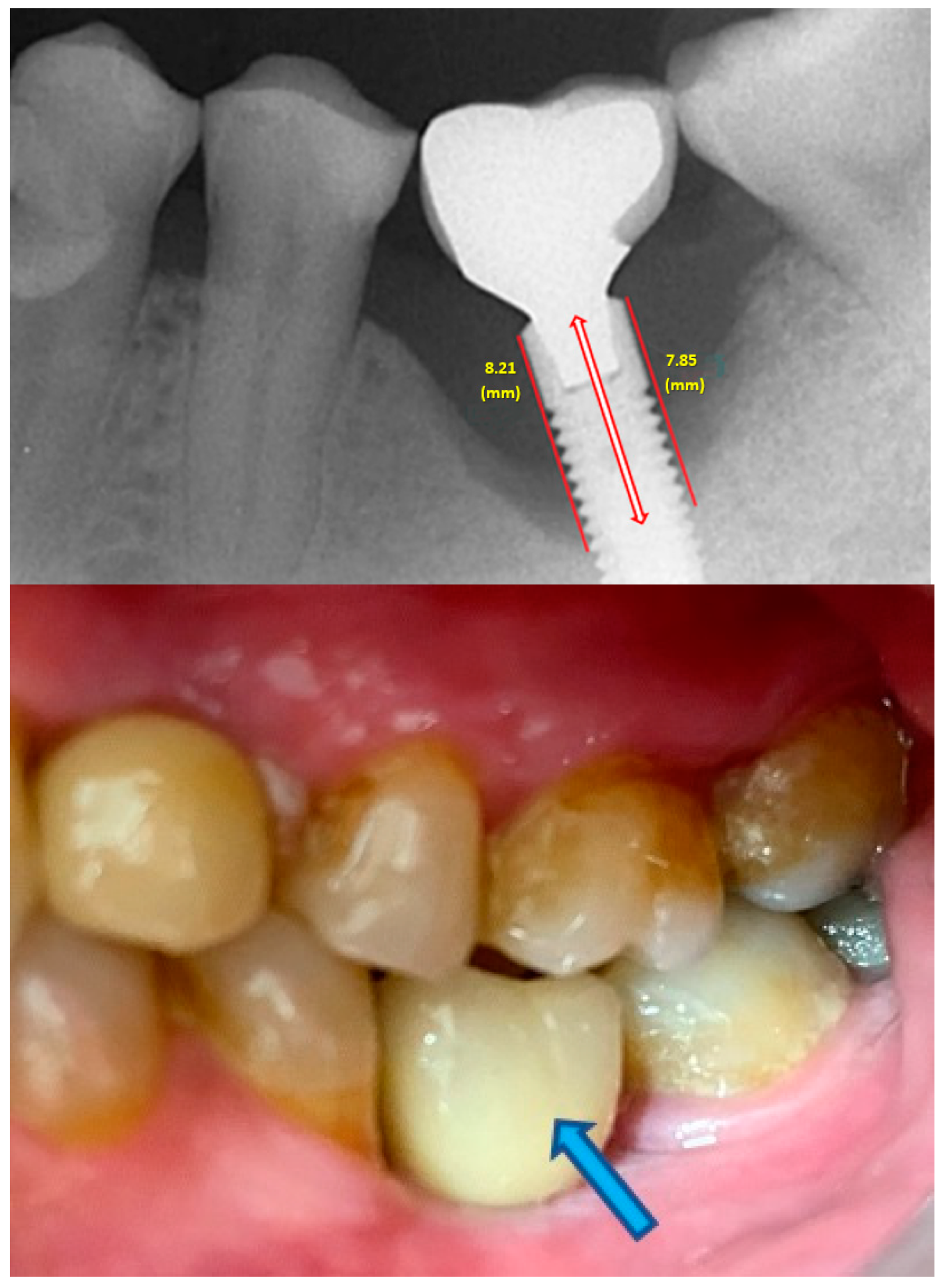
- Measurement of mesial and distal bone loss: Clinically, an increase in the probing depth of the peri-implant pockets, both mesial and distal, was recorded using a calibrated probe with a force of 0.25 N. Radiographically, mesial and distal peri-implant bone height loss was measured using properly performed radiological projections. In fewer than five patients, statistically significant findings in cases of bone loss were difficult to achieve.
- Measurement of the mesial and distal cantilever: The mesial cantilever was significantly greater for the bilateral bone loss group (1833.5 ± 1531.4 µm) compared to the group without MBL (1029.5 ± 968.6 µm) (F = 2.77; p < 0.05). Concerning the effect of the mesial versus distal cantilever, although the mesial cantilever appears to be more favorable, the only article found in the literature regarding this finding is a study by Romeo et al. (2003), which showed that this effect is not always consistent, although the difference is minimal [31].
- Measurement of occlusal load using the PRESCALE® (Fujifilm, Japan) is the standard parameter to objectively evaluate masticatory function. This system is supported by several publications as it is reproducible, accurate, objective, internationally scientifically validated, easy to use, and does not increase the vertical dimension [32,33,34,35,36]. While it may not reveal the sequence and timing of dental contact, making it difficult to identify each individual tooth, PRESCALE® effectively integrated the occlusal analysis and provided the required information for this study. In this clinical study, it is evident that there is a higher occlusal load in the left anterior region, and there is a linear association between this load and MBL (r = 0.47; p < 0.01).
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boedeker, D.; Dyer, J.; Kraut, R. Clinical outcome of immediately loaded maxillary implants: A 2-year retrospective study. J. Oral. Maxillofac. Surg. 2011, 69, 1335–1343. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, T.; Yamamoto, K.; Horita, S.; Murakami, K.; Tsutsumi, S.; Kirita, T. Effects of implant tilting and the loading direction on the displacement and micromotion of immediately loaded implants: An in vitro experiment and finite element analysis. J. Periodontal Implant Sci. 2017, 47, 251–262. [Google Scholar] [CrossRef]
- Delgado-Ruiz, R.A.; Calvo-Guirado, J.L.; Romanos, G.E. Effects of occlusal forces on the peri-implant-bone interface stability. Periodontology 2000 2019, 81, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Schmid, J. Pathogenesis of implant failures. Periodontology 2000 1994, 4, 127–138. [Google Scholar] [CrossRef]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.C.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A new classification scheme for periodontal and peri-implant diseases and conditions—Introduction and key changes from the 1999 classification. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S1–S8. [Google Scholar] [CrossRef]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45 (Suppl. S20), 286–291. [Google Scholar] [CrossRef]
- Araujo, M.G.; Lindhe, J. Peri-implant health. J. Clin. Periodontol. 2018, 45 (Suppl. S20), 230–236. [Google Scholar] [CrossRef] [PubMed]
- Manea, A.; Bran, S.; Dinu, C.; Rotaru, H.; Barbur, I.; Crisan, B.; Armencea, G.; Onisor, F.; Lazar, M.; Ostas, D.; et al. Principles of biomechanics in oral implantology. Med. Pharm. Rep. 2019, 92 (Suppl. S3), S14–S19. [Google Scholar] [CrossRef] [PubMed]
- Brunski, J.B. Biomaterial and biomechanics in dental implant design. Int. J. Oral. Maxillofac. Implants 1988, 3, 85–97. [Google Scholar]
- Brånemark, P.I. Osseointegration and its experimental background. J. Prosthet. Dent. 1983, 50, 399–410. [Google Scholar] [CrossRef]
- Duyck, J.; Naert, I.E.; Van Oosterwyck, H.; Van der Sloten, J.; De Cooman, M.; Lievens, S.; Puers, B. Biomechanics of oral implants: A review of the literature. Technol. Health Care 1997, 5, 253–273. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.M.; Wilson, T.G., Jr.; Jensen, O.T. Minimum criteria for immediate provisionalization of single-tooth dental implants in extraction sites: A 1-year retrospective study of 100 consecutive cases. J. Oral. Maxillofac. Surg. 2011, 69, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Sohn, B.S.; Heo, S.J.; Koak, J.Y.; Kim, S.K.; Lee, S.Y. Strain of implants depending on occlusion types in mandibular implant-supported fixed prostheses. J. Adv. Prosthodont. 2011, 3, 1–9. [Google Scholar] [CrossRef]
- Pjetursson, B.E.; Thoma, D.; Jung, R.; Zwahlen, M.; Zembic, A. A systematic review of the survival and complication rates of implant-supported fixed dental prostheses (FDPs) after a mean observation period of at least 5 years. Clin. Oral. Implants Res. 2012, 23 (Suppl. S6), 22–38. [Google Scholar] [CrossRef]
- Shackleton, J.L.; Carr, L.; Slabbert, J.C.; Becker, P.J. Survival of fixed implant-supported prostheses related to cantilever lengths. J. Prosthet. Dent. 1994, 71, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, R.A.; Decker, A.M.; Plonka, A.B.; Wang, H.L. The Role of Occlusion in Implant Therapy: A Comprehensive Updated Review. Implant. Dent. 2016, 25, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, M.M.; Del Bel Cury, A.A.; Pizzoloto, L.; Acapa, I.R.H.; Shibli, J.A.; Bordin, D. Does traumatic occlusal forces lead to peri-implant bone loss? A systematic review. Braz. Oral. Res. 2019, 33 (Suppl. S1), e069. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, M.S. Mechanical complications of dental implants. Clin. Oral. Implants Res. 2000, 11 (Suppl. S1), 156–158. [Google Scholar] [CrossRef]
- Chambrone, L.; Chambrone, L.A.; Lima, L.A. Effects of occlusal overload on peri-implant tissue health: A systematic review of animal-model studies. J. Periodontol. 2010, 81, 1367–1378. [Google Scholar] [CrossRef]
- Kozlovsky, A.; Tal, H.; Laufer, B.Z.; Leshem, R.; Rohrer, M.D.; Weinreb, M.; Artzi, Z. Impact of implant overloading on the peri-implant bone in inflamed and non-inflamed peri-implant mucosa. Clin. Oral. Implants Res. 2007, 18, 601–610. [Google Scholar] [CrossRef]
- Kourtis, S.G.; Sotiriadou, S.; Voliotis, S.; Challas, A. Private practice results of dental implants. Part I: Survival and evaluation of risk factors--Part II: Surgical and prosthetic complications. Implant Dent. 2004, 13, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.M. Cantilever fixed prostheses utilizing dental implants: A 10-year retrospective analysis. Quintessence Int. 2004, 35, 437–441. [Google Scholar] [PubMed]
- Khorshid, H.E.; Issa, N.O.; Ekram, A.M. Effect of implant diameter and cantilever length on the marginal bone height changes and stability of implants supporting screw retained prostheses: A randomized double blinded control trial. J. Adv. Prosthodont. 2023, 15, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, M.; Maeda, I.; Kokubo, Y.; Tanaka, Y.; Ueno, T.; Ohara, Y.; Motokawa, K.; Hayakawa, M.; Shirobe, M.; Edahiro, A.; et al. Standard Values and Concurrent Validity of a Newly Developed Occlusal Force-Measuring Device among Community-Dwelling Older Adults: The Otassha Study. Int. J. Environ. Res. Public Health 2022, 19, 5588. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Derks, J.; Monje, A.; Wang, H.L. Peri-implantitis. J. Clin. Periodontol. 2018, 45 (Suppl. S20), 246–266. [Google Scholar] [CrossRef] [PubMed]
- Heitz-Mayfield, L.J. Peri-implant diseases: Diagnosis and risk indicators. J. Clin. Periodontol. 2008, 35 (Suppl. S8), 292–304. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Gárces, M.A.; Gay-Escoda, C. Periimplantitis. Med. Oral Patol. Oral Cir. Bucal 2004, 9 (Suppl. S69–S74), 63–69. [Google Scholar]
- Renvert, S.; Persson, G.R.; Pirih, F.Q.; Camargo, P.M. Peri-implant health, peri-implant mucositis, and peri-implantitis: Case definitions and diagnostic considerations. J. Periodontol. 2018, 89 (Suppl. S1), S304–S312. [Google Scholar] [CrossRef]
- Scribante, A.; Gallo, S.; Pascadopoli, M.; Frani, M.; Butera, A. Ozonized gels vs chlorhexidine in non-surgical periodontal treatment: A randomized clinical trial. Oral Dis. 2023, 1–8. [Google Scholar] [CrossRef]
- Elbay, M.; Elbay, Ü.Ş.; Kaya, E.; Kalkan, Ö.P. Effects of photobiomodulation with different application parameters on injection pain in children: A randomized clinical trial. J. Clin. Pediatr. Dent. 2023, 47, 54–62. [Google Scholar]
- Romeo, E.; Lops, D.; Margutti, E.; Ghisolfi, M.; Chiapasco, M.; Vogel, G. Implant-supported fixed cantilever prostheses in partially edentulous arches. A seven-year prospective study. Clin. Oral Implants Res. 2003, 14, 303–311. [Google Scholar] [CrossRef]
- Shiga, H.; Komino, M.; Yokoyama, M.; Sano, M.; Arakawa, I.; Nakajima, K.; Fujii, S. Relationship between age and occlusal force in adults with natural dentition. Odontology 2023, 111, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Okiyama, S.; Ikebe, K.; Nokubi, T. Association between masticatory performance and maximal occlusal force in young men. J. Oral. Rehabil. 2003, 30, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Horibe, Y.; Matsuo, K.; Ikebe, K.; Minakuchi, S.; Sato, Y.; Sakurai, K.; Ueda, T. Relationship between two pressure-sensitive films for testing reduced occlusal force in diagnostic criteria for oral hypofunction. Gerodontology 2022, 39, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhao, Z.; Zhou, M.; Zheng, X.; An, N.; Niu, L.; Tay, F.R.; Ma, C.; Wang, F. In vivo evaluation of the reliability and validity of three digital occlusion analysis methods. J. Dent. 2022, 127, 104355. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.F.; Wang, C.M.; Shieh, W.Y.; Liao, Y.F.; Hong, H.H.; Chang, C.T. The correlation between two occlusal analyzers for the measurement of bite force. BMC Oral Health 2022, 22, 472. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Liu, H.; Liu, G.; Jin, Z.; Wang, L.; Ma, J.; Li, H. Analysis of brain activity involved in chewing-side preference during chewing: An fMRI study. J. Oral Rehabil. 2015, 42, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Barcellos, D.C.; Gonçalves, S.E.; da Silva, M.A.; Batista, G.R.; Pleffken, P.R.; Pucci, C.R.; Borges, A.B.; Rocha Gomes Torres, C. Prevalence of chewing side preference in the deciduous, mixed and permanent dentitions. J. Contemp. Dent. Pract. 2011, 12, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Athab Abduljabbar, Z.; Svensson, K.G.; Hjalmarsson, L.; Franke Stenport, V.; Eliasson, A. Chewing side preference and laterality in patients treated with unilateral posterior implant-supported fixed partial prostheses. J. Oral Rehabil. 2022, 49, 1080–1086. [Google Scholar] [CrossRef]
- Peñarrocha-Diago, M.A.; Flichy-Fernández, A.J.; Alonso-González, R.; Peñarrocha-Oltra, D.; Balaguer-Martínez, J.; Peñarrocha-Diago, M. Influence of implant neck design and implant-abutment connection type on peri-implant health. Radiological study. Clin. Oral Implants Res. 2013, 24, 1192–1200. [Google Scholar] [CrossRef]
- Koo, K.T.; Lee, E.J.; Kim, J.Y.; Seol, Y.J.; Han, J.S.; Kim, T.I.; Lee, Y.M.; Ku, Y.; Wikesjö, U.M.; Rhyu, I.C. The effect of internal versus external abutment connection modes on crestal bone changes around dental implants: A radiographic analysis. J. Periodontol. 2012, 83, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Koutouzis, T.; Gholami, F.; Reynolds, J.; Lundgren, T.; Kotsakis, G.A. Abutment Disconnection/Reconnection Affects Peri-implant Marginal Bone Levels: A Meta-Analysis. Int. J. Oral Maxillofac. Implants 2017, 32, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Montero, J. A Review of the Major Prosthetic Factors Influencing the Prognosis of Implant Prosthodontics. J. Clin. Med. 2021, 10, 816. [Google Scholar] [CrossRef] [PubMed]
- Peñarrocha, M.; Palomar, M.; Sanchis, J.M.; Guarinos, J.; Balaguer, J. Radiologic study of marginal bone loss around 108 dental implants and its relationship to smoking, implant location, and morphology. Int. J. Oral Maxillofac. Implants 2004, 19, 861–867. [Google Scholar] [PubMed]
- Brånemark, P.I.; Zarb, G.A.; Albrektsson, T. Tissue-Integrated Prostheses: Osseointegration in Clinical Dentistry; Quintessence: Chicago, IL, USA, 1985; pp. 111–197. [Google Scholar]
- Wennström, J.; Zurdo, J.; Karlsson, S.; Ekestubbe, A.; Gröndahl, K.; Lindhe, J. Bone level change at implant-supported fixed partial dentures with and without cantilever extension after 5 years in function. J. Clin. Periodontol. 2004, 31, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Toljanic, J.A.; Banakis, M.L.; Willes, L.A.; Graham, L. Soft tissue exposure of endosseous implants between stage I and stage II surgery as a potential indicator of early crestal bone loss. Int. J. Oral Maxillofac. Implants 1999, 14, 436–441. [Google Scholar] [PubMed]
- Zandim-Barcelos, D.L.; Carvalho, G.G.; Sapata, V.M.; Villar, C.C.; Hämmerle, C.; Romito, G.A. Implant-based factor as possible risk for peri-implantitis. Braz. Oral Res. 2019, 33 (Suppl. S1), e067. [Google Scholar] [CrossRef] [PubMed]
- Bilhan, H.; Mumcu, E.; Geçkili, O.; Atalay, B. The evaluation of the success of immediately placed single implants: A retrospective clinical study. Implant Dent. 2011, 20, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Ramanauskaite, A. It is all about peri-implant tissue health. Periodontology 2000 2022, 88, 9–12. [Google Scholar] [CrossRef]
- Schou, S.; Holmstrup, P.; Worthington, H.V.; Esposito, M. Outcome of implant therapy in patients with previous tooth loss due to periodontitis. Clin. Oral Implants Res. 2006, 17 (Suppl. S2), 104–123. [Google Scholar] [CrossRef]
- Karoussis, I.K.; Salvi, G.E.; Heitz-Mayfield, L.J.; Brägger, U.; Hämmerle, C.H.; Lang, N.P. Long-term implant prognosis in patients with and without a history of chronic periodontitis: A 10-year prospective cohort study of the ITI Dental Implant System. Clin. Oral Implants Res. 2003, 14, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Levin, L.; Hertzberg, R.; Har-Nes, S.; Schwartz-Arad, D. Long-term marginal bone loss around single dental implants affected by current and past smoking habits. Implant Dent. 2008, 17, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.; Spille, J.H.; Wiltfang, J.; Naujokat, H. Systematic review on diabetes mellitus and dental implants: An update. Int. J. Implant Dent. 2022, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Lindhe, J.; Meyle, J.; Group D of European Workshop on Periodontology. Peri-implant diseases: Consensus Report of the Sixth European Workshop on Periodontology. J. Clin. Periodontol. 2008, 35 (Suppl. S8), 282–285. [Google Scholar] [CrossRef] [PubMed]
- De Bruyn, H.; Vandeweghe, S.; Ruyffelaert, C.; Cosyn, J.; Sennerby, L. Radiographic evaluation of modern oral implants with emphasis on crestal bone level and relevance to peri-implant health. Periodontology 2000 2013, 62, 256–270. [Google Scholar] [CrossRef] [PubMed]
- Renvert, S.; Hirooka, H.; Polyzois, I.; Kelekis-Cholakis, A.; Wang, H.L.; Working Group 3. Diagnosis and non-surgical treatment of peri-implant diseases and maintenance care of patients with dental implants-Consensus report of working group 3. Int. Dent. J. 2019, 69 (Suppl. S2), 12–17. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P.; Berglundh, T.; Working Group 4 of Seventh European Workshop on Periodontology. Periimplant diseases: Where are we now?--Consensus of the Seventh European Workshop on Periodontology. J. Clin. Periodontol. 2011, 38 (Suppl. S11), 178–181. [Google Scholar] [CrossRef] [PubMed]
- Lindquist, L.W.; Carlsson, G.E.; Jemt, T. A prospective 15-year follow-up study of mandibular fixed prostheses supported by osseointegrated implants. Clinical results and marginal bone loss. Clin. Oral Implants Res. 1996, 7, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Serino, G.; Ström, C. Peri-implantitis in partially edentulous patients: Association with inadequate plaque control. Clin. Oral Implants Res. 2009, 20, 169–174. [Google Scholar] [CrossRef]
- Heitz-Mayfield, L.J.; Schmid, B.; Weigel, C.; Gerber, S.; Bosshardt, D.D.; Jönsson, J.; Lang, N.P.; Jönsson, J. Does excessive occlusal load affect osseointegration? An experimental study in the dog. Clin. Oral Implants Res. 2004, 15, 259–268. [Google Scholar] [CrossRef]
- Lang, N.P.; Wilson, T.G.; Corbet, E.F. Biological complications with dental implants: Their prevention, diagnosis and treatment. Clin. Oral Implants Res. 2000, 11 (Suppl. S1), 146–155. [Google Scholar] [CrossRef] [PubMed]




| NO MBL (n = 5 Patients with a Total of 19 Implants) | UNILATERAL MBL (n = 10 Patients with a Total of 30 Implants) | BILATERAL MBL (n = 26 Patients with a Total of 86 Implants) | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Clinical Profile | Mean | SD | Mean | SD | Mean | SD | |
| Mesial cantilever (µm) | 1029.5 | 968.6 | 1521.0 | 1149.4 | 1833.5 | 1531.4 | F = 2.77; p < 0.05 |
| Distal cantilever (µm) | 1645.8 | 1341.5 | 1592.7 | 2148.9 | 1483.1 | 1563.9 | F = 0.09; p = 0.90 |
| Implant diameter (mm) | 3.80 | 0.5 | 3.90 | 0.5 | 3.8 | 0.4 | F = 0.16; p = 0.85 |
| Implant length (mm) | 10.0 | 1.2 | 10.7 | 1.4 | 10.6 | 1.7 | F = 1.33; p = 0.268 |
| Follow-up (months) | 45.1 | 18.8 | 45.1 | 19.6 | 48.9 | 22.0 | F = 0.50; p = 0.60 |
| CLINICAL VARIABLES (Implant-Related) | MBL (p-Value) |
|---|---|
| Implant diameter | r = 0.27 (p = 0.001) |
| Implant length | r = 0.20 (p = 0.021) |
| Follow-up time | r = 0.29 (p = 0.001) |
| Occlusal load in the anterior region | r = 0.47 (p = 0.002) |
| NO MBL (n = 5 with 19 Implants) | UNILATERAL MBL (n = 10 Patients with 30 Implants) | BILATERAL MBL (n = 26 Patients with 86 Implants) | COMPARISON p-Value | ||||
|---|---|---|---|---|---|---|---|
| CLINICAL VARIABLES | n | % | n | % | n | % | % |
| Periodontal disease | 1 | 20.0 | 1 | 10.0 | 4 | 15.4 | χ2 = 0.30; p = 0.861 |
| Diabetes | 0 | 0 | 0 | 0 | 2 | 7.7 | χ2 = 1.21; p = 0.545 |
| Other diseases | 1 | 20.0 | 0 | 0 | 4 | 15.4 | χ2 = 1.92; p = 0.383 |
| SYMPTOMS | n | % | n | % | n | % | |
| Asymptomatic | 19 | 100.0 | 30 | 100.0 | 84 | 97.7 | χ2 = 1.16; p = 0.56 |
| Symptomatic | 0 | 0.0 | 0 | 0.0 | 2 | 2.3 | |
| BEHAVIORAL VARIABLE | n | % | n | % | n | % | |
| Regular maintenance visits | 5 | 100.0 | 9 | 90.0 | 20 | 76.9 | χ2 = 2.04; p = 0.36 |
| PLAQUE INDEX | n | % | n | % | n | % | |
| No plaque | 8 | 42.1 | 12 | 40.0 | 34 | 39.5 | χ2 = 5.18; p = 0.52 |
| 1/3 plaque | 8 | 42.1 | 15 | 50.0 | 33 | 38.4 | |
| 2/3 plaque | 2 | 10.5 | 1 | 3.3 | 16 | 18.6 | |
| >2/3 plaque | 1 | 5.3 | 2 | 6.7 | 3 | 3.5 | |
| BLEEDING ON PROBING | n | % | n | % | n | % | |
| No | 12 | 63.2 | 18 | 60.0 | 47 | 54.7 | χ2 = 0.60; p = 0.74 |
| Yes | 7 | 36.8 | 12 | 40.0 | 39 | 45.3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceresuela, P.; Montero, J. Biomechanical Factors in the Prognosis of Implants: A Clinical Study. Prosthesis 2024, 6, 896-912. https://doi.org/10.3390/prosthesis6040065
Ceresuela P, Montero J. Biomechanical Factors in the Prognosis of Implants: A Clinical Study. Prosthesis. 2024; 6(4):896-912. https://doi.org/10.3390/prosthesis6040065
Chicago/Turabian StyleCeresuela, Paola, and Javier Montero. 2024. "Biomechanical Factors in the Prognosis of Implants: A Clinical Study" Prosthesis 6, no. 4: 896-912. https://doi.org/10.3390/prosthesis6040065
APA StyleCeresuela, P., & Montero, J. (2024). Biomechanical Factors in the Prognosis of Implants: A Clinical Study. Prosthesis, 6(4), 896-912. https://doi.org/10.3390/prosthesis6040065








