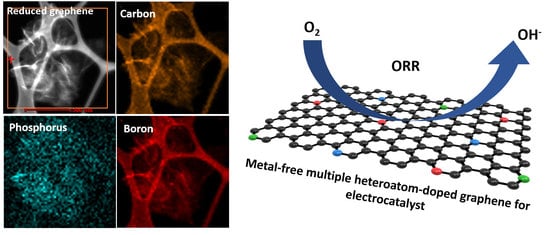
Importance of Doping Sequence in Multiple Heteroatom-Doped Reduced Graphene Oxide as Efficient Oxygen Reduction Reaction Electrocatalysts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Ternary-Doped RGO
2.2. Structural Characterizations
2.3. Electrochemical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Takanabe, K. Photocatalytic water splitting: Quantitative approaches toward photocatalyst by design. ACS Catal. 2017, 7, 8006–8022. [Google Scholar] [CrossRef]
- Strasser, P. Free electrons to molecular bonds and back: Closing the energetic oxygen reduction (ORR)-oxygen evolution (OER) cycle using core-shell nanoelectrocatalysts. Acc. Chem. Res. 2016, 49, 2658–2668. [Google Scholar] [CrossRef]
- Yadegari, H.; Norouzi Banis, M.; Lushington, A.; Sun, Q.; Li, R.; Sham, T.K.; Sun, X. A bifunctional solid state catalyst with enhanced cycling stability for Na and Li-O2 cells: Revealing the role of solid state catalysts. Energy Env. Sci. 2017, 10, 286–295. [Google Scholar] [CrossRef]
- Wang, Z.L.; Xu, D.; Xu, J.J.; Zhang, X.B. Oxygen electrocatalysts in metal-air batteries: From aqueous to nonaqueous electrolytes. Chem. Soc. Rev. 2014, 43, 7746–7786. [Google Scholar] [CrossRef]
- Yan, Y.; Shin, W.I.; Chen, H.; Lee, S.-M.; Manickam, S.; Hanson, S.; Zhao, H.; Lester, E.; Wu, T.; Pang, C.H. A recent trend: Application of graphene in catalyst. Carbon Lett. 2021, 31, 177–199. [Google Scholar] [CrossRef]
- Debe, M.K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 2012, 486, 43–51. [Google Scholar] [CrossRef]
- Steele, B.C.H.; Heinzel, A. Materials for fuel-cell technologies. Nature 2001, 414, 345–352. [Google Scholar] [CrossRef]
- Serov, A.; Kwak, C. Review of non-platinum anode catalysts for DMFC and PEMFC application. Appl. Catal. 2009, 90, 313–320. [Google Scholar] [CrossRef]
- Sun, Y.Q.; Wu, Q.O.; Shi, G.Q. Graphene based new energy materials. Energy Environ. Sci. 2011, 4, 1113–1132. [Google Scholar] [CrossRef]
- Liu, X.; Dai, L. Carbon-based metal-free catalysts. Nat. Rev. Mater. 2016, 1, 16064. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, C.G.; Hu, Y.; Cheng, H.H.; Shi, G.Q.; Qu, L.T. A versatile, ultralight, nitrogen-doped graphene framework. Angew. Chem. Int. Ed. 2012, 51, 11371–11375. [Google Scholar] [CrossRef]
- Lai, L.; Potts, J.R.; Zhan, D.; Wang, L.; Poh, C.K.; Tang, C.; Gong, H.; Shen, Z.; Lin, J.; Ruoff, R.S. Exploration of the active center structure of nitrogen-doped graphene-based catalysts for oxygen reduction reaction. Energy Environ. Sci. 2012, 5, 7936–7942. [Google Scholar] [CrossRef]
- Zhou, Y.Z.; Yen, C.H.; Fu, S.F.; Yang, G.H.; Zhu, C.Z.; Du, D.; Wo, P.C.; Cheng, X.N.; Yang, J.; Wai, C.M.; et al. One-pot synthesis of b-doped three-dimensional reduced graphene oxide via supercritical fluid for oxygen reduction reaction. Green Chem. 2015, 17, 3552–3560. [Google Scholar] [CrossRef]
- Li, R.; Wei, Z.D.; Gou, X.L.; Xu, W. Phosphorus-doped graphene nanosheets as efficient metal-free oxygen reduction electrocatalysts. RSC Adv. 2013, 3, 9978–9984. [Google Scholar] [CrossRef]
- Zhang, C.Z.; Mahmood, N.; Yin, H.; Liu, F.; Hou, Y.L. Synthesis of phosphorus-doped graphene and its multifunctional applications for oxygen reduction reaction and lithium ion batteries. Adv. Mater. 2013, 25, 4932–4937. [Google Scholar] [CrossRef]
- Yang, Z.; Yao, Z.; Li, G.F.; Fang, G.Y.; Nie, H.G.; Liu, Z.; Zhou, X.M.; Chen, X.; Huang, S.M. Sulfur-doped graphene as an efficient metal-free cathode catalyst for oxygen reduction. ACS Nano 2012, 6, 205–211. [Google Scholar] [CrossRef]
- Chen, X.; Chen, X.H.; Xu, X.; Yang, Z.; Liu, Z.; Zhang, L.J.; Xu, X.J.; Chen, Y.; Huang, S.M. Sulfur-doped porous reduced graphene oxide hollow nanosphere frameworks as metal-free electrocatalysts for oxygen reduction reaction and as supercapacitor electrode materials. Nanoscale 2014, 6, 13740–13747. [Google Scholar] [CrossRef]
- Gong, Y.J.; Fei, H.L.; Zou, X.L.; Zhou, W.; Yang, S.B.; Ye, G.L.; Liu, Z.; Peng, Z.W.; Lou, J.; Vajtai, R.; et al. Boron- and nitrogen-substituted graphene nanoribbons as efficient catalysts for oxygen reduction reaction. Chem. Mater. 2015, 27, 1181–1186. [Google Scholar] [CrossRef]
- Pei, Y.; Song, H.; Liu, Y.; Cheng, Y.; Li, W.; Chen, Y.; Fan, Y.; Liu, B.; Lu, S. Boron-nitrogen-doped carbon dots on multi-walled carbon nanotubes for efficient electrocatalysis of oxygen reduction reactions. J. Colloid Interface Sci. 2021, 600, 865–871. [Google Scholar] [CrossRef]
- Ai, W.; Luo, Z.M.; Jiang, J.; Zhu, J.H.; Du, Z.Z.; Fan, Z.X.; Xie, L.H.; Zhang, H.; Huang, W.; Yu, T. Nitrogen and sulfur codoped graphene: Multifunctional electrode materials for high performance Li-ion batteries and oxygen reduction reaction. Adv. Mater. 2014, 26, 6186–6192. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, C.G.; Song, L.; Wang, L.X.; Shi, G.Q.; Dai, L.M.; Qu, L.T. Functional graphene nanomesh foam. Energy Environ. Sci. 2014, 7, 1913–1918. [Google Scholar] [CrossRef]
- Xu, J.X.; Dong, G.F.; Jin, C.H.; Huang, M.H.; Guan, L.H. Sulfur and nitrogen co-doped, few-layered graphene oxide as a highly efficient electrocatalyst for the oxygen reduction reaction. ChemSusChem 2013, 6, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.H.; Chung, M.W.; Kwon, H.C.; Park, S.H.; Woo, S.I. B, N- and P, N-doped graphene as highly active catalysts for oxygen reduction reactions in acidic media. J. Mater. Chem. A 2013, 1, 3694–3699. [Google Scholar] [CrossRef]
- Wang, C.L.; Zhou, Y.; Sun, L.; Zhao, Q.; Zhang, X.; Wan, P.; Qiu, J.S. N/P-codoped thermally reduced graphene for high-performance supercapacitor applications. J. Phys. Chem. C 2013, 117, 14912–14919. [Google Scholar] [CrossRef]
- Li, R.; Wei, Z.D.; Gou, X.L. Nitrogen and phosphorus dual-doped graphene/carbon nanosheets as bifunctional electrocatalysts for oxygen reduction and evolution. ACS Catal. 2015, 5, 4133–4142. [Google Scholar] [CrossRef]
- Lin, H.L.; Chu, L.; Wang, X.J.; Yao, Z.Q.; Liu, F.; Ai, Y.N.; Zhuang, X.D.; Han, S. Boron, nitrogen, and phosphorus ternary doped graphene aerogel with hierarchically porous structure as highly efficient electrocatalysts for oxygen reduction reaction. N. J. Chem. 2016, 40, 6022–6029. [Google Scholar] [CrossRef]
- Feicht, P.; Eigler, S. Defects in graphene oxide as structural motifs. ChemNanoMat 2018, 4, 244–252. [Google Scholar] [CrossRef]
- Wang, S.Y.; Yu, D.S.; Dai, L.M.; Chang, D.W.; Baek, J.B. Polyelectrolyte-functionalized graphene as metal-free electrocatalysts for oxygen reduction. ACS Nano 2011, 5, 6202–6209. [Google Scholar] [CrossRef]
- Paulus, U.A.; Wokaun, A.; Scherer, G.G.; Schmidt, T.J.; Stamenkovic, V.; Radmilovic, V.; Markovic, N.M.; Ross, P.N. Oxygen reduction on carbon-supported Pt-Ni and Pt-Co alloy catalyst. J. Phys. Chem. B 2002, 106, 4181–4191. [Google Scholar] [CrossRef]
- Fujisawa, K.; Hayashi, T.; Endo, M.; Terrones, M.; Kim, J.H.; Kim, Y.A. Effect of boron doping on the electrical conductivity of metallicity-separated single walled carbon nanotubes. Nanoscale 2018, 10, 12723. [Google Scholar] [CrossRef]
- Maldonado, S.; Morin, S.; Stevenson, K.J. Structure, composition, and chemical reactivity of carbon nanotubes by selective nitrogen doping. Carbon 2006, 44, 1429–1437. [Google Scholar] [CrossRef]
- Pels, J.; Kapteijn, F.; Moulijn, J.; Zhu, Q.; Thomas, K. Evolution of nitrogen functionalities in carbonaceous materials during pyrolysis. Carbon 1995, 33, 1641–1653. [Google Scholar] [CrossRef]
- Wu, J.; Zheng, X.; Jin, C.; Tian, J.; Yang, R. Ternary doping of phosphorus, nitrogen, and sulfur into porous carbon for enhancing electrocatalytic reduction. Carbon 2015, 92, 327–338. [Google Scholar] [CrossRef]
- Chadha, N.; Sharma, R.; Saini, P. A new insight into the structural modulation of graphene oxide upon chemical reduction probed by Raman spectroscopy and X-ray diffraction. Carbon Lett. 2021. [Google Scholar] [CrossRef]
- Cuesta, A.; Dhamelincourt, P.; Laureyns, J.; Martinez-Alonso, A.; Tascon, J.M.D. Raman microprobe studies on carbon materials. Carbon 1994, 32, 1523–1532. [Google Scholar] [CrossRef]
- Panomsuwan, G.; Saito, N.; Isizaki, T. Electrocatalytic oxygen reduction on nitrogen-doped carbon nanoparticles derived from cyano-aromatic molecules via a solution plasma approach. Carbon 2016, 98, 411–420. [Google Scholar] [CrossRef]
- Ha, S.; Choi, G.B.; Hong, S.; Kim, D.W.; Kim, Y.A. Substitutional boron doping on carbon materials. Carbon Lett. 2018, 27, 1–11. [Google Scholar]
- Shin, Y.; Park, S. Production of B-doped reduced graphene oxide using wet-process in teterhydrofuran. Carbon Lett. 2020, 27, 1–11. [Google Scholar] [CrossRef]
- Liu, Z.W.; Peng, F.; Wang, H.J.; Yu, H.; Xheng, W.X.; Yang, J. Phosphorus-doped graphite layers with high electrocatalytic activity for the O2 reduction in an alkaline medium. Angew. Chem. Int. Ed. 2011, 50, 3257–3261. [Google Scholar] [CrossRef]
- Zhao, G.; Shi, L.; Xu, J.B.; Yan, X.O.; Zhao, T.S. Role of phosphorus in nitrogen, phosphorus dual-doped ordered mesoporous carbon electrocatalyst for oxygen reduction reaction in alkaline media. Int. J. Hydrogen. Energy 2018, 43, 1470–1478. [Google Scholar] [CrossRef]
- Fujisawa, K.; Cruz-Silva, R.; Yang, K.-S.; Kim, Y.A.; Hayashi, T.; Endo, M.; Terrones, M.; Dresselhaus, M.S. Importance of open, heteroatom-decorated edges in chemically doped-graphene for supercapacitor applications. J. Mater. Chem. A 2014, 2, 9532–9540. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, D.; He, J.; Wang, Y.; Wang, K.; Li, H.; Wang, Y.; Miao, L.; Ren, R.; Xie, M. Simple and green fabrication of a biomass-derived N and O self-doped hierarchical porous carbon via a self-activation route for supercapacitor application. Carbon Lett. 2020, 30, 709–719. [Google Scholar] [CrossRef]
- Zhang, J.T.; Xia, Z.H.; Dai, L.M. Carbon-based electrocatalysts for advanced energy conversion and storage. Sci. Adv. 2015, 1, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jonsson, H. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Xu, H.; Cheng, D.; Cao, D.; Zeng, X.C. A universal principle for a rational design of single-atom electrocatalysts. Nat. Catal. 2018, 1, 339–348. [Google Scholar] [CrossRef]
- Jiang, Z.; Alexandrov, V. Enhancing Oxygen Electroreduction Activity of Single-Site Fe–N–C Catalysts by a Metal Support. J. Phys. Chem. C 2019, 123, 30335–30340. [Google Scholar] [CrossRef]




| I.D. | Atomic % | ||||
|---|---|---|---|---|---|
| C | O | N | P | B | |
| Single-step | 68.77 | 14.93 | 6.85 | 1.01 | 8.44 |
| Two-step | 77.50 | 13.23 | 3.02 | 2.36 | 3.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.H.; Han, J.H.; Wee, J.-H.; Choi, G.B.; Hong, S.; Kim, Y.A. Importance of Doping Sequence in Multiple Heteroatom-Doped Reduced Graphene Oxide as Efficient Oxygen Reduction Reaction Electrocatalysts. Appl. Nano 2021, 2, 267-277. https://doi.org/10.3390/applnano2030019
Kim JH, Han JH, Wee J-H, Choi GB, Hong S, Kim YA. Importance of Doping Sequence in Multiple Heteroatom-Doped Reduced Graphene Oxide as Efficient Oxygen Reduction Reaction Electrocatalysts. Applied Nano. 2021; 2(3):267-277. https://doi.org/10.3390/applnano2030019
Chicago/Turabian StyleKim, Jin Hee, Jong Hun Han, Jae-Hyung Wee, Go Bong Choi, Seungki Hong, and Yoong Ahm Kim. 2021. "Importance of Doping Sequence in Multiple Heteroatom-Doped Reduced Graphene Oxide as Efficient Oxygen Reduction Reaction Electrocatalysts" Applied Nano 2, no. 3: 267-277. https://doi.org/10.3390/applnano2030019
APA StyleKim, J. H., Han, J. H., Wee, J.-H., Choi, G. B., Hong, S., & Kim, Y. A. (2021). Importance of Doping Sequence in Multiple Heteroatom-Doped Reduced Graphene Oxide as Efficient Oxygen Reduction Reaction Electrocatalysts. Applied Nano, 2(3), 267-277. https://doi.org/10.3390/applnano2030019