Transition Metal Oxides (WO3-ZrO2) as Promoters and Hydrogen Adsorption Modulators in Pt/WO3-ZrO2-C Electrocatalyst for the Reduction of NOx
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of the ZWC Support
2.2. Synthesis of the PtC and PtZWC Materials
2.3. Materials Characterization
2.4. Electrochemical Evaluation
3. Results and Discussion
3.1. Textural Properties; Nitrogen Adsorption
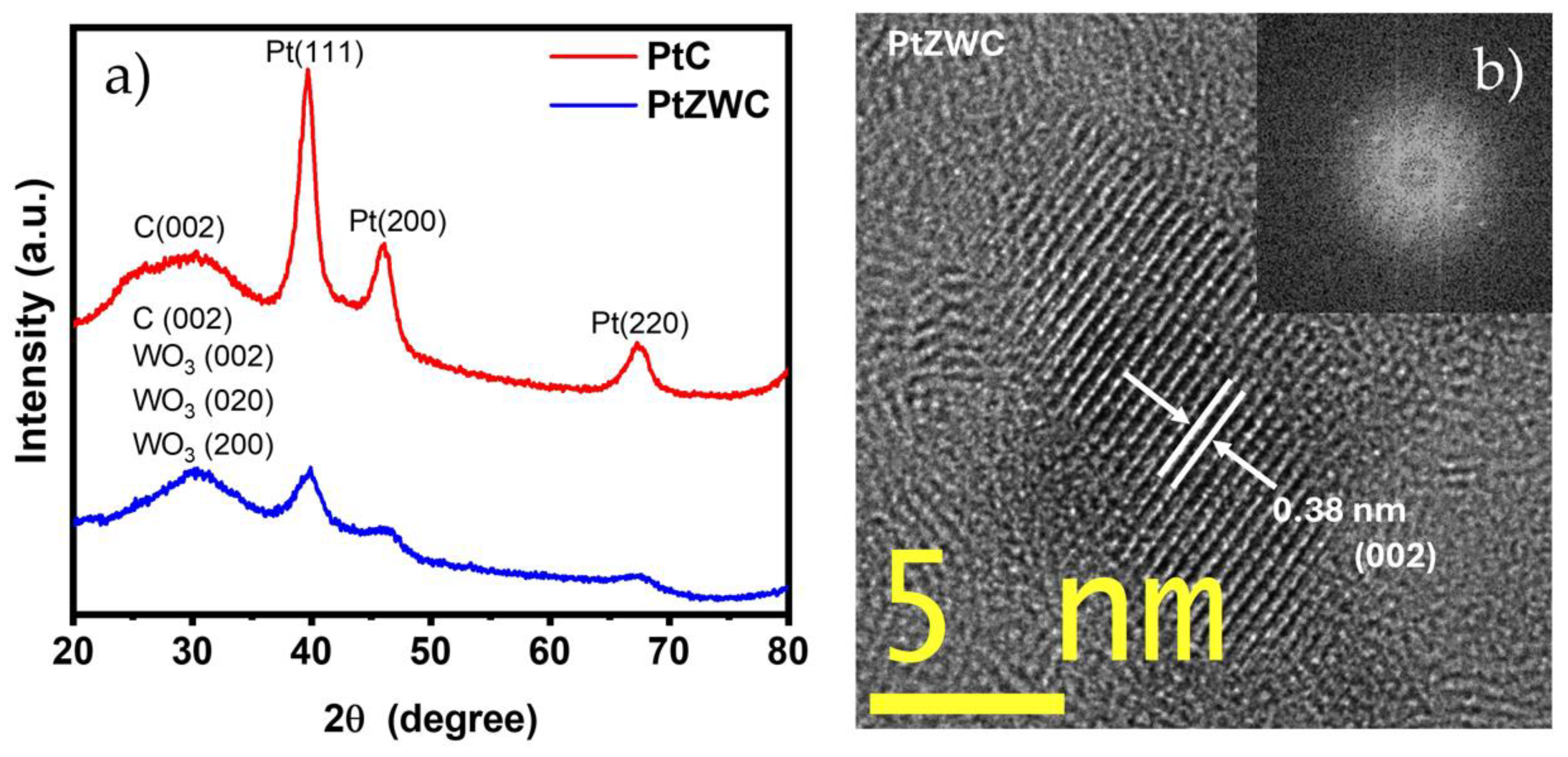
3.2. Crystal Phases; XRD and HRTEM Analysis
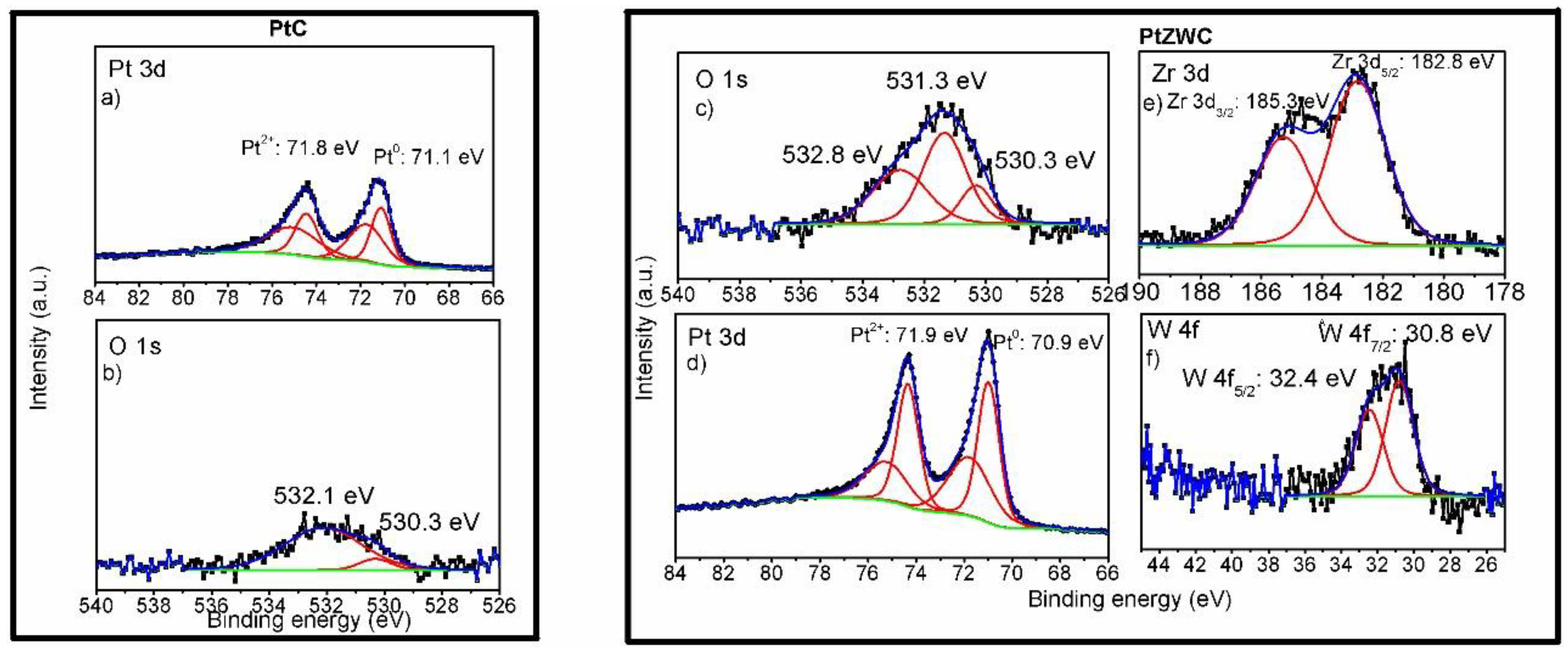
3.3. Surface Properties; X-Ray Photoelectron Spectroscopy
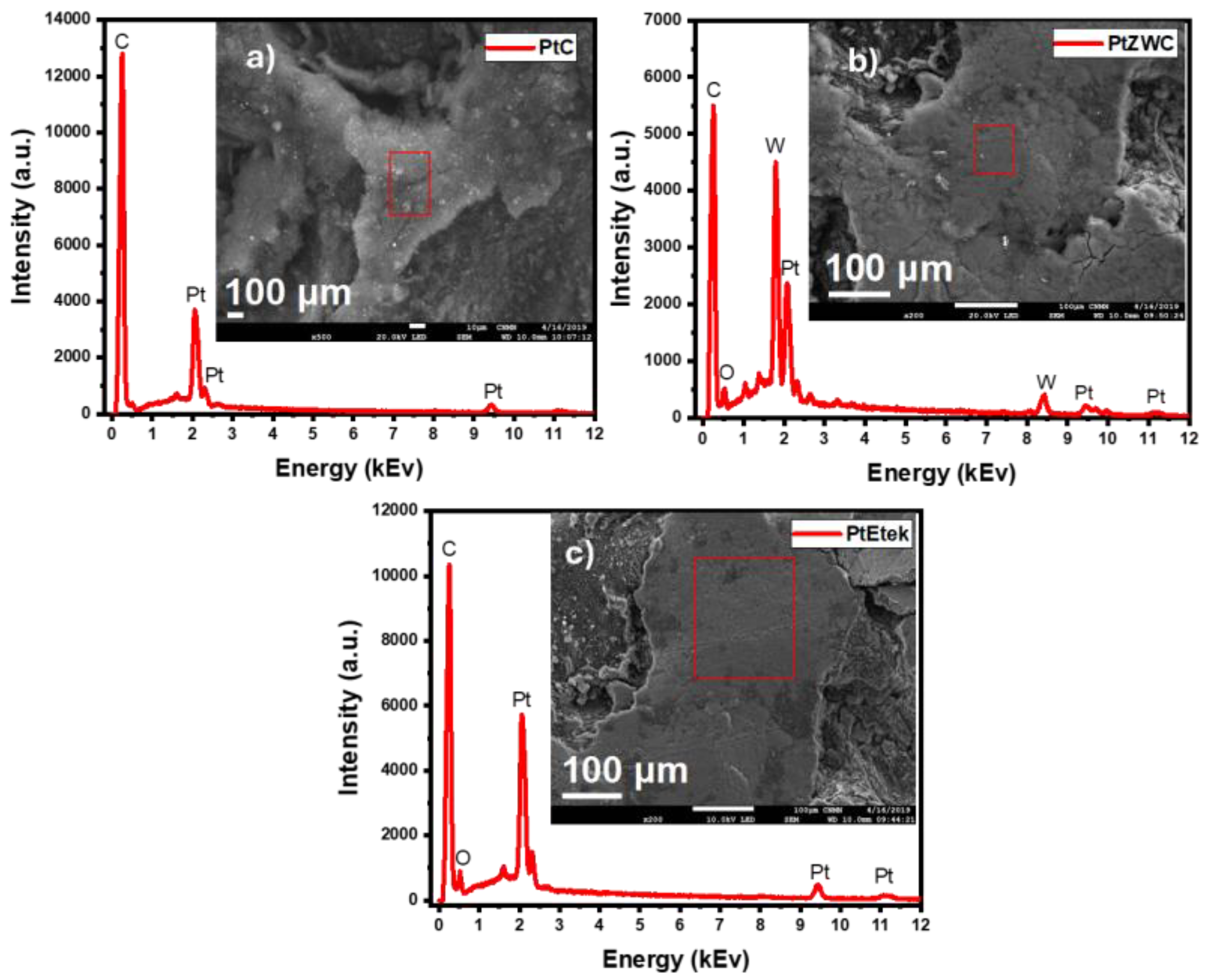
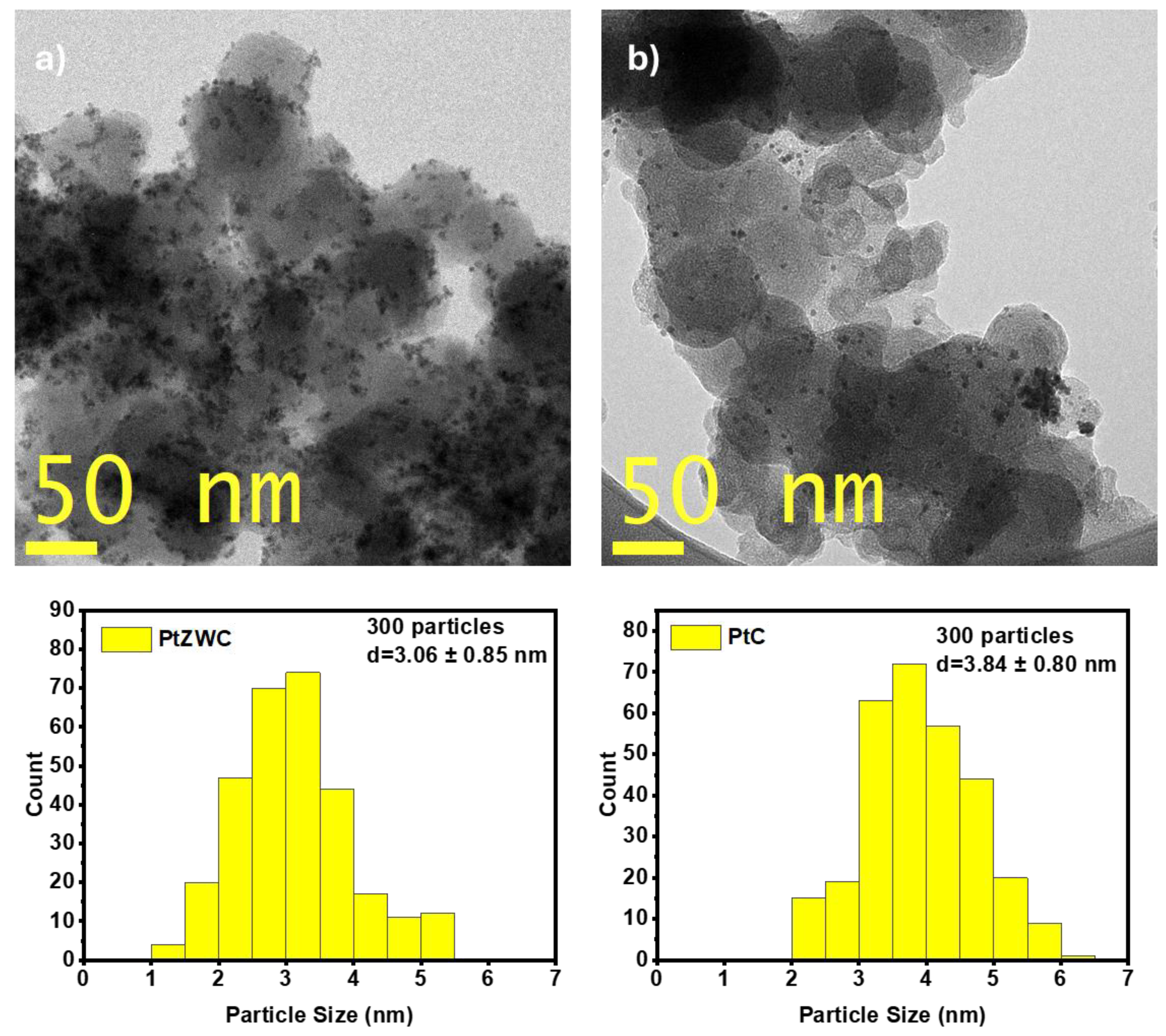
3.4. Morphology and Dispersion; SEM and TEM
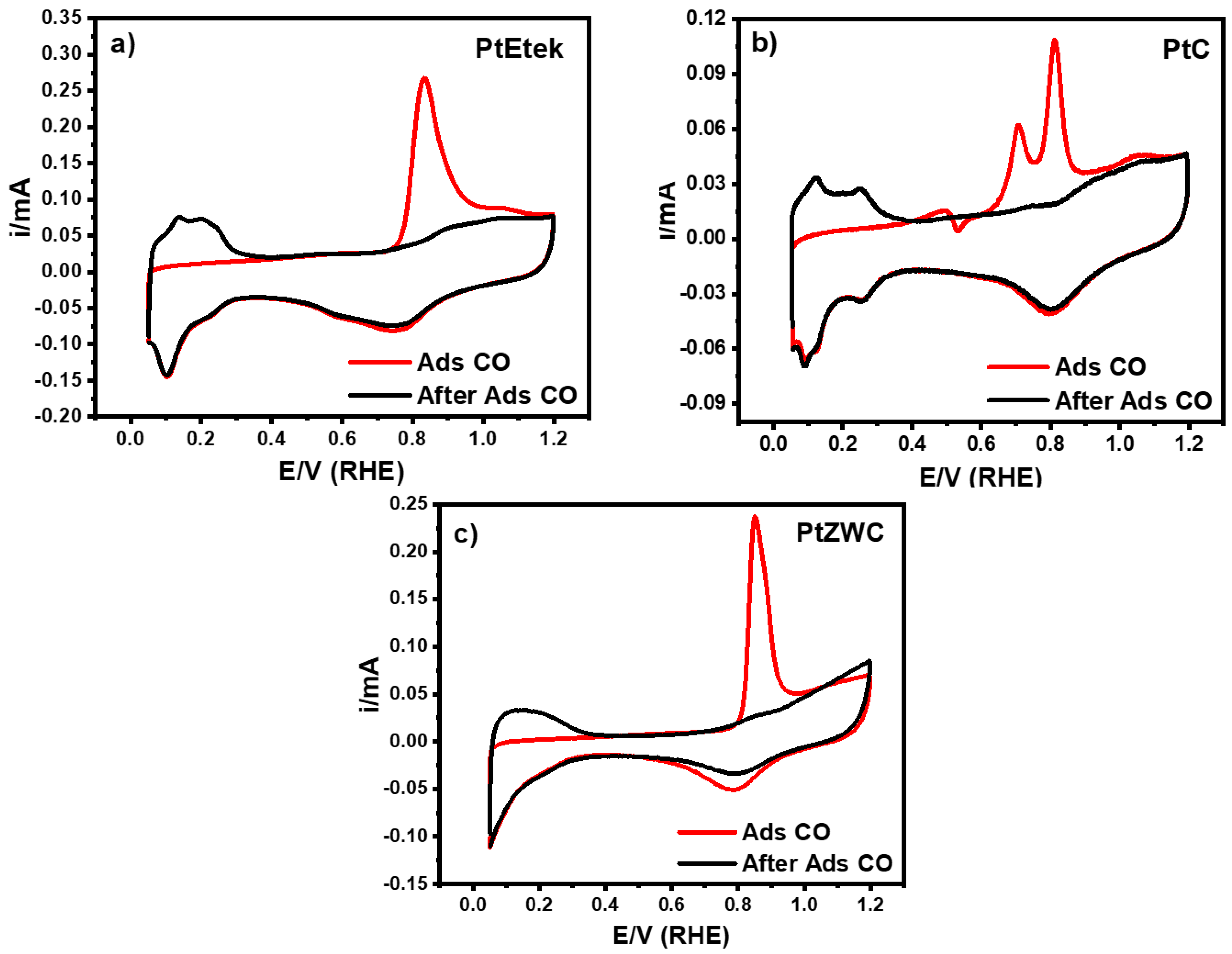
3.5. Electrochemical Properties
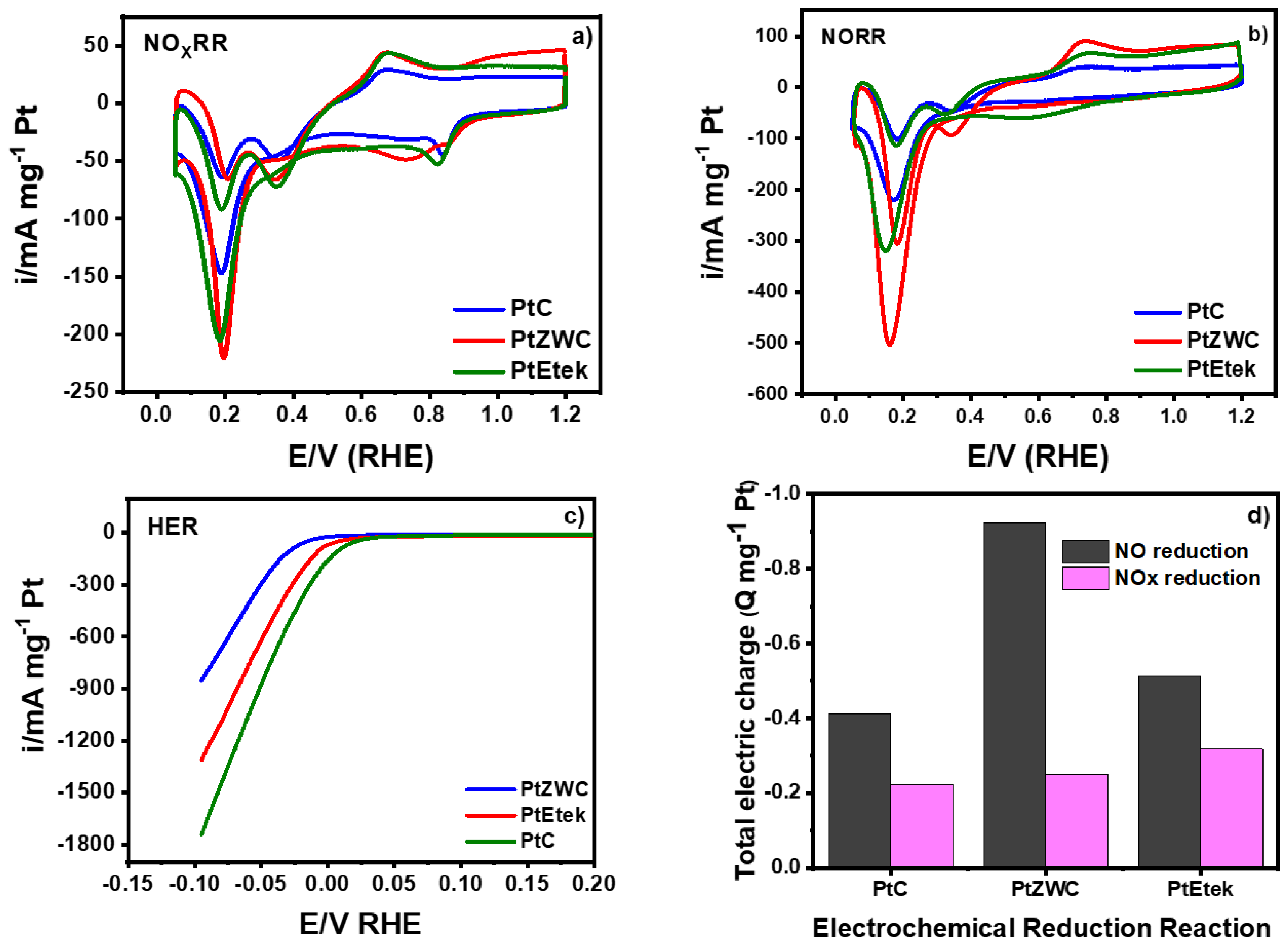
3.6. Reaction Reduction of Nitric Oxide and Nitrogen Oxides
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EDGAR Emission Database for Global Atmospheric Research. EDGAR Emission Database for Global Atmospheric Research (2024). Available online: https://edgar.jrc.ec.europa.eu/ (accessed on 18 November 2024).
- International Energy Agency (IEA). Trends in Electric Cars—Global EV Outlook 2024—Analysis. Available online: https://www.iea.org/reports/global-ev-outlook-2024/trends-in-electric-cars (accessed on 22 February 2025).
- U. Epa, C. Change Division. Inventory of U.S. Greenhouse Gas Emissions and Sinks: 1990–2018—Main Text. Available online: https://www.epa.gov/ghgemissions/inventory-us-greenhouse-gas-emissions-and-sinks (accessed on 22 February 2025).
- World Health Organization. Air Quality, Energy and Health. 2024. Available online: https://www.who.int/teams/environment-climate-change-and-health/air-quality-energy-and-health/about (accessed on 22 February 2025).
- Wang, X.; Westermann, A.; Shi, Y.X.; Cai, N.S.; Rieu, M.; Viricelle, J.-P.; Vernoux, P. Electrochemical Removal of NOx on Ceria-Based Catalyst-Electrodes. Catalysts 2017, 7, 61. [Google Scholar] [CrossRef]
- Santiago-Ramírez, C.; Vera-Iturriaga, J.; del Angel, P.; Manzo-Robledo, A.; Hernández-Pichardo, M.; Soto-Hernández, J. DEMS and RAMAN study of the monatomic hydrogen adsorption during electro-reduction of NO3− and NO2− at Pt nanoparticles supported at W18O49-ZrO2-C nanocomposite. Appl. Catal. B Environ. 2020, 282, 119545. [Google Scholar] [CrossRef]
- Bayan, Y.; Paperzh, K.; Pankov, I.; Alekseenko, A. Influence of a carbon support on the catalytic activity and durability of the Pt-based electrocatalysts. Mater. Lett. 2024, 368. [Google Scholar] [CrossRef]
- Carro, P.; Choi, J.; Macfarlane, D.R.; Simonov, A.N.; Doña-Rodríguez, J.M.; Azofra, L.M. Competition between metal-catalysed electroreduction of dinitrogen, protons, and nitrogen oxides: A DFT perspective. Catal. Sci. Technol. 2022, 9, 2856–2864. [Google Scholar] [CrossRef]
- Choi, C.; Gu, G.H.; Noh, J.; Park, H.S.; Jung, Y. Understanding potential-dependent competition between electrocatalytic dinitrogen and proton reduction reactions. Nat. Commun. 2021, 12, 1–11. [Google Scholar] [CrossRef]
- Matias, M.L.; Carlos, E.; Branquinho, R.; Valle, H.D.; Marcelino, J.; Morais, M.; Pimentel, A.; Rodrigues, J.; Monteiro, T.; Fortunato, E.; et al. A Comparison between Solution-Based Synthesis Methods of ZrO2 Nanomaterials for Energy Storage Applications. Energies 2022, 15, 6452. [Google Scholar] [CrossRef]
- Wei, J.; Jing, Y.; Zhao, Z.; Fan, Z.; Liang, Z.; Huang, J.; Wu, H.; Xie, Z.; Liu, D.; Qu, D.; et al. Catalyst-Support interactions enhanced electrochemical nitrogen reduction on Au/ZrO2. Electrochim. Acta 2021, 381, 138222. [Google Scholar] [CrossRef]
- Chen, J.; Chen, C.; Qin, M.; Li, B.; Lin, B.; Mao, Q.; Yang, H.; Liu, B.; Wang, Y. Reversible hydrogen spillover in Ru-WO3-x enhances hydrogen evolution activity in neutral pH water splitting. Nat. Commun. 2022, 13, 1–10. [Google Scholar] [CrossRef]
- Mineo, G.; Bruno, L.; Bruno, E.; Mirabella, S. WO3 Nanorods Decorated with Very Small Amount of Pt for Effective Hydrogen Evolution Reaction. Nanomaterials 2023, 13, 1071. [Google Scholar] [CrossRef]
- Jaiswal, M.K.; Deka, S.; Kalita, T.; Kalita, D.J.; Choudhury, B. The Role of W ↔ N Electron Transport Channel in Potassium-Doped WO3/g-C3N4 Nanocomposites for Light-Assisted Electrocatalytic Oxygen and Hydrogen Evolution Reactions: Decoding the S-Scheme Mechanism. ACS Sustain. Chem. Eng. 2024, 12, 14670–14683. [Google Scholar] [CrossRef]
- Gong, Z.; Chen, Z.; Li, H.; Yang, G.; Yuan, M.; Li, Z.; Wan, X.; Cui, Y. Palladium single atoms from melting nanoparticles on WO3− for boosted hydrogen evolution reaction. J. Energy Chem. 2023, 91, 637–644. [Google Scholar] [CrossRef]
- Jacquline, L.S.; Elamurugu, E. Tungsten based nanostructured hybrid electrocatalysts in neutral medium for hydrogen evolution reaction. Int. J. Hydrogen Energy 2024, 89, 1049–1059. [Google Scholar] [CrossRef]
- Jeon, S.; Im, M.; Kim, K.; Kim, D.; Han, J.-I. Electrochemically-mediated reactive separation of nitric oxide into nitrate using iron chelate. Chemosphere 2023, 341, 140026. [Google Scholar] [CrossRef]
- Soto-Hernández, J.; Santiago-Ramirez, C.; Ramirez-Meneses, E.; Luna-Trujillo, M.; Wang, J.-A.; Lartundo-Rojas, L.; Manzo-Robledo, A. Electrochemical reduction of NOx species at the interface of nanostructured Pd and PdCu catalysts in alkaline conditions. Appl. Catal. B Environ. 2019, 259. [Google Scholar] [CrossRef]
- Vázquez-Bautista, S.; Ramírez-Meneses, E.; Manzo-Robledo, A.; Zacahua-Tlacuatl, G.; Lartundo-Rojas, L.; Acosta-Jara, J.; Pedraza-Segura, L.; Luna-Trujillo, M. Electro-reduction of NOx-species in alkaline medium at modified carbon-supported palladium nanoparticles with variable concentration of C-sp2: An in-situ mass-spectrometry approach. Appl. Catal. B Environ. 2022, 320. [Google Scholar] [CrossRef]
- Dorhout, J.M.; Anderson, A.S.; Batista, E.; Carlson, R.K.; Currier, R.P.; Martinez, R.K.; Clegg, S.M.; Wilkerson, M.P.; Nowak-Lovato, K. NOx speciation from copper dissolution in nitric acid/water solutions using FTIR spectroscopy. J. Mol. Spectrosc. 2020, 372, 111334. [Google Scholar] [CrossRef]
- Sander, R. Compilation of Henry’s law constants (version 5.0.0) for water as solvent. Atmos. Chem. Phys. 2023, 23, 10901–12440. [Google Scholar] [CrossRef]
- Sakthivel, M.; Drillet, J.-F. An extensive study about influence of the carbon support morphology on Pt activity and stability for oxygen reduction reaction. Appl. Catal. B Environ. 2018, 231, 62–72. [Google Scholar] [CrossRef]
- Holade, Y.; Morais, C.; Servat, K.; Napporn, T.W.; Kokoh, K.B. Enhancing the available specific surface area of carbon supports to boost the electroactivity of nanostructured Pt catalysts. Phys. Chem. Chem. Phys. 2014, 16, 25609–25620. [Google Scholar] [CrossRef]
- Storck, S.; Bretinger, H.; Maier, W.F. Characterization of micro- and mesoporous solids by physisorption methods and pore-size analysis. Appl. Catal. A Gen. 1998, 174, 137–146. [Google Scholar] [CrossRef]
- Liu, S.-C.; Yue, Z.-F.; Liu, Y. Mesoporous carbon–ZrO2 composites prepared using thermolysis of zirconium based metal–organic frameworks and their adsorption properties. J. Porous Mater. 2015, 22, 465–471. [Google Scholar] [CrossRef]
- Scherrer, P. Bestimmung der Grosse und Inneren Struktur von Kolloidteilchen Mittels Rontgenstrahlen, Nach Ges Wiss Gottingen 2 (1918) 8–100. Available online: https://cir.nii.ac.jp/crid/1573105975714168704 (accessed on 16 February 2025).
- Ferrero, G.A.; Preuss, K.; Marinovic, A.; Jorge, A.B.; Mansor, N.; Brett, D.J.L.; Fuertes, A.B.; Sevilla, M.; Titirici, M.-M. Fe–N-Doped Carbon Capsules with Outstanding Electrochemical Performance and Stability for the Oxygen Reduction Reaction in Both Acid and Alkaline Conditions. ACS Nano 2016, 10, 5922–5932. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Dong, Y.; Zhang, Y.; Wang, B.; Yuan, Q.; Du, H.; Zhao, J. Insights into the role of an Fe–N active site in the oxygen reduction reaction on carbon-supported supramolecular catalysts. RSC Adv. 2020, 10, 8709–8716. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.M.; Cao, G.-P.; Al-Fatesh, A.S.; Alreshaidan, S.B.; Ali, N.; Shehu, M.S.; Yan, J.-Y. Highly efficient platinum nano-particles decorated multi-walled carbon nanotubes (Pt/MWCNTs) catalyst for catalytic hydrogenation of styrene–butadiene–styrene (SBS) copolymer. Arab. J. Chem. 2024, 17. [Google Scholar] [CrossRef]
- Abdulaziz, M.; Petruk, A.; George, T.; Shaw, N.; Sciaini, G.; Trevani, L. Exploring Platinum Thin Films as Electrodes for High-Temperature and -Pressure Electrochemical Studies in Aqueous Systems. Electrochem. Sci. Adv. 2024. [Google Scholar] [CrossRef]
- Bumajdad, A.; Nazeer, A.A.; Al Sagheer, F.; Nahar, S.; Zaki, M.I. Controlled Synthesis of ZrO2 Nanoparticles with Tailored Size, Morphology and Crystal Phases via Organic/Inorganic Hybrid Films. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Xu, T.; Adamsen, K.C.; Li, Z.; Lammich, L.; Lauritsen, J.V.; Wendt, S. WO3 Monomers Supported on Anatase TiO2(101), −(001), and Rutile TiO2(110): A Comparative STM and XPS Study. J. Phys. Chem. C 2022, 126, 2493–2502. [Google Scholar] [CrossRef]
- Kim, H.-W.; Jin, J.; Kim, M.; Kim, K.; Han, J.-I. Direct recovery of electro-synthesized ammonia from low-concentration nitric oxide using pulse electrodeposited Cu/C catalyst in a catholyte-free system. Chem. Eng. J. 2024, 485. [Google Scholar] [CrossRef]
- de Vooys, A.; Beltramo, G.; van Riet, B.; van Veen, J.; Koper, M. Mechanisms of electrochemical reduction and oxidation of nitric oxide. Electrochim. Acta 2004, 49, 1307–1314. [Google Scholar] [CrossRef]
- Beltramo, G.L.; Koper, M.T.M. Nitric Oxide Reduction and Oxidation on Stepped Pt[n(111)×(111)] Electrodes. Langmuir 2003, 19, 8907–8915. [Google Scholar] [CrossRef]
- Liu, H.; Bai, L.; Bergmann, A.; Cuenya, B.R.; Luo, J. Electrocatalytic reduction of nitrogen oxide species to ammonia. Chem 2024, 10, 2963–2986. [Google Scholar] [CrossRef]
- Saadatjou, N.; Jafari, A.; Sahebdelfar, S. Ruthenium Nanocatalysts for Ammonia Synthesis: A Review. Chem. Eng. Commun. 2014, 202, 420–448. [Google Scholar] [CrossRef]
- Delagrange, S.; Schuurman, Y. HCN synthesis from methane and ammonia over platinum. Catal. Today 2007, 121, 204–209. [Google Scholar] [CrossRef]
- Huang, Z.; Yang, B.; Zhou, Y.; Luo, W.; Chen, G.; Liu, M.; Liu, X.; Ma, R.; Zhang, N. Tungsten Nitride/Tungsten Oxide Nanosheets for Enhanced Oxynitride Intermediate Adsorption and Hydrogenation in Nitrate Electroreduction to Ammonia. ACS Nano 2023, 17, 25091–25100. [Google Scholar] [CrossRef]
- Sahu, V.; Shekhar, S.; Ahuja, P.; Gupta, G.; Singh, S.K.; Sharma, R.K.; Singh, G. Synthesis of hydrophilic carbon black; role of hydrophilicity in maintaining the hydration level and protonic conduction. RSC Adv. 2013, 3, 3917–3924. [Google Scholar] [CrossRef]
- Li, H.; Abdelgaid, M.; Paudel, J.R.; Holzapfel, N.P.; Augustyn, V.; McKone, J.R.; Mpourmpakis, G.; Crumlin, E.J. Operando Unveiling of Hydrogen Spillover Mechanisms on Tungsten Oxide Surfaces. J. Am. Chem. Soc. 2025. [Google Scholar] [CrossRef]
- Papakonstantinou, G.D.; Jaksic, J.M.; Labou, D.; Siokou, A.; Jaksic, M.M. Spillover Phenomena and Its Striking Impacts in Electrocatalysis for Hydrogen and Oxygen Electrode Reactions. Adv. Phys. Chem. 2011, 2011. [Google Scholar] [CrossRef]
- Rajalakshmi, R.; Srividhya, G.; Viswanathan, C.; Ponpandian, N. Hydrogen spillover effect–harnessing hydrogen evolution reaction from diverse carbon-based supports with a tungsten oxide catalyst. J. Mater. Chem. A 2023, 11, 15889–15905. [Google Scholar] [CrossRef]

| Material | BET Specific Area (m2g−1) | BJH Pore Diameter (nm) |
|---|---|---|
| Vulcan carbon | 240 [22] | 2 [22] |
| Vulcan carbon 350 °C | 352 | 2.5 |
| ZWC | 96 | 3 |
| Material | PtEtek | PtC | PtZWC |
|---|---|---|---|
| Pt wt.% (EDS) | 15.02 | 10.4 | 8.218 |
| ECSAH (m2g−1pt) | 39.56 | 27.14 | 37.51 |
| ECSACO (m2g−1pt) | 81.55 | 57.43 | 73.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santiago-Ramírez, C.R.; Hernández-Pichardo, M.L.; Manzo-Robledo, A.; Acuña-Leal, D.A.; Gracia-Pinilla, M.A. Transition Metal Oxides (WO3-ZrO2) as Promoters and Hydrogen Adsorption Modulators in Pt/WO3-ZrO2-C Electrocatalyst for the Reduction of NOx. Electrochem 2025, 6, 7. https://doi.org/10.3390/electrochem6010007
Santiago-Ramírez CR, Hernández-Pichardo ML, Manzo-Robledo A, Acuña-Leal DA, Gracia-Pinilla MA. Transition Metal Oxides (WO3-ZrO2) as Promoters and Hydrogen Adsorption Modulators in Pt/WO3-ZrO2-C Electrocatalyst for the Reduction of NOx. Electrochem. 2025; 6(1):7. https://doi.org/10.3390/electrochem6010007
Chicago/Turabian StyleSantiago-Ramírez, Claudia R., Martha L. Hernández-Pichardo, Arturo Manzo-Robledo, Daniel A. Acuña-Leal, and Miguel A. Gracia-Pinilla. 2025. "Transition Metal Oxides (WO3-ZrO2) as Promoters and Hydrogen Adsorption Modulators in Pt/WO3-ZrO2-C Electrocatalyst for the Reduction of NOx" Electrochem 6, no. 1: 7. https://doi.org/10.3390/electrochem6010007
APA StyleSantiago-Ramírez, C. R., Hernández-Pichardo, M. L., Manzo-Robledo, A., Acuña-Leal, D. A., & Gracia-Pinilla, M. A. (2025). Transition Metal Oxides (WO3-ZrO2) as Promoters and Hydrogen Adsorption Modulators in Pt/WO3-ZrO2-C Electrocatalyst for the Reduction of NOx. Electrochem, 6(1), 7. https://doi.org/10.3390/electrochem6010007






