Electrolyte Additives in Lithium Ion EV Batteries and the Relationship of the SEI Composition to Cell Resistance and Lifetime
Abstract
:1. Introduction
2. Materials and Methods
3. Results
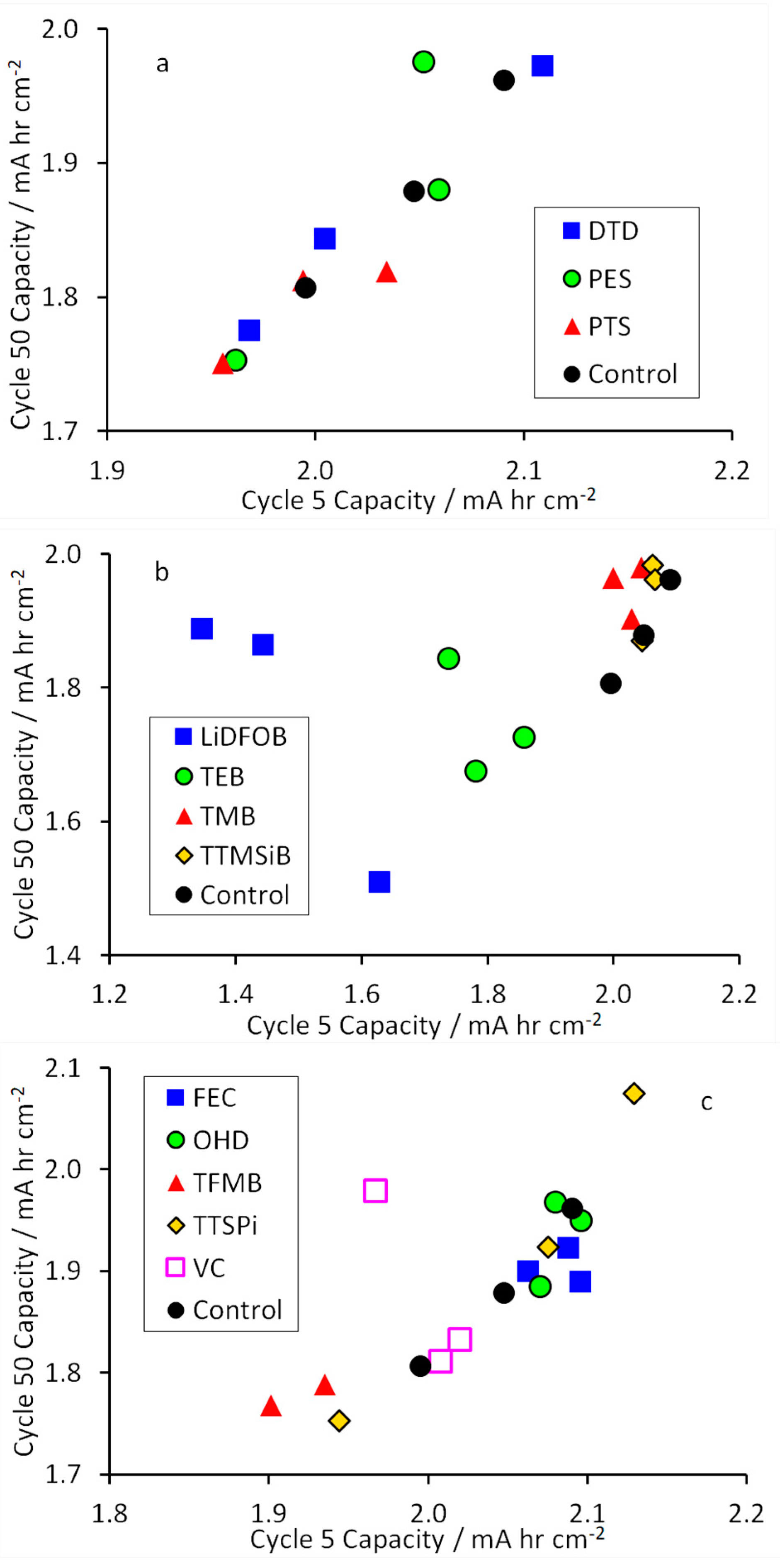
3.1. Electrochemical Testing Results
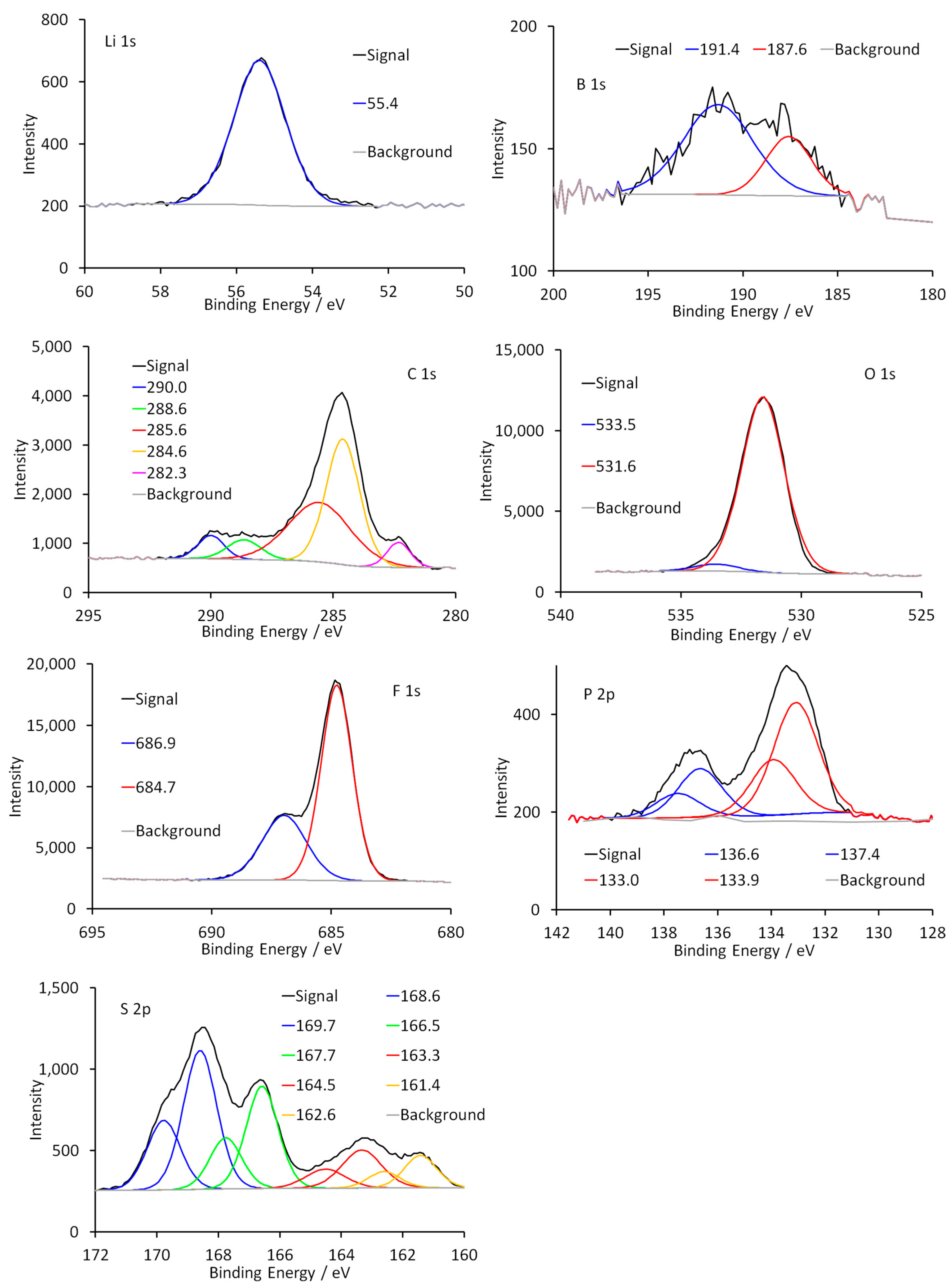
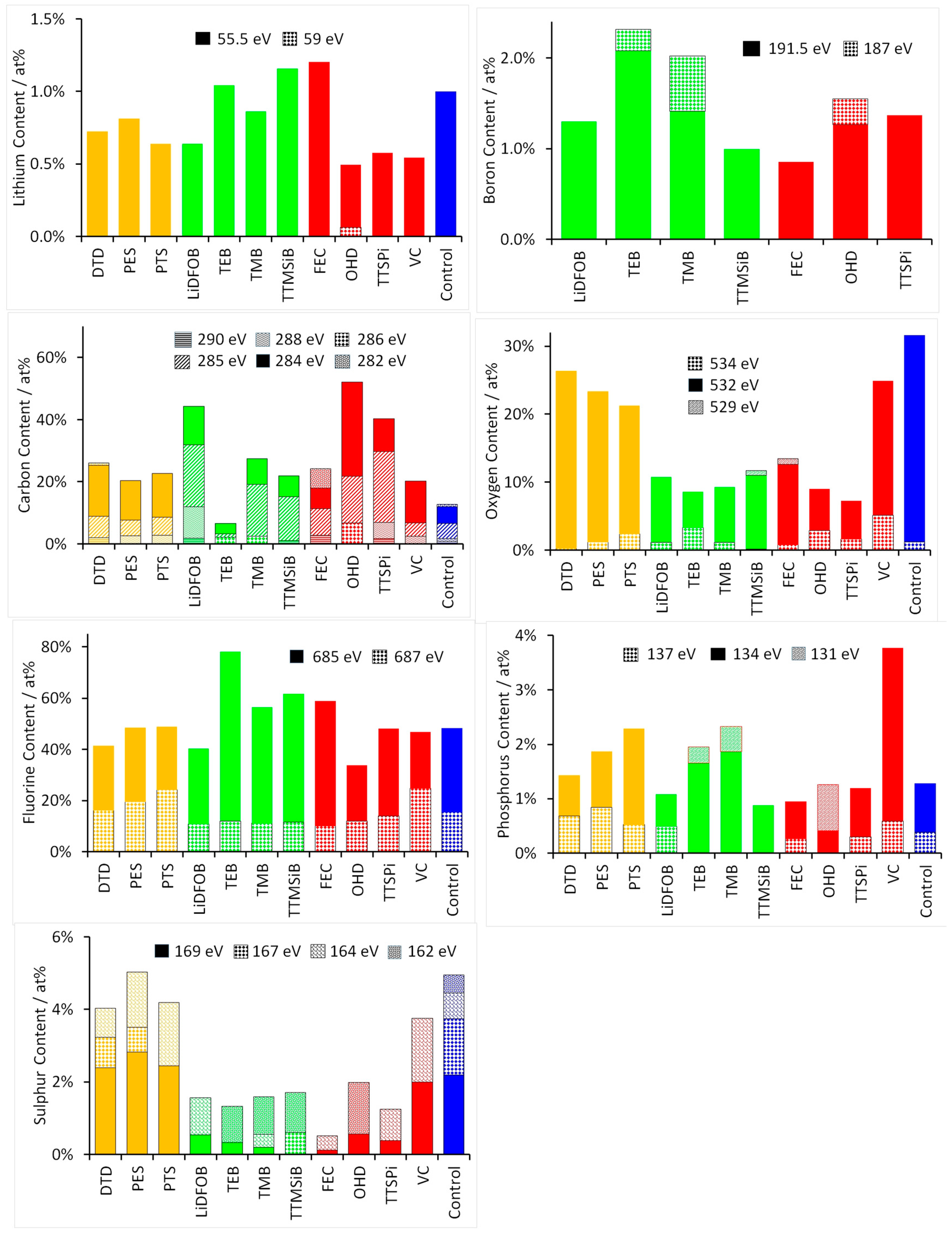
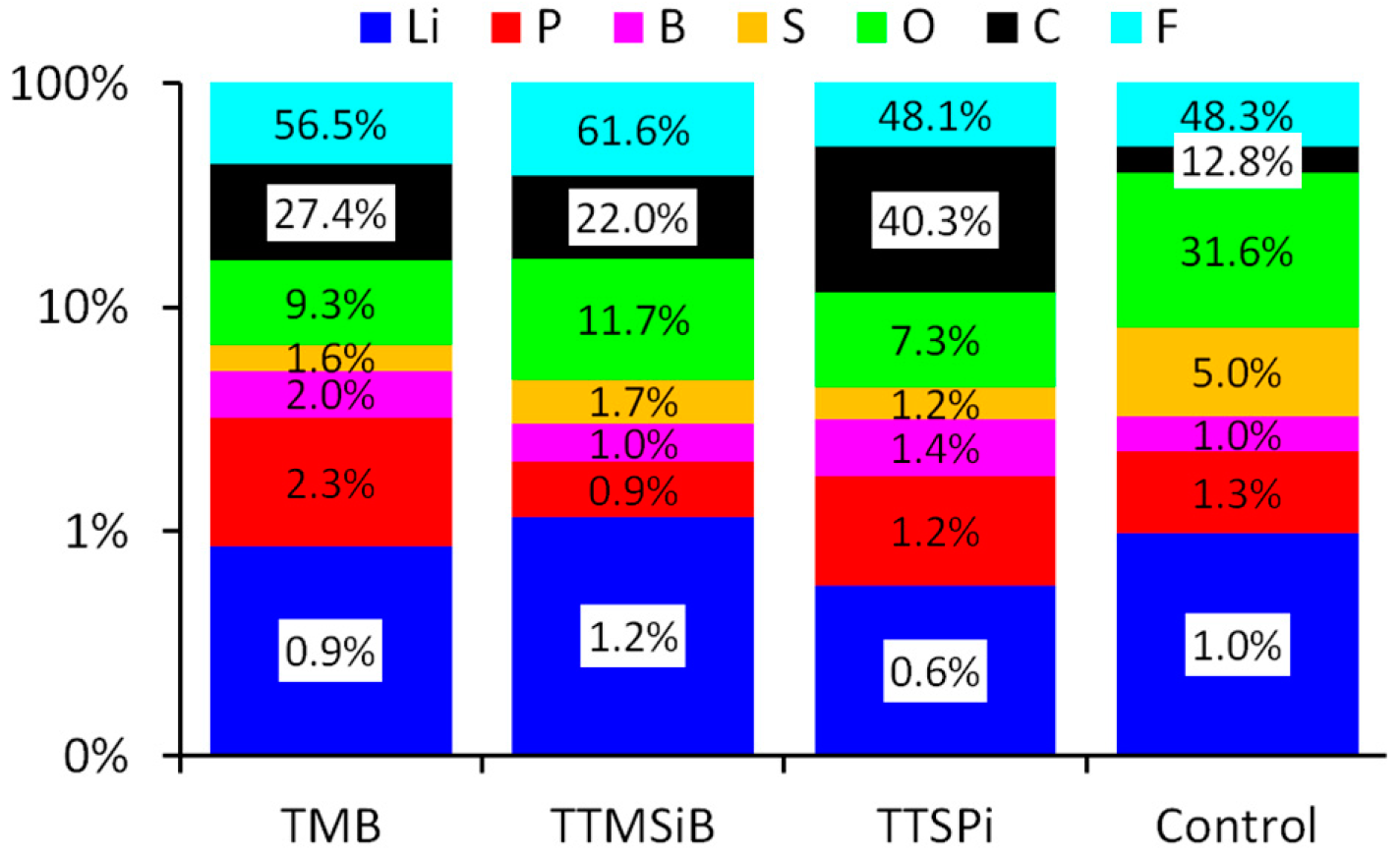
3.2. Chemical Analysis of the SEI Surface





4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Blomgren, G.E. The development and future of lithium ion batteries. J. Electrochem. Soc. 2017, 164, A5019–A5025. [Google Scholar] [CrossRef] [Green Version]
- Aurbach, D.; Talyosef, Y.; Markovsky, B.; Markevich, E.; Zinigrad, E.; Asraf, L.; Gnanaraj, J.S.; Kim, H.-J. Design of electrolyte solutions for lithium and lithium ion batteries: A review. Electrochim. Acta 2004, 50, 247–254. [Google Scholar] [CrossRef]
- Balbuena, P.B. Electrolyte materials—Issues and challenges. AIP Conf. Proc. 2014, 1597, 82–97. [Google Scholar] [CrossRef]
- Li, Q.; Chen, J.; Fan, L.; Kong, X.; Lu, Y. Progress in electrolytes for rechargeable lithium based batteries and beyond. Green Energy Environ. 2016, 1, 18–42. [Google Scholar] [CrossRef] [Green Version]
- Verma, P.; Maire, P.; Novák, P. A review of the features and analyses of the SEI in lithium ion batteries. Electrochim. Acta 2010, 55, 6332–6341. [Google Scholar] [CrossRef]
- Zhang, S.S. A review of electrolyte additives for lithium ion batteries. J. Power Sources 2016, 162, 1379–1394. [Google Scholar] [CrossRef]
- Maleki, H.; Deng, G.; Anani, A.; Howard, J. Thermal stability studies of lithium ion cells and components. J. Electrochem. Soc. 1999, 146, 3224–3229. [Google Scholar] [CrossRef]
- Wang, Q.; Ping, P.; Zhao, X.; Chu, G.; Sun, J.; Chen, C. Thermal runaway caused fire and explosion of lithium ion battery. J. Power Sources 2012, 208, 210–224. [Google Scholar] [CrossRef]
- Belov, D.; Yang, M.-H. Investigation of the kinetic mechanism in over-charge process for lithium ion battery. Solid State Ionics 2008, 179, 1816–1821. [Google Scholar] [CrossRef]
- Aurbach, D. Review of selected electrode—Solution interactions which determine the performance of lithium and lithium ion batteries. J. Power Sources 2000, 89, 206–218. [Google Scholar] [CrossRef]
- Parimalam, B.S.; Lucht, B.L. Reduction reactions of electrolyte salts for lithium ion batteries: LiPF6, LiBF4, LiDFOB, LiBOB and LiTFSI. J. Electrochem. Soc. 2018, 165, A251–A255. [Google Scholar] [CrossRef] [Green Version]
- Peled, E.; Menkin, S. Review. SEI: Past, present and future. J. Electrochem. Soc. 2017, 164, A1703–A1719. [Google Scholar] [CrossRef]
- Agubra, V.A.; Fergus, J.W. The formation and stability of the SEI on the graphite anodes. J. Power Sources 2014, 268, 153–162. [Google Scholar] [CrossRef]
- An, S.J.; Li, J.; Daniel, C.; Mohanty, D.; Nagpure, S.; Wood, D.L. The state of understanding of the lithium ion battery graphite SEI and its relationship to formation cycling. Carbon 2016, 105, 52–76. [Google Scholar] [CrossRef] [Green Version]
- El Ouatani, L.; Dedryvère, R.; Siret, C.; Biensan, P.; Reynaud, S.; Iratçabal, P.; Gonbeau, D. The effect of vinylene carbonate additive on surface film formation on both electrodes in lithium ion batteries. J. Electrochem. Soc. 2009, 156, A103–A113. [Google Scholar] [CrossRef]
- Xiong, D.; Burns, J.C.; Smith, A.J.; Sinha, N.; Dahn, J.R. A high precision study of the effect of VC additive in lithium/graphite cells. J. Electrochem. Soc. 2011, 158, A1431–A1435. [Google Scholar] [CrossRef]
- Xu, K. Non-aqueous liquid electrolytes for lithium based rechargeable batteries. Chem. Rev. 2004, 104, 4303–4417. [Google Scholar] [CrossRef]
- Wang, I.Y.; Zhang, M.; von Sacken, U.; Way, B.M. Additives for Improving Cycle Life of Non-Aqueous Rechargeable Lithium Batteries. U.S. Patent 6,045,948, 4 April 2000. [Google Scholar]
- Ue, M. Role-assigned Electrolyte Additives. In Lithium Ion Batteries; Yoshio, M., Brodd, R.J., Kozawa, A., Eds.; Springer: New York, NY, USA, 2009. [Google Scholar]
- Xu, K. Electrolytes and interphases in lithium ion batteries and beyond. Chem. Rev. 2014, 114, 11504–11618. [Google Scholar] [CrossRef]
- Retrieved 2016. Available online: http://www.eco-aesc-lb.com/en/product/liion_ev/ (accessed on 20 April 2020).
- Barker, J.; Cochran, S. Methods of Fabricating Electrochemical Cells. U.S. Patent 5,871,865, 16 February 1999. [Google Scholar]
- Yasunami, S. Non-Aqueous Secondary Battery and Method for Preparing Same. U.S. Patent 6,371,995, 16 April 2002. [Google Scholar]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Perkin Elmer: Norwalk, CT, USA, 1992. [Google Scholar]
- Utsugi, K.; Kusachi, Y.; Katou, T. Electrolyte Solution for a Secondary Battery. U.S. Patent 9,012,071, 21 April 2015. [Google Scholar]
- Hagiyama, K.; Matsuo, A.; Yasuda, H.; Miyakubo, H. Non-Aqueous Electrolyte Composition and Non-Aqueous Electrolyte Secondary Battery. U.S. Patent 9,160,033, 13 October 2015. [Google Scholar]
- Ma, L.; Wang, D.Y.; Downie, L.E.; Xia, J.; Nelson, K.J.; Sinha, N.N.; Dahn, J.R. Ternary and quaternary electrolyte additive mixtures for lithium ion cells that promote long lifetime, high discharge rate and better safety. J. Electrochem. Soc. 2014, 161, A1261–A1265. [Google Scholar] [CrossRef]
- Kang, K.S.; Choi, S.; Song, J.; Woo, S.-G.; Jo, Y.N.; Choi, J.; Yim, T.; Yi, J.-S.; Kim, Y.-J. Effect of additives on electrochemical performance of NMC at high temperature. J. Power Sources 2014, 253, 48–54. [Google Scholar] [CrossRef]
- Self, J.; Hall, D.S.; Madec, L.; Dahn, J.R. The role of PES as an additive in lithium ion cells. J. Power Sources 2015, 298, 369–378. [Google Scholar] [CrossRef]
- Huang, W.; Xing, L.; Zhang, R.; Wang, X.; Li, W. A novel electrolyte additive for improving the interfacial stability of high voltage LNMO cathode. J. Power Sources 2015, 293, 71–77. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, Y.; Bettge, M.; Abraham, D.P. Positive electrode passivation by LiDFOB electrolyte additive in high capacity lithium ion cells. J. Electrochem. Soc. 2012, 159, A2109–A2117. [Google Scholar] [CrossRef]
- Wang, Z.; Xing, L.; Li, J.; Xu, M.; Li, W. Triethylborate as an electrolyte additive for high voltage layered NMC cathode of lithium ion battery. J. Power Sources 2016, 307, 587–592. [Google Scholar] [CrossRef]
- Li, J.; Xing, L.; Zhang, L.; Yu, L.; Fan, W.; Xu, M.; Li, W. Insight into self-discharge of layered lithium rich cathode in carbonate based electrolytes with and without additive. J. Power Sources 2016, 324, 17–25. [Google Scholar] [CrossRef]
- Rong, H.; Xu, M.; Xie, B.; Liao, X.; Huang, W.; Xing, L.; Li, W. Tris trimethylsilyl borate (TMSB) as a cathode surface film forming additive for 5 V LNMO lithium ion cells. Electrochim. Acta 2014, 147, 31–39. [Google Scholar] [CrossRef]
- Li, J.; Xing, L.; Zhang, R.; Chen, M.; Wang, Z.; Xu, M.; Li, W. Tris trimethylsilyl borate as an electrolyte additive for improving interfacial stability of high voltage layered lithium rich oxide cathode/carbonate based electrolyte. J. Power Sources 2015, 285, 360–366. [Google Scholar] [CrossRef]
- Xia, J.; Nie, M.; Burns, J.C.; Xiao, A.; Lamanna, W.M.; Dahn, J.R. Fluorinated electrolyte for 4.5 V NCM-424/graphite lithium ion cells. J. Power Sources 2016, 307, 340–350. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, J.; Youssef, K.; Redfern, P.C.; Curtiss, L.A.; Amine, K.; Zhang, Z. Molecular engineering toward stabilised interface: An electrolyte additive for high performance lithium ion battery. J. Electrochem. Soc. 2014, 161, A2262–A2267. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Xing, L.; Wang, W.; Xu, M.; Li, W.; Xie, F.; Xia, S. 4-trifluoromethyl benzonitrile: A novel electrolyte additive for LNMO cathode of high voltage lithium ion battery. J. Power Sources 2014, 267, 560–565. [Google Scholar] [CrossRef]
- Zhu, Y.; Luo, X.; Xu, M.; Zhang, L.; Yu, L.; Fan, W.; Li, W. Failure mechanism of layered lithium rich oxide/graphite cell and its solution by using electrolyte additives. J. Power Sources 2016, 317, 65–73. [Google Scholar] [CrossRef]
- Somerville, L.; Bareno, J.; Jennings, P.; McGordon, A.; Lyness, C.; Bloom, I. The effect of pre-analysis washing on the surface film of graphite electrodes. Electrochim. Acta 2016, 206, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.-K.; Yoo, J.; Yim, T. Why is tris trimethylsilyl phosphite effective as an additive for high voltage lithium ion batteries? J. Mater. Chem. A 2015, 3, 10900–10909. [Google Scholar] [CrossRef]
- Peebles, C.; Sahore, R.; Gilbert, J.A.; Garcia, J.C.; Tornheim, A.; Bareño, J.; Iddir, H.; Liao, C.; Abraham, D.P. TMSPi and TEPi as electrolyte additives for lithium ion batteries: Mechanistic insights into differences during NMC-532/graphite full cell cycling. J. Electrochem. Soc. 2017, 164, A1579–A1586. [Google Scholar] [CrossRef]
- Qi, X.; Tao, L.; Hahn, H.; Schultz, C.; Gallus, D.R.; Cao, X.; Nowak, S.; Röser, S.; Li, J.; Cekic-Laskovic, I.; et al. Lifetime limit of tris (trimethylsilyl) phosphite as electrolyte additive for high voltage lithium ion batteries. RSC Adv. 2016, 6, 38342–38349. [Google Scholar] [CrossRef] [Green Version]
- Yim, T.; Han, Y.-K. Tris (trimethylsilyl) phosphite as an efficient electrolyte additive to improve the surface stability of graphite anodes. ACS Appl. Mater. Interfaces 2017, 9, 32851–32858. [Google Scholar] [CrossRef]
- Bothe-Almquist, C.L.; Ettireddy, R.P.; Bobst, A.; Smirniotis, P.G. An XRD, XPS and EPR study of Li/MgO catalysts: Case of oxidative methylation of acetonitrile to acrylonitrile with methane. J. Catal. 2000, 192, 174–184. [Google Scholar] [CrossRef]
- Xiao, A.; Yang, L.; Lucht, B.L.; Kang, S.-H.; Abraham, D.P. Examining the SEI on binder free graphite electrodes. J. Electrochem. Soc. 2009, 156, A318–A327. [Google Scholar] [CrossRef]
- Blyth, R.I.R.; Buqa, H.; Netzer, F.P.; Ramsey, M.G.; Besenhard, J.O.; Golb, P.; Winter, M. XPS studies of graphite electrode materials for lithium ion batteries. Appl. Surf. Sci. 2000, 167, 99–106. [Google Scholar] [CrossRef]
- Nie, M.; Demeaux, J.; Young, B.T.; Heskett, D.R.; Chen, Y.; Bose, A.; Woicik, J.C.; Lucht, B.L. Effect of VC and FEC on SEI formation on graphitic anodes in lithium ion batteries. J. Electrochem. Soc. 2015, 162, A7008–A7014. [Google Scholar] [CrossRef] [Green Version]
- Kanamura, K.; Shiraishi, S.; Takezawa, H.; Takehara, Z. XPS analysis of the surface of a carbon electrode intercalated by lithium ions. Chem. Mater. 1997, 9, 1797–1804. [Google Scholar] [CrossRef]
- Yazami, R. Surface chemistry and lithium storage capability of the graphite lithium electrode. Electrochim. Acta 1999, 45, 87–97. [Google Scholar] [CrossRef]
- Edström, K.; Herstedt, M.; Abraham, D.P. A new look at the solid electrolyte interphase on graphite anodes in lithium ion batteries. J. Power Sources 2006, 153, 380–384. [Google Scholar] [CrossRef]
- Andersson, A.M.; Abraham, D.P.; Haasch, R.; MacLaren, S.; Liu, J.; Amine, K. Surface characterisation of electrodes from high power lithium ion batteries. J. Electrochem. Soc. 2002, 149, A1358–A1369. [Google Scholar] [CrossRef]
- Ota, H.; Akai, T.; Namita, H.; Yamaguchi, S.; Nomura, M. XAFS and TOF-SIMS analysis of SEI layers on electrodes. J. Power Sources 2003, 119–121, 567–571. [Google Scholar] [CrossRef]
- Chen, S.; Dai, F.; Gordin, M.L.; Yu, Z.; Gao, Y.; Song, J.; Wang, D. Functional organo-sulphide electrolyte promotes and alternative reaction pathway to achieve high performance in lithium sulphur batteries. Angew. Chem. Int. Ed. 2016, 55, 4231–4235. [Google Scholar] [CrossRef]
- Li, B.; Xu, M.; Li, B.; Liu, Y.; Yang, L.; Li, W.; Hu, S. Properties of SEI formed by PES on graphite anode of lithium ion batteries. Electrochim. Acta 2013, 105, 1–6. [Google Scholar] [CrossRef]
- Leggesse, E.G.; Jiang, J.-C. Theoretical study of the reductive decomposition of ethylene sulphite: A film forming electrolyte additive in lithium ion batteries. J. Phys. Chem. A 2012, 116, 11025–11033. [Google Scholar] [CrossRef]
- Ein-Eli, Y.; Thomas, S.R.; Koch, V.R. The role of SO2 as an additive to organic lithium ion battery electrolytes. J. Electrochem. Soc. 1997, 144, 1159–1165. [Google Scholar] [CrossRef]






| Type | Acronym | Name | Concn./wt % | Ref |
|---|---|---|---|---|
| Sulphur Containing | DTD | Dioxathilane-2,2-dioxide | 1.0 | [27] |
| PES | Propene sulfone | 1.0 | [28,29] | |
| PTS | Phenyl trifluoromethyl sulphide | 0.5 | [30] | |
| Boron Containing | LiDFOB | Lithium difluoro oxalato borate | 2.0 | [31] |
| TEB | Triethyl borate | 2.5 | [32] | |
| TMB | Trimethyl borate | 1.0 | [33] | |
| TTMSiB | Tris trimethylsilyl borate | 0.6 | [34,35] | |
| Other | FEC | Fluoroethylene carbonate | 1.0 | [36] |
| OHD | 3-oxabicyclo [3.1.0]hexane-2,4-dione | 0.2 | [37] | |
| TFMB | 4-Trifluoromethyl benzonitrile | 1.0 | [38] | |
| TTSPi | Tris trimethyl silyl phosphite | 1.0 | [27,39] | |
| VC | Vinylene carbonate | 1.0 | [32] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lain, M.J.; Rubio Lopez, I.; Kendrick, E. Electrolyte Additives in Lithium Ion EV Batteries and the Relationship of the SEI Composition to Cell Resistance and Lifetime. Electrochem 2020, 1, 200-216. https://doi.org/10.3390/electrochem1020014
Lain MJ, Rubio Lopez I, Kendrick E. Electrolyte Additives in Lithium Ion EV Batteries and the Relationship of the SEI Composition to Cell Resistance and Lifetime. Electrochem. 2020; 1(2):200-216. https://doi.org/10.3390/electrochem1020014
Chicago/Turabian StyleLain, Micheal J., Irene Rubio Lopez, and Emma Kendrick. 2020. "Electrolyte Additives in Lithium Ion EV Batteries and the Relationship of the SEI Composition to Cell Resistance and Lifetime" Electrochem 1, no. 2: 200-216. https://doi.org/10.3390/electrochem1020014





