Inspiratory Muscle Training Intensity in Patients Living with Cardiovascular Diseases: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
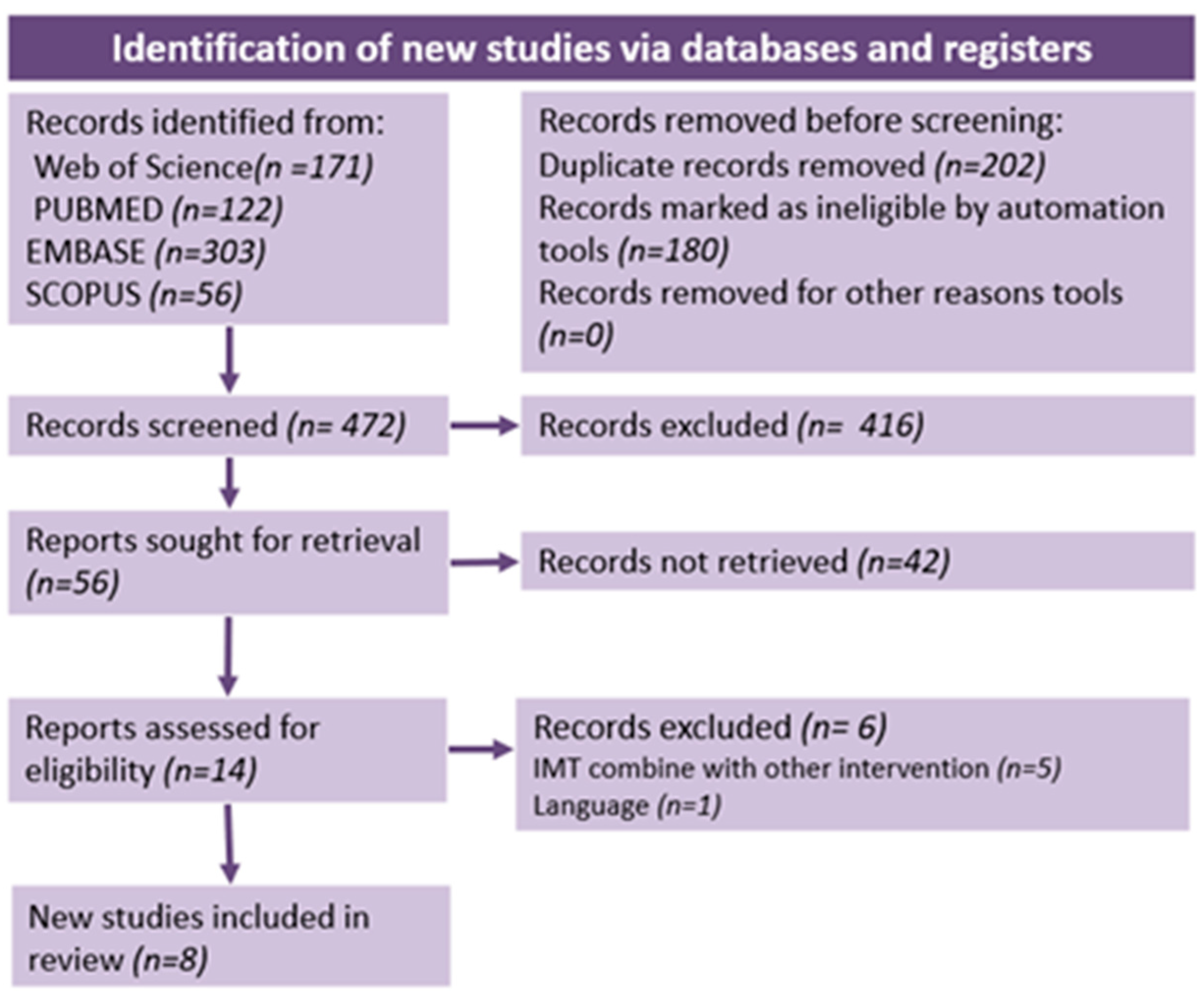
2.1. Search Strategy and Study Selection
2.2. Eligibility Criteria
2.3. Data Extraction
2.4. Quality Assessment
2.5. Certainty of Evidence
3. Results
3.1. Characteristic of Studies
3.2. Characteristics of Intervention
3.3. Primary Outcomes Results
3.4. Secondary Outcome Results
3.5. Quality Assessment
3.6. Certainty of Evidence
4. Discussion
4.1. Primary Outcomes
4.1.1. Inspiratory Muscle Strength
4.1.2. Inspiratory Muscle Endurance
4.1.3. Exercise Capacity
4.2. Secondary Outcomes
4.2.1. Dyspnoea
4.2.2. Quality of Life
4.2.3. Lung Function
4.3. Comparison with the Literature
4.4. Methodological Quality of Studies
4.5. Limitations
4.6. Future Perspective
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Detail. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases (accessed on 7 July 2023).
- Bosnak-Guclu, M.; Arikan, H.; Savci, S.; Inal-Ince, D.; Tulumen, E.; Aytemir, K.; Tokgözoglu, L. Effects of Inspiratory Muscle Training in Patients with Heart Failure. Respir. Med. 2011, 105, 1671–1681. [Google Scholar] [CrossRef]
- Chiappa, G.R.; Roseguini, B.T.; Vieira, P.J.C.; Alves, C.N.; Tavares, A.; Winkelmann, E.R.; Ferlin, E.L.; Stein, R.; Ribeiro, J.P. Inspiratory Muscle Training Improves Blood Flow to Resting and Exercising Limbs in Patients With Chronic Heart Failure. J. Am. Coll. Cardiol. 2008, 51, 1663–1671. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Zhang, Q.; Li, S.; Li, S.; Yao, Q.; Zheng, X.; Li, G.; Zeng, Y.; Chen, L.; Chen, S.; et al. Effects of Inspiratory Muscle Training in Patients with Hypertension: A Meta-Analysis. Front. Cardiovasc. Med. 2023, 10, 1113509. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zheng, Y.; Dang, Y.; Wang, L.; Cheng, Y.; Zhang, X.; Mao, M.; Lu, X. Can Inspiratory Muscle Training Benefit Patients after Stroke? A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Clin. Rehabil. 2020, 34, 866–876. [Google Scholar] [CrossRef] [PubMed]
- Shei, R.-J.; Paris, H.L.R.; Wilhite, D.P.; Chapman, R.F.; Mickleborough, T.D. The Role of Inspiratory Muscle Training in the Management of Asthma and Exercise-Induced Bronchoconstriction. Physician Sportsmed. 2016, 44, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Sogard, A.S.; Mickleborough, T.D. The Therapeutic Role of Inspiratory Muscle Training in the Management of Asthma: A Narrative Review. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2023, 325, R645–R663. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B.T.; O’Halloran, K.D. Strength in Breath: Respiratory Metaboreflex Response to Training and Detraining. Exp. Physiol. 2023, 108, 541–542. [Google Scholar] [CrossRef] [PubMed]
- Dubey, P. Effect of Metaboreflex on Cardiovascular System in Subjects of Metabolic Syndrome. J. Clin. Diagn. Res. 2017, 11, CC01. [Google Scholar] [CrossRef] [PubMed]
- Hoffman1, M. Inspiratory Muscle Training in Interstitial Lung Disease: A Systematic Scoping Review. J. Bras. Pneumol. 2021, 47, e20210089. [Google Scholar] [CrossRef]
- Larson, J.L.; Kim, M.J.; Sharp, J.T.; Larson, D.A. Inspiratory Muscle Training with a Pressure Threshold Breathing Device in Patients with Chronic Obstructive Pulmonary Disease. Am. Rev. Respir. Dis. 1988, 138, 689–696. [Google Scholar] [CrossRef]
- Parreiras De Menezes, K.K.; Nascimento, L.R.; Ada, L.; Avelino, P.R.; Polese, J.C.; Mota Alvarenga, M.T.; Barbosa, M.H.; Teixeira-Salmela, L.F. High-Intensity Respiratory Muscle Training Improves Strength and Dyspnea Poststroke: A Double-Blind Randomized Trial. Arch. Phys. Med. Rehabil. 2019, 100, 205–212. [Google Scholar] [CrossRef]
- Weiner, P.; Waizman, J.; Magadle, R.; Berar-Yanay, N.; Pelled, B. The Effect of Specific Inspiratory Muscle Training on the Sensation of Dyspnea and Exercise Tolerance in Patients with Congestive Heart Failure. Clin. Cardiol. 1999, 22, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.R.; Taylor, B.J. Inspiratory Muscle Weakness in Cardiovascular Diseases: Implications for Cardiac Rehabilitation. Prog. Cardiovasc. Dis. 2022, 70, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Azambuja, A.D.C.M.; De Oliveira, L.Z.; Sbruzzi, G. Inspiratory Muscle Training in Patients With Heart Failure: What Is New? Systematic Review and Meta-Analysis. Phys. Ther. 2020, 100, 2099–2109. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Kuang, L.; Fu, L. Effects of Inspiratory Muscle Training in Chronic Heart Failure Patients: A Systematic Review and Meta-Analysis. Congenit. Heart Dis. 2018, 13, 194–202. [Google Scholar] [CrossRef]
- Matos, A.P.; Pegorari, M.S. How to Classify Clinical Trials Using the PEDro Scale? J. Lasers Med. Sci. 2020, 11, 1–2. [Google Scholar] [CrossRef]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; deBeer, H. GRADE Guidelines: 1. Introduction—GRADE Evidence Profiles and Summary of Findings Tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. PLoS Med. 2021, 18, e1003583. [Google Scholar] [CrossRef]
- Dall’Ago, P.; Chiappa, G.R.S.; Guths, H.; Stein, R.; Ribeiro, J.P. Inspiratory Muscle Training in Patients with Heart Failure and Inspiratory Muscle Weakness: A Randomized Trial. J. Am. Coll. Cardiol. 2006, 47, 757–763. [Google Scholar] [CrossRef]
- Johnson, P. A Randomized Controlled Trial of Inspiratory Muscle Training in Stable Chronic Heart Failure. Eur. Heart J. 1998, 19, 1249–1253. [Google Scholar] [CrossRef]
- Marco, E.; Ramírez-Sarmiento, A.L.; Coloma, A.; Sartor, M.; Comin-Colet, J.; Vila, J.; Enjuanes, C.; Bruguera, J.; Escalada, F.; Gea, J.; et al. High-Intensity vs. Sham Inspiratory Muscle Training in Patients with Chronic Heart Failure: A Prospective Randomized Trial. Eur. J. Heart Fail. 2013, 15, 892–901. [Google Scholar] [CrossRef]
- Palau, P.; Domínguez, E.; Núñez, E.; Schmid, J.-P.; Vergara, P.; Ramón, J.M.; Mascarell, B.; Sanchis, J.; Chorro, F.J.; Núñez, J. Effects of Inspiratory Muscle Training in Patients with Heart Failure with Preserved Ejection Fraction. Eur. J. Prev. Cardiol. 2014, 21, 1465–1473. [Google Scholar] [CrossRef]
- Tran, D.; Munoz, P.; Lau, E.M.T.; Alison, J.A.; Brown, M.; Zheng, Y.; Corkery, P.; Wong, K.; Lindstrom, S.; Celermajer, D.S.; et al. Inspiratory Muscle Training Improves Inspiratory Muscle Strength and Functional Exercise Capacity in Pulmonary Arterial Hypertension and Chronic Thromboembolic Pulmonary Hypertension: A Pilot Randomised Controlled Study. Heart Lung Circ. 2021, 30, 388–395. [Google Scholar] [CrossRef]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef]
- Seixas, M.B.; Almeida, L.B.; Trevizan, P.F.; Martinez, D.G.; Laterza, M.C.; Vanderlei, L.C.M.; Silva, L.P. Effects of Inspiratory Muscle Training in Older Adults. Respir. Care 2020, 65, 535–544. [Google Scholar] [CrossRef]
- Meyer, F.J.; Borst, M.M.; Zugck, C.; Kirschke, A.; Schellberg, D.; Kübler, W.; Haass, M. Respiratory Muscle Dysfunction in Congestive Heart Failure: Clinical Correlation and Prognostic Significance. Circulation 2001, 103, 2153–2158. [Google Scholar] [CrossRef]
- Hamazaki, N.; Kamiya, K.; Yamamoto, S.; Nozaki, K.; Ichikawa, T.; Matsuzawa, R.; Tanaka, S.; Nakamura, T.; Yamashita, M.; Maekawa, E.; et al. Changes in Respiratory Muscle Strength Following Cardiac Rehabilitation for Prognosis in Patients with Heart Failure. J. Clin. Med. 2020, 9, 952. [Google Scholar] [CrossRef]
- Kelley, R.C.; Ferreira, L.F. Diaphragm Abnormalities in Heart Failure and Aging: Mechanisms and Integration of Cardiovascular and Respiratory Pathophysiology. Heart Fail. Rev. 2017, 22, 191–207. [Google Scholar] [CrossRef]
- Jaenisch, R.B.; Hentschke, V.S.; Quagliotto, E.; Cavinato, P.R.; Schmeing, L.A.; Xavier, L.L.; Dal Lago, P. Respiratory Muscle Training Improves Hemodynamics, Autonomic Function, Baroreceptor Sensitivity, and Respiratory Mechanics in Rats with Heart Failure. J. Appl. Physiol. 2011, 111, 1664–1670. [Google Scholar] [CrossRef]
- Weiner, P.; Magadle, R.; Beckerman, M.; Weiner, M.; Berar-Yanay, N. Maintenance of Inspiratory Muscle Training in COPD Patients: One Year Follow-Up. Eur. Respir. J. 2004, 23, 61–65. [Google Scholar] [CrossRef]
- Zoll, J.; Sanchez, H.; N’Guessan, B.; Ribera, F.; Lampert, E.; Bigard, X.; Serrurier, B.; Fortin, D.; Geny, B.; Veksler, V.; et al. Physical Activity Changes the Regulation of Mitochondrial Respiration in Human Skeletal Muscle. J. Physiol. 2002, 543, 191–200. [Google Scholar] [CrossRef]
- Chung, Y.; Huang, T.-Y.; Liao, Y.-H.; Kuo, Y.-C. 12-Week Inspiratory Muscle Training Improves Respiratory Muscle Strength in Adult Patients with Stable Asthma: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2021, 18, 3267. [Google Scholar] [CrossRef]
- Porter, C.; Reidy, P.T.; Bhattarai, N.; Sidossis, L.S.; Rasmussen, B.B. Resistance Exercise Training Alters Mitochondrial Function in Human Skeletal Muscle. Med. Sci. Sports Exerc. 2015, 47, 1922–1931. [Google Scholar] [CrossRef]
- Huertas, J.R.; Casuso, R.A.; Agustín, P.H.; Cogliati, S. Stay Fit, Stay Young: Mitochondria in Movement: The Role of Exercise in the New Mitochondrial Paradigm. Oxidative Med. Cell. Longev. 2019, 2019, 1–18. [Google Scholar] [CrossRef]
- Harms, C.A. Insights into the Role of the Respiratory Muscle Metaboreflex. J. Physiol. 2007, 584, 711. [Google Scholar] [CrossRef]
- Salah, H.M.; Goldberg, L.R.; Molinger, J.; Felker, G.M.; Applefeld, W.; Rassaf, T.; Tedford, R.J.; Mirro, M.; Cleland, J.G.F.; Fudim, M. Diaphragmatic Function in Cardiovascular Disease. J. Am. Coll. Cardiol. 2022, 80, 1647–1659. [Google Scholar] [CrossRef]
- Gama, G.; Farinatti, P.; Rangel, M.V.D.S.; Mira, P.A.D.C.; Laterza, M.C.; Crisafulli, A.; Borges, J.P. Muscle Metaboreflex Adaptations to Exercise Training in Health and Disease. Eur. J. Appl. Physiol. 2021, 121, 2943–2955. [Google Scholar] [CrossRef]
- Handelzalts, S.; Melzer, I.; Soroker, N. Analysis of Brain Lesion Impact on Balance and Gait Following Stroke. Front. Hum. Neurosci. 2019, 13, 149. [Google Scholar] [CrossRef]
- Sbruzzi, G.; Dal Lago, P.; Ribeiro, R.A.; Plentz, R.D.M. Inspiratory Muscle Training and Quality of Life in Patients with Heart Failure: Systematic Review of Randomized Trials. Int. J. Cardiol. 2012, 156, 120–121. [Google Scholar] [CrossRef][Green Version]
- Del Corral, T.; Fabero-Garrido, R.; Plaza-Manzano, G.; Fernández-de-las-Peñas, C.; Navarro-Santana, M.; López-de-Uralde-Villanueva, I. Home-Based Respiratory Muscle Training on Quality of Life and Exercise Tolerance in Long-Term Post-COVID-19: Randomized Controlled Trial. Ann. Phys. Rehabil. Med. 2023, 66, 101709. [Google Scholar] [CrossRef]
- Hare, D.L.; Toukhsati, S.R.; Johansson, P.; Jaarsma, T. Depression and Cardiovascular Disease: A Clinical Review. Eur. Heart J. 2014, 35, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- HajGhanbari, B.; Yamabayashi, C.; Buna, T.R.; Coelho, J.D.; Freedman, K.D.; Morton, T.A.; Palmer, S.A.; Toy, M.A.; Walsh, C.; Sheel, A.W.; et al. Effects of Respiratory Muscle Training on Performance in Athletes: A Systematic Review With Meta-Analyses. J. Strength. Cond. Res. 2013, 27, 1643–1663. [Google Scholar] [CrossRef] [PubMed]
- Sadek, Z.; Salami, A.; Joumaa, W.H.; Awada, C.; Ahmaidi, S.; Ramadan, W. Best Mode of Inspiratory Muscle Training in Heart Failure Patients: A Systematic Review and Meta-Analysis. Eur. J. Prev. Cardiol. 2018, 25, 1691–1701. [Google Scholar] [CrossRef]
- Menezes, K.K.; Nascimento, L.R.; Ada, L.; Polese, J.C.; Avelino, P.R.; Teixeira-Salmela, L.F. Respiratory Muscle Training Increases Respiratory Muscle Strength and Reduces Respiratory Complications after Stroke: A Systematic Review. J. Physiother. 2016, 62, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Pozuelo-Carrascosa, D.P.; Carmona-Torres, J.M.; Laredo-Aguilera, J.A.; Latorre-Román, P.Á.; Párraga-Montilla, J.A.; Cobo-Cuenca, A.I. Effectiveness of Respiratory Muscle Training for Pulmonary Function and Walking Ability in Patients with Stroke: A Systematic Review with Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 5356. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Arias, R.; Hinojosa-Riadi, J.; Sandoval-Cañío, A.; Santana-Garrido, H.; Valdovinos-Guerrero, N.; Seron, P. Effectiveness of Respiratory Muscle Training in Adults With Pulmonary Hypertension. A Systematic Review and Meta-Analysis. Heart Lung Circ. 2023, 32, 315–329. [Google Scholar] [CrossRef]
- Luo, Z.; Qian, H.; Zhang, X.; Wang, Y.; Wang, J.; Yu, P. Effectiveness and Safety of Inspiratory Muscle Training in Patients with Pulmonary Hypertension: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2022, 9, 999422. [Google Scholar] [CrossRef]
- Rehder-Santos, P.; Minatel, V.; Milan-Mattos, J.C.; Signini, É.D.F.; De Abreu, R.M.; Dato, C.C.; Catai, A.M. Critical Inspiratory Pressure—A New Methodology for Evaluating and Training the Inspiratory Musculature for Recreational Cyclists: Study Protocol for a Randomized Controlled Trial. Trials 2019, 20, 258. [Google Scholar] [CrossRef]
| First Author, Year | Country | Population (Disease) | Groups and Sample Size | Sex Distribution (M/F) | Age (Years), Mean ± SD * | BMI Baseline (Kg/m2) | PEDro Total Score |
|---|---|---|---|---|---|---|---|
| Bosnak-Guclu, 2011 [2] | Turkey | HF (LVEF < 40%) | IMT (n = 16) CON (n = 14) | IMT (12/4) CON (12/2) | IMT (69.50 ± 7.96) CON (65.71 ± 10.52) | IMT (26.76 ± 4.30) CON (25.08 ± 3.17) | 6/10 (High) |
| Dall’Ago, 2006 [20] | Brazil | CHF | IMT (n = 16) CON (n = 16) | IMT (11/5) CON (10/6) | IMT (54 ± 3) CON (58 ± 2) | IMT (27 ± 4) CON (27 ± 5) | 6/10 (High) |
| Johnson, 1998 [21] | UK | CHF | IMT (n = 9) CON (n = 9) | ALL (15/3) | ALL (66.5 ± 5.6) | NR | 6/10 (High) |
| Marco, 2013 [22] | Spain | CHF | IMT (n = 11) CON (n = 11) | IMT (7/4) CON (10/1) | IMT (68.5 ± 8.88) CON (70.1 ± 10.75) | IMT (28.4 ± 3.64) CON (26.3 ± 2.4) | 9/10 (High) |
| Palau, 2014 [23] | Spain | HFpEF | IMT (n = 14) CON (n = 12) | IMT (7/7) CON (6/6) | IMT (68 (60–76)) CON (74 (73–77)) | IMT (34.3 (28.2; 38)) CON (30 (26; 32) | 7/10 (High) |
| Parreiras de Menezes, 2019 [12] | Brazil | Stroke | IMT (n = 19) CON (n = 19) | IMT (8/11) CON (8/11) | IMT (60 ± 14) CON (67 ± 11) | NR | 6/10 (High) |
| Tran, 2021 [24] | Australia | PAH | IMT (n = 6) CON (n = 6) | IMT (1/5) CON (1/5) | IMT (55 ± 17) CON (66 ± 10) | IMT (22.6 ± 4.2) CON (27.7 ± 5.7) | 6/10 (High) |
| Weiner, 1999 [13] | Israel | HF (LVEF < 40%) | IMT (n = 10) CON (n = 10) | ALL (18/2) | ALL (68 ± 6.2) | NR | 6/10 (High) |
| First Author, Year | Device | Intensity | Session Duration and Frequency | Duration of Intervention | Supervised Intervention | Progression | Follow-Up |
|---|---|---|---|---|---|---|---|
| Bosnak-Guclu, 2011 [2] | Threshold IMT (Respironics, Murrysville, PA, USA) | IMT: 40% of MIP CON: 15% of MIP | ALL: 30 min per day, 7 days per week | 6 weeks | 1 session/week | IMT: Workload adjusted weekly to maintain 40% of the MIP CON: fixed workload | |
| Dall’Ago, 2006 [12] | Threshold IMT (Healthscan Products Inc., Cedar Grove, NJ, USA) | IMT: 30% of MIP CON: 0% of MIP | ALL: 30 min per day, 7 days per week | 12 weeks | 1 session/week | IMT: Workload adjusted weekly to maintain 30% of the MIP CON: No workload | 1 year after entering the study |
| Johnson, 1998 [13] | Threshold IMT (Respironics, Murrysville, PA, USA) | IMT: 30% of MIP CON: 15% of MIP | ALL: 30 min per day (15 min twice daily), 7 days per week | 8 weeks | None | IMT: Workload adjusted weekly to maintain 30% of the MIP CON: Fixed workload | |
| Marco, 2013 [14] | Respiratory trainer (Orygen-Dual valve, Girona, Spain) | IMT: Adjusted based on 100% of their 10 RM CON: 10 cmH20 | ALL: 5 sets × 10 reps, twice a day, 7 days per week | 4 weeks | 1 session/week | IMT: Workloads adjusted weekly according to 10 RM CON: Workload increased weekly of 2.5 cmH2O | |
| Palau, 2014 [15] | Threshold IMT (Respironics, Respironics, Murrysville, PA, USA) | IMT: 25–30% of MIP CON: Usual care | IMT: 40 min per day (20 min twice daily), 7 days per week | 12 weeks | None | IMT: Workload adjusted weekly to be within the training threshold range | Baseline Weekly +Diary card |
| Parreiras de Menezes, 2019 [9] | Respiratory trainer (Orygen-Dual valve, Girona, Spain) | IMT: 50% of MIP CON: 0% of MIP | ALL: 40 min per day (20 min twice daily), 7 days per week | 8 weeks | None | IMT: Workloads adjusted weekly to maintain 50% of MIP CON: Fixed workload | Baseline 8-week (end of intervention) 12-week +Diary |
| Tran, 2021 [16] | Electronic KHP2 respiratory muscle training (POWERbreathe International Ltd. Warwickshire, UK) | IMT: 2 × 30 breaths at 30–40% of MIP CON: Usual care | IMT: 5 days per week | 8 weeks | 1 session/week. Compliance to unsupervised sessions monitored through KHP2 device data extraction | IMT: Training intensity adjusted weekly to be within the training threshold range | Training data were extracted from the POWERbreathe to monitor compliance |
| Weiner, 1999 [10] | Threshold IMT (Healthscan, Cedar Grove, NJ, USA) | IMT: 15% of MIP the first week, increased incrementally, 5% each session, to reach 60% at the end of the 1st month. Then continued for the next two months at 60% of MIP. CON: 0% of MIP | ALL: 30 min per day, 6 days per week | 12 weeks | All sessions | IMT: Training intensity adjusted every week to the new MIP achieved. | Baseline MIP weekly 3-month |
| Intensity | First Author, Year | Main Outcomes | Results | Secondary Outcomes | Results |
|---|---|---|---|---|---|
| Low (25–30% MIP) | Palau, 2014 [15] | Inspiratory muscle strength: MIP Exercise capacity: 6MWT (distance, HRrest, HRmax), CPET (VO2 peak, VO2AT, VE/VCO2 slope, METs, RER) | The IMT group showed significantly greater improvement in MIP, VO2 peak, VO2 AT, VE/VCO2 slope, METs, 6MWD compared to the CON group. | QoL: the MLHF questionnaire | The IMT group showed significantly greater improvement of QoL compared to the CON group. |
| Low (30% MIP) | Dall’Ago, 2006 [12] | Inspiratory muscle strength: MIP Inspiratory muscle endurance: Pthmax Exercise capacity: CPET (VO2max, blood pressure, VE peak, maximal circulatory power), 6MWT (distance + dyspnoea Borg scale) | The IMT group showed significantly greater improvement in MIP, Pthmax, VE peak, VO2peak, maximal circulatory power, and 6MWD compared to the CON group. | Dyspnoea: the Borg scale QoL: the MLHF Questionnaire Lung function: FVC, FEV1 | The IMT group showed significantly greater improvement in dyspnoea and QoL compared to the CON group. No significant improvement in lung function in any of the groups. |
| Low (30% MIP) | Johnson, 1998 [13] | Inspiratory muscle strength: MIP Exercise capacity: treadmill stress test (modified Bruce protocol), Corridor walk test (time) | IMT showed significantly greater improvement in MIP compared to the CON group. No significant improvement in treadmill test time, corridor walk test time in both groups. | Dyspnoea: the Borg scale (during activity) QoL: disease-specific questionnaire | No significant improvement in dyspnoea and QoL scores in both groups. |
| Low/Moderate (30–40% MIP) | Tran, 2021 [16] | Inspiratory muscle strength: MIP Exercise capacity: CPET on ergometer (resting VO2, VO2 peak, resting SpO2, SpO2 peak, peak HR, O2 pulse, VE/VCO2, OUES, peak RER), 6MWT (mean change in distance) | The IMT group showed significantly greater improvement in MIP compared to the CON group. Significant improvement in 6MWD in the IMT group, with no significant improvement observed in the CON group. No significant differences In peak VO2 between groups. | Lung function: FVC, FEV1 | No significant improvement in lung function in any of the groups. |
| Moderate (40% MIP) | Bosnak-Guclu, 2011 [2] | Inspiratory muscle strength: MIP Exercise capacity: 6MWT (distance + % predicted distance + HRmax%) | The IMT group showed significantly greater improvements in MIP and 6MWD compared to the CON group. | QoL: Turkish version of the SF-36, Fatigue Severity Scale, Montgomery Âsberg Depression Rating Scale Dyspnoea: MMRC dyspnoea scale + Borg scale (during activity) Lung function: FEV1, FVC, PEF | Significant decreases in depression and dyspnoea in the IMT group compared to CON group. Improvements in lung function, QoL and fatigue perception are significant but similar in both groups. |
| Moderate (50% MIP) | Parreiras de Menezes, 2019 [9] | Inspiratory muscle strength: MIP Inspiratory muscle endurance: number of breaths Exercise capacity: 6MWT (distance) | The IMT group showed significantly greater improvement in MIP and inspiratory endurance compared to the CON group. No significant difference in 6MWD between groups. | Dyspnoea: the MRC scale | The IMT group showed significantly greater improvement in dyspnoea compared to the CON group. |
| High (100% of 10 RM) | Marco, 2013 [14] | Inspiratory muscle strength: MIP Inspiratory muscle endurance: 10 RM | The IMT group showed significantly greater improvement in MIP and 10 RM compared to the CON group. | Dyspnoea: the MMRC dyspnoea scale QoL: the MLHF questionnaire, SF-36 | No significant differences between groups. |
| High (60% MIP) | Weiner, 1999 [10] | Inspiratory muscle strength: MIP Inspiratory muscle endurance: PmPeak Exercise capacity: 12MWT (distance), exercise tolerance test (VO2max + RR) | Significant improvement in MIP, inspiratory muscle endurance, twelve-minute distance walk in the IMT group, with no significant improvement observed in the CON group. No significant changes in VO2max in both groups. | Lung function: FVC, FEV1 Dyspnoea: dyspnoea index described by Mahler and Harver | Significant improvement in dyspnoea and minimal but significant increase in FVC in the IMT group compared with the CON group. No significant improvement in FEV1 in any of the groups. |
| First Author, Year | PEDro Ratings | Quality of Evidence | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Total | ||
| Bosnak-Guclu, 2011 [2] | Yes | ✔ | ✘ | ✔ | ✔ | ✘ | ✔ | ✘ | ✘ | ✔ | ✔ | 6 | High |
| Dall’Ago, 2006 [20] | Yes | ✔ | ✔ | ✔ | ✘ | ✘ | ✔ | ✘ | ✘ | ✔ | ✔ | 6 | High |
| Johnson, 1998 [21] | No | ✔ | ✘ | ✔ | ✔ | ✘ | ✘ | ✔ | ✘ | ✔ | ✔ | 6 | High |
| Marco, 2013 [22] | Yes | ✔ | ✔ | ✔ | ✔ | ✘ | ✔ | ✔ | ✔ | ✔ | ✔ | 9 | High |
| Palau, 2014 [23] | Yes | ✔ | ✘ | ✔ | ✘ | ✘ | ✔ | ✔ | ✔ | ✔ | ✔ | 7 | High |
| Parreiras de Menezes, 2019 [12] | Yes | ✔ | ✔ | ✘ | ✔ | ✘ | ✘ | ✘ | ✔ | ✔ | ✔ | 6 | High |
| Tran, 2021 [24] | Yes | ✔ | ✘ | ✔ | ✘ | ✘ | ✘ | ✔ | ✔ | ✔ | ✔ | 6 | High |
| Weiner, 1999 [13] | Yes | ✔ | ✘ | ✔ | ✘ | ✘ | ✘ | ✔ | ✔ | ✔ | ✔ | 6 | High |
| Number of Studies (Design) | Comparison | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Intervention (n) | Comparator (n) | Certainty |
|---|---|---|---|---|---|---|---|---|---|
| MIP | |||||||||
| 3 RCTs [2,21,22] | IMT vs SHAM IMT | Not serious | Serious a | Not serious | Serious d | None | 36 | 34 | ⨁⨁◯◯ Low |
| 5 RCTs [12,13,20,23,24] | IMT vs no intervention | Not serious | Not serious | Not serious | Serious d | None | 65 | 63 | ⨁⨁⨁◯ Moderate |
| Walking distance | |||||||||
| 4 RCTs [12,20,23,24] | IMT vs no intervention | Not serious | Serious b | Not serious | Very serious d,e | None | 55 | 53 | ⨁◯◯◯ Very low |
| 1 RCT [2] | IMT vs SHAM IMT | Not serious | Not serious | Not serious | Serious d | None | 16 | 14 | ⨁⨁⨁◯ Moderate |
| VO2 peak | |||||||||
| 3 RCTs [20,23,24] | IMT vs no intervention | Not serious | Serious c | Not serious | Serious d,f | None | 36 | 34 | ⨁⨁◯◯ Low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beaujolin, A.; Mané, J.; Presse, C.; Barbosa-Silva, J.; Bernini, M.; Corbellini, C.; de Abreu, R.M. Inspiratory Muscle Training Intensity in Patients Living with Cardiovascular Diseases: A Systematic Review. Hearts 2024, 5, 75-90. https://doi.org/10.3390/hearts5010006
Beaujolin A, Mané J, Presse C, Barbosa-Silva J, Bernini M, Corbellini C, de Abreu RM. Inspiratory Muscle Training Intensity in Patients Living with Cardiovascular Diseases: A Systematic Review. Hearts. 2024; 5(1):75-90. https://doi.org/10.3390/hearts5010006
Chicago/Turabian StyleBeaujolin, Anaïs, Jessica Mané, Céline Presse, Jordana Barbosa-Silva, Michela Bernini, Camilo Corbellini, and Raphael Martins de Abreu. 2024. "Inspiratory Muscle Training Intensity in Patients Living with Cardiovascular Diseases: A Systematic Review" Hearts 5, no. 1: 75-90. https://doi.org/10.3390/hearts5010006
APA StyleBeaujolin, A., Mané, J., Presse, C., Barbosa-Silva, J., Bernini, M., Corbellini, C., & de Abreu, R. M. (2024). Inspiratory Muscle Training Intensity in Patients Living with Cardiovascular Diseases: A Systematic Review. Hearts, 5(1), 75-90. https://doi.org/10.3390/hearts5010006







