Binary Biocompatible CNC–Gelatine Hydrogel as 3D Scaffolds Suitable for Cell Culture Adhesion and Growth
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
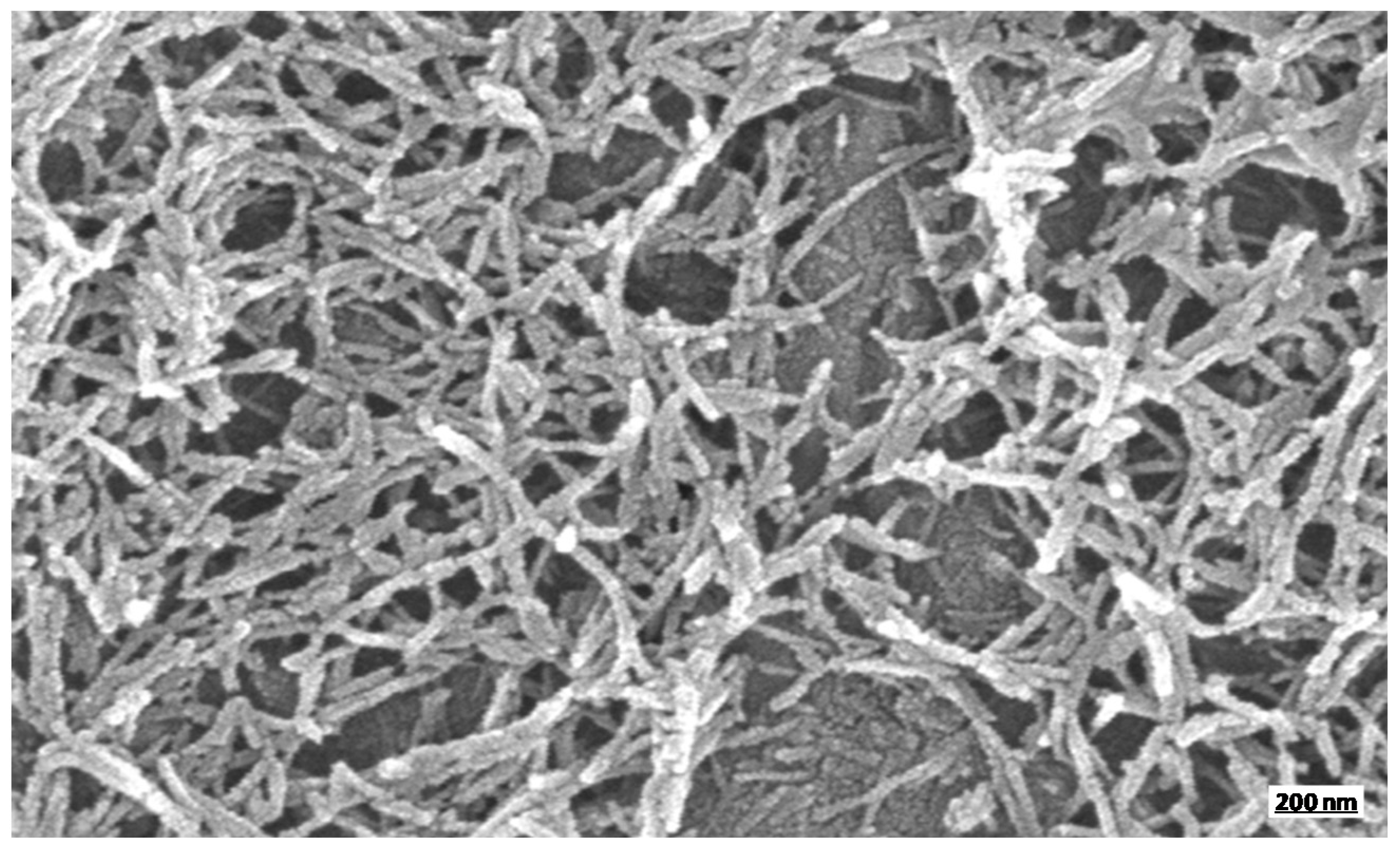
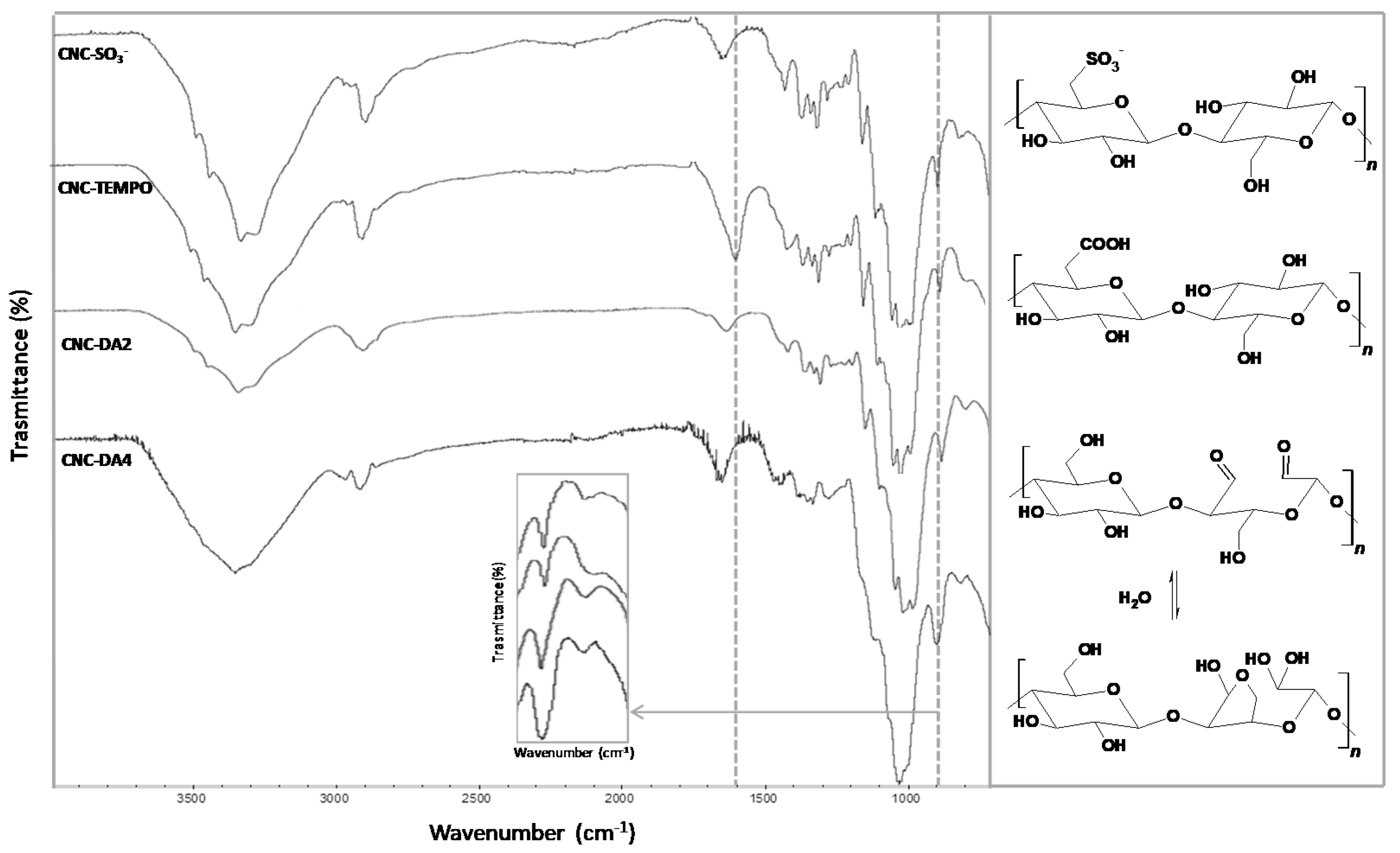
3.1. CNCs Preparation
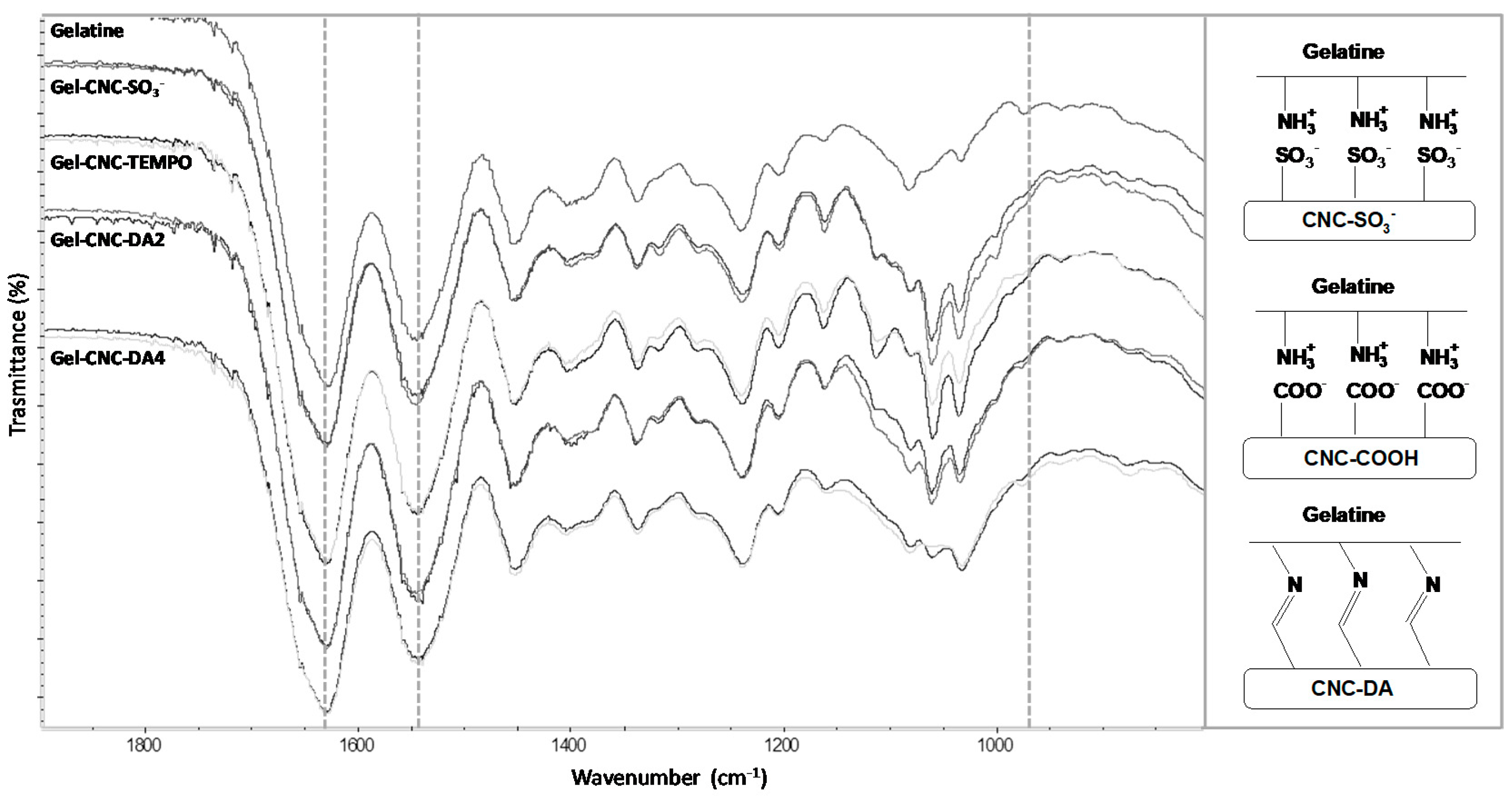
3.2. Hydrogels
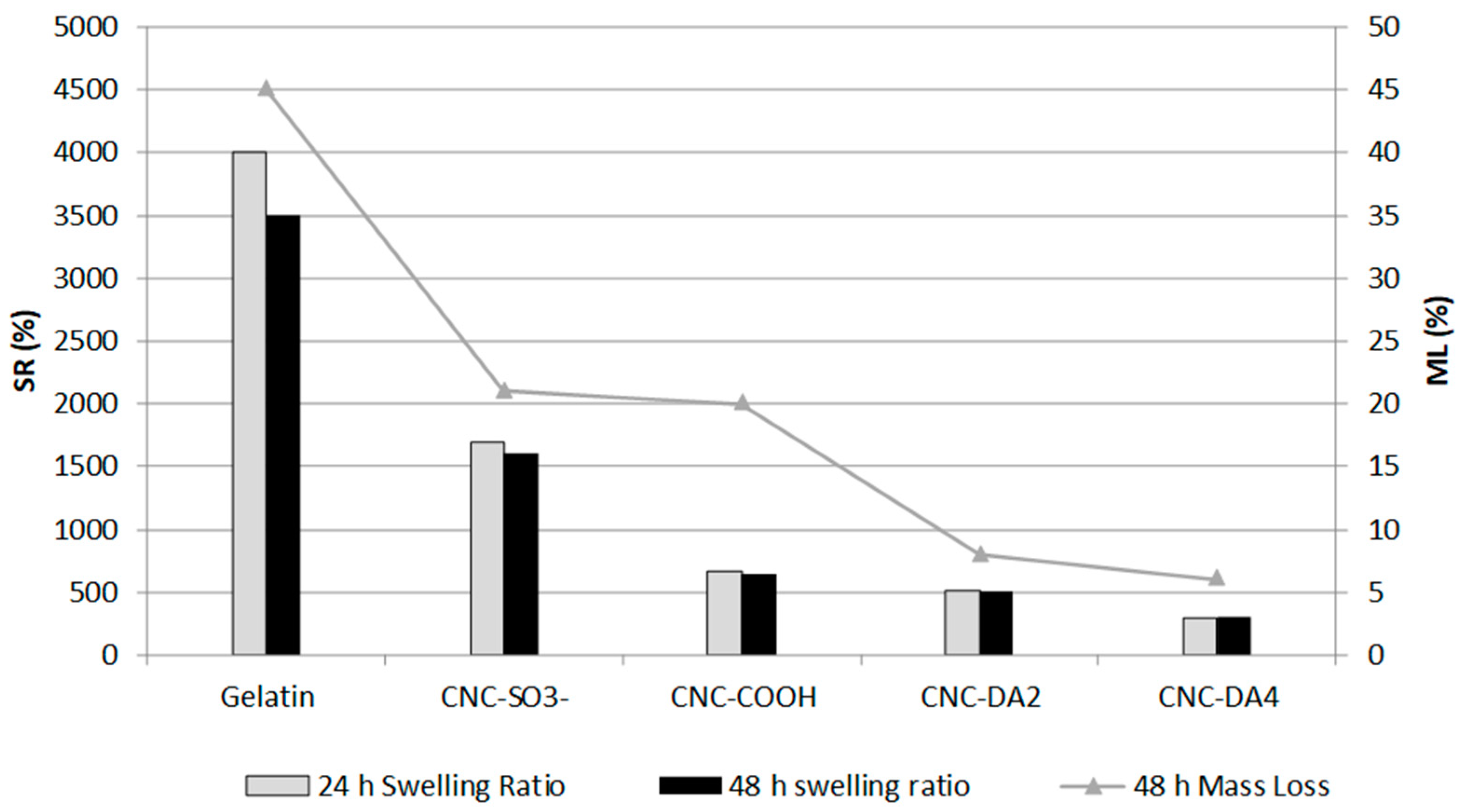
3.3. Swelling Behavior and Stability
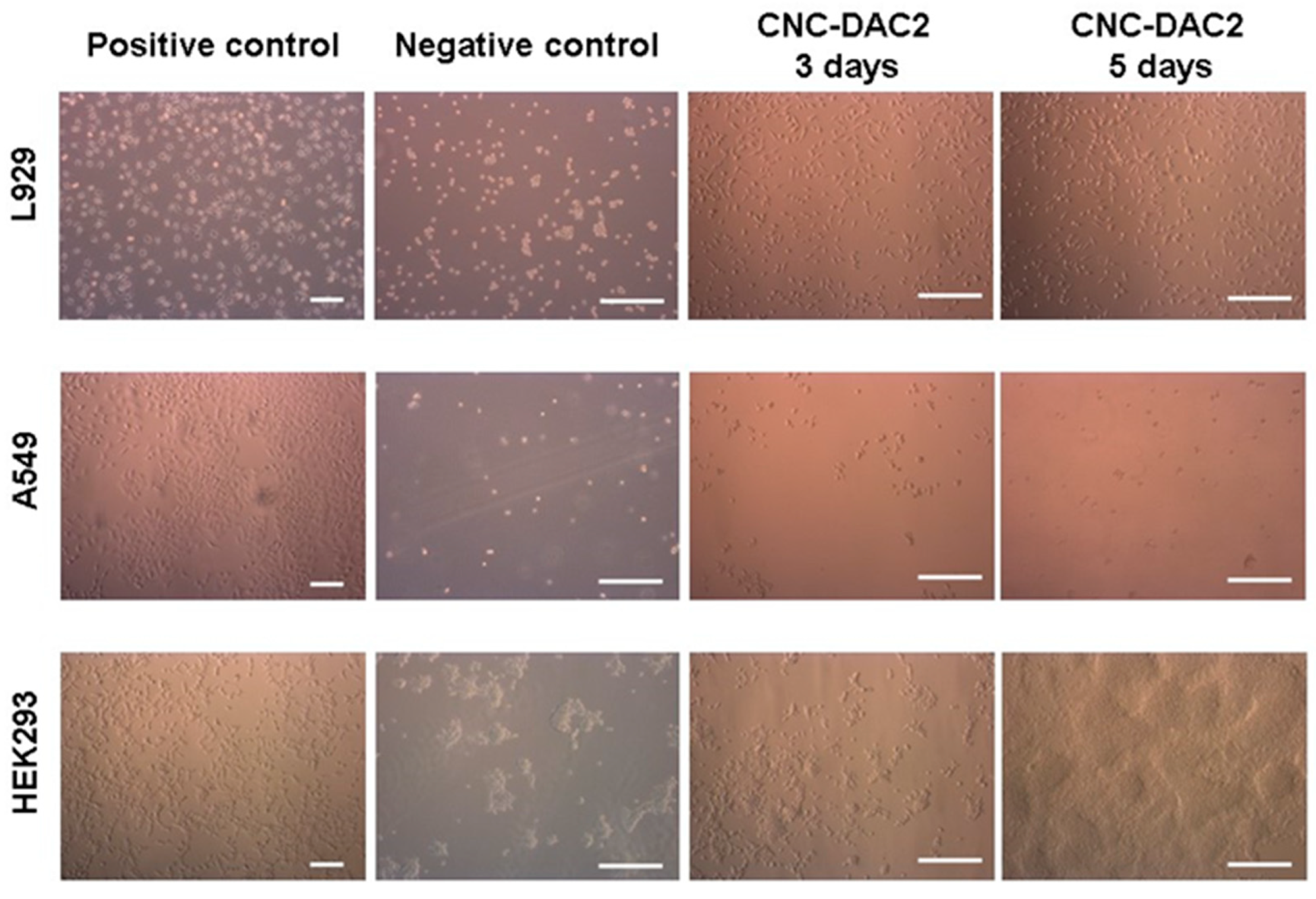
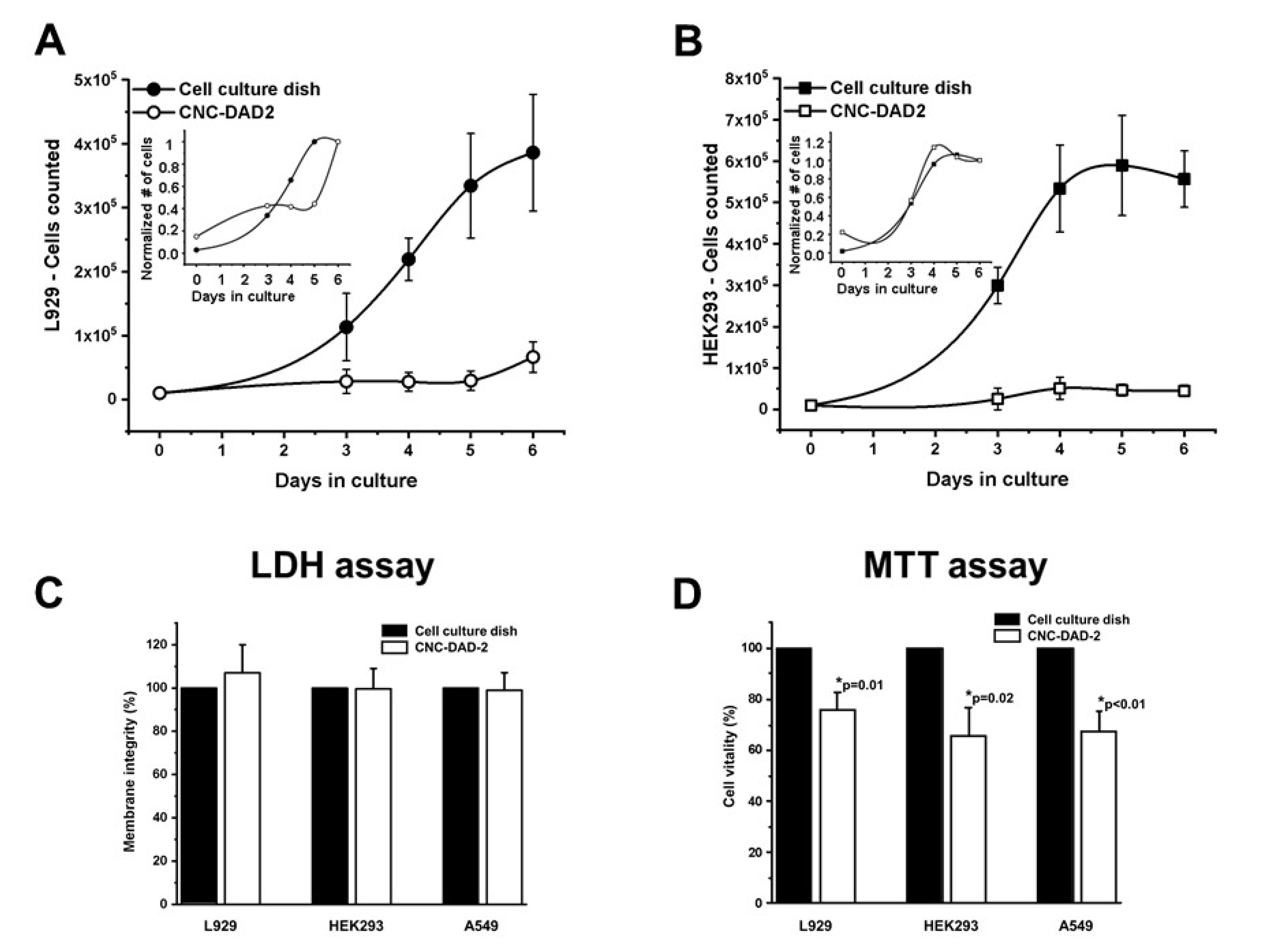
3.4. Cell Adhesion and Growth
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sharma, C.; Dinda, A.K.; Mishra, N.C. Fabrication and characterization of natural origin chitosan-gelatin-alginate composite scaffold by foaming method without using surfactant. J. Appl. Polym. Sci. 2013, 127, 3228–3241. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, X.; Yuk, H.; Lin, S.; Liu, X.; Parada, G. Soft Materials by Design: Unconventional Polymer Networks Give Extreme Properties. Chem. Rev. 2021, 121, 4309–4372. [Google Scholar] [CrossRef]
- Ramos, T.; Moroni, L. The Role of Biofabrication—A Year in Review. Tissue Eng. Regen. Med. 2019, 26, 2. [Google Scholar]
- Fagerholm, P.; Lagali, N.S.; Ong, J.A.; Merrett, K.; Jackson, W.B.; Polarek, J.W.; Suuronen, E.J.; Liu, Y.; Brunette, I.; Griffith, M. Stable corneal regeneration four years after implantation of a cell-free recombinant human collagen scaffold. Biomaterials 2014, 35, 2420–2427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacques, E.; Hosoyama, K.; Biniam, B.; Eren Cimenci, C.; Sedlakova, V.; Steeves, A.J.; Variola, F.; Davis, D.R.; Stewart, D.J.; Suuronen, E.J.; et al. Collagen-Based Microcapsules As Therapeutic Materials for Stem Cell Therapies in Infarcted Myocardium. ACS Biomater. Sci. Eng. 2020, 6, 4614–4622. [Google Scholar] [CrossRef]
- Kilmer, C.E.; Battistoni, C.M.; Cox, A.; Breur, G.J.; Panitch, A.; Liu, J.C. Collagen Type I and II Blend Hydrogel with Autologous Mesenchymal Stem Cells as a Scaffold for Articular Cartilage Defect Repair. ACS Biomater. Sci. Eng. 2020, 6, 3464–3476. [Google Scholar] [CrossRef] [PubMed]
- Stevens, K.R.; Einerson, N.J.; Burmania, J.A.; Kao, W.J. In vivo biocompatibility of gelatin-based hydrogels and interpenetrating networks. J. Biomater. Sci. Polym. Ed. 2002, 13, 1353. [Google Scholar] [CrossRef]
- Liu, X.; Smith, L.A.; Hu, J.; Ma, P.X. Biomimetic nanofibrous gelatin/apatite composite scaffolds for bone tissue engineering. Biomaterials 2009, 30, 2252–2258. [Google Scholar] [CrossRef] [Green Version]
- Gulrez, S.K.; Al-Assaf, S.; Phillips, G.O. Hydrogels: Methods of preparation, characterisation and applications. In Progress in Molecular and Environmental Bioengineering from Analysis and Modelling to Technology Applications; InTech: London, UK, 2011. [Google Scholar]
- Patel, D.K.; Dutta, S.D.; Shin, W.-C.; Ganguly, K.; Lim, K.-T. Fabrication and characterization of 3D printable nanocellulose-based hydrogels for tissue engineering. RSC Adv. 2021, 11, 7466–7478. [Google Scholar] [CrossRef]
- Yang, C.; Xu, L.; Zhou, Y.; Zhang, X.; Huang, X.; Wang, M.; Han, Y.; Zhai, M.; Wei, S.; Li, J. A green fabrication approach of gelatin/CM-chitosan hybrid hydrogel for wound healing. Carbohydr. Polym. 2010, 82, 1297–1305. [Google Scholar] [CrossRef]
- Colombo, L.; Zoia, L.; Violatto, M.B.; Previdi, S.; Talamini, L.; Sitia, L.; Nicotra, F.; Orlandi, M.; Salmona, M.; Bigini, P.; et al. Organ Distribution and Bone Tropism of Cellulose Nanocrystals in Living Mice. Biomacromolecules 2015, 16, 2862–2871. [Google Scholar] [CrossRef]
- Zoia, L.; Morelli, A.; Talamini, L.; Violatto, M.B.; Lovati, A.B.; Lopa, S.; Recordati, C.; Toffanin, C.; Salanti, A.; Russo, L.; et al. Cellulose Nanocrystals: A multimodal tool to enhance the targeted drug delivery against bone disorders. Nanomedicine 2020, 15, 2271–2285. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, G.; Munizza, L.; Zampolli, J.; Forcella, M.; Zoia, L.; Fusi, P.; Di Gennaro, P.; La Ferla, B. Cellulose Nano Crystals as Effective Inhibitors of host cell bacteria adhesion. J. Mater. Chem. B. 2017, 5, 7018–7020. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Zhou, J.-P.; Xu, X.-D.; Jiang, Y.-N.; Shi, H.-C.; Zhao, G.-Q. Research on 3D bio-printing molding technology of tissue engineering scaffold by nanocellulose/gelatin hydrogel composite. BioResource 2019, 14, 9244–9257. [Google Scholar]
- Hivechi, A.; Bahrami, S.H.; Siegel, R.A. Investigation of morphological, mechanical and biological properties of cellulose nanocrystal reinforced electrospun gelatin nanofibers. Int. J. Biol. Macromol. 2019, 124, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Ishak, W.H.W.; Ahmad, I.; Ramli, S.; Amin, M.C.I.M. Gamma Irradiation-Assisted Synthesis of Cellulose Nanocrystal-Reinforced Gelatin Hydrogels. Nanomaterials 2018, 8, 749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwak, H.W.; Lee, H.; Park, S.; Lee, M.F.; Jin, H.-J. Chemical and physical reinforcement of hydrophilic gelatin film with dialdehyde nanocellulose. Int. J. Biol. Macromol. 2020, 146, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhou, J.; Yang, Z.; Liu, D.; Xv, X.; Zhao, G.; Shi, H.; Zhang, Q. Dialdehyde cellulose nanocrystal/gelatin hydrogel optimized for 3D printing applications. J. Mater. Sci. 2018, 53, 11883–11900. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, J.; Jiang, J.; Zhang, Q.; Shi, H.; Liu, D. 3D printing process of oxidized nanocellulose and gelatin scaffold. J. Biomater. Sci. Polym. Ed. 2018, 29, 1498–1513. [Google Scholar] [CrossRef]
- Saito, T.; Isogai, A. Tempo-mediated oxidation of native cellulose. The effect of oxidation conditions on chemical and crystal structures of the water-insoluble fractions. Biomacromolecules 2004, 5, 1983–1989. [Google Scholar] [CrossRef]
- Huang, C.-F.; Chen, J.-K.; Tsai, T.-Y.; Hsieh, Y.-A.; Andrew Lin, K.-Y. Dual-functionalized cellulose nanofibrils prepared through TEMPO-mediated oxidation and surface-initiated ATRP. Polymer 2015, 72, 395–405. [Google Scholar] [CrossRef]
- Stepanenko, A.A.; Dmitrenko, V.V. HEK293 in cell biology and cancer research: Phenotype, karyotype, tumorigenicity, and stress-induced genome-phenotype evolution. Gene 2015, 569, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Orlando, A.; Re, F.; Sesana, S.; Rivolta, I.; Panariti, A.; Brambilla, D.; Nicolas, J.; Couvreur, P.; Andrieux, K.; Masserini, M.; et al. Effect of nanoparticles binding β-amyloid peptide on nitric oxide production by cultured endothelial cells and macrophages. Int. J. Nanomed. 2013, 8, 1335–1347. [Google Scholar]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose Nanocrystals: Chemistry, Self-Assembly, and Applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, J.; Zheng, X.; Zhang, A.; Zhang, X.; Tang, K. Pullulan dialdehyde crosslinked gelatin hydrogels with high strength for biomedical applications. Carbohydr. Polym. 2019, 216, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Plappert, S.F.; Quraishi, S.; Pircher, N.; Mikkonen, S.K.; Veigel, S.; Klinger, K.M.; Potthast, A.; Rosenau, T.; Liebner, F.W. Transparent, Flexible, and Strong 2,3-Dialdehyde Cellulose Films with High Oxygen Barrier Properties. Biomacromolecules 2018, 19, 2969–2978. [Google Scholar] [CrossRef]
- Wong, T.; McGrath, J.A.; Navsaria, H. The role of fibroblasts in tissue engineering and regeneration. Br. J. Dermatol. 2007, 156, 1149–1155. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zoia, L.; Binda, A.; Cipolla, L.; Rivolta, I.; La Ferla, B. Binary Biocompatible CNC–Gelatine Hydrogel as 3D Scaffolds Suitable for Cell Culture Adhesion and Growth. Appl. Nano 2021, 2, 118-127. https://doi.org/10.3390/applnano2020010
Zoia L, Binda A, Cipolla L, Rivolta I, La Ferla B. Binary Biocompatible CNC–Gelatine Hydrogel as 3D Scaffolds Suitable for Cell Culture Adhesion and Growth. Applied Nano. 2021; 2(2):118-127. https://doi.org/10.3390/applnano2020010
Chicago/Turabian StyleZoia, Luca, Anna Binda, Laura Cipolla, Ilaria Rivolta, and Barbara La Ferla. 2021. "Binary Biocompatible CNC–Gelatine Hydrogel as 3D Scaffolds Suitable for Cell Culture Adhesion and Growth" Applied Nano 2, no. 2: 118-127. https://doi.org/10.3390/applnano2020010
APA StyleZoia, L., Binda, A., Cipolla, L., Rivolta, I., & La Ferla, B. (2021). Binary Biocompatible CNC–Gelatine Hydrogel as 3D Scaffolds Suitable for Cell Culture Adhesion and Growth. Applied Nano, 2(2), 118-127. https://doi.org/10.3390/applnano2020010








