Recent Advances in the Electro-Active Therapeutic Phytochemical-Based Sensors
Abstract
1. Introduction
2. Therapeutic Value
2.1. Phytomedicine
2.2. Nutritive Content Estimation
2.3. Therapeutic Protein Expression Using Plants
3. Phytochemical Based Analysis and Sensor Systems
3.1. Phenotyping Tool/Phylogenic Classification
3.2. Antioxidant Screening
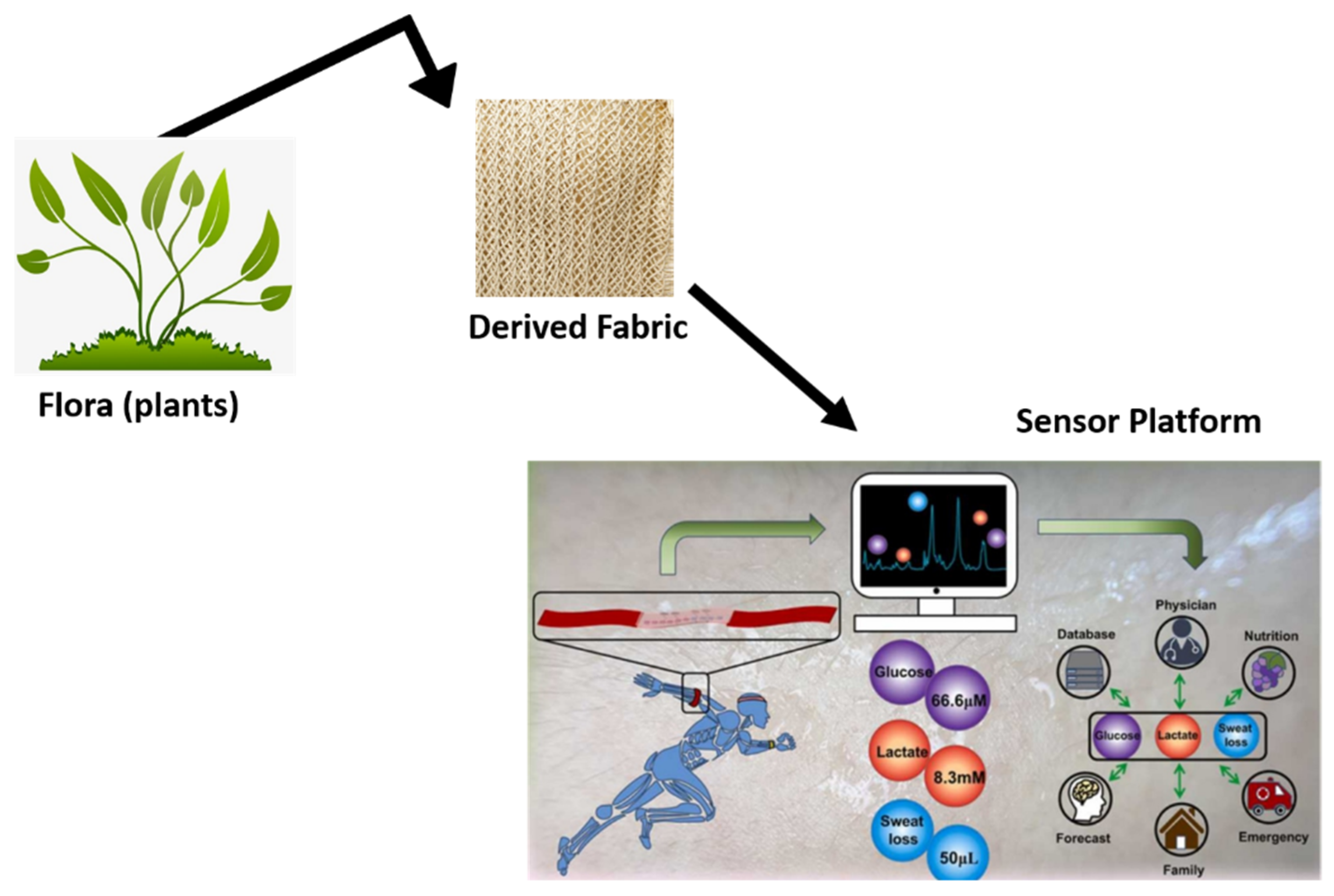
3.3. Sensor Platform Fabrication
3.4. Disease Diagnostic Functions/Disease Biomarker
4. Bottlenecks/Drawbacks
5. Advantages
6. Conclusions and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Katona, P.; Katona-Apte, J. The interaction between nutrition and infection. Clin. Infect. Dis. 2008, 46, 1582–1588. [Google Scholar] [CrossRef] [PubMed]
- Nasri, H.; Baradaran, A.; Shirzad, H.; Rafieian-Kopaei, M. New Concepts in Nutraceuticals as Alternative for Pharmaceuticals. Int. J. Prev. Med. 2014, 5, 1487. [Google Scholar] [PubMed]
- Bavishi, K.; Laursen, T.; Martinez, K.L.; Møller, B.L.; Della Pia, E.A. Application of nanodisc technology for direct electrochemical investigation of plant cytochrome P450s and their NADPH P450 oxidoreductase. Sci. Rep. 2016, 6, 29459. [Google Scholar] [CrossRef] [PubMed]
- Udit, A.K.; Hill, M.G.; Gray, H.B. Electrochemistry of cytochrome P450 BM3 in sodium dodecyl sulfate films. Langmuir 2006, 22, 10854–10857. [Google Scholar] [CrossRef] [PubMed]
- Mcquaid, K.E.; Keenan, A.K. Physiological Society Symposium: Impaired Endothelial and Smooth Muscle Cell Function in Oxidative Stress Endothelial Barrier Dysfunction and Oxidative Stress: Roles for Nitric Oxide? Exp. Physiol. 1997, 82, 369–376. [Google Scholar] [CrossRef]
- Nath, S.; Tamuli, K.J.; Gogoi, B.; Bordoloi, M.; Das, A.; Barua, C.C.; Barua, I.C. Antioxidant properties, phenolic and mineral profiling, assessment of angiotensin I converting enzyme (ACE) inhibitory potential of Elsholtzia communis (Collett & Hemsl.) Diels from North East India. Eur. J. Integr. Med. 2020, 40, 101247. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Barros, L.; Cabrita, L.; Boas, M.V.; Carvalho, A.M.; Ferreira, I.C.F.R. Chemical, biochemical and electrochemical assays to evaluate phytochemicals and antioxidant activity of wild plants. Food Chem. 2011, 127, 1600–1608. [Google Scholar] [CrossRef]
- Chen, C.Y.; Stemberger, R.S.; Klaue, B.; Blum, J.D.; Pickhardt, P.C.; Folt, C.L. Accumulation of heavy metals in food web components across a gradient of lakes. Limnol. Oceanogr. 2000, 45, 1525–1536. [Google Scholar] [CrossRef]
- Dales, J.P.; Desplat-Jégo, S. Metal imbalance in neurodegenerative diseases with a specific concern to the brain of multiple sclerosis patients. Int. J. Mol. Sci. 2020, 21, 9105. [Google Scholar] [CrossRef]
- He, Z.; Xu, Q.; Newland, B.; Foley, R.; Lara-Sáez, I.; Curtin, J.F.; Wang, W. Reactive oxygen species (ROS): Utilizing injectable antioxidative hydrogels and ROS-producing therapies to manage the double-edged sword. J. Mater. Chem. B 2021, 9, 6326–6346. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Q.; Dong, Y.; Gong, J.; Li, Z.; Zhang, J. Core-shell structured gold nanorods on thread-embroidered fabric-based microfluidic device for Ex Situ detection of glucose and lactate in sweat. Sens. Actuators B Chem. 2021, 353, 131154. [Google Scholar] [CrossRef]
- Jideani, A.I.O.; Silungwe, H.; Takalani, T.; Omolola, A.O.; Udeh, H.O.; Anyasi, T.A. Antioxidant-rich natural fruit and vegetable products and human health. Int. J. Food Prop. 2021, 24, 41–67. [Google Scholar] [CrossRef]
- Boeing, H.; Bechthold, A.; Bub, A.; Ellinger, S.; Haller, D.; Kroke, A.; Leschik-Bonnet, E.; Müller, M.J.; Oberritter, H.; Schulze, M.; et al. Critical review: Vegetables and fruit in the prevention of chronic diseases. Eur. J. Nutr. 2012, 51, 637–663. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Amaral, G.; Dantas Dos Santos, H.; Oliveira Da Conceição, A.; Faustino De Oliveira, F.; Aparecida De Oliveira, R. Evaluation of triterpenes isolated from stems of Pouteria macahensis TD. Trends Phytochem. Res. 2019, 3, 181–188. [Google Scholar]
- Gandhi, M.; Amreen, K. Electrochemical Profiling of Plants. Electrochem 2022, 3, 434–450. [Google Scholar] [CrossRef]
- Tzima, K.; Brunton, N.P.; Rai, D.K. Qualitative and quantitative analysis of polyphenols in lamiaceae plants—A review. Plants 2018, 7, 25. [Google Scholar] [CrossRef]
- Chen, P.; Shakhnovich, E.I. Thermal adaptation of viruses and bacteria. Biophys. J. 2010, 98, 1109–1118. [Google Scholar] [CrossRef]
- Domingo, E. Rna Virus Mutations. Annu. Rev. Microbiol. 1997, 51, 151–178. [Google Scholar] [CrossRef]
- Balol, G.B.; Divya, B.L.; Basavaraj, S.; Sundaresha, S.; Mahesh, Y.S.; Erayya HS, D. Sources of genetic variation in plant virus populations. J. Pure Appl. Microbiol. 2010, 4, 803–808. [Google Scholar]
- Srivastava, J.K.; Shankar, E.; Gupta, S. Chamomile: A herbal medicine of the past with a bright future (review). Mol. Med. Rep. 2010, 3, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.V.; Gan, S.H. Cinnamon: A multifaceted medicinal plant. Evid.-Based Complement. Altern. Med. 2014, 2014, 642942. [Google Scholar] [CrossRef] [PubMed]
- Nassar, R.; Eid, S.; Chahine, R.; Chabi, B.; Bonnieu, A.; El Sabban, M.; Najjar, F.; Hamade, A. Antioxidant effects of lebanese Crocus sativus L. and its main components, crocin and safranal, on human skeletal muscle cells. Eur. J. Integr. Med. 2020, 40, 101250. [Google Scholar] [CrossRef]
- DeGeorge, K.C.; Ring, D.J.; Dalrymple, S.N. Treatment of the common cold. Am. Fam. Physician 2019, 100, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Awang, D.V.C. Prescribing therapeutic feverfew (Tanacetum parthenium (L.) Schultz bip., syn. Chrysanthemum parthenium (L.) Bernh.). Integr. Med. 1998, 1, 11–13. [Google Scholar] [CrossRef]
- Bayan, L.; Koulivand, P.H.; Gorji, A. Garlic: A review of potential therapeutic effects. Avicenna J. Phytomed. 2014, 4, 1–14. [Google Scholar] [CrossRef]
- Mahomoodally, M.; Aumeeruddy, M.; Rengasamy, K.R.; Roshan, S.; Hammad, S.; Pandohee, J.; Hu, X.; Zengin, G. Ginger and its active compounds in cancer therapy: From folk uses to nano-therapeutic applications. Semin. Cancer Biol. 2021, 69, 140–149. [Google Scholar] [CrossRef]
- Rojas, P.; Montes, P.; Rojas, C.; Serrano-García, N.; Rojas-Castañeda, J.C. Effect of a phytopharmaceutical medicine, Ginko biloba extract 761, in an animal model of Parkinson’s disease: Therapeutic perspectives. Nutrition 2012, 28, 1081–1088. [Google Scholar] [CrossRef]
- Choi, M.K.; Song, I.S. Interactions of ginseng with therapeutic drugs. Arch. Pharmacal Res. 2019, 42, 862–878. [Google Scholar] [CrossRef]
- Gurley, B.J.; Swain, A.; Hubbard, M.A.; Hartsfield, F.; Thaden, J.; Williams, D.K.; Gentry, W.B.; Tong, Y. Supplementation with goldenseal (Hydrastis canadensis), but not kava kava (Piper methysticum), inhibits human CYP3A activity in vivo. Clin. Pharmacol. Ther. 2008, 83, 61–69. [Google Scholar] [CrossRef]
- Singh, Y.N.; Singh, N.N. Therapeutic potential of kava in the treatment of anxiety disorders. CNS Drugs 2002, 16, 731–743. [Google Scholar] [CrossRef] [PubMed]
- Adetuyi, B.O.; Omolabi, F.K.; Olajide, P.A. Pharmacological, Biochemical and Therapeutic Potential of Milk Thistle (Silymarin): A Review. World News Nat. Sci. 2021, 37, 75–91. [Google Scholar]
- Bloch, M.H.; Mulqueen, J. Nutritional supplements for the treatment of ADHD. Child Adolesc. Psychiatr. Clin. N. Am. 2014, 23, 883–897. [Google Scholar] [CrossRef]
- Presley, C.L.; Kolodziejczyk, T.C.; Pulsipher, K.J.; Maghfour, J.; Militello, M.; Rietcheck, H.R.; Fonseca, A.; Olayinka, T.J.; Rundle, C.W.; Waller, J.D.; et al. A Scoping Review of Pharmacotherapy, Complementary, and Alternative Medicine (CAM), and Surgical Therapies for Androgenic Alopecia. Curr. Dermatol. Rep. 2021, 10, 48–54. [Google Scholar] [CrossRef]
- Tammadon, M.R.; Nobahar, M.; Hydarinia-Naieni, Z.; Ebrahimian, A.; Ghorbani, R.; Vafaei, A.A. The Effects of Valerian on Sleep Quality, Depression, and State Anxiety in Hemodialysis Patients: A Randomized, Double-blind, Crossover Clinical Trial. Oman Med. J. 2021, 36, e255. [Google Scholar] [CrossRef]
- Lone, R.; Shuab, R.; Kamili, A.N. Plant Phenolics in Sustainable Agriculture; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Shoja, M.M.; Tubbs, R.S.; Bosmia, A.N.; Fakhree, M.A.A.; Jouyban, A.; Balch, M.W.; Loukas, M.; Khodadoust, K.; Khalili, M.; Eknoyan, G. Herbal diuretics in medieval Persian and Arabic medicine. J. Altern. Complement. Med. 2015, 21, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Tapsell, L.C.; Hemphill, I.; Cobiac, L.; Patch, C.S.; Sullivan, D.R.; Fenech, M.; Roodenrys, S.; Keogh, J.B.; Clifton, P.M.; Williams, P.G.; et al. Health benefits of herbs and spices: The past, the present, the future. Med. J. Aust. 2006, 185, S1–S24. [Google Scholar] [CrossRef]
- Shimelis, T. Spices production and marketing in Ethiopia: A review. Cogent. Food Agric. 2021, 7, 1915558. [Google Scholar] [CrossRef]
- Masic, I.; Skrbo, A.; Naser, N.; Tandir, S.; Zunic, L.; Medjedovic, S.; Sukalo, A. Contribution of Arabic Medicine and Pharmacy to the Development of Health Care Protection in Bosnia and Herzegovina—The First Part. Med. Arch. 2017, 71, 364–372. [Google Scholar] [CrossRef]
- Alongi, M.; Anese, M. Re-thinking functional food development through a holistic approach. J. Funct. Foods 2021, 81, 104466. [Google Scholar] [CrossRef]
- Haytowitz, D.B.; Eldridge, A.L.; Bhagwat, S.; Gebhardt, S.E.; Holden, J.M.; Beecher, G.R.; Peterson, J.; Dwyer, J. Flavonoid Content of Vegetables; Agricultural Research Service: Washington, DC, USA, 2002.
- Pinto, J.T.; Rivlin, R.S. Recent Advances on the Nutritional Effects Associated with the Use of Garlic. J. Nutr. 2001, 131, 1058–1060. [Google Scholar] [CrossRef]
- Lii, C.K.; Tsai, C.W.; Wu, C.C. Garlic allyl sulfides display differential modulation of rat cytochrome P450 2B1 and the placental form glutathione S-transferase in various organs. J. Agric. Food Chem. 2006, 54, 5191–5196. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, H.; Tijerina, M.T.; Tobola, A.S. Preferential overexpression of a class MU glutathione S-transferase subunit in mouse liver by myristicin. Biochem. Biophys. Res. Commun. 1997, 236, 825–828. [Google Scholar] [CrossRef]
- Awad, R.; Levac, D.; Cybulska, P.; Merali, Z.; Trudeau, V.L.; Arnason, J.T. Effects of traditionally used anxiolytic botanicals on enzymes of the γ-aminobutyric acid (GABA) system. Can. J. Physiol. Pharmacol. 2007, 85, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Kaltner, F. Fate of Food-Relevant Toxic Plant Alkaloids during Food Processing or Storing and Analytical Strategies to Unveil Potential Transformation Products. J. Agric. Food Chem. 2022, 70, 5975–5981. [Google Scholar] [CrossRef]
- Hadad, M.; Gattuso, S.; Gattuso, M.; Feresin, G.; Tapia, A. Anatomical studies of Baccharis grisebachii Hieron. (Asteraceae). Used in folk medicine of San Juan province, Argentina. Dominguezia 2013, 29, 41–47. [Google Scholar]
- Akomolafe, R.O.; Adeoshun, I.O.; Ayoka, A.O.; Elujoba, A.A.; Iwalewa, E.O. An in vitro study of the effects of Cassia podocarpa fruit on the intestinal motility of rats. Phytomedicine 2004, 11, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Luthra, P.M.; Singh, R.; Chandra, R. Therapeutic uses of curcuma longa (Turmeric). Indian J. Clin. Biochem. 2001, 16, 153–160. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mandal, S.K.; Maji, A.K.; Mishra, S.K.; Ishfaq, P.M.; Devkota, H.P.; Silva, A.S.; Das, N. Goldenseal (Hydrastis canadensis L.) and its active constituents: A critical review of their efficacy and toxicological issues. Pharmacol. Res. 2020, 160, 105085. [Google Scholar] [CrossRef] [PubMed]
- Alvarez Perez Gil, A.L.; Barbosa Navarro, L.; Patipo Vera, M.; Petricevich, V.L. Anti-inflammatory and antinociceptive activities of the ethanolic extract of Bougainvillea xbuttiana. J. Ethnopharmacol. 2012, 144, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Battino, M.; Giampieri, F.; Cianciosi, D.; Ansary, J.; Chen, X.; Zhang, D.; Gil, E.; Forbes-Hernández, T. The roles of strawberry and honey phytochemicals on human health: A possible clue on the molecular mechanisms involved in the prevention of oxidative stress and inflammation. Phytomedicine 2020, 86, 153170. [Google Scholar] [CrossRef]
- Hani, K.; Zairi, A.; Tangy, F.; Bouassida, K. Dermaseptins and magainins: Antimicrobial peptides from frogs’ skin-new sources for a promising spermicides microbicides-a mini review. J. Biomed. Biotechnol. 2009, 2009, 452567. [Google Scholar] [CrossRef]
- Sajankumar, R.P.; Hegde, V.; Shetty, P. Antimicrobial effectiveness of Neem (Azadirachta indica) and Babool (Acacia nilotica) on Streptococcus mutans: An in vitro study. J. Indian Assoc. Public Health Dent. 2015, 13, 517. [Google Scholar] [CrossRef]
- Perumal Samy, R.; Gopalakrishnakone, P. Therapeutic potential of plants as anti-microbials for drug discovery. Evid.-Based Complement. Altern. Med. 2010, 7, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.G.; Apetrei, C. A Review on Electrochemical Sensors and Biosensors Used in Assessing Antioxidant Activity. Antioxidants 2022, 11, 584. [Google Scholar] [CrossRef] [PubMed]
- Williamson, E.M. Synergy and other interactions in phytomedicines. Phytomedicine 2001, 8, 401–409. [Google Scholar] [CrossRef]
- Coppedè, N.; Janni, M.; Bettelli, M.; Maida, C.L.; Gentile, F.; Villani, M.; Ruotolo, R.; Iannotta, S.; Marmiroli, N.; Marmiroli, M.; et al. An in vivo biosensing, biomimetic electrochemical transistor with applications in plant science and precision farming. Sci. Rep. 2017, 7, 16195. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, M.; Rajagopal, D.; Parthasarathy, S.; Raja, S.; Huang, S.; Kumar, A.S. In Situ Immobilized Sesamol-Quinone/Carbon Nanoblack-Based Electrochemical Redox Platform for E fficient Bioelectrocatalytic and Immunosensor Applications. ACS Omega 2018, 3, 10823–10835. [Google Scholar] [CrossRef]
- Vishnu, N.; Gandhi, M.; Badhulika, S.; Kumar, A.S. Tea quality testing using 6B pencil lead as an electrochemical sensor. Anal. Methods 2018, 10, 2327–2336. [Google Scholar] [CrossRef]
- Vishnu, N.; Gandhi, M.; Rajagopal, D.; Kumar, A.S. Pencil graphite as an elegant electrochemical sensor for separation-free and simultaneous sensing of hypoxanthine, xanthine and uric acid in fish samples. Anal Methods 2017, 9, 2265–2274. [Google Scholar] [CrossRef]
- Araújo, D.A.; Camargo, J.R.; Pradela-Filho, L.A.; Lima, A.P.; Muñoz, R.A.; Takeuchi, R.M.; Janegitz, B.C.; Santos, A.L. A lab-made screen-printed electrode as a platform to study the effect of the size and functionalization of carbon nanotubes on the voltammetric determination of caffeic acid. Microchem. J. 2020, 158, 105297. [Google Scholar] [CrossRef]
- Lobsey, C.R.; Rossel, R.A.V.; Mcbratney, A.B.; Minasny, B. Proximal Soil Sensing; Springer: Dordrecht, The Netherlands, 2010. [Google Scholar] [CrossRef]
- Sahoo, S.; Kariya, T.; Ishikawa, K. Targeted delivery of therapeutic agents to the heart. Nat. Rev. Cardiol. 2021, 18, 389–399. [Google Scholar] [CrossRef]
- Fahad, S.; Khan, F.A.; Pandupuspitasari, N.S.; Ahmed, M.M.; Liao, Y.C.; Waheed, M.T.; Sameeullah, M.; Hussain, S.; Saud, S.; Hassan, S.; et al. Recent developments in therapeutic protein expression technologies in plants. Biotechnol. Lett. 2015, 37, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Kurup, V.M.; Thomas, J. Edible Vaccines: Promises and Challenges. Mol. Biotechnol. 2020, 62, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Rosano, G.L.; Ceccarelli, E.A. Recombinant protein expression in Escherichia coli: Advances and challenges. Front. Microbiol. 2014, 5, 172. [Google Scholar] [CrossRef] [PubMed]
- Sempere, R.N.; Gómez, P.; Truniger, V.; Aranda, M.A. Development of expression vectors based on pepino mosaic virus. Plant Methods 2011, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- De Marchis, F.; Wang, Y.; Stevanato, P.; Arcioni, S.; Bellucci, M. Genetic transformation of the sugar beet plastome. Transgenic Res. 2009, 18, 17–30. [Google Scholar] [CrossRef]
- Rosales-Mendoza, S.; Soria-Guerra, R.E.; Olivera-Flores, M.T.D.J.; López-Revilla, R.; Argüello-Astorga, G.R.; Jiménez-Bremont, J.F.; la Cruz, R.F.G.-D.; Loyola-Rodríguez, J.P.; Alpuche-Solís, G. Expression of Escherichia coli heat-labile enterotoxin b subunit (LTB) in carrot (Daucus carota L.). Plant Cell Rep. 2007, 26, 969–976. [Google Scholar] [CrossRef]
- Peyret, H.; Lomonossoff, G.P. When plant virology met Agrobacterium: The rise of the deconstructed clones. Plant Biotechnol. J. 2015, 13, 1121–1135. [Google Scholar] [CrossRef]
- Hennegan, K.; Yang, D.; Nguyen, D.; Wu, L.; Goding, J.; Huang, J.; Guo, F.; Huang, N.; Watkins, S. Improvement of human lysozyme expression in transgenic rice grain by combining wheat (Triticum aestivum) puroindoline b and rice (Oryza sativa) Gt1 promoters and signal peptides. Transgenic Res. 2005, 14, 583–592. [Google Scholar] [CrossRef]
- Buhrow, L.M.; Clark, S.M.; Loewen, M.C. Identification of an attenuated barley stripe mosaic virus for the virus-induced gene silencing of pathogenesis-related wheat genes. Plant Methods 2016, 12, 12. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tansil, N.C.; Xie, H.; Xie, F.; Gao, Z. Direct detection of DNA with an electrocatalytic threading intercalator. Anal. Chem. 2005, 77, 126–134. [Google Scholar] [CrossRef]
- Doménech-Carbó, A.; Ibars, A.M.; Prieto-Mossi, J.; Estrelles, E.; Scholz, F.; Cebrián-Torrejón, G.; Martini, M. Electrochemistry-based chemotaxonomy in plants using the voltammetry of microparticles methodology. New J. Chem. 2015, 39, 7421–7428. [Google Scholar] [CrossRef]
- Fu, L.; Zheng, Y.; Zhang, P.; Zhang, H.; Wu, M.; Zhang, H.; Wang, A.; Su, W.; Chen, F.; Yu, J.; et al. An electrochemical method for plant species determination and classification based on fingerprinting petal tissue. Bioelectrochemistry 2019, 129, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Teig-Sussholz, O.; Schuster, S.; Avni, A.; Shacham-Diamand, Y. Integrated electrochemical Chip-on-Plant functional sensor for monitoring gene expression under stress. Biosens. Bioelectron. 2018, 117, 493–500. [Google Scholar] [CrossRef]
- Yang, B.; Kotani, A.; Arai, K.; Kusu, F. Estimation of the Antioxidant Activities of Flavonoids from Their Oxidation Potentials. Anal. Sci. 2001, 17, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Brainina, K.Z.; Ivanova, A.V.; Sharafutdinova, E.N.; Lozovskaya, E.L.; Shkarina, E.I. Potentiometry as a method of antioxidant activity investigation. Talanta 2007, 71, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Doménech-Carbó, A.; Machado De Carvalho, L.; Martini, M.; Valencia, D.P.; Cebrián-Torrejón, G. Electrochemical monitoring of the pharmacological activity of natural products. In Studies in Natural Products Chemistry; Elsevier: Berlin/Heidelberg, Germany, 2015; Volume 45, pp. 59–84. [Google Scholar] [CrossRef]
- Teixeira, J.; Oliveira, C.; Amorim, R.; Cagide, F.; Garrido, J.; Ribei-ro, J.A.; Pereira, C.M.; Silva, A.F.; Andrade, P.B.; Oliveira, P.J.; et al. Development of hydroxybenzoic-based plat-forms as a solution to deliver dietary antioxi-dants to mitochondria. Sci. Rep. 2017, 7, 6842. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Kozlova, E.; Budnikov, H. Polyquercetin/MWNT-modified Electrode for the Determination of Natural Phenolic Antioxidants. Electroanalysis 2017, 29, 2610–2619. [Google Scholar] [CrossRef]
- Hoyos-Arbeláez, J.; Vázquez, M.; Contreras-Calderón, J. Electrochemical methods as a tool for determining the antioxidant capacity of food and beverages: A review. Food Chem. 2017, 221, 1371–1381. [Google Scholar] [CrossRef] [PubMed]
- Samoticha, J.; Jara, M.J.; José, P.; Hernández, M.; Francisco, H.; Aneta, J.H. Phenolic compounds and antioxidant activity of twelve grape cultivars measured by chemical and electrochemical methods. Eur. Food Res. Technol. 2018, 244, 1933–1943. [Google Scholar] [CrossRef]
- Lanfer-marquez, U.M.; Barros, R.M.C.; Sinnecker, P. Antioxidant activity of chlorophylls and their derivatives. Food Res. Int. 2005, 38, 885–891. [Google Scholar] [CrossRef]
- Cosio, M.S.; Buratti, S.; Mannino, S.; Benedetti, S. Use of an electrochemical method to evaluate the antioxidant activity of herb extracts from the Labiatae family. Food Chem. 2006, 97, 725–731. [Google Scholar] [CrossRef]
- Szczepaniak, O.M.; Ligaj, M.; Kobus-Cisowska, J.; Maciejewska, P.; Tichoniuk, M.; Szulc, P. Application for novel electrochemical screening of antioxidant potential and phytochemicals in Cornus mas extracts. CYTA J. Food 2019, 17, 781–789. [Google Scholar] [CrossRef]
- Juárez-Gómez, J.; Ramírez-Silva, M.T.; Guzmán-Hernández, D.S.; Romero-Romo, M.; Palomar-Pardavé, M. Novel electrochemical method to evaluate the antioxidant capacity of infusions and beverages, based on in situ formation of free superoxide radicals. Food Chem. 2020, 332, 127409. [Google Scholar] [CrossRef]
- Wu, T.; Li, L.; Jiang, X.; Liu, F.; Liu, Q.; Liu, X. Construction of silver-cotton carbon fiber sensing interface and study on the protective effect of antioxidants on hypoxia-induced cell damage. Microchem. J. 2020, 159, 105345. [Google Scholar] [CrossRef]
- Mohtar, L.G.; Messina, G.A.; Bertolino, F.A.; Pereira, S.V.; Raba, J.; Nazareno, M.A. Comparative study of different methodologies for the determination the antioxidant activity of Venezuelan propolis. Microchem. J. 2020, 158, 105244. [Google Scholar] [CrossRef]
- Tomac, I.; Šeruga, M.; Labuda, J. Evaluation of antioxidant activity of chlorogenic acids and coffee extracts by an electrochemical DNA-based biosensor. Food Chem. 2020, 325, 126787. [Google Scholar] [CrossRef]
- Aguirre, M.J.; Chen, Y.Y.; Isaacs, M.; Matsuhiro, B.; Mendoza, L.; Torres, S. Electrochemical behaviour and antioxidant capacity of anthocyanins from Chilean red wine, grape and raspberry. Food Chem. 2010, 121, 44–48. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, J.; Zhang, H.; Gai, P.; Zhang, X.; Chen, J. Electrochemical evaluation of total antioxidant capacities in fruit juice based on the guanine/graphene nanoribbon/glassy carbon electrode. Talanta 2013, 106, 206–211. [Google Scholar] [CrossRef]
- Rodrí, E. Electrochemical Quantification of the Antioxidant Capacity of Medicinal Plants Using Biosensors. Sensors 2014, 14, 14423–14439. [Google Scholar] [CrossRef]
- Xie, H.; Zhang, C.; Gao, Z. Amperometric Detection of Nucleic Acid at Femtomolar Levels with a Nucleic Acid/Electrochemical Activator Bilayer on Gold Electrode. Anal. Chem. 2004, 76, 1611–1617. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Lillehoj, P.B.; Ho, C.M. DNA diagnostics: Nanotechnology-enhanced electrochemical detection of nucleic acids. Pediatr. Res. 2010, 67, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Gamella, M.; Bueno-Díaz, C.; Montiel, V.R.-V.; Povedano, E.; Reviejo, A.; Villalba, M.; Campuzano, S.; Pingarrón, J. First electrochemical immunosensor for the rapid detection of mustard seeds in plant food extracts. Talanta 2020, 219, 121247. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Park, J.S.; Jo, D.G.; Cho, M.; Lee, Y. Curcumin-based electrochemical sensor of amyloid-Β oligomer for the early detection of Alzheimer’s disease. Sens. Actuators B Chem. 2018, 273, 1593–1599. [Google Scholar] [CrossRef]
- Amreen, K.; Shukla, V.K.; Shukla, S.; Rajagopal, D.; Kumar, A.S. Redox behaviour and surface-confinement of electro active species of ginger extract on graphitized mesoporous carbon surface and its copper complex for H2O2 sensing. Nano-Struct. Nano-Objects 2017, 11, 56–64. [Google Scholar] [CrossRef]
- Deroco, P.B.; Fatibello-Filho, O.; Arduini, F.; Moscone, D. Electrochemical determination of capsaicin in pepper samples using sustainable paper-based screen-printed bulk modified with carbon black. Electrochim. Acta 2020, 354, 136628. [Google Scholar] [CrossRef]
- Gandhi, M.; Indiramma, J.; Jayaprakash, N.S.; Kumar, A.S. An efficient electrochemical sandwich ELISA for urinary human serum albumin-biomarker based on highly redox-active thionine surface-confined MWCNT/PEDOT.PSS platform. J. Electroanal. Chem. 2022, 906, 116018. [Google Scholar] [CrossRef]
- Khater, M.; de la Escosura-Muñiz, A.; Quesada-González, D.; Merkoçi, A. Electrochemical detection of plant virus using gold nanoparticle-modified electrodes. Anal. Chim. Acta 2019, 1046, 123–131. [Google Scholar] [CrossRef]
- Fang, Y.; Umasankar, Y.; Ramasamy, R.P. A novel bi-enzyme electrochemical biosensor for selective and sensitive determination of methyl salicylate. Biosens. Bioelectron. 2016, 81, 39–45. [Google Scholar] [CrossRef]
- Mars, A.; Hamami, M.; Bechnak, L.; Patra, D.; Raouafi, N. Curcumin-graphene quantum dots for dual mode sensing platform: Electrochemical and fluorescence detection of APOe4, responsible of Alzheimer’s disease. Anal. Chim. Acta 2018, 1036, 141–146. [Google Scholar] [CrossRef]
- Li, X.; Wu, D.; Ma, H.; Wang, H.; Wang, Y.; Fan, D.; Du, B.; Wei, Q.; Zhang, N. Ultrasensitive amyloid-β proteins detection based on curcumin conjugated ZnO nanoparticles quenching electrochemiluminescence behavior of luminol immobilized on Au@MoS2/Bi2S3 nanorods. Biosens. Bioelectron. 2019, 131, 136–142. [Google Scholar] [CrossRef]
- Chen, L.; Liu, X.; Chen, C. Impedimetric biosensor modified with hydrophilic material of tannic acid/polyethylene glycol and dopamine-assisted deposition for detection of breast cancer-related BRCA1 gene. J. Electroanal. Chem. 2017, 791, 204–210. [Google Scholar] [CrossRef]
- Alipour, E.; Shahabi, H.; Mahmoudi-Badiki, T. Introducing curcumin as an electrochemical DNA hybridization indicator and its application for detection of human interleukin-2 gene. J. Solid State Electrochem. 2016, 20, 1645–1653. [Google Scholar] [CrossRef]
- Roushani, M.; Valipour, A. Using electrochemical oxidation of Rutin in modeling a novel and sensitive immunosensor based on Pt nanoparticle and graphene–ionic liquid–chitosan nanocomposite to detect human chorionic gonadotropin. Sens. Actuators B Chem. 2016, 222, 1103–1111. [Google Scholar] [CrossRef]
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar] [CrossRef]
- Zhang, Y.; Kim, H.H.; Heller, A. Enzyme-amplified amperometric detection of 3000 copies of DNA in a 10-μL droplet at 0.5 fM concentration. Anal. Chem. 2003, 75, 3267–3269. [Google Scholar] [CrossRef]
- Konieczyński, P. Electrochemical fingerprint studies of selected medicinal plants rich in flavonoids. Acta Pol. Pharm. Drug Res. 2015, 72, 655–661. [Google Scholar]
- Gao, Z.; Yu, Y.H. A microRNA biosensor based on direct chemical ligation and electrochemically amplified detection. Sens. Actuators B Chem. 2007, 121, 552–559. [Google Scholar] [CrossRef]
- Naghdi, T.; Faham, S.; Mahmoudi, T.; Pourreza, N.; Ghavami, R.; Golmohammadi, H. Phytochemicals toward Green (Bio)sensing. ACS Sens. 2020, 5, 3770–3805. [Google Scholar] [CrossRef]
- Olvera, D.; Monaghan, M.G. Electroactive material-based biosensors for detection and drug delivery. Adv. Drug Deliv. Rev. 2021, 170, 396–424. [Google Scholar] [CrossRef]


| S. No. | Name of Herbs | Part of the Plant | Treatment of Diseases | Disadvantages/Side Effects | Ref |
|---|---|---|---|---|---|
| 1. | Chamomile (used as a tea) | Flower | Skin irritation due to radiation, anxiety and relaxation, wound healing | May interfere with some medicines | [22] |
| 2. | Cinnamon | Bark | Diabetic treatment (insulin–enhancing activity) | Not to be used with medicines | [23] |
| 3. | Crocus sativus | Petals | Antitumor, Anti-inflammatory and anti-oxidant | Can cause allergic reactions | [24] |
| 4. | Echinacea | Leaf, Stalk, Root | Prevents infections, and promotes wound healing | Can be an allergen | [25] |
| 5. | Feverfew | Leaf | Treatment of fever, migraines and arthritis | Digestive irritation | [26] |
| 6. | Garlic | Clove, Root | Antimicrobial, cardioprotective, anticancer, anti-inflammatory, lowers blood pressure | Not to be used with warfarin | [27] |
| 7. | Ginger | Root | Easing nausea and motion sickness. Anticancer agent | Bloating, gas, heartburn | [28] |
| 8. | Gingko | Leaf | Treatment of asthma, bronchitis, fatigue | Can cause seizures, has ginkgo toxin | [29] |
| 9. | Ginseng | Root | Tonic and aphrodisiac | High bp and tachycardia | [30] |
| 10. | Goldenseal | Root, Rhizome | Treat diarrhea and eye skin irritation, antiseptic, diarrhea | Can be poisonous in high doses and can cause gastric irritation | [31] |
| 11. | Kava | Rhizome, roots | Treat anxiety, nervous tension, and agitation and act as a sedative | Digestive issues | [32] |
| 12. | Milk Thistle | Fruit | Liver conditions and high cholesterol reduces the growth of cancer cells | Uncertain about side-effects | [33] |
| 13. | Saint John’s Wort | Flower, Leaf | Antidepressant | Sensitivity to light | [34] |
| 14. | Saw Palmetto | Fruit | Treat urine symptoms from benign prostatic hypertrophy | Digestive upset, headache | [35] |
| 15. | Valerian | Root | Treatment of sleeplessness and reduce anxiety | Consultation with a doctor is necessary | [36] |
| S. No. | Scientific Names | Parts | Constituent Class | Compounds | Mechanism of Medicine | Ref |
|---|---|---|---|---|---|---|
| 1. | Baccharis grisebachii Hieron | Resinous exudate | Diterpenes, p-coumaric acid, derivatives, flavones | 3 and 3,5-Prenyl-p-coumaric acid | Argentinian traditional medicine showed activity toward dermatophytes and bacteria | [49] |
| 2. | Cassia podocarpa Guill et Perr. | Leaf and Flower | Glycosides | Anthraquinone glycosides, free aglycone | Optimum laxative activity and reduced toxicity | [50] |
| 3. | Curcuma longa L. | Rhizome | Flavonoids | Curcumin and curcuminoids | Several different molecules are involved in inflammation that is inhibited by curcumin including lipo-oxygenase, phospholipase and elastase. | [51] |
| 4. | Hydrastis Canadensis L. | Whole plant | Alkaloid | Berberine-entrahydroberberine and 8-oxoberberine | Chinese herb, exhibited vasodilator activity, has been attributed to multiple cellular mechanisms. Its derivatives are attributed to the blockade of K+ channels and exchangers. | [52] |
| 5. | Bougainvillea xbuttiana | Leaf | Protein | Lysine | The inhibitor showed N-glycosidase activity on 25S rRNA of tobacco ribosomes, which interfere with virus multiplication through ribosome interaction. | [53] |
| S. No. | Chemically Modified Electrode | pH | Technique | Nutritive Estimation | Comment | Ref No. |
|---|---|---|---|---|---|---|
| 1. | GCE/CB | PBS | DPV | Sesamol content | Direct analysis with buffer dilution | [61] |
| 2. | Pencil graphite Electrode | PBS | DPV | 1,2-, 1,3-, and 1,4-Di hydroxyl benzoic acid | Tea quality assays were calculated | [62] |
| 3. | Pencil graphite electrode | PBS | DPV | Xanthine, Hypoxanthine and Uric acid | Fish freshness was accounted | [63] |
| 4. | SPCE | HClO4− | CV | Caffeic acid | Tea samples | [64] |
| 5. | Ion Selective Electrode | - | Based on ion-selective field emission transistors | Soil nutrient sensing | Suffer various interferences | [65] |
| S. No. | Type of Transformation | Host Plant | Vector/Promoter | Recombinant Protein | Ref |
|---|---|---|---|---|---|
| 1. | Agroinfiltration | Tomato | Pepino Mosaic Virus PepMV | FMDV 2A catalytic peptide | [70] |
| 2. | Chloroplast transformation | Sugar beet | Prrn promoter- | GFP | [71] |
| 3. | Nuclear transformation | Carrot | CaMV-35S | Heat labile enterotoxin B | [72] |
| 4. | magniCON | Tobacco | pTBSV | HBc (VLPs), GFP | [73] |
| 5. | Target Specific Expression | Rice endosperm | Tapur promoter | Human lysozyme | [74] |
| 6. | Virus-induced gene silencing (VIGS) | Wheat Spike | Barley stripe mosaic virus | NA | [75] |
| S. No. | Chemically Modified Electrode | pH | Phytochemical/Chemical Moiety | Technique | Comment | Ref No. |
|---|---|---|---|---|---|---|
| 1. | GCE | Sodium acetate acid buffer | Labiatae family | CV; Amperometry | The pH of 4 was maintained and oxidation-reduction potential was sued for antioxidant activity measurement | [88] |
| 2. | SPCE/MWCNT | 0.1 mol L−1 HClO4− | Caffeic acid | CV | Tea and other samples were tested for total polyphenolic content. | [64] |
| 3. | Carbon Paste electrode | PBS | Cornelian cherry was tested against reducing peroxyl radical | SWV; CV | Antioxidant potential was tested for Cornus mas extracts | [89] |
| 4. | Carbon Paste Electrode | Acetate buffer | Infusions (green tea), beverages (red wine and coffee) | CV | Antioxidant activity via in situ formation of free superoxide radical was analyzed | [89] |
| 5. | GCE/Ag-NCF | PBS | Glioma cells were tested | Amp i-t | Superoxide ions released from glioma cells were monitored | [90] |
| 6. | GCE | Acetonitrile + Bu4NPF6 | Venezuelan Propolis | CV | Total phenolic content and flavonoid content were determined | [91] |
| 7. | SPCE/SWCNT-COOH | PBS | Chlorogenic acids and coffee extract | CV | Antioxidant activity based on pro-oxidant content (OH.) quantification | [92] |
| 8. | GCE | NaCl + NaOH | Chilean red wine, grape and raspberry | DPV | The pH of 3.6 was maintained (N2 atm) | [93] |
| 9. | Guanine/GNR/GCE | PBS | Fruit juices | SWV; CV | Antioxidant capacity was tested based on ascorbic acid oxidation of free radicals | [94] |
| 10. | SPE/Tyr/HSA/GA | Acetate buffer | Salvia microphylla; Lippia dulcis; Lippia alba | Amp i-t; EIS | Indirect evaluation based on catechol formation using enzyme has been studied at 4.5 pH | [95] |
| S. No. | Chemically Modified Electrode | pH | Reactant Moiety | Technique | Sensor Development | Ref |
|---|---|---|---|---|---|---|
| 1. | GCE/CB@Ses-Qn | PBS | Sesamol-phytonutrient-based | CV | White spot syndrome virus detection | [60] |
| 2. | SPCE/HQ | PBS | Mustard protein via sandwich immuno-sensing | Amperometry | Trace mustard protein determination in food samples | [98] |
| 3. | Ni foam/curcumin-based | PBS | Curcumin-based phytonutrient for Amyloid β oligomer detection | EIS | Alzheimer’s disease detection | [99] |
| 4. | GCE/GMC@Ginger-Cu2+ | pH 2 HCl-KCl | Ginger/Gingerol-phyto compound | CV | H2O2 sensing | [100] |
| 5. | CB-SPE | 0.1 mol H2SO4 | capsaicin | SWV | Determination of capsaicin in pepper samples | [101] |
| S. No. | Chemically Modified Electrode | pH | Disease/Virus Targeted | Technique | Comment | Ref |
|---|---|---|---|---|---|---|
| 1. | CM-GQD’s-ITO-APO-e4-DNA sensor | 0.1 mM PBS | Alzheimer’s Disease (cardiac Troponin l biomarker) | EIS | No redox peak was associated with the CU modified platform | [105] |
| 2. | Ab1/Luminol-Au@MoS2/Bi2S3 | PBS | Amyloid-β-protein | EIS | No CV or redox studies | [106] |
| 3. | PEG/TA/pDA | 0.2 M PBS | (BRCA1) Mutation of breast cancer | EIS | No CV or redox characteristics | [107] |
| 4. | Cur-oligonucleotide modified/PGE | Tris-EDTA | Interleukine-2 | DPV | As the different contact time changes, the current value changes | [108] |
| 5. | PtNP/Gr-IL-Chit/GCE | 0.1 M PBS | Human Chronic Gonadotrophin | DPV | Ill-defined peak response | [109] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gandhi, M.; Amreen, K.; Tiwari, B.K. Recent Advances in the Electro-Active Therapeutic Phytochemical-Based Sensors. Electrochem 2022, 3, 613-632. https://doi.org/10.3390/electrochem3040041
Gandhi M, Amreen K, Tiwari BK. Recent Advances in the Electro-Active Therapeutic Phytochemical-Based Sensors. Electrochem. 2022; 3(4):613-632. https://doi.org/10.3390/electrochem3040041
Chicago/Turabian StyleGandhi, Mansi, Khairunnisa Amreen, and Brahm Kumar Tiwari. 2022. "Recent Advances in the Electro-Active Therapeutic Phytochemical-Based Sensors" Electrochem 3, no. 4: 613-632. https://doi.org/10.3390/electrochem3040041
APA StyleGandhi, M., Amreen, K., & Tiwari, B. K. (2022). Recent Advances in the Electro-Active Therapeutic Phytochemical-Based Sensors. Electrochem, 3(4), 613-632. https://doi.org/10.3390/electrochem3040041







