Bioaccumulation of Trace Elements in Myctophids in the Oxygen Minimum Zone Ecosystem of the Gulf of California
Abstract
:1. Introduction
2. Materials and Methods
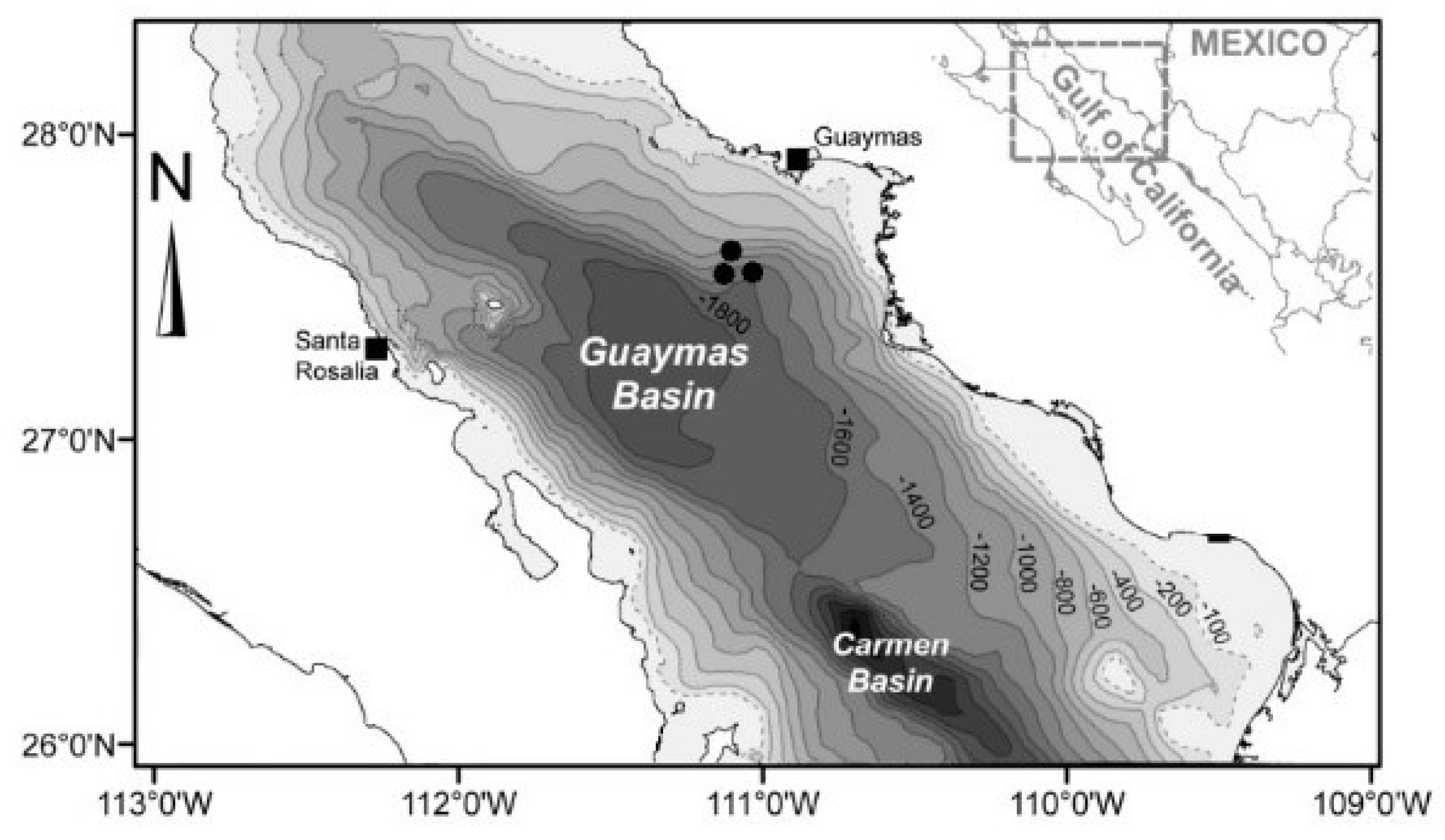
2.1. Sampling
2.2. Trace Element Analysis
2.3. Statistical Analyses
2.4. Biomagnification Factor
3. Results
4. Discussion
4.1. Geographical Differences in Elemental Composition
4.2. Interspecific Differences
4.3. Myctophids as Potential Vectors of Trace Elements
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Catul, V.; Gauns, M.; Karuppasamy, P. A review on mesopelagic fishes belonging to family Myctophidae. Rev. Fish Biol. Fish. 2011, 21, 339–354. [Google Scholar] [CrossRef]
- Hulley, P. Lanternfishes. In Encyclopedia of Fishes; Academic Press: San Diego, CA, USA, 1994; pp. 127–128. [Google Scholar]
- Robison, B.H. Distribution of the midwater fishes of the Gulf of California. Copeia 1972, 3, 448–461. [Google Scholar] [CrossRef]
- Williams, A.; Koslow, J.; Terauds, A.; Haskard, K. Feeding ecology of five fishes from the mid-slope micronekton community off southern Tasmania, Australia. Mar. Biol. 2001, 139, 1177–1192. [Google Scholar]
- Luo, J.; Ortner, P.B.; Forcucci, D.; Cummings, S.R. Diel vertical migration of zooplankton and mesopelagic fish in the Arabian Sea. Deep Sea Res. B 2000, 47, 1451–1473. [Google Scholar] [CrossRef]
- Hopkins, T.L.; Baird, R.C. Aspects of the trophic ecology of the mesopelagic fish Lampanyctus alatus (Family Myctophidae) in the eastern Gulf of Mexico. Biol. Oceanogr. 1985, 3, 285–313. [Google Scholar]
- Kinzer, J.; Schulz, K. Vertical distribution and feeding patterns of midwater fish in the central equatorial Atlantic. Mar. Biol. 1985, 85, 313–322. [Google Scholar] [CrossRef] [Green Version]
- Brierley, A.S. Diel vertical migration. Curr. Biol. 2014, 24, 1074–1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hays, G.C. A review of the adaptative significance and ecosystem consequences of zooplankton diel vertical migrations. In Migrations and Dispersal of Marine Organisms; Springer: Dordrecht, The Netherlands, 2003; Volume 163, p. 170. [Google Scholar]
- Cartes, J.E. Adaptations to Life in the Oceans. In Marine Ecology; Eolss Publishers Co Ltd.: Oxford, UK, 2009; pp. 168–169. [Google Scholar]
- Gjøsaeter, J.; Kawaguchi, K. A review of the world resources of mesopelagic fish. In A review of the World Resources of Mesopelagic Fish; Food & Agriculture Organization of the United Nations: Rome, Italy, 1980; pp. 1–151. [Google Scholar]
- Lopes, A.R.; Trubenbach, K.; Teixeira, T.; Lopes, V.M.; Pires, V.; Baptista, M.; Repolho, T.; Calado, R.; Diniz, M.; Rosa, R. Oxidative stress in deep scattering layers: Heat shock response and antioxidant enzymes activities of myctophid fishes thriving in oxygen minimum zones. Deep Sea Res. A 2013, 82, 10–16. [Google Scholar] [CrossRef]
- Radchenko, V.I. Mesopelagic fish community supplies “biological pump”. Raffles Bull. Zool. Suppl. 2007, 14, 247–253. [Google Scholar]
- Subotić, S.; Spasić, S.; Višnjić-Jeftić, Ž.; Hegediš, A.; Krpo-Ćetković, J.; Mićković, B.; Skorić, S.; Lenhardt, M. Heavy metal and trace element bioaccumulation in target tissues of four edible fish species from the Danube River (Serbia). Ecotoxicol. Environ. Saf. 2013, 98, 196–202. [Google Scholar]
- Bai, J.; Zhao, Q.; Lu, Q.; Wang, J.; Reddy, K.R. Effects of freshwater input on trace element pollution in salt marsh soils of a typical coastal estuary, China. J. Hydrol. 2015, 520, 186–192. [Google Scholar] [CrossRef]
- Purves, D. Trace element Contamination of the Environment; Elsevier: New York, NY, USA, 2012; pp. 150–182. [Google Scholar]
- Adriano, D.C. Trace Elements in the Terrestrial Environment; Springer Science & Business Media: South Carolina, SC, USA, 2013. [Google Scholar]
- Kojadinovic, J.; Potier, M.; Le Corre, M.; Cosson, R.P.; Bustamante, P. Bioaccumulation of trace elements in pelagic fish from the Western Indian Ocean. Environ. Pollut. 2007, 146, 548–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benito, V.; Devesa, V.; Muñoz, O.; Suñer, M.A.; Montoro, R.; Baos, R.; Hiraldo, F.; Ferrer, M.; Fernández, M.; González, M.J. Trace elements in blood collected from birds feeding in the area around Doñana National Park affected by the toxic spill from the Aznalcóllar mine. Sci. Total Environ. 1999, 242, 309–323. [Google Scholar] [CrossRef]
- Enríquez-Andrade, R.; Anaya-Reyna, G.; Barrera-Guevara, J.C.; Carvajal-Moreno, M.A.; Martínez-Delgado, M.E.; Vaca-Rodrígues, J.; Valdés-Casillas, C. An analysis of critical areas for biodiversity conservation in the Gulf of California region. Ocean Coast. Manag. 2005, 48, 31–50. [Google Scholar] [CrossRef]
- Raimundo, J.; Vale, C.; Rosa, R. Trace element concentrations in the top predator jumbo squid (Dosidicus gigas) from the Gulf of California. Ecotoxicol. Environ. Saf. 2014, 102, 179–186. [Google Scholar] [CrossRef]
- Calvert, S. Accumulation of diatomaceous silica in the sediments of the Gulf of California. Geol. Soc. Am. Bull. 1966, 77, 569–596. [Google Scholar] [CrossRef]
- Gieskes, J.; Kastner, M.; Einsele, G.; Kelts, K.; Niemitz, J. Hydrothermal activity in the Guaymas Basin, Gulf of California: A synthesis. Initial Rep. Deep Sea Drill. Proj. 1982, 64, 1159–1167. [Google Scholar]
- Demina, L.L.; Galkin, S.V.; Shumilin, E.N. Bioaccumulation of some trace elements in the biota of hydrothermal fields of the Guaymas Basin (Gulf of California). Boletín Soc. Geológica Mex. 2009, 61, 31–45. [Google Scholar] [CrossRef]
- Otero, X.L.; Huerta-Diaz, M.A.; Macías, F. Influence of a turbidite deposit on the extent of pyritization of iron, manganese and trace metals in sediments from the Guaymas Basin, Gulf of California (Mexico). Appl. Geochem. 2003, 18, 1149–1163. [Google Scholar] [CrossRef]
- García-Rico, L.; Tejeda-Valenzuela, L.; Jara-Marini, M.E.; Gómez-Álvarez, A. Dissolved and particulate metals in water from Sonora Coast: A pristine zone of Gulf of California. Environ. Monit. Assess. 2011, 176, 109–123. [Google Scholar] [CrossRef]
- Childress, J.J.; Barnes, A.T.; Quetin, L.B.; Robison, B.H. Thermally protecting cod ends for the recovery of living deep-sea animals. Deep Sea Res. 1978, 25, 419–542. [Google Scholar] [CrossRef]
- Seibel, B.A.; Thuesen, E.V.; Childress, J.J.; Gorodezky, L.A. Decline in pelagic cephalopod metabolism with habitat depth reflects differences in locomotory efficiency. Biol. Bull. 1997, 192, 262–278. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.; Cortesão, C.; Castro, O.; Vale, C. Accumulation of metals and organochlorines in tissues of the oyster Crassostrea angulata from the Sado Estuary, Portugal. Sci. Total Environ. 1990, 97, 627–639. [Google Scholar] [CrossRef]
- Gray, J.S. Biomagnification in marine systems: The perspective of an ecologist. Mar. Pollut. Bull. 2002, 45, 46–52. [Google Scholar] [CrossRef]
- Fatemi, M.H.; Baher, E. A novel quantitative structure–activity relationship model for prediction of biomagnification factor of some organochlorine pollutants. Mol. Divers. 2009, 13, 343–352. [Google Scholar] [CrossRef]
- Hoekstra, P.F.; O’hara, T.M.; Fisk, A.T.; Borgå, K.; Solomon, K.R.; Muir, D.C.G. Trophic transfer of persistent organochlorine contaminants (OCs) within an Arctic marine food web from the southern Beaufort–Chukchi Seas. Environ. Pollut. 2003, 124, 509–522. [Google Scholar] [CrossRef]
- Dehn, L.A.; Follmann, E.H.; Thomas, D.L.; Sheffield, G.G.; Rosa, C.; Duffy, L.K.; O’Hara, T.M. Trophic relationships in an Arctic food web and implications for trace metal transfer. Sci. Total Environ. 2006, 362, 103–123. [Google Scholar] [CrossRef]
- Jæger, I.; Hop, H.; Gabrielsen, G.W. Biomagnification of mercury in selected species from an Arctic marine food web in Svalbard. Sci. Total Environ. 2009, 407, 4744–4751. [Google Scholar] [CrossRef]
- Shilla, D.; Pajala, G.; Routh, J.; Dario, M.; Kristoffersson, P. Trophodynamics and biomagnification of trace metals in aquatic food webs: The case of Rufiji estuary in Tanzania. Appl. Geochem. 2019, 100, 160–168. [Google Scholar] [CrossRef]
- Mertz, W. The essential trace elements. Science 1981, 213, 1332–1338. [Google Scholar] [CrossRef] [Green Version]
- Prashanth, L.; Kattapagari, K.K.; Chitturi, R.T.; Baddam, V.R.R.; Prasad, L.K. A review on role of essential trace elements in health and disease. J. NTR Univ. Health Sci. 2015, 4, 75–85. [Google Scholar]
- Rainbow, P.S. The biology of heavy metals in the sea. Int. J. Environ. Stud. 1985, 25, 195–211. [Google Scholar] [CrossRef]
- Chowdhury, B.A.; Chandra, R.K. Biological and health implications of toxic heavy metal and essential trace element interactions. Prog. Food Nutr. Sci. 1986, 11, 55–113. [Google Scholar]
- Pauly, D.; Trites, A.W.; Capuli, E.; Cristensen, V. Diet composition and trophic levels of marine mammals. ICES J. Mar. Sci. 1998, 55, 467–481. [Google Scholar] [CrossRef]
- Field, J.C.; Baltz, K.E.N.; Phillips, A.J.; Walker, W.A. Range expansion and trophic interactions of the jumbo squid, Dosidicus gigas, in the California Current. Calif. Coop. Ocean. Fish. Investig. Rep. 2007, 48, 131–146. [Google Scholar]
- Froese, R.; Pauly, D. 2019. FishBase. World Wide Web Electronic Publication. Available online: https://www.fishbase.se/summary/2739 (accessed on 3 February 2020).
- Froese, R.; Pauly, D. 2019. FishBase. World Wide Web Electronic Publication. Available online: https://www.fishbase.se/Summary/50707 (accessed on 3 February 2020).
- Ruelas, J.; Páez-Osuna, F. Distribution of cadmium, copper, iron, manganese, lead, and zinc in spinner dolphins Stenella longirostris stranded in La Paz Lagoon, Southwest Gulf of California. Bull. Environ. Contam. Toxicol. 2002, 69, 408–414. [Google Scholar] [CrossRef]
- Ruelas-Inzunza, J.; Páez-Osuna, F. Distribution of Cd, Cu, Fe, Mn, Pb and Zn in selected tissues of juvenile whales stranded in the SE Gulf of California (Mexico). Environ. Int. 2002, 28, 325–329. [Google Scholar] [CrossRef]
- Méndez, L.; Alvarez-Castañeda, S.; Acosta, B.; Sierra-Beltrán, A. Trace metals in tissues of gray whale (Eschrichtius robustus) carcasses from the Northern Pacific Mexican Coast. Mar. Pollut. Bull. 2002, 44, 217–221. [Google Scholar] [CrossRef]
- Fernandez, T.J.; Pradeep, K.; Anandan, R.; Zynudheen, A. Comparison of nutritional characteristics of myctophid fishes (Diaphus effulgens and D. hudsoni) with common Indian food fishes. Fish. Tech. 2014, 51, 173–178. [Google Scholar]
- Asante, K.; Kubota, R.; Agusa, T.; Subramanian, A.; Tanabe, S.; Nishida, S.; Yamaguchi, M.; Suetsugu, K.; Ohta, S.; Yeh, H. Trace element and stable isotope analyses of deep sea fish from the Sulu sea, Philippines. West Afr. J. Appl. Ecol. 2009, 14, 35–46. [Google Scholar] [CrossRef]
- Bustamante, P.; Bocher, P.; Cherel, Y.; Miramand, P.; Caurant, F. Distribution of trace elements in the tissues of benthic and pelagic fish from the Kerguelen Islands. Sci. Total Environ. 2003, 313, 25–39. [Google Scholar] [CrossRef] [Green Version]
- Bocher, P.; Caurant, F.; Miramand, P.; Cherel, Y.; Bustamante, P. Influence of the diet on the bioaccumulation of heavy metals in zooplankton-eating petrels at Kerguelen archipelago, Southern Indian Ocean. Polar Biol. 2003, 26, 759–767. [Google Scholar] [CrossRef]
- Cutshall, N.; Naidu, J.; Pearcy, W. Zinc and cadmium in the Pacific hake Merluccius productus off the western US coast. Mar. Biol. 1977, 44, 195–201. [Google Scholar] [CrossRef]
- Schulz-Baldes, M. Baseline study on Cd, Cu and Pb concentrations in Atlantic neuston organisms. Mar. Biol. 1992, 112, 211–222. [Google Scholar] [CrossRef]
- Windom, H.; Stickney, R.; Smith, R.; White, D.; Taylor, F. Arsenic, cadmium, copper, mercury, and zinc in some species of North Atlantic finfish. J. Fish. Board Can. 1973, 30, 275–279. [Google Scholar] [CrossRef]
- Fowler, S. Trace metal monitoring of pelagic organisms from the open Mediterranean Sea. Environ. Monit. Assess. 1986, 7, 59–78. [Google Scholar] [CrossRef]
- Cipro, C.V.; Cherel, Y.; Bocher, P.; Caurant, F.; Miramand, P.; Bustamante, P. Trace elements in invertebrates and fish from Kerguelen waters, southern Indian Ocean. Polar Biol. 2018, 41, 175–191. [Google Scholar] [CrossRef]
- Storelli, M.; Marcotrigiano, G. Interspecific variation in total arsenic body concentrations in elasmobranch fish from the Mediterranean Sea. Mar. Pollut. Bull. 2004, 48, 1145–1149. [Google Scholar] [CrossRef]
- Delgadillo-Hinojosa, F.; Macıas-Zamora, J.; Segovia-Zavala, J.; Torres-Valdés, S. Cadmium enrichment in the Gulf of California. Mar. Chem. 2001, 75, 109–122. [Google Scholar] [CrossRef]
- Páez-Osuna, F.; Álvarez-Borrego, S.; Ruiz-Fernández, A.C.; García-Hernández, J.; Jara-Marini, M.E.; Bergés-Tiznado, M.E.; Piñón-Gimate, A.; Alonso-Rodríguez, R.; Soto-Jiménez, M.F.; Frías-Espericueta, M.G.; et al. Environmental status of the Gulf of California: A pollution review. Earth-Sci. Rev. 2017, 166, 181–205. [Google Scholar] [CrossRef]
- Lavín, M.; Marinone, S. An Overview of the Physical Oceanography of the Gulf of California in Nonlinear Processes in Geophysical Fluid Dynamics; Kluver Academic Publishers: Dordrecht, The Netherlands, 2003; pp. 173–204. [Google Scholar]
- Barham, E.G. Deep sea fishes: Lethargy and vertical orientation. In Proceedings of an International Symposium on Biological Sound Scattering in the Ocean; Report of the Maury Center Ocean Science, MC-005: Washington, DC, USA, 1971; pp. 100–118. [Google Scholar]
- Raimundo, J.; Vale, C.; Martins, I.; Fontes, J.; Graça, G.; Caetano, M. Elemental composition of two ecologically contrasting seamount fishes, the bluemouth (Helicolenus dactylopterus) and blackspot seabream (Pagellus bogaraveo). Mar. Pollut. Bull. 2015, 100, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Sassa, C.; Kawaguchi, K.; Kinoshita, T.; Watanabe, C. Assemblages of vertical migratory mesopelagic fish in the transitional region of the western North Pacific. Fish. Oceanogr. 2002, 11, 193–204. [Google Scholar] [CrossRef]
- Phillips, K.L.; Jackson, G.D.; Nichols, P.D. Predation on myctophids by the squid Moroteuthis ingens around Macquarie and Heard Islands: Stomach contents and fatty acid analyses. Mar. Ecol. Prog. Ser. 2001, 215, 179–189. [Google Scholar] [CrossRef]
- Pereira, J.N.; Neves, V.C.; Prieto, R.; Silva, M.A.; Cascão, I.; Oliveira, C.; Cruz, M.J.; Medeiros, J.V.; Barreiros, J.P.; Porteiro, F.M.; et al. Diet of mid-Atlantic Sowerby’s beaked whales Mesoplondon bidens. Deep Sea Res. A 2011, 58, 1084–1090. [Google Scholar] [CrossRef] [Green Version]
- Daneri, G.; Carlini, A. Fish prey of southern elephant seals, Mirounga leonina, at King George Island. Polar Biol. 2002, 25, 739–743. [Google Scholar] [CrossRef]
- O’Dwyer, P.; Berrow, S.; López-Suárez, P.; Oujo Lamao, C. Insights into the diet of a poorly known species: Pygmy killer whale Feresa attenuata from Cape Verde, West Africa. Afr. J. Mar. Sci. 2015, 37, 427–430. [Google Scholar] [CrossRef]
- Ohizumi, H.; Kuramochi, T.; Kubodera, T.; Yoshioka, M.; Miyazaki, N. Feeding habits of Dall’s porpoises (Phocoenoides dalli) in the subarctic North Pacific and the Bering Sea basin and the impact of predation on mesopelagic micronekton. Deep Sea Res. A 2003, 50, 593–610. [Google Scholar] [CrossRef]
- Casaux, R.; Baroni, A.; Carlini, A. The diet of the Antarctic fur seal Arctocephalus gazella at Harmony Point, Nelson Island, South Shetland Islands. Polar Biol. 1998, 20, 424–428. [Google Scholar] [CrossRef]
- Cherel, Y.; Duhamel, G. Diet of the squid Moroteuthis ingens (Teuthoidea: Onychoteuthidae) in the upper slope waters of the Kerguelen Islands. Mar. Ecol. Prog. Ser. 2003, 250, 197–203. [Google Scholar] [CrossRef]
- Markaida, U.; Sosa-Nishizaki, O. Food and feeding habits of jumbo squid Dosidicus gigas (Cephalopoda: Ommastrephidae) from the Gulf of California, Mexico. J. Mar. Biol. Assoc. UK 2003, 83, 507–522. [Google Scholar] [CrossRef]
- Moteki, M.; Arai, M.; Tsuchiya, K.; Okamoto, H. Composition of piscine prey in the diet of large pelagic fish in the eastern tropical Pacific Ocean. Fish. Sci. 2001, 67, 1063–1074. [Google Scholar] [CrossRef]
- Pethybridge, H.; Daley, R.K.; Nichols, P.D. Diet of demersal sharks and chimaeras inferred by fatty acid profiles and stomach content analysis. J. Exp. Mar. Biol. Ecol. 2011, 409, 290–299. [Google Scholar] [CrossRef]
- Croxall, J.; Prince, P.; Reid, K. Dietary segregation of krill-eating South Georgia seabirds. J. Zool. 1997, 242, 531–556. [Google Scholar] [CrossRef]
- Cherel, Y.; Verdon, C.; Ridoux, V. Seasonal importance of oceanic myctophids in king penguin diet at Crozet Islands. Polar Biol. 1993, 13, 355–357. [Google Scholar] [CrossRef] [Green Version]



| Cr | Mn | Co | Ni | Cu | Zn | As | Se | Cd | Pb | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (µg.g−1, DW) | |||||||||||
| IAEA - 452 | Obtained | - | 250 ± 6.0 | 1.79 ± 0.30 | - | 11 ± 1.5 | 165 ± 30 | 18.5 ± 3.6 | 7.7 ± 0.3 | 31.7 ± 4.4 | - |
| Certified | - | 273 ± 34 | 1.62 ± 0.20 | - | 10.8 ± 1.3 | 166 ± 21 | 17.5 ± 2.2 | 6.55 ± 0.82 | 29.6 ± 3.7 | - | |
| DORM-4 | Obtained | 2.2 ± 0.85 | - | - | 1.4 ± 0.36 | 16 ± 2.9 | 55 ± 13 | 7.4 ± 1.5 | 4.2 ± 1.5 | 0.31 ± 0.058 | 0.32 ± 0.10 |
| Certified | 1.87 ± 0.16 | - | - | 1.36 ± 0.22 | 15.9 ± 0.9 | 52.2 ± 3.2 | 6.80 ± 0.64 | 3.56 ± 0.34 | 0.306 ± 0.015 | 0.416 ± 0.053 | |
| DOLT-4 | Obtained | 1.2 ± 0.69 | - | 0.23 ± 0.03 | 0.71 ± 0.28 | 32 ± 3.8 | 125 ± 12 | 9.1 ± 1.8 | 9.1 ± 1.1 | 24 ± 3.9 | 0.22 ± 0.15 |
| Certified | 1.4 * | - | 0.25 * | 0.97 ± 0.11 | 31.2 ± 1.1 | 116 ± 6 | 9.66 ± 0.62 | 8.3 ± 1.3 | 24.3 ± 0.8 | 0.16 ± 0.04 | |
| id | Region | n | Cr | Mn | Co | Ni | Cu | Zn | As | Se | Cd | Pb | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (µg.g−1, dw) | |||||||||||||
| Triphoturus mexicanus | Guaymas Basin | 45 | 1.3 (1–2.5) | 1.5 (1.2–2.6) | 0.039 (0.0085–0.055) | 0.33 (0.2–1.5) | 2.6 (2.3–3.3) | 40 (31–98) | 6.8 (5.8–9.3) | 4.1 (3.2–4.4) | 1.02 (0.55–1.7) | 0.66 (0.12–4.5) | a |
| Benthosema panamense | 45 | 0.97 (0.70–1.2) | 1.6 (1.2–2.3) | 0.034 (0.017–0.049) | 0.32 (0.17–0.40) | 2.1 (1.7–2.6) | 31 (26–37) | 7.1 (5.3–8.3) | 3.7 (3.0–4.6) | 1.2 (0.57–1.9) | 0.27 (0.07–0.47) | ||
| Diaphus effulgens | India | - | - | ND | - | - | ND | ND | - | - | ND | - | b |
| Diaphus hudsoni | - | - | 1 ± 0.2 | - | - | ND | ND | - | - | ND | - | ||
| Myctophidae | Kerguelen archipelago | 45 | - | - | - | - | 1.0 ± 0.3 | 9 ± 2 | - | - | 0.011 ± 0.007 | - | c |
| Myctophidae | Western United States | 9 | - | - | - | - | - | 10 | - | - | 0.060 | - | d |
| Myctophidae | Atlantic Ocean 48N and 40S | 76 | - | - | - | - | 6.2 | - | - | - | 1.5 | - | e |
| Hygophum hygomi | Sargasso Sea | 2 | - | - | - | - | 3.4 | 15 | <1.0 | - | <1.0 | - | f |
| Cerastocopelus warmingii | 2 | - | - | - | - | 2.2 | 35 | <1.0 | - | 0.7 | - | ||
| Notoscopelus caudispinous | 2 | - | - | - | - | 3.2 | 81 | <1.0 | - | 0.4 | - | ||
| Lobianchia dofleini | 1 | - | - | - | - | 23.0 | 49 | <1.0 | - | 1.6 | - | ||
| Lepidophanes indicas | 1 | - | - | - | - | 13.0 | 56 | <1.0 | - | 0.9 | - | ||
| Diaphus mollis | 1 | - | - | - | - | 7.0 | 34 | <1.0 | - | 0.8 | - | ||
| Lampanyctus pusillus | 1 | - | - | - | - | 23.0 | 48 | <1.0 | - | 1.6 | - | ||
| Lampanyctus pusillus | 1 | - | - | - | - | 2.7 | 27 | <1.0 | - | 0.4 | - | ||
| Myctophum glaciale | Mediterranean | 4.1–11 | 0.03–0.24 | - | 2–6.4 | 1.3–44.8 | 0.10–0.28 | g | |||||
| Ceratoscopelus warmingii | Sulu Sea | 3 | 3–8.4 | 5.6–6.5 | 0.13 | - | 3.8–5.2 | 39–47 | 28–46 | 2.2–3.1 | 0.75–0.99 | 0.19–0.21 | |
| Diaphus problematicus | 1 | 0.23 | 3.4 | 0.081 | - | 3.8 | 39.9 | 25.1 | 2.5 | 0.78 | 0.091 | h | |
| Diaphus regani | 1 | 1.2 | 6.9 | 0.11 | - | 5.6 | 36.1 | 15.9 | 1.9 | 0.76 | 0.099 | ||
| Gymnoscopelus nicholsi | Kerguelen Islands | 4 | - | - | - | - | 1.9–3.4 | 6.6–15.0 | - | - | 0.004–0.021 | - | i |
| Gymnoscopelus piabilis | 5 | - | - | - | - | 0.8–1.7 | 8.4–11.3 | - | - | 0.004–0.021 | - | ||
| Electrona antarctica | Kerguelen Islands | 15 | - | - | - | - | 2.1 ± 0.5 (1.6 - 3.5) | 22 ± 3 (17-28) | - | - | 0.270 ± 0.101 (0.132–0.506) | - | j |
| Gymnoscopelus fraseri | 15 | - | - | - | - | 3.2 ± 0.6 (2.4–4.8) | 27 ± 2 (24–31) | - | - | 0.496 ± 0.233 (0.256–0.929) | - | ||
| Gymnoscopelus nicholsi | 4 | - | - | - | - | 2.2 ± 0.7 (1.4–2.9) | 19 ± 1 (17–20) | - | - | 0.251 ± 0.098 (0.180–0.392) | - | ||
| Gymnoscopelus piabilis | 14 | - | - | - | - | 2.3 ± 0.3 (1.6–2.9) | 28 ± 4 (20–35) | - | - | 0.887 ± 0.454 (0.453–1.826) | - | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figueiredo, C.; Baptista, M.; Grilo, T.F.; Caetano, M.; Markaida, U.; Raimundo, J.; Rosa, R. Bioaccumulation of Trace Elements in Myctophids in the Oxygen Minimum Zone Ecosystem of the Gulf of California. Oceans 2020, 1, 34-46. https://doi.org/10.3390/oceans1010004
Figueiredo C, Baptista M, Grilo TF, Caetano M, Markaida U, Raimundo J, Rosa R. Bioaccumulation of Trace Elements in Myctophids in the Oxygen Minimum Zone Ecosystem of the Gulf of California. Oceans. 2020; 1(1):34-46. https://doi.org/10.3390/oceans1010004
Chicago/Turabian StyleFigueiredo, Cátia, Miguel Baptista, Tiago F. Grilo, Miguel Caetano, Unai Markaida, Joana Raimundo, and Rui Rosa. 2020. "Bioaccumulation of Trace Elements in Myctophids in the Oxygen Minimum Zone Ecosystem of the Gulf of California" Oceans 1, no. 1: 34-46. https://doi.org/10.3390/oceans1010004
APA StyleFigueiredo, C., Baptista, M., Grilo, T. F., Caetano, M., Markaida, U., Raimundo, J., & Rosa, R. (2020). Bioaccumulation of Trace Elements in Myctophids in the Oxygen Minimum Zone Ecosystem of the Gulf of California. Oceans, 1(1), 34-46. https://doi.org/10.3390/oceans1010004








