Pathological Studies and Postmortem Computed Tomography of Dolphins with Meningoencephalomyelitis and Osteoarthritis Caused by Brucella ceti
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dolphins
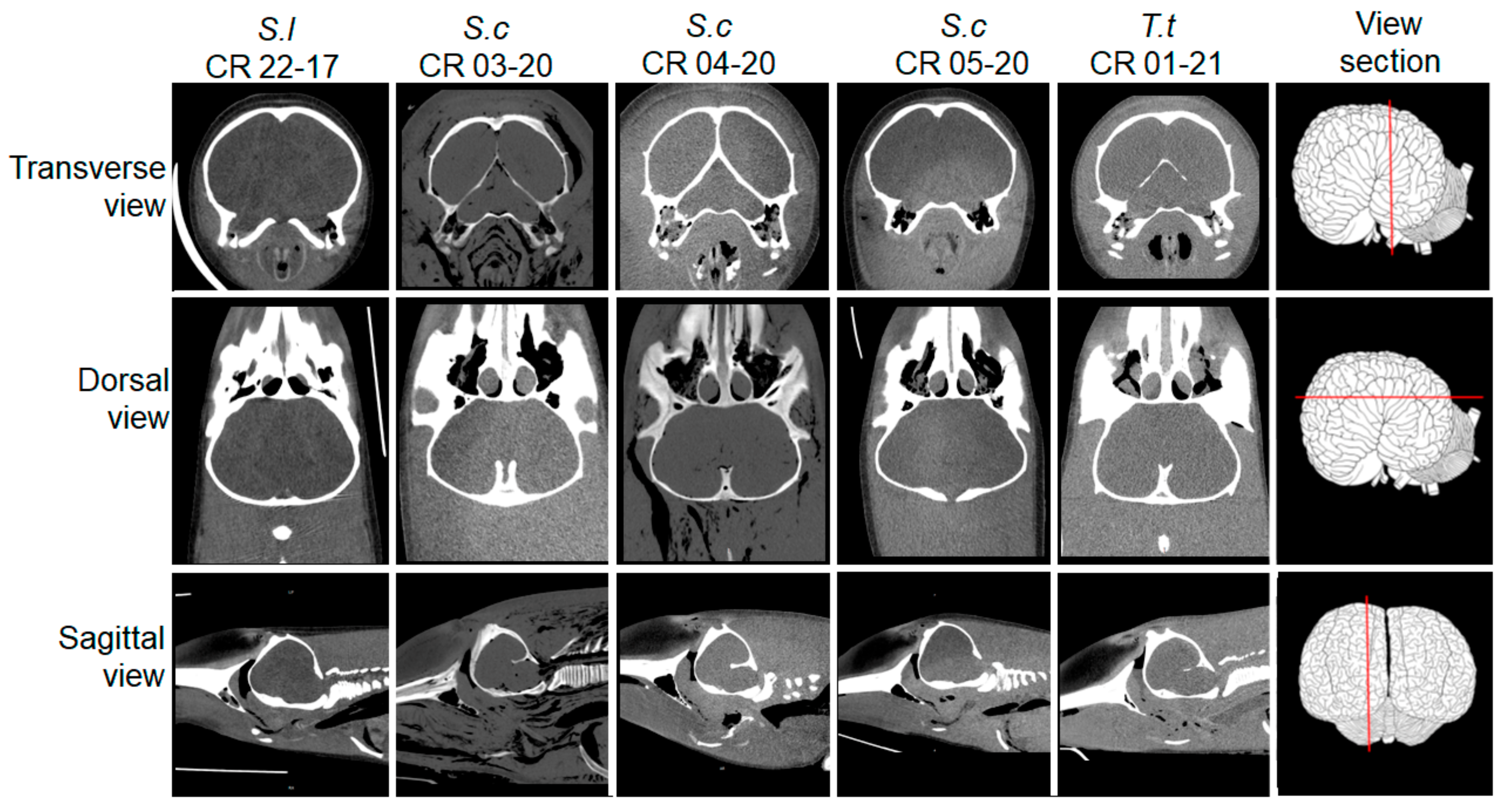
2.2. Postmortem Computed Tomography (PMCT)
2.3. Necropsy, Sample Collection, and Histopathology
2.4. Detection of Antibodies and Bacterial Isolation
3. Results
3.1. Dolphins
3.2. PMCT, Necropsy, Histopathology, Serology, and Bacterial Isolation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- May-Collado, L.; Amador-Caballero, M.; Casa, J.; Gamboa-Poveda, M. Ecology and Conservation of Cetaceans of Costa Rica and Panama. In Advances in Marine Vertebrate Research in Latin America; Rossi-Santos, M., Finkl, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 293–319. [Google Scholar]
- Rosel, P.E.; Wilcox, L.A.; Yamada, T.K.; Mullin, K.D. A new species of baleen whale (Balaenoptera) from the Gulf of Mexico, with a review of its geographic distribution. Mar. Mam. Sci. 2021, 37, 577–610. [Google Scholar] [CrossRef]
- Dubey, J.P.; Morales, J.A.; Sundar, N.; Velmurugan, G.V.; González-Barrientos, C.R.; Hernández-Mora, G.; Su, C. Isolation and genetic characterization of Toxoplasma gondii from striped dolphin (Stenella coeruleoalba) from Costa Rica. J. Parasitol. 2007, 93, 710–711. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Mora, G.; González-Barrientos, R.; Morales, J.A.; Chaves-Olarte, E.; Guzmán-Verri, C.; Barquero-Calvo, E.; De-Miguel, M.J.; Marín, C.M.; Blasco, J.M.; Moreno, E. Neurobrucellosis in stranded dolphins, Costa Rica. Emerg. Infect. Dis. 2008, 14, 1430–1433. [Google Scholar] [CrossRef] [PubMed]
- Teman, S.J.; Gaydos, J.K.; Norman, S.A.; Huggins, J.L.; Lambourn, D.M.; Calambokidis, J.; Ford, J.; Hanson, M.B.; Haulena, M.; Zabek, E.; et al. Epizootiology of a Cryptococcus gattii outbreak in porpoises and dolphins from the Salish Sea. Dis. Aquat. Organ. 2021, 146, 129–143. [Google Scholar] [CrossRef]
- Rubio-Guerri, C.; Melero, M.; Esperón, F.; Bellière, E.N.; Arbelo, M.; Crespo, J.L.; Sierra, E.; García-Párraga, D.; Sánchez-Vizcaíno, J.M. Unusual striped dolphin mass mortality episode related to cetacean morbillivirus in the Spanish Mediterranean Sea. BMC Vet. Res. 2013, 9, 106. [Google Scholar] [CrossRef] [Green Version]
- González-Barrientos, R.; Morales, J.A.; Hernández-Mora, G.; Barquero-Calvo, E.; Guzmán-Verri, C.; Chaves-Olarte, E.; Moreno, E. Pathology of striped dolphins (Stenella coeruleoalba) infected with Brucella ceti. J. Comp. Pathol. 2010, 142, 347–352. [Google Scholar] [CrossRef]
- Guzmán-Verri, C.; González-Barrientos, R.; Hernández-Mora, G.; Morales, J.A.; Baquero-Calvo, E.; Chaves-Olarte, E.; Moreno, E. Brucella ceti and brucellosis in cetaceans. Front. Cell. Infect. Microbiol. 2012, 2, 3. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Mora, G.; González-Barrientos, R.; Víquez-Ruíz, E.; Palacios-Alfaro, J.D.; Bettoni-Rodríguez, G.; Gendre, M.; Vincent, C.; Roca-Monge, K.; Ruiz-Villalobos, N.; Suárez-Esquivel, M.; et al. Brucella sp. sequence-type 27 associated with abortion in dwarf sperm whale Kogia Sima. Eur. J. Wild. Res. 2021, 67, 1–5. [Google Scholar] [CrossRef]
- Isidoro-Ayza, M.; Ruiz-Villalobos, N.; Pérez, L.; Guzmán-Verri, C.; Muñoz, P.M.; Alegre, F.; Barberán, M.; Chacón-Díaz, C.; Chaves-Olarte, E.; González-Barrientos, R.; et al. Brucella ceti infection in dolphins from the Western Mediterranean Sea. BMC Vet. Res. 2014, 10, 206. [Google Scholar] [CrossRef] [Green Version]
- Whatmore, A.M.; Dawson, C.E.; Groussaud, P.; Koylass, M.S.; King, A.C.; Shankster, S.J.; Sohn, A.H.; Probert, W.S.; McDonald, W.L. Marine mammal Brucella genotype associated with zoonotic infection. Emerg. Infect. Dis. 2008, 14, 517–518. [Google Scholar] [CrossRef]
- Suárez-Esquivel, M.; Baker, K.S.; Ruiz-Villalobos, N.; Hernández-Mora, G.; Barquero-Calvo, E.; González-Barrientos, R.; Castillo-Zeledón, A.; Jiménez-Rojas, C.; Chacón-Díaz, C.; Cloeckaert, A.; et al. Brucella Genetic variability in wildlife marine mammals populations relates to host preference and ocean distribution. Genome Biol. Evol. 2017, 9, 1901–1912. [Google Scholar] [CrossRef] [PubMed]
- González, L.; Patterson, I.A.; Reid, R.J.; Foster, G.; Barberán, M.; Blasco, J.M.; Kennedy, S.; Howie, F.E.; Godfroid, J.; MacMillan, A.P.; et al. Chronic meningoencephalitis associated with Brucella sp. infection in live-stranded striped dolphins (Stenella coeruleoalba). J. Comp. Pathol. 2002, 126, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Dagleish, M.P.; Barley, J.; Howie, F.E.; Reid, R.J.; Herman, J.; Foster, G. Isolation of Brucella species from a diseased atlanto-occipital joint of an Atlantic white-sided dolphin (Lagenorhynchus acutus). Vet. Rec. 2007, 160, 876–878. [Google Scholar] [CrossRef] [PubMed]
- Esmaeilnejad-Ganji, S.M.; Esmaeilnejad-Ganji, S. Osteoarticular manifestations of human brucellosis: A review. World J. Orthop. 2019, 10, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Turgut, M.; Haddad, F.S.; Divitis, O. Neurobrucellosis. In Clinical, Diagnostic and Therapeutic Features, 1st ed.; Springer: New York, NY, USA, 2016. [Google Scholar]
- Erdem, H.; Senbayrak, S.; Meriç, K.; Batirel, A.; Karahocagil, M.K.; Hasbun, R.; Sengoz, G.; Karsen, H.; Kaya, S.; Inal, A.S.; et al. Cranial imaging findings in neurobrucellosis: Results of Istanbul-3 study. Infection 2016, 44, 623–631. [Google Scholar] [CrossRef]
- Langan, J.N.; Ivančić, M.; Adkesson, M.J.; Chinnadurai, S.K.; Houser, D.; Stacey, R.; Whitehead, H.; Chu, C.; Morell, M.; Colegrove, C.M. Antemortem diagnosis of hydrocephalus and hearing loss associated with chronic Brucella infection in a stranded rehabilitated bottlenose dolphin (Tursiops truncatus). In Proceedings of the IAAAM Meeting & Conference, Virginia, VA, USA, 21–26 May 2016. [Google Scholar]
- Leger, J.A.; Schmitt, T.; Reidarson, T.; Scadeng, M.; Dubowitz, D.; Danil, K. Hydrocephalus and nonsuppurative meningoencephalitis associated with Brucella sp. infection in two live-stranded dolphins. In Proceedings of the IAAAM Meeting & Conference, Orlando, FL, USA, 5–9 May 2007. [Google Scholar]
- Tsui, H.C.L.; Kot, B.C.W.; Chung, T.Y.T.; Chan, D.K.P. Virtopsy as a revolutionary tool for Cetacean Stranding Programs: Implementation and Management. Front. Mar. Sci. 2020, 7, 542015. [Google Scholar] [CrossRef]
- Kot, B.C.W.; Chung, T.Y.T.; Chan, D.K.P.; Tsui, H.C.L. Image rendering techniques in postmortem computed tomography: Evaluation of biological health and profile in stranded cetaceans. J. Vis. Exp. 2020, 163, e61701. [Google Scholar] [CrossRef]
- Kot, B.C.W.; Tsui, H.C.L.; Chung, T.Y.T.; Lau, A.P.Y. Postmortem neuroimaging of cetacean brains using computed tomography and magnetic resonance imaging. Front. Mar. Sci. 2020, 7, 544037. [Google Scholar] [CrossRef]
- USDA. National Veterinary Services Laboratories Guidelines for Necropsy. United States Department of Agriculture. 2021; p. 3, Retrieved November 2021. Available online: www.aphis.usda.gov/animal_health/lab_info_services/downloads/NecropsyGuideline.pdf (accessed on 18 December 2021).
- Hernández-Mora, G.; Manire, C.A.; González-Barrientos, R.; Barquero-Calvo, E.; Guzmán-Verri, C.; Staggs, L.; Thompson, R.; Chaves-Olarte, E.; Moreno, E. Serological diagnosis of Brucella infections in odontocetes. Clin. Vaccine Immunol. 2009, 16, 906–915. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Mora, G.; Bonilla-Montoya, R.; Barrantes-Granados, O.; Esquivel-Suárez, A.; Montero-Caballero, D.; González-Barrientos, R.; Fallas-Monge, Z.; Palacios-Alfaro, J.D.; Baldi, M.; Campos, E.; et al. Brucellosis in mammals of Costa Rica: An epidemiological survey. PLoS ONE 2017, 12, e0182644. [Google Scholar] [CrossRef] [Green Version]
- De Miguel, M.J.; Marín, C.M.; Muñoz, P.M.; Dieste, L.; Grilló, M.J.; Blasco, J.M. Development of a selective culture medium for primary isolation of the main Brucella species. J. Clin. Microbiol. 2011, 49, 1458–1463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alton, G.G.; Jones, L.M.; Angus, R.D.; Verger, J.M. Techniques for Brucellosis Laboratory; INRA: Clermont-Ferrand, France, 1988. [Google Scholar]
- Hong, C.B.; Donahue, J.M.; Giles, R.C., Jr.; Poonacha, K.B.; Tuttle, P.A.; Cheville, N.F. Brucella abortus-associated meningitis in aborted bovine fetuses. Vet. Pathol. 1991, 28, 492–496. [Google Scholar] [CrossRef] [PubMed]
- O’Brown, N.M.; Pfau, S.J.; Gu, C. Bridging barriers: A comparative look at the blood-brain barrier across organisms. Genes dev. 2018, 32, 466–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ridgway, S.H.; Carlin, K.P.; Van Alstyne, K.R.; Hanson, A.C.; Tarpley, R.J. Comparison of dolphins’ body and brain measurements with four other groups of cetaceans reveals great diversity. Brain Behav. Evol. 2016, 88, 235–257. [Google Scholar] [CrossRef]
- Hofman, M.A. Design principles of the human brain: An evolutionary perspective. Prog. Brain Res. 2012, 195, 373–390. [Google Scholar] [CrossRef]
- Miraglia, M.C.; Rodriguez, A.M.; Barrionuevo, P.; Rodriguez, J.; Kim, K.S.; Dennis, V.A.; Delpino, M.V.; Giambartolomei, G.H. Brucella abortus traverses brain microvascular endothelial cells using infected monocytes as a Trojan horse. Front. Cell. Infect. Microbial. 2018, 8, 200. [Google Scholar] [CrossRef]
- Türel, O.; Sanli, K.; Hatipoğlu, N.; Aydoğmuş, C.; Hatipoğlu, H.; Siraneci, R. Acute meningoencephalitis due to Brucella: Case report and review of neurobrucellosis in children. Turk. J. Pediatr. 2010, 5252, 426–429. [Google Scholar]
- Brix, M.K.; Westman, E.; Simmons, A.; Ringstad, G.A.; Eide, P.K.; Wagner-Larsen, K.; Page, C.M.; Vitelli, V.; Beyer, M.K. The Evans’ Index revisited: New cutoff levels for use in radiological assessment of ventricular enlargement in the elderly. Eur. J. Radiol. 2017, 95, 28–32. [Google Scholar] [CrossRef] [Green Version]
- Hamidu, A.U.; Olarinoye-Akorede, S.A.; Ekott, D.S.; Danborno, B.; Mahmud, M.R.; Balogun, M.S. Computerized tomographic study of normal Evans index in adult Nigerians. J. Neurosci. Rural Pract. 2015, 66, 55–58. [Google Scholar] [CrossRef]
- Cassle, S.E.; Jensen, E.D.; Smith, C.R.; Meegan, J.M.; Johnson, S.P.; Lutmerding, B.; Ridgway, S.H.; Francis-Floyd, R. Diagnosis and successful treatment of a lung abscess associated with Brucella species infection in a bottlenose dolphin (Tursiops truncatus). J. Zoo Wildl. Med. 2013, 44, 495–499. [Google Scholar] [CrossRef]
- Aziz-Ahari, A.; Mamishi, S.; Dadkhah, A.; Ghazinejadian-Sh, F.S. Neurobrucellosis in a 9-year-old girl. J. Radiol. Case Rep. 2019, 13, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Scian, R.; Barrionuevo, P.; Fossati, C.A.; Giambartolomei, G.H.; Delpino, M.V. Brucella abortus invasion of osteoblasts inhibits bone formation. Infect. Immun. 2012, 80, 2333–2345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueno, Y.; Yanagisawa, M.; Kino, S.; Shigeno, S.; Osaki, M.; Takamatsu, D.; Katsuda, K.; Maruyama, T.; Ohishi, K. Molecular characterization of Brucella ceti from a bottlenose dolphin (Tursiops truncatus) with osteomyelitis in the western Pacific. J. Vet. Med. Sci. 2020, 82, 754–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Sous, M.W.; Bohlega, S.; Al-Kawi, M.Z.; Alwatban, J.; McLean, D.R. Neurobrucellosis: Clinical and neuroimaging correlation. AJNR. Am. J. Neuroradiol. 2004, 25, 395–401. [Google Scholar] [PubMed]
- Buckle, K.; Roe, W.D.; Howe, L.; Michael, S.; Duignan, P.J.; Burrows, E.; Ha, H.J.; Humphrey, S.; McDonald, W.L. Brucellosis in endangered Hector’s dolphins (Cephalorhynchus hectori). Vet. Pathol. 2017, 54, 838–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Code | Species | Age | Sex | Stranding | Ocean | PMCT Days b | RBT/ cELISA | B. cetic |
|---|---|---|---|---|---|---|---|---|
| CR 22-17 | Stenella longirostris | C | M | 2017 | P | 10 | Negative | Negative |
| CR 03-20 | Stenella coeruleoalba | A | M | 2020 | P | 0 | Negative | Negative |
| CR 04-20 | Stenella coeruleoalba | A | F | 2020 | P | 3 | Negative | Negative |
| CR 05-20 | Stenella coeruleoalba | C | F | 2020 | P | 3 | Negative | Negative |
| CR 01-21 | Tursiops truncatus | A | F | 2021 | P | 0 | Negative | Negative |
| CR 09-20 | Lagenodelphis hosei | A | M | 2020 | Cb | 20 | Positive | Negative |
| CR 03-18 | Stenella coeruleoalba | J | M | 2018 | P | 8 | Positive | Positive |
| CR 10-20 | Stenella coeruleoalba | A | M | 2020 | P | 3 | Positive | Positive |
| CR 05-19 | Stenella coeruleoalba | A | F | 2019 | P | 1 | Positive | Positive |
| CR 07-19 | Stenella coeruleoalba | A | F | 2019 | P | 2 | Positive | Positive |
| CR 08-20 | Stenella coeruleoalba | A | M | 2020 | P | 24 | Positive | Positive |
| CR 01-18 | Stenella coeruleoalba | C | F | 2018 | P | 2 | Positive | Positive |
| CR 08-17 | Delphinus delphis | A | M | 2017 | P | ND | Positive | Positive |
| CR 15-18 | Stenella coeruleoalba | A | F | 2018 | P | ND | Positive | Positive |
| CR 20-18 | Stenella coeruleoalba | A | M | 2018 | P | ND | Positive | Positive |
| CR 06-17 | Stenella coeruleoalba | A | M | 2017 | P | ND | Positive | Positive |
| CR 13-17 | Stenella coeruleoalba | A | M | 2017 | P | ND | Positive | Positive |
| CR 16-17 | Stenella coeruleoalba | A | M | 2017 | P | ND | Positive | Positive |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Granados-Zapata, A.; Robles-Malagamba, M.J.; González-Barrientos, R.; Kot, B.C.-W.; Barquero-Calvo, E.; Cordero-Chavaría, M.; Suárez-Esquivel, M.; Guzmán-Verri, C.; Palacios-Alfaro, J.D.; Tien-Sung, C.; et al. Pathological Studies and Postmortem Computed Tomography of Dolphins with Meningoencephalomyelitis and Osteoarthritis Caused by Brucella ceti. Oceans 2022, 3, 189-203. https://doi.org/10.3390/oceans3020014
Granados-Zapata A, Robles-Malagamba MJ, González-Barrientos R, Kot BC-W, Barquero-Calvo E, Cordero-Chavaría M, Suárez-Esquivel M, Guzmán-Verri C, Palacios-Alfaro JD, Tien-Sung C, et al. Pathological Studies and Postmortem Computed Tomography of Dolphins with Meningoencephalomyelitis and Osteoarthritis Caused by Brucella ceti. Oceans. 2022; 3(2):189-203. https://doi.org/10.3390/oceans3020014
Chicago/Turabian StyleGranados-Zapata, Andrés, María José Robles-Malagamba, Rocío González-Barrientos, Brian Chin-Wing Kot, Elías Barquero-Calvo, Minor Cordero-Chavaría, Marcela Suárez-Esquivel, Caterina Guzmán-Verri, Jose David Palacios-Alfaro, Connie Tien-Sung, and et al. 2022. "Pathological Studies and Postmortem Computed Tomography of Dolphins with Meningoencephalomyelitis and Osteoarthritis Caused by Brucella ceti" Oceans 3, no. 2: 189-203. https://doi.org/10.3390/oceans3020014
APA StyleGranados-Zapata, A., Robles-Malagamba, M. J., González-Barrientos, R., Kot, B. C. -W., Barquero-Calvo, E., Cordero-Chavaría, M., Suárez-Esquivel, M., Guzmán-Verri, C., Palacios-Alfaro, J. D., Tien-Sung, C., Moreno, E., & Hernández-Mora, G. (2022). Pathological Studies and Postmortem Computed Tomography of Dolphins with Meningoencephalomyelitis and Osteoarthritis Caused by Brucella ceti. Oceans, 3(2), 189-203. https://doi.org/10.3390/oceans3020014








