Abstract
Gold nanoparticles have been increasingly used in several electronic, material fabrication, and biomedical applications. Several methods have been reported to prepare gold nanoparticles of various shapes and sizes with different photophysical properties. Although useful to prepare gold nanoparticles, most of the methods are not stable enough, which leads to the degradation of the nanoparticles, if they are stored at room temperatures (up to 30 °C) for a few days. In this paper, we report a novel and environmentally friendly method to synthesize self-assembled gold nanoparticles in cruciform shapes by using leaf extract of Cannabis indica as a reducing agent without the aid of any polymers or additional chemicals. The nanoparticles are found to be stable for more than a month (45 days) when stored at room temperature (up to 30 °C). They were able to form stable conjugates with bovine α-lactalbumin protein that may possess anti-cancerous properties.
1. Introduction
The use of gold nanoparticles has increased exponentially in several biomedical and non-biomedical applications [1]. Gold nanoparticles have been shown to possess anti-cancerous activity by several studies and, recently, they have been clinically shown to treat localized prostate tumors [2]. Due to their excellent photophysical properties, they have been explored to be used as theranostic agents [3]. Gold nanoparticles have emerged as one of the most promising agents for the treatment of various types of cancer due to several advantages associated with them such as (1) ease of cellular uptake by endocytosis, (2) ease of large-scale synthesis and characterization, (3) the ability to form stable complexes with a wide range of biomolecules, (4) biocompatibility, and (5) unique physicochemical properties. Gold nanoparticles of different shapes and sizes have different unique properties with different effects on cancer cells [4]. Conjugates of bovine α-lactalbumin (BLA) protein with oleic acid, commonly known as BAMLET (bovine alpha-lactalbumin made lethal to tumors), have been successfully shown to be anti-cancerous; however, oleic acid is toxic to healthy cells as well [5,6]. Biocompatible gold nanoparticles have been shown to replace oleic acid in the BAMLET conjugate to form anti-cancerous gold nanoparticle–BLA conjugates in vivo [7]. One of the significant challenges of synthesizing assembled gold nanoparticles is making them stable at temperatures up to 30 °C, which will give them a long shelf life and make them biocompatible to form stable conjugates with proteins [8]. Several nanoparticle synthesis methodologies require high temperatures and toxic chemicals, which can be harmful to the proteins. In this study, we report the synthesis of stable, cruciformly assembled gold nanoparticles using Cannabis indica leaves and the formation of its conjugates with the BLA protein. Normally, assembled gold nanoparticles are prepared with the aid of polymers or other thiol-assisted chemicals; however, in this report, we did not use any such synthetic chemicals to form the cruciform assembly of the gold nanoparticles [1,9].
2. Materials and Methods
Materials: Cetyl trimethylammonium bromide (CTAB) was obtained from Himedia, India. Oleic acid was obtained from RFCL Limited, India. Auric chloride hydrate and bovine α-lactalbumin (BLA) were obtained from Sigma Aldrich, India. All the glassware was purchased from Borosil, India. Milli-Q (HPLC grade) water was used in all preparations.
Synthesis of cruciform assembly of gold nanoparticles by using Cannabis indica leaves extract: To prepare the reducing agent, the Cannabis indica leaves were (2.80 g) dissolved in 5 mL of water followed by centrifugation at 5000 rpm for 10 min. The resultant supernatant was filtered using a 0.22 µm syringe filter (Whatman), and the pellet was discarded. A solution of auric chloride (5 mL, 2.5 mM) in water was mixed with 18.65 mL of 12.5 mM of cetyl trimethylammonium bromide (CTAB) aqueous solution, and the mixture was stirred at 100 °C. After 2 minutes, the filtered leaf extract (supernatant) was added to the reaction mixture. The reaction was quenched by putting the reaction mixture flask in an ice bath soon after the color of the mixture turned pinkish-red, which is the characteristic color of gold nanoparticles.
Dynamic light scattering (DLS) particle size analysis: The DLS particle size analysis was performed to determine the average size and polydispersity index of the nanoparticles using a Malvern Zetasizer Nano-ZS instrument.
Scanning electron microscope (SEM) and energy dispersive X-ray (EDX) analyses: The high-resolution SEM image was taken through a Jeol JSM-6480 LV instrument. Thin films of the sample were prepared on the grid for imaging. The EDX analysis was carried out by the JEOL-JSM 5800 instrument to confirm the presence of gold.
UV-Vis spectra analysis of GNPs: The UV-Vis spectroscopic (PerkinElmer, Lambda35) analysis of the reaction mixture was performed at wavelengths 400 nm–700 nm to test the stability of the nanoparticles. The nanoparticles were stored at room temperature (up to 30 °C) in the dark between the measurements.
Preparation of BAMLET and GNP–BLA conjugates: The stock solution of bovine α-lactalbumin (BLA) was prepared in a 20 mM phosphate buffer of 7.2 pH. To prepare the conjugates, the gold nanoparticles’ suspension (0.35 mL) was mixed with the 0.15 mL BLA solution to get a final concentration of BLA at 1.0 mg/mL and GNPs at 1.75 mM followed by incubation at 60 °C for 10 min. To prepare BAMLET, 10 µL of oleic acid was mixed with 0.3 mL of stock solution of BLA (final concentration was 1 mg/mL) and 0.69 mL of buffer and incubated at 60 °C for 10 min [10].
Tryptophan fluorescence emission spectra: The bovine α-lactalbumin (BLA) protein solution was prepared in a 20 mM phosphate buffer (pH = 7.2) at a concentration of 1 mg/mL. Tryptophan fluorescence spectra of the GNP–BLA conjugates and only BLA solution were recorded in a Perkin-Elmer LS-55 Luminescence spectrometer. An excitation wavelength of 280 nm was used, and emission spectra were recorded from 280 to 550 nm.
3. Results
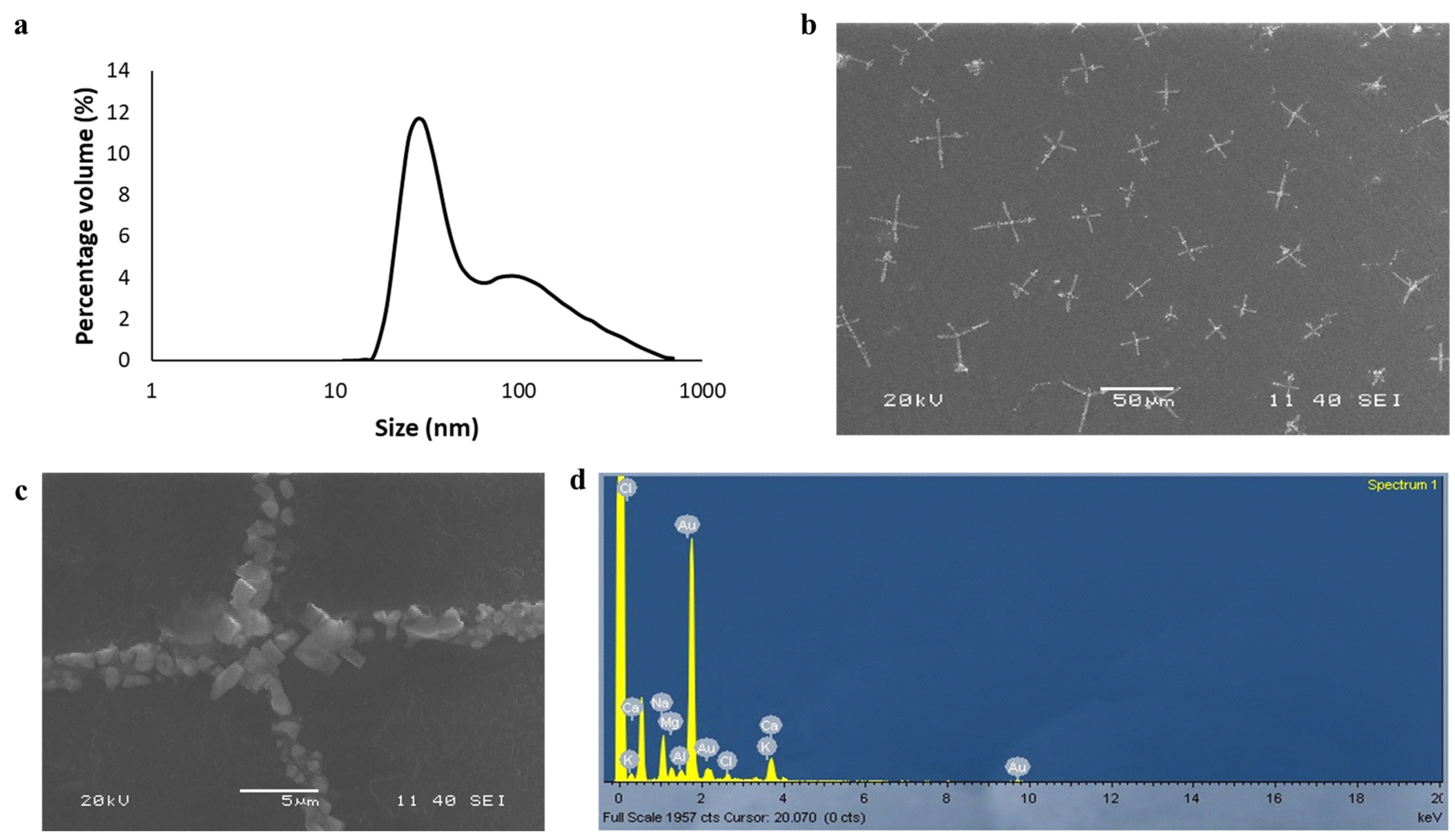
Gold nanoparticles are commonly prepared by the chemical reduction method [11]. Different plant extracts such as Hibiscus rosa-sinensis and Ocimum tenuiflorum have been used as reducing agents for the synthesis of gold nanoparticles from auric chloride [12,13]. Here, we synthesized a stable cruciform assembly of gold nanoparticles by using water extract from the leaves of Cannabis indica plants. We confirmed through dynamic light scattering that the particles prepared by our method were monodispersed (poly-dispersity index of 0.28) and nanosized (Figure 1a). The sizes and shapes of the nanoparticles were confirmed by scanning electron microscopy (SEM) experiments (Figure 1b,c). The SEM experiments revealed a rare cruciform assembly of the nanoparticles. The EDX analysis (Figure 1d) confirmed the presence of gold in the sample.
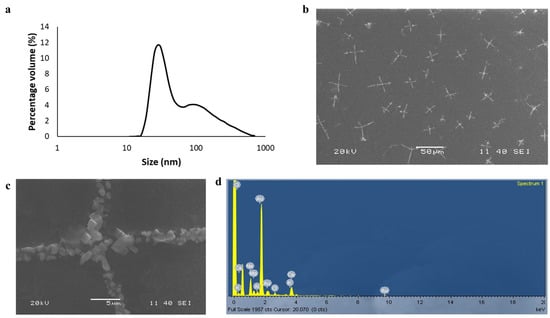
Figure 1.
(a) The dynamic light scattering experiment showed that particles were mostly monodispersed (polydispersity index = 0.28). A majority of the number of nanoparticles was found to be less than 100 nm. (b) Cruciform assembly of gold nanoparticles synthesized from Cannabis indica leaf water extract method. (c) A magnified image of the cruciform assembly. (d) EDX analysis of the sample confirmed the presence of gold.
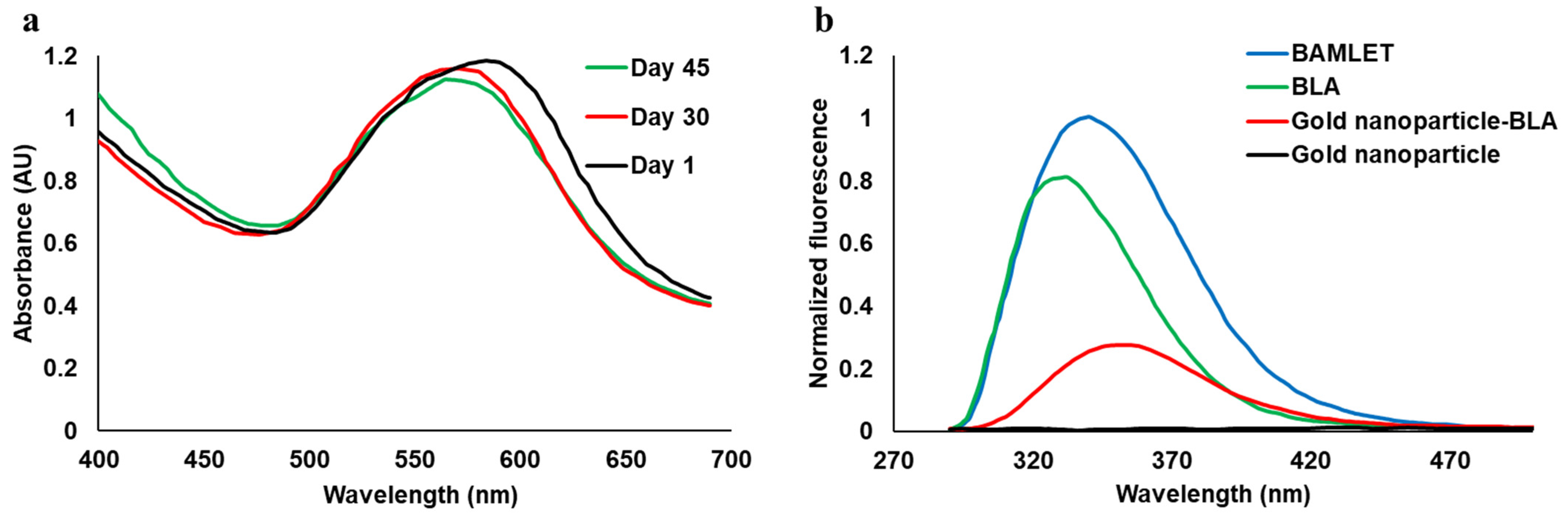
We did not observe any significant change in its absorbance spectra in the visible light region (400–700 nm) over the range of 45 days when stored at room temperature with temperatures reaching up to 30 °C, proving the stability of the nanoparticles (Figure 2a). No precipitation was observed over 45 days. The light absorbance maxima at around 580 nm due to surface plasmon resonance further demonstrates the formation of gold nanoparticles. The change of full-width half maxima (FWHM) of the spectrum reduced by about 5 nm from day 1 to day 30 and 3 nm from day 30 to day 45, which is not significant and further demonstrates the stability of the nanoparticles. After synthesizing stable gold nanoparticles, we prepared its conjugate with the BLA protein by a heat-shock method and monitored the tryptophan fluorescence spectrum (Figure 2b) [14]. The fluorescence intensities of all the samples were normalized with respect to the BAMLET. The tryptophan fluorescence intensity of the BLA protein changed significantly after binding to gold nanoparticles (to form a nanoparticle–BLA conjugate) and oleic acid (to form BAMLET), thus confirming the formation of the conjugates. BLA exhibited its strong intrinsic tryptophan fluorescence emission spectra with an emission maximum at 334.5 nm, which corresponds to a partially buried configuration of tryptophan [15]. The emission maximum of tryptophan in the gold nanoparticle–BLA conjugate was at around 355 nm, which corresponds to more exposure to the water solvent, thus confirming a conformational change in the protein structure [14]. The gold nanoparticles did not show any fluorescence emission (Figure 2b).
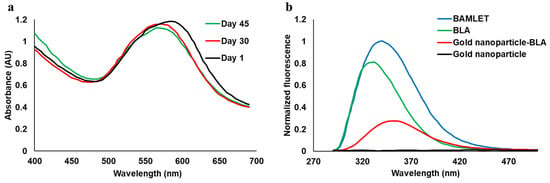
Figure 2.
(a) Visible light spectra of gold nanoparticles measured over 45 days, proving their stability. (b) Tryptophan fluorescence emission spectra of BLA and the conjugates with tryptophan excitation at 280 nm.
4. Discussion
This work reports only the synthesis and characterization of gold nanoparticles using Cannabis indica leaves’ extract and its conjugate with BLA, which was found to be anti-cancerous against breast cancer cells in preliminary studies in vitro, as previously reported as part of an undergraduate thesis [16]. We synthesized a stable cruciform assembly of gold nanoparticles using water extract of Cannabis indica leaves as a reducing agent without the usage of any additional synthetic chemicals such as polymers. To the best of our knowledge, such a cruciform assembly of individual gold nanoparticles has not been reported before. The self-assembly of nanoparticles is a result of several types of interfacial interactions, such as van der Walls attractions, magnetic and Coulombic forces, steric repulsions, hydrophobic and hydrophilic interactions, etc., dependent on the surface chemistry of the nanoparticles [17,18]. The water extract of the Cannabis indica leaves contains several chemicals that may have assisted in forming the cruciform assembly of the gold nanoparticles in our experimental conditions. In the future, it may be interesting to isolate the chemicals and test the effects of each of them individually or in the presence of one another in forming self-assembled gold nanoparticles. Given the presence of hundreds of chemicals in the leaves, carrying out such work was beyond the scope of the current study. Self-assembled gold nanoparticles have huge applications in the field of sensors, optics, and electronics. Self-assembled nanoparticles have also found usage in enhanced brain tumor targeting, which remains one of the toughest challenges to solve in medical science [19].
The use of a water extract of the Cannabis indica leaves as a reducing agent ensures that no toxic waste is generated during the synthesis procedure, as opposed to using harmful chemicals. This finding gives insight into a new class of compounds, which can be used to synthesize stable (at temperatures up to 30 °C), self-assembled gold nanoparticles. Upon studying the fluorescence emission spectra, we found a red-shift as well as a reduction in the tryptophan fluorescence intensity of the BLA protein, which indicates a conformational change in the protein. The change in the BLA protein folding due to the nanoparticle conjugation seems to have exposed the tryptophan from a partially buried configuration in the hydrophobic core to a more water-exposed configuration; however, the exact reason for the decrease in the quantum yield, which causes energy relaxation after light excitation, is difficult to predict, as previously suggested [14]. The nanoparticle–BLA conjugation takes place due to hydrophobic interactions between the BLA and the gold nanoparticle [20]. A discussion of the exact and detailed chemistry behind the formation of the nanoparticle–BLA conjugate is beyond the scope of this work. This work is of significance because different types of self-assembled gold nanoparticles with unique photophysical and physicochemical properties can be first synthesized; then, their conjugates with the BLA protein can be formed. The clinical trials that utilized gold nanoparticles to treat tumors have used photodynamic therapy, which relies on using light of specific wavelengths to excite the nanoparticles and generate heat or reactive oxygenated species [2,21,22]. Although photodynamic therapy has been beneficial to treat localized tumors, it cannot be practically used to kill metastatic tumors that have spread to several body parts because it is not always possible to shine a light on different, deeper parts of the human body. Chemotherapy-based techniques that utilize drugs such as doxorubicin and paclitaxel are gold-standard clinical methods to kill metastatic cancer cells, but they are also harmful to healthy cells. Hence, alternatives are required, such as gold nanoparticle–BLA conjugates, which are not toxic to healthy cells [7,23]. Gold nanoparticles can be combined with other natural chemicals, such as pyropheophorbide-a and related molecules that have been shown to possess anti-cancerous and photophysical properties, to form conjugates with enhanced efficacies [24,25]. Apart from cancer biology, gold nanoparticles have been used for nonlinear optical imaging such as second harmonic generation [26]. It may be interesting to study the nonlinear optical properties of these nanoparticles in live cells and lipid monolayer droplets by conjugating them with porphyrins, which have been shown to possess extremely high nonlinear optical properties [27]. Gold nanoparticles have also found usage in neuronal modulation as an alternative to optogenetics. Additionally, it may be exciting to make their conjugates with different optical dyes to make a single agent, which can modulate neurons and sense action potentials simultaneously [28,29]. Overall, the application of gold nanoparticles is rising exponentially, and new, greener methods that can synthesize stable, self-assembled nanoparticles are highly required, which we attempted to target through this work.
Author Contributions
A.K. and S.P. designed and conceived the project. A.K. designed the gold nanoparticle synthesis protocol, synthesized all the samples, and characterized them. S.P. supervised the project. A.K. wrote the paper. All authors contributed to writing the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received funding from the Department of Biotechnology, Government of India, and the National Institute of Technology, Rourkela, India.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are present in the manuscript. Additional data can be provided upon reasonable request from the corresponding authors.
Acknowledgments
We thank Sunil Kumar Sarangi, Deependra K. Ban, Kunal Pal, Sirsendu Sekhar Ray at the National Institute of Technology, Rourkela for useful discussions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Daniel, M.-C.; Astruc, D. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications toward Biology, Catalysis, and Nanotechnology. Chem. Rev. 2004, 104, 293. [Google Scholar] [CrossRef] [PubMed]
- Rastinehad, A.R.; Anastos, H.; Wajswol, E.; Winoker, J.S.; Sfakianos, J.P.; Doppalapudi, S.K.; Carrick, M.R.; Knauer, C.J.; Taouli, B.; Lewis, S.C.; et al. Gold nanoshell-localized photothermal ablation of prostate tumors in a clinical pilot device study. Proc. Natl. Acad. Sci. USA 2019, 116, 18590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Q.; Zhang, J.; Gao, J.; Zhang, Z.; Zhu, H.; Wang, D. Gold Nanoparticles in Cancer Theranostics. Front. Bioeng. Biotechnol. 2021, 9, 647905. [Google Scholar] [CrossRef]
- Sztandera, K.; Gorzkiewicz, M.; Klajnert-Maculewicz, B. Gold Nanoparticles in Cancer Treatment. Mol. Pharm. 2019, 16, 1–23. [Google Scholar] [CrossRef]
- Svensson, M.; Sabharwal, H.; Håkansson, A.; Mossberg, A.-K.; Lipniunas, P.; Leffler, H.; Svanborg, C.; Linse, S. Molecular Characterization of α–Lactalbumin Folding Variants That Induce Apoptosis in Tumor Cells*. J. Biol. Chem. 1999, 274, 6388. [Google Scholar] [CrossRef] [Green Version]
- Shabbirahmed, A.M.; Kumaravel, M.; Somu, P.; Paul, S.; Khadria, A. Recent Advancements in Nanomaterials for Photodynamic Therapy of Cancers BT—Handbook of Oxidative Stress in Cancer: Therapeutic Aspects; Chakraborti, S., Ed.; Springer: Singapore, 2021; pp. 1–24. [Google Scholar]
- Yang, J.; Wang, T.; Zhao, L.; Rajasekhar, V.K.; Joshi, S.; Andreou, C.; Pal, S.; Hsu, H.; Zhang, H.; Cohen, I.J.; et al. Gold/alpha-lactalbumin nanoprobes for the imaging and treatment of breast cancer. Nat. Biomed. Eng. 2020, 4, 686. [Google Scholar] [CrossRef]
- Wang, A.; Ng, H.P.; Xu, Y.; Li, Y.; Zheng, Y.; Yu, J.; Han, F.; Peng, F.; Fu, L. Gold nanoparticles: Synthesis, stability test, and application for the rice growth. J. Nanomater. 2014, 2014, 451232. [Google Scholar] [CrossRef]
- Ofir, Y.; Samanta, B.; Rotello, V.M. Polymer and biopolymer mediated self-assembly of gold nanoparticles †. Chem. Soc. Rev. 2008, 37, 1814. [Google Scholar] [CrossRef] [Green Version]
- Kamijima, T.; Ohmura, A.; Sato, T.; Akimoto, K.; Itabashi, M.; Mizuguchi, M.; Kamiya, M.; Kikukawa, T.; Aizawa, T.; Takahashi, M.; et al. Heat-treatment method for producing fatty acid-bound alpha-lactalbumin that induces tumor cell death. Biochem. Biophys. Res. Commun. 2008, 376, 211. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55. [Google Scholar] [CrossRef]
- Philip, D.; Unni, C. Extracellular biosynthesis of gold and silver nanoparticles using Krishna tulsi (Ocimum sanctum) leaf. Phys. E Low-Dimens. Syst. Nanostruct. 2011, 43, 1318. [Google Scholar] [CrossRef]
- Philip, D. Green synthesis of gold and silver nanoparticles using Hibiscus rosa sinensis. Phys. E Low-Dimens. Syst. Nanostruct. 2010, 42, 1417. [Google Scholar] [CrossRef]
- Royer, C.A. Probing Protein Folding and Conformational Transitions with Fluorescence. Chem. Rev. 2006, 106, 1769. [Google Scholar] [CrossRef] [PubMed]
- Royer, C.A.; Hinck, A.P.; Loh, S.N.; Prehoda, K.E.; Peng, X.; Jonas, J.; Markley, J.L. Effects of amino acid substitutions on the pressure denaturation of staphylococcal nuclease as monitored by fluorescence and nuclear magnetic resonance spectroscopy. Biochemistry 1993, 32, 5222. [Google Scholar] [CrossRef]
- Khadria, A. Preparation of Gold Nanoparticles-α Lactalbumin Binary Complex for Breast Cancer Therapy. Bachelor’s Thesis, National Institute of Technology, Rourkela, India, 2012. [Google Scholar]
- Bian, K.; Schunk, H.; Ye, D.; Hwang, A.; Luk, T.S.; Li, R.; Wang, Z.; Fan, H. Formation of self-assembled gold nanoparticle supercrystals with facet-dependent surface plasmonic coupling. Nat. Commun. 2018, 9, 2365. [Google Scholar]
- Wang, C.; Siu, C.; Zhang, J.; Fang, J. Understanding the forces acting in self-assembly and the implications for constructing three-dimensional (3D) supercrystals. Nano Res. 2015, 8, 2445. [Google Scholar] [CrossRef]
- Feng, Q.; Shen, Y.; Fu, Y.; Muroski, M.E.; Zhang, P.; Wang, Q.; Xu, C.; Lesniak, M.S.; Li, G.; Cheng, Y. Self-Assembly of Gold Nanoparticles Shows Microenvironment-Mediated Dynamic Switching and Enhanced Brain Tumor Targeting. Theranostics 2017, 7, 1875. [Google Scholar] [CrossRef]
- Lystvet, S.M.; Volden, S.; Halskau, O.; Glomm, W.R. Immobilization onto gold nanoparticles alters α-lactalbumin interaction with pure and mixed phospholipid monolayers. Soft Matter 2011, 7, 11501. [Google Scholar] [CrossRef]
- Hu, F.; Xu, S.; Liu, B. Photosensitizers with Aggregation-Induced Emission: Materials and Biomedical Applications. Adv. Mater. 2018, 30, 1801350. [Google Scholar]
- Moan, J.; Peng, Q. An outline of the history of PDT. Anticancer Res. 2003, 23, 3591. [Google Scholar]
- Sudha, T.; Bharali, D.J.; Yalcin, M.; Darwish, N.H.E.; Coskun, M.D.; Keating, K.A.; Lin, H.-Y.; Davis, P.J.; Mousa, S.A. Targeted delivery of paclitaxel and doxorubicin to cancer xenografts via the nanoparticle of nano-diamino-tetrac. Int. J. Nanomed. 2017, 12, 1305. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.H.; Nafiujjaman, M.; Nurunnabi, M.; Li, L.; Khan, H.A.; Cho, K.J.; Huh, K.M.; Lee, Y. Hybrid photoactive nanomaterial composed of gold nanoparticles, pheophorbide-A and hyaluronic acid as a targeted bimodal phototherapy. Macromol. Res. 2015, 23, 474. [Google Scholar] [CrossRef]
- Khadria, A.; de Coene, Y.; Gawel, P.; Roche, C.; Clays, K.; Anderson, H.L. Push–pull pyropheophorbides for nonlinear optical imaging †. Org. Biomol. Chem. 2017, 15, 947. [Google Scholar] [CrossRef] [PubMed]
- Clark, H.A.; Campagnola, P.J.; Wuskell, J.P.; Lewis, A.; Loew, L.M. Second Harmonic Generation Properties of Fluorescent Polymer-Encapsulated Gold Nanoparticles. J. Am. Chem. Soc. 2000, 122, 10234. [Google Scholar] [CrossRef]
- Khadria, A.; Fleischhauer, J.; Boczarow, I.; Wilkinson, J.D.; Kohl, M.M.; Anderson, H.L. Porphyrin Dyes for Nonlinear Optical Imaging of Live Cells. iScience 2018, 4, 153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, K.; Dou, Q.; Loh, X.J. Nanomaterial mediated optogenetics: Opportunities and challenges. RSC Adv. 2016, 6, 60896. [Google Scholar] [CrossRef] [Green Version]
- Khadria, A. Tools to measure membrane potential of neurons. Biomed. J. 2022; in press. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).