A Focus on Heart Failure Management through Diet and Nutrition: A Comprehensive Review
Abstract
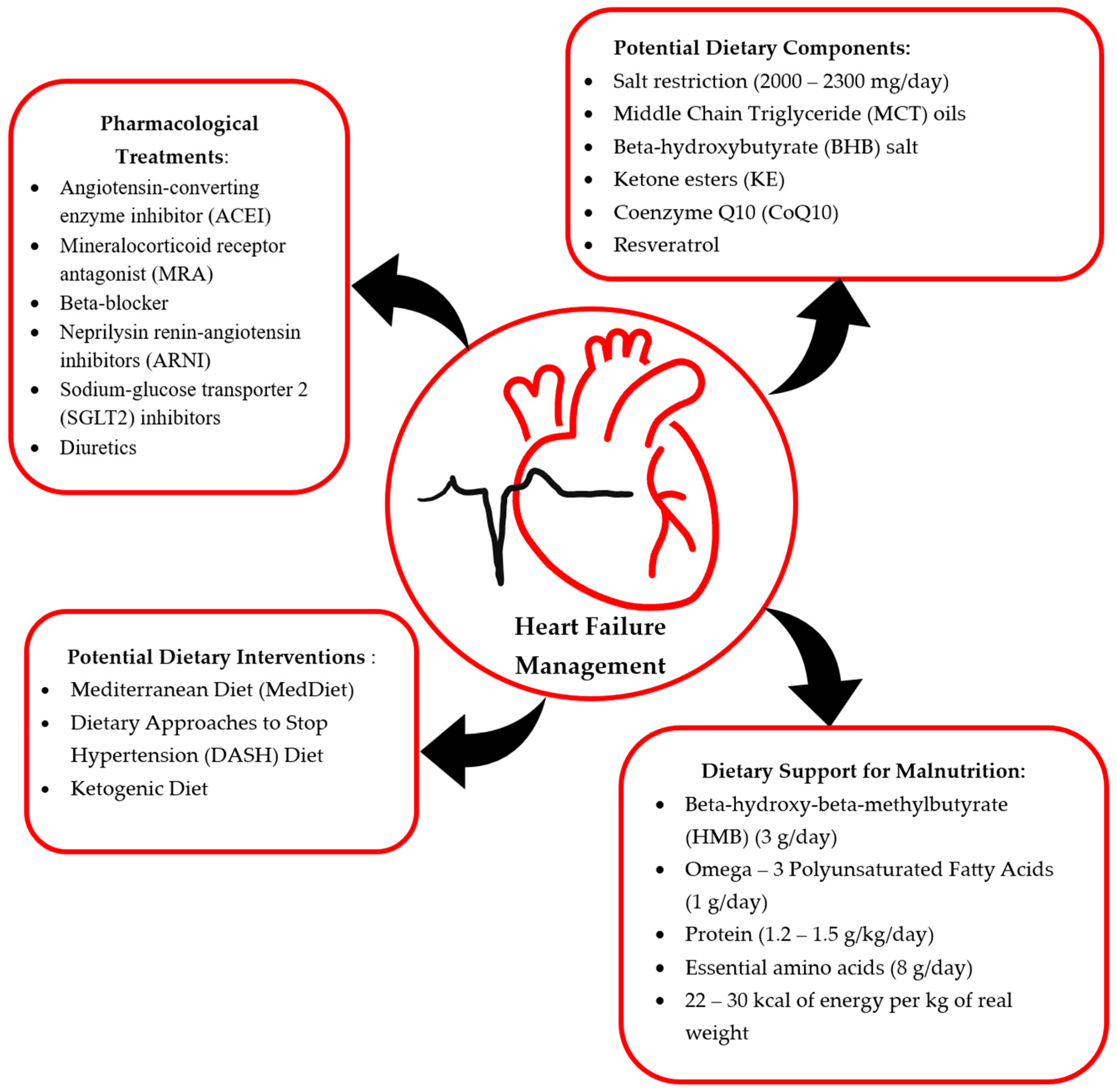
1. Introduction
2. Definition and Current Pharmacotherapies in HF
3. Dietary Components in HF
3.1. Limiting Salt Intake
3.2. Dietary Supplementation
3.2.1. MCT Oil
3.2.2. Beta-Hydroxybutyrate (BHB) Salt
3.2.3. Ketone Esters
3.2.4. Coenzyme Q10
3.2.5. Resveratrol
4. Dietary Pattern Alterations in Heart Failure Management
4.1. Dietary Approaches to Stop Hypertension (DASH)
4.2. Mediterranean Diet
4.3. Ketogenic Diet
5. Comorbidity Influence on Dietary Modifications in Heart Failure
6. Malnutrition in Advanced Heart Failure
7. Conclusions and Future Directions in Heart Failure Dietary Management
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dassanayaka, S.; Jones, S.P. Recent developments in heart failure. Circ. Res. 2015, 117, e58–e63. [Google Scholar] [CrossRef] [PubMed]
- Savarese, G.; Becher, P.M.; Lund, L.H.; Seferovic, P.; Rosano, G.M.; Coats, A.J. Global burden of heart failure: A comprehensive and updated review of epidemiology. Cardiovasc. Res. 2022, 118, 3272–3287. [Google Scholar] [CrossRef]
- Heart, Stroke and Vascular Disease: Australian Facts. Available online: https://www.aihw.gov.au/reports/heart-stroke-vascular-diseases/hsvd-facts/contents/risk-factors/overweight-and-obesity (accessed on 13 May 2024).
- Hariharaputhiran, S.; Peng, Y.; Ngo, L.; Ali, A.; Hossain, S.; Visvanathan, R.; Adams, R.; Chan, W.; Ranasinghe, I. Long-term survival and life expectancy following an acute heart failure hospitalization in Australia and New Zealand. Eur. J. Heart Fail. 2022, 24, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Atherton, J.J.; Sindone, A.; De Pasquale, C.G.; Driscoll, A.; MacDonald, P.S.; Hopper, I.; Kistler, P.M.; Briffa, T.; Wong, J.; Abhayaratna, W. National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand: Guidelines for the prevention, detection, and management of heart failure in Australia 2018. Heart Lung Circ. 2018, 27, 1123–1208. [Google Scholar] [CrossRef] [PubMed]
- Colin-Ramirez, E.; Sepehrvand, N.; Rathwell, S.; Ross, H.; Escobedo, J.; Macdonald, P.; Troughton, R.; Saldarriaga, C.; Lanas, F.; Doughty, R.; et al. Sodium Restriction in Patients With Heart Failure: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Circ. Heart. Fail. 2023, 16, e009879. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Khan, F.; Fonarow, G.C.; Sreenivasan, J.; Greene, S.J.; Khan, S.U.; Usman, M.S.; Vaduganathan, M.; Fudim, M.; Anker, S.D.; et al. Dietary interventions and nutritional supplements for heart failure: A systematic appraisal and evidence map. Eur. J. Heart Fail. 2021, 23, 1468–1476. [Google Scholar] [CrossRef] [PubMed]
- Luong, T.V.; Abild, C.B.; Bangshaab, M.; Gormsen, L.C.; Søndergaard, E. Ketogenic Diet and Cardiac Substrate Metabolism. Nutrients 2022, 14, 1322. [Google Scholar] [CrossRef] [PubMed]
- Takahara, S.; Soni, S.; Maayah, Z.H.; Ferdaoussi, M.; Dyck, J.R. Ketone therapy for heart failure: Current evidence for clinical use. Cardiovasc. Res. 2022, 118, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Burnett, H.; Earley, A.; Voors, A.A.; Senni, M.; McMurray, J.J.; Deschaseaux, C.; Cope, S. Thirty Years of Evidence on the Efficacy of Drug Treatments for Chronic Heart Failure With Reduced Ejection Fraction: A Network Meta-Analysis. Circ. Heart. Fail. 2017, 10, e003529. [Google Scholar] [CrossRef]
- Docherty, K.F.; Vaduganathan, M.; Solomon, S.D.; McMurray, J.J.V. Sacubitril/Valsartan: Neprilysin Inhibition 5 Years After PARADIGM-HF. JACC Heart Fail. 2020, 8, 800–810. [Google Scholar] [CrossRef]
- Okumura, N.; Jhund, P.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Swedberg, K.; Zile, M.R.; Solomon, S.D. Effects of sacubitril/valsartan in the PARADIGM-HF trial (Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure) according to background therapy. Circ. Heart. Fail. 2016, 9, e003212. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner-La Rocca, H.P.; Choi, D.J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- Soldin, O.P.; Mattison, D.R. Sex differences in pharmacokinetics and pharmacodynamics. Clin. Pharmacokinet. 2009, 48, 143–157. [Google Scholar] [CrossRef]
- Rosano, G.M.C.; Lewis, B.; Agewall, S.; Wassmann, S.; Vitale, C.; Schmidt, H.; Drexel, H.; Patak, A.; Torp-Pedersen, C.; Kjeldsen, K.P.; et al. Gender differences in the effect of cardiovascular drugs: A position document of the Working Group on Pharmacology and Drug Therapy of the ESC. Eur. Heart J. 2015, 36, 2677–2680. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.S.P.; Arnott, C.; Beale, A.L.; Chandramouli, C.; Hilfiker-Kleiner, D.; Kaye, D.M.; Ky, B.; Santema, B.T.; Sliwa, K.; Voors, A.A. Sex differences in heart failure. Eur. Heart J. 2019, 40, 3859–3868c. [Google Scholar] [CrossRef]
- Lennie, T.A.; Song, E.K.; Wu, J.R.; Chung, M.L.; Dunbar, S.B.; Pressler, S.J.; Moser, D.K. Three gram sodium intake is associated with longer event-free survival only in patients with advanced heart failure. J. Card. Fail. 2011, 17, 325–330. [Google Scholar] [CrossRef]
- Arcand, J.; Ivanov, J.; Sasson, A.; Floras, V.; Al-Hesayen, A.; Azevedo, E.R.; Mak, S.; Allard, J.P.; Newton, G.E. A high-sodium diet is associated with acute decompensated heart failure in ambulatory heart failure patients: A prospective follow-up study. Am. J. Clin. Nutr. 2011, 93, 332–337. [Google Scholar] [CrossRef]
- Patel, Y.; Joseph, J. Sodium Intake and Heart Failure. Int. J. Mol. Sci. 2020, 21, 9474. [Google Scholar] [CrossRef]
- Ezekowitz, J.A.; Colin-Ramirez, E.; Ross, H.; Escobedo, J.; Macdonald, P.; Troughton, R.; Saldarriaga, C.; Alemayehu, W.; McAlister, F.A.; Arcand, J.; et al. Reduction of dietary sodium to less than 100 mmol in heart failure (SODIUM-HF): An international, open-label, randomised, controlled trial. Lancet 2022, 399, 1391–1400. [Google Scholar] [CrossRef]
- Stein, C.; Helal, L.; Migliavaca, C.B.; Sangalli, C.N.; Colpani, V.; da Rosa, P.R.; Beck-da-Silva, L.; Rohde, L.E.; Polanczyk, C.A.; Falavigna, M. Are the recommendation of sodium and fluid restriction in heart failure patients changing over the past years? A systematic review and meta-analysis. Clin. Nutri. ESPEN 2022, 49, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Cheng, M.; Su, Y.; Ma, T.; Lei, X.; Hou, Y. Effect of Dietary Sodium Restriction on the Quality of Life of Patients with Heart Failure: A Systematic Review of Randomized Controlled Trials. J. Cardiovasc. Nurs. 2022, 37, 570–580. [Google Scholar] [CrossRef]
- Philipson, H.; Ekman, I.; Forslund, H.B.; Swedberg, K.; Schaufelberger, M. Salt and fluid restriction is effective in patients with chronic heart failure. Eur. J. Heart Fail. 2013, 15, 1304–1310. [Google Scholar] [CrossRef]
- Kalogeropoulos, A.; Papadimitriou, L.; Georgiopoulou, V.V.; Dunbar, S.B.; Skopicki, H.; Butler, J. Low- Versus Moderate-Sodium Diet in Patients With Recent Hospitalization for Heart Failure: The PROHIBIT (Prevent Adverse Outcomes in Heart Failure by Limiting Sodium) Pilot Study. Circ. Heart Fail. 2020, 13, e006389. [Google Scholar] [CrossRef]
- Cappuccio, F.P.; Campbell, N.R.C.; He, F.J.; Jacobson, M.F.; MacGregor, G.A.; Antman, E.; Appel, L.J.; Arcand, J.; Blanco-Metzler, A.; Cook, N.R.; et al. Sodium and Health: Old Myths and a Controversy Based on Denial. Curr. Nutr. Rep. 2022, 11, 172–184. [Google Scholar] [CrossRef] [PubMed]
- He, F.J.; Ma, Y.; Campbell, N.R.C.; MacGregor, G.A.; Cogswell, M.E.; Cook, N.R. Formulas to Estimate Dietary Sodium Intake From Spot Urine Alter Sodium-Mortality Relationship. Hypertension 2019, 74, 572–580. [Google Scholar] [CrossRef]
- Ma, Y.; He, F.J.; Sun, Q.; Yuan, C.; Kieneker, L.M.; Curhan, G.C.; MacGregor, G.A.; Bakker, S.J.; Campbell, N.R.; Wang, M. 24-hour urinary sodium and potassium excretion and cardiovascular risk. N. Engl. J. Med. 2022, 386, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Mente, A.; O’Donnell, M.; Rangarajan, S.; McQueen, M.; Dagenais, G.; Wielgosz, A.; Lear, S.; Ah, S.T.L.; Wei, L.; Diaz, R. Urinary sodium excretion, blood pressure, cardiovascular disease, and mortality: A community-level prospective epidemiological cohort study. Lancet 2018, 392, 496–506. [Google Scholar] [CrossRef]
- Graudal, N.; Hubeck-Graudal, T.; Jürgens, G.; Taylor, R.S. Dose-response relation between dietary sodium and blood pressure: A meta-regression analysis of 133 randomized controlled trials. Am. J. Clin. Nutr. 2019, 109, 1273–1278. [Google Scholar] [CrossRef]
- Basuray, A.; Dolansky, M.; Josephson, R.; Sattar, A.; Grady, E.M.; Vehovec, A.; Gunstad, J.; Redle, J.; Fang, J.; Hughes, J.W. Dietary sodium adherence is poor in chronic heart failure patients. J. Card. Fail. 2015, 21, 323–329. [Google Scholar] [CrossRef]
- Heidenreich, P.; Bozkurt, B.; Aguilar, D.; Allen, L.; Byun, J.; Colvin, M.; Deswal, A.; Drazner, M.; Dunlay, S.; Evers, L. Yancy CW 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation 2022, 145, e895. [Google Scholar] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar]
- Ezekowitz, J.A.; O’Meara, E.; McDonald, M.A.; Abrams, H.; Chan, M.; Ducharme, A.; Giannetti, N.; Grzeslo, A.; Hamilton, P.G.; Heckman, G.A. 2017 Comprehensive update of the Canadian Cardiovascular Society guidelines for the management of heart failure. Can. J. Cardiol. 2017, 33, 1342–1433. [Google Scholar] [CrossRef] [PubMed]
- Simão, D.O.; Júlia da Costa, R.; Fonseca Verneque, B.J.; Ferreira do Amaral, J.; Chagas, G.M.; Duarte, C.K. Sodium and/or fluid restriction and nutritional parameters of adult patients with heart failure: A systematic review and meta-analysis of randomized controlled trial. Clin. Nutri. ESPEN 2021, 45, 33–44. [Google Scholar] [CrossRef]
- The Australian Government and the New Zealand Ministry of Health. Australian and New Zealand Nutrient Reference Values for Sodium: A Report Prepared for the Australian Government Department of Health and the New Zealand Ministry of Health. Available online: www.eatforhealth.gov.au/nutrient-reference-values/nutrients/sodium (accessed on 27 May 2024).
- Xu, X.; Zeng, L.; Jha, V.; Cobb, L.K.; Shibuya, K.; Appel, L.J.; Neal, B.; Schutte, A.E. Potassium-enriched salt substitutes: A review of recommendations in clinical management guidelines. Hypertension 2024, 81, 400–414. [Google Scholar] [CrossRef] [PubMed]
- Neal, B.; Wu, Y.; Feng, X.; Zhang, R.; Zhang, Y.; Shi, J.; Zhang, J.; Tian, M.; Huang, L.; Li, Z.; et al. Effect of Salt Substitution on Cardiovascular Events and Death. N. Engl. J. Med. 2021, 385, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Bistola, V.; Arfaras-Melainis, A.; Trogkanis, E.; Bakosis, G.; Polyzogopoulou, E.; Karavidas, I.-N.; Ikonomidis, I.; Parissis, J.; Karavidas, A. Safety and efficacy of salt substitution with a low sodium-potassium enriched dietary salt in patients with heart failure with reduced ejection fraction: A pilot study. Clin. Nutri. ESPEN 2020, 35, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Greer, R.C.; Marklund, M.; Anderson, C.A.; Cobb, L.K.; Dalcin, A.T.; Henry, M.; Appel, L.J. Potassium-enriched salt substitutes as a means to lower blood pressure: Benefits and risks. Hypertension 2020, 75, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, H.B.; Annapure, U.S. Triglycerides of medium-chain fatty acids: A concise review. J. Food Sci. Technol. 2023, 60, 2143–2152. [Google Scholar] [CrossRef]
- St-Pierre, V.; Vandenberghe, C.; Lowry, C.M.; Fortier, M.; Castellano, C.A.; Wagner, R.; Cunnane, S.C. Plasma Ketone and Medium Chain Fatty Acid Response in Humans Consuming Different Medium Chain Triglycerides During a Metabolic Study Day. Front. Nutr. 2019, 6, 46. [Google Scholar] [CrossRef]
- McKenzie, K.M.; Lee, C.M.; Mijatovic, J.; Haghighi, M.M.; Skilton, M.R. Medium-Chain Triglyceride Oil and Blood Lipids: A Systematic Review and Meta-Analysis of Randomized Trials. J. Nutr. 2021, 151, 2949–2956. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Wang, Z.; Yu, P.; Yan, X.; Zhao, J.; Zhang, G.; Gong, D.; Zeng, Z. Effect of Different Medium-Chain Triglycerides on Glucose Metabolism in High-Fat-Diet Induced Obese Rats. Foods 2024, 13, 241. [Google Scholar] [CrossRef] [PubMed]
- Murano, C.; Binda, A.; Palestini, P.; Baruscotti, M.; DiFrancesco, J.C.; Rivolta, I. Effect of the ketogenic diet in excitable tissues. Am. J. Physiol. Cell Physiol. 2021, 320, C547–C553. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Zhang, C.; Xie, M. Beta-Hydroxybutyrate, Friend or Foe for Stressed Hearts. Front. Aging. 2021, 2, 681513. [Google Scholar] [CrossRef]
- Flores-Guerrero, J.L.; Westenbrink, B.D.; Connelly, M.A.; Otvos, J.D.; Groothof, D.; Shalaurova, I.; Garcia, E.; Navis, G.; de Boer, R.A.; Bakker, S.J.L.; et al. Association of beta-hydroxybutyrate with development of heart failure: Sex differences in a Dutch population cohort. Eur. J. Clin. Investig. 2021, 51, e13468. [Google Scholar] [CrossRef] [PubMed]
- Lommi, J.; Kupari, M.; Koskinen, P.; Näveri, H.; Leinonen, H.; Pulkki, K.; Härkönen, M. Blood ketone bodies in congestive heart failure. J. Am. Coll. Cardiol. 1996, 28, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Murashige, D.; Jang, C.; Neinast, M.; Edwards, J.J.; Cowan, A.; Hyman, M.C.; Rabinowitz, J.D.; Frankel, D.S.; Arany, Z. Comprehensive quantification of fuel use by the failing and nonfailing human heart. Science 2020, 370, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, R.; Møller, N.; Gormsen, L.C.; Tolbod, L.P.; Hansson, N.H.; Sorensen, J.; Harms, H.J.; Frøkiær, J.; Eiskjaer, H.; Jespersen, N.R.; et al. Cardiovascular Effects of Treatment With the Ketone Body 3-Hydroxybutyrate in Chronic Heart Failure Patients. Circulation 2019, 139, 2129–2141. [Google Scholar] [CrossRef] [PubMed]
- Oneglia, A.P.; Young, B.E.; Cipher, D.J.; Zaha, V.; Nelson, M.D. Acute effects of β-hydroxybutyrate on left ventricular function in young, healthy adults. J. Appl. Physiol. 2023, 135, 1440–1445. [Google Scholar] [CrossRef]
- Takahara, S.; Soni, S.; Phaterpekar, K.; Kim, T.T.; Maayah, Z.H.; Levasseur, J.L.; Silver, H.L.; Freed, D.H.; Ferdaoussi, M.; Dyck, J.R.B. Chronic exogenous ketone supplementation blunts the decline of cardiac function in the failing heart. ESC Heart Fail. 2021, 8, 5606–5612. [Google Scholar] [CrossRef]
- Monzo, L.; Sedlacek, K.; Hromanikova, K.; Tomanova, L.; Borlaug, B.A.; Jabor, A.; Kautzner, J.; Melenovsky, V. Myocardial ketone body utilization in patients with heart failure: The impact of oral ketone ester. Metabolism 2021, 115, 154452. [Google Scholar] [CrossRef] [PubMed]
- Berg-Hansen, K.; Gopalasingam, N.; Christensen, K.H.; Ladefoged, B.; Andersen, M.J.; Poulsen, S.H.; Borlaug, B.A.; Nielsen, R.; Møller, N.; Wiggers, H. Cardiovascular Effects of Oral Ketone Ester Treatment in Patients With Heart Failure With Reduced Ejection Fraction: A Randomized, Controlled, Double-Blind Trial. Circulation 2024, 149, 1474–1489. [Google Scholar] [CrossRef] [PubMed]
- Alcázar-Fabra, M.; Navas, P.; Brea-Calvo, G. Coenzyme Q biosynthesis and its role in the respiratory chain structure. Biochim. Biophys. Acta Bioenerg. 2016, 1857, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeldt, F.; Marasco, S.; Lyon, W.; Wowk, M.; Sheeran, F.; Bailey, M.; Esmore, D.; Davis, B.; Pick, A.; Rabinov, M.; et al. Coenzyme Q10 therapy before cardiac surgery improves mitochondrial function and in vitro contractility of myocardial tissue. J. Thorac. Cardiovasc. Surg. 2005, 129, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Belardinelli, R.; Muçaj, A.; Lacalaprice, F.; Solenghi, M.; Seddaiu, G.; Principi, F.; Tiano, L.; Littarru, G.P. Coenzyme Q10 and exercise training in chronic heart failure. Eur. Heart J. 2006, 27, 2675–2681. [Google Scholar] [CrossRef]
- Molyneux, S.L.; Florkowski, C.M.; George, P.M.; Pilbrow, A.P.; Frampton, C.M.; Lever, M.; Richards, A.M. Coenzyme Q10: An independent predictor of mortality in chronic heart failure. J. Am. Coll. Cardiol. 2008, 52, 1435–1441. [Google Scholar] [CrossRef] [PubMed]
- Alehagen, U.; Johansson, P.; Björnstedt, M.; Rosén, A.; Dahlström, U. Cardiovascular mortality and N-terminal-proBNP reduced after combined selenium and coenzyme Q10 supplementation: A 5-year prospective randomized double-blind placebo-controlled trial among elderly Swedish citizens. Int. J. Cardiol. 2013, 167, 1860–1866. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, S.A.; Rosenfeldt, F.; Kumar, A.; Dolliner, P.; Filipiak, K.J.; Pella, D.; Alehagen, U.; Steurer, G.; Littarru, G.P. The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure: Results from Q-SYMBIO: A randomized double-blind trial. JACC Heart Fail. 2014, 2, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, A.L.; Rosenfeldt, F.; Filipiak, K.J. Effect of coenzyme Q10 in Europeans with chronic heart failure: A sub-group analysis of the Q-SYMBIO randomized double-blind trial. Cardiol. J. 2019, 26, 147–156. [Google Scholar] [CrossRef]
- Raj, P.; Louis, X.L.; Thandapilly, S.J.; Movahed, A.; Zieroth, S.; Netticadan, T. Potential of resveratrol in the treatment of heart failure. Life Sci. 2014, 95, 63–71. [Google Scholar] [CrossRef]
- Gal, R.; Praksch, D.; Kenyeres, P.; Rabai, M.; Toth, K.; Halmosi, R.; Habon, T. Hemorheological Alterations in Patients with Heart Failure with Reduced Ejection Fraction Treated by Resveratrol. Cardiovasc. Ther. 2020, 2020, 7262474. [Google Scholar] [CrossRef] [PubMed]
- Gal, R.; Deres, L.; Horvath, O.; Eros, K.; Sandor, B.; Urban, P.; Soos, S.; Marton, Z.; Sumegi, B.; Toth, K.; et al. Resveratrol Improves Heart Function by Moderating Inflammatory Processes in Patients with Systolic Heart Failure. Antioxidants 2020, 9, 1108. [Google Scholar] [CrossRef] [PubMed]
- Magyar, K.; Halmosi, R.; Palfi, A.; Feher, G.; Czopf, L.; Fulop, A.; Battyany, I.; Sumegi, B.; Toth, K.; Szabados, E. Cardioprotection by resveratrol: A human clinical trial in patients with stable coronary artery disease. Clin. Hemorheol. Microcirc. 2012, 50, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Sacks, F.M.; Svetkey, L.P.; Vollmer, W.M.; Appel, L.J.; Bray, G.A.; Harsha, D.; Obarzanek, E.; Conlin, P.R.; Miller, E.R.; Simons-Morton, D.G. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N. Engl. J. Med. 2001, 344, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Juraschek, S.P.; Kovell, L.C.; Appel, L.J.; Miller, E.R., 3rd; Sacks, F.M.; Chang, A.R.; Christenson, R.H.; Rebuck, H.; Mukamal, K.J. Effects of Diet and Sodium Reduction on Cardiac Injury, Strain, and Inflammation: The DASH-Sodium Trial. J. Am. Coll. Cardiol. 2021, 77, 2625–2634. [Google Scholar] [CrossRef] [PubMed]
- Pirouzeh, R.; Heidarzadeh-Esfahani, N.; Morvaridzadeh, M.; Izadi, A.; Yosaee, S.; Potter, E.; Heshmati, J.; Pizarro, A.B.; Omidi, A.; Heshmati, S. Effect of DASH diet on oxidative stress parameters: A systematic review and meta-analysis of randomized clinical trials. Diabetes Metab. Syndr. 2020, 14, 2131–2138. [Google Scholar] [CrossRef] [PubMed]
- Ibsen, D.B.; Levitan, E.B.; Åkesson, A.; Gigante, B.; Wolk, A. The DASH diet is associated with a lower risk of heart failure: A cohort study. Eur. J. Prev. Cardiol. 2022, 29, 1114–1123. [Google Scholar] [CrossRef]
- Goyal, P.; Balkan, L.; Ringel, J.B.; Hummel, S.L.; Sterling, M.R.; Kim, S.; Arora, P.; Jackson, E.A.; Brown, T.M.; Shikany, J.M.; et al. The Dietary Approaches to Stop Hypertension (DASH) Diet Pattern and Incident Heart Failure. J. Card. Fail. 2021, 27, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Hummel, S.L.; Karmally, W.; Gillespie, B.W.; Helmke, S.; Teruya, S.; Wells, J.; Trumble, E.; Jimenez, O.; Marolt, C.; Wessler, J.D.; et al. Home-Delivered Meals Postdischarge From Heart Failure Hospitalization. Circ. Heart Fail. 2018, 11, e004886. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Morze, J.; Hoffmann, G. Mediterranean diet and health status: Active ingredients and pharmacological mechanisms. Br. J. Pharmacol. 2020, 177, 1241–1257. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G. Mediterranean dietary pattern, inflammation and endothelial function: A systematic review and meta-analysis of intervention trials. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Fitó, M.; Estruch, R.; Salas-Salvadó, J.; Martínez-Gonzalez, M.A.; Arós, F.; Vila, J.; Corella, D.; Díaz, O.; Sáez, G.; de la Torre, R.; et al. Effect of the Mediterranean diet on heart failure biomarkers: A randomized sample from the PREDIMED trial. Eur. J. Heart Fail. 2014, 16, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Martínez, A.D.; Muñoz Jiménez, C.; López Aguilera, J.; Crespin, M.C.; Manzano García, G.; Gálvez Moreno, M.; Calañas Continente, A.; Molina Puerta, M.J. Mediterranean Diet, Vitamin D, and Hypercaloric, Hyperproteic Oral Supplements for Treating Sarcopenia in Patients with Heart Failure-A Randomized Clinical Trial. Nutrients 2023, 16, 110. [Google Scholar] [CrossRef] [PubMed]
- Papadaki, A.; Martínez-González, M.; Alonso-Gómez, A.; Rekondo, J.; Salas-Salvadó, J.; Corella, D.; Ros, E.; Fitó, M.; Estruch, R.; Lapetra, J.; et al. Mediterranean diet and risk of heart failure: Results from the PREDIMED randomized controlled trial. Eur. J. Heart Fail. 2017, 19, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Lopaschuk, G.D.; Karwi, Q.G.; Tian, R.; Wende, A.R.; Abel, E.D. Cardiac energy metabolism in heart failure. Circ. Res. 2021, 128, 1487–1513. [Google Scholar] [CrossRef] [PubMed]
- Bedi, K.C., Jr.; Snyder, N.W.; Brandimarto, J.; Aziz, M.; Mesaros, C.; Worth, A.J.; Wang, L.L.; Javaheri, A.; Blair, I.A.; Margulies, K.B.; et al. Evidence for Intramyocardial Disruption of Lipid Metabolism and Increased Myocardial Ketone Utilization in Advanced Human Heart Failure. Circulation 2016, 133, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Horton, J.L.; Davidson, M.T.; Kurishima, C.; Vega, R.B.; Powers, J.C.; Matsuura, T.R.; Petucci, C.; Lewandowski, E.D.; Crawford, P.A.; Muoio, D.M.; et al. The failing heart utilizes 3-hydroxybutyrate as a metabolic stress defense. JCI Insight 2019, 4, e124079. [Google Scholar] [CrossRef] [PubMed]
- Yurista, S.R.; Chong, C.R.; Badimon, J.J.; Kelly, D.P.; de Boer, R.A.; Westenbrink, B.D. Therapeutic Potential of Ketone Bodies for Patients With Cardiovascular Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 77, 1660–1669. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, F.; Wang, J.; Zhang, D.; Zhao, X. Ketogenic diet attenuates aging-associated myocardial remodeling and dysfunction in mice. Exp. Gerontol. 2020, 140, 111058. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, X.; Li, T.; Zhao, J.; Yang, Y.; Yao, Y.; Wang, L.; Yang, B.; Ren, G.; Tan, Y. Alternate-day ketogenic diet feeding protects against heart failure through preservation of ketogenesis in the liver. Ox. Med. Cell. Long. 2022, 2022, 4253651. [Google Scholar] [CrossRef]
- Ho, K.L.; Karwi, Q.; Wang, F.; Wagg, C.; Zhang, L.; Panidarapu, S.; Chen, B.; Pherwani, S.; Greenwell, A.A.; Oudit, G.; et al. The ketogenic diet does not improve cardiac function and blunts glucose oxidation in ischemic heart failure. Cardiovasc. Res. 2024, cvae092. [Google Scholar] [CrossRef]
- Zhu, H.; Bi, D.; Zhang, Y.; Kong, C.; Du, J.; Wu, X.; Wei, Q.; Qin, H. Ketogenic diet for human diseases: The underlying mechanisms and potential for clinical implementations. Signal Transduct. Target. Ther. 2022, 7, 11. [Google Scholar] [CrossRef]
- Cicero, A.F.; Benelli, M.; Brancaleoni, M.; Dainelli, G.; Merlini, D.; Negri, R. Middle and Long-Term Impact of a Very Low-Carbohydrate Ketogenic Diet on Cardiometabolic Factors: A Multi-Center, Cross-Sectional, Clinical Study. High. Blood Press. Cardiovasc. Prev. 2015, 22, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Bueno, N.B.; de Melo, I.S.V.; de Oliveira, S.L.; da Rocha Ataide, T. Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2013, 110, 1178–1187. [Google Scholar] [CrossRef] [PubMed]
- Koutentakis, M.; Kuciński, J.; Świeczkowski, D.; Surma, S.; Filipiak, K.J.; Gąsecka, A. The Ketogenic Effect of SGLT-2 Inhibitors-Beneficial or Harmful? J. Cardiovasc. Dev. Dis. 2023, 10, 465. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, L.S.; Jose, M.M.; Sallam, H.; Serag, H.; Golovko, G.; Khanipov, K.; Hamilton, M.A.; Fonarow, G.C. High-protein vs. standard-protein diets in overweight and obese patients with heart failure and diabetes mellitus: Findings of the Pro-HEART trial. ESC Heart Fail. 2021, 8, 1342–1348. [Google Scholar] [CrossRef]
- Baragetti, I.; De Simone, I.; Biazzi, C.; Buzzi, L.; Ferrario, F.; Luise, M.C.; Santagostino, G.; Furiani, S.; Alberghini, E.; Capitanio, C.; et al. The low-protein diet for chronic kidney disease: 8 years of clinical experience in a nephrology ward. Clin. Kidney J. 2020, 13, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Bechthold, A.; Boeing, H.; Schwedhelm, C.; Hoffmann, G.; Knüppel, S.; Iqbal, K.; De Henauw, S.; Michels, N.; Devleesschauwer, B.; Schlesinger, S.; et al. Food groups and risk of coronary heart disease, stroke and heart failure: A systematic review and dose-response meta-analysis of prospective studies. Crit. Rev. Food Sci. Nutr. 2019, 59, 1071–1090. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Samman Tahhan, A.; Vaduganathan, M.; Greene, S.J.; Alrohaibani, A.; Anker, S.D.; Vardeny, O.; Fonarow, G.C.; Butler, J. Trends in prevalence of comorbidities in heart failure clinical trials. Eur. J. Heart Fail. 2020, 22, 1032–1042. [Google Scholar] [CrossRef]
- Screever, E.M.; van der Wal, M.H.L.; van Veldhuisen, D.J.; Jaarsma, T.; Koops, A.; van Dijk, K.S.; Warink-Riemersma, J.; Coster, J.E.; Westenbrink, B.D.; van der Meer, P.; et al. Comorbidities complicating heart failure: Changes over the last 15 years. Clin. Res. Cardiol. 2023, 112, 123–133. [Google Scholar] [CrossRef]
- Takimura, H.; Hada, T.; Kawano, M.; Yabe, T.; Takimura, Y.; Nishio, S.; Nakano, M.; Tsukahara, R.; Muramatsu, T. A novel validated method for predicting the risk of re-hospitalization for worsening heart failure and the effectiveness of the diuretic upgrading therapy with tolvaptan. PLoS ONE 2018, 13, e0207481. [Google Scholar] [CrossRef] [PubMed]
- Kleissl-Muir, S.; Owen, A.; Rasmussen, B.; Zinn, C.; Driscoll, A. Effects of a low carbohydrate diet on heart failure symptoms and quality of life in patients with diabetic cardiomyopathy: A randomised controlled trial pilot study. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 2455–2463. [Google Scholar] [CrossRef]
- Elagizi, A.; Carbone, S.; Lavie, C.J.; Mehra, M.R.; Ventura, H.O. Implications of obesity across the heart failure continuum. Prog. Cardiovasc. Dis. 2020, 63, 561–569. [Google Scholar] [CrossRef] [PubMed]
- El Hajj, E.C.; El Hajj, M.C.; Sykes, B.; Lamicq, M.; Zile, M.R.; Malcolm, R.; O’Neil, P.M.; Litwin, S.E. Pragmatic weight management program for patients with obesity and heart failure with preserved ejection fraction. J. Am. Heart Assoc. 2021, 10, e022930. [Google Scholar] [CrossRef] [PubMed]
- Kitzman, D.W.; Brubaker, P.; Morgan, T.; Haykowsky, M.; Hundley, G.; Kraus, W.E.; Eggebeen, J.; Nicklas, B.J. Effect of Caloric Restriction or Aerobic Exercise Training on Peak Oxygen Consumption and Quality of Life in Obese Older Patients With Heart Failure With Preserved Ejection Fraction: A Randomized Clinical Trial. JAMA 2016, 315, 36–46. [Google Scholar] [CrossRef]
- Fordham, T.M.; Morelli, N.S.; Garcia-Reyes, Y.; Ware, M.A.; Rahat, H.; Sundararajan, D.; Fuller, K.N.; Severn, C.; Pyle, L.; Malloy, C.R. Metabolic effects of an essential amino acid supplement in adolescents with PCOS and obesity. Obesity 2024, 32, 678–690. [Google Scholar] [CrossRef]
- Aquilani, R.; Viglio, S.; Iadarola, P.; Opasich, C.; Testa, A.; Dioguardi, F.S.; Pasini, E. Oral amino acid supplements improve exercise capacities in elderly patients with chronic heart failure. Am. J. Cardiol. 2008, 101, S104–S110. [Google Scholar] [CrossRef]
- Libby, P.; Theroux, P. Pathophysiology of coronary artery disease. Circulation 2005, 111, 3481–3488. [Google Scholar] [CrossRef] [PubMed]
- Jonnalagadda, S.S.; Harnack, L.; Liu, R.H.; McKeown, N.; Seal, C.; Liu, S.; Fahey, G.C. Putting the whole grain puzzle together: Health benefits associated with whole grains--summary of American Society for Nutrition 2010 Satellite Symposium. J. Nutr. 2011, 141, 1011s–1022s. [Google Scholar] [CrossRef]
- Das, U.N. Nutritional factors in the prevention and management of coronary artery disease and heart failure. Nutrition 2015, 31, 283–291. [Google Scholar] [CrossRef]
- Ryan, D.K.; Banerjee, D.; Jouhra, F. Management of Heart Failure in Patients with Chronic Kidney Disease. Eur. Cardiol. 2022, 17, e17. [Google Scholar] [CrossRef] [PubMed]
- Garneata, L.; Mircescu, G. Effect of Low-Protein Diet Supplemented With Keto Acids on Progression of Chronic Kidney Disease. J. Ren. Nutr. 2013, 23, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Sze, S.; Pellicori, P.; Zhang, J.; Clark, A.L. Malnutrition, congestion and mortality in ambulatory patients with heart failure. Heart 2019, 105, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Wawrzeńczyk, A.; Anaszewicz, M.; Wawrzeńczyk, A.; Budzyński, J. Clinical significance of nutritional status in patients with chronic heart failure-a systematic review. Heart Fail. Rev. 2019, 24, 671–700. [Google Scholar] [CrossRef] [PubMed]
- Grossniklaus, D.A.; O’Brien, M.C.; Clark, P.C.; Dunbar, S.B. Nutrient intake in heart failure patients. J. Cardiovasc. Nurs. 2008, 23, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Kinugawa, S.; Fukushima, A. Malnutrition in Heart Failure: Important But Undervalued Issue. JACC Heart Fail. 2018, 6, 487–488. [Google Scholar] [CrossRef]
- von Haehling, S.; Ebner, N.; Dos Santos, M.R.; Springer, J.; Anker, S.D. Muscle wasting and cachexia in heart failure: Mechanisms and therapies. Nat. Rev. Cardiol. 2017, 14, 323–341. [Google Scholar] [CrossRef]
- Anker, S.D.; Ponikowski, P.; Varney, S.; Chua, T.P.; Clark, A.L.; Webb-Peploe, K.M.; Harrington, D.; Kox, W.J.; Poole-Wilson, P.A.; Coats, A.J. Wasting as independent risk factor for mortality in chronic heart failure. Lancet 1997, 349, 1050–1053. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A. Sarcopenia: Revised European consensus on definition and diagnosis. Age. Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Fülster, S.; Tacke, M.; Sandek, A.; Ebner, N.; Tschöpe, C.; Doehner, W.; Anker, S.D.; von Haehling, S. Muscle wasting in patients with chronic heart failure: Results from the studies investigating co-morbidities aggravating heart failure (SICA-HF). Eur. Heart J. 2013, 34, 512–519. [Google Scholar] [CrossRef]
- Valentova, M.; Anker, S.D.; von Haehling, S. Cardiac Cachexia Revisited: The Role of Wasting in Heart Failure. Cardiol. Clin. 2022, 40, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Pombo, A.; Rodríguez-Carnero, G.; Castro, A.I.; Cantón-Blanco, A.; Seoane, L.M.; Casanueva, F.F.; Crujeiras, A.B.; Martínez-Olmos, M.A. Relevance of nutritional assessment and treatment to counteract cardiac cachexia and sarcopenia in chronic heart failure. Clin. Nutr. 2021, 40, 5141–5155. [Google Scholar] [CrossRef] [PubMed]
- Thanapholsart, J.; Khan, E.; Lee, G.A. A Current Review of the Uses of Bioelectrical Impedance Analysis and Bioelectrical Impedance Vector Analysis in Acute and Chronic Heart Failure Patients: An Under-valued Resource? Biol. Res. Nurs. 2023, 25, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Casey, P.; Alasmar, M.; McLaughlin, J.; Ang, Y.; McPhee, J.; Heire, P.; Sultan, J. The current use of ultrasound to measure skeletal muscle and its ability to predict clinical outcomes: A systematic review. J. Cachexia Sarcopenia Muscle 2022, 13, 2298–2309. [Google Scholar] [CrossRef] [PubMed]
- Bonilla-Palomas, J.L.; Gámez-López, A.L.; Castillo-Domínguez, J.C.; Moreno-Conde, M.; López Ibáñez, M.C.; Alhambra Expósito, R.; Ramiro Ortega, E.; Anguita-Sánchez, M.; Villar-Ráez, A. Nutritional Intervention in Malnourished Hospitalized Patients with Heart Failure. Arch. Med. Res. 2016, 47, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Hersberger, L.; Dietz, A.; Bürgler, H.; Bargetzi, A.; Bargetzi, L.; Kägi-Braun, N.; Tribolet, P.; Gomes, F.; Hoess, C.; Pavlicek, V.; et al. Individualized Nutritional Support for Hospitalized Patients With Chronic Heart Failure. J. Am. Coll. Cardiol. 2021, 77, 2307–2319. [Google Scholar] [CrossRef] [PubMed]
- Habaybeh, D.; de Moraes, M.B.; Slee, A.; Avgerinou, C. Nutritional interventions for heart failure patients who are malnourished or at risk of malnutrition or cachexia: A systematic review and meta-analysis. Heart Fail. Rev. 2021, 26, 1103–1118. [Google Scholar] [CrossRef]
- Deutz, N.E.; Matheson, E.M.; Matarese, L.E.; Luo, M.; Baggs, G.E.; Nelson, J.L.; Hegazi, R.A.; Tappenden, K.A.; Ziegler, T.R. Readmission and mortality in malnourished, older, hospitalized adults treated with a specialized oral nutritional supplement: A randomized clinical trial. Clin. Nutr. 2016, 35, 18–26. [Google Scholar] [CrossRef]
- Chrysohoou, C.; Metallinos, G.; Georgiopoulos, G.; Mendrinos, D.; Papanikolaou, A.; Magkas, N.; Pitsavos, C.; Vyssoulis, G.; Stefanadis, C.; Tousoulis, D. Short term omega-3 polyunsaturated fatty acid supplementation induces favorable changes in right ventricle function and diastolic filling pressure in patients with chronic heart failure; A randomized clinical trial. Vascul. Pharmacol. 2016, 79, 43–50. [Google Scholar] [CrossRef]
- 2016 ACC/AHA/HFSA Focused Update on New Pharmacological Therapy for Heart Failure: An Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J. Card. Fail. 2016, 22, 659–669. [CrossRef]
- Streng, K.W.; Hillege, H.L.; Ter Maaten, J.M.; van Veldhuisen, D.J.; Dickstein, K.; Ng, L.L.; Samani, N.J.; Metra, M.; Ponikowski, P.; Cleland, J.G.; et al. Clinical implications of low estimated protein intake in patients with heart failure. J. Cachexia Sarcopenia Muscle 2022, 13, 1762–1770. [Google Scholar] [CrossRef]
- Rozentryt, P.; von Haehling, S.; Lainscak, M.; Nowak, J.U.; Kalantar-Zadeh, K.; Polonski, L.; Anker, S.D. The effects of a high-caloric protein-rich oral nutritional supplement in patients with chronic heart failure and cachexia on quality of life, body composition, and inflammation markers: A randomized, double-blind pilot study. J. Cachexia Sarcopenia Muscle 2010, 1, 35–42. [Google Scholar] [CrossRef]
- Narita, K.; Amiya, E. Is branched-chain amino acid nutritional supplementation beneficial or detrimental in heart failure? World J. Cardiol. 2021, 13, 163–169. [Google Scholar] [CrossRef]
- Nichols, S.; McGregor, G.; Al-Mohammad, A.; Ali, A.N.; Tew, G.; O’Doherty, A.F. The effect of protein and essential amino acid supplementation on muscle strength and performance in patients with chronic heart failure: A systematic review. Eur. J. Nutr. 2020, 59, 1785–1801. [Google Scholar] [CrossRef]
- Salmani, M.; Alipoor, E.; Navid, H.; Farahbakhsh, P.; Yaseri, M.; Imani, H. Effect of l-arginine on cardiac reverse remodeling and quality of life in patients with heart failure. Clin. Nutr. 2021, 40, 3037–3044. [Google Scholar] [CrossRef]
- Lombardi, C.; Carubelli, V.; Lazzarini, V.; Vizzardi, E.; Bordonali, T.; Ciccarese, C.; Castrini, A.I.; Dei Cas, A.; Nodari, S.; Metra, M. Effects of oral administration of orodispersible levo-carnosine on quality of life and exercise performance in patients with chronic heart failure. Nutrition 2015, 31, 72–78. [Google Scholar] [CrossRef]
| Comorbidity | Proportion of HF Patients (%) [92] | Relationship to HF | Dietary Recommendation |
|---|---|---|---|
| DM | 45 |
| |
| Obesity | 29 |
|
|
| CAD | 48 |
|
|
| CKD | 60 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, L.P.; Pant, A.; Marschner, S.; Talbot, P.; Zaman, S. A Focus on Heart Failure Management through Diet and Nutrition: A Comprehensive Review. Hearts 2024, 5, 293-307. https://doi.org/10.3390/hearts5030022
Liao LP, Pant A, Marschner S, Talbot P, Zaman S. A Focus on Heart Failure Management through Diet and Nutrition: A Comprehensive Review. Hearts. 2024; 5(3):293-307. https://doi.org/10.3390/hearts5030022
Chicago/Turabian StyleLiao, Lee P., Anushriya Pant, Simone Marschner, Peter Talbot, and Sarah Zaman. 2024. "A Focus on Heart Failure Management through Diet and Nutrition: A Comprehensive Review" Hearts 5, no. 3: 293-307. https://doi.org/10.3390/hearts5030022
APA StyleLiao, L. P., Pant, A., Marschner, S., Talbot, P., & Zaman, S. (2024). A Focus on Heart Failure Management through Diet and Nutrition: A Comprehensive Review. Hearts, 5(3), 293-307. https://doi.org/10.3390/hearts5030022







