An Update on the Use of Artificial Intelligence in Cardiovascular Medicine
Abstract
1. Introduction
2. Artificial Intelligence: A Brief Overview
Materials and Methods
3. Applications and Utility of Artificial Intelligence in Cardiovascular Medicine Major Subspecialties (Figure 2)
3.1. Preventive Cardiology
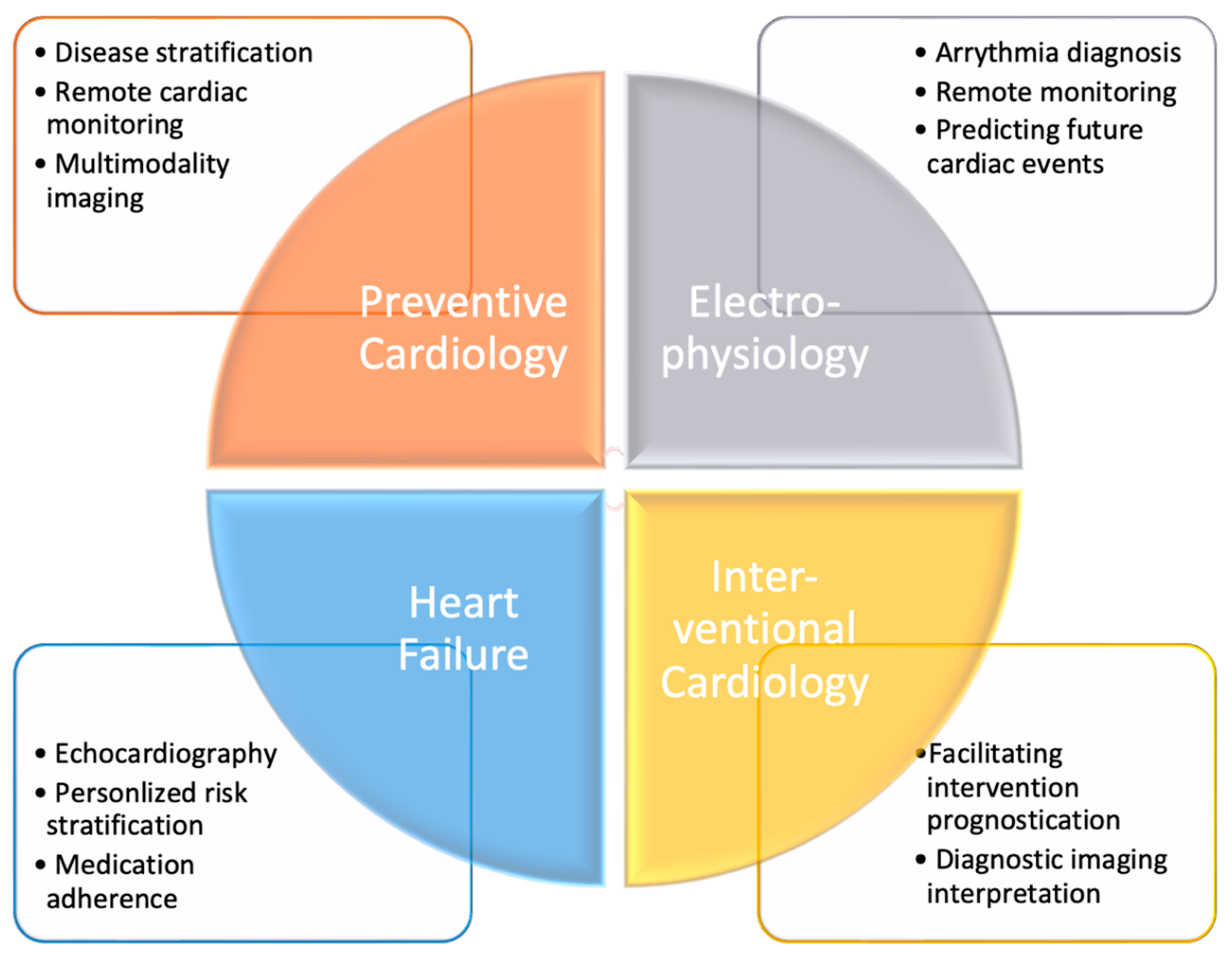
3.2. Cardiac Electrophysiology
3.3. Advanced Heart Failure and Circulatory Support
3.4. Interventional Cardiology and Cardiovascular Imaging
4. Future Direction for Applications of Artificial Intelligence and ChatGPT in Cardiovascular Medicine
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sallam, M. ChatGPT Utility in Healthcare Education, Research, and Practice: Systematic Review on the Promising Perspectives and Valid Concerns. Healthcare 2023, 11, 887. [Google Scholar] [CrossRef]
- Korteling, J.E.H.; van de Boer-Visschedijk, G.C.; Blankendaal, R.a.M.; Boonekamp, R.C.; Eikelboom, A.R. Human- versus Artificial Intelligence. Front. Artif. Intell. 2021, 4, 622364. [Google Scholar] [CrossRef] [PubMed]
- Sarker, I.H. AI-Based Modeling: Techniques, Applications and Research Issues Towards Automation, Intelligent and Smart Systems. SN Comput. Sci. 2022, 3, 158. [Google Scholar] [CrossRef] [PubMed]
- OpenAI. OpenAI: Models GPT-3. Available online: https://beta.openai.com/docs/models (accessed on 9 May 2023).
- Brown, T.B.; Mann, B.; Ryder, N.; Subbiah, M.; Kaplan, J.; Dhariwal, P.; Neelakantan, A.; Shyam, P.; Sastry, G.; Askell, A.; et al. Language Models are Few-Shot Learners. arXiv 2020, arXiv:2005.14165. [Google Scholar] [CrossRef]
- King, M.R.; ChatGPT. A Conversation on Artificial Intelligence, Chatbots, and Plagiarism in Higher Education. Cell. Mol. Bioeng. 2023, 16, 1–2. [Google Scholar] [CrossRef]
- Haq, I.U.; Chhatwal, K.; Sanaka, K.; Xu, B. Artificial Intelligence in Cardiovascular Medicine: Current Insights and Future Prospects. Vasc. Health Risk Manag. 2022, 18, 517–528. [Google Scholar] [CrossRef]
- Johnson, K.W.; Torres Soto, J.; Glicksberg, B.S.; Shameer, K.; Miotto, R.; Ali, M.; Ashley, E.; Dudley, J.T. Artificial Intelligence in Cardiology. J. Am. Coll. Cardiol. 2018, 71, 2668–2679. [Google Scholar] [CrossRef]
- Lin, A.; Kolossváry, M.; Motwani, M.; Išgum, I.; Maurovich-Horvat, P.; Slomka, P.J.; Dey, D. Artificial Intelligence in Cardiovascular Imaging for Risk Stratification in Coronary Artery Disease. Radiol. Cardiothorac. Imaging 2021, 3, e200512. [Google Scholar] [CrossRef]
- German, C.A.; Baum, S.J.; Ferdinand, K.C.; Gulati, M.; Polonsky, T.S.; Toth, P.P.; Shapiro, M.D. Defining preventive cardiology: A clinical practice statement from the American Society for Preventive Cardiology. Am. J. Prev. Cardiol. 2022, 12, 100432. [Google Scholar] [CrossRef] [PubMed]
- Dawber, T.R.; Kannel, W.B.; Revotskie, N.; Stokes, J.; Kagan, A.; Gordon, T. Some Factors Associated with the Development of Coronary Heart Disease—Six Years’ Follow-Up Experience in the Framingham Study. Am. J. Public Health Nations Health 1959, 49, 1349–1356. [Google Scholar] [CrossRef]
- Kannel, W.B. Factors of Risk in the Development of Coronary Heart Disease—Six-Year Follow-Up Experience: The Framingham Study. Ann. Intern. Med. 1961, 55, 33. [Google Scholar] [CrossRef]
- Kannel, W.B. Habitual level of physical activity and risk of coronary heart disease: The Framingham study. Can. Med. Assoc. J. 1967, 96, 811–812. [Google Scholar]
- Kannel, W.B.; Lebauer, E.J.; Dawber, T.R.; Mcnamara, P.M. Relation of Body Weight to Development of Coronary Heart Disease: The Framingham Study. Circulation 1967, 35, 734–744. [Google Scholar] [CrossRef]
- Kannel, W.B.; McGee, D.L. Diabetes and cardiovascular risk factors: The Framingham study. Circulation 1979, 59, 8–13. [Google Scholar] [CrossRef]
- Sng, G.G.R.; Tung, J.Y.M.; Lim, D.Y.Z.; Bee, Y.M. Potential and Pitfalls of ChatGPT and Natural-Language Artificial Intelligence Models for Diabetes Education. Diabetes Care 2023, 46, e103–e105. [Google Scholar] [CrossRef]
- Ciccarelli, M.; Giallauria, F.; Carrizzo, A.; Visco, V.; Silverio, A.; Cesaro, A.; Calabrò, P.; De Luca, N.; Mancusi, C.; Masarone, D.; et al. Artificial intelligence in cardiovascular prevention: New ways will open new doors. J. Cardiovasc. Med. 2023, 24 (Suppl. S2), e106–e115. [Google Scholar] [CrossRef]
- Liao, B.; Jia, X.; Zhang, T.; Sun, R. DHDIP: An interpretable model for hypertension and hyperlipidemia prediction based on EMR data. Comput. Methods Programs Biomed. 2022, 226, 107088. [Google Scholar] [CrossRef]
- Elgendi, M.; Fletcher, R.; Liang, Y.; Howard, N.; Lovell, N.H.; Abbott, D.; Lim, K.; Ward, R. The use of photoplethysmography for assessing hypertension. NPJ Digit. Med. 2019, 2, 60. [Google Scholar] [CrossRef]
- Tangri, N.; Ferguson, T.W. Artificial Intelligence in the Identification, Management, and Follow-Up of CKD. Kidney360 2022, 3, 554–556. [Google Scholar] [CrossRef]
- Kabra, R.; Israni, S.; Vijay, B.; Baru, C.; Mendu, R.; Fellman, M.; Sridhar, A.; Mason, P.; Cheung, J.W.; DiBiase, L.; et al. Emerging role of artificial intelligence in cardiac electrophysiology. Cardiovasc. Digit. Health J. 2022, 3, 263–275. [Google Scholar] [CrossRef]
- Lee, P.; Bubeck, S.; Petro, J. Benefits, Limits, and Risks of GPT-4 as an AI Chatbot for Medicine. N. Engl. J. Med. 2023, 388, 1233–1239. [Google Scholar] [CrossRef]
- Kataoka, Y.; So, R. Benefits, Limits, and Risks of GPT-4 as an AI Chatbot for Medicine. N. Engl. J. Med. 2023, 388, 2399–2400. [Google Scholar] [CrossRef]
- Dassen, W.R.M.; Mulleneers, R.; Smeets, J.; Den Dulk, K.; Cruz, F.; Brugada, P.; Wellens, H.J.J. Self-learning neural networks in electrocardiography. J. Electrocardiol. 1990, 23, 200–202. [Google Scholar] [CrossRef]
- Dassen, W.R.M.; Mulleneers, R.G.A.; Dulk, K.D.; Smeets, J.R.L.M.; Cruz, F.; Penn, O.C.K.M.; Wellens, H.J.J. An Artificial Neural Network to Localize Atrioventricular Accessory Pathways in Patients Suffering from the Wolff-Parkinson-White Syndrome. Pacing Clin. Electrophysiol. 1990, 13, 1792–1796. [Google Scholar] [CrossRef]
- January, C.T.; Wann, L.S.; Calkins, H.; Chen, L.Y.; Cigarroa, J.E.; Cleveland, J.C.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; Furie, K.L.; et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients with Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration with the Society of Thoracic Surgeons. Circulation 2019, 140, e125–e151. [Google Scholar] [CrossRef]
- Giudicessi, J.R.; Schram, M.; Bos, J.M.; Galloway, C.D.; Shreibati, J.B.; Johnson, P.W.; Carter, R.E.; Disrud, L.W.; Kleiman, R.; Attia, Z.I.; et al. Artificial Intelligence–Enabled Assessment of the Heart Rate Corrected QT Interval Using a Mobile Electrocardiogram Device. Circulation 2021, 143, 1274–1286. [Google Scholar] [CrossRef]
- Hannun, A.Y.; Rajpurkar, P.; Haghpanahi, M.; Tison, G.H.; Bourn, C.; Turakhia, M.P.; Ng, A.Y. Cardiologist-level arrhythmia detection and classification in ambulatory electrocardiograms using a deep neural network. Nat. Med. 2019, 25, 65–69. [Google Scholar] [CrossRef]
- Acharya, U.R.; Fujita, H.; Lih, O.S.; Hagiwara, Y.; Tan, J.H.; Adam, M. Automated detection of arrhythmias using different intervals of tachycardia ECG segments with convolutional neural network. Inf. Sci. 2017, 405, 81–90. [Google Scholar] [CrossRef]
- Kashou, A.H.; Ko, W.-Y.; Attia, Z.I.; Cohen, M.S.; Friedman, P.A.; Noseworthy, P.A. A comprehensive artificial intelligence–enabled electrocardiogram interpretation program. Cardiovasc. Digit. Health J. 2020, 1, 62–70. [Google Scholar] [CrossRef]
- Hughes, J.W.; Olgin, J.E.; Avram, R.; Abreau, S.A.; Sittler, T.; Radia, K.; Hsia, H.; Walters, T.; Lee, B.; Gonzalez, J.E.; et al. Performance of a Convolutional Neural Network and Explainability Technique for 12-Lead Electrocardiogram Interpretation. JAMA Cardiol. 2021, 6, 1285. [Google Scholar] [CrossRef]
- Xiong, Z.; Stiles, M.; Zhao, J. Robust ECG Signal Classification for the Detection of Atrial Fibrillation Using Novel Neural Networks. In Proceedings of the 2017 Computing in Cardiology (CinC), Rennes, France, 24–27 September 2017. [Google Scholar] [CrossRef]
- Attia, Z.I.; Noseworthy, P.A.; Lopez-Jimenez, F.; Asirvatham, S.J.; Deshmukh, A.J.; Gersh, B.J.; Carter, R.E.; Yao, X.; Rabinstein, A.A.; Erickson, B.J.; et al. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: A retrospective analysis of outcome prediction. Lancet 2019, 394, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Tison, G.H.; Zhang, J.; Delling, F.N.; Deo, R.C. Automated and Interpretable Patient ECG Profiles for Disease Detection, Tracking, and Discovery. Circ. Cardiovasc. Qual. Outcomes 2019, 12, e005289. [Google Scholar] [CrossRef] [PubMed]
- Grogan, M.; Lopez-Jimenez, F.; Cohen-Shelly, M.; Dispenzieri, A.; Attia, Z.I.; Abou Ezzedine, O.F.; Lin, G.; Kapa, S.; Borgeson, D.D.; Friedman, P.A.; et al. Artificial Intelligence–Enhanced Electrocardiogram for the Early Detection of Cardiac Amyloidosis. Mayo Clin. Proc. 2021, 96, 2768–2778. [Google Scholar] [CrossRef] [PubMed]
- Siontis, K.C.; Liu, K.; Bos, J.M.; Attia, Z.I.; Cohen-Shelly, M.; Arruda-Olson, A.M.; Zanjirani Farahani, N.; Friedman, P.A.; Noseworthy, P.A.; Ackerman, M.J. Detection of hypertrophic cardiomyopathy by an artificial intelligence electrocardiogram in children and adolescents. Int. J. Cardiol. 2021, 340, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Shelly, M.; Attia, Z.I.; Friedman, P.A.; Ito, S.; Essayagh, B.A.; Ko, W.-Y.; Murphree, D.H.; Michelena, H.I.; Enriquez-Sarano, M.; Carter, R.E.; et al. Electrocardiogram screening for aortic valve stenosis using artificial intelligence. Eur. Heart J. 2021, 42, 2885–2896. [Google Scholar] [CrossRef] [PubMed]
- Attia, Z.I.; Kapa, S.; Dugan, J.; Pereira, N.; Noseworthy, P.A.; Jimenez, F.L.; Cruz, J.; Carter, R.E.; DeSimone, D.C.; Signorino, J.; et al. Rapid Exclusion of COVID Infection With the Artificial Intelligence Electrocardiogram. Mayo Clin. Proc. 2021, 96, 2081–2094. [Google Scholar] [CrossRef] [PubMed]
- Bos, J.M.; Attia, Z.I.; Albert, D.E.; Noseworthy, P.A.; Friedman, P.A.; Ackerman, M.J. Use of Artificial Intelligence and Deep Neural Networks in Evaluation of Patients with Electrocardiographically Concealed Long QT Syndrome from the Surface 12-Lead Electrocardiogram. JAMA Cardiol. 2021, 6, 532. [Google Scholar] [CrossRef]
- Perez, M.V.; Mahaffey, K.W.; Hedlin, H.; Rumsfeld, J.S.; Garcia, A.; Ferris, T.; Balasubramanian, V.; Russo, A.M.; Rajmane, A.; Cheung, L.; et al. Large-Scale Assessment of a Smartwatch to Identify Atrial Fibrillation. N. Engl. J. Med. 2019, 381, 1909–1917. [Google Scholar] [CrossRef]
- Pereira, T.; Tran, N.; Gadhoumi, K.; Pelter, M.M.; Do, D.H.; Lee, R.J.; Colorado, R.; Meisel, K.; Hu, X. Photoplethysmography based atrial fibrillation detection: A review. NPJ Digit. Med. 2020, 3, 3. [Google Scholar] [CrossRef]
- Abdou, A.; Krishnan, S. Horizons in Single-Lead ECG Analysis From Devices to Data. Front. Signal Process. 2022, 2, 866047. [Google Scholar] [CrossRef]
- Willcox, M.E.; Compton, S.J.; Bardy, G.H. Continuous ECG monitoring versus mobile telemetry: A comparison of arrhythmia diagnostics in human- versus algorithmic-dependent systems. Heart Rhythm. O2 2021, 2, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Westphal, P.; Luo, H.; Shahmohammadi, M.; Prinzen, F.W.; Delhaas, T.; Cornelussen, R.N. Machine learning-powered, device-embedded heart sound measurement can optimize AV delay in patients with CRT. Heart Rhythm 2023, 20, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Missel, R.; Gyawali, P.K.; Murkute, J.V.; Li, Z.; Zhou, S.; AbdelWahab, A.; Davis, J.; Warren, J.; Sapp, J.L.; Wang, L. A hybrid machine learning approach to localizing the origin of ventricular tachycardia using 12-lead electrocardiograms. Comput. Biol. Med. 2020, 126, 104013. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, X.; Chen, X.; Guo, J. Automatic Premature Ventricular Contraction Detection Using Deep Metric Learning and KNN. Biosensors 2021, 11, 69. [Google Scholar] [CrossRef]
- Liu, C.-M.; Chang, S.-L.; Chen, H.-H.; Chen, W.-S.; Lin, Y.-J.; Lo, L.-W.; Hu, Y.-F.; Chung, F.-P.; Chao, T.-F.; Tuan, T.-C.; et al. The Clinical Application of the Deep Learning Technique for Predicting Trigger Origins in Patients With Paroxysmal Atrial Fibrillation With Catheter Ablation. Circ. Arrhythm. Electrophysiol. 2020, 13, e008518. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kwon, O.-S.; Shim, J.; Lee, J.; Han, H.-J.; Yu, H.T.; Kim, T.-H.; Uhm, J.-S.; Joung, B.; Lee, M.-H.; et al. Left Atrial Wall Stress and the Long-Term Outcome of Catheter Ablation of Atrial Fibrillation: An Artificial Intelligence-Based Prediction of Atrial Wall Stress. Front. Physiol. 2021, 12, 686507. [Google Scholar] [CrossRef] [PubMed]
- Zou, F.; Brahier, M.; Migliarese, F.; Ma, X.; Wu, C.; Taylor, A.; Sandfort, V.; Thomaides, A.; Vargas, J. B-PO03-076 a machine learning-derived recurrence risk model for atrial fibrillation after catheter ablation. Heart Rhythm 2021, 18, S219–S220. [Google Scholar] [CrossRef]
- Schwartz, A.L.; Chorin, E.; Mann, T.; M., Y.L.; Hochstadt, A.; Margolis, G.; Viskin, S.; Banai, S.; Rosso, R. Reconstruction of the left atrium for atrial fibrillation ablation using the machine learning CARTO 3 m-FAM software. J. Interv. Card. Electrophysiol. 2022, 64, 39–47. [Google Scholar] [CrossRef]
- Juhola, M.; Penttinen, K.; Joutsijoki, H.; Varpa, K.; Saarikoski, J.; Rasku, J.; Siirtola, H.; Iltanen, K.; Laurikkala, J.; Hyyrö, H. Signal analysis and classification methods for the calcium transient data of stem cell-derived cardiomyocytes. Comput. Biol. Med. 2015, 61, 1–7. [Google Scholar] [CrossRef]
- Visco, V.; Radano, I.; Campanile, A.; Ravera, A.; Silverio, A.; Masarone, D.; Pacileo, G.; Correale, M.; Mazzeo, P.; Dattilo, G.; et al. Predictors of sacubitril/valsartan high dose tolerability in a real world population with HFrEF. ESC Heart Fail. 2022, 9, 2909–2917. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Romiti, S.; Vinciguerra, M.; Saade, W.; Anso Cortajarena, I.; Greco, E. Artificial Intelligence (AI) and Cardiovascular Diseases: An Unexpected Alliance. Cardiol. Res. Pract. 2020, 2020, 4972346. [Google Scholar] [CrossRef]
- Ortiz, J.; Ghefter, C.G.; Silva, C.E.; Sabbatini, R.M. One-year mortality prognosis in heart failure: A neural network approach based on echocardiographic data. J. Am. Coll. Cardiol. 1995, 26, 1586–1593. [Google Scholar] [CrossRef] [PubMed]
- Binder, T.; Süssner, M.; Moertl, D.; Strohmer, T.; Baumgartner, H.; Maurer, G.; Porenta, G. Artificial neural networks and spatial temporal contour linking for automated endocardial contour detection on echocardiograms: A novel approach to determine left ventricular contractile function. Ultrasound Med. Biol. 1999, 25, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.E.; Kim, S.; Chang, H.J. Artificial Intelligence and Echocardiography. J. Cardiovasc. Imaging 2021, 29, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Arshad, M.S.; Greene, S.J.; Van Spall, H.G.C.; Pandey, A.; Vemulapalli, S.; Perakslis, E.; Butler, J. Artificial intelligence and heart failure: A state-of-the-art review. Eur. J. Heart Fail. 2023, 25, 1507–1525. [Google Scholar] [CrossRef]
- Medvedofsky, D.; Mor-Avi, V.; Amzulescu, M.; Fernández-Golfín, C.; Hinojar, R.; Monaghan, M.J.; Otani, K.; Reiken, J.; Takeuchi, M.; Tsang, W.; et al. Three-dimensional echocardiographic quantification of the left-heart chambers using an automated adaptive analytics algorithm: Multicentre validation study. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 47–58. [Google Scholar] [CrossRef]
- Sun, J.; Qiu, Y.; Guo, H.; Hua, Y.; Shao, B.; Qiao, Y.; Guo, J.; Ding, H.; Zhang, Z.; Miao, L.; et al. A method to screen left ventricular dysfunction through ECG based on convolutional neural network. J. Cardiovasc. Electrophysiol. 2021, 32, 1095–1102. [Google Scholar] [CrossRef]
- Ouyang, D.; He, B.; Ghorbani, A.; Yuan, N.; Ebinger, J.; Langlotz, C.P.; Heidenreich, P.A.; Harrington, R.A.; Liang, D.H.; Ashley, E.A.; et al. Video-based AI for beat-to-beat assessment of cardiac function. Nature 2020, 580, 252–256. [Google Scholar] [CrossRef]
- Jiang, B.; Guo, N.; Ge, Y.; Zhang, L.; Oudkerk, M.; Xie, X. Development and application of artificial intelligence in cardiac imaging. Br. J. Radiol. 2020, 93, 20190812. [Google Scholar] [CrossRef]
- Shade, J.K.; Prakosa, A.; Popescu, D.M.; Yu, R.; Okada, D.R.; Chrispin, J.; Trayanova, N.A. Predicting risk of sudden cardiac death in patients with cardiac sarcoidosis using multimodality imaging and personalized heart modeling in a multivariable classifier. Sci. Adv. 2021, 7, eabi8020. [Google Scholar] [CrossRef]
- Son, Y.-J.; Kim, H.-G.; Kim, E.-H.; Choi, S.; Lee, S.-K. Application of Support Vector Machine for Prediction of Medication Adherence in Heart Failure Patients. Healthc. Inform. Res. 2010, 16, 253. [Google Scholar] [CrossRef]
- Cikes, M.; Sanchez-Martinez, S.; Claggett, B.; Duchateau, N.; Piella, G.; Butakoff, C.; Pouleur, A.C.; Knappe, D.; Biering-Sørensen, T.; Kutyifa, V.; et al. Machine learning-based phenogrouping in heart failure to identify responders to cardiac resynchronization therapy. Eur. J. Heart Fail. 2019, 21, 74–85. [Google Scholar] [CrossRef]
- Stehlik, J.; Schmalfuss, C.; Bozkurt, B.; Nativi-Nicolau, J.; Wohlfahrt, P.; Wegerich, S.; Rose, K.; Ray, R.; Schofield, R.; Deswal, A.; et al. Continuous Wearable Monitoring Analytics Predict Heart Failure Hospitalization: The LINK-HF Multicenter Study. Circ. Heart Fail. 2020, 13, e006513. [Google Scholar] [CrossRef]
- Kang, Y.; McHugh, M.D.; Chittams, J.; Bowles, K.H. Utilizing Home Healthcare Electronic Health Records for Telehomecare Patients With Heart Failure: A Decision Tree Approach to Detect Associations With Rehospitalizations. CIN Comput. Inform. Nurs. 2016, 34, 175–182. [Google Scholar] [CrossRef]
- Piening, B.D.; Dowdell, A.K.; Zhang, M.; Loza, B.-L.; Walls, D.; Gao, H.; Mohebnasab, M.; Li, Y.R.; Elftmann, E.; Wei, E.; et al. Whole transcriptome profiling of prospective endomyocardial biopsies reveals prognostic and diagnostic signatures of cardiac allograft rejection. J. Heart Lung Transplant. 2022, 41, 840–848. [Google Scholar] [CrossRef]
- Peyster, E.G.; Arabyarmohammadi, S.; Janowczyk, A.; Azarianpour-Esfahani, S.; Sekulic, M.; Cassol, C.; Blower, L.; Parwani, A.; Lal, P.; Feldman, M.D.; et al. An automated computational image analysis pipeline for histological grading of cardiac allograft rejection. Eur. Heart J. 2021, 42, 2356–2369. [Google Scholar] [CrossRef]
- Lipkova, J.; Chen, T.Y.; Lu, M.Y.; Chen, R.J.; Shady, M.; Williams, M.; Wang, J.; Noor, Z.; Mitchell, R.N.; Turan, M.; et al. Deep learning-enabled assessment of cardiac allograft rejection from endomyocardial biopsies. Nat. Med. 2022, 28, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Medved, D.; Ohlsson, M.; Höglund, P.; Andersson, B.; Nugues, P.; Nilsson, J. Improving prediction of heart transplantation outcome using deep learning techniques. Sci. Rep. 2018, 8, 3613. [Google Scholar] [CrossRef] [PubMed]
- Gotlieb, N.; Azhie, A.; Sharma, D.; Spann, A.; Suo, N.-J.; Tran, J.; Orchanian-Cheff, A.; Wang, B.; Goldenberg, A.; Chassé, M.; et al. The promise of machine learning applications in solid organ transplantation. NPJ Digit. Med. 2022, 5, 89. [Google Scholar] [CrossRef] [PubMed]
- Lisboa, P.J.G.; Jayabalan, M.; Ortega-Martorell, S.; Olier, I.; Medved, D.; Nilsson, J. Enhanced survival prediction using explainable artificial intelligence in heart transplantation. Sci. Rep. 2022, 12, 19525. [Google Scholar] [CrossRef]
- Hoda, M.R.; Grimm, M.; Laufer, G. Prediction of Cyclosporine Blood Levels in Heart Transplantation Patients Using a Pharmacokinetic Model Identified by Evolutionary Algorithms. J. Heart Lung Transplant. 2005, 24, 1855–1862. [Google Scholar] [CrossRef]
- Woillard, J.; Labriffe, M.; Debord, J.; Marquet, P. Tacrolimus Exposure Prediction Using Machine Learning. Clin. Pharmacol. Ther. 2021, 110, 361–369. [Google Scholar] [CrossRef]
- Al-Ani, M.A.; Bai, C.; Hashky, A.; Parker, A.M.; Vilaro, J.R.; Aranda, J.M., Jr.; Shickel, B.; Rashidi, P.; Bihorac, A.; Ahmed, M.M.; et al. Artificial intelligence guidance of advanced heart failure therapies: A systematic scoping review. Front. Cardiovasc. Med. 2023, 10, 1127716. [Google Scholar] [CrossRef]
- Henglin, M.; Stein, G.; Hushcha, P.V.; Snoek, J.; Wiltschko, A.B.; Cheng, S. Machine Learning Approaches in Cardiovascular Imaging. Circ. Cardiovasc. Imaging 2017, 10, e005614. [Google Scholar] [CrossRef]
- Subhan, S.; Malik, J.; Haq, A.U.; Qadeer, M.S.; Zaidi, S.M.J.; Orooj, F.; Zaman, H.; Mehmoodi, A.; Majeedi, U. Role of Artificial Intelligence and Machine Learning in Interventional Cardiology. Curr. Probl. Cardiol. 2023, 48, 101698. [Google Scholar] [CrossRef] [PubMed]
- Du, T.; Xie, L.; Liu, X. Intelligent recognition of coronary angiography by deep learning technology: A novel computer-aided diagnostic system. J. Am. Coll. Cardiol. 2018, 72, B98. [Google Scholar] [CrossRef]
- Molenaar, M.A.; Selder, J.L.; Nicolas, J.; Claessen, B.E.; Mehran, R.; Bescós, J.O.; Schuuring, M.J.; Bouma, B.J.; Verouden, N.J.; Chamuleau, S.A.J. Current State and Future Perspectives of Artificial Intelligence for Automated Coronary Angiography Imaging Analysis in Patients with Ischemic Heart Disease. Curr. Cardiol. Rep. 2022, 24, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Ciusdel, C.; Turcea, A.; Puiu, A. An artificial intelligence based solution for fully auto- mated cardiac phase and end-diastolic frame detection on coronary angiographies. J. Am. Coll. Cardiol. 2018, 72, B96–B97. [Google Scholar] [CrossRef]
- Molony, D.; Hosseini, H.; Samady, H. Deep IVUS: A machine learning framework for fully automatic IVUS segmentation. J. Am. Coll. Cardiol. 2018, 72, B1. [Google Scholar] [CrossRef]
- Liao, J.; Huang, L.; Qu, M.; Chen, B.; Wang, G. Artificial Intelligence in Coronary CT Angiography: Current Status and Future Prospects. Front. Cardiovasc. Med. 2022, 9, 896366. [Google Scholar] [CrossRef] [PubMed]
- Wolterink, J.M.; Leiner, T.; Takx, R.A.P.; Viergever, M.A.; Išgum, I. An automatic machine learning system for coronary calcium scoring in clinical non-contrast enhanced, ECG-triggered cardiac CT. Proceedings of SPIE: Medical Imaging 2014—Computer-Aided Diagnosis, San Diego, CA, USA, 15–20 February 2014; Aylward, S., Hadjiiski, L.M., Eds.; International Society for Optics and Photonics: Bellingham, WA, USA, 2014; Volume 9035, p. 90350. [Google Scholar] [CrossRef]
- Mediratta, A.; Addetia, K.; Medvedofsky, D.; Schneider, R.J.; Kruse, E.; Shah, A.P.; Nathan, S.; Paul, J.D.; Blair, J.E.; Ota, T.; et al. 3D echocardiographic analysis of aortic annulus for transcatheter aortic valve replacement using novel aortic valve quantification software: Comparison with computed tomography. Echocardiography 2017, 34, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Guez, D.; Boroumand, G.; Ruggiero, N.J.; Mehrotra, P.; Halpern, E.J. Automated and Manual Measurements of the Aortic Annulus with ECG-Gated Cardiac CT Angiography Prior to Transcatheter Aortic Valve Replacement. Acad. Radiol. 2017, 24, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Kagiyama, N.; Toki, M.; Hara, M.; Fukuda, S.; Aritaka, S.; Miki, T.; Ohara, M.; Hayashida, A.; Hirohata, A.; Yamamoto, K.; et al. Efficacy and Accuracy of Novel Automated Mitral Valve Quantification: Three-Dimensional Transesophageal Echocardiographic Study. Echocardiography 2016, 33, 756–763. [Google Scholar] [CrossRef]
- Baumgartner, C. The potential impact of ChatGPT in clinical and translational medicine. Clin. Transl. Med. 2023, 13, e1206. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rao, S.J.; Iqbal, S.B.; Isath, A.; Virk, H.U.H.; Wang, Z.; Glicksberg, B.S.; Krittanawong, C. An Update on the Use of Artificial Intelligence in Cardiovascular Medicine. Hearts 2024, 5, 91-104. https://doi.org/10.3390/hearts5010007
Rao SJ, Iqbal SB, Isath A, Virk HUH, Wang Z, Glicksberg BS, Krittanawong C. An Update on the Use of Artificial Intelligence in Cardiovascular Medicine. Hearts. 2024; 5(1):91-104. https://doi.org/10.3390/hearts5010007
Chicago/Turabian StyleRao, Shiavax J., Shaikh B. Iqbal, Ameesh Isath, Hafeez Ul Hassan Virk, Zhen Wang, Benjamin S. Glicksberg, and Chayakrit Krittanawong. 2024. "An Update on the Use of Artificial Intelligence in Cardiovascular Medicine" Hearts 5, no. 1: 91-104. https://doi.org/10.3390/hearts5010007
APA StyleRao, S. J., Iqbal, S. B., Isath, A., Virk, H. U. H., Wang, Z., Glicksberg, B. S., & Krittanawong, C. (2024). An Update on the Use of Artificial Intelligence in Cardiovascular Medicine. Hearts, 5(1), 91-104. https://doi.org/10.3390/hearts5010007






