Review of the Design of Current Collectors for Improving the Battery Performance in Lithium-Ion and Post-Lithium-Ion Batteries
Abstract
:1. Introduction
2. Selection of Materials for CCs
3. Surface Modification of CCs for Improving and Maintaining Battery Performance
3.1. Surface Morphology Modification to Improve the Adhesion of the Active Material Layer to the CC
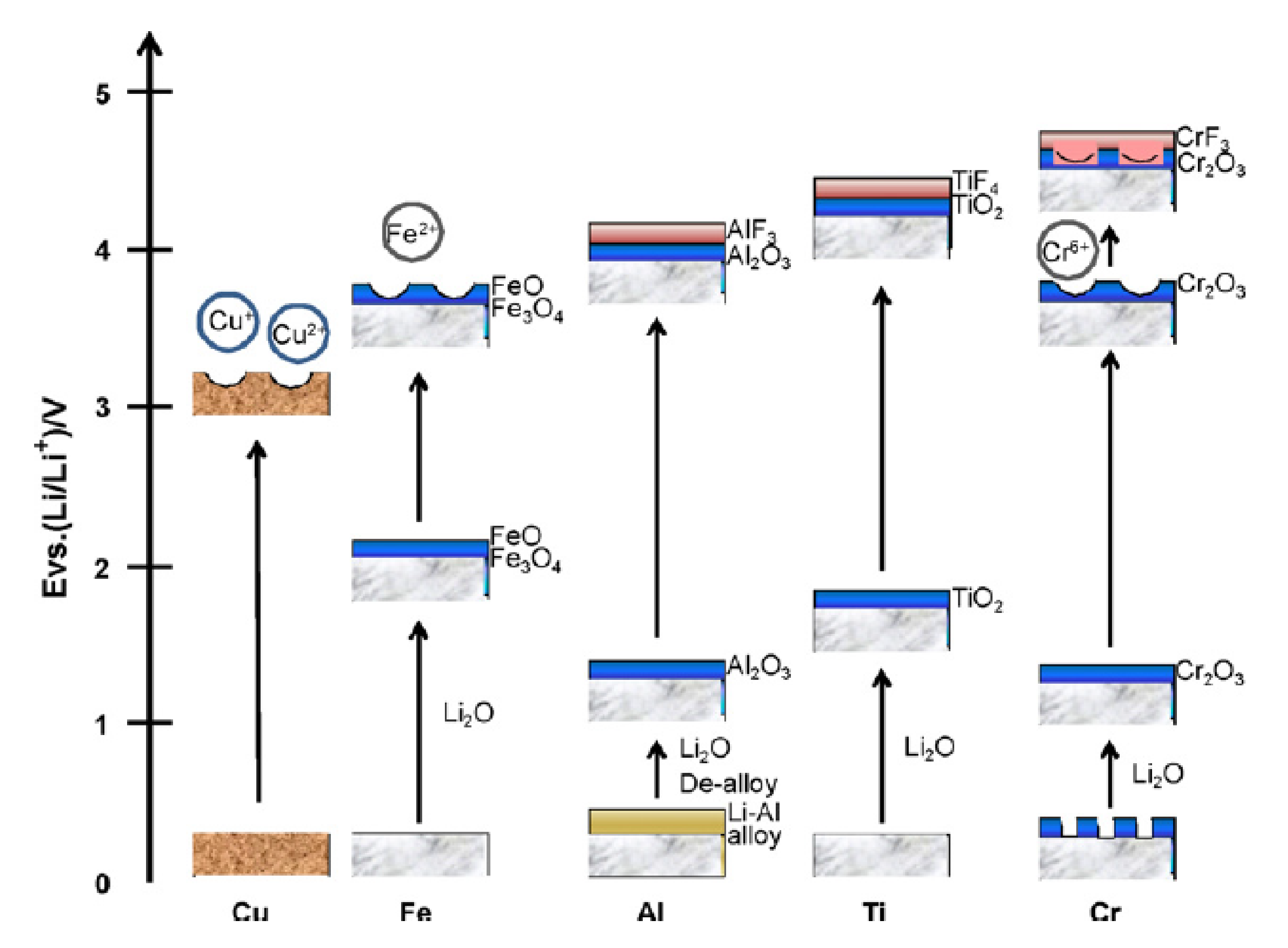
3.2. Inhibition of CC Corrosion for Chemical Stability of CCs: Selection of Li Salts, Solvents and Additives
3.3. Primer Layer Coatings on CCs to Inhibit Their Corrosion and to Decrease the Contact Resistance
4. 3D CCs
4.1. CC Structures that Inhibit the Formation of Li Dendrites
4.2. 3D Structures for Volumetric High Energy Density in a Cell
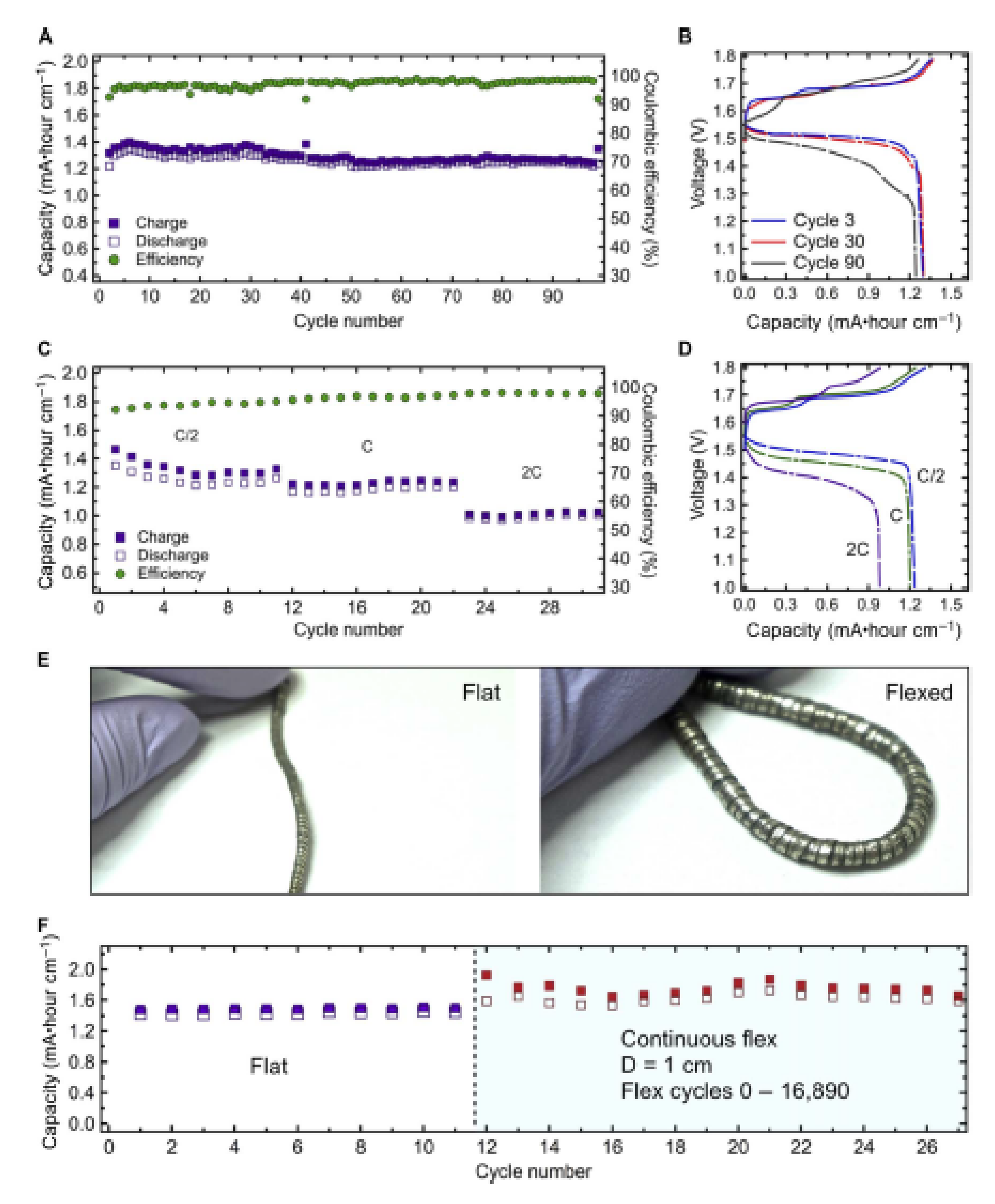
4.3. Flexible and Free-Standing CCs
5. Improvement of Durability of CCs against Mechanical Stress and Corrosion Caused by Contact with Water
5.1. CCs for Si Anodes
5.2. CCs for Water Processing in Anode and Cathode Fabrication and Aqueous LIBs
6. Structure of CCs to Ensure the Safety of LIBs
7. CCs for All Solid-State LIBs
8. CCs for Post-LIBs
8.1. Na-Ion and Na Metal Batteries
8.2. Mg-Ion and Mg Metal Batteries
8.3. Ca-Ion and Ca Metal Batteries
8.4. Al Batteries
8.5. Sulfur Batteries
9. Conclusions and Perspectives
- (1)
- To provide anticorrosion properties and a wide potential window for CCs, stainless steel and Ni, which are costly and have complicated coating processes, should not be employed. In contrast, additives that can be added to electrolytes should be developed because they can adsorb onto CC surfaces and form protective layers to inhibit corrosion and electrochemical dissolution at higher potentials. The employment of additives may avoid an increase in the number of processes in the LIB fabrication process. We should turn our attention towards the development of additives that can easily form a desirable surface layer.
- (2)
- Many 3D CC structures that contain thick electrodes and produce a cell with a high energy density have been proposed in many papers and have been found to exhibit excellent battery performance. To achieve 3D anodes and cathodes with 3D CCs in commercially available batteries, the mass production of 3D anodes and cathodes is fundamentally required. The recent fabrication of 3D structures with lasers may shed light on solving the problem [198]. Laser processes for cutting, annealing, structuring, and printing battery materials that show great potential for battery mass production have been developed to minimize fabrication costs and to increase the electrochemical performance and operational lifetime of batteries. It is especially important to develop highly scalable methods by shortening laser processing time.
- (3)
- In currently available LIBs, Cu and Al CCs are used because of their light weight, low cost, and chemical and electrochemical stability. In the future, it will be highly necessary for CCs to be composed light materials, and the content ratio of the CCs in a cell will need to be extremely close to zero. Recently, the words “free-standing” have often been seen in the titles of papers related to batteries. This term can refer to a CC-free or metal-free CC and carbon CCs. In addition, the number of papers researching carbon-based CCs has recently increased. Highly functional carbon materials are being applied to prepare CCs with high electron conductivity and anticorrosion properties. Carbon materials are good for the construction of 3D CCs that can produce thick anode and cathode layers and that improve the energy density of batteries. Additionally, in the area of flexible batteries, which will be demanded and progressively developed, carbon CCs will play an important role and realize the upcoming commercialization of flexible batteries as the power sources for wearable devices.
- (4)
- Three-dimensional anodes and cathodes are becoming increasingly important factors to remarkably improve battery performance. To date, 3D CCs can be formed with various strategies. However, the fabrication process of 3D anodes and cathodes with 3D CCs has not been developed. In current LIB fabrication processes, the anode and cathode are prepared by casting a slurry containing anode or cathode materials on planar CC surfaces and drying the slurry thin layer on the CC surface. However, in 3D anodes and cathodes with 3D CCs, dense anode and cathode layers cannot be formed without voids in the active material layers at the mass production level. To the best of our knowledge, coating machines for 3D CCs have not yet been developed. Thus, the development of coating machines is urgent.
- (5)
- To date, the development of all-solid-state batteries and post-LIBs is encouraging because dedicated research efforts are required to commercialize batteries. Conventional CCs are used for batteries. However, a deep examination of matching CCs and electrolytes for batteries has not yet been performed. As done in the case of organic liquids containing Li salts, the chemical and electrochemical stability of CCs should be analyzed with various experimental techniques. In particular, a discussion of the reaction of CCs with solid-state electrolytes is insufficient. The data regarding CCs for solid-state electrolytes should be published as soon as possible because the resistance between CCs and active material layers should be removed so that research on electron and Li+ transfers in the interface between active material surfaces and electrolytes can be conducted. After optimizing CCs for each battery, future research should focus on considering strategies to incorporate CCs into the mass production of all-solid-state batteries and post-LIBs.
Author Contributions
Funding
Conflicts of Interest
References
- Winter, M.; Barnett, B.; Xu, K. Before Li ion batteries. Chem. Rev. 2018, 118, 11433–11456. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 years of lithium-ion batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Xu, J.; Yin, D.; Du, Y. Electrolytes for batteries with earth-abundant metal anodes. Chem. Eur. J. 2018, 24, 18220–18234. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Roy, P.; Srivastava, S.K. Nanostructured anode materials for lithium ion batteries. J. Mater. Chem. A 2015, 3, 2454–2484. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Park, K.-S. The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef]
- Yuan, M.; Liu, K. Rational design on separators and liquid electrolytes for safer lithiumion batteries. J. Energy Chem. 2020, 43, 58–70. [Google Scholar] [CrossRef] [Green Version]
- Kato, Y.; Hori, S.; Saito, T.; Suzuki, K.; Hirayama, M.; Mitsui, A.; Yonemura, M.; Iba, H.; Kanno, R. High-power all-solid-state batteries using sulfide superionic conductors. Nat. Energy 2016, 1, 16030. [Google Scholar] [CrossRef]
- Chen, H.; Ling, M.; Hencz, L.; Ling, H.Y.; Li, G.; Lin, Z.; Liu, G.; Zhang, S. Exploring chemical, mechanical, and electrical functionalities of binders for advanced energy-storage devices. Chem. Rev. 2018, 118, 8936–8982. [Google Scholar] [CrossRef]
- Yang, Z.; Ren, J.; Zhang, Z.; Chen, X.; Guan, G.; Qiu, L.; Zhang, Y.; Peng, H. Recent advancement of nanostructured carbon for energy applications. Chem. Rev. 2015, 115, 5159–5223. [Google Scholar] [CrossRef]
- Ming, J.; Cao, Z.; Wu, Y.; Wahyudi, Y.; Wang, W.; Guo, X.; Cavallo, L.; Hwang, J.-Y.; Shamim, A.; Li, L.-J.; et al. New insight on the role of electrolyte additives in rechargeable lithium ion batteries. ACS Energy Lett. 2019, 4, 2613–2622. [Google Scholar] [CrossRef]
- Myung, S.-T.; Sasaki, Y.; Sakurada, S.; Sun, Y.-K.; Yashiro, H. Electrochemical behavior of current collectors for lithium batteries in non-aqueous alkyl carbonate solution and surface analysis by ToF-SIMS. Electrochim. Acta 2009, 55, 288–297. [Google Scholar] [CrossRef]
- Bard, A.J.; Parsons, R.; Joedan, J. Standard Potentials in Aqueous Solutions, International Union of Pure and Applied Chemistry; Marcel Dekker, Inc.: New York, NY, USA, 1985. [Google Scholar]
- Dai1, S.; Chen, J.; Ren, Y.; Liu, Z.; Chen, J. Electrochemical corrosion behavior of the copper current collector in the electrolyte of lithium-ion batteries. Int. J. Electrochem. Sci. 2017, 12, 10589–10598. [Google Scholar] [CrossRef]
- Chen, J.; Li, C.; Zhang, J.; Li, C.; Chen, J.; Ren, Y. First-principles study on the adsorption and dissociation of impurities on copper current collector in electrolyte for lithium-ion batteries. Materials 2018, 11, 1256. [Google Scholar] [CrossRef] [Green Version]
- Fear, C.; Juarez-Robles, D.; Jeevarajan, J.A.; Mukherjee, P.P. Elucidating copper dissolution phenomenon in Li-ion cells under overdischarge extremes. J. Electrochem. Soc. 2018, 165, A1639–A1647. [Google Scholar] [CrossRef]
- Onodera, S.; Kato, N.; Ito, T.; Ito, T.; Tachibana, K.; Nishina, N. The Effect of dispersion degree of positive electrodes containing carbon nanotubes with identical composition on contact resistance in lithium ion secondary batteries. Electrochemistry 2015, 83, 386–388. [Google Scholar] [CrossRef]
- Honda, C.; Kikuchi, S.; Ito, T.; Onodera, S.; Nagai, A.; Ito, T.; Tachibana, K.; Nishina, T. Evaluation of contact resistance of aluminum current collector surfaces for energy storage systems. Electrochemistry 2014, 82, 328–330. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.-C.; Wu, H.-C.; Lee, E.; Wu, N.-L. High-temperature carbon-coated aluminum current collector for enhanced power performance of LiFePO4 electrode of Li-ion batteries. Electrochem. Commun. 2010, 12, 488–491. [Google Scholar] [CrossRef]
- Myung, S.-T.; Yashiro, H.; Sun, Y.-K. Electrochemical behavior and passivation of current collectors in lithium-ion batteries. J. Mater. Chem. 2011, 21, 9891–9911. [Google Scholar] [CrossRef]
- Streipert, B.; Röser, S.; Kasnatscheew, J.; Janßen, P.; Cao, X.; Wagner, R.; Cekic-Laskovic, I.; Winter, M. Influence of LiPF6 on the aluminum current collector dissolution in high voltage lithium ion batteries after long-term charge/discharge experiments. J. Electrochem. Soc. 2017, 164, A1474–A1479. [Google Scholar] [CrossRef]
- Ma, T.; Xu, G.-L.; Li, Y.; Wang, L.; He, X.; Zheng, J.; Liu, J.; Engelhard, M.H.; Zapol, P.; Curtiss, L.A.; et al. Revisiting the corrosion of the aluminum current collector in lithium-ion batteries. J. Phys. Chem. Lett. 2017, 8, 1072–1077. [Google Scholar] [CrossRef]
- Sayed, F.N.; Rodrigues, M.-T.F.; Kalaga, K.; Gullapalli, H.; Ajayan, P.M. Curious case of positive current collectors: Corrosion and passivation at high temperature. ACS Appl. Mater. Interfaces 2017, 9, 43623–43631. [Google Scholar] [CrossRef]
- Ni, S.; Li, T.; Lv, X.; Yang, X.; Zhang, L. Designed constitution of NiO/Ni nanostructured electrode for high performance lithium ion battery. Electrochim. Acta 2013, 91, 267–274. [Google Scholar] [CrossRef]
- Yao, M.; Okuno, K.; Iwaki, T.; Kato, M.; Tanase, S.; Emura, K.; Sakai, T. LiFePO4-based electrode using micro-porous current collector for high power lithium ion battery. J. Power Sources 2007, 173, 545–549. [Google Scholar] [CrossRef]
- Song, K.Y.; Jang, G.S.; Tao, J.; Lee, J.H.; Joo, S.K. Influence of conductive carbon content using a three-dimensional foam-type current collector for lithium ion battery. J. Electrochem. Soc. 2016, 163, A2981–A2987. [Google Scholar] [CrossRef]
- Kang, C.W.; Choi, J.; Ko, Y.-J.; Lee, S.M.; Kim, H.J.; Kim, J.P.; Son, S.U. Thin coating of microporous organic network makes a big difference: Sustainability issue of Ni electrodes on the PET textile for flexible lithium-ion batteries. ACS Appl. Mater. Interfaces 2017, 9, 36936–36943. [Google Scholar] [CrossRef]
- Liu, L.-J.; Chen, Y.; Zhang, Z.-F.; You, X.-L.; Walle, M.D.; Li, Y.-J.; Liu, Y.-N. Electrochemical reaction of sulfur cathodes with Ni foam current collector in Li-S batteries. J. Power Sources 2016, 325, 301–305. [Google Scholar] [CrossRef]
- Furukawa, K.; Yoshimoto, N.; Egashira, M.; Morita, M. Anodic behavior of stainless-steel substrate in organic electrolytesolutions containing different lithium salts. Electrochim. Acta 2014, 140, 125–131. [Google Scholar] [CrossRef]
- Myunga, S.-T.; Sasaki, Y.; Saito, T.; Sun, Y.-K.; Yashiro, H. Passivation behavior of Type 304 stainless steel in a non-aqueous alkyl carbonate solution containing LiPF6 salt. Electrochim. Acta 2009, 54, 5804–5812. [Google Scholar] [CrossRef] [Green Version]
- Shironita, S.; Ihsan, N.; Konakawa, K.; Souma, K.; Umeda, M. Investigation of nitriding treated Ni-free stainless steel as current collector for 5 V-class Li-ion secondary cell. Electrochim. Acta 2019, 295, 1052–1056. [Google Scholar] [CrossRef]
- Aliahmad, N.; Liu, Y.; Xie, J.; Agarwal, M. V2O5/graphene hybrid supported on paper current collectors for flexible ultrahigh-capacity electrodes for lithium-ion batteries. ACS Appl. Mater. Interfaces 2018, 10, 16490–16499. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.-C.; Lin, Y.-C.; Chiu, K.-F.; Leu, H.-J.; Ko, T.-H. Advanced carbon cloth as current collector for enhanced electrochemical performance of lithium-rich layered oxide cathodes. ChemistrySelect 2017, 2, 4419–4427. [Google Scholar] [CrossRef]
- Martha, S.K.; Dudney, N.J.; Kiggans, J.O.; Nanda, J. Electrochemical stability of carbon fibers compared to aluminum as current collectors for lithium-ion batteries. J. Electrochem. Soc. 2012, 159, A1652–A1658. [Google Scholar] [CrossRef]
- Cui, L.-F.; Hu, L.; Choi, J.W.; Cui, Y. Light-weight free-standing carbon nanotube-silicon films for anodes of lithium ion batteries. ACS Nano 2010, 4, 3671–3678. [Google Scholar] [CrossRef]
- Hu, J.W.; Wu, Z.P.; Zhong, S.W.; Zhang, W.B.; Suresh, S.; Mehta, A.; Koratkar, N. Folding insensitive, high energy density lithium-ion battery featuring carbon nanotube current collectors. Carbon 2015, 87, 292–298. [Google Scholar] [CrossRef]
- Wang, K.; Wu, Y.; Wu, H.; Luo, Y.; Wang, D.; Jiang, K.; Li, Q.; Li, Y.; Fan, S.; Wang, J. Super-aligned carbon nanotube films with a thin metal coating as highly conductive and ultralight current collectors for lithium-ion batteries. J. Power Sources 2017, 351, 160–168. [Google Scholar] [CrossRef]
- Whitehead, A.H.; Schreiber, H. Current collectors for positive electrodes of lithium-based batteries. J. Electrochem. Soc. 2005, 152, A2105–A2113. [Google Scholar] [CrossRef]
- Iwakura, C.; Fukumoto, Y.; Inoue, H.; Ohashi, S.; Kobayashi, S.; Tada, H.; Abe, H. Electrochemical characterization of various metal foils as a current collector of positive electrode for rechargeable lithium batteries. J. Power Sources 1997, 68, 301–303. [Google Scholar] [CrossRef]
- Wang, S.; Kravchyk, K.V.; Filippin, A.N.; Widmer, R.; Tiwari, A.N.; Buecheler, S.; Bodnarchuk, M.I.; Kovalenko, M.V. Overcoming the high-voltage limitations of Li-ion batteries using a titanium nitride current collector. ACS Appl. Energy Mater. 2019, 2, 974–978. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Kravchyk, K.V.; Filippin, A.N.; Müller, U.; Tiwari, A.N.; Buecheler, S.; Bodnarchuk, M.I.; Kovalenko, M.V. Aluminum chloride-graphite batteries with flexible current collectors prepared from earth-abundant elements. Adv. Sci. 2018, 5, 1700712. [Google Scholar] [CrossRef]
- Jeong, C.U.; Lee, S.Y.; Kim, J.; Cho, K.Y.; Yoon, S. Embossed aluminum as a current collector for high-rate lithium cathode performance. J. Power Sources 2018, 398, 193–200. [Google Scholar] [CrossRef]
- Nakamura, T.; Okano, S.; Yaguma, N.; Morinaga, Y.; Takahara, H.; Yamada, Y. Electrochemical performance of cathodes prepared on current collector with different surface morphologies. J. Power Sources 2013, 244, 532–537. [Google Scholar] [CrossRef]
- Loghavi, M.M.; Askari, M.; Babaiee, M.; Ghasemi, A. Improvement of the cyclability of Li-ion battery cathode using a chemical-modified current collector. J. Electroanal. Chem. 2019, 841, 107–110. [Google Scholar] [CrossRef]
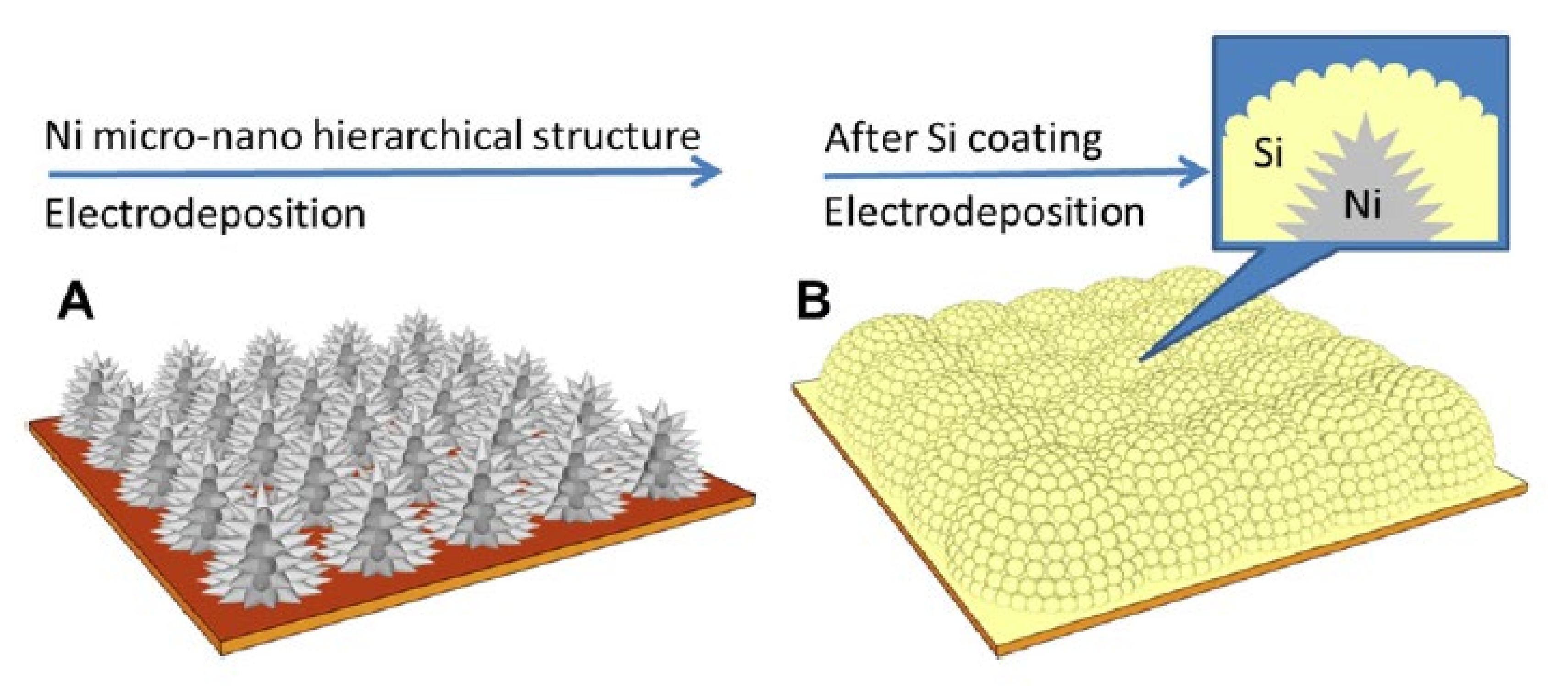
- Hang, T.; Nara, H.; Yokoshima, T.; Momma, T.; Osaka, T. Silicon composite thick film electrodeposited on a nickel micro-nanocones hierarchical structured current collector for lithium batteries. J. Power Sources 2013, 222, 503–509. [Google Scholar] [CrossRef]
- Long, J.; Liu, H.; Xie, Y.; Tang, W.; Fu, T.; Tang, Y.; Lu, L.; Ding, X.; Tang, X. Three-dimensional copper foil-powder sintering current collector for a silicon-based anode lithium-ion battery. Materials 2018, 11, 1338. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.; Kim, T.-H.; Lee, J.-I.; Kim, J.; Song, H.-K.; Park, S. General approach for high-power Li-ion batteries: Multiscale lithographic patterning of electrodes. ChemSusChem 2014, 7, 3483–3490. [Google Scholar] [CrossRef]
- Kanamura, K. Anodic oxidation of nonaqueous electrolytes on cathode materials and current collectors for rechargeable lithium batteries. J. Power Sources 1999, 81, 123–129. [Google Scholar] [CrossRef]
- Meister, P.; Qi, X.; Kloepsch, R.; Kramer, E.; Streipert, B.; Winter, M.; Placke, T. Anodic behavior of the aluminum current collector in imide-based electrolytes: Influence of solvent, operating temperature, and native oxide-layer thickness. ChemSusChem 2017, 10, 804–814. [Google Scholar] [CrossRef]
- Armond, M.B.; Moursli, F. Bis Perhalogenoacyl -or Sulfonyl-Imides of Alkali Metals, Their Solid Solutions with Plastic Materials and Their Use to the Constitution of Conductor Elements for Electrochemical Generators. U.S. Patent 4,505,997, 19 March 1985. [Google Scholar]
- Dudley, J.T.; Wilkinson, D.P.; Thomas, G.; Le Vae, R.; Woo, S.; Blom, H.; Horvath, C.; Juzkow, M.W.; Denis, B.; Juric, P.; et al. Conductivity of electrolytes for rechargeable lithium batteries. J. Power Sources 1991, 35, 59–82. [Google Scholar] [CrossRef]
- Webber, A. Conductivity and viscosity of solutions of LiCF3SO3, Li(CF3SO2)2N, and their mixtures. J. Electrochem. Soc. 1991, 138, 2586–2590. [Google Scholar] [CrossRef]
- Kawamura, T.; Tanaka, T.; Egashira, M.; Watanabe, I.; Okada, S.; Yamaki, J. Methyl difluoroacetate inhibits corrosion of aluminum cathode current collector for lithium ion cells. Electrochem. Solid-State Lett. 2005, 8, A459–A463. [Google Scholar] [CrossRef]
- Oldiges, K.; Aspern, N.v.; Cekic-Laskovic, I.; Winter, M.; Brunklaus, G. Impact of trifluoromethylation of adiponitrile on aluminum dissolution behavior in dinitrile-based electrolytes. J. Electrochem. Soc. 2018, 165, A3773–A3781. [Google Scholar] [CrossRef]
- Shkrob, I.A.; Pupek, K.Z.; Abraham, D.P. Allotropic control: How certain fluorinated carbonate electrolytes protect aluminum current collectors by promoting the formation of insoluble coordination polymers. J. Phys. Chem. C 2016, 120, 18435–18444. [Google Scholar] [CrossRef]
- Park, K.; Yu, S.; Lee, C.; Lee, H. Comparative study on lithium borates as corrosion inhibitors of aluminum current collector in lithium bis(fluorosulfonyl)imide electrolytes. J. Power Sources 2015, 296, 197–203. [Google Scholar] [CrossRef]
- Liu, X.; Shen, C.; Gao, N.; Hou, Q.; Song, F.; Tian, X.; He, Y.; Huang, J.; Fang, Z.; Xie, K. Concentrated electrolytes based on dual salts of LiFSI and LiODFB for lithium-metal battery. Electrochim. Acta 2018, 289, 422–427. [Google Scholar] [CrossRef]
- Kühnel, R.-S.; Lübke, M.; Winter, M.; Passerini, S.; Balducci, A. Suppression of aluminum current collector corrosion in ionic liquid containing electrolytes. J. Power Sources 2012, 214, 178–184. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.-W.; Khan, S.A.; Fedkiw, P.S. Attenuation of aluminum current collector corrosion in LiTFSI electrolytes using fumed silica nanoparticles. Electrochem. Solid-State Lett. 2004, 7, A228–A230. [Google Scholar] [CrossRef]
- Zhang, S.S. A review on electrolyte additives for lithium-ion batteries. J. Power Sources 2006, 162, 1379–1394. [Google Scholar] [CrossRef]
- Wanga, R.; Lia, W.; Liuc, L.; Qian, Y.; Liu, F.; Chen, M.; Guo, Y.; Liu, L. Carbon black/graphene-modified aluminum foil cathode current collectors for lithium ion batteries with enhanced electrochemical performances. J. Electroanal. Chem. 2019, 833, 63–69. [Google Scholar] [CrossRef]
- Busson, C.; Blin, M.-A.; Guichard, P.; Soudan, P.; Crosnier, O.; Guyomard, D.; Lestriez, B. A primed current collector for high performance carbon-coated LiFePO4 electrodes with no carbon additive. J. Power Sources 2018, 406, 7–17. [Google Scholar] [CrossRef]
- Lepage, D.; Savignac, L.; Saulnier, M.; Gervais, S.; Schougaard, S.B. Modification of aluminum current collectors with a conductive polymer for application in lithium batteries. Electrochem. Commun. 2019, 102, 1–4. [Google Scholar] [CrossRef]
- Swain, P.; Viji, M.; Mocherla, P.S.V.; Sudakar, C. Carbon coating on the current collector and LiFePO4 nanoparticles -Influence of sp2 and sp3-like disordered carbon on the electrochemical properties. J. Power Sources 2015, 293, 613–625. [Google Scholar] [CrossRef]
- Liu, T.; Cao, F.; Ren, L.; Li, X.; Sun, S.; Sun, X.; Zang, Z.; Niu, Q.; Wu, J. A theoretical study of different carbon coatings effect on the depolarization effect and electrochemical performance of LiFePO4 cathode. J. Electroanal. Chem. 2017, 807, 52–58. [Google Scholar] [CrossRef]
- Kuenzel, M.; Bresser, D.; Kim, G.-T.; Axmann, P.; Wohlfahrt-Mehrens, M.; Passerini, S. Unveiling and amplifying the benefits of carbon-coated aluminum current collectors for sustainable LiNi0.5Mn1.5O4 cathodes. ACS Appl. Energy Mater. 2020, 3, 218–230. [Google Scholar] [CrossRef] [Green Version]
- Tong, X.; Zhang, F.; Ji, B.; Sheng, M.; Tang, Y. Carbon-coated porous aluminum foil anode for high-rate, long-term cycling stability, and high energy density dual-ion batteries. Adv. Mater. 2016, 28, 9979–9985. [Google Scholar] [CrossRef]
- Li, X.; Deng, S.; Banis, M.N.; Doyle-Davis, K.; Zhang, D.; Zhang, T.; Yang, J.; Divigalpitiya, R.; Brandys, F.; Li, R.; et al. Suppressing corrosion of aluminum foils via highly conductive graphene-like carbon coating in high-performance lithium-based batteries. ACS Appl. Mater. Interfaces 2019, 11, 32826–32832. [Google Scholar] [CrossRef]
- Doberdò, I.; Löffler, N.; Laszczynski, N.; Cericola, D.; Penazzi, N.; Bodoardo, S.; Kim, G.-T.; Passerini, S. Enabling aqueous binders for lithium battery cathodes—Carbon coating of aluminum current collector. J. Power Sources 2014, 248, 1000–1006. [Google Scholar] [CrossRef]
- Heckmann, A.; Krott, M.; Streipert, B.; Uhlenbruck, S.; Winter, M.; Placke, T. Suppression of aluminum current collector dissolution by protective ceramic coatings for better high-voltage battery performance. ChemPhysChem 2017, 18, 156–163. [Google Scholar] [CrossRef]
- Ely, T.O.; Kamzabek, D.; Chakraborty, D. Batteries safety: Recent progress and current challenges. Front. Energy Res. 2019, 7, 71. [Google Scholar]
- Li, Q.; Zhu, S.; Lu, Y. 3D porous Cu current collector/Li-metal composite anode for stable lithium-metal batteries. Adv. Funct. Mater. 2017, 27, 1606422. [Google Scholar] [CrossRef]
- Lu, Z.; Zhang, Z.; Chen, X.; Chen, Q.; Ren, F.; Wang, M.; Wu, S.; Peng, Z.; Wang, D.; Ye, J. Improving Li anode performance by a porous 3D carbon paper host with plasma assisted sponge carbon coating. Energy Storage Mater. 2018, 11, 47–56. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, Z.; Lan, M.; Yang, S.; Cheng, J.; Cai, J.; Shen, J.; Zhu, Y.; Zhang, K.; Zhang, W. Lithiophilic Cu-CuO-Ni hybrid structure: Advanced current collectors toward stable lithium metal anodes. Adv. Mater. 2018, 30, 1705830. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, A.; Liu, X.; Luo, J. Dendrites in lithium metal anodes: Suppression, regulation, and elimination. Acc. Chem. Res. 2019, 52, 3223–3232. [Google Scholar] [CrossRef] [PubMed]
- Zolin, L.; Chandesris, M.; Porcher, W.; Lestriez, B. An innovative process for ultra-thick electrodes elaboration: Toward low-cost and high-energy batteries. Energy Technol. 2019, 7, 1900025. [Google Scholar] [CrossRef]
- Yang, G.-F.; Song, K.-Y.; Joo, S.-K. Ultra-thick Li-ion battery electrodes using different cell size of metal foam current collectors. RSC Adv. 2015, 5, 16702–16706. [Google Scholar] [CrossRef] [Green Version]
- Aguilό-Aguayo, N.; Espiñeira, P.P.; Manian, A.P.; Bechtold, T. Three-dimensional embroidered current collectors for ultra-thick electrodes in batteries. RSC Adv. 2016, 6, 69685–69690. [Google Scholar] [CrossRef] [Green Version]
- Noelle, D.J.; Wang, M.; Qiao, Y. Improved safety and mechanical characterizations of thick lithium-ion battery electrodes structured with porous metal current collectors. J. Power Sources 2018, 399, 125–132. [Google Scholar] [CrossRef]
- Yang, G.-F.; Joo, S.-K. Calendering effect on the electrochemical performances of the thick Li-ion battery electrodes using a three dimensional Ni alloy foam current collector. Electrochim. Acta 2015, 170, 263–268. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, R.; Yu, W.; Yang, Y.; Wang, Z.; Zhang, C.; Bando, Y.; Golberg, D.; Wang, X.; Ding, Y. Three-dimensional electrode with conductive Cu framework for stable and fast Li-ion storage. Energy Storage Mater. 2018, 11, 83–90. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Shen, K.; Li, B.; Li, S.; Yang, S. Continuously 3D printed quantum dot-based electrodes for lithium storage with ultrahigh capacities. J. Mater. Chem. A 2018, 6, 19960–19966. [Google Scholar] [CrossRef]
- Tsuda, T.; Ando, N.; Nakamura, S.; Ishihara, Y.; Hayashi, N.; Soma, N.; Gunji, T.; Tanabe, T.; Ohsaka, T.; Matsumoto, F. Improvement of high-rate discharging performance of LiFePO4 cathodes by forming micrometer-sized through-holed electrode structures with a pico-second pulsed laser. Electrochim. Acta 2019, 296, 27–38. [Google Scholar] [CrossRef]
- Cha, H.; Kim, J.; Lee, Y.; Cho, J.; Park, M. Issues and challenges facing flexible lithium-ion batteries for practical application. Small 2018, 14, 1702989. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Wu, H.; Mantia, F.L.; Yang, Y.; Cui, Y. Thin, flexible secondary Li-ion paper batteries. ACS Nano 2010, 4, 5843–5848. [Google Scholar] [CrossRef] [PubMed]
- Yazici, M.S.; Krassowski, D.; Prakash, J. Flexible graphite as battery anode and current collector. J. Power Sources 2005, 141, 171–176. [Google Scholar] [CrossRef]
- Long, B.; Yang, H.; Wang, F.; Mao, Y.; Balogun, M.-S.; Song, S.; Tong, Y. Chemically-modified stainless steel mesh derived substrate-free iron-based composite as anode materials for affordable flexible energy storage devices. Electrochim. Acta 2018, 284, 271–278. [Google Scholar] [CrossRef]
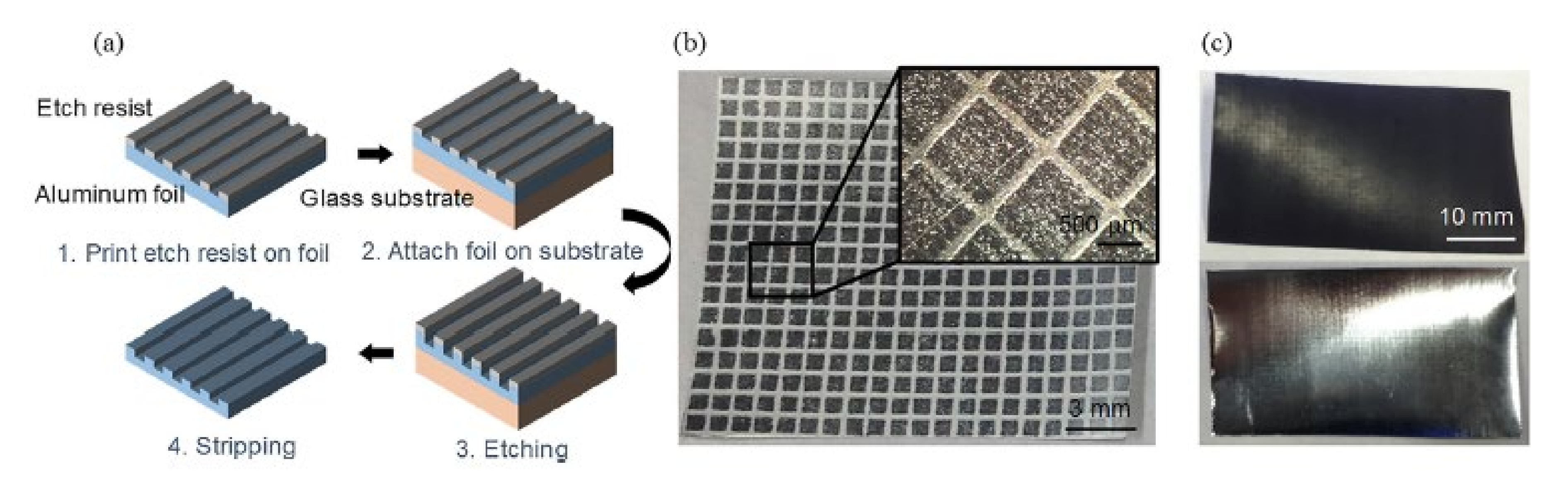
- Gu, Y.; Federici, J.F. Fabrication of a flexible current collector for lithium ion batteries by inkjet printing. Batteries 2018, 4, 42. [Google Scholar] [CrossRef] [Green Version]
- Zamarayeva, A.M.; Ostfeld, A.E.; Wang, M.; Duey, J.K.; Deckman, I.; Lechêne, B.P.; Davies, G.; Steingart, D.A.; Arias, A.C. Flexible and stretchable power sources for wearable electronics. Sci. Adv. 2017, 3, e1602051. [Google Scholar] [CrossRef] [Green Version]
- Zhou, G.; Li, F.; Cheng, H.-M. Progress in flexible lithium batteries and future prospects. Energy Environ. Sci. 2014, 7, 1307–1338. [Google Scholar] [CrossRef]
- Zuo, X.; Zhu, J.; Müller-Buschbaum, P.; Cheng, Y.-J. Silicon based lithium-ion battery anodes: A chronicle perspective review. Nano Energy 2017, 31, 113–143. [Google Scholar] [CrossRef]
- Park, C.-M.; Kim, J.-H.; Kim, H.; Sohn, H.-J. Li-alloy based anode materials for Li secondary batteries. Chem. Soc. Rev. 2010, 39, 3115–3141. [Google Scholar] [CrossRef]
- Su, X.; Wu, Q.; Li, J.; Xiao, X.; Lott, A.; Lu, W.; Sheldon, B.W.; Wu, J. Silicon-based nanomaterials for lithium-ion batteries: A review. Adv. Energy Mater. 2014, 4, 1300882. [Google Scholar] [CrossRef]
- Wu, H.; Chan, G.; Choi, J.W.; Ryu, I.; Yao, Y.; McDowell, M.T.; Lee, S.W.; Jackson, A.; Yang, Y.; Hu, L.; et al. Stable cycling of double-walled silicon nanotube battery anodes through solid–electrolyte interphase control. Nat. Nanotech. 2012, 7, 310–315. [Google Scholar] [CrossRef]
- Yao, Y.; McDowell, M.T.; Ryu, I.; Wu, H.; Liu, N.; Hu, L.; Nix, W.D.; Cui, Y. Interconnected silicon hollow nanospheres for lithium-ion battery anodes with long cycle life. Nano Lett. 2011, 11, 2949–2954. [Google Scholar] [CrossRef] [PubMed]
- Unno, H.; Nagata, T.; Fujimoto, N.; Fukuda, M. Fe-based metal foils for current collectors in Li-secondary batteries. Nippon Steel Gihou 2019, 412, 173–183. [Google Scholar]
- Kataoka, R.; Oda, Y.; Inoue, R.; Kawasaki, N.; Takeichi, N.; Kiyobayashi, T. Silicon micropowder negative electrode endures more than 1000 cycles when a surface-roughened clad current collector is used. J. Power Sources 2017, 346, 128–133. [Google Scholar] [CrossRef]
- Guo, J.; Sun, A.; Wang, C. A porous silicon–carbon anode with high overall capacity on carbon fiber current collector. Electrochem. Commun. 2010, 12, 981–984. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, Y.; Du, C.; Ren, Y.; Li, X.; Zuo, P.; Yin, G.; Ma, Y.; Cheng, X.; Gao, Y. Free-standing sandwich-type graphene/nanocellulose/silicon laminar anode for flexible rechargeable lithium ion batteries. ACS Appl. Mater. Interfaces 2018, 10, 29638–29646. [Google Scholar] [CrossRef]
- Cho, G.-B.; Kim, J.-K.; Lee, S.-H.; Kim, G.-T.; Noh, J.-P.; Cho, K.-K.; Kim, K.-W.; Nam, T.-H.; Ahn, H.-J. Facile fabrication of patterned Si film electrodes containing trench-structured Cu current collectors for thin-film batteries. Electrochim. Acta 2017, 224, 649–659. [Google Scholar] [CrossRef]
- Luais, E.; Sakai, J.; Desplobain, S.; Gautier, G.; Tran-Van, F.; Ghamouss, F. Thin and flexible silicon anode based on integrated microporous silicon film onto electrodeposited copper current collector. J. Power Sources 2013, 242, 166–170. [Google Scholar] [CrossRef]
- Park, J.H.; Ku, J.; Lim, J.W.; Choi, J.-M.; Son, I.H. Adhesive interlayer between active film and current collector for improving the performance of silicon anodes of Li-ion batteries. J. Electroanal. Chem. 2016, 778, 53–56. [Google Scholar] [CrossRef]
- Chen, Z.; Kim, G.-T.; Chao, D.; Loeffler, N.; Copley, M.; Lin, J.; Shen, Z.; Passerini, S. Toward greener lithium-ion batteries: Aqueous binder-based LiNi0.4Co0.2Mn0.4O2 cathode material with superior electrochemical performance. J. Power Sources 2017, 372, 180–187. [Google Scholar] [CrossRef]
- Du, Z.; Rollag, K.M.; Li, J.; An, S.J.; Wood, M.; Sheng, Y.; Mukherjee, P.P.; Daniel, C.; Wood, D.L., III. Enabling aqueous processing for crack-free thick electrodes. J. Power Sources 2017, 354, 200–206. [Google Scholar] [CrossRef] [Green Version]
- Church, B.C.; Kaminski, D.T.; Jiang, J. Corrosion of aluminum electrodes in aqueous slurries for lithium ion batteries. J. Mater. Sci. 2014, 49, 3234–3241. [Google Scholar] [CrossRef]
- Tanabe, T.; Liu, Y.B.; Miyamoto, K.; Irii, Y.; Maki, F.; Gunji, T.; Kaneko, S.; Ugawa, S.; Lee, H.; Ohsaka, T.; et al. Synthesis of water-resistant thin TiOx layer-coated high-voltage and high-capacity LiNiaCobAl1-a-bO2 (a > 0.85) cathode and its cathode performance to apply a water-based hybrid polymer binder to Li-ion batteries. Electrochim. Acta 2017, 258, 1348–1355. [Google Scholar] [CrossRef]
- Li, S.Y.; Church, B.C. Effect of aqueous-based cathode slurry pH and immersion time on corrosion of aluminum current collector in lithium-ion batteries. Mater. Corros. 2016, 67, 978–987. [Google Scholar] [CrossRef]
- Wakao, T.; Gunji, T.; Jeevagan, A.J.; Mochizuki, Y.; Kaneko, S.; Baba, K.; Watanabe, M.; Kanda, Y.; Murakami, K.; Omura, M.; et al. Stable charge/discharge cycle performance of a LiFePO4 cathode prepared with a carboxymethyl cellulose binder. ECS Trans. 2014, 58, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Posada, J.O.G.; Rennie, A.J.R.; Villar, S.P.; Martins, V.L.; Marinaccio, J.; Barnes, A.; Glover, C.F.; Worsley, D.A.; Hall, P.J. Aqueous batteries as grid scale energy storage solutions. Renew. Sustain. Energy Rev. 2017, 68, 1174–1182. [Google Scholar] [CrossRef] [Green Version]
- Bin, D.; Wen, Y.; Wang, Y.; Xia, Y. The development in aqueous lithium-ion batteries. J. Energy Chem. 2018, 27, 1521–1535. [Google Scholar] [CrossRef]
- Eftekhari, A. High-energy aqueous lithium batteries. Adv. Energy Mater. 2018, 8, 1801156. [Google Scholar] [CrossRef]
- Gheytani, S.; Liang, Y.; Jing, Y.; Xu, J.Q.; Yao, Y. Chromate conversion coated aluminium as a lightweight and corrosion-resistant current collector for aqueous lithium-ion batteries. J. Mater. Chem. A 2016, 4, 395–399. [Google Scholar] [CrossRef]
- Kühnel, R.-S.; Reber, D.; Remhof, A.; Figi, R.; Bleiner, D.; Battaglia, C. “Water-in-salt” electrolytes enable the use of cost-effective aluminum current collectors for aqueous high-voltage batteries. Chem. Commun. 2016, 52, 10435–10438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, Y.H.; Shao, L.; Zhao, P.C.; Wang, B.Y.; Cao, G.P.; Yang, Y.S. Carbon coated stainless steel mesh as a low-cost and corrosion-resistant current collector for aqueous rechargeable batteries. J. Mater. Chem. A 2017, 5, 15752–15758. [Google Scholar] [CrossRef]
- Wanga, Q.; Ping, P.; Zhao, X.; Chu, G.; Sun, J.; Chen, C. Thermal runaway caused fire and explosion of lithium ion battery. J. Power Sources 2012, 208, 210–224. [Google Scholar] [CrossRef]
- Wu, X.; Song, K.; Zhang, X.; Hu, N.; Li, L.; Li, W.; Zhang, L.; Zhang, H. Safety issues in lithium ion batteries: Materials and cell design. Front. Energy Res. 2019, 7, 65. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Le, A.V.; Shi, Y.; Noelle, D.J.; Qiao, Y. Heterogeneous current collector in lithium-ion battery for thermal-runaway mitigation. Appl. Phys. Lett. 2017, 110, 083902. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Le, A.V.; Noelle, D.J.; Shi, Y.; Meng, Y.S.; Qiao, Y. Internal-short-mitigating current collector for lithium-ion battery. J. Power Sources 2017, 349, 84–93. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Shi, Y.; Noelle, D.J.; Le, A.V.; Yoon, H.; Chung, H.; Zhang, M.; Meng, Y.S.; Qiao, Y. Internal short circuit mitigation of high-voltage lithium-ion batteries with functional current collectors. RSC Adv. 2017, 7, 45662–45667. [Google Scholar] [CrossRef] [Green Version]
- Naguib, M.; Allu, S.; Simunovic, S.; Li, J.; Wang, H.; Dudney, N.J. Limiting internal short-circuit damage by electrode partition for impact-tolerant Li-ion batteries. Joule 2018, 2, 155–167. [Google Scholar] [CrossRef] [Green Version]
- Sakuda, A. Favorable composite electrodes for all-solid-state batteries. J. Ceram. Soc. Jpn. 2018, 126, 675–683. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.; Sun, H.; Fu, L.; Ye, F.; Zhang, Y.; Luo, W.; Huang, Y. Promises, challenges, and recent progress of inorganic solid-state electrolytes for all-solid-state lithium batteries. Adv. Mater. 2018, 30, 1705702. [Google Scholar] [CrossRef]
- Zhang, Z.; Shao, Y.; Lotsch, B.; Hu, Y.-S.; Li, H.; Janek, J.; Nazar, L.F.; Nan, C.W.; Maier, J.; Armand, M.; et al. New horizons for inorganic solid state ion conductors. Energy Environ. Sci. 2018, 11, 1945–1976. [Google Scholar] [CrossRef] [Green Version]
- Park, K.H.; Bai, Q.; Kim, D.H.; Oh, D.Y.; Zhu, Y.; Mo, Y.; Jung, Y.S. Design strategies, practical considerations, and new solution processes of sulfide solid electrolytes for all-solid-state batteries. Adv. Energy Mater. 2018, 8, 1800035. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, L.; Qi, X.; Lu, Y.; Wu, F.; Zhao, J.; Yu, Y.; Hu, Y.-S.; Chen, L. Solid-state sodium batteries. Adv. Energy Mater. 2018, 8, 1703012. [Google Scholar] [CrossRef]
- Lu, Y.; Li, L.; Zhang, Q.; Niu, Z.Q.; Chen, J. Electrolyte and interface engineering for solid-state sodium batteries. Joule 2018, 2, 1747–1770. [Google Scholar] [CrossRef] [Green Version]
- Deivanayagam, R.; Cheng, M.; Wang, M.; Vasudevan, V.; Foroozan, T.; Medhekar, N.V.; Shahbazian-Yassar, R. Composite polymer electrolyte for highly cyclable room-temperature solid-state magnesium batteries. ACS Appl. Energy Mater. 2019, 2, 7980–7990. [Google Scholar] [CrossRef]
- Zhou, W.; Li, Y.; Xin, S.; Goodenough, J.B. Rechargeable sodium all-solid-state battery. ACS Cent. Sci. 2017, 3, 52–57. [Google Scholar] [CrossRef]
- Choi, S.; Kim, J.; Eom, M.; Meng, X.; Shin, D. Application of a carbon nanotube (CNT) sheet as a current collector for all-solid-state lithium batteries. J. Power Sources 2015, 299, 70–75. [Google Scholar] [CrossRef]
- De, B.; Yadav, A.; Khan, S.; Kar, K.K. A Facile methodology for the development of a printable and flexible all-solid-state rechargeable battery. ACS Appl. Mater. Interfaces 2017, 9, 19870–19880. [Google Scholar] [CrossRef]
- Filippin, A.N.; Rawlence, M.; Wäckerlin, A.; Feurer, T.; Zünd, T.; Kravchyk, K.; Kovalenko, M.V.; Romanyuk, Y.E.; Tiwaria, A.N.; Buecheler, S. Chromium nitride as a stable cathode current collector for all-solid-state thin film Li-ion batteries. RSC Adv. 2017, 7, 26960–26967. [Google Scholar] [CrossRef] [Green Version]
- Filippin, A.N.; Lin, T.-Y.; Rawlence, M.; Zünd, T.; Kravchyk, K.; Sastre-Pellicer, J.; Haass, S.G.; Wäckerlin, A.; Kovalenko, M.V.; Buecheler, S. Ni–Al–Cr superalloy as high temperature cathode current collector for advanced thin film Li batteries. RSC Adv. 2018, 8, 20304–20313. [Google Scholar] [CrossRef] [Green Version]
- Pervez, S.A.; Cambaz, M.A.; Thangadurai, V.; Fichtner, M. Interface in solid-state lithium battery: Challenges, progress, and outlook. ACS Appl. Mater. Interfaces 2019, 11, 22029–22050. [Google Scholar] [CrossRef] [PubMed]
- Hao, F.; Han, F.; Liang, Y.; Wang, C.; Yao, Y. Architectural design and fabrication approaches for solid-state batteries. MRS Bull. 2018, 43, 775–781. [Google Scholar] [CrossRef]
- Alexander, G.V.; Indu, M.S.; Kamakshy, S.; Murugan, R. Development of stable and conductive interface between garnet structured solid electrolyte and lithium metal anode for high performance solid-state battery. Electrochim. Acta 2020, 332, 135511. [Google Scholar] [CrossRef]
- Chen, L.; Huang, Z.; Pang, W.; Jin, Z.; Li, Y.; Wang, C.-A. Dual interface layers for solid-state Li metal battery with low interfacial resistance and small polarization based on garnet electrolyte. Electrochim. Acta 2020, 330, 135352. [Google Scholar] [CrossRef]
- López-Aranguren, P.; Judez, X.; Chakir, M.; Armand, M.; Buannic, L. High voltage solid state batteries: Targeting high energy density with polymer composite electrolytes. J. Electrochem. Soc. 2020, 167, 020548. [Google Scholar] [CrossRef]
- Luo, J.; Zhong, S.; Huang, Z.; Huang, B.; Wang, C.-A. High Li+-conductive perovskite Li3/8Sr7/16Ta3/4Zr1/4O3 electrolyte prepared by hot-pressing for all-solid-state Li-ion batteries. Solid State Ion. 2019, 338, 1–4. [Google Scholar] [CrossRef]
- Yoshinari, T.; Koerver, R.; Hofmann, P.; Uchimoto, Y.; Zeier, W.G.; Janek, J. Interfacial stability of phosphate-NASICON solid electrolytes in Ni-rich NCM cathode-based solid-state batteries. ACS Appl. Mater. Interfaces 2019, 11, 23244–23253. [Google Scholar] [CrossRef]
- Li, W.J.; Hirayama, M.; Suzuki, K.; Kanno, R. Fabrication and all solid-state battery performance of TiS2/Li10GeP2S12 Composite Electrodes. Mater. Trans. 2016, 57, 549–552. [Google Scholar] [CrossRef] [Green Version]
- Taminato, S.; Okumura, T.; Takeuchi, T.; Kobayashi, H. Fabrication and charge-discharge reaction of all solid-state lithium battery using Li4-2xGe1-xSxO4 electrolyte. Solid State Ion. 2018, 326, 52–57. [Google Scholar] [CrossRef]
- Choi, S.; Jeon, M.; Jung, W.D.; Yang, S.; Park, S.; Ji, H.-I.; Lee, J.-H.; Kim, B.-K.; Sang, B.-I.; Kim, H. Robust solid-state interface with a deformable glass interlayer in sulfide-based all-solid-state batteries. Solid State Ion. 2020, 346, 115217. [Google Scholar] [CrossRef]
- Banerjee, A.; Tang, H.; Wang, X.; Cheng, J.-H.; Nguyen, H.; Zhang, M.; Tan, D.H.S.; Wynn, T.A.; Wu, E.A.; Doux, J.-M.; et al. Revealing nanoscale solid–solid interfacial phenomena for long-life and high-energy all-solid-state batteries. ACS Appl. Mater. Interfaces 2019, 11, 43138–43145. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.S.; Park, H.W.; Park, J.-Y.; Lim, H.-T. Effect of electrode design on electrochemical performance of all-solid-state lithium secondary battery using lithium-silicide anodes. Electrochim. Acta 2015, 185, 242–249. [Google Scholar] [CrossRef]
- Long, L.; Wang, S.; Xiao, M.; Meng, Y. Polymer electrolytes for lithium polymer batteries. J. Mater. Chem. A 2016, 4, 10038–10069. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, J.; He, P.; Zhang, Y.; Xu, J.; Wu, X. Corrosion suppression of aluminum metal by optimizing lithium salt concentration in solid-state imide salt-based polymer plastic crystal electrolyte membrane. ACS Appl. Energy Mater. 2018, 1, 7022–7027. [Google Scholar] [CrossRef]
- Cong, L.; Liu, J.; Armand, M.; Mauger, A.; Julien, C.M.; Xie, H.; Sun, L. Role of perfluoropolyether-based electrolytes in lithium metal batteries: Implication for suppressed Al current collector corrosion and the stability of Li metal/electrolytes interfaces. J. Power Sources 2018, 380, 115–125. [Google Scholar] [CrossRef]
- Marsh, R.A.; Russell, P.G.; Reddy, T.B. Bipolar lithium-ion battery development. J. Power Sources 1997, 65, 133–141. [Google Scholar] [CrossRef]
- Takami, N.; Yoshima, K.; Harada, Y. 12 V-class bipolar lithium-ion batteries using Li4Ti5O12 anode for low-voltage system applications. J. Electrochem. Soc. 2017, 141, A6254–A6259. [Google Scholar] [CrossRef]
- Jung, K.-N.; Shin, H.-S.; Park, M.-S.; Lee, J.-W. Solid-state lithium batteries: Bipolar design, fabrication, and electrochemistry. ChemElectroChem 2019, 6, 3842–3859. [Google Scholar] [CrossRef]
- Gambe, Y.; Sun, Y.; Honma, I. Development of bipolar all-solid-state lithium battery based on quasi-solid-state electrolyte containing tetraglyme-LiTFSA equimolar complex. Sci. Rep. 2015, 5, 8869. [Google Scholar] [CrossRef] [Green Version]
- Shin, H.S.; Ryu, W.-G.; Park, M.-S.; Jung, K.-N.; Kim, H.; Lee, J.-W. Multilayered, bipolar, all-solid-state battery enabled by a perovskite-based biphasic solid electrolyte. ChemSusChem 2018, 11, 3184–3190. [Google Scholar] [CrossRef]
- Kim, S.-H.; Kim, J.-H.; Cho, S.-J.; Lee, S.-Y. All-solid-state printed bipolar Li–S batteries. Adv. Energy Mater. 2019, 9, 1901841. [Google Scholar] [CrossRef]
- Dugas, R.; Forero-Saboya, J.D.; Ponrouch, A. Methods and protocols for reliable electrochemical testing in post-Li batteries (Na, K, Mg, and Ca). Chem. Mater. 2019, 31, 8613–8628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muldoon, J.; Bucur, C.B.; Gregory, T. Quest for nonaqueous multivalent secondary batteries: Magnesium and beyond. Chem. Rev. 2014, 114, 11683–11720. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Aurbach, D. Promise and reality of post-lithium-ion batteries with high energy densities. Nat. Rev. Mater. 2016, 1, 1–16. [Google Scholar] [CrossRef]
- Massé, R.C.; Uchaker, E.; Cao, G. Beyond Li-ion: Electrode materials for sodium- and magnesium-ion batteries. Sci. China. Mater. 2015, 58, 715–766. [Google Scholar] [CrossRef] [Green Version]
- Delmas, C. Sodium and sodium-ion batteries: 50 years of research. Adv. Energy Mater. 2018, 8, 1703137. [Google Scholar] [CrossRef]
- Nayak, P.K.; Yang, L.; Brehm, W.; Adelhelm, P. From lithium-ion to sodium-ion batteries: Advantages, challenges, and surprises. Angew. Chem. Int. Ed. 2018, 57, 102–120. [Google Scholar] [CrossRef]
- Liu, S.; Tang, S.; Zhang, X.; Wang, A.; Yang, Q.-H.; Luo, J. Porous Al current collector for dendrite-free Na metal anodes. Nano Lett. 2017, 17, 5862–5868. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Q.; Han, M.; Chen, J. Stable Na plating/stripping electrochemistry realized by a 3D Cu current collector with thin nanowires. Chem. Commun. 2017, 53, 12910–12913. [Google Scholar] [CrossRef]
- Chi, S.-S.; Qi, X.-G.; Hu, Y.-S.; Fan, L.-Z. 3D flexible carbon felt host for highly sable sodium metal anodes. Adv. Energy Mater. 2018, 8, 1702764. [Google Scholar] [CrossRef]
- Wang, Z.; Li, M.; Ruan, C.; Liu, C.; Zhang, C.; Xu, C.; Edström, K.; Strømme, M.; Nyholm, L. Conducting polymer paper-derived mesoporous 3D N-doped carbon current collectors for Na and Li metal anodes: A combined experimental and theoretical study. J. Phys. Chem. C 2018, 122, 23352–23363. [Google Scholar] [CrossRef]
- Jung, J.; Hwang, D.Y.; Kristanto, I.; Kwak, S.K.; Kang, S.J. Deterministic growth of a sodium metal anode on a pre-patterned current collector for highly rechargeable seawater batteries. J. Mater. Chem. A 2019, 7, 9773–9781. [Google Scholar] [CrossRef]
- Hou, Z.; Wang, W.; Yu, Y.; Zhao, X.; Chen, Q.; Zhao, L.; Di, Q.; Ju, H.; Quan, Z. Poly(vinylidene difluoride) coating on Cu current collector for high-performance Na metal anode. Energy Storage Mater. 2020, 24, 588–593. [Google Scholar] [CrossRef]
- Otaegui, L.; Goikolea, E.; Aguesse, F.; Armand, M.; Rojo, T.; Singh, G. Effect of the electrolytic solvent and temperature on aluminum current collector stability: A case of sodium-ion battery cathode. J. Power Sources 2015, 297, 168–173. [Google Scholar] [CrossRef]
- Song, J.; Sahadeo, E.; Noked, M.; Lee, S.B. Mapping the challenges of magnesium battery. J. Phys. Chem. Lett. 2016, 7, 1736–1749. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Liu, T.; Shao, Y.; Engelhard, M.H.; Liu, J.; Li, G. Electrochemically stable cathode current collectors for rechargeable magnesium batteries. J. Mater. Chem. A 2014, 2, 2473–2477. [Google Scholar] [CrossRef]
- Aurbach, D.; Lu, Z.; Schechter, A.; Gofer, Y.; Gizbar, H.; Turgeman, R.; Cohen, Y.; Moshkovich, M.; Levi, E. Prototype systems for rechargeable magnesium batteries. Nature 2000, 407, 724–727. [Google Scholar] [CrossRef]
- Wall, C.; Zhao-Karger, Z.; Fichtner, M. Corrosion resistance of current collector materials in bisamide based electrolyte for magnesium batteries. ECS Electrochem. Lett. 2015, 4, C8–C10. [Google Scholar] [CrossRef] [Green Version]
- Lv, D.; Xu, T.; Saha, P.; Datta, M.K.; Gordin, M.L.; Manivannan, A.; Kumta, P.N.; Wang, D. A scientific study of current collectors for Mg batteries in Mg(AlCl2EtBu)2/THF electrolyte. J. Electrochem. Soc. 2013, 160, A351–A355. [Google Scholar] [CrossRef] [Green Version]
- Yagi, S.; Tanaka, A.; Ichikawa, Y.; Ichitsubo, T.; Matsubara, E. Electrochemical stability of magnesium battery current collectors in a grignard reagent-based electrolyte. J. Electrochem. Soc. 2013, 160, C83–C88. [Google Scholar] [CrossRef]
- Cheng, Y.; Choi, D.; Han, K.S.; Mueller, K.T.; Zhang, J.-G.; Sprenkle, V.L.; Liu, J.; Li, G. Toward the design of high voltage magnesium–lithium hybrid batteries using dual-salt electrolytes. Chem. Commun. 2016, 52, 5379–5382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Jiang, C.; Zhang, S.; Song, X.; Tang, Y.; Cheng, H.-M. Reversible calcium alloying enables a practical room-temperature rechargeable calcium-ion battery with a high discharge voltage. Nat. Chem. 2018, 10, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Gummow, R.J.; Vamvounis, G.; Kannan, M.B.; He, Y. Calcium-ion batteries: Current state-of-the-art and future perspectives. Adv. Mater. 2018, 30, 1801702. [Google Scholar] [CrossRef] [PubMed]
- Lipson, A.L.; Proffit, D.L.; Pan, B.; Fister, T.T.; Liao, C.; Burrell, A.K.; Vaughey, J.T.; Ingram, B.J. Current collector corrosion in Ca-ion batteries. J. Electrochem. Soc. 2015, 162, A1574–A1578. [Google Scholar] [CrossRef]
- Verrelli, R.; Black, A.; Dugas, R.; Tchitchekova, D.; Ponrouch, A.; Palacin, M.R. Steps towards the use of TiS2 electrodes in Ca batteries. J. Electrochem. Soc. 2020, 167, 070532. [Google Scholar] [CrossRef]
- Ponrouch, A.; Tchitchekova, D.; Frontera, C.; Bardé, F.; Dompablo, M.E.A.; Palacín, M.R. Assessing Si-based anodes for Ca-ion batteries: Electrochemical decalciation of CaSi2. Electrochem. Commun. 2016, 66, 75–78. [Google Scholar] [CrossRef] [Green Version]
- Cabello, M.; Nacimiento, F.; Alcántara, R.; Lavela, P.; Vicente, C.P.; Tirado, J.L. Applicability of molybdite as an electrode material in calcium batteries: A structural study of layer-type CaxMoO3. Chem. Mater. 2018, 30, 5853–5861. [Google Scholar] [CrossRef]
- Walter, M.; Kovalenko, M.V.; Kravchyk, K.V. Challenges and benefits of post-lithium-ion batteries. New J. Chem. 2020, 44, 1677–1683. [Google Scholar] [CrossRef] [Green Version]
- Angell, M.; Pan, C.-J.; Rong, Y.; Yuan, C.; Lin, M.-C.; Hwang, B.-J.; Dai, H. High Coulombic efficiency aluminum-ion battery using an AlCl3-urea ionic liquid analog electrolyte. Proc. Natl. Acad. Sci. USA 2017, 114, 834–839. [Google Scholar] [CrossRef] [Green Version]
- Kravchyk, K.V.; Wang, S.; Piveteau, L.; Kovalenko, M.V. Efficient aluminum chloride–natural graphite battery. Chem. Mater. 2017, 29, 4484–4492. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Wang, W.; Yu, Z.; Yuan, Y.; Wang, S.; Jiao, S. A new aluminium-ion battery with high voltage, high safety and low cost. Chem. Commun. 2015, 51, 11892–11895. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Torrero, D.; Anderson, M.; Palma, J.; Marcilla, R.; Ventosa, E. Unexpected contribution of current collector to the cost of rechargeable Al-ion batteries. ChemElectroChem 2019, 6, 2766–2770. [Google Scholar] [CrossRef]
- Muñoz-Torrero, D.; Palma, J.; Marcilla, R.; Ventosa, E. A critical perspective on rechargeable Al-ion battery technology. Dalton Trans. 2019, 48, 9906–9911. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, K. Low-cost, lightweight electrodes based on carbon fibers as current collectors for aluminum-ion batteries. J. Electroanal. Chem. 2019, 849, 113374. [Google Scholar] [CrossRef]
- Zhang, S.; Tan, X.; Meng, Z.; Tian, H.; Xu, F.; Han, W.-Q. Naturally abundant high-performance rechargeable aluminum/iodine batteries based on conversion reaction chemistry. J. Mater. Chem. A 2018, 6, 9984–9996. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, Z.; Li, N.; Jiao, S.; Chen, H.; Song, W.-L.; Fang, D. Thick electrodes upon biomass-derivative carbon current collectors: High-areal capacity positive electrodes for aluminum-ion batteries. Electrochim. Acta 2019, 323, 134805. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, G.; Cui, Y. Nanostructured sulfur cathodes. Chem. Soc. Rev. 2013, 42, 3018–3032. [Google Scholar] [CrossRef]
- Bresser, D.; Passerini, S.; Scrosati, B. Recent progress and remaining challenges in sulfur-based lithium secondary batteries—A review. Chem. Commun. 2013, 49, 10545–10562. [Google Scholar] [CrossRef]
- Yu, X.; Manthiram, A. Performance enhancement and mechanistic studies of room-temperature sodium–sulfur batteries with a carbon-coated functional Nafion separator and a Na2S/activated carbon nanofiber cathode. Chem. Mater. 2016, 28, 896–905. [Google Scholar] [CrossRef]
- Vinayan, B.P.; Zhao-Karger, Z.; Diemant, T.; Chakravadhanula, V.S.K.; Schwarzburger, N.I.; Cambaz, M.A.; Behm, R.J.; Kübel, C.; Fichtner, M. Performance study of magnesium–sulfur battery using a graphene based sulfur composite cathode electrode and a non-nucleophilic Mg electrolyte. Nanoscale 2016, 8, 3296–3306. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Ma, L.; Wu, T.; Ye, H.; Zhou, J.; Zhao, F.; Han, N.; Wang, Y.; Wu, Y.; Li, Y.; et al. Chemical immobilization and conversion of active polysulfides directly by copper current collector: A new approach to enabling stable room-temperature Li-S and Na-S batteries. Adv. Energy Mater. 2018, 8, 1800624. [Google Scholar] [CrossRef]
- Muthuraj, D.; Ghosh, A.; Kumar, A.; Mitra, S. Nitrogen and sulfur doped carbon cloth as current collector and polysulfide immobilizer for magnesium-sulfur batteries. ChemElectroChem 2019, 6, 684–689. [Google Scholar] [CrossRef]
- Robba, A.; Mežnar, M.; Vizintin, A.; Bitenc, J.; Bobnar, J.; Arčon, I.; Randon-Vitanova, A.; Dominko, R. Role of Cu current collector on electrochemical mechanism of Mg–S battery. J. Power Sources 2020, 450, 227672. [Google Scholar] [CrossRef]
- Wang, Y.; Liao, H.; Wang, J.; Qian, X.; Zhu, Y.; Cheng, S. Effects of current collectors on electrochemical performance of FeS2 for Li-ion battery. Int. J. Electrochem. Sci. 2013, 8, 4002–4009. [Google Scholar]
- Raguzin, I.; Choudhury, S.; Simon, F.; Stamm, M.; Ionov, L. Effect of current collector on performance of Li-S batteries. Adv. Mater. Interfaces 2017, 4, 1600811. [Google Scholar] [CrossRef]
- Pfleging, W. A review of laser electrode processing for development and manufacturing of lithium-ion batteries. A review of laser electrode processing for development and manufacturing of lithium-ion batteries. Nanophotonics 2018, 7, 549–573. [Google Scholar] [CrossRef]











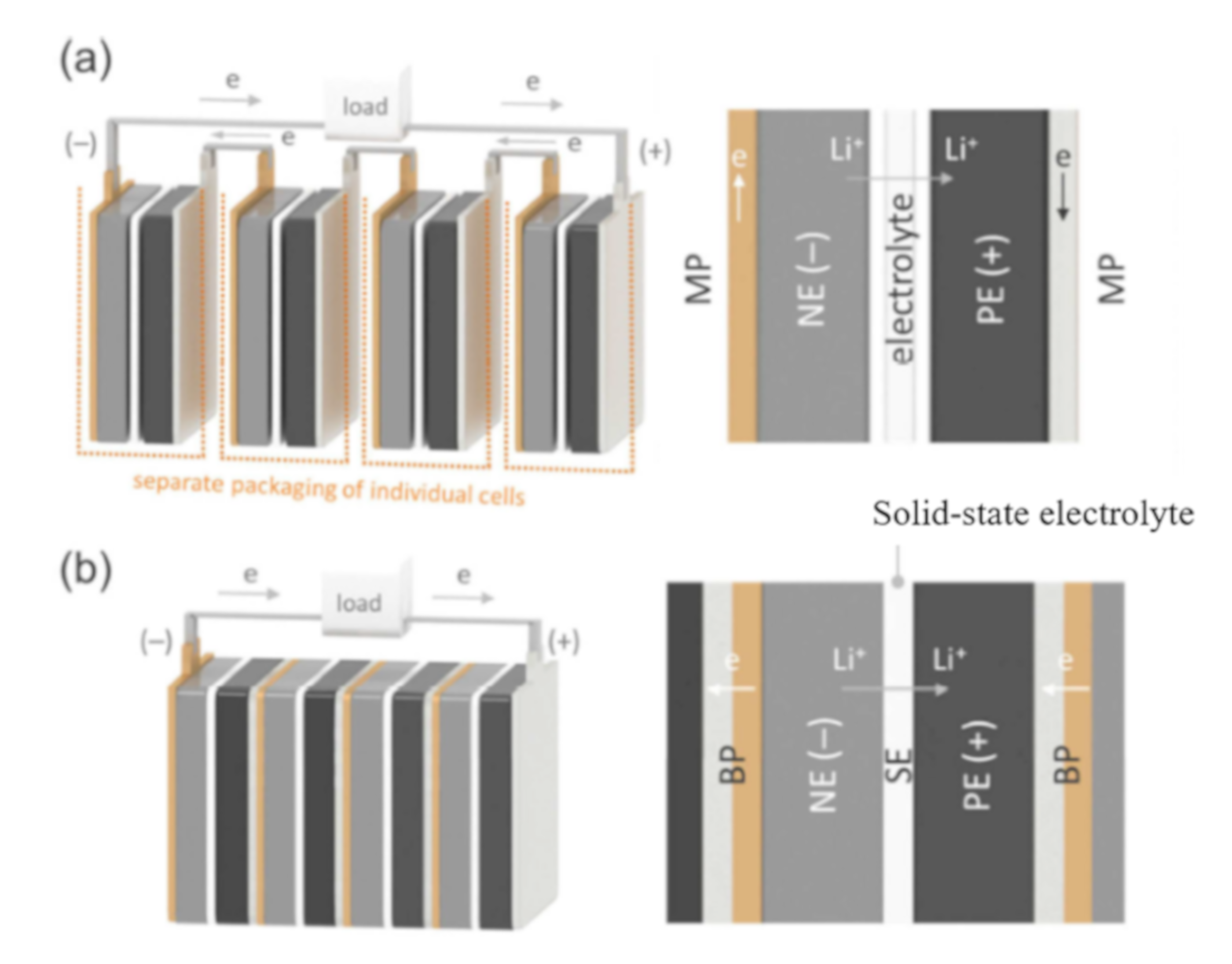
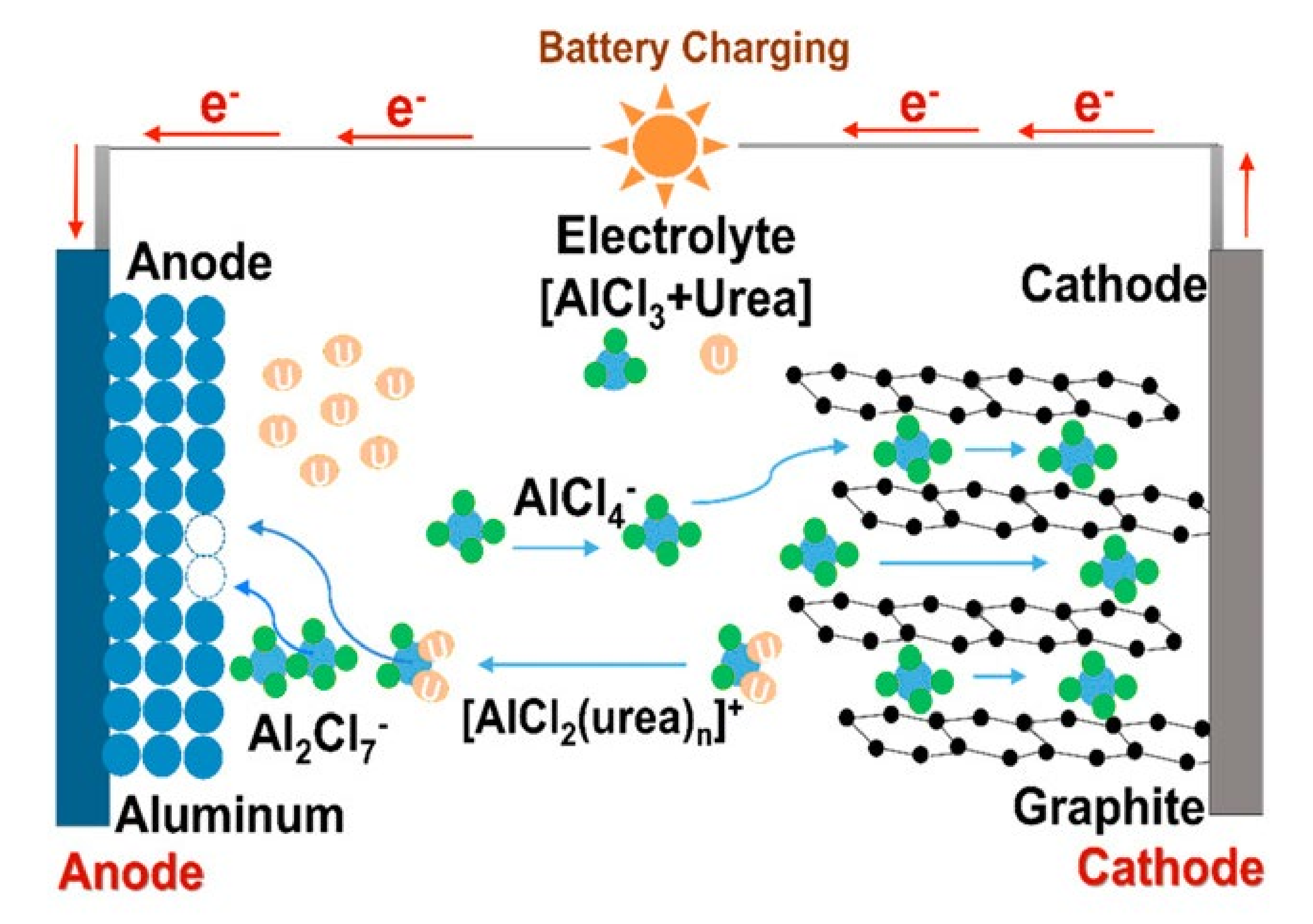
| Battery Type | Active Material | Electrolyte | Operation Voltage Range (V) | CC | Ref. | ||
|---|---|---|---|---|---|---|---|
| Anode | Cathode | Anode | Cathode | ||||
| Na metal battery | Na metal | NaTi2(PO4)3 | Na3Zr2(PO4)(SiO4)2 | −0.5–5 | ─ | Carbon-coated Al | [128] |
| Li battery | Indium foil | LiCoO2 | 78Li2S-22P2S5 | 1.9–3.68 | ─ | Carbon nanotube sheet | [129] |
| Li-ion battery | TiO2 | LiFePO4 | PEO (60 wt.%)/LiCF3SO3 (20 wt.%)/chopped glass fibers(20 wt.%) | 0.04–2.67 | Al | Cu-coated carbon fiber | [130] |
| Li metal battery | Li metal | LiMn2O4 | 1 M LiPF6/dimethylcarbonate-ethylene carbonate (1:1 wt.)/3 wt.% 4-fuoro-1,3-dioxolan-2-one/glass microfiber | 3–5 | ─ | Cr2N/stainless steel | [131] |
| Li metal battery | Graphite-deposited Li metal | LiNi0.33Mn0.33Co0.33O2 | Li6.28Al0.24La3Zr2O12/ poly (vinylidene fluoride-co-hexafluropropylene) containing 1 M LiTFSI ethylene carbonate and dimethyl carbonate (1:1) (cathode side) | 2–4.8 | Stainless steel | Al | [135] |
| Li metal battery | Li metal | LiFePO4 | PEO-LiTFSI/Li6.4La3Zr1.4Ta0.6O12/PEO-LiTFSI | 2.8–3.8 | ─ | Al | [136] |
| Li metal battery | Li metal | LiNi0.6Mn0.2Co0.2O2 | PEO-LiTFSI/Li7La3Zr2O12/PEO-LiTFSI | 2.7–4.2 | ─ | Al | [137] |
| Li metal battery | Li metal | LiFePO4 | PEO-LiTFSI/Li3/8Sr7/16Ta3/4Zr1/4O3/PEO-LiTFSI | 2.8–3.85 | ─ | Al | [138] |
| Li-ion battery | Li4Ti5O12 | LiNi0.8Co0.1Mn0.1O2 | β-Li3PS4 | 2.65–4.35 | Stainless steel | Stainless steel | [139] |
| Li-ion battery | Li4Ti5O12 | LiNi0.8Co0.1Mn0.1O2 | Li1.5Al0.5Ti1.5(PO4)3 | 2.65–4.35 | Stainless steel | Stainless steel | [139] |
| Li metal battery | Li metal | TiS2 | Li10GeP2S12 | 1–2.5 | ─ | Al | [140] |
| Li metal battery | Li metal | LiNi1/3Mn1/3Co1/3O2 | PEO-LiTFSI(LiTFSI/EO=0.06)(anode side)/Li3.6Ge0.8S0.2O4 | 2.0–4.2 | ─ | Au (by DC sputtering) | [141] |
| Li-ion battery | In metal | LiNi0.6Co0.2Mn0.2O2 | (Li2S)0.75(P2S5)0.25 | 2.0–3.6 | ─ | Stainless steel | [142] |
| Li metal battery | Li0.5In alloy | LiNbO3-coated LiNi0.85Co0.1Al0.05O2 | Li6PS5Cl | 2.5–4.3 | ─ | Al | [143] |
| Li-ion battery | Li4.4Si | TiS2 | Li2S-P2S5 | 1.1–2.4 | Stainless steel | Stainless steel | [144] |
| Li-ion battery | Li4Ti5O12 | LiCoO2 | LiTFSI-poly(vinylidene fluoride-hexafluoro propylene)-succinonitrile | 1.5–3 | Al | Al | [146] |
| Li metal battery | Li metal | LiFePO4 | LiTFSI-methyl carbonate-capped perfluoropolyethers-ethylene/dimethyl carbonate (EC and DMC, 1:1, v/v) | 2.5–4.2 | ─ | Al | [147] |
| Battery Type | Active Materials | Electrolyte | Operation Voltage Range (V) | CCs | Ref. | ||
|---|---|---|---|---|---|---|---|
| Anode | Cathode | Anode | Cathode | ||||
| Na metal battery | Na metal | ─ | 1 M NaPF6/diglyme | 0–0.5 | Porous Al | ─ | [159] |
| Na metal | Na3V2(PO4)3 | 1 M NaPF6/dimethyl ether | 2.5–3.8 | 3D Cu | Carbon | [160] | |
| Na metal | Na0.67Ni0.33Mn0.67O2 | 1 M NaClO4/ethylene carbonate/propylene carbonate (EC:PC = 1:1 by volume) | 2.5–3.8 | Carbon felt | Al | [162] | |
| Na battery | Na deposition/dissolution | ─ | 1.5 M NaSO3CF3(Na(OTf))/diglyme | 0–1 | N-doped mesoporous carbon nanofiber | ─ | [163] |
| Na deposition/dissolution | ─ | 1 M Na(OTf)/dimethyl ether | 0–0.5 | Cu micropatterns on Al foil | ─ | [164] | |
| Na deposition/dissolution | ─ | 1 M NaPF6/diethylene glycol dimethyl ether | 0–0.2 | PVdF@Cu, PVdF-HFP@Cu, and PEO@Cu | ─ | [165] | |
| Na deposition/dissolution | NaFe0.4Ni0.3Ti0.3O2 | NaFSI-N-propyl-N-methylpyrrolidinium bis(fluorosulfonyl)imide | 2.6–3.7 | ─ | Al | [166] | |
| Mg battery | Mg deposition/dissolution | ─ | MgCl2–AlEtCl2–THF | −0.5–3.5 | ─ | Mo and W | [168] |
| Mg metal battery | Mg metal | ─ | Magnesium bis(hexamethyldisilazide)-AlCl3/tetraglyme | 2.2–3.1 | ─ | Inconel 625, Hastelloy B, graphite and carbon coated Al foil | [170] |
| Mg metal battery Mg–Li hybrid battery | Mg metal | ─ | Mg(AlCl2EtBu)2/THF | 1.8–2.4 | ─ | Ni | [171] |
| Mg metal | ─ | 0.50 M PhMgCl and 0.25 M AlCl3/THF | 0–4 | ─ | Glassy carbon | [172] | |
| Mg metal | LiFePO4, LiMn2O4 | 0.2 M [Mg2Cl2(DME)4][AlCl4]2 and 1.0 M LiTFSI | 1.6–3.2 | ─ | Mo | [173] | |
| Ca-ion battery | Sn | Graphite | 0.8 M Ca(PF6)2/ethylene carbonate and ethyl methyl carbonate | 3–5 | Sn | Al | [174] |
| Ca battery | Ca deposition/dissolution | CaMn2O4 | 0.1M Ca(OTf)2 and 1M TBA(OTf)/DMF | −1–1.5 | Stainless Steel | Graphite and stainless steel | [176] |
| Ca metal battery | Ca metal | CaSi2 | 0.45M Ca(BF4)2/EC:PC (50/50 wt.%) | 0.5–3.5 | ─ | Al | [178] |
| Ca metal battery | Ca metal | CaxMoO3 | 0.5 M Ca(TFSI)2/1,2-dimethoxyethane | 0.5–2.2 | steel grid | Ti | [179] |
| Al metal battery | Al metal | Graphite | AlCl3/urea = 1.3 mol ratio | 1–2.2 | ─ | Carbon fiber paper | [181] |
| Al metal battery Al battery | Al metal | Graphite | AlCl3/EMIC = 1.1–2.0 mol ratio | 0–2.5 | ─ | W and glassy carbon | [182] |
| Al metal | Graphite | AlCl3/EMIC = 1.3 mol ratio | 0.5–2.5 | ─ | Mo | [183] | |
| Al deposition/dissolution | I2 | AlCl3/EMIC = 1.3 mol ratio | 0.6–1.2 | Al foil | active carbon cloth | [187] | |
| Al metal battery | Al metal | Graphite | AlCl3/EMIC = 1.3 mol ratio | 0.1–2.3 | ─ | biomass-derivative carbon | [188] |
| Na–S battery | Na metal | Na2S | Na-Nafion (anode side)//1.5 M NaClO4 and 0.2 M NaNO3/tetraglyme(cathode side) | 1.25–3 | ─ | Carbo nanofiber | [191] |
| Mg–S battery | Mg–carbon composite pellets | sulfur-reduced graphene oxide nanocomposite | Magnesium-bis(hexamethyldisilazide)/AlCl3/MgCl2/tetraglyme | 0.5–2.5 | ─ | Inconel 625 | [192] |
| Li–S battery | Li metal | polysulfide | 1 M LiTFSI/1,3-dioxolane-1,2-dimethoxyethane (1:1 v/v) | 1.3–2.6 | ─ | Cu foam | [193] |
| Mg–S battery | Mg metal | sulfur-reduced graphene oxide nanocomposite | Magnesium bis(hexamethyldisilazide)/AlCl3/MgCl2/tetraethylene glycol dimethyl ether | 0.5–2.75 | ─ | N, S dual-doped carbon cloth | [194] |
| Li–S battery | Li metal | FeS2 | 1 M LiClO4/dimethoxy ethane-1,3-dioxolane (2:1, v/v) | 1.0–2.5 | ─ | Ni foil | [196] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamada, M.; Watanabe, T.; Gunji, T.; Wu, J.; Matsumoto, F. Review of the Design of Current Collectors for Improving the Battery Performance in Lithium-Ion and Post-Lithium-Ion Batteries. Electrochem 2020, 1, 124-159. https://doi.org/10.3390/electrochem1020011
Yamada M, Watanabe T, Gunji T, Wu J, Matsumoto F. Review of the Design of Current Collectors for Improving the Battery Performance in Lithium-Ion and Post-Lithium-Ion Batteries. Electrochem. 2020; 1(2):124-159. https://doi.org/10.3390/electrochem1020011
Chicago/Turabian StyleYamada, Mitsuru, Tatsuya Watanabe, Takao Gunji, Jianfei Wu, and Futoshi Matsumoto. 2020. "Review of the Design of Current Collectors for Improving the Battery Performance in Lithium-Ion and Post-Lithium-Ion Batteries" Electrochem 1, no. 2: 124-159. https://doi.org/10.3390/electrochem1020011
APA StyleYamada, M., Watanabe, T., Gunji, T., Wu, J., & Matsumoto, F. (2020). Review of the Design of Current Collectors for Improving the Battery Performance in Lithium-Ion and Post-Lithium-Ion Batteries. Electrochem, 1(2), 124-159. https://doi.org/10.3390/electrochem1020011






