Abstract
Objectives: To investigate primary and secondary surgical outcomes following transcaval repair (TCR), modified Warden repair, and transatrial repair techniques for partial anomalous pulmonary venous connections (PAPVCs) and sinus venosus atrial septal defects (ASDs). Methods: This is an observational cohort clinical study. Patients who underwent TCR, modified Warden repair, and transatrial surgical repair for PAPVC and ASD between January 2003 and October 2019 at our institution were included in the study. Patients had one of the surgical procedures based on the anatomy of the defect. Results: Ten patients, seven (70%) males and three (30%) females, were included in the analysis. Seven patients underwent TCR, two patients the modified Warden technique, and one patient underwent transatrial surgical repair. Mean age was 57 years ± 14.7. Mean EuroScore II was 3.4 ± 3.5. The baseline left ventricle ejection fraction was 45 ± 6.5%. No patient had previous stroke, pacemaker (PM) implantation, or myocardial infarction. Total cardiopulmonary bypass and cross-clamping time were 123 ± 72.5 and 100 ± 48.5 min, respectively. Mean mechanical ventilation, mean intensive care unit, and mean hospital length of stay for the transcaval, modified Warden, and transatrial groups were 4.6 ± 10.7, 5.7 ± 8.8, and 10.5 ± 9.2 days, respectively. Superior caval or pulmonary venous obstruction, sinus node dysfunction, and PM implantation were not present at follow-up. The patient who underwent transatrial repair had died at 5.5-year follow-up due to myocardial infarction. Total survival rate at 6 years was 90%. Conclusions: The findings from this study elicit that all three techniques have low postoperative morbidity and are feasible and reliable procedures.
1. Introduction
Surgical repair of partial anomalous venous connection (PAPVC) remains one of the most performed congenital cardiac surgery procedures. The ‘’Achilles heel’’ for the correction of PAPVC is the high rate of pacemaker (PM) implantation due to sinus node dysfunction and superior caval or pulmonary vein obstruction during surgery [1,2]. The important point is the selection of an appropriate surgical procedure based on the anatomy of the defect. Adult patients operated upon with the Warden technique had an increased rate of early complications and reoperation [3]. However, complications such as direct implantation of PAPVC (that has been associated with pulmonary hypertension) or superior vena cava (SVC) obstruction [4,5] are not easily mitigated. Surgical techniques to mitigate the risk of SVC obstruction or sinus node dysfunction include transcaval (TCR), transatrial, and modified Warden approaches [6,7]. Most studies do not supply information regarding the surgical outcomes of these techniques, such as incidence of myocardial infarction (MI), stroke rate, or long-term follow-up [8,9]. On the other hand, PAPVC with ASDs is a rare anomaly, and most studies in the international literature mainly describe case reports of these techniques including only between 3 and 20 patients [10,11,12]. To increase the number of patients, previous and recent publications tend to group adults and children, expecting similar outcomes [13,14]. Due to the persistent increased pulmonary and intracardiac pressures in the adult population compared to children, which leads to chronic physiological changes, we cannot expect similar outcomes between the two populations after cardiac surgery. Unfortunately, prospective clinical studies have not been conducted to compare the outcomes of these techniques; thus, the risks of these surgical approaches have not been systematically weighed against their proposed benefits. Moreover, the impact of a high EuroScore II value has not been fully investigated. The goal of this study is to investigate and compare primary and secondary outcomes following surgical repair of PAPVC through TCR, transatrial, or modified Warden techniques.
2. Material and Methods
2.1. Study Design
This is an observational cohort, single center clinical study with data collected and entered in an adult congenital heart disease (ACHD) institutional database. Patients with bilateral partial anomalous pulmonary venous connection, patients who refused treatment, or patients who were missing information were excluded from the study. Surgical indications were obtained according to the European Society of Cardiology’s clinical guidelines [15].
2.2. Surgical Techniques
All surgical operations were performed by the same surgeon (AD). TCR consisted of a lateral aspect incision of the SVC extending up to the superior board of the pulmonary vein. This incision was extended in a cephalad manner and inferiorly to incorporate the junction of all anomalous pulmonary veins in continuity with the SVC. The incision did not cross the cavoatrial junction. This allows for excellent exposure both to the anomalous pulmonary venous orifices in the SVC and to the ASD. There are three important landmarks in the repair (Figure 1): the ASD rim, the junction of the most superior anomalous pulmonary vein with the SVC, and the junction of the most inferior anomalous pulmonary vein with the SVC [16]. Transatrial repair consists of an incision of the right atrium and extension of the incision to the inferior vena cava to increase surgical exposure. The posterior pericardium just superior to the dome of the left atrium was incised, and the pulmonary venous confluence was identified with a longitudinal incision made along the entire length of the confluence to create a wide opening. A matching incision was made on the superior aspect of the roof of the left atrium, placing gentle traction leftward on the left atrial appendage [17]. The modified Warden technique consists of a longitudinal incision made along the anterior wall of the cephalic end of the divided SVC up to the innominate vein–SVC junction, which is then anastomosed to the posterior edge of the right atrial appendage. The native SVC orifice in the right atrium is rerouted to the left atrium through the ASD with a pericardial patch to drain the anomalous pulmonary veins into the left atrium [18]. Preoperative planning was based on the anomaly location. In the event the PAPVC drained up to 1 cm from the cavoatrial junction, a transatrial technique was applied. In the event that PAPVC drainage was higher in the SVC, depending on the presence of a left persistent superior vena cava and its development, a modified Warden or TCR technique was applied. Patients with a large SVC underwent the TCR technique, while patients with non-large SVC underwent the modified Warden technique. This was done to avoid SVC obstruction [13] (Figure 2). Surgical exposure was performed through median sternotomy in all patients. The pericardium was opened, thus exposing the heart. The SVC was dissected up to the azygos vein confluence to obtain adequate surgical exposure. Regarding the cannulation technique, cardiopulmonary bypass was established through ascending aortic and bicaval vein cannulation. The superior venous cannula was placed close to the innominate vein confluence. A third venous cannula was inserted in the presence of a left SVC. A single-patch technique was used to direct the flow from the anomalous vein through the ASD to the left atrium. In all patients, an autologous pericardial patch pretreated with glutaraldehyde was used for the repair. The incision at the SVC was closed primarily.
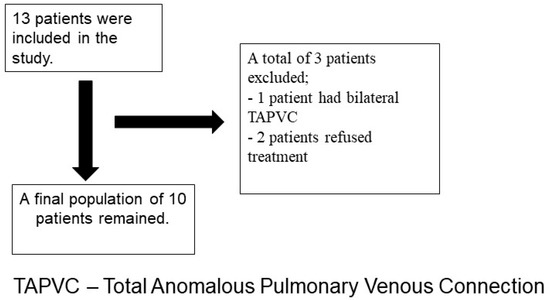
Figure 1.
Flowchart of the patient selection process.
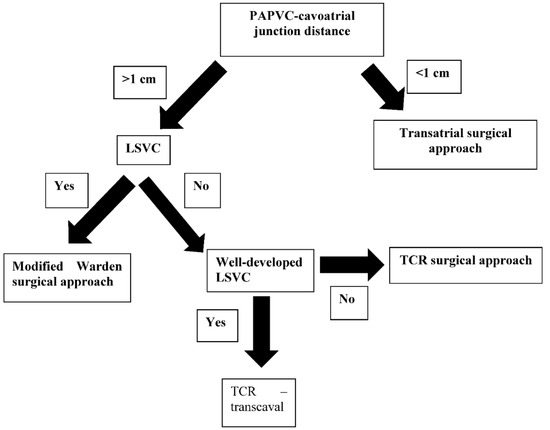
Figure 2.
Procedural selection process. LSVC—left superior vena cava present; well-developed LSVC—big enough to allow for the procedure without leading to post-procedural stenosis.
2.3. Primary and Secondary Outcomes
The primary outcomes were cardiac death, death from any other cause, PM implantation, SVC or pulmonary vein obstruction, and MI. Secondary outcomes were infection, stroke, and NYHA class at discharge. With respect to in-hospital outcomes, the analyzed variables were cardiopulmonary bypass time, aortic cross-clamp time, intensive care unit (ICU) and in-hospital length of stay, and infection rate. Early, mid, and long-term survival analyses were performed during check-ups at our hospital.
2.4. Statistical Analysis
Data were extracted manually from the database and analyzed using SPSS version 26 (SAS Institute, Cary, NC, USA). The Ethical Committee approved this manuscript in accordance with the 1975 Declaration of Helsinki as revised in 2013, IRB 311223. This research was conducted with the informed and appropriate consent of each participant.
2.5. Clinical Data
Preoperative/postoperative data and complications were based on definitions set forth in the Society of Thoracic Surgeons’ adult database [19]. Stroke and TIA were defined according to the Stroke Council of the American Heart Association—American Stroke Association [20]. Primary pulmonary hypertension is defined by a mean pulmonary artery pressure >25 mmHg at rest or >30 mmHg during exercise in the presence of pulmonary capillary wedge pressure ≤15 mmHg [21]. Unilateral total pulmonary venous connection was defined as two anomalous pulmonary venous connections coming from the same side. Based on the preoperative data, EuroScore II was calculated to predict the risk of postoperative mortality. With respect to mortality, we included all deaths after cardiac surgery, regardless of the cause. Early mortality was defined as mortality occurring during the first 30 days after operation [22]. The composite endpoint of in-hospital safety (all-cause mortality, stroke, life-threatening bleeding, acute kidney injury (RIFLE Stage 2 or 3 or renal replacement therapy)), coronary artery obstruction requiring intervention, major vascular complication, and valve-related dysfunction were evaluated. The patient selection process was performed according to STROBE II recommendations.
2.6. Preoperative Evaluation
Preoperative diagnostic check-ups included medication records, routine blood analysis, an electrocardiogram (ECG), and a chest X-ray. The diagnosis of PAPVC and ASD for all patients was confirmed by echocardiography and CT-scan imaging. Coronary angiography was performed in patients older than 40 who presented significant risk factors for coronary artery disease.
2.7. Postoperative Evaluation
Patients had a postoperative ECG upon ICU arrival and on postoperative day 1, 2, and 3. Postoperative chest X-rays and echocardiography were performed on all patients.
2.8. Definitions
Persistent left superior vena cava (PLSVC) is a rare vascular anomaly that begins at the junction of the left subclavian and internal jugular veins and passes through the left side of the mediastinum adjacent to the arcus aorta. It mostly drains into the right atrium via the coronary sinus (CS). Although PLSVC is infrequent among all vascular anomalies, it is the most common thoracic venous anomaly. SVC obstruction is a narrowing or blockage of the superior vena cava (SVC). Pulmonary valve stenosis is a narrowing of the valve located between the lower right heart chamber (right ventricle) and the lung arteries.
3. Results
Thirteen patients were initially screened for inclusion in this analysis. Three patients were excluded from the study due to bilateral partial anomalous pulmonary venous connection (one patient) or the patient refusing treatment (two patients). The final study population included ten patients (seven males and three females) with PAPVCs and ASDs that underwent surgical repair (Table 1). The mean age of the patients was 57 ± 14.7 years. Pulmonary artery hypertension (PAPs > 35 mmHg) was present in six patients. Three patients had had previous cardiac surgery. Mean EuroScore II was 3.4 ± 3.5. The baseline left ventricle ejection fraction was 45 ± 6.5%. No patient had previous stroke, PM implantation, or MI. Patient #1 had permanent atrial fibrillation, persistent left superior vena cava, and a small right superior vena cava. Patient #2 had a previous ASD closure, while patient #10 had a previous ASD and a ventricular septal defect repair at 7 months. Seven patients were treated by TCR (four males and three females, mean age of 51.8), two by modified Warden procedure (two males, mean age 68), and one by transatrial repair with unilateral total drainage directly into the right atrium (a 71-year-old male). In five patients, associated procedures were required to treat valvular disease and/or coronary artery disease. Patients #1, #3, #7, and #10 required an additional surgical valve procedure. Patients #3 and #8 underwent concomitant coronary artery bypass.

Table 1.
Patients’ characteristics and surgical procedures.
3.1. In-Hospital Outcomes
Primary outcomes in terms of cardiac death, death from other causes, SVC and pulmonary vein obstruction, PM implantation, and MI rate were 0% at hospital discharge. Secondary outcomes included cardiopulmonary bypass and cross-clamp time, which were 123 ± 72.5 min and 100 ± 48.5 min, respectively. Mean mechanical ventilation, mean ICU, and mean hospital length of stay for the three groups were 4.6 ± 10.7, 5.7 ± 8.8, and 10.5 ± 9.2 days, respectively. Stroke rate was 0% (Table 2). Patient #1 with permanent atrial fibrillation had early postoperative bradycardia, which resolved without PM implantation. All patients survived the surgical procedure and were discharged from the hospital. NYHA class at discharge was III for patient #2 and II for the other patients. There was one urinary tract in-hospital infection in the TCR group, due to Escherichia Coli, which was treated medically (Table 3).

Table 2.
Patients in-hospital outcomes.

Table 3.
Postoperative data based on single patients’ outcomes.
3.2. Follow-Up
3.2.1. Primary Outcomes
SVC or pulmonary venous obstruction, sinus node dysfunction, and PM implantation were not present at follow-up. At follow-up, patient #2, who underwent transatrial repair, had died of MI by the 5.5-year follow-up. Overall, mean survival at six years was 90% (Table 4).

Table 4.
Primary outcomes at follow-up.
3.2.2. Secondary Outcomes
The impact of a high EuroScore II value on long-term survival was significant, as demonstrated by the death of patient #2 (Table 5).

Table 5.
The impact of EuroScore II ˃ 7 on long-term survival.
4. Discussion
The number of adults diagnosed with congenital cardiac abnormalities is higher than the number of children due to an improved assessment of cardiac anatomy and medical imaging diagnosis in adults [23]. The main findings from this study can be summarized as follows: Firstly, TCR was utilized in 70% of patients with ACHD, including patients with unilateral total anomalous venous connection and ASDs. This technique was shown to have a high survival rate (100%) and few postoperative complications. However, one patient had an episode of postoperative bradycardia that was medically treated. Secondly, the surgical incision at the SVC during TCR is performed away from the sinus node and its artery, compared to the transatrial technique [24]. As a result, patients who were treated with TCR (seven patients) presented with persistent sinus rhythm post-surgery. Thirdly, the TCR, modified Warden, and transatrial techniques resulted in no incidence of SVC or pulmonary vein obstruction. This finding is in contrast with data reported by Park et al. [8], who described a high incidence of caval obstruction and stenosis among 3 out of 30 patients during the first postoperative year following TCR. In addition, Shariari et al. [25] reported pulmonary venous stenosis among their patient population following TCR repair. Our study did not evidence these complications, which further emphasizes the complexity of this procedure and the importance of a surgeon’s experience. On the other hand, Chandra et al. [26] showed good surgical outcomes with the modified Warden technique. Fourthly, we had good outcomes at follow-up in terms of survival rate compared to other clinical studies [26]. Due to the small number of patients undergoing cardiac surgery with this specific indication for congenital PAPVC repair, most publications tend to group adults and children, expecting similar outcomes [13]. Due to persistent increased pulmonary and intracardiac pressure in the adult population compared to children, which leads to chronic physiological changes, we cannot expect similar outcomes between the two populations after cardiac surgery.
Fifthly, the appropriate technique must be chosen depending on the anatomy of the PAPVC.
We performed research on postoperative echocardiographic outcomes after PAPVC repair in the literature (Table 6).

Table 6.
Postoperative echocardiographic outcomes after PAPVC repair in the literature.
Surgical outcomes in adult patients with PAPVC are important. An experienced heart-team including cardiologists, anesthesiologists, perfusionists, and nurses is essential for good results.
When using other techniques, a higher incidence of rhythm disturbance should be considered. Due to recent technical developments, some surgeons have been able to perform a minimally invasive approach through either right or left minithoracotomy to repair this anomaly with good results. However, this type of approach is based on a surgeon’s experience and the learning curve associated with this procedure. High-volume centers may prefer this approach due to the potential benefit to the patient. Nevertheless, single-lung ventilation may be required to achieve appropriate surgical exposure. In line with other studies, we have included patients who underwent additional surgical procedures. The outcome of this study may help to identify those patients who would benefit from referral to experienced cardiac centers.
5. Limitations
Although this study elicits significant findings on PAPVC and ASD treatment methods, several limitations need to be acknowledged. Given the retrospective design of the investigation, a bias with respect to data collection exists. Furthermore, confounding variables such as patient ethnicity, body mass index, history of diabetes, and acquired diseases, and the long-standing left-to-right shunt, could have impacted patient complications and survival rates. The size of the patient population is an additional limitation as only 10 patients were included in this study. The STS score includes multiple cofactors, such as illicit drug use, alcohol use, severity of carotid stenosis, etc., that were not present in our database. Therefore, we used EuroScore II as a predictive risk score. In addition, we did not report a frailty score, symptoms of the patients, SPO2 in RA, right ventricle end-diastolic volume, pulmonary artery pressure, pulmonary valve flow velocity, or Qp/Qs because these parameters were not available in our database. Multi-institutional studies performed over a long postoperative period on a larger patient population should be conducted to further validate the findings from this investigation.
6. Conclusions
Our findings suggest that the three surgical approaches may each play a role, although a definitive answer cannot be given at present in terms of technique superiority. A multi-center study would be required for validation purposes.
Author Contributions
Conceptualization, A.D., H.K.; methodology, A.D., M.C., M.M., H.K.; software; validation, A.D., H.K., S.S., M.B.; formal analysis, A.D., M.C., H.K., M.M., S.S., M.B.; investigation, A.V., A.D.; resources, A.V.; data curation, A.V., A.D.; writing—original draft preparation, A.V., A.D., S.S.; writing—review and editing, A.V., A.D., S.S., M.B.; visualization, A.V., A.D., S.S., M.B.; supervision, A.V., A.D., M.M., M.C., S.S., M.B.; project administration, A.V., A.D., H.K., S.S., M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
The Ethical Committee approved this manuscript in accordance with the 1975 Declaration of Helsinki, revised in 2013. This research was conducted with the informed and appropriate consent of each participant. IRB n. 311223. University of Siena, Italy.
Informed Consent Statement
Informed consent was obtained from each of the patients.
Data Availability Statement
The data can be provided by the corresponding author upon reasonable request.
Acknowledgments
We thank the floor nurses for their dedication.
Conflicts of Interest
The authors declare that there is no conflict of interest.
Scientific Meeting Presentation
E-Poster, American Association of Thoracic Surgeons Meeting, Toronto, Canada, 2019.
Abbreviations
| partial anomalous venous connection | PAPVC |
| superior vena cava | SVC |
| transcaval repair | TCR |
| atrial septal defect | ASD |
| pacemaker | PM |
| intensive care unit | ICU |
| adult congenital heart defect | ACHD |
References
- Gustafson, R.A.; Warden, H.E.; Murray, G.F. Partial anomalous pulmonary venous connection to the superior vena cava. Ann. Thorac. Surg. 1995, 60 (Suppl. S6), S614–S617. [Google Scholar] [CrossRef]
- William, W.G.; Rowe, R.D. Late results following repair of partial anomalous pulmonary venous connection with sinus venosus atrial septal defect. J. Thorac. Cardiovasc. Surg. 1980, 79, 776–781. [Google Scholar]
- Warden, H.E.; Gustafson, R.A.; Tarnay, T.J.; Neal, W.A. An alternative method for repair of partial anomalous pulmonary venous connection to the superior vena cava. Ann. Thorac. Surg. 1984, 38, 601–605. [Google Scholar] [CrossRef]
- Chartrand, C.; Payot, M.; Davignon, A.; Guerin, R.; Stanley, P.; Bruneau, J. A new surgical approach for correction of partial anomalous pulmonary venous drainage into the superior vena cava. J. Thorac. Cardiovasc. Surg. 1976, 71, 29–34. [Google Scholar] [CrossRef]
- Friedli, B.; Guerin, R.; Davignon, A.; Fouron, J.C.; Stanley, P. Surgical treatment of partial anomalous pulmonary venous drainage: A long-term follow-up study. Circulation 1972, 45, 159–170. [Google Scholar] [CrossRef]
- Nassar, M.; Fouilloux, V.; Macé, L.; Kreitmann, B.; Metras, D. Transcaval correction of partial anomalous pulmonary venous drainage into the superior vena cava. Ann. Thorac. Surg. 2012, 93, 193–196. [Google Scholar] [CrossRef]
- Perri, G.; Graziani, F.; Bruno, P.; Grandinetti, M.; Lanzillo, C.; Marziali, M.; Amodeo, A.; Massetti, M. Modified Warden procedure in adult with partial anomalous pulmonary venous connection after previous atrial septal defect repair. Cor Vasa 2016, 58, e501–e504. [Google Scholar] [CrossRef]
- Park, C.S.; Kwak, J.G.; Lee, C.; Lee, C.-H.; Lee, S.Y.; Choi, E.Y.; Song, J.Y.; Kim, S.-J. Partial anomalous pulmonary venous connection to the superior vena cava; the outcome after the warden procedure. Eur. J. Cardiothorac Surg. 2012, 41, 261–265. [Google Scholar] [CrossRef]
- Stewart, R.D.; Bailliard, F.; Kelle, A.M.; Backer, C.L.; Young, L.; Mavroudis, C. Evolving surgical strategy for sinus venosus atrial septal defect: Effect on sinus node function and late venous obstruction. Ann. Thorac. Surg. 2007, 84, 1651–1655. [Google Scholar] [CrossRef]
- Kottayil, B.P.; Dharan, B.S.; Menon, S.; Bijulal, S.; Neema, P.K.; Gopalakrishnan, S.K.; Jayakumar, K. Anomalous pulmonary venous connection to superior vena cava: Warden technique. Eur. J. Cardiothorac Surg. 2011, 39, 388–391. [Google Scholar] [CrossRef]
- Baron, O.; Roussel, J.C.; Videcoq, M.; Guerin, P.; Gournay, V.; Lefevre, M. Partial anomalous pulmonary venous connection; correction by intra-atrial baffle and cavoatrial anastomosis. J. Card. Surg. 2002, 17, 166–169. [Google Scholar] [CrossRef] [PubMed]
- DiBardino, D.J.; McKenzie, E.D.; Heinle, J.S.; Su, J.T.; Fraser, C.D. The Warden procedure for partially anomalous pulmonary venous connection to the superior caval vein. Cardiol. Young 2004, 14, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Dearani, J.A.; Connolly, H.M.; Martinez, R.; Fontanet, H.; Webb, G.D. Caring for adults with congenital cardiac disease: Successes and challenges for 2007 and beyond. Cardiol. Young 2007, 17 (Suppl. S2), 87–96. [Google Scholar] [CrossRef] [PubMed]
- Said, S.M.; Burkhart, H.M.; Dearani, J.A.; Eidem, B.; Stensrud, P.; Phillips, S.D.; Schaff, H.V. Outcomes of caval division for partial anomalous pulmonary venous connections to the superior vena cava. Ann. Thorac. Surg. 2011, 92, 980–984, discussion 985. [Google Scholar] [CrossRef]
- Nicholson, I.A.; Chard, R.B.; Nunn, G.R.; Cartmill, T.B. Transcaval repair of the sinus venosus syndrome. J. Thorac. Cardiovasc. Surg. 2000, 119, 741–744. [Google Scholar] [CrossRef]
- Baumgartner, H.; de Backer, J.; Babu-Narayan, S.V.; Budts, W.; Chessa, M.; Diller, G.; Lung, B.; Kluin, J.; Lang, I.M.; Meijboom, F.; et al. 2020 ESC Guidelines for the management of adult congenital heart disease: The Task Force for the management of adult congenital heart disease of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Adult Congenital Heart Disease (ISACHD). Eur. Heart J. 2021, 42, 563–645. [Google Scholar] [PubMed]
- Okonta, K.E.; Agarwal, V.; Abubakar, U. Superior repair: A useful approach for some anatomic variants of total anomalous pulmonary venous connection. Afr. J. Paediatr. Surg. 2013, 10, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Tao, K.; Pan, W.; Lin, K.; Shi, Y.; Zhu, P.; Guo, Y.; Gan, C.; An, Q. Modified cavoatrial anastomosis in Warden procedure. Ann. Thorac. Surg. 2010, 89, 2047–2048. [Google Scholar] [CrossRef]
- Fleisher, L.A.; Jneid, F.H.; Sundt III, T.M.; Thompson, A. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Collage Cardiol. 2017, 70, 252–289. [Google Scholar]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2018, 49, e46–e110. [Google Scholar] [CrossRef]
- McLaughin, V.V.; Archer, S.L.; Badesch, D.B.; Barst, R.J.; Farber, H.W.; Lindner, J.R.; Mathier, M.A.; McGoon, M.D.; Park, M.H.; Rosenson, R.S.; et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J. Am. Collega Cardiol. 2009, 53, 1573–1619. [Google Scholar]
- Marang-van de Mheen, P.J.; Stadlander, M.C.; Kievit, J. Adverse outcomes in surgical patients. Implementation of a nationwide routine reporting system. Qual. Saf. Health Care 2006, 15, 320–324. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stout, K.K.; Daniels, C.J.; Aboulhosn, J.A.; Bozkurt, B.; Broberg, C.S.; Colman, J.M.; Crumb, S.R.; Dearani, J.A.; Fuller, S.; Gurvitz, M.; et al. 2018 AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e698–e800. [Google Scholar] [PubMed]
- Napoleone, C.P.; Mariucci, E.; Angeli, E.; Oppido, G.; Gargiulo, G.D. Sinus node dysfunction after partial anomalous pulmonary venous connection repair. J. Thorac. Cardiovasc. Surg. 2008, 136, 329–334. [Google Scholar] [CrossRef]
- Shahriari, A.; Rodefeld, M.D.; Turrentine, M.W.; Brown, J.W. Caval division technique for sinus venosus atrial septal defect with partial anomalous pulmonary venous connection. Ann. Thorac. Surg. 2006, 81, 224–229, discussion 229-30. [Google Scholar] [CrossRef]
- Chandra, D.; Gupta, A.; Nath, R.K.; Grover, V.; Gupta, V.K. Surgical management of anomalous pulmonary venous connection to the superior vena cava—Early results. Indian Heart J. 2013, 65, 561–565. [Google Scholar] [CrossRef][Green Version]
- Touray, M.; Bouchardy, J.; Ladouceur, M.; Schwerzmann, M.; Greutmann, M.; Tobler, D.; Harald, R.; Engel, R.; Pruvot, E.; Blanche, C.; et al. Long-term outcome of adult patients with partial anomalous pulmonary venous connection treated surgically and conservatively: Data from the SACHER registry and a French center. Eur. Heart J. 2020, 41, ehaa946.2222. [Google Scholar] [CrossRef]
- Rodríguez-Collado, J.; Attie, F.; Zabal, C.; Troyo, P.; Olvera, S.; Vázquez, J.; Gutiérrez, B.; Vargas-Barrón, J. Total anomalous pulmonary venous connection in adults. Long-term follow-up. J. Thorac. Cardiovasc. Surg. 1992, 103, 877–880. [Google Scholar] [CrossRef]
- Majdalany, D.S.; Phillips, S.D.; Dearani, J.A.; Connolly, H.M.; Warnes, C.A. Isolated partial anomalous pulmonary venous connections in adults: Twenty-year experience. Congenit. Heart Dis. 2010, 5, 537–545. [Google Scholar] [CrossRef]
- Griffeth, E.M.; Dearani, J.A.; Mathew, J.; Graham, G.C.; Connolly, H.M.; King, K.S.; Schaff, H.V.; Stephens, E.H. Early and Late Outcomes of the Warden and Modified Warden Procedure. Ann. Thorac. Surg. 2022, 114, 1723–1729. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).