A Nitrogen- and Carbon-Present Tin Dioxide-Supported Palladium Composite Catalyst (Pd/N-C-SnO2)
Abstract
1. Introduction
2. Experiments
2.1. Materials
2.2. Preparation of the Nitrogen- and Carbon-Present SnO2-Supported Pd Composite Catalyst (Pd/N-C-SnO2)
2.3. Preparation of Pd/N-C-SnO2-Coated Glassy Carbon (GC) Electrodes
2.4. Characterization
3. Results and Discussion
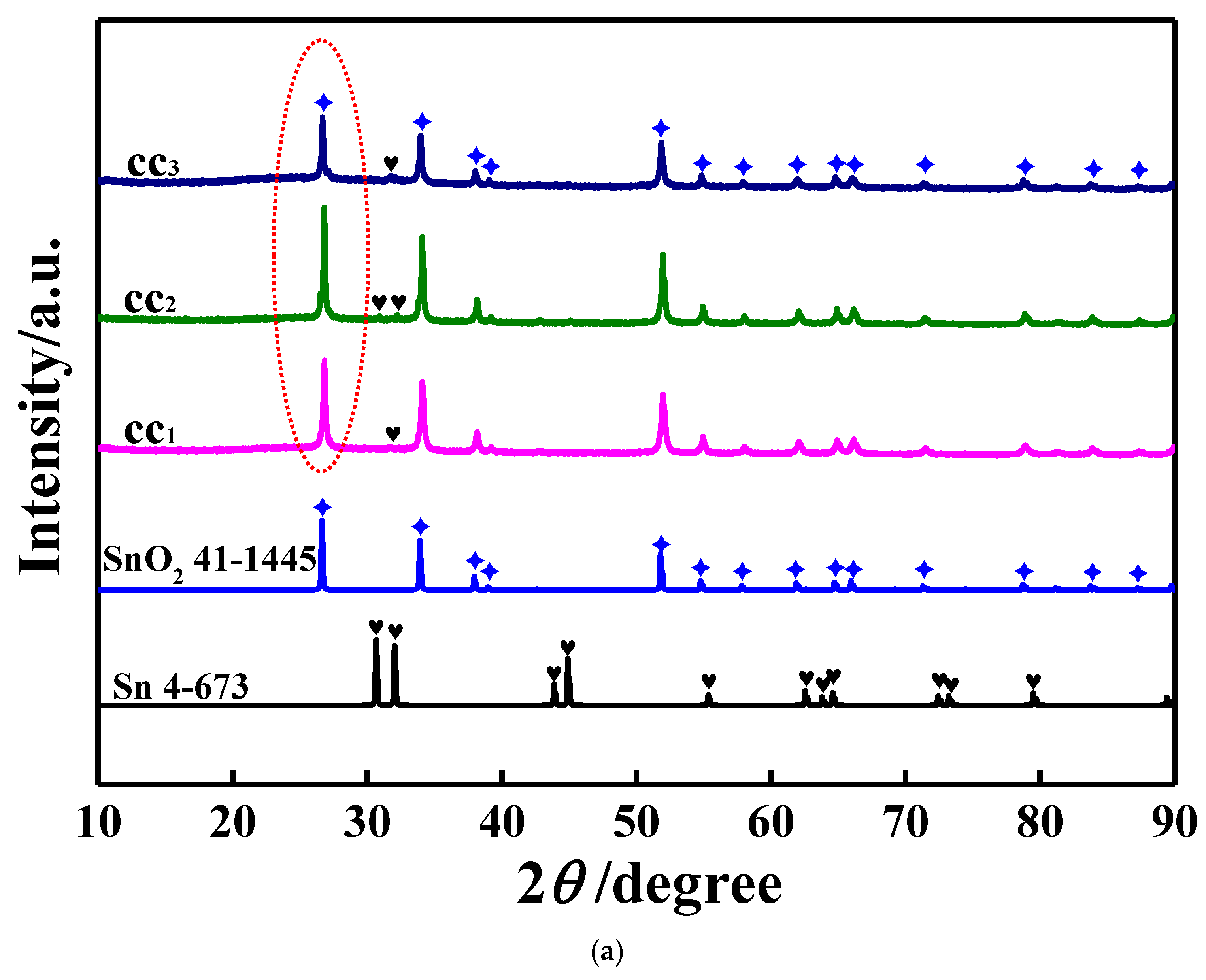
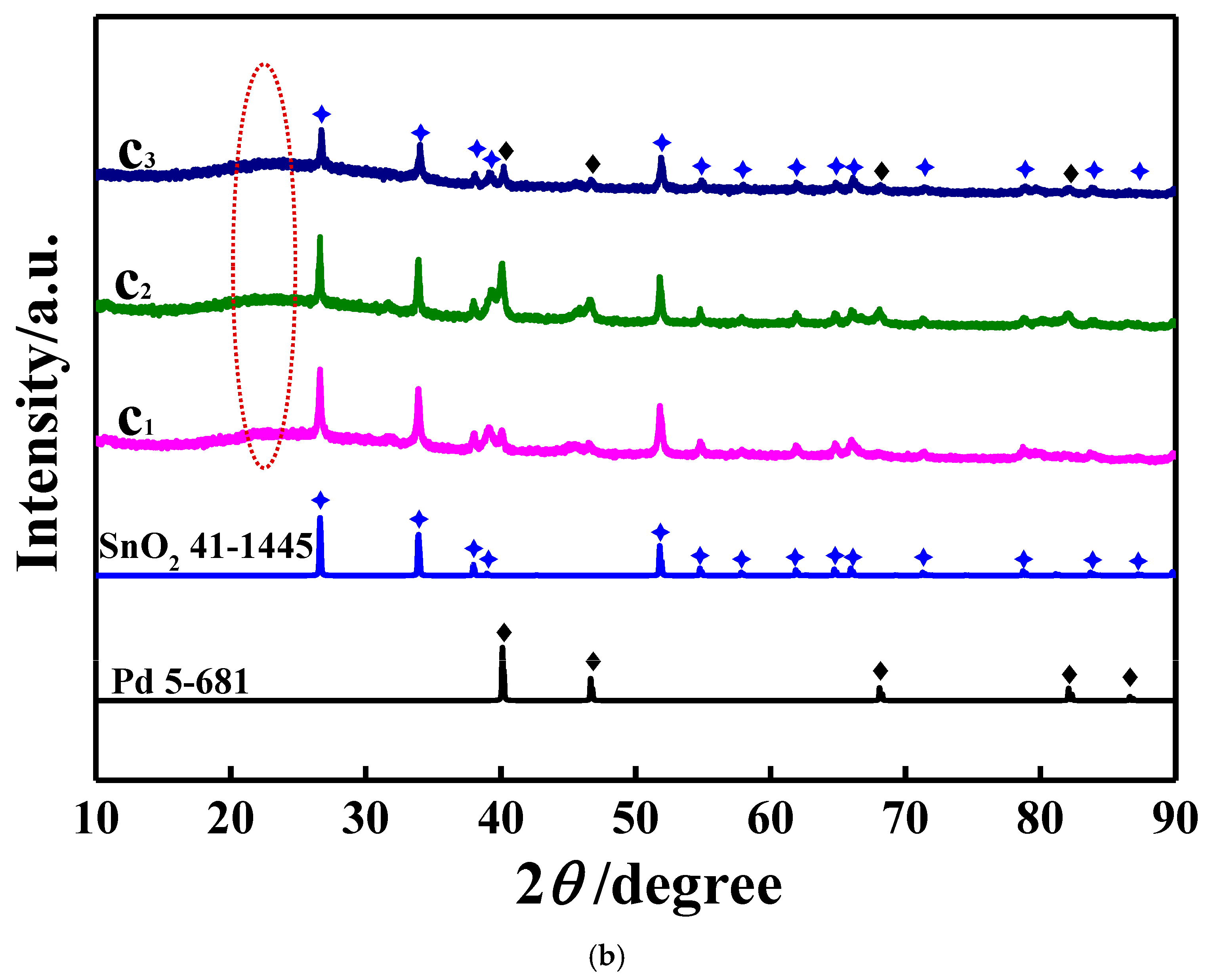
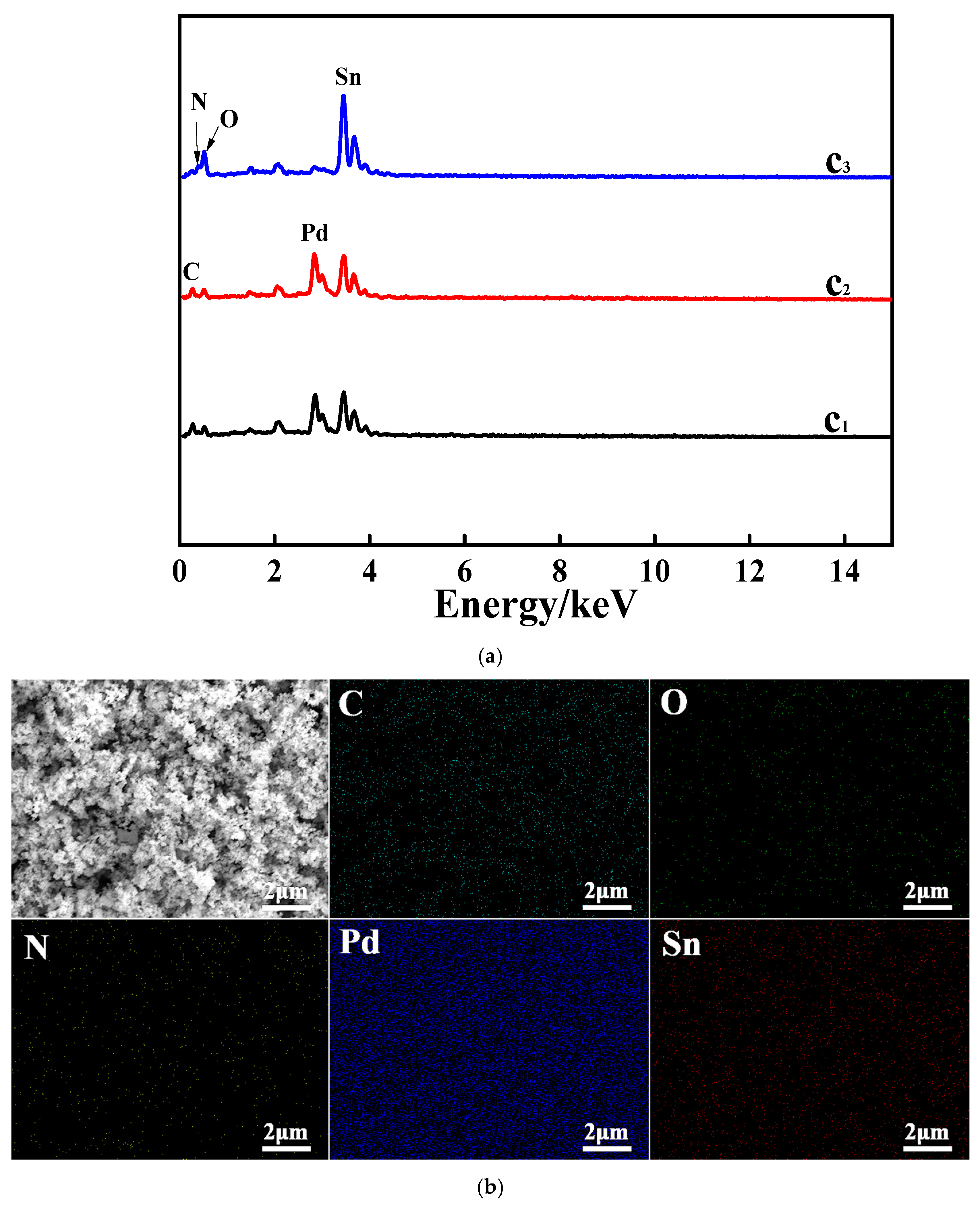
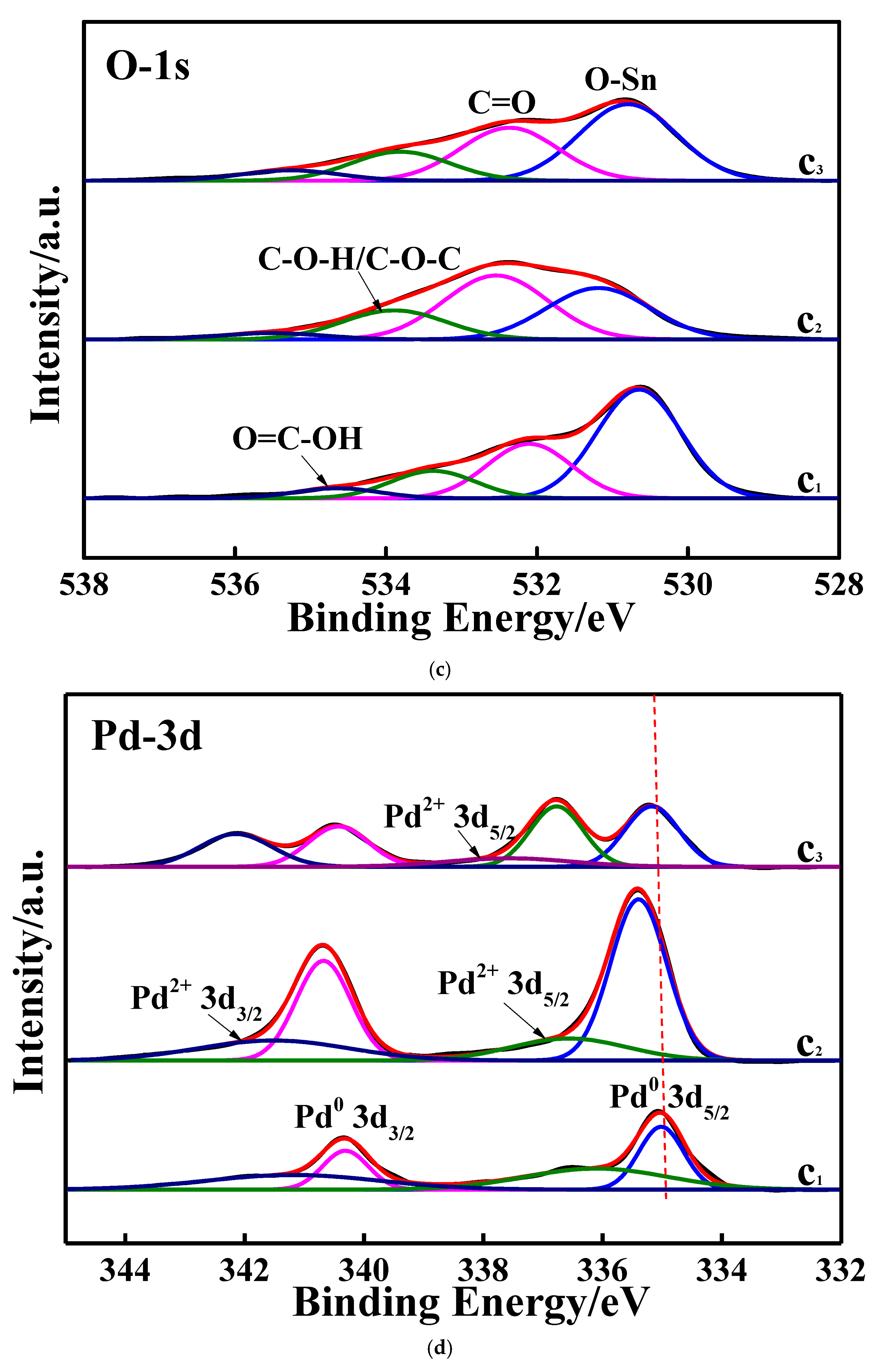
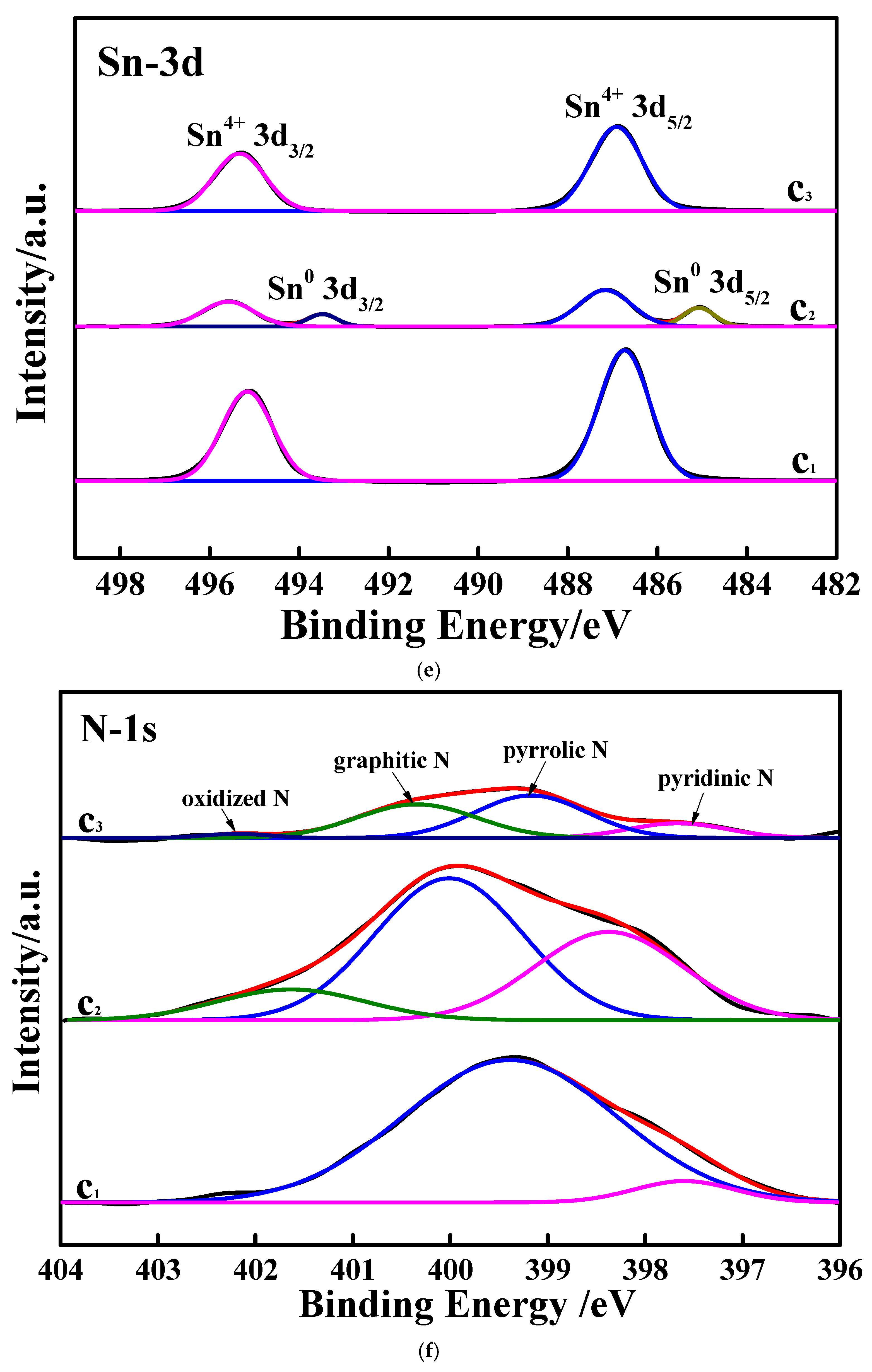
3.1. Characterizations of the Studied Samples
3.2. SEM Characterization
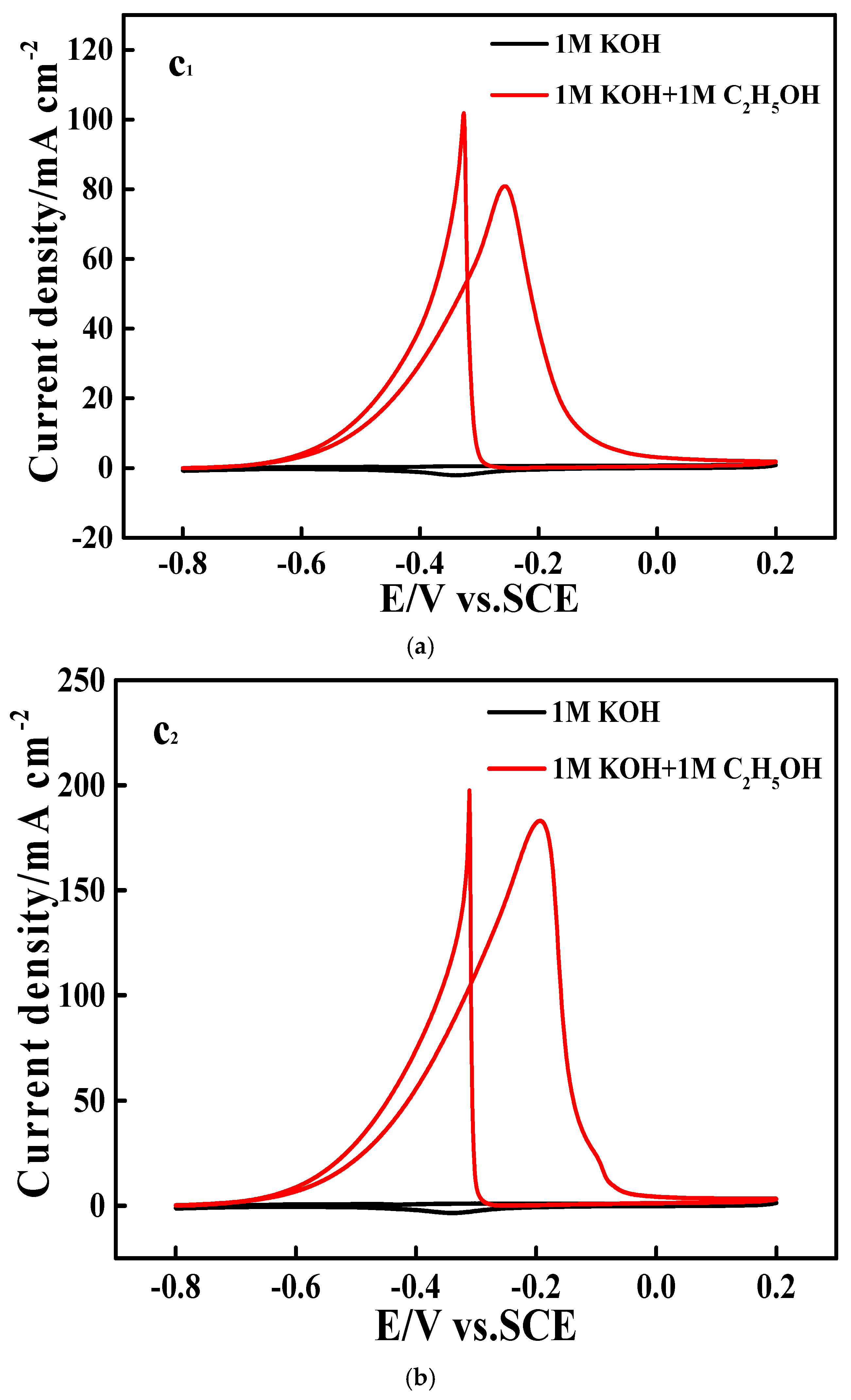
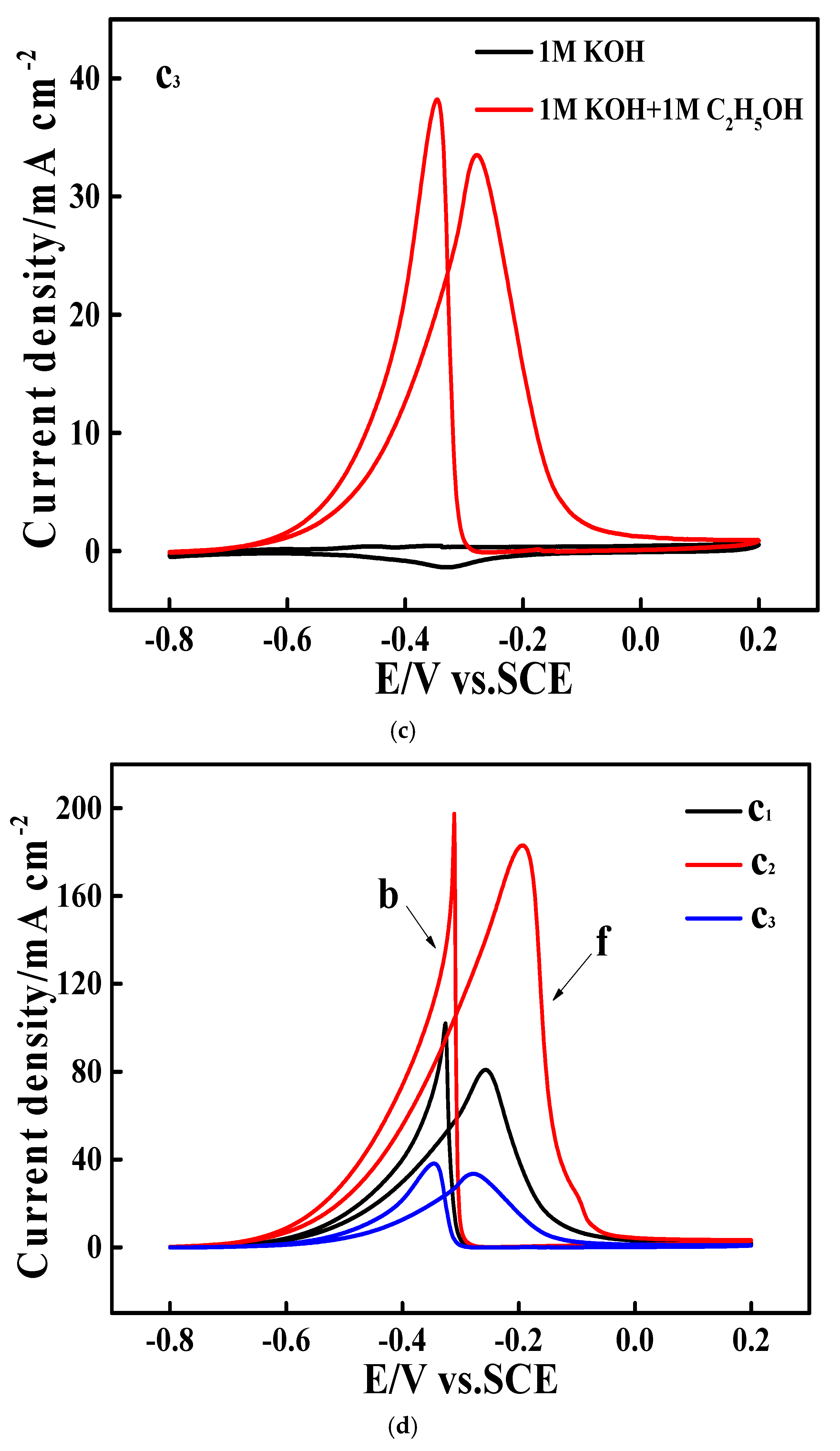
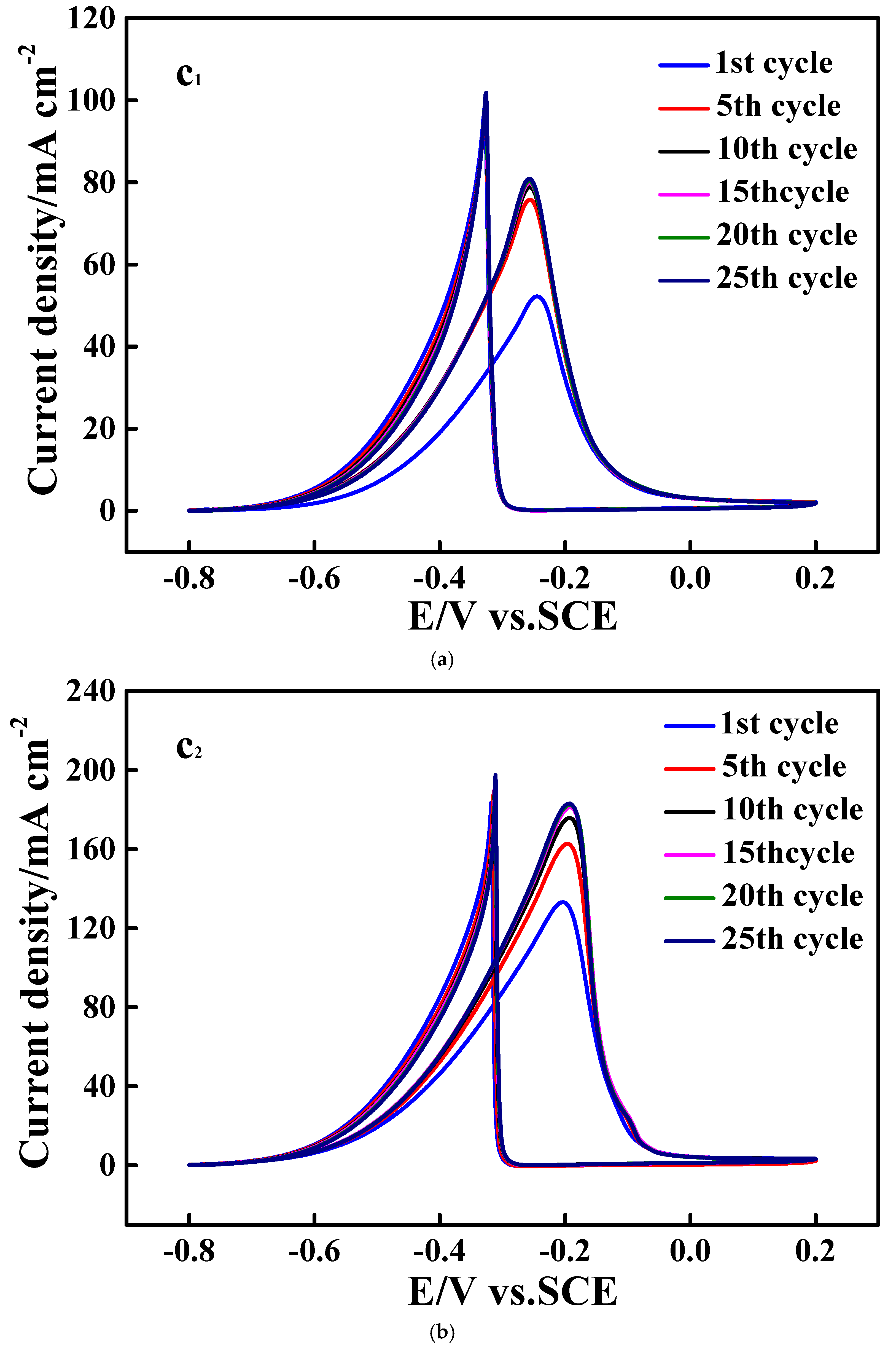
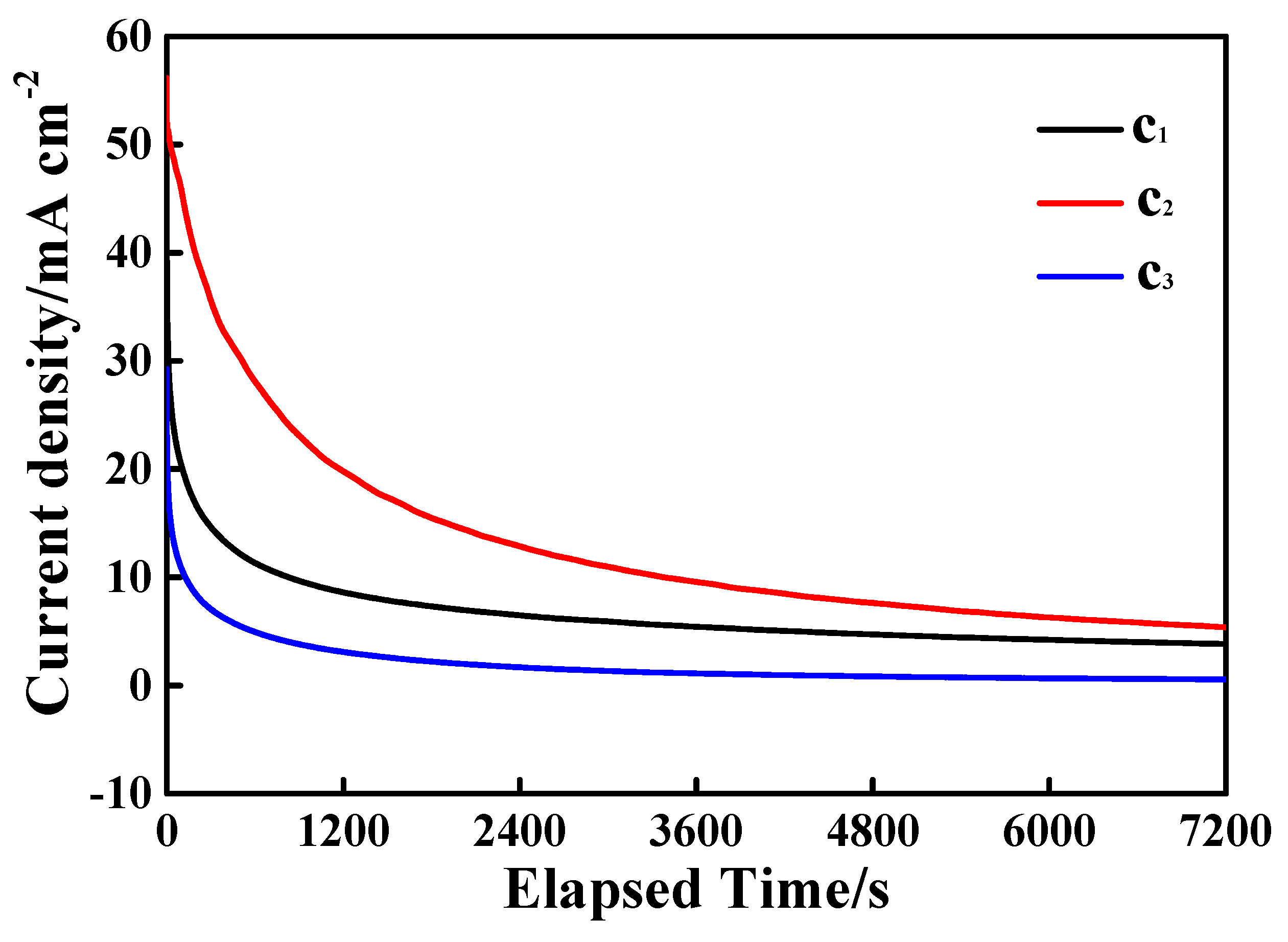
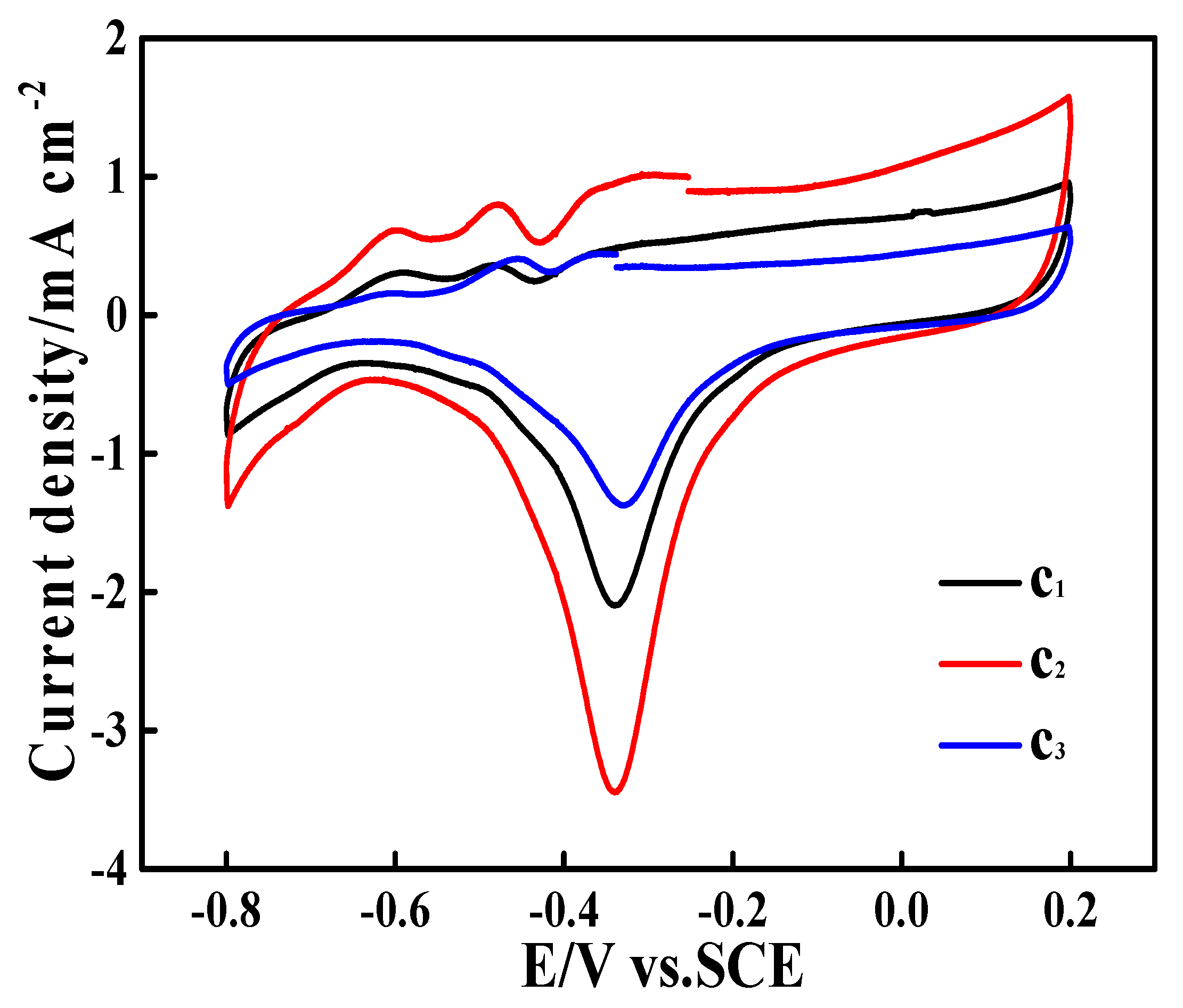
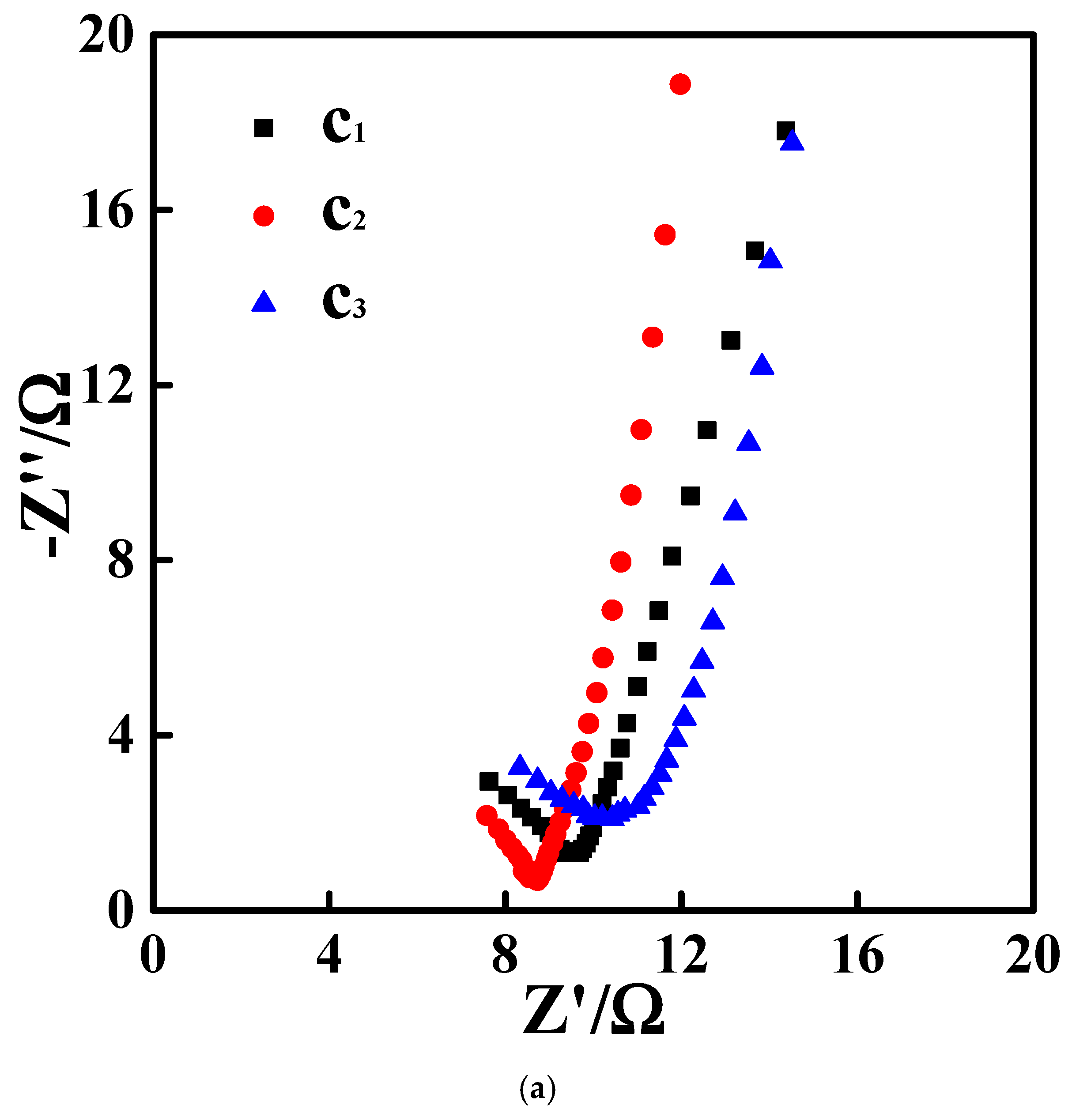
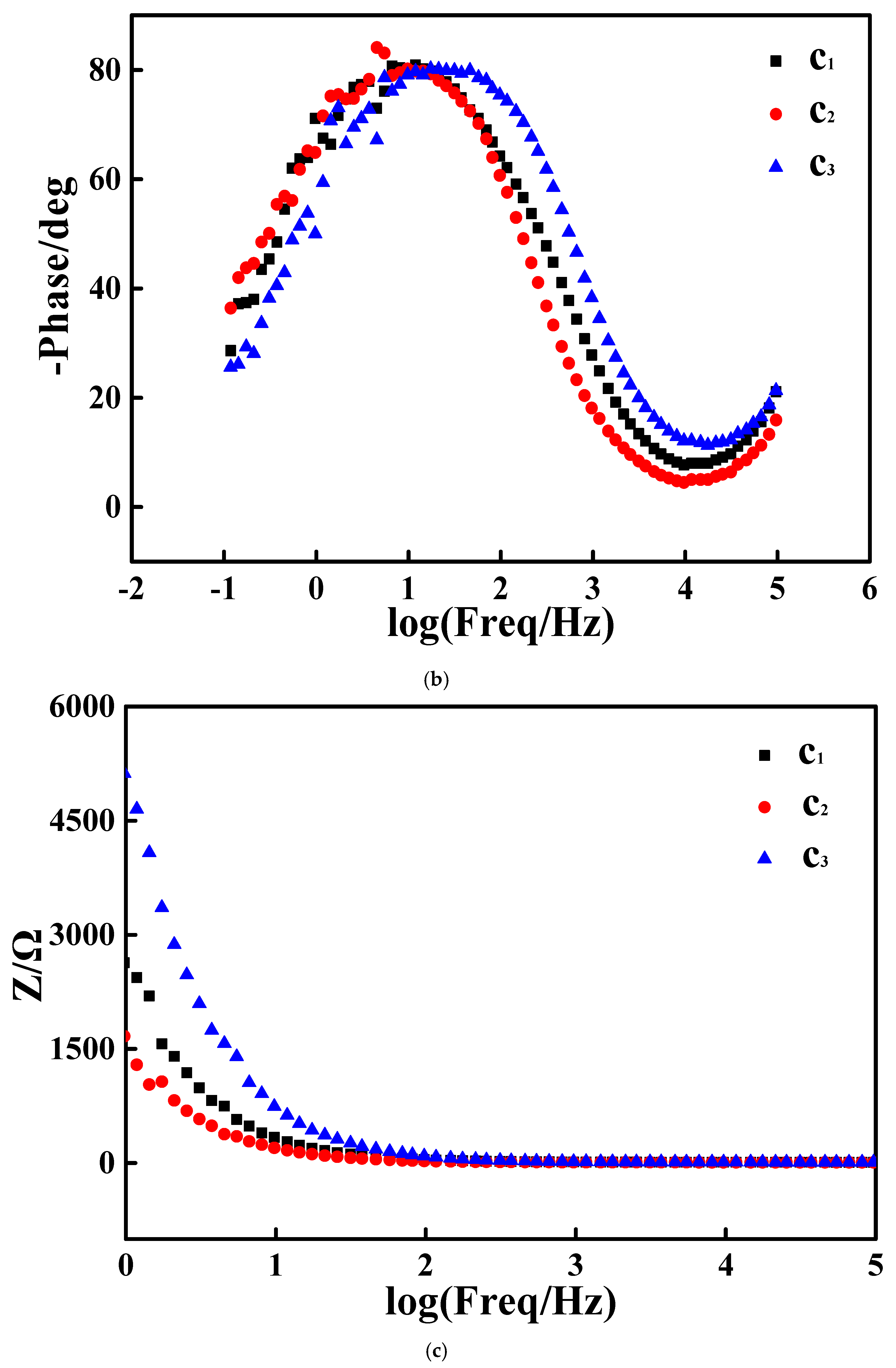
3.3. Electrocatalytic Performances Towards EOR Achieved by the Pd/N-C-SnO2 Catalysts
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wei, K.; Lin, H.; Zhao, X.; Zhao, Z.; Marinkovic, N.; Morales, M.; Huang, Z.; Perlmutter, L.; Guan, H.; Harris, C.; et al. Au/Pt bimetallic nanowires with stepped Pt sites for enhanced C-C cleavage in C2+ alcohol electro-oxidation reactions. J. Am. Chem. Soc. 2023, 145, 19076–19085. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Luo, S.; Sun, M.; Wu, X.; Zhou, Y.; Liao, Y.; Tang, M.; Fan, X.; Huang, B.; Quan, Z. High-entropy intermetallic PtRhBiSnSb nanoplates for highly efficient alcohol oxidation electrocatalysis. Adv. Mater. 2022, 34, 2206276. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Li, G.; Cui, S.; Park, G.-S.; Oh, R.; Chen, W.; Cheng, X.; Zhang, J.-M.; Li, W.; Ji, L.-F.; et al. Ga-modification near-surface composition of Pt−Ga/C catalyst facilitates high-efficiency electrochemical ethanol oxidation through a C2 intermediate. J. Am. Chem. Soc. 2023, 145, 17220–17231. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Liu, T.; Liang, X.; Dou, W.; Geng, H.; Yu, Z.; Li, Y.; Zhang, Y.; Shao, Q.; Fan, J.; et al. Pd-Sb rhombohedra with an unconventional rhombohedral phase as a trifunctional electrocatalyst. Adv. Mater. 2022, 34, 2206528. [Google Scholar] [CrossRef]
- Ao, W.; Ren, H.; Cheng, C.; Fan, Z.; Yin, P.; Qin, Q.; Zhang, Q.; Dai, L. Mesoporous PtPb nanosheets as efficient electrocatalysts for hydrogen evolution and ethanol oxidation. Angew. Chem. Int. Ed. 2023, 62, e202305158. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, M.; Li, Y.; Ye, C.; Chen, J.; Ye, J.; Zhang, Q.; Li, J.; Zhou, Z.; Fu, X.-Z.; et al. p-d orbital hybridization induced by a monodispersed Ga site on a Pt3Mn nanocatalyst boosts ethanol electrooxidation. Angew. Chem. Int. Ed. 2022, 61, e202115735. [Google Scholar] [CrossRef]
- Zhu, L.D.; Zhao, T.S.; Xu, J.B.; Liang, Z.X. Preparation and characterization of carbon-supported sub-monolayer palladium decorated gold nanoparticles for the electro-oxidation of ethanol in alkaline media. J. Power Sources 2009, 187, 80–84. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.Z.; Liu, T.Y.; Ma, X.Y.; Feng, Y.G.; Xu, B.Y.; Cai, W.B.; Li, Y.F.; Su, D.; Shao, Q.; et al. Rhombohedral Pd-Sb nanoplates with Pd-terminated surface: An efficient bifunctional fuel-cell catalyst. Adv. Mater. 2022, 34, 2202333. [Google Scholar] [CrossRef]
- Antoniassi, R.M.; Erikson, H.; Solla-Gullón, J.; Torresi, R.M.; Feliu, J.M. Formic acid electrooxidation on small, {1 0 0} structured, and Pd decorated carbon-supported Pt nanoparticles. J. Catal. 2021, 400, 140–147. [Google Scholar] [CrossRef]
- Baruah, S.; Kumar, A.; Peela, N.R. Role of ZSM-5/AC hybrid support on the catalytic activity of Pd-Ag electrocatalysts towards ethanol oxidation: An experimental and kinetic study. Electrochim. Acta 2023, 453, 142357. [Google Scholar] [CrossRef]
- Bai, G.; Yang, X.; Jia, S.; Lv, Y.; Tong, X. Controlling the size of Ag@Pd catalysts to boost ethanol oxidation. J. Electron. Mater. 2023, 52, 3841–3847. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, S.; Zhu, C.; Zhang, H.; Zhu, L.; Feng, Y.; Wang, J.; Yang, Q.; Liu, B.; Xu, W. Pyrolysis of ZIF-8 to PdZn alloy nanoparticles on carbon doping with nitrogen for highly efficient electrocatalytic alcohol oxidation. J. Alloys Compd. 2023, 965, 171281. [Google Scholar] [CrossRef]
- Alfonso, A.N.R.; Villanueva, M.L.; Laxamana, J.R.P.; Necesito, H.G.G.; Tongol, B.J.V. Enhanced electrocatalytic activity of palladium electrocatalysts supported on corncob biochar for ethanol oxidation reaction in alkaline medium. J. Chin. Chem. Soc. 2024, 71, 324–332. [Google Scholar] [CrossRef]
- Yu, K.; Lin, Y.; Fan, J.; Li, Q.; Shi, P.; Xu, Q.; Min, Y. Ternary N, S, and P-doped hollow carbon spheres derived from polyphosphazene as Pd supports for ethanol oxidation reaction. Catalysts 2019, 9, 114. [Google Scholar] [CrossRef]
- Wu, B.; Meng, H.; Morales, D.M.; Zeng, F.; Zhu, J.; Wang, B.; Risch, M.; Xu, Z.J.; Petit, T. Nitrogen-rich carbonaceous materials for advanced oxygen electrocatalysis: Synthesis, characterization, and activity of nitrogen sites. Adv. Funct. Mater. 2022, 32, 2204137. [Google Scholar] [CrossRef]
- Xiao, S.; Zhu, L.; Osman, S.M.; Feng, Y.; Zhang, S.; Li, G.; Zeng, S.; Luque, R.; Chen, B.H. Ultrahigh stable covalent organic framework-derived carbon-nitrogen-supported palladium nanoparticles for highly efficient electrocatalytic methanol and ethanol oxidation reactions. Green Chem. 2022, 24, 5813–5821. [Google Scholar] [CrossRef]
- Su, Y.; Li, C.; Xu, L.; Xue, J.; Yuan, W.; Yao, C.; Liu, J.; Cheng, M.; Hou, S. Palladium nanoparticles supported on flower-like boron, nitrogen doped carbon for electrochemical oxidation ethanol reaction. J. Alloys Compd. 2022, 901, 163333. [Google Scholar] [CrossRef]
- Li, S.; Wu, L.; Zhao, J.; Li, R.; Yang, H.; Zhao, L.; Jin, R. Nitrogen-doped carbon nanotubes embedded with nitrogen-doped carbon black anchoring Pd nanocrystals to boost ethanol electrooxidation. Green Chem. 2023, 25, 10033–10042. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, N.; Zhao, Y.; Wu, Y.; Wang, X.; Zhao, Y. Nanosized Pd-loaded the Fe3C encapsulated by N-doped carbon nanotube as an electrocatalyst for oxygen reduction and ethanol oxidation reactions. Appl. Surf. Sci. 2022, 595, 153512. [Google Scholar] [CrossRef]
- Shekhawat, A.; Samanta, R.; Panigrahy, S.; Barman, S. Electrocatalytic oxidation of urea and ethanol on two-dimensional amorphous nickel oxide encapsulated on N-doped carbon nanosheets. ACS Appl. Energy Mater. 2023, 6, 3135–3146. [Google Scholar] [CrossRef]
- Quílez-Bermejo, J.; Morallon, E.; Cazorla-Amoros, D. On the deactivation of N-doped carbon materials active sites during oxygen reduction reaction. Carbon 2022, 189, 548–560. [Google Scholar] [CrossRef]
- Yang, M.M.; Kong, Q.Q.; Feng, W.; Yao, W.T. N/O double-doped biomass hard carbon material realizes fast and stable potassium ion storage. Carbon 2021, 176, 71–82. [Google Scholar] [CrossRef]
- Jongsomjit, S.; Prapainainar, P.; Sombatmankhong, K. Synthesis and characterisation of Pd-Ni-Sn electrocatalyst for use in direct ethanol fuel cells. Solid State Ion. 2016, 288, 147–153. [Google Scholar] [CrossRef]
- Li, Q.Y.; Zhou, X.X.; Lu, M.N.; Pan, S.Q.; Ajmal, S.; Xiang, D.; Sun, Z.J.; Zhu, M.Z.; Li, P. In-situ synthesis of carbon-supported ultrafine trimetallic PdSnAg nanoparticles for highly efficient alcohols electrocatalysis. J. Colloid Interface Sci. 2024, 653, 1264–1271. [Google Scholar] [CrossRef]
- Nan, L.; Fan, Z.; Yue, W.; Dong, Q.; Zhu, L.; Yang, L.; Fan, L. Graphene-based porous carbon-Pd/SnO2 nanocomposites with enhanced electrocatalytic activity and durability for methanol oxidation. J. Mater. Chem. A 2016, 4, 8898–8904. [Google Scholar] [CrossRef]
- Pinheiro, V.S.; Souza, F.M.; Gentil, T.C.; Nascimento, A.N.; Bὃhnstedt, P.; Parreira, L.S.; Paz, E.C.; Hammer, P.; Sairre, M.I.; Batista, B.L.; et al. Sn-containing electrocatalysts with a reduced amount of palladium for alkaline direct ethanol fuel cell applications. Renew. Energy 2020, 158, 49–63. [Google Scholar] [CrossRef]
- Gao, Q.; Mou, T.; Liu, S.; Johnson, G.; Han, X.; Yan, Z.; Ji, M.; He, Q.; Zhang, S.; Xin, H.; et al. Monodisperse PdSn/SnOx core/shell nanoparticles with superior electrocatalytic ethanol oxidation performance. J. Mater. Chem. A 2020, 8, 20931–20938. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, C.; Chi, M.; Wei, M.; Dong, X.; Zhu, A.; Zhang, Q.; Liu, Q. Two-dimensional PdSn/TiO2-GO towards ethanol electrooxidation catalyst with high stability. Int. J. Hydrogen Energy 2021, 46, 19129–19139. [Google Scholar] [CrossRef]
- Pinheiro, V.S.; Souza, F.M.; Gentil, T.C.; Nascimento, A.N.; Parreira, L.S.; Hammer, P.; Sairre, M.I.; Batista, B.L.; Santos, M.C. Electrocatalysts based on low amounts of palladium combined with tin nanoparticles and cerium dioxide nanorods for application as ADEFC anodes. Int. J. Hydrogen Energy 2021, 46, 39438–39456. [Google Scholar] [CrossRef]
- Ding, K.; Gu, H.; Zheng, C.; Liu, L.; Liu, L.; Yan, X.; Guo, Z. Octagonal prism shaped lithium iron phosphate composite particlesas positive electrode materials for rechargeable lithium-ion battery. Electrochim. Acta 2014, 146, 585–590. [Google Scholar] [CrossRef]
- Li, Y.; Yao, L.; Zhang, L.-Q.; Liu, A.-R.; Zhang, Y.-J.; Liu, S.-Q. Palladium nanoparticles supported on nitrogen-doped carbon spheres as enhanced catalyst for ethanol electro-oxidation. J. Electroanal. Chem. 2014, 730, 65–68. [Google Scholar] [CrossRef]
- Wu, J.; Jin, C.; Yang, Z.; Tian, J.; Yang, R. Synthesis of phosphorus-doped carbon hollow spheres as efficient metal-free electrocatalysts for oxygen reduction. Carbon 2015, 82, 562–571. [Google Scholar] [CrossRef]
- Sun, X.; Li, Y. Colloidal carbon spheres and their core/shell structures with noble-metal nanoparticles. Angew. Chem. Int. Ed. 2004, 43, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Priya, P.S.D.; Philip, A.; Kumar, A.R. An investigation on the compositional effects of 3D graphite on theelectrochemical performance of NiO-Co3O4 composite. Diam. Relat. Mater. 2024, 141, 110597. [Google Scholar]
- Yadav, V.; Singh, N.; Meena, D. Investigation of structural and optical properties of pure SnO2, ZnO and SnO2/ZnO composite nanorods. Mater. Today Proc. 2022, 62, 3368–3375. [Google Scholar] [CrossRef]
- Sambasivam, S.; Obaidat, I.M. Effect of iron doping on ESR and Raman spectra of SnO2 nanomaterials. Mater. Today Proc. 2020, 28, 587–590. [Google Scholar] [CrossRef]
- Farithkhan, A.; John, S.A. Investigation of biomass-derived heteroatom-doped carbon fiber aerogel decorated with NiCo2S4 flowers as a binder-free anode for urea electrooxidation: From a sustainable standpoint. ACS Appl. Eng. Mater. 2023, 1, 458–468. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, G.; Xue, C.; Zhang, C.; Xue, J.; Zhang, X. Self-nitrogen-doped hierarchical porous carbon spheres as metal-free catalyst for efficient photocatalytic CO2 reduction. Sep. Purif. Technol. 2024, 342, 127008. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Y.; Zhang, X.; Dong, Z.; Wu, W.; Fan, C. Self-nitrogen-doped carbon spheres assisted CeO2 composites as a bifunctional adsorbent/photocatalyst for CO2 photoreduction. Fuel 2024, 362, 130848. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, S.; Cui, Z.; Li, Z.; Wu, S.; Liang, Y. Tuning the π-electron delocalization degree of mesoporous carbon for hydrogen peroxide electrochemical generation. J. Catal. 2020, 392, 1–7. [Google Scholar] [CrossRef]
- Babu, B.; Reddy, I.N.; Yoo, K.; Kim, D.; Shim, J. Bandgap tuning and XPS study of SnO2 quantum dots. Mater. Lett. 2018, 221, 211–215. [Google Scholar] [CrossRef]
- Selepe, C.T.; Gwebu, S.S.; Matthews, T.; Mashola, T.A.; Sikeyi, L.L.; Zikhali, M.; Mbokazi, S.P.; Makhunga, T.S.; Adegoke, K.A.; Maxakato, N.W. Electro-catalytic properties of palladium and palladium alloy electro-catalysts supported on carbon nanofibers for electro-oxidation of methanol and ethanol in alkaline medium. Catalysts 2022, 12, 608. [Google Scholar] [CrossRef]
- Sabzehmeidani, M.M.; Kazemzad, M.; Ebadzadeh, T. Bimetallic Au-Pd nanoparticles decorated electrospun spinel CoFe2O4 nanostructures as efficient electrocatalysts for ethanol fuel oxidation in alkaline media. Int. J. Hydrogen Energy 2024, 51, 517–528. [Google Scholar] [CrossRef]
- Song, T.; Gao, F.; Zhang, Y.; Yu, P.; Wang, C.; Shiraishi, Y.; Li, S.; Wang, C.; Guo, J.; Du, Y. Shape-controlled PdSn alloy as superior electrocatalysts for alcohol oxidation reactions. J. Taiwan Inst. Chem. Eng. 2019, 101, 167–176. [Google Scholar] [CrossRef]
- Chellasamy, V.; Thangadurai, P. Design, synthesis and investigations of PdxSn1−x nanoparticle-impregnated NiTiO3 as highly active electrocatalyst for methanol oxidation reaction in alkaline medium. J. Mater. Sci. 2023, 58, 2721–2739. [Google Scholar] [CrossRef]
- Song, X.; Chen, X.; Chen, W.; Ao, T. Fe-N/C anode with pseudocapacitive behavior derived from Fe-modified ZIF-8/C composites for phosphorus capture. Colloids Surf. A 2023, 673, 131867. [Google Scholar] [CrossRef]
- Ding, K.; Wang, Y.; Yang, H.; Zheng, C.; Cao, Y.; Wei, H.; Wang, Y.; Guo, Z. Electrocatalytic activity of multi-walled carbon nanotubes-supported PtxPdy catalysts prepared by a pyrolysis process toward ethanol oxidation reaction. Electrochim. Acta 2013, 100, 147–156. [Google Scholar] [CrossRef]
- Liang, Z.X.; Zhao, T.S.; Xu, J.B.; Zhu, L.D. Mechanism study of the ethanol oxidation reaction on palladium in alkaline media. Electrochim. Acta 2009, 54, 2203–2208. [Google Scholar] [CrossRef]
- Ding, K.; Zhao, Y.; Liu, L.; Li, Y.; Liu, L.; Wang, Y.; Gu, H.; Wei, H.; Guo, Z. Multi-walled carbon nanotubes supported Pd composite nanoparticles hydrothermally produced from technical grade PdO precursor. Electrochim. Acta 2015, 176, 1256–1265. [Google Scholar] [CrossRef]
- Ding, K.; Li, B.; Shi, F.; Di, M.; Yan, M.; Xu, L.; Wang, X.; Wang, H. A palladium and nickel composite catalyst composed of huge spherical particles and sawdust-shaped particles showing significant electrocatalytic activities towards ethanol oxidation reaction (EOR). Int. J. Electrochem. Sci. 2022, 17, 220639. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, S.; Peng, H.; Yang, Z.; Martin, D.J.; Bund, A.; Nanjundan, A.K.; Yamauchi, Y. The interphase electrochemical characteristics of the cobaltosic oxide in organic electrolyte by bode plots: Double layer capacitance and pseudocapacitance. ChemElectroChem 2019, 6, 2456–2463. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, K.; Li, W.; Li, M.; Di, M.; Bai, Y.; Liang, X.; Wang, H. A Nitrogen- and Carbon-Present Tin Dioxide-Supported Palladium Composite Catalyst (Pd/N-C-SnO2). Electrochem 2024, 5, 482-505. https://doi.org/10.3390/electrochem5040032
Ding K, Li W, Li M, Di M, Bai Y, Liang X, Wang H. A Nitrogen- and Carbon-Present Tin Dioxide-Supported Palladium Composite Catalyst (Pd/N-C-SnO2). Electrochem. 2024; 5(4):482-505. https://doi.org/10.3390/electrochem5040032
Chicago/Turabian StyleDing, Keqiang, Weijia Li, Mengjiao Li, Mengyao Di, Ying Bai, Xiaoxuan Liang, and Hui Wang. 2024. "A Nitrogen- and Carbon-Present Tin Dioxide-Supported Palladium Composite Catalyst (Pd/N-C-SnO2)" Electrochem 5, no. 4: 482-505. https://doi.org/10.3390/electrochem5040032
APA StyleDing, K., Li, W., Li, M., Di, M., Bai, Y., Liang, X., & Wang, H. (2024). A Nitrogen- and Carbon-Present Tin Dioxide-Supported Palladium Composite Catalyst (Pd/N-C-SnO2). Electrochem, 5(4), 482-505. https://doi.org/10.3390/electrochem5040032




