Recent Advancements in Selenium-Based Cathode Materials for Lithium Batteries: A Mini-Review
Abstract
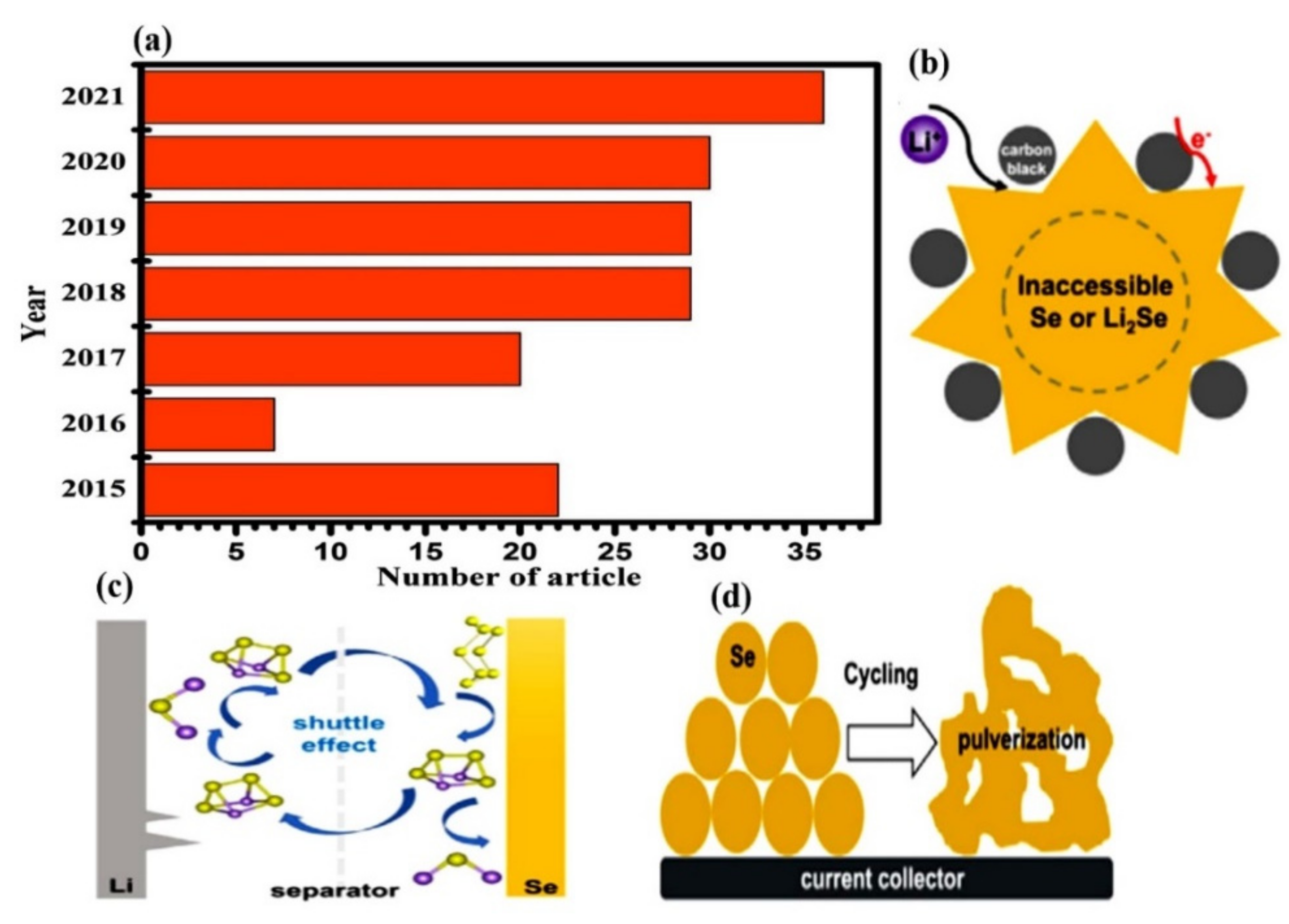
:1. Introduction
2. Choice of the Proper Electrolyte for Li-Se Batteries
3. Carbon-Selenium Composites for Li-Se Batteries
3.1. Porous Carbon/Se Composites
3.1.1. Mesoporous Carbon/Se Composite
3.1.2. Microporous Carbon/Se Composite
3.1.3. Hierarchical Porous Carbon/Se Composites
3.2. CNTs-CNFs/Se Composites
3.3. Se/C Core-Shell Composite and Free-Standing Carbon/Se Composite
4. Metal Selenide-Based Electrodes
5. Interlayers and Separators
6. Li2Se Cathode and Se Toxicity
7. Conclusions and Future Outlook
- (1)
- Cost-effective carbon-based materials should be chosen. Se cathodes have limited applications despite their high adsorptive and catalytic activity due to the exorbitant expense of their constituent elements.
- (2)
- The commercial implementations of Li-Se batteries are hindered by the instability of electrodes and the suppression of the shuttling effect during the charge-discharge process. Constructing a stable, effective solid electrolyte interphase (SEI) layer could restrict the dissolution of polyselenide and improve cycle performance.
- (3)
- The As Se cathode is a dynamic electrochemical system whose electrochemical characteristics are intimately connected to the arrangements of the entities. Thus, the effectiveness of any single carbon material is highly dependent on the cathode’s architecture and its ability to respond to minor adjustments. In light of the vast amount of research data reported, the screening of numerous outcomes, and the in-depth data analysis, it is highly suggested to use machine learning to determine that the incorporation is not only a simple material but also an integral component and arrangement of the entire network.
- (4)
- The thickness of interlayers typically spans from a few tens to hundreds of microns. The substantial thickness and high areal mass loading can lead to a significant increase in the weight of the interlayers, hence decreasing the practical, specific energy. Therefore, prospective interlayer technology research should construct lightweight interlayers employing various techniques. Undoubtedly, the destiny of interlayer technology for high-energy Li-Se batteries depends on the development of ultralight interlayers that can still perform their role in narrower arrangements.
- (5)
- The interlayers have been converted from a carbon-based substance to oxides and other chemicals. The interlayer structure of the future will presumably be considerably more diverse.
- (6)
- The usage of interlayers is hampered by the potential for high costs or a dearth of raw materials. To achieve the industrialization of Li-Se batteries, they must contend with established LIBs and other energy storage devices. Therefore, we must spare no effort in the development of functional systems.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leccardi, F.; Nodari, D.; Spada, D.; Ambrosetti, M.; Bini, M. Synergistic Effect of Polymorphs in Doped NaNi0. 5Mn0. 5O2 Cathode Material for Improving Electrochemical Performances in Na-Batteries. Electrochem 2021, 2, 335–346. [Google Scholar] [CrossRef]
- Darbar, D.; Bhattacharya, I. Application of Machine Learning in Battery: State of Charge Estimation Using Feed Forward Neural Network for Sodium-Ion Battery. Electrochem 2022, 3, 42–57. [Google Scholar] [CrossRef]
- Yamada, M.; Watanabe, T.; Gunji, T.; Wu, J.; Matsumoto, F. Review of the design of current collectors for improving the battery performance in lithium-ion and post-lithium-ion batteries. Electrochem 2020, 1, 124–159. [Google Scholar] [CrossRef]
- Madani, S.S.; Schaltz, E.; Kær, S.K. Thermal Simulation of Phase Change Material for Cooling of a Lithium-Ion Battery Pack. Electrochem 2020, 1, 439–449. [Google Scholar] [CrossRef]
- Hameed, S.A.; Petnikota, S.; Hassan, N.S.; Al-Qaradawi, S.Y.; Karim, Z.; Reddy, M. Synthesis of Nickel Fumarate and Its Electrochemical Properties for Li-Ion Batteries. Electrochem 2021, 2, 439–451. [Google Scholar] [CrossRef]
- Byeon, Y.-W.; Kim, H. Review on Interface and Interphase Issues in Sulfide Solid-State Electrolytes for All-Solid-State Li-Metal Batteries. Electrochem 2021, 2, 452–471. [Google Scholar] [CrossRef]
- Joy, R.; Balakrishnan, N.T.M.; Das, A.; Shafeek, S.; Thakur, V.K.; Zaghib, K.; Jaffarali, J.F.M.; Reddy, M.V.V.; Raghavan, P. Graphene: Chemistry and Applications for Lithium-Ion Batteries. Electrochem 2022, 3, 143–183. [Google Scholar] [CrossRef]
- Darbar, D.; Reddy, M.; Bhattacharya, I. Understanding the effect of Zn doping on stability of cobalt-free P2-Na0. 60Fe0. 5Mn0. 5O2 cathode for sodium ion batteries. Electrochem 2021, 2, 323–334. [Google Scholar] [CrossRef]
- Zhao, M.; Li, B.-Q.; Zhang, X.-Q.; Huang, J.-Q.; Zhang, Q. A perspective toward practical lithium–sulfur batteries. ACS Cent. Sci. 2020, 6, 1095–1104. [Google Scholar] [CrossRef]
- Wang, R.; Wang, K.; Tao, H.; Zhao, W.; Jiang, M.; Yan, J.; Jiang, K. Controllable electrolytic formation of Ti2O as an efficient sulfur host in lithium–sulfur (Li–S) batteries. J. Mater. Chem. A 2020, 8, 11224–11232. [Google Scholar] [CrossRef]
- Zhao, M.; Chen, X.; Li, X.Y.; Li, B.Q.; Huang, J.Q. An organodiselenide comediator to facilitate sulfur redox kinetics in lithium–sulfur batteries. Adv. Mater. 2021, 33, 2007298. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, W.; Xu, J.; Guo, Z. Advances in polar materials for lithium–sulfur batteries. Adv. Funct. Mater. 2018, 28, 1707520. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Chang, Z.; Wu, S.; Zhou, H. Effective strategies for long-cycle life lithium–sulfur batteries. J. Mater. Chem. A 2018, 6, 6155–6182. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, F.; Huang, J.Q.; Li, H. Lithium–Sulfur Batteries: Co-Existence of Challenges and Opportunities. Adv. Funct. Mater. 2018, 28, 1804589. [Google Scholar] [CrossRef]
- Zhao, M.; Li, B.Q.; Peng, H.J.; Yuan, H.; Wei, J.Y.; Huang, J.Q. Lithium–sulfur batteries under lean electrolyte conditions: Challenges and opportunities. Angew. Chem. Int. Ed. 2020, 59, 12636–12652. [Google Scholar] [CrossRef]
- Su, D.; Zhou, D.; Wang, C.; Wang, G. Toward high performance lithium–sulfur batteries based on Li2S cathodes and beyond: Status, challenges, and perspectives. Adv. Funct. Mater. 2018, 28, 1800154. [Google Scholar] [CrossRef]
- Wang, L.; Ye, Y.; Chen, N.; Huang, Y.; Li, L.; Wu, F.; Chen, R. Development and challenges of functional electrolytes for high-performance lithium–sulfur batteries. Adv. Funct. Mater. 2018, 28, 1800919. [Google Scholar] [CrossRef]
- Hou, L.-P.; Zhang, X.-Q.; Li, B.-Q.; Zhang, Q. Challenges and promises of lithium metal anode by soluble polysulfides in practical lithium–sulfur batteries. Mater. Today 2021, 45, 62–76. [Google Scholar] [CrossRef]
- Deshpande, S.S.; Deshpande, M.D.; Alhameedi, K.; Ahuja, R.; Hussain, T. Carbon Nitride Monolayers as Efficient Immobilizers toward Lithium Selenides: Potential Applications in Lithium–Selenium Batteries. ACS Appl. Energy Mater. 2021, 4, 3891–3904. [Google Scholar] [CrossRef]
- Park, H.; Kim, J.; Lee, D.; Park, J.; Jo, S.; Kim, J.; Song, T.; Paik, U. Epitaxial Growth of Nanostructured Li2Se on Lithium Metal for All Solid-State Batteries. Adv. Sci. 2021, 8, 2004204. [Google Scholar] [CrossRef]
- Bui, H.T.; Jang, H.; Ahn, D.; Han, J.; Sung, M.; Kutwade, V.; Patil, M.; Sharma, R.; Han, S.-H. High-performance Li–Se battery: Li2Se cathode as intercalation product of electrochemical in situ reduction of multilayer graphene-embedded 2D-MoSe2. Electrochim. Acta 2021, 368, 137556. [Google Scholar] [CrossRef]
- Li, W.; Liu, F.; Shen, Z.; Hu, H.; Fu, Z. High-Rate Performance All-Solid-State Li-SeS2 Battery with Selenium sulphide/three-dimensional graphene as cathode material. Int. J. Electrochem. Sci. 2021, 16, 210759. [Google Scholar] [CrossRef]
- Jin, W.-w.; Li, H.-j.; Zou, J.-z.; Inguva, S.; Zhang, Q.; Zeng, S.-z.; Xu, G.-z.; Zeng, X.-r. Metal organic framework-derived carbon nanosheets with fish-scale surface morphology as cathode materials for lithium–selenium batteries. J. Alloy. Compd. 2020, 820, 153084. [Google Scholar] [CrossRef]
- Abouimrane, A.; Dambournet, D.; Chapman, K.W.; Chupas, P.J.; Weng, W.; Amine, K. A new class of lithium and sodium rechargeable batteries based on selenium and selenium–sulfur as a positive electrode. J. Am. Chem. Soc. 2012, 134, 4505–4508. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Abouimrane, A.; Sun, C.-J.; Ren, Y.; Amine, K. Li–Se battery: Absence of lithium polyselenides in carbonate based electrolyte. Chem. Commun. 2014, 50, 5576–5579. [Google Scholar] [CrossRef]
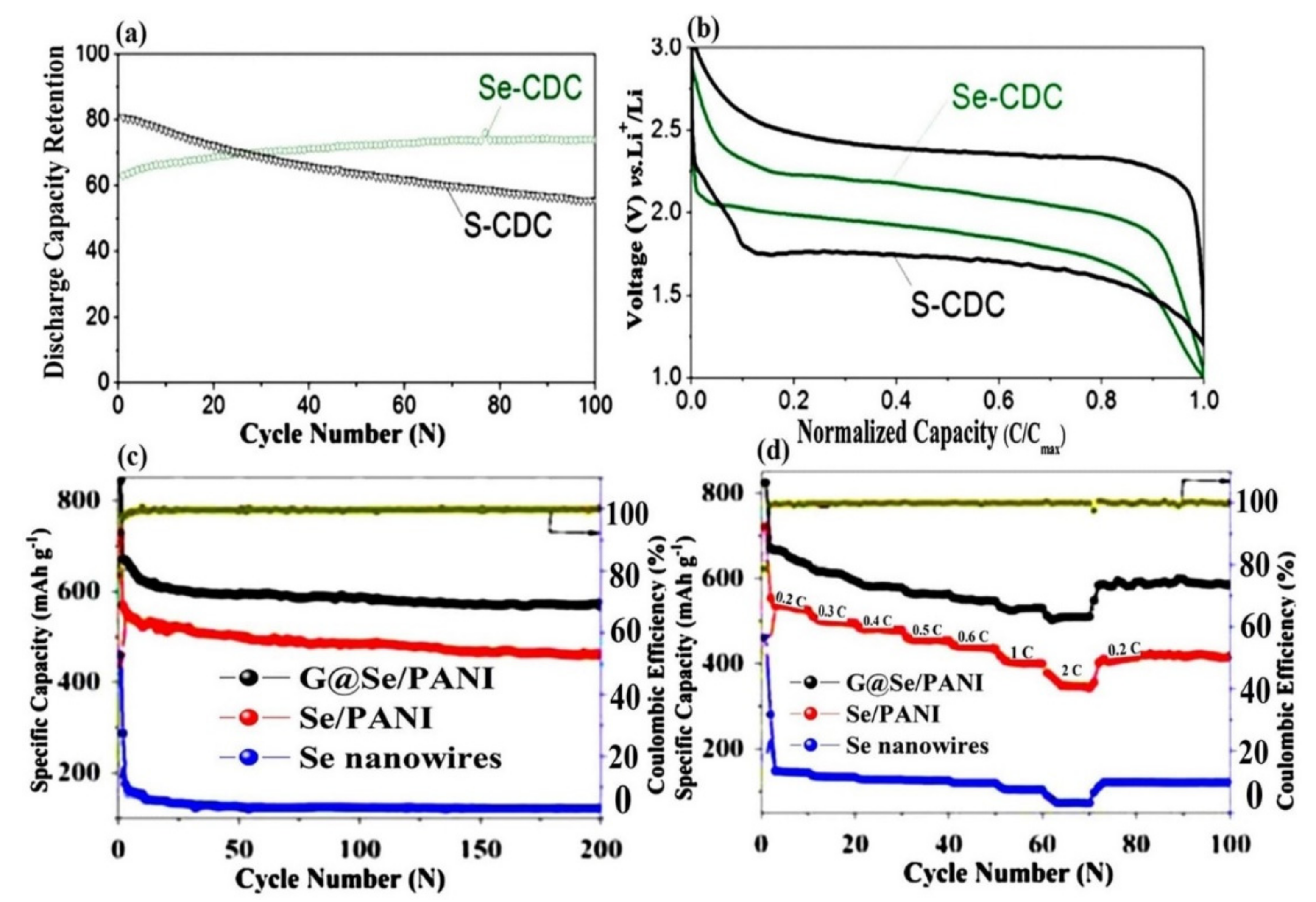
- Lee, J.T.; Kim, H.; Oschatz, M.; Lee, D.C.; Wu, F.; Lin, H.T.; Zdyrko, B.; Cho, W.I.; Kaskel, S.; Yushin, G. Micro-and Mesoporous Carbide-Derived Carbon–Selenium Cathodes for High-Performance Lithium Selenium Batteries. Adv. Energy Mater. 2015, 5, 1400981. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Y.; Fan, L.; Zhu, Y.; Liang, J.; Qian, Y. Graphene–encapsulated selenium/polyaniline core–shell nanowires with enhanced electrochemical performance for Li–Se batteries. Nano Energy 2015, 13, 592–600. [Google Scholar] [CrossRef]
- Dong, Y.; Lu, P.; Ding, Y.; Shi, H.; Feng, X.; Wu, Z.S. Advanced design of cathodes and interlayers for high-performance lithium-selenium batteries. SusMat 2021, 1, 393–412. [Google Scholar] [CrossRef]
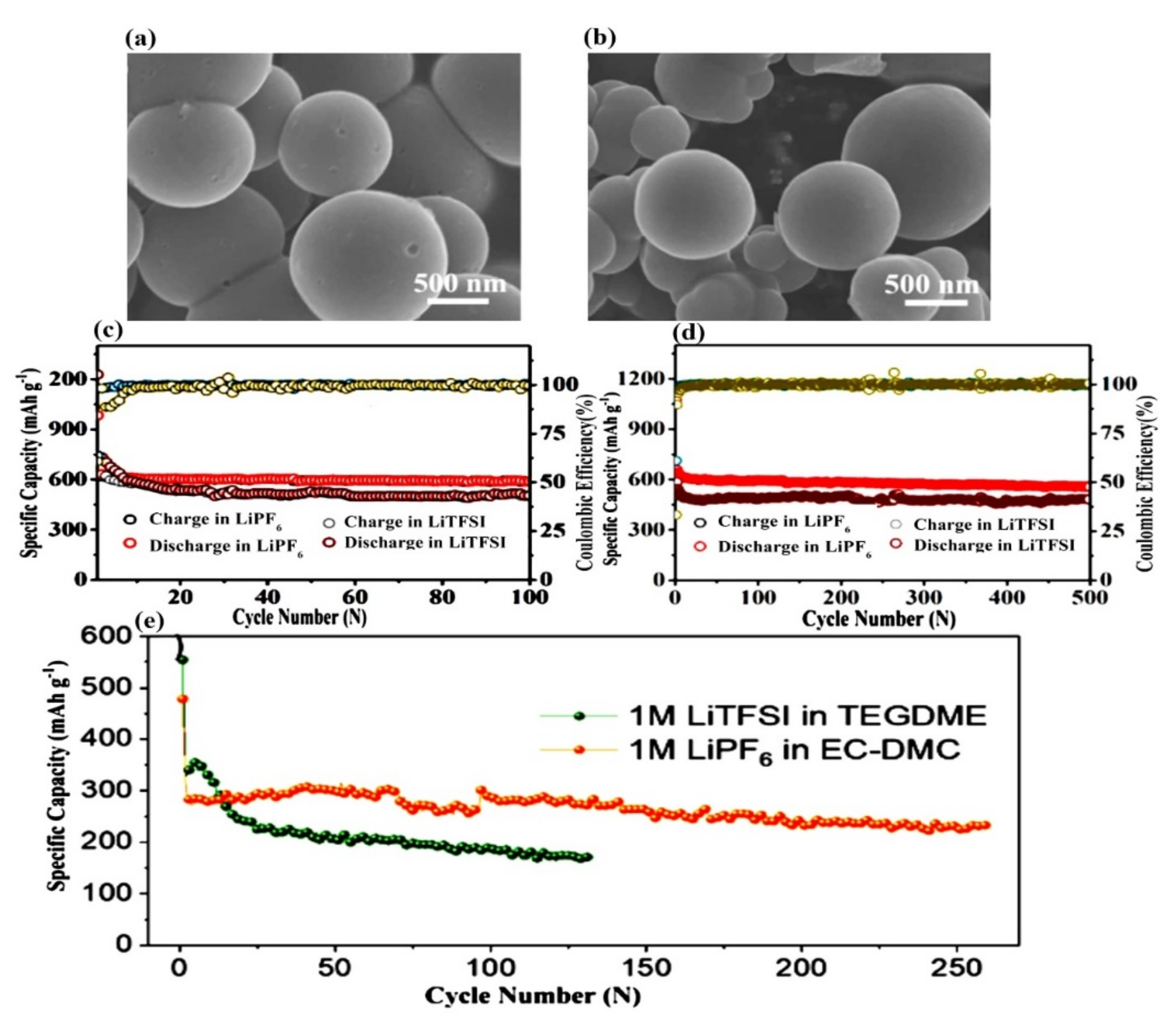
- Mutlu, T.; Demir-Cakan, R. Carbonate or ether based electrolyte for Li-Se batteries: An in-situ study of intermediate polyselenide formation. Electrochim. Acta 2021, 390, 138825. [Google Scholar] [CrossRef]
- He, B.; Liu, D.; Cheng, Z.; Miao, Z.; Rao, Z.; Zhang, H.; Yuan, L.; Li, Z.; Huang, Y. Enabling Selenium-Rich SexSy Cathodes to Work in Carbonate-Based Electrolytes. Adv. Energy Mater. 2022, 12, 2102832. [Google Scholar] [CrossRef]
- Qi, X.; Yang, Y.; Jin, Q.; Yang, F.; Xie, Y.; Sang, P.; Liu, K.; Zhao, W.; Xu, X.; Fu, Y. Two-Plateau Li-Se Chemistry for High Volumetric Capacity Se Cathodes. Angew. Chem. 2020, 132, 14012–14018. [Google Scholar] [CrossRef]
- Lei, Y.; Liang, X.; Yang, L.; Chen, J.; Qu, L.; Xu, K.; Feng, J. Li–Se batteries: Insights to the confined structure of selenium in hierarchical porous carbon and discharge mechanism in the carbonate electrolyte. Carbon 2022, 191, 122–131. [Google Scholar] [CrossRef]
- Lei, Z.; Lei, Y.; Liang, X.; Yang, L.; Feng, J. High stable rate cycling performances of microporous carbon spheres/selenium composite (MPCS/Se) cathode as lithium–selenium battery. J. Power Sources 2020, 473, 228611. [Google Scholar] [CrossRef]
- Eftekhari, A. The rise of lithium–selenium batteries. Sustain. Energy Fuels 2017, 1, 14–29. [Google Scholar] [CrossRef]
- Cui, Y.; Abouimrane, A.; Lu, J.; Bolin, T.; Ren, Y.; Weng, W.; Sun, C.; Maroni, V.A.; Heald, S.M.; Amine, K. (De) Lithiation mechanism of Li/SeS x (x = 0–7) batteries determined by in situ synchrotron X-ray diffraction and X-ray absorption spectroscopy. J. Am. Chem. Soc. 2013, 135, 8047–8056. [Google Scholar] [CrossRef]
- Yi, Z.; Yuan, L.; Sun, D.; Li, Z.; Wu, C.; Yang, W.; Wen, Y.; Shan, B.; Huang, Y. High-performance lithium–selenium batteries promoted by heteroatom-doped microporous carbon. J. Mater. Chem. A 2015, 3, 3059–3065. [Google Scholar] [CrossRef]
- Li, Z.; Yuan, L.; Yi, Z.; Liu, Y.; Huang, Y. Confined selenium within porous carbon nanospheres as cathode for advanced Li–Se batteries. Nano Energy 2014, 9, 229–236. [Google Scholar] [CrossRef]
- Zhou, X.; Gao, P.; Sun, S.; Bao, D.; Wang, Y.; Li, X.; Wu, T.; Chen, Y.; Yang, P. Amorphous, crystalline and crystalline/amorphous selenium nanowires and their different (De) lithiation mechanisms. Chem. Mater. 2015, 27, 6730–6736. [Google Scholar] [CrossRef]
- Liu, L.; Wei, Y.; Zhang, C.; Zhang, C.; Li, X.; Wang, J.; Ling, L.; Qiao, W.; Long, D. Enhanced electrochemical performances of mesoporous carbon microsphere/selenium composites by controlling the pore structure and nitrogen doping. Electrochim. Acta 2015, 153, 140–148. [Google Scholar] [CrossRef]
- Zhang, Q.; Cai, L.; Liu, G.; Li, Q.; Jiang, M.; Yao, X. Selenium-infused ordered mesoporous carbon for room-temperature all-solid-state lithium–selenium batteries with ultrastable cyclability. ACS Appl. Mater. Interfaces 2020, 12, 16541–16547. [Google Scholar] [CrossRef]
- Xue, P.; Zhai, Y.; Wang, N.; Zhang, Y.; Lu, Z.; Liu, Y.; Bai, Z.; Han, B.; Zou, G.; Dou, S. Selenium@ Hollow mesoporous carbon composites for high-rate and long-cycling lithium/sodium-ion batteries. Chem. Eng. J. 2020, 392, 123676. [Google Scholar] [CrossRef]
- Luo, C.; Xu, Y.; Zhu, Y.; Liu, Y.; Zheng, S.; Liu, Y.; Langrock, A.; Wang, C. Selenium@ mesoporous carbon composite with superior lithium and sodium storage capacity. ACS Nano 2013, 7, 8003–8010. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.P.; Xin, S.; Yin, Y.X.; Ye, H.; Zhang, J.; Guo, Y.G. An advanced selenium–carbon cathode for rechargeable lithium–selenium batteries. Angew. Chem. Int. Ed. 2013, 52, 8363–8367. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Jia, M.; Zhang, Y.; Han, J.; Li, Y.; Bao, S.; Liu, D.; Jiang, J.; Xu, M. Confined selenium within metal-organic frameworks derived porous carbon microcubes as cathode for rechargeable lithium–selenium batteries. J. Power Sources 2017, 341, 53–59. [Google Scholar] [CrossRef]
- Lai, Y.; Gan, Y.; Zhang, Z.; Chen, W.; Li, J. Metal-organic frameworks-derived mesoporous carbon for high performance lithium–selenium battery. Electrochim. Acta 2014, 146, 134–141. [Google Scholar] [CrossRef]
- Shiraz, M.H.A.; Hu, Y.; Liu, J. A Stable Lithium-ion Selenium Batteries Enabled by Microporous Carbon/Se and Fluoroethylene Carbonate Additive. ECS Trans. 2020, 97, 279. [Google Scholar] [CrossRef]
- Liu, Y.; Si, L.; Zhou, X.; Liu, X.; Xu, Y.; Bao, J.; Dai, Z. A selenium-confined microporous carbon cathode for ultrastable lithium–selenium batteries. J. Mater. Chem. A 2014, 2, 17735–17739. [Google Scholar] [CrossRef]
- Wu, H.B.; Wei, S.; Zhang, L.; Xu, R.; Hng, H.H.; Lou, X.W. Embedding sulfur in MOF-derived microporous carbon polyhedrons for lithium–sulfur batteries. Chem. A Eur. J. 2013, 19, 10804–10808. [Google Scholar] [CrossRef]
- Liu, Y.; Si, L.; Du, Y.; Zhou, X.; Dai, Z.; Bao, J. Strongly bonded selenium/microporous carbon nanofibers composite as a high-performance cathode for lithium–selenium batteries. J. Phys. Chem. C 2015, 119, 27316–27321. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, J.; Xu, Z.; Zhang, T.; Chen, Z.; Wang, J. A high performance lithium–selenium battery using a microporous carbon confined selenium cathode and a compatible electrolyte. J. Mater. Chem. A 2017, 5, 9350–9357. [Google Scholar] [CrossRef]
- Song, J.-P.; Wu, L.; Dong, W.-D.; Li, C.-F.; Chen, L.-H.; Dai, X.; Li, C.; Chen, H.; Zou, W.; Yu, W.-B. MOF-derived nitrogen-doped core–shell hierarchical porous carbon confining selenium for advanced lithium–selenium batteries. Nanoscale 2019, 11, 6970–6981. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Shiraz, M.H.A.; Zhu, H.; Liu, Y.; Tao, L.; Liu, J. Hierarchically porous carbon from waste coffee grounds for high-performance Li–Se batteries. Electrochim. Acta 2019, 325, 134931. [Google Scholar] [CrossRef]
- Liu, T.; Dai, C.; Jia, M.; Liu, D.; Bao, S.; Jiang, J.; Xu, M.; Li, C.M. Selenium embedded in metal–organic framework derived hollow hierarchical porous carbon spheres for advanced lithium–selenium batteries. ACS Appl. Mater. Interfaces 2016, 8, 16063–16070. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Zhang, Z.; Lai, Y.; Qu, Y.; Wang, X.; Li, J. Selenium encapsulated into 3D interconnected hierarchical porous carbon aerogels for lithium–selenium batteries with high rate performance and cycling stability. J. Power Sources 2014, 267, 394–404. [Google Scholar] [CrossRef]
- Qu, Y.; Zhang, Z.; Jiang, S.; Wang, X.; Lai, Y.; Liu, Y.; Li, J. Confining selenium in nitrogen-containing hierarchical porous carbon for high-rate rechargeable lithium–selenium batteries. J. Mater. Chem. A 2014, 2, 12255–12261. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, F.; Kang, W.; Shen, Q. Encapsulating selenium into macro-/micro-porous biochar-based framework for high-performance lithium-selenium batteries. Carbon 2015, 95, 354–363. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Qu, Y.; Wang, G.; Lai, Y.; Li, J. Solution-based synthesis of multi-walled carbon nanotube/selenium composites for high performance lithium–selenium battery. J. Power Sources 2015, 287, 247–252. [Google Scholar] [CrossRef]
- Balakumar, K.; Kalaiselvi, N. Selenium containing Tube-in-Tube carbon: A one dimensional carbon frame work for selenium cathode in Li-Se battery. Carbon 2017, 112, 79–90. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Z.; Li, Q.; Qu, Y.; Jiang, S. Selenium encapsulated into interconnected polymer-derived porous carbon nanofiber webs as cathode materials for lithium-selenium batteries. J. Electrochem. Soc. 2014, 161, A2093. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Q.; Qi, C.; Jin, J.; Zhu, Y.; Wen, Z. One-step microwave synthesized core–shell structured selenium@ carbon spheres as cathode materials for rechargeable lithium batteries. Chem. Commun. 2016, 52, 5613–5616. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Yang, X.; Guo, Z.; Qu, Y.; Li, J.; Lai, Y. Selenium/carbon-rich core–shell composites as cathode materials for rechargeable lithium–selenium batteries. J. Power Sources 2015, 279, 88–93. [Google Scholar] [CrossRef]
- Zhang, J.; Fan, L.; Zhu, Y.; Xu, Y.; Liang, J.; Wei, D.; Qian, Y. Selenium/interconnected porous hollow carbon bubbles composites as the cathodes of Li–Se batteries with high performance. Nanoscale 2014, 6, 12952–12957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, X.; Wang, L.; Zhang, X.; Gao, B.; Fu, J.; Xiao, S.; Huo, K.; Chu, P.K. Reduced graphene oxide encapsulated selenium nanoparticles for high-power lithium–selenium battery cathode. J. Power Sources 2015, 288, 214–220. [Google Scholar] [CrossRef]
- Ding, J.; Zhou, H.; Zhang, H.; Tong, L.; Mitlin, D. Selenium impregnated monolithic carbons as free-standing cathodes for high volumetric energy lithium and sodium metal batteries. Adv. Energy Mater. 2018, 8, 1701918. [Google Scholar] [CrossRef]
- Han, K.; Liu, Z.; Shen, J.; Lin, Y.; Dai, F.; Ye, H. A free-standing and ultralong-life lithium-selenium battery cathode enabled by 3D mesoporous carbon/graphene hierarchical architecture. Adv. Funct. Mater. 2015, 25, 455–463. [Google Scholar] [CrossRef]
- Huang, D.; Li, S.; Luo, Y.; Xiao, X.; Gao, L.; Wang, M.; Shen, Y. Graphene oxide-protected three dimensional Se as a binder-free cathode for Li-Se battery. Electrochim. Acta 2016, 190, 258–263. [Google Scholar] [CrossRef]
- Han, K.; Liu, Z.; Ye, H.; Dai, F. Flexible self-standing graphene–Se@ CNT composite film as a binder-free cathode for rechargeable Li–Se batteries. J. Power Sources 2014, 263, 85–89. [Google Scholar] [CrossRef]
- He, J.; Chen, Y.; Lv, W.; Wen, K.; Li, P.; Wang, Z.; Zhang, W.; Qin, W.; He, W. Three-dimensional hierarchical graphene-CNT@ Se: A highly efficient freestanding cathode for Li–Se batteries. ACS Energy Lett. 2016, 1, 16–20. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Wang, L.; Wang, J.; Jiang, D.; Li, G.; Zhang, Y.; Zhong, H.; Jiang, Y. Phase transition mechanism and electrochemical properties of nanocrystalline MoSe2 as anode materials for the high performance lithium-ion battery. J. Phys. Chem. C 2015, 119, 10197–10205. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Park, G.O.; Choi, Y.S.; Shon, J.K.; Yoon, J.; Kim, K.H.; Yoon, W.-S.; Kim, H.; Kim, J.M. Mesoporous transition metal dichalcogenide ME 2 (M = Mo, W; E = S, Se) with 2-D layered crystallinity as anode materials for lithium ion batteries. RSC Adv. 2016, 6, 14253–14260. [Google Scholar] [CrossRef]
- Jin, F.; Li, M.; Xie, L.; Jiang, J. Selenium-doped carbon nanotubes/nickel selenide coaxial nanocables for energy storage. J. Power Sources 2021, 514, 230587. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, M.; Chen, D. Sheet-like MoSe 2/C composites with enhanced Li-ion storage properties. J. Mater. Chem. A 2015, 3, 11857–11862. [Google Scholar] [CrossRef]
- Zhao, X.; Sui, J.; Li, F.; Fang, H.; Wang, H.; Li, J.; Cai, W.; Cao, G. Lamellar MoSe 2 nanosheets embedded with MoO 2 nanoparticles: Novel hybrid nanostructures promoted excellent performances for lithium ion batteries. Nanoscale 2016, 8, 17902–17910. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wu, Y.; Chen, X.; Ma, Y.; Xu, M.; Wei, W.; Pan, J.; Xiong, X. Rational design and preparation of few-layered MoSe 2 nanosheet@ C/TiO 2 nanobelt heterostructures with superior lithium storage performance. RSC Adv. 2016, 6, 23161–23168. [Google Scholar] [CrossRef]
- Xu, H.; Chen, G.; Jin, R.; Chen, D.; Wang, Y.; Pei, J. Green synthesis of Bi 2 Se 3 hierarchical nanostructure and its electrochemical properties. Rsc Adv. 2014, 4, 8922–8929. [Google Scholar] [CrossRef]
- Zhao, Q.; Hu, X.; Zhang, K.; Zhang, N.; Hu, Y.; Chen, J. Sulfur nanodots electrodeposited on Ni foam as high-performance cathode for Li–S batteries. Nano Lett. 2015, 15, 721–726. [Google Scholar] [CrossRef]
- Zhang, Z.; Fu, Y.; Yang, X.; Qu, Y.; Li, Q. Nanostructured ZnSe anchored on graphene nanosheets with superior electrochemical properties for lithium ion batteries. Electrochim. Acta 2015, 168, 285–291. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, Z.; Du, K.; Qu, Y.; Li, Q.; Yang, X. Spherical-like ZnSe with facile synthesis as a potential electrode material for lithium ion batteries. Mater. Lett. 2015, 146, 96–98. [Google Scholar] [CrossRef]
- Kwon, H.-T.; Park, C.-M. Electrochemical characteristics of ZnSe and its nanostructured composite for rechargeable Li-ion batteries. J. Power Sources 2014, 251, 319–324. [Google Scholar] [CrossRef]
- Xu, Y.; Liang, J.; Zhang, K.; Zhu, Y.; Wei, D.; Qian, Y. Origin of additional capacities in selenium-based ZnSe@ C nanocomposite Li-ion battery electrodes. Electrochem. Commun. 2016, 65, 44–47. [Google Scholar] [CrossRef]
- Valldor, M.; Mikhailova, D.; Giebeler, L.; Lai, K.T.; Spillecke, L.; Grafe, H.-J.; Büchner, B. Synthesis, Characterization, and Electrochemistry of Layered Chalcogenides LiCu Ch (Ch = Se, Te). Inorg. Chem. 2018, 57, 7201–7207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lu, L.; Zhang, D.; Hu, W.; Wang, N.; Xu, B.; Li, Y.; Zeng, H. Dual-buffered SnSe@ CNFs as negative electrode with outstanding lithium storage performance. Electrochim. Acta 2016, 209, 423–429. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.-S.; Manthiram, A. A new approach to improve cycle performance of rechargeable lithium–sulfur batteries by inserting a free-standing MWCNT interlayer. Chem. Commun. 2012, 48, 8817–8819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, S.H.; Manthiram, A. Bifunctional separator with a light-weight carbon-coating for dynamically and statically stable lithium-sulfur batteries. Adv. Funct. Mater. 2014, 24, 5299–5306. [Google Scholar] [CrossRef]
- Yang, Z.; Zhu, K.; Dong, Z.; Jia, D.; Jiao, L. Stabilization of Li–Se Batteries by Wearing PAN Protective Clothing. ACS Appl. Mater. Interfaces 2019, 11, 40069–40077. [Google Scholar] [CrossRef]
- Wu, F.; Lee, J.T.; Xiao, Y.; Yushin, G. Nanostructured Li2Se cathodes for high performance lithium-selenium batteries. Nano Energy 2016, 27, 238–246. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Z.; Zhang, K.; Yang, X.; Li, Q. Improvement of electrochemical performance of rechargeable lithium–selenium batteries by inserting a free-standing carbon interlayer. RSC Adv. 2014, 4, 15489–15492. [Google Scholar] [CrossRef]
- Si, L.; Wang, J.; Li, G.; Hong, X.; Wei, Q.; Yang, Y.; Zhang, M.; Cai, Y. High energy density lithium-selenium batteries enabled by a covalent organic framework-coated separator. Mater. Lett. 2019, 246, 144–148. [Google Scholar] [CrossRef]
- Zhang, F.; Guo, X.; Xiong, P.; Zhang, J.; Song, J.; Yan, K.; Gao, X.; Liu, H.; Wang, G. Interface engineering of MXene composite separator for high-performance Li–Se and Na–Se batteries. Adv. Energy Mater. 2020, 10, 2000446. [Google Scholar] [CrossRef]
- Fang, R.; Zhou, G.; Pei, S.; Li, F.; Cheng, H.-M. Localized polyselenides in a graphene-coated polymer separator for high rate and ultralong life lithium–selenium batteries. Chem. Commun. 2015, 51, 3667–3670. [Google Scholar] [CrossRef]
- Gu, X.; Xin, L.; Li, Y.; Dong, F.; Fu, M.; Hou, Y. Highly reversible Li–Se batteries with ultra-lightweight N, S-codoped graphene blocking layer. Nano-Micro Lett. 2018, 10, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Hong, X.-J.; Song, C.-L.; Li, G.-H.; Zheng, Y.-X.; Zhou, D.-D.; Zhang, M.; Cai, Y.-P.; Wang, H. Lithium bis (trifluoromethanesulfonyl) imide assisted dual-functional separator coating materials based on covalent organic frameworks for high-performance lithium–selenium sulfide batteries. J. Mater. Chem. A 2019, 7, 16323–16329. [Google Scholar] [CrossRef]
- Mukkabla, R.; Killi, K.; Shivaprasad, S.M.; Deepa, M. Metal Oxide Interlayer for Long-Lived Lithium–Selenium Batteries. Chem. –A Eur. J. 2018, 24, 17327–17338. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Chen, R.; Wang, X.; Hu, Y.; Wang, Y.; Chen, T.; Ma, L.; Zhu, G.; Liang, J.; Tie, Z. High-performance Li–Se batteries enabled by selenium storage in bottom-up synthesized nitrogen-doped carbon scaffolds. ACS Appl. Mater. Interfaces 2017, 9, 25232–25238. [Google Scholar] [CrossRef]
- Pang, J.; Mendes, R.G.; Bachmatiuk, A.; Zhao, L.; Ta, H.Q.; Gemming, T.; Liu, H.; Liu, Z.; Rummeli, M.H. Applications of 2D MXenes in energy conversion and storage systems. Chem. Soc. Rev. 2019, 48, 72–133. [Google Scholar] [CrossRef]
- Jayan, R.; Islam, M.M. Functionalized MXenes as effective polyselenide immobilizers for lithium–selenium batteries: A density functional theory (DFT) study. Nanoscale 2020, 12, 14087–14095. [Google Scholar] [CrossRef]
- Song, J.; Guo, X.; Zhang, J.; Chen, Y.; Zhang, C.; Luo, L.; Wang, F.; Wang, G. Rational design of free-standing 3D porous MXene/rGO hybrid aerogels as polysulfide reservoirs for high-energy lithium–sulfur batteries. J. Mater. Chem. A 2019, 7, 6507–6513. [Google Scholar] [CrossRef]
- Liang, X.; Rangom, Y.; Kwok, C.Y.; Pang, Q.; Nazar, L.F. Interwoven MXene nanosheet/carbon-nanotube composites as Li–S cathode hosts. Adv. Mater. 2017, 29, 1603040. [Google Scholar] [CrossRef]
- Fan, Y.; Yang, Z.; Hua, W.; Liu, D.; Tao, T.; Rahman, M.M.; Lei, W.; Huang, S.; Chen, Y. Functionalized Boron Nitride Nanosheets/Graphene Interlayer for Fast and Long-Life Lithium–Sulfur Batteries. Adv. Energy Mater. 2017, 7, 1602380. [Google Scholar] [CrossRef]
- Shao, H.; Ai, F.; Wang, W.; Zhang, H.; Wang, A.; Feng, W.; Huang, Y. Crab shell-derived nitrogen-doped micro-/mesoporous carbon as an effective separator coating for high energy lithium–sulfur batteries. J. Mater. Chem. A 2017, 5, 19892–19900. [Google Scholar] [CrossRef]
- Zeng, P.; Huang, L.; Zhang, X.; Zhang, R.; Wu, L.; Chen, Y. Long-life and high-areal-capacity lithium-sulfur batteries realized by a honeycomb-like N, P dual-doped carbon modified separator. Chem. Eng. J. 2018, 349, 327–337. [Google Scholar] [CrossRef]
- Balach, J.; Singh, H.K.; Gomoll, S.; Jaumann, T.; Klose, M.; Oswald, S.; Richter, M.; Eckert, J.r.; Giebeler, L. Synergistically enhanced polysulfide chemisorption using a flexible hybrid separator with N and S dual-doped mesoporous carbon coating for advanced lithium–sulfur batteries. ACS Appl. Mater. Interfaces 2016, 8, 14586–14595. [Google Scholar] [CrossRef] [PubMed]
- Ponraj, R.; Kannan, A.G.; Ahn, J.H.; Lee, J.H.; Kang, J.; Han, B.; Kim, D.-W. Effective trapping of lithium polysulfides using a functionalized carbon nanotube-coated separator for lithium–sulfur cells with enhanced cycling stability. ACS Appl. Mater. Interfaces 2017, 9, 38445–38454. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Wei, J.; Wang, Y.; Xia, Y. Synergetic protective effect of the ultralight MWCNTs/NCQDs modified separator for highly stable lithium–sulfur batteries. Adv. Energy Mater. 2018, 8, 1702288. [Google Scholar] [CrossRef]
- Son, Y.; Lee, J.S.; Son, Y.; Jang, J.H.; Cho, J. Recent advances in lithium sulfide cathode materials and their use in lithium sulfur batteries. Adv. Energy Mater. 2015, 5, 1500110. [Google Scholar] [CrossRef]
- Li, M.; Chen, Z.; Wu, T.; Lu, J. Li2S-or S-Based Lithium-Ion Batteries. Adv. Mater. 2018, 30, 1801190. [Google Scholar] [CrossRef]
- Su, D.; Zhou, D.; Wang, C.; Wang, G. Lithium-Sulfur Batteries: Toward High Performance Lithium–Sulfur Batteries Based on Li2S Cathodes and Beyond: Status, Challenges, and Perspectives (Adv. Funct. Mater. 38/2018). Adv. Funct. Mater. 2018, 28, 1870273. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; He, L.; Liu, W.; Fan, C.; Zheng, W.; Wong, Y.-S.; Chen, T. Selective cellular uptake and induction of apoptosis of cancer-targeted selenium nanoparticles. Biomaterials 2013, 34, 7106–7116. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Xu, T. Elemental selenium at nano size (Nano-Se) as a potential chemopreventive agent with reduced risk of selenium toxicity: Comparison with se-methylselenocysteine in mice. Toxicol. Sci. 2008, 101, 22–31. [Google Scholar] [CrossRef] [Green Version]
- Kundu, D.; Krumeich, F.; Nesper, R. Investigation of nano-fibrous selenium and its polypyrrole and graphene composite as cathode material for rechargeable Li-batteries. J. Power Sources 2013, 236, 112–117. [Google Scholar] [CrossRef]
- Lee, S.; Lee, H.; Ha, N.; Lee, J.T.; Jung, J.; Eom, K. In Batteria Electrochemical Polymerization to Form a Protective Conducting Layer on Se/C Cathodes for High-Performance Li–Se Batteries. Adv. Funct. Mater. 2020, 30, 2000028. [Google Scholar] [CrossRef]
- Luo, C.; Wang, J.; Suo, L.; Mao, J.; Fan, X.; Wang, C. In situ formed carbon bonded and encapsulated selenium composites for Li–Se and Na–Se batteries. J. Mater. Chem. A 2015, 3, 555–561. [Google Scholar] [CrossRef]
- Li, J.; Zhao, X.; Zhang, Z.; Lai, Y. Facile synthesis of hollow carbonized polyaniline spheres to encapsulate selenium for advanced rechargeable lithium–selenium batteries. J. Alloy. Compd. 2015, 619, 794–799. [Google Scholar] [CrossRef]
- Jia, M.; Lu, S.; Chen, Y.; Liu, T.; Han, J.; Shen, B.; Wu, X.; Bao, S.-J.; Jiang, J.; Xu, M. Three-dimensional hierarchical porous tubular carbon as a host matrix for long-term lithium-selenium batteries. J. Power Sources 2017, 367, 17–23. [Google Scholar] [CrossRef]
- Sha, L.; Gao, P.; Ren, X.; Chi, Q.; Chen, Y.; Yang, P. A Self-Repairing Cathode Material for Lithium–Selenium Batteries: Se− C Chemically Bonded Selenium–Graphene Composite. Chem. –A Eur. J. 2018, 24, 2151–2156. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, X.; Wang, X.; Li, Q.; Zhang, Z. TiO2–Se composites as cathode material for rechargeable lithium–selenium batteries. Solid State Ion. 2014, 260, 101–106. [Google Scholar] [CrossRef]
- Dutta, D.; Gope, S.; Negi, D.S.; Datta, R.; Sood, A.; Bhattacharyya, A.J. Pressure-induced capillary encapsulation protocol for ultrahigh loading of sulfur and selenium inside carbon nanotubes: Application as high performance cathode in Li–S/se rechargeable batteries. J. Phys. Chem. C 2016, 120, 29011–29022. [Google Scholar] [CrossRef]
- Mukkabla, R.; Deshagani, S.; Meduri, P.; Deepa, M.; Ghosal, P. Selenium/graphite platelet nanofiber composite for durable Li–Se batteries. ACS Energy Lett. 2017, 2, 1288–1295. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zhang, C.; Wu, C.-J.; Tao, Y.; Zhang, L.; Yang, Q.-H. Improved performance of Li–Se battery based on a novel dual functional CNTs@ graphene/CNTs cathode construction. Rare Met. 2017, 36, 425–433. [Google Scholar] [CrossRef]
- Youn, H.-C.; Jeong, J.H.; Roh, K.C.; Kim, K.-B. Graphene–selenium hybrid microballs as cathode materials for high-performance lithium–selenium secondary battery applications. Sci. Rep. 2016, 6, 30865. [Google Scholar] [CrossRef] [Green Version]
- Zeng, L.; Chen, X.; Liu, R.; Lin, L.; Zheng, C.; Xu, L.; Luo, F.; Qian, Q.; Chen, Q.; Wei, M. Green synthesis of a Se/HPCF–rGO composite for Li–Se batteries with excellent long-term cycling performance. J. Mater. Chem. A 2017, 5, 22997–23005. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Q.; Jin, J.; Chen, C.; Wen, Z. Analysis of structure and electrochemistry of selenium-containing conductive polymer materials for rechargeable lithium batteries. J. Electrochem. Soc. 2016, 163, A654. [Google Scholar] [CrossRef]
- Manthiram, A.; Yu, X.; Wang, S. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2017, 2, 16103. [Google Scholar] [CrossRef]
- Wang, D.-G.; Wang, Y.; Song, M.; Kuang, G.-C.; Han, K. Chemical anchoring of SeS 2 on a fluoro-substituted covalent organic framework as a high-performance cathode material. Chem. Commun. 2019, 55, 13247–13250. [Google Scholar] [CrossRef] [PubMed]

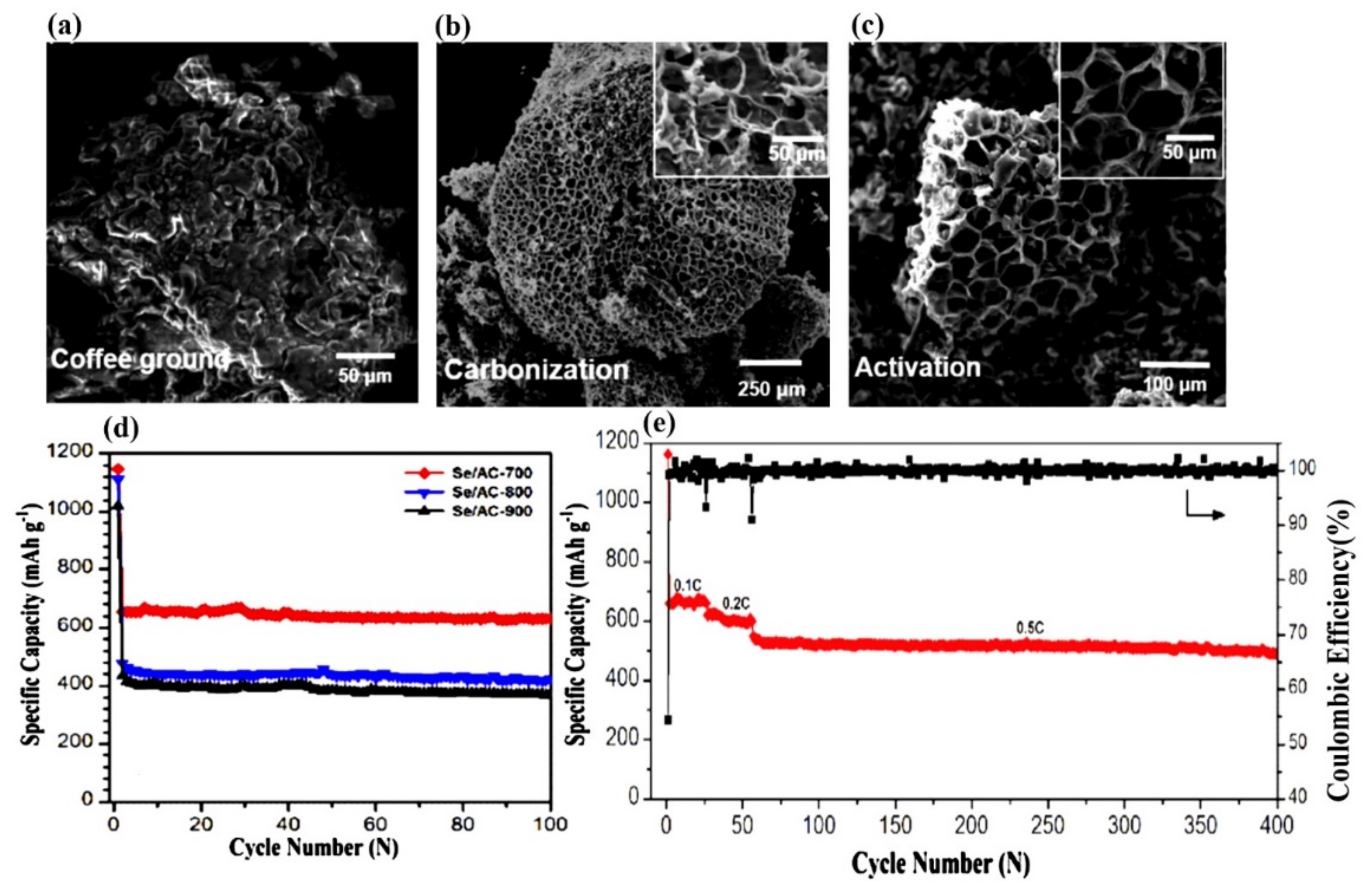

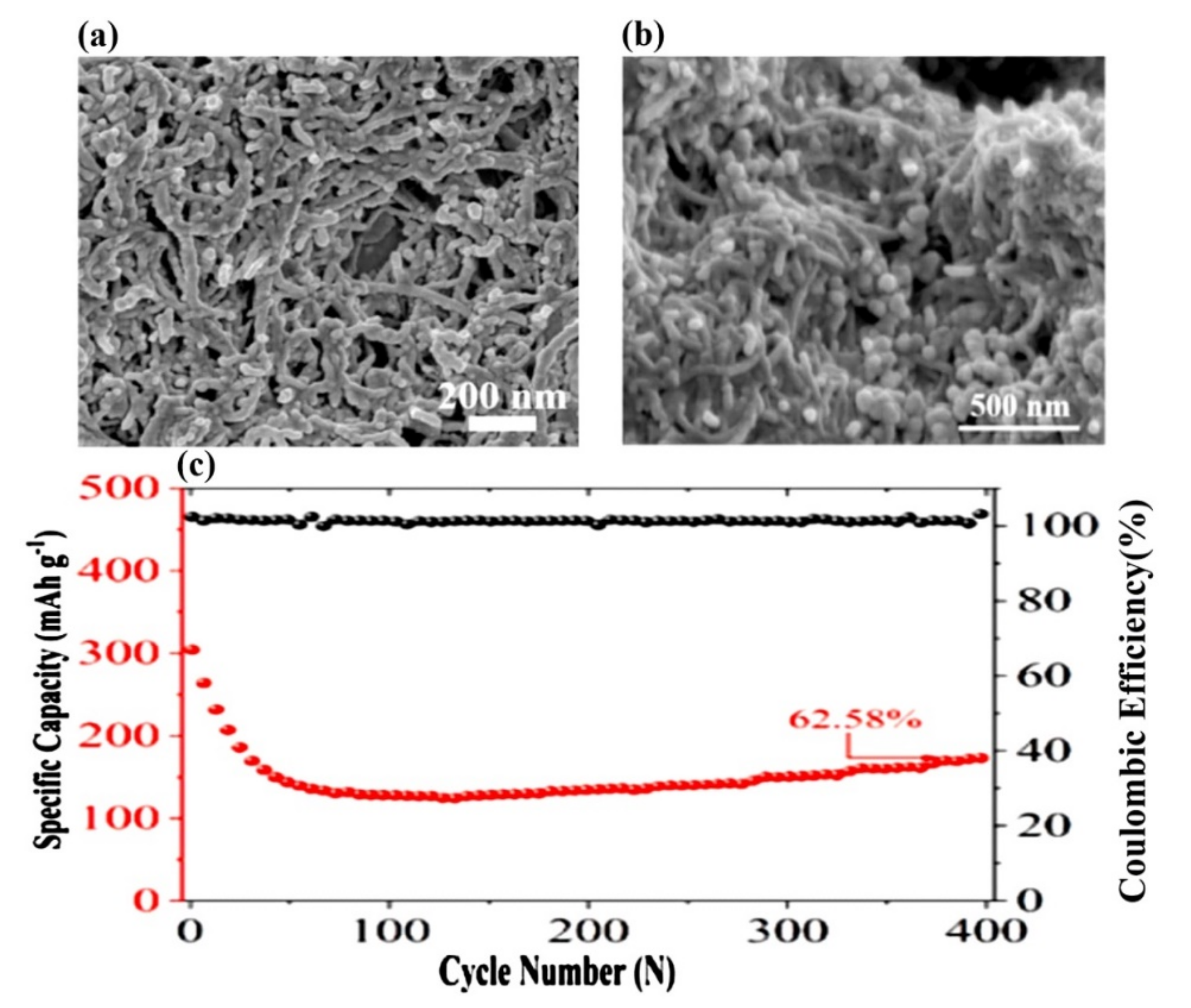
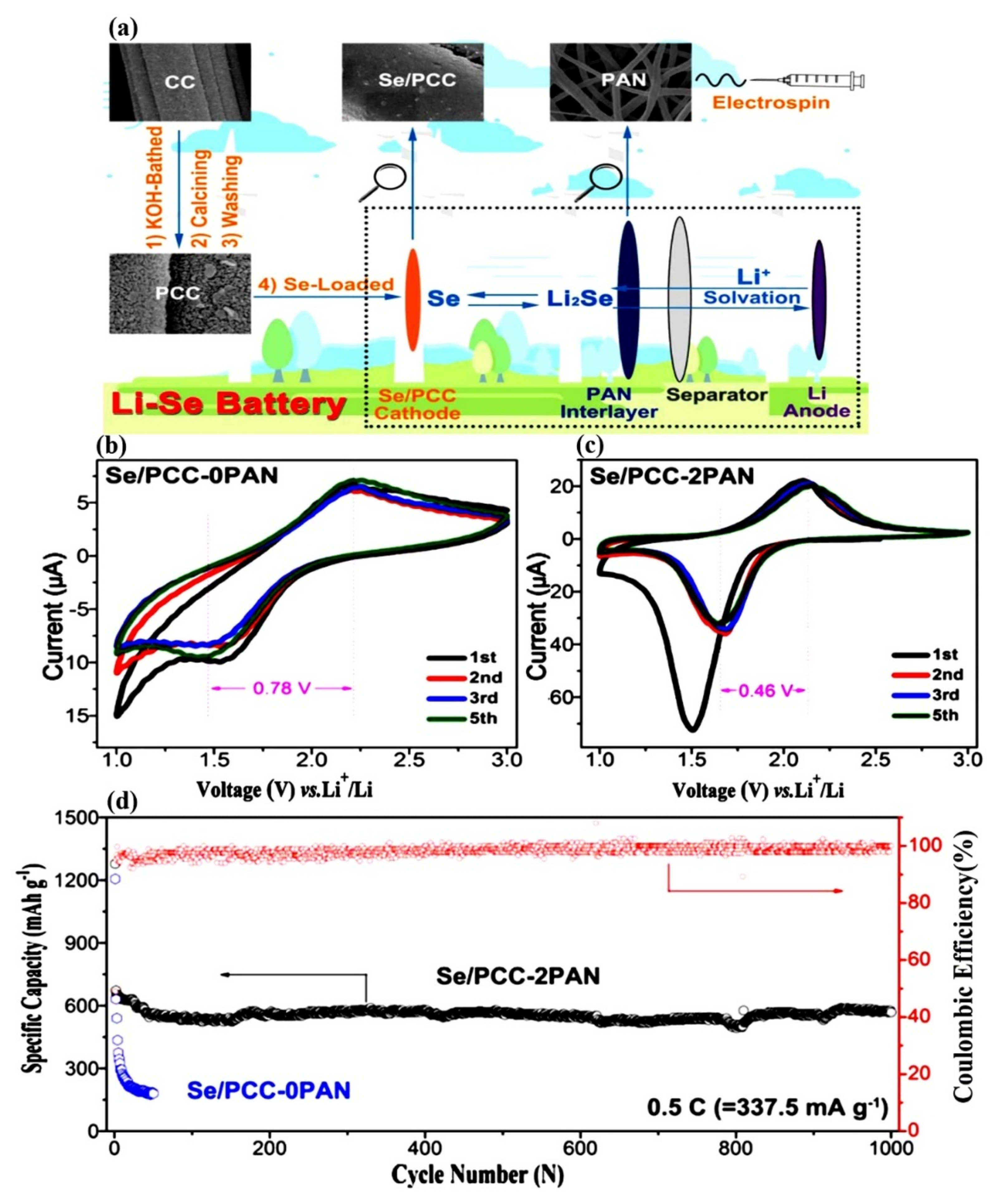

| Properties | Sulfur (S) | Selenium (Se) | LiCoO2 |
|---|---|---|---|
| Theoretical gravimetric capacity (mAh g−1) | 1675 | 675 | 274 |
| Electrical conductivity (S cm−1) | 5 × 10−30 (25 °C) | 1 × 10−5 (25 °C) | 1 × 10−4 |
| Theoretical energy density (WhL−1) | 2800 | 2528 | 1190 |
| Redox potential (V vs. Li+/Li) | 2.2 | 2 | 4.2 |
| Theoretical specific energy (Whkg−1) | 2267 | 1155 | 420 |
| Separator | Film Mass Loading (mg cm−2) | Active Material Loading (mg cm−2) | Specific Capacity (mAh g−1) | Ref. |
|---|---|---|---|---|
| DMTA-COF + ceramic | NA | 3 | ~125 (100th cycles) | [88] |
| PP/CTAB-CNTs-Ti3C2Tx | 0.09/0.27 | 2.1 | ~480 (100th cycles) | [89] |
| PP/Graphene | 0.3/5 | 4 | ~330 (1000th cycles) | [90] |
| PP + N,S-doped Graphene | 0.65/32 | 5 | ~330 (500th cycles) | [91] |
| Sample | Se (wt.%) | Current Rate | Specific Capacity (mAh g−1) | Ref. |
|---|---|---|---|---|
| Se@PPy | 90 | 0.07 C | ~60 (50 cycles) | [110] |
| Se@rGO | 80 | 1 C | ~270 (500 cycles) | [63] |
| Se/C@PANI | 66 | 0.2 C | ~520 (200 cycles) | [111] |
| Se@C | 54 | 0.15 C | ~430 (250 cycles) | [112] |
| Se@N-C | 41.2 | 0.5 C | ~300 (100 cycles) | [113] |
| Se/MiPCs | 1.4 | 5 C | ~511 (1000 cycles) | [50] |
| Se/MePCs | 48 | 0.5 C | ~310 (100 cycles) | [45] |
| Se/HPCs | 53 | 2 C | ~320 (900 cycles) | [114] |
| Se/G | 80 | 0.1 C | ~970 (500 cycles) | [115] |
| Se/TiO2 | 70.8 | 0.1 C | ~155 (50 cycles) | [116] |
| Se/CNTs | 85 | 0.1 C | ~350 (100 cycles) | [117] |
| Se/CNFs | 75 | 0.1 C | ~380 (350 cycles) | [118] |
| Se/CNTs/rGO | 70 | 0.74 C | ~535 (80 cycles) | [119] |
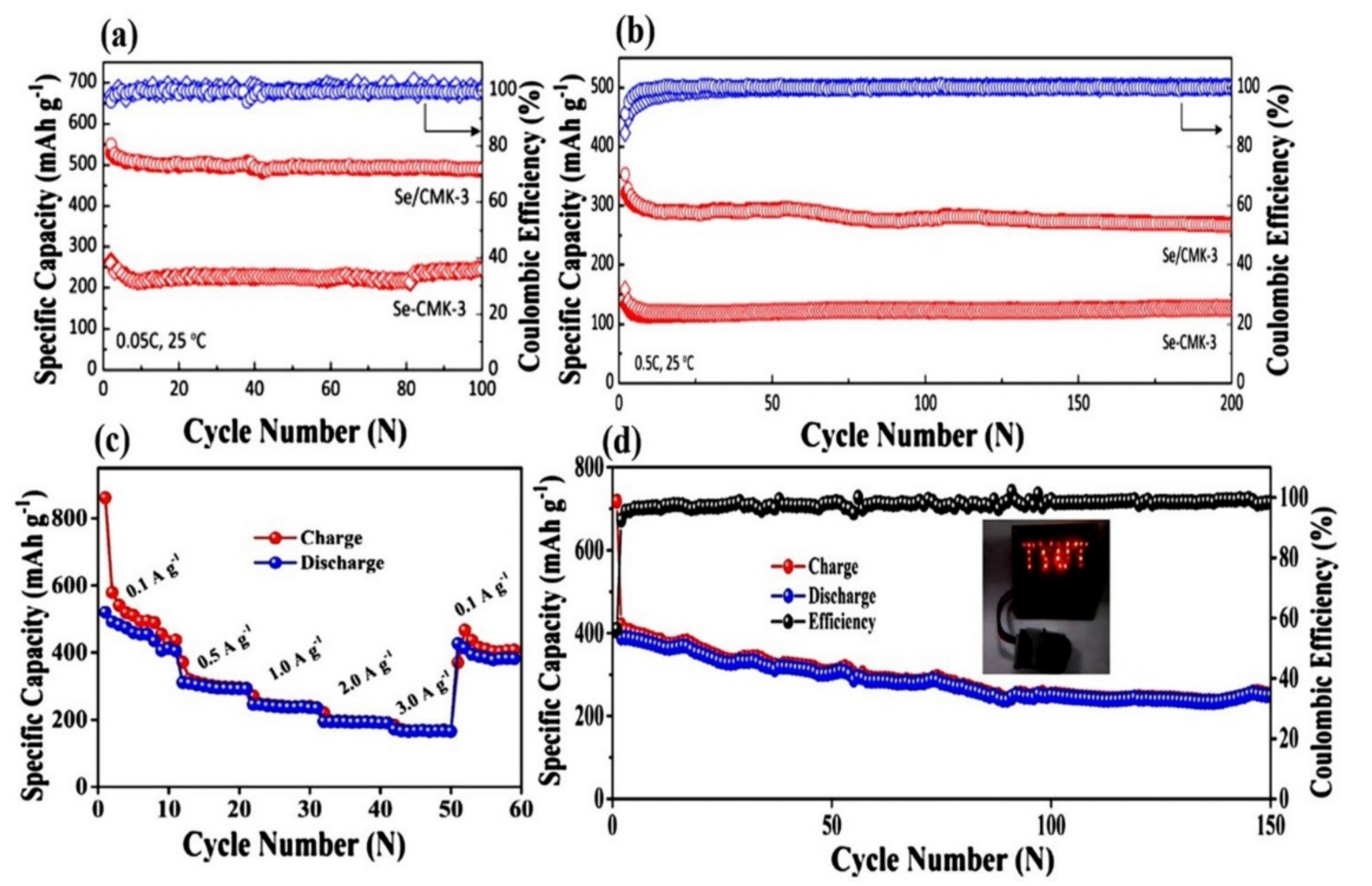
| Se/CMK-3 | 49 | 0.1 C | ~590 (50 cycles) | [43] |
| Se@rGO | 80 | 0.1 C | ~530 (100 cycles) | [120] |
| Se/HPCFs/rGO | 57 | 0.5 C | ~520 (200 cycles) | [121] |
| SePAN | 20 | 0.3 C | ~160 (3400 cycles) | [122] |
| Li2Se@C | 59 | 0.17 C | ~300 (100 cycles) | [86] |
| Li2Se@NC@C | 63 | 0.17 C | ~290 (100 cycles) | [123] |
| Se@COF | 40 | 0.1 C | ~950 (100 cycles) | [124] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.; Ding, X.; Zhao, H.; Wang, Y.; Zhang, N.; Chen, X.; Xu, J. Recent Advancements in Selenium-Based Cathode Materials for Lithium Batteries: A Mini-Review. Electrochem 2022, 3, 285-308. https://doi.org/10.3390/electrochem3020020
Khan M, Ding X, Zhao H, Wang Y, Zhang N, Chen X, Xu J. Recent Advancements in Selenium-Based Cathode Materials for Lithium Batteries: A Mini-Review. Electrochem. 2022; 3(2):285-308. https://doi.org/10.3390/electrochem3020020
Chicago/Turabian StyleKhan, Mustafa, Xuli Ding, Hongda Zhao, Yuxin Wang, Ning Zhang, Xiaojing Chen, and Jiahao Xu. 2022. "Recent Advancements in Selenium-Based Cathode Materials for Lithium Batteries: A Mini-Review" Electrochem 3, no. 2: 285-308. https://doi.org/10.3390/electrochem3020020
APA StyleKhan, M., Ding, X., Zhao, H., Wang, Y., Zhang, N., Chen, X., & Xu, J. (2022). Recent Advancements in Selenium-Based Cathode Materials for Lithium Batteries: A Mini-Review. Electrochem, 3(2), 285-308. https://doi.org/10.3390/electrochem3020020








