Abstract
Endocarditis is a severe infection affecting the heart’s inner layer, the endocardium. Its pathophysiology may involve heart valve damage, bacteria adhesion and biofilm formation, potentially leading to fatal complications. Bacteria from various sources, including from endodontic diseases and its treatments may enter the bloodstream provoking this condition. This systematic review aimed to explore the influence of endodontic factors on endocarditis. Searches across PubMed, Embase, Cochrane Library and manual sources yielded 14 relevant articles from 1562 screened studies. Assessment platforms from JBI Critical Appraisal Tools evaluated studies biases. Findings mainly focused on transient bacteraemia as a key indicator of risk correlating bacterial virulence and counts with endocarditis development. Worryingly, multi-species bacteraemia post-endodontic treatment was noted including the genera Enterococcus, Parvimonas, Streptococcus and Staphylococcus. Conclusive validation of the incidence and association between endodontic patients and endocarditis was limited due to a lack of robust longitudinal investigations, such as randomized controlled trials. This emphasizes the need for further research with well-designed methodologies to provide a full understanding of the causative bacterial population and its pathological mechanisms. A current guideline (2023 European Society of Cardiology) was developed to support healthcare professionals in diagnosing and managing infective endocarditis; this 2023 version is introducing a new diagnostic algorithm to aid in patient classification aiming to improve outcomes for this challenging disease. The study was a priori registered on PROSPERO (CRD42023407736).
1. Introduction
The pathophysiology of endocarditis comprises the occurrence of at least three fundamental events namely the damage to the heart valves, the adhesion of circulating bacteria to its surfaces, and the survival of bacteria adhering to the molecular matrix and platelets resulting in layered depositions on these surfaces [1]. As an inflammatory disease, this condition can affect one or more of the aortic, mitral, or tricuspid valves; besides, three crucial steps are involved in the development of endocarditis: adherence of bacteria, platelet activation and fibrin overlaying [2]. Progressively, this infected platelet-fibrin complex increases in mass as cells colonize and expand the layering upon the vegetation [3].
This bacterial-related damage can be caused by changes in blood flow associated with heart-related congenital or acquired diseases that promote platelet and fibrin deposition [4,5] favoring bacterial deposition referred to as ‘vegetation’ [5,6,7,8]. Thus, the infection by microorganisms with the potential to colonize these damaged areas may be related to transient bacteraemia resulting from medical or dental procedures in patients with complex congenital heart defects, prosthetic heart valves or a history of endocarditis [9,10]. This pathology has an incidence ranging from one to five cases per 100,000 person/year [11], but with low prevalence [12]. The short-term mortality rate ranges from 10% to 30% [13] with a mortality rate of 65.1% being attributed to men [14].
In physiological conditions, transient bacteraemia can be cleared within a few minutes and usually does not pose a threat in otherwise healthy patients [15]. However, clinically compromised patients are at risk of developing endocarditis, especially patients with prosthetic heart valves, previous history of endocarditis, complex cyanotic congenital heart disease, surgically constructed systemic pulmonary conduits, congenital heart malformations, acquired valve dysfunction, hypertrophic cardiomyopathy and mitral valve prolapse with valve regurgitation [2,16]. In these specific conditions, antibiotic prophylaxis [17] should be considered to provide some degree of systemic protection—from bacteraemia—whenever these patients are subjected to procedures that would potentially cause the breakdown of the heart mucosal barrier favouring bacterial infection [18]. In clinical practice, bacterial resistance—subject of thousands of publications each year [19]—occurs when a microorganism is no longer susceptible to antimicrobial medication, thus reducing the effectiveness of treatment. Conversely, antimicrobial susceptibility, refers to the sensitivity of the microorganism to the medication, resulting in the elimination of the pathogen in therapeutically achievable medication concentrations [20,21].
It is noteworthy that the complex processes related to bacterial resistance contribute to the difficulty in preventing and controlling the development of resistance, representing a global concern [22,23]. A survey study [24] reported that the knowledge of dentists about antibiotic prescriptions for endocarditis was identified as low, as they were usually not familiar with the guidelines of the American Heart Association, for example. Although the majority prescribed the correct antimicrobial agent, there was a low rate of prescriptions with the correct dose and time of administration, as well as for its indication for the appropriate procedures.
In a metagenomic analysis [25], several bacterial species were detected in the aortic valve tissues, including 10 phyla, 69 genera and 219 bacterial species. Of these, nine bacterial species were common between oral plaque and aortic valve tissue samples. However, the most frequently detected microorganism in cases of endocarditis was Staphylococcus aureus, followed by Staphylococcus epidermidis, Streptococcus mitis, Enterococcus faecalis and Escherichia coli [9,26].
Evidence that pathogens present in the oral cavity could migrate to other parts of the body was pointed out since 1891 indicating a correlative potential role in the etiology of systemic diseases [27]. Transient bacteraemia is directly influenced by the degree and extension of trauma caused by certain dental procedures, such as extraction, periodontal therapies [2], endodontic treatment, tooth brushing and the use of dental floss [28,29]. These procedures are considered potential risk factors for endocarditis due to the possibility of laceration of venules and capillaries in the gingival sulcus, periodontal ligament and alveolar bone-provoking bacteraemia [30,31,32,33]. The concentration of bacterial inoculum and the duration of bacteraemia resulting from a dental procedure is directly proportional to the degree of trauma and the level of inflammation at the surgical site. For this reason, bacteraemia is most observed after tooth extractions followed by a lower frequency of dental scaling procedures [30,34] indicating that the procedure extension is a crucial parameter in this sense. In addition, the time of exposure to bacteria present in the oral cavity can be prolonged in cases of multiple extractions sites, due to the longer duration of the procedure [35,36].
Non-surgical endodontic procedures produce a lower incidence of bacteraemia, in comparison with the reflection of flaps, periapical curettage or dental extractions [37]. This might be justified by the limited manipulated area during this procedure and the local number of blood vessels involved is smaller than that of other procedures. The possible relationship of endodontic pathology with human endocarditis has been widely investigated for a century now. Although the theory of focal infection and elective localization have already been discredited, it remains to be elucidated whether, even in rare cases, the passage of remaining bacteria in the root canal system of the endodontically treated tooth into the bloodstream can trigger infective endocarditis. Moreover, the development of endodontic medicine in recent decades has shown that endodontic pathology is interrelated with systemic pathologies [38,39].
Although there are studies on different dental procedures associating them with the risk of developing endocarditis, no previous systematic reviews intended to correlate endodontic infection with endocarditis. Thus, the present systematic review aimed to investigate whether any endodontic disease or treatment (considering a treatment-provoked bacteraemia) exerts any influence on endocarditis in humans.
2. Materials and Methods
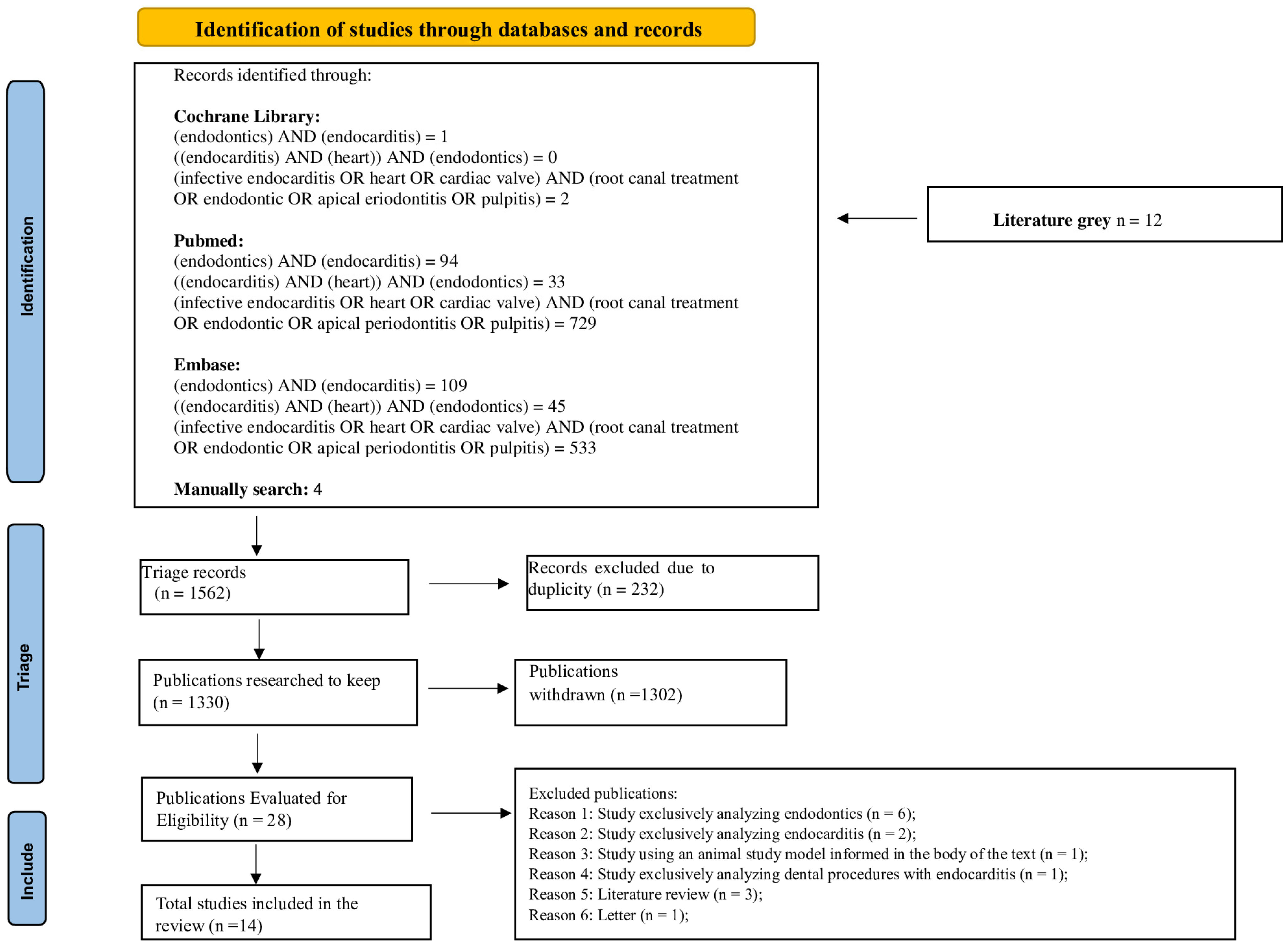
The present systematic review adopted planning and execution rules following the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). The PRISMA Checklist and the systematic review protocol were registered a priori in the International Prospective Register of Systematic Reviews (PROSPERO) and identified by the registration number CRD42023407736. The PRISMA flowchart of the process of selection, inclusion and exclusion of studies is detailed in Figure 1.
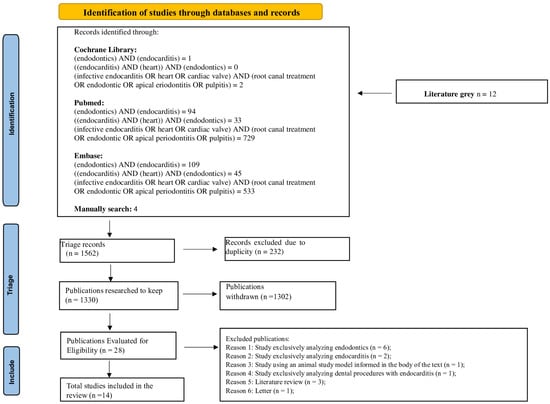
Figure 1.
Flowchart of the systematic review search strategy following the Preferred Reporting Items for Systematic Reviews and meta-Analyses (PRISMA) guidelines 2020.
2.1. Study Design
The systematic review question elaboration followed a previously reported guide [40] for ‘Aetiology and/or Risk’ which include the elements ‘Population, Exposure, Outcome (PEO)’. Thus, the proposed question is “does the endodontic treatment (Exposure) have any influence on endocarditis (Outcome) in humans (Population)?”.
2.2. Eligibility Criteria
Studies were only included when they involved any human participant, excluding those that used animal models or had participants with other associated systemic conditions. The investigation focused only on endodontics as the main intervention. Results that indicated any influence of an endodontic disease or treatment on the development of endocarditis were considered. All study designs were eligible; however, specific types of publications were excluded, such as literature reviews, conference abstracts, studies aiming for a protocol design, letters to the editor, personal opinions, book chapters or institutional manuals.
2.3. Search Strategies and Sources of Information
The search strategy covered electronic databases: PubMed, Embase, Cochrane Library and Gray literature until 7 May 2024. Additionally, a hand search was performed focusing on the reference lists of the included papers and all the issues of specific endodontic and cardiac journals of the last 20 years were searched for relevant articles. No language restrictions were applied. The descriptors were identified using the Medical Subject Headings (MESH), with the following search terms:
- (endodontics) AND (endocarditis)
- (endocarditis) AND (heart) AND (endodontics)
- (infective endocarditis OR heart OR cardiac valve) AND (root canal treatment OR endodontic OR apical periodontitis OR pulpitis)
2.4. Study Selection and Data Extraction
All search results were exported as compatible files for the Zotero reference manager (Version 6 for Windows) and duplicates were removed. The titles and abstracts of the articles were independently analyzed by two reviewers (JSP and ACNL), evaluating the inclusion and exclusion criteria and in case of disagreements, a third reviewer was consulted. As a calibration exercise, reviewers discussed the eligibility criteria and applied them to a 10% sample of titles to determine the inter-rater agreement. After obtaining a Kappa level of agreement above 0.83, the titles of the studies were then analyzed.
After the initial screening, potentially relevant studies were re-evaluated by full-text reading. A spreadsheet (Excel Microsoft Professional 365, Build 17531.20152) was previously developed for data extraction including citation (first author and year of publication), type of study, number of sample participants, objective/approach and influence/outcome. Studies rejected at this stage were recorded separately, making clear the reasons for its exclusion.
2.5. Risk of Bias Assessment
The methodological quality analysis of observational studies was performed with tools of the Joanna Briggs Institute (JBI) according to the design of each study [40,41]. Two authors independently assessed (a third author was consulted for divergencies) each domain for the risk of bias and each question could be answered—according to each tool—as follows: “Yes”, if the study did not present biases towards the question; “No”, if the study presented biases; “Unclear” if the study did not provide sufficient information for assessment; and “Not Applicable”, if the question did not fit in the characteristic of the study. A previously reported guide was observed for the selection of each tool [42].
2.6. Evaluation of Heterogeneity
Due to the heterogeneity between the different study designs included, it was not possible to conduct the synthesis of quantitative data for a meta-analysis. Therefore, clinical results were extracted, summarized and a descriptive review was performed.
3. Results
3.1. Study Selection Results
Initially, 1562 articles were included resulting in 232 after duplicate removal. Of these, 1302 were excluded for different reasons [i.e., exclusive focus on endodontics (553) or endocarditis (95), studies in animal models (9), other dental procedures associated with endocarditis (21), literature reviews (101), letters to readers (3), studies on other subjects (485), assessment of professionals’ knowledge (25), book chapters (2) and cases in which the articles could not be found (8)]. Thus, in total, 28 studies were identified according to eligibility. Of these, 14 articles were included in this review. Hand search resulted in zero relevant articles.
3.2. Characteristics of Eligible Studies
Most of the studies were published after 1962 (50% between 2012 and 2024) and 92.8% of the studies were published in English. The minimum individual age was 16 years-old and the maximum age was 90 years-old. The total of enrolled individuals was 2394 participants and eight root canals (there is no specification as to whether the results belong to the same patient or to different individuals).
The identified studies included the following study designs: seven observational cross-sectional studies, two prevalence studies, two cohort study, one prospective observational study, one case report and one case–control. The main characteristics of the 15 included studies are shown in Table 1.

Table 1.
Distribution in reverse chronological order of selected studies according to author, year, number of participants, results of the influence of endodontic treatment on human endocarditis.
3.3. Risk of Bias Assessment

Table 2.
Risk of bias assessment for analytical cross-sectional studies.

Table 3.
Risk of bias assessment for a case report type study.

Table 4.
Risk of bias assessment for cohort study.

Table 5.
Risk of bias assessment for case–control study.

Table 6.
Risk of bias assessment for prevalence study.
- Q1. Were the criteria for inclusion in the sample clearly defined?
- Q2. Were the study subjects and the setting described in detail?
- Q3. Was the exposure measured in a valid and reliable way?
- Q4. Were objective, standard criteria used for measurement of the condition?
- Q5. Were confounding factors identified?
- Q6. Were strategies to deal with stated confounding factors?
- Q7. Were the outcomes measured in a valid and reliable way?
- Q8. Was suitable statistical analysis used?
- Q1. Were the patient’s demographic characteristics clearly described?
- Q2. Was the patient’s history clearly described and presented as a timeline?
- Q3. Was the current clinical condition of the patient on presentation clearly described?
- Q4. Were diagnostic tests or assessment methods and the results clearly described?
- Q5. Was the intervention(s) or treatment procedure(s) clearly described?
- Q6. Was the post-intervention clinical condition clearly described?
- Q7. Were adverse events (harms) or unanticipated events identified and described?
- Q8. Does the case report provide takeaway lessons?
- Q1. Were the two groups similar and recruited from the same population?
- Q2. Were the exposures measured similarly to assign people to both exposed and unexposed groups?
- Q3. Was the exposure measured in a valid and reliable way?
- Q4. Were confounding factors identified?
- Q5. Were there strategies to deal with stated confounding factors?
- Q6. Were the groups/participants free of the outcome at the start of the study (or at the moment of exposure)?
- Q7. Were the outcomes measured in a valid and reliable way?
- Q8. Was the follow up time reported and sufficient to be long enough for outcomes to occur?
- Q9. Was follow up complete, and if not, were the reasons for loss to follow up described and explored?
- Q10. Were strategies to address incomplete follow up utilized?
- Q11. Was suitable statistical analysis used?
- Q1. Were the groups comparable other than the presence of disease in cases or the absence of disease in controls?
- Q2. Were cases and controls matched appropriately?
- Q3. Were the same criteria used for identification of cases and controls?
- Q4. Was exposure measured in a standard, valid and reliable way?
- Q5. Was exposure measured in the same way for cases and controls?
- Q6. Were confounding factors identified?
- Q7. Were there strategies to deal with stated confounding factors?
- Q8. Were outcomes evaluated in a standard, valid and reliable way for cases and controls?
- Q9. Was the exposure period of interest long enough to be meaningful?
- Q10. Was suitable statistical analysis used?
- Q1. Was the sample frame appropriate to address the target population?
- Q2. Were study participants sampled in an appropriate way?
- Q3. Was the sample size adequate?
- Q4. Were the study subjects and the setting described in detail?
- Q5. Was the data analysis conducted with sufficient coverage of the identified sample?
- Q6. Were valid methods used for the identification of the condition?
- Q7. Was the condition measured in a standard, reliable way for all participants?
- Q8. Was there appropriate statistical analysis?
- Q9. Was the response rate adequate, and if not, was the low response rate managed appropriately?
4. Discussion
Endocarditis can be a consequence of invasive procedures of dental procedures, including endodontic treatments. The review question “does the endodontic treatment influence human endocarditis?” could be partially answered, since the present systematic review included 14 studies reporting this association. The analyzed studies showed that bacteraemia occurs after endodontic treatment, but for this infection to cause systemic effects other factors besides the presence of bacteria (i.e., virulence capacity and bacterial counts) in the bloodstream were correlated with the development of the disease [54]. The inclusion of the considerably low number of studies regarding this crucial association between endodontic treatment and endocarditis poses a concern mainly associated with the urgency of endocarditis conditions. Unfortunately, due to the heterogeneity between the studies, a meta-analysis could not be possible to be performed at this moment.
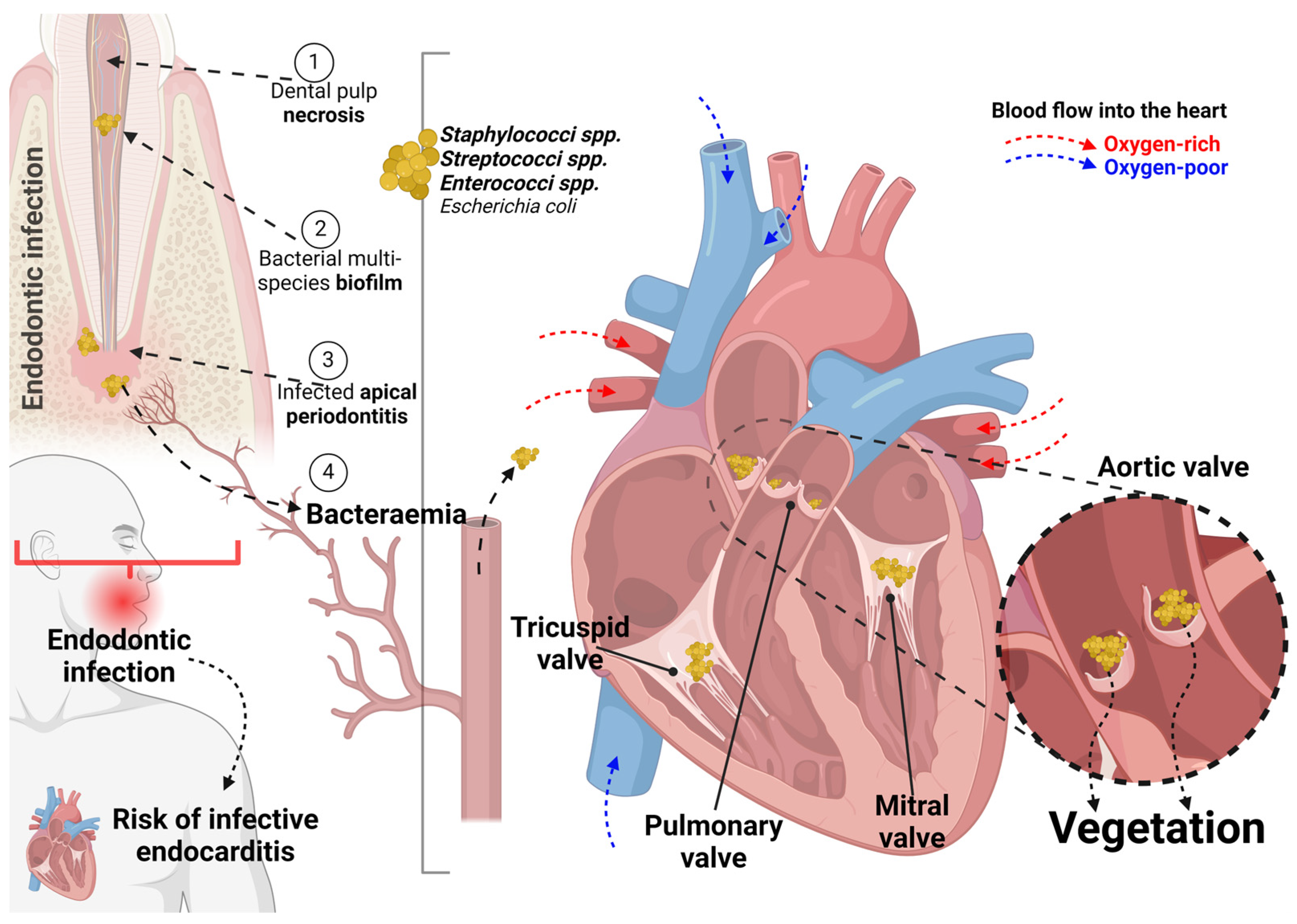
Since 1962, cases of non-surgical endodontics have been reported correlating bacteraemia with consequential bacterial endocarditis [53]. Another report, in 1963, described a transient—for 10 min—blood bacteraemia following non-surgical endodontic procedures [52]; however, the implications of this bacteraemia in patients with heart conditions prone to develop endocarditis deserves discussion [51]. A hypothesized mechanism indicating the endodontic infection as the bacterial source for bacteraemia and, potentially, leading to valve vegetation is shown in Figure 2. The included studies have divergent opinions, as they failed to identify a clear relationship between dental procedures and endocarditis, verifying only a trend of risk associated with endodontic treatment [9,26,44]. It is important to highlight here that endodontic treatments include vital pulp treatments, primary root canal treatment, non-surgical retreatment and surgical retreatment aiming to treat a vital pulp tissue or—after pulp tissue necrosis—a condition of infected apical periodontitis [55]. These treatments may provoke bacteraemia specially in cases of pulp tissue necrosis and apical periodontitis [38].
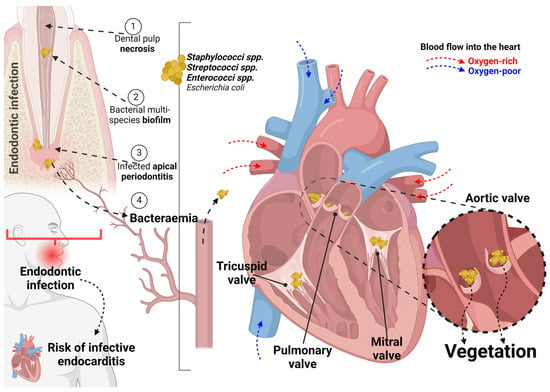
Figure 2.
A hypothesized mechanism indicating the endodontic infection following dental pulp necrosis with biofilm formation and apical infection. This bacterial source leads to bacteraemia and, potentially, results in valve vegetation specially in the aortic valve (highlighted). Worryingly, multi-species bacteraemia post-endodontic treatment was reported. Created using BioRender.com.
The findings here revised must be carefully interpreted, since there may have been a recruitment bias in the control groups that were originally from the community or hospitalized in cardiology wards for reasons other than endocarditis [9,10,56]. In a study that minimized this bias, it was found that factors associated with endocarditis were also correlated with hygiene habits, besides pulp necrosis and dental procedures [57]. Recent studies have expanded the understanding of the possible connections between dental infections and medical conditions, such as prosthetic valve endocarditis and rejection of transplanted organs [58,59]. Thus, the elimination of chronic infections of the oral cavity plays a crucial role in the prevention of endocarditis [60] and preoperative oral care positively impacts the outcome of cardiovascular surgery [48].
The identification of the causative microorganisms is crucial for an adequate and effective indication of antimicrobial therapy and the absence of a microbiological diagnosis is an independent predictor of in-hospital mortality [61]. A genomic study associated with bioinformatics allowed the evaluation of the microbial community and identified that the genera Parvimonas, Streptococcus and Enterococcus were the main ones detected in root canals associated with endocarditis [43]. Corroborating with these findings, other studies indicated that the species of Streptococcus, Staphylococcus and Enterococcus represent 80% to 90% of the microorganisms associated with endocarditis [26,45,62,63].
Oral Viridans streptococci are implicated as causative organisms in 35–45% of cases with endodontic-associated endocarditis [9,26]. The great disparity between the microbiological causes of endocarditis found in the studies can be partially explained by the different acquisition routes [64,65]. Microbiological profiles of root canals and periodontal pockets may not be the only risk factors for endocarditis but may also be associated with systemic diseases including myocarditis, human cytomegalovirus infection, bacterial invasion of epithelial cells, Huntington’s disease, lateral sclerosis amyotrophic and hypertrophic cardiomyopathy [2,43]. This is a clear indication that further studies are needed to stablish a fully understood association between endodontic conditions and endocarditis.
Other important studies, not included as a full text in this systematic review, indicated that Parvimonas (anaerobic Gram-positive cocci) is a commensal organism of the human oral cavity and gastrointestinal tract [66]. However, this microorganism is associated with serious infections such as: prosthetic joint infections, diabetic foot, liver and splenic abscesses, spondylodiscitis, empyema, pericarditis and meningitis [67,68,69,70,71]. In addition, bacterial endocarditis from Parvimonas micra causing severe damage to the heart valves and leading to fatal complications was reported [72]. The pathogenicity of Parvimonas micra in the oral cavity has been attributed to its adhesion to epithelial cells, cell morphotype and/or proteolytic activity in addition to the response of human macrophages; however, in isolated infections these factors are not clear [73].
There is a change in the characteristics of community-associated endocarditis, with an increase in the mortality rate when caused by Staphylococcus aureus and a decrease in comparison with endocarditis streptococcal infection of Viridans [74,75,76,77]. Staphylococcal spp. endocarditis cases are mainly attributed to hospital health care, injectable drugs and outpatient clinics [78]. Besides, this condition is more commonly observed in patients receiving haemodialysis [79]. Staphylococcus aureus can form a biofilm by adhering to the endothelial cell surface due to the presence of a protein factor that is involved in cell aggregation and biofilm formation [80].
Staphylococcus, Streptococcus, and Enterococcus carry the microbial surface component that reacts with matrix adhesive molecules that mediate adhesion to damaged heart valves and bacterial vegetations [81]. When endothelial damage occurs, there may occur activation of the blood clotting system, resulting in the formation of thrombi in the affected area, which can serve as favourable sites for the implantation of infected biofilm, which consist of aggregates of bacteria, blood cells and tissue debris, covered by fibrin [82]. This multilayer cluster is surrounded by an extracellular matrix of polysaccharides and proteins that protect against the immune system and hinders the penetration of antibiotics [22,83]. This pathogen also tends to increase antibiotic resistance with methicillin-resistant strains emerging as a serious concern worldwide [84].
Streptococcus viridans is a species of Gram-positive bacteria that is normally part of the normal human microbiota, colonizing mainly the oral cavity, throat and gastrointestinal tract [85]. Under normal conditions, they are considered commensal, not causing disease in healthy individuals [86]. However, under certain circumstances, these bacteria can become pathogenic and trigger infections, such as endocarditis, especially in people with compromised immune systems or in situations that facilitate their entry into the bloodstream [87]. This can occur after invasive procedures, such as dental treatments, medical surgeries or also skin piercings [88]. An exceptional complication of endocarditis, involving the mitral valve, was reported in the literature caused by the intramedullary abscess of the medulla [89].
Bacterial attachment in exposed collagen tissue from compromised endothelium plays a crucial role in the onset of endocarditis [90]. Viridans streptococci exhibit properties of adhesion and invasion in human vascular endothelial cells [91,92]. Opposingly, some Streptococcus mutans (associated with human dental caries and infected root canals [93] strains have a virulence factor related to collagen binding, as they have a collagen-binding protein on their cell surface [94]. In addition, the lack of a protein antigen makes them more adherent and invasive [95]. The inactivation of these collagen-binding proteins results in a defect in adhesion to the matrix meaning that bacteria lose the ability to attach properly to exposed collagen tissue in compromised endothelium [96].
Enterococci are part of the gastrointestinal flora [97] and have a remarkable ability to adapt to the environment, in certain situations they can cause infections in different parts of the body, such as urinary infections, intra-abdominal infections, wounds and pelvic infections [98]. Enterococcal endocarditis was associated with urological procedures [99]. Besides, studies have linked this pathogen to the occurrence of invasive conditions, such as colorectal neoplasia [100] and cardiovascular infections [101]. Worryingly, enterococcal endocarditis is characterized by a higher frequency of relapses than other endocarditis, being often asymptomatic and usually occurring more than 6 months after the initial episode [102]. In addition, this microorganism was also associated with unusual manifestations in the form of pemphigus foliaceus [103], postpartum infective endocarditis without underlying disease [104] and in a case of endocarditis with vancomycin-resistant complicated by splenic infarction and embolic stroke [47,105].
An included study regarding the investigation of Enterococcus faecalis indicated that a potent virulence gene and endocardial adhesion factor antigen in bacteraemia of this microorganism may pose risks for systemic complications such as endocarditis in at-risk patients. When analyzing the genotype of enterococci isolated from root canals, this study indicated that the phenotypic and genotypic evidence of potential virulence factors were identified in endodontic Enterococcus spp., specifically the production of gelatinase and its response to pheromones playing an important role in biofilm formation [50].
Conducting studies on bacterial counts in the incidence of bacteraemia after dental procedures is crucial for the development of prophylaxis guidelines for endocarditis [106,107]. In dental procedures, the peak of bacteraemia was detected within 5 min after the end of the procedure, decreasing significantly between 6 and 20 min suggesting that the initial elimination of bacteria from the bloodstream is rapid [108]. In this review, two studies [46,49] identified bacteraemia through cultures. The first found that bacteraemia was present 5 and 30 min after endodontic treatment. This agrees with another study [109] which also did not find bacteraemia when the endodontic instrumentation was short of the apical foramen. However, these results differ from other culture studies [37,110] that reported the occurrence of bacteraemia in cases where instrumentation reached beyond the foramen [39] and these bacteraemia do not last more than 60 min after their generation, but intentional or accidental over-instrument—especially with the presence of a periapical lesion (Figure 2)—can expose high-risk patients to a greater tendency to develop endocarditis, since there is a manipulation of the periapical tissue in a vascularized site [45,111]. The second study reported that five minutes after endodontic treatment, the presence of bacteraemia was verified, without difference between primary treatments and retreatments. Microorganisms were identified in the root canals, but only two aerobic species showed the same genotype in the root canal and the blood samples. This suggests that microorganisms can cause transient bacteraemia and increase the risk of cardiovascular complications [49]. The presence of apical periodontitis [43] and periodontal diseases [2] could increase the risk of developing endocarditis in susceptible patients. These findings corroborate a previous study that also indicated this possibility when analyzing the Enterococcus faecalis endocarditis antigen, a potent virulence gene, which was detected in the root canals of endodontic infections as being similar to reports of medically relevant strains [47].
Variations in the results of the incidence of bacteraemia between studies should be considered when interpreting the type of surgical treatment and the methods used to isolate microorganisms from the blood. Some microorganisms may need specific conditions or nutrients to survive and develop, which may limit their detection in conventional blood culture systems [30]. Studies using qPCR methods may present a detection bias since they cannot distinguish between DNA from viable and dead bacterial cells which may affect the interpretation of results [46].
There are few published studies on the magnitude of bacteraemia after a dental procedure and only one study was included [46] using qPCR. The authors reported that the average level of total bacteria and Streptococci in the bacteraemia (also for 5 min) was of the order of 101 to 102/mL in the blood and could be considered a relatively low concentration when compared to the range of the inoculum of 103 to 109 bacterial cells/mL of blood necessary to cause experimental endocarditis [54]. The range of inoculum to cause endocarditis in humans is still unknown [5]. This information assumes crucial relevance in determining the degree of invasiveness of a procedure and the identification of potential risks for the patient that leads to the potential beneficial effect of administering antibiotic prophylaxis in selected patients to reduce bacteraemia. Although it is possible to speculate that the duration and, possibly, even the incidence of bacteraemia may be influenced by its magnitude, this relationship still lacks validation [108].
In a retrospective analysis of 739 patients in Taiwan, no increased probability of exposure to dental procedures was found in the three months before hospitalization for endocarditis, compared to a control period [14]. These findings suggest that invasive dental procedures, including endodontic treatment, may not be the triggering event for most cases of endocarditis [112] as the studies may have been conducted in populations that already used antibiotic prophylaxis potentially masking a plausible association [57]. It is known that the use of antibiotics may be less frequent for procedures such as scaling and endodontic treatment, as shown in studies on the knowledge of dentists [113,114,115].
Another possible association could be inferred by events that are triggered by low-level exposures, such as bacteraemia associated with cumulative daily activities such as toothbrushing, flossing and chewing [5,107,116,117]. Additionally, neglected hygiene habits in clinically compromised patients alters the defense barrier to infection and is weakened due to the local inflammation [118]. However, these results should be interpreted with caution due to the low number of patients and study design [119]. It is crucial to also consider that dental preventive check-ups in patients undergoing cardio-thoracic surgery and interventional cardiovascular procedures needs close attention by both the heart and the dental teams in the pre-interventional preparation phase [120]. Besides, molecular tools (i.e., Karius non-invasive pathogen blood test) can confirm, in endocarditis affected patients, Gram-positive anaerobic cocci (Anaerococcus hydrogenalis) microbial cell-free DNA (mcfDNA) is rarely detected, indicating its applicability in endocarditis cases [121].
Finally, a case-by-case evaluation would be the best scenario in patients with intermediate host-related risk factors as one significant gap in evidence is the association between bacteremia and endocarditis in these cases. A current guideline (2023 European Society of Cardiology [ESC], available and periodically revised at https://www.escardio.org/Guidelines/Clinical-Practice-Guidelines/Endocarditis-Guidelines, accessed on 15 July 2024) was developed to support healthcare professionals in diagnosing and managing infective endocarditis; this 2023 version is introducing a new diagnostic algorithm to aid in patient classification aiming to improve outcomes for this challenging disease. The guideline includes the observation under topic 3.3.1: “At-risk dental procedures include dental extractions, oral surgery procedures (including periodontal surgery, implant surgery, and oral biopsies), and dental procedures involving manipulation of the gingival or periapical region of the teeth (including scaling and root canal procedures).” Here, we highlight the inclusion of endodontic treatments in the 2023 ESC guidelines observation which demands a careful anamnesis to be performed by the dental professional focusing on the risk conditions.
Risk of Bias
The cross-sectional studies included in this review do not allow the establishment of a definitive cause–effect relationship between endodontics and endocarditis due to evaluation period biases, since the data were collected in a single moment [43,45,46,47,49,50,51,52]. Two studies presented interpretation bias due to the lack of clear establishment of a direct causal relationship between the studied factors and endocarditis [43,50]. One study showed confounding bias, as it did not control for other factors that could influence the association found [45]. Four studies showed information bias due to different issues, such as diagnostic classification based on ICD-9-CM codes instead of Duke criteria, lack of sufficient detail on cases, use of records and reports to collect data and subjective interpretation of data from dental procedures and their related causes [14,26,45,53]. One study also showed loss-to-follow-up bias due to reduced effective sample size and possible recall biases [48].
This systematic review focused on any influence between endodontic disease and treatment, and endocarditis; however, the small number of studies, low certainty in evidence due to risk of bias issues with included data involved confounding factors and lack of randomized clinical trials made it difficult to explore the influencing factors for the synthesis of scientific evidence that would be obtained in a meta-analysis.
5. Conclusions
Evidence, to date, transient bacteraemia was used as a surrogate indicator to assess the risk of endocarditis in patients undergoing endodontic treatment. However, definitive validation of the association between the magnitude of the bacteraemia, its duration, the incidence of the disease and, mainly, its direct link with endocarditis remains inconclusive and deserves investigation.
Author Contributions
J.S.P. was involved in conceptualization, review, data acquisition and writing. A.C.N.L. was involved in data acquisition, writing and review. L.E.P. was involved in conceptualization, methodology, and writing. M.A.M. and B.P.F.A.G. were involved in writing, funding obtention and supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Capes), grant number: code 001 and by The Sao Paulo Research Foundation FAPESP 2022/03093-9.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
BioRender.com is acknowledged as the design tool for Figure 2.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sullam, P.M.; Drake, T.A.; Sande, M.A. Pathogenesis of endocarditis. Am. J. Med. 1985, 78, 110–115. [Google Scholar] [CrossRef]
- Tonelli, A.; Lumngwena, E.N.; Ntusi, N.A.B. The oral microbiome in the pathophysiology of cardiovascular disease. Nat. Rev. Cardiol. 2023, 20, 386–403. [Google Scholar] [CrossRef]
- Carapetis, J.R.; Beaton, A.; Cunningham, M.W.; Guilherme, L.; Karthikeyan, G.; Mayosi, B.M.; Sable, C.; Steer, A.; Wilson, N.; Wyber, R.; et al. Acute rheumatic fever and rheumatic heart disease. Nat. Rev. Dis. Primers 2016, 2, 15084. [Google Scholar] [CrossRef]
- Sun, L.C.; Lai, C.C.; Wang, C.Y.; Wang, Y.H.; Wang, J.Y.; Hsu, Y.L.; Hu, Y.L.; Wu, E.T.; Lin, M.T.; Sy, L.B.; et al. Risk factors for infective endocarditis in children with congenital heart diseases—A nationwide population-based case control study. Int. J. Cardiol. 2017, 248, 126–130. [Google Scholar] [CrossRef]
- Wilson, W.; Taubert, K.A.; Gewitz, M.; Lockhart, P.B.; Baddour, L.M.; Levison, M.; Bolger, A.; Cabell, C.H.; Takahashi, M.; Baltimore, R.S.; et al. Prevention of Infective Endocarditis: Guidelines from the American Heart Association: A Guideline From the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation 2007, 116, 1736–1754. [Google Scholar]
- Baehr, G. Glomerular lesions of subacute bacterial endocarditis. J. Exp. Med. 1912, 15, 330–347. [Google Scholar] [CrossRef]
- Blake, F.G. The etiology of rat-bite fever. J. Exp. Med. 1916, 23, 39–60. [Google Scholar] [CrossRef][Green Version]
- Maccallum, W.G.; Hastings, T.W. A case of acute endocarditis caused by micrococcus zymogenes (nov. spec.), with a description of the microorganism. J. Exp. Med. 1899, 4, 521–534. [Google Scholar] [CrossRef]
- Lacassin, F.; Hoen, B.; Leport, C.; Selton-Suty, C.; Delahaye, F.; Goulet, V.; Etienne, J.; Briançon, S. Procedures associated with infective endocarditis in adults—A case control study. Eur. Heart J. 1995, 16, 1968–1974. [Google Scholar] [CrossRef]
- Strom, B.L. Dental and Cardiac Risk Factors for Infective Endocarditis: A Population-Based, Case-Control Study. Ann. Intern. Med. 1998, 129, 761. [Google Scholar] [CrossRef]
- Albakri, A.; Ahsan, A.; Vengal, M.; Ramacham Parambathu, A.K.; Majeed, A.; Siddiq, H. Antibiotic prophylaxis before invasive dental procedures for patients at high risk of infective endocarditis—A systematic review. Indian J. Dent. Res. 2022, 33, 452. [Google Scholar] [PubMed]
- McGowan, D.A. Dental treatment of patients with valvular heart disease. Br. Dent. J. 1968, 124, 519–520. [Google Scholar]
- Hasbun, R.; Vikram, H.R.; Barakat, L.A.; Buenconsejo, J.; Quagliarello, V.J. Complicated Left-Sided Native Valve Endocarditis in Adults: Risk Classification for Mortality. JAMA 2003, 289, 1933–1940. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.C.; Tung, Y.C.; Wu, P.W.; Wu, L.S.; Lin, Y.S.; Chang, C.J.; Kung, S.; Chu, P.H. Dental Procedures and the Risk of Infective Endocarditis. Medicine 2015, 94, e1826. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.A.; Harrison, L.M.; Biswas, J.; Telford, D.R. Transient bacteraemia: A possible cause of sudden life threatening events. Med. Hypotheses 2007, 69, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- Dajani, A.S.; Taubert, K.A.; Wilson, W.; Bolger, A.F.; Bayer, A.; Ferrieri, P.; Gewitz, M.H.; Shulman, S.T.; Nouri, S.; Newburger, J.W.; et al. Prevention of bacterial endocarditis: Recommendations by the American Heart Association. J. Am. Dent. Assoc. 1997, 128, 1142–1151. [Google Scholar] [CrossRef] [PubMed]
- Cloitre, A.; Lesclous, P.; Trochu, Q.; Selton-Suty, C.; Boutoille, D.; Le Tourneau, T.; Delahaye, F.; Thomas, D.; Iung, B.; Gaudin, A.; et al. Antibiotic prophylaxis of infective endocarditis in patients with predisposing cardiac conditions: French cardiologists’ implementation of current guidelines. Int. J. Cardiol. 2020, 299, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.P.; Del Zotti, F.; Dulgheru, R.; El Khoury, G.; Erba, P.A.; Iung, B.; et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC)Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur. Heart J. 2015, 36, 3075–3128. [Google Scholar] [PubMed]
- Flynn, C.E.; Guarner, J. Emerging Antimicrobial Resistance. Mod. Pathol. 2023, 36, 100249. [Google Scholar] [CrossRef]
- Bengtsson-Palme, J.; Kristiansson, E.; Larsson, D.G.J. Environmental factors influencing the development and spread of antibiotic resistance. FEMS Microbiol. Rev. 2018, 42, fux053. [Google Scholar] [CrossRef]
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. Virulence Mech. Bact. Pathogens 2016, 4, 481–511. [Google Scholar]
- Davies, J.; Davies, D. Origins and Evolution of Antibiotic Resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef]
- Larsson, D.G.J.; Flach, C.-F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Adeyemo, W.L.; Oderinu, O.H.; Olojede, A.C.; Ayodele, A.O.; Fashina, A.A. Nigerian dentists’ knowledge of the current guidelines for preventing infective endocarditis. Community Dent. Health 2011, 28, 178–181. [Google Scholar]
- Yoshiba, S.; Nakagawa, H.; Kuwata, H.; Nabuchi, A.; Yaso, A.; Shirota, T. Metagenomic analysis of oral plaques and aortic valve tissues reveals oral bacteria associated with aortic stenosis. Clin. Oral Investig. 2023, 27, 4335–4344. [Google Scholar] [CrossRef]
- Suárez-García, S.; Berrío-Solarte, R.; Marín-Monsalve, C.; Abadía-Zapata, J.; Botero, J. Prevalence of infective endocarditis from dental procedures. Rev. Colomb. Cardiol. 2023, 30, 3–9. [Google Scholar] [CrossRef]
- Miller, W.D. The human mouth as a focus of infection. Lancet 1891, 138, 340–342. [Google Scholar] [CrossRef]
- Cotti, E.; Mercuro, G. Apical periodontitis and cardiovascular diseases: Previous findings and ongoing research. Int. Endod. J. 2015, 48, 926–932. [Google Scholar] [CrossRef]
- Korn, N.A.; Schaffer, E.M. A Comparison of the Postoperative Bacteremias Induced Following Different Periodontal Procedures. J. Periodontol. 1962, 33, 226–231. [Google Scholar] [CrossRef]
- Heimdahl, A.; Hall, G.; Hedberg, M.; Sandberg, H.; Söder, P.O.; Tunér, K.; Nord, C.E. Detection and quantitation by lysis-filtration of bacteremia after different oral surgical procedures. J. Clin. Microbiol. 1990, 28, 2205–2209. [Google Scholar] [CrossRef]
- Lineberger, L.T.; De Marco, T.J. Evaluation of Transient Bacteremia Following Routine Periodontal Procedures. J. Periodontol. 1973, 44, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Seymour, R.A.; Lowry, R.; Whitworth, J.M.; Martin, M.V. Infective endocarditis, dentistry and antibiotic prophylaxis; time for a rethink? Br. Dent. J. 2000, 189, 610–616. [Google Scholar]
- Thornhill, M.H.; Gibson, T.B.; Yoon, F.; Dayer, M.J.; Prendergast, B.D.; Lockhart, P.B.; O’Gara, P.T.; Baddour, L.M. Endocarditis, invasive dental procedures, and antibiotic prophylaxis efficacy in US Medicaid patients. Oral Dis. 2024, 30, 1591–1605. [Google Scholar] [CrossRef] [PubMed]
- Reis, L.C.; Rôças, I.N.; Siqueira, J.F., Jr.; de Uzeda, M.; Lacerda, V.S.; Domingues, R.; Miranda, K.R.; Saraiva, R.M. Bacteremia after supragingival scaling and dental extraction: Culture and molecular analyses. Oral Dis. 2018, 24, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Bender, I.B.; Naidorf, I.J.; Garvey, G.J. Bacterial endocarditis: A consideration for physician and dentist. J. Am. Dent. Assoc. 1984, 109, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Coffin, F.; Thompson, R.E.M. Factors influencing bacteræmia following dental extraction. Lancet 1956, 268, 654–656. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, J.C.; Heggers, J.P.; Harrison, J.W. Incidence of bacteremias related to endodontic procedures.: II. Surgical endodontics. J. Endod. 1977, 3, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Niazi, S.A.; Bakhsh, A. Association between Endodontic Infection, Its Treatment and Systemic Health: A Narrative Review. Medicina 2022, 58, 931. [Google Scholar] [CrossRef]
- Baumgartner, J.C.; Heggers, J.P.; Harrison, J.W. The incidence of bacteremias related to endodontic procedures I. Nonsurgical endodontics. J. Endod. 1976, 2, 135–140. [Google Scholar] [CrossRef]
- Munn, Z.; Moola, S.; Lisy, K.; Riitano, D.; Tufanaru, C. Chapter 5: Systematic reviews of prevalence and incidence. In JBI Manual for Evidence Synthesis; JBI: Adelaide, Australia, 2020. [Google Scholar] [CrossRef]
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.; Lisy, K.; Qureshi, R.; Mattis, P.; et al. Chapter 7: Systematic reviews of etiology and risk. In JBI Manual for Evidence Synthesis; JBI: Adelaide, Australia, 2020. [Google Scholar] [CrossRef]
- Ma, L.L.; Wang, Y.Y.; Yang, Z.H.; Huang, D.; Weng, H.; Zeng, X.T. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: What are they and which is better? Mil. Med. Res. 2020, 7, 7. [Google Scholar] [CrossRef]
- Gomes, B.P.; Berber, V.B.; Chiarelli-Neto, V.M.; Aveiro, E.; Chapola, R.C.; Passini, M.R.; Lopes, E.M.; Chen, T.; Paster, B.J. Microbiota present in combined endodontic-periodontal diseases and its risks for endocarditis. Clin. Oral Investig. 2023, 27, 4757–4771. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, G.W.; Glaudemans, A.W.; Erba, P.A.; Wouthuyzen-Bakker, M.; Sinha, B.; Vállez García, D.; van der Sluis, L.W.; Slart, R.H. Relationship between 18F-FDG Uptake in the Oral Cavity, Recent Dental Treatments, and Oral Inflammation or Infection: A Retrospective Study of Patients with Suspected Endocarditis. Diagnostics 2020, 10, 625. [Google Scholar] [CrossRef] [PubMed]
- Chirillo, F.; Faggiano, P.; Cecconi, M.; Moreo, A.; Squeri, A.; Gaddi, O.; Cecchi, E.; Italian Registry on Infective Endocarditis (RIEI) Investigators. Predisposing cardiac conditions, interventional procedures, and antibiotic prophylaxis among patients with infective endocarditis. Am. Heart J. 2016, 179, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Reis, L.C.; Rôças, I.N.; Siqueira, J.F., Jr.; de Uzeda, M.; Lacerda, V.S.; Domingues, R.M.; Moraes, S.R.; Saraiva, R.M. Bacteremia after Endodontic Procedures in Patients with Heart Disease: Culture and Molecular Analyses. J. Endod. 2016, 42, 1181–1185. [Google Scholar] [CrossRef] [PubMed]
- Preethee, T.; Kandaswamy, D.; Hannah, R. Molecular identification of an Enterococcus faecalis endocarditis antigen efaA in root canals of therapy-resistant endodontic infections. J. Conserv. Dent. 2012, 15, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Deppe, H.; Auer-Bahrs, J.; Kolk, A.; Hall, D.; Wagenpfeil, S. Need for dental treatment following cardiac valve surgery: A clinical study. J. Cranio-Maxillofac. Surg. 2007, 35, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Savarrio, L.; Mackenzie, D.; Riggio, M.; Saunders, W.P.; Bagg, J. Detection of bacteraemias during non-surgicalroot canal treatment. J. Dent. 2005, 33, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Sedgley, C.M.; Molander, A.; Flannagan, S.E.; Nagel, A.C.; Appelbe, O.K.; Clewell, D.B.; Dahlén, G. Virulence, phenotype and genotype characteristics of endodontic Enterococcus spp. Oral Microbiol. Immunol. 2005, 20, 10–19. [Google Scholar] [CrossRef]
- Bate, A.L.; Ma, J.K.; Ford, T.R.P. Detection of bacterial virulence genes associated with infective endocarditis in infected root canals. Int. Endod. J. 2000, 33, 194–203. [Google Scholar] [CrossRef]
- Bender, I.B.; Seltzer, S.; Tashman, S.; Meloff, G. Dental procedures in patients with rheumatic heart disease. Oral Surg. Oral Med. Oral Pathol. 1963, 16, 466–473. [Google Scholar] [CrossRef]
- Eisenbud, L. Subacute bacterial endocarditis precipitated by nonsurgical dental procedures. Report of two cases. Oral Surg. Oral Med. Oral Pathol. 1962, 15, 624–627. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.J.; Wilson, W.R. Experimental animal endocarditis. In Mayo Clinic Proceedings; Mayo Foundation for Medical Education and Research: Rochester, UK, 1982; Volume 57, pp. 10–14. [Google Scholar]
- Duncan, H.F.; Kirkevang, L.; Peters, O.A.; El-Karim, I.; Krastl, G.; Del Fabbro, M.; Chong, B.S.; Galler, K.M.; Segura-Egea, J.J.; Kebschull, M.; et al. Treatment of pulpal and apical disease: The European Society of Endodontology (ESE) S3-level clinical practice guideline. Int. Endod. J. 2023, 56, 238–295. [Google Scholar] [CrossRef] [PubMed]
- Van Der Meer, J.T.M.; Michel, M.F.; Valkenburg, H.A.; van Wijk, W.; Thompson, J.; Vandenbroucke, J.P. Efficacy of antibiotic prophylaxis for prevention of native-valve endocarditis. Lancet 1992, 339, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Duval, X.; Millot, S.; Chirouze, C.; Selton-Suty, C.; Moby, V.; Tattevin, P.; Strady, C.; Euvrard, E.; Agrinier, N.; Thomas, D. Oral Streptococcal Endocarditis, Oral Hygiene Habits, and Recent Dental Procedures: A Case-Control Study. Clin. Infect. Dis. 2017, 64, 1678–1685. [Google Scholar] [CrossRef] [PubMed]
- Picu, C.; Mille, C.; Popescu, G.A.; Bret, L.; Prazuck, T. Aortic Prosthetic Endocarditis with Neisseria elongata Subspecies nitroreducens. Scand. J. Infect. Dis. 2003, 35, 280–282. [Google Scholar] [CrossRef] [PubMed]
- Vogel, M.; Knirsch, W.; Lange, P.E. Schwere Komplikationen durch Nichtbeachtung der Endokarditisprophylaxe während zahnärztlicher Eingriffe bei Erwachsenen mit angeborenen Herzfehlern. Dtsch. Med. Wochenschr. 2008, 125, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Terezhalmy, G.T.; Safadi, T.J.; Longworth, D.L.; Muehrcke, D.D. Oral Disease Burden in Patients Undergoing Prosthetic Heart Valve Implantation. Ann. Thorac. Surg. 1997, 63, 402–404. [Google Scholar] [CrossRef] [PubMed]
- Díez-Villanueva, P.; Muñoz, P.; Marín, M.; Bermejo, J.; de Alarcón González, A.; Fariñas, M.C.; Gutiérrez-Cuadra, M.; Pericás-Pulido, J.M.; Lepe, J.A.; Castelo, L.; et al. Infective endocarditis: Absence of microbiological diagnosis is an independent predictor of inhospital mortality. Int. J. Cardiol. 2016, 220, 162–165. [Google Scholar] [CrossRef]
- Montano, T.C.P.; Wanderley, M.I.A.; Sampaio, R.O.; Alves, C.G.B.; Neves, I.L.I.; Lopes, M.A.; Tarasoutchi, F.; Strabelli, T.M.V.; Neves, R.S.; Grinberg, M.; et al. Demographic, cardiological, microbiologic, and dental profiles of Brazilian patients who developed oral bacteria–related endocarditis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021, 132, 418–425. [Google Scholar] [CrossRef]
- Peterson, G.E.; Crowley, A.L. Antibiotic Prophylaxis for Infective Endocarditis: A Pound of Prevention and an Ounce of Cure. Circulation 2019, 140, 181–183. [Google Scholar] [CrossRef]
- Bouza, E.; Menasalvas, A.; Muñoz, P.; Vasallo, F.J.; Moreno, M.D.M.; Fernandez, M.A.G. Infective Endocarditis—A Prospective Study at the End of the Twentieth Century: New Predisposing Conditions, New Etiologic Agents, and Still a High Mortality. Medicine 2001, 80, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Ferreiros, E.; Nacinovich, F.; Casabé, J.H.; Modenesi, J.C.; Swieszkowski, S.; Cortes, C.; Hernan, C.A.; Kazelian, L.; Varini, S.; Eira-2 Investigators. Epidemiologic, clinical, and microbiologic profile of infective endocarditis in Argentina: A national survey. The Endocarditis Infecciosa en la República Argentina–2 (EIRA-2) Study. Am. Heart J. 2006, 151, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.; Ang, G.; Er, C.; Yap, S.F.; Aravamudan, V.M. An Unusual Presentation of Parvimonas micra Infective Endocarditis. Cureus 2018, 10, e3447. [Google Scholar] [CrossRef] [PubMed]
- Brook, I.; Frazier, E.H. Microbiology of Liver and Spleen Abscesses. J. Med. Microbiol. 1998, 47, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.H.; Baek, J.Y.; Kang, C.I.; Lee, W.J.; Lee, J.Y.; Cho, S.Y.; Ha, Y.E.; Kim, S.H.; Chung, D.R.; Peck, K.R.; et al. Bacteremic meningitis caused by Parvimonas micra in an immunocompetent host. Anaerobe 2015, 34, 161–163. [Google Scholar] [CrossRef] [PubMed]
- Leder, K.S.; Barlam, T.F. A Case of Paraspinal Abscess and Diskitis Due to Peptostreptococcus micros. Clin. Infect. Dis. 2000, 30, 622–623. [Google Scholar] [CrossRef] [PubMed]
- Poetter, C.; Pithois, C.; Caty, S.; Petit, V.; Combier, J.P.; Mourtialon, P.; Mattner, F. Hiding Behind Confusion: Pleural Empyema Caused by Parvimonas micra. Surg. Infect. 2014, 15, 356–357. [Google Scholar]
- Wheat, L.J. Diabetic Foot Infections: Bacteriologic Analysis. Arch. Intern. Med. 1986, 146, 1935. [Google Scholar] [CrossRef] [PubMed]
- Gomez, C.A.; Gerber, D.A.; Zambrano, E.; Banaei, N.; Deresinski, S.; Blackburn, B.G. First case of infectious endocarditis caused by Parvimonas micra. Anaerobe 2015, 36, 53–55. [Google Scholar] [CrossRef]
- Cobo, F.; Rodríguez-Granger, J.; Sampedro, A.; Aliaga-Martinez, L.; Navarro-Marí, J.M. Pleural effusion due to Parvimonas micra. A case report and a literature review of 30 cases. Rev. Esp. Quimioter. 2017, 30, 285–292. [Google Scholar]
- Cahill, T.J.; Prendergast, B.D. Infective endocarditis. Lancet 2016, 387, 882–893. [Google Scholar] [CrossRef] [PubMed]
- Hill, E.E.; Herijgers, P.; Claus, P.; Vanderschueren, S.; Herregods, M.C.; Peetermans, W.E. Infective endocarditis: Changing epidemiology and predictors of 6-month mortality: A prospective cohort study. Eur. Heart J. 2006, 28, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Hoen, B. Changing Profile of Infective Endocarditis Results of a 1-Year Survey in France. JAMA 2002, 288, 75. [Google Scholar] [CrossRef] [PubMed]
- Martín-Dávila, P.; Fortún, J.; Navas, E.; Cobo, J.; Jiménez-Mena, M.; Moya, J.L.; Moreno, S. Nosocomial Endocarditis in a Tertiary Hospital. Chest 2005, 128, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, N.; Chikwe, J.; Itagaki, S.; Gelijns, A.C.; Adams, D.H.; Egorova, N.N. Trends in Infective Endocarditis in California and New York State, 1998–2013. JAMA 2017, 317, 1652. [Google Scholar] [CrossRef] [PubMed]
- Benito, N. Health Care–Associated Native Valve Endocarditis: Importance of Non-nosocomial Acquisition. Ann. Intern. Med. 2009, 150, 586. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, K.; Jularic, M.; Horsburgh, S.M.; Hirschhausen, N.; Neumann, C.; Bertling, A.; Schulte, A.; Foster, S.; Kehrel, B.E.; Peters, G.; et al. Molecular Characterization of a Novel Staphylococcus aureus Surface Protein (SasC) Involved in Cell Aggregation and Biofilm Accumulation. PLoS ONE 2009, 4, e7567. [Google Scholar] [CrossRef]
- Patti, J.M.; Allen, B.L.; McGavin, M.J.; Hook, M. MSCRAMM-Mediated adherence of microorganisms to host tissues. Annu. Rev. Microbiol. 1994, 48, 585–617. [Google Scholar] [CrossRef]
- Nappi, F.; Avtaar Singh, S.S. Host–Bacterium Interaction Mechanisms in Staphylococcus aureus Endocarditis: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 11068. [Google Scholar] [CrossRef]
- Martinez, J.L. General principles of antibiotic resistance in bacteria. Drug Discov. Today Technol. 2014, 11, 33–39. [Google Scholar] [CrossRef]
- Segal, B.; Langham, A.; Klevansky, R.; Patel, N.; Mokoena, T.; Nassiep, M.; Ramatlo, O.; Ahmad, A.; Duse, A.G. Analysis of the Trends of Methicillin-Resistant Staphylococcus aureus in Gauteng Public Hospitals from 2009 to 2018. Microbiol. Spectr. 2023, 11, e03623-22. [Google Scholar] [CrossRef] [PubMed]
- Carinci, F.; Martinelli, M.; Contaldo, M.; Santoro, R.; Pezzetti, F.; Lauritano, D.; Candotto, V.; Mucchi, D.; Palmieri, A.; Tagliabue, A.; et al. Focus on periodontal disease and development of endocarditis. J. Biol. Regul. Homeost. Agents 2018, 32, 143–147. [Google Scholar] [PubMed]
- Corredoira, J.; Alonso, M.P.; Coira, A.; Casariego, E.; Arias, C.; Alonso, D.; Pita, J.; Rodriguez, A.; Lopez, M.J.; Varela, J. Characteristics of Streptococcus bovis endocarditis and its differences with Streptococcus viridans endocarditis. Eur. J. Clin. Microbiol. Infect. Dis. 2008, 27, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.R.; Gewitz, M.; Lockhart, P.B.; Bolger, A.F.; DeSimone, D.C.; Kazi, D.S.; Couper, D.J.; Beaton, A.; Kilmartin, C.; Miro, J.M.; et al. Prevention of Viridans Group Streptococcal Infective Endocarditis: A Scientific Statement From the American Heart Association. Circulation 2021, 143, e963–e978. [Google Scholar] [CrossRef] [PubMed]
- Barkan, D.; Fanne, R.A.; Elazari-Scheiman, A.; Maayan, S.; Beeri, R. Navel Piercing as a Cause for Streptococcus viridans Endocarditis: Case Report, Review of the Literature and Implications for Antibiotic Prophylaxis. Cardiology 2007, 108, 159–160. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ruiz, M.; López-Medrano, F.; García-Montero, M.; Hornedo-Muguiro, J.; Aguado, J.M. Intramedullary Cervical Spinal Cord Abscess by Viridans Group Streptococcus Secondary to Infective Endocarditis and Facilitated by Previous Local Radiotherapy. Intern. Med. 2009, 48, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Nomura, R.; Nakano, K.; Naka, S.; Nemoto, H.; Masuda, K.; Lapirattanakul, J.; Alaluusua, S.; Matsumoto, M.; Kawabata, S.; Ooshima, T. Identification and characterization of a collagen-binding protein, Cbm, in Streptococcus mutans: Characterization of Cbm in S. mutans. Mol. Oral Microbiol. 2012, 27, 308–323. [Google Scholar] [CrossRef]
- Nagata, E.; De Toledo, A.; Oho, T. Invasion of human aortic endothelial cells by oral viridans group streptococci and induction of inflammatory cytokine production: HAEC cytokine induction by oral streptococci. Mol. Oral Microbiol. 2011, 26, 78–88. [Google Scholar] [CrossRef]
- Stinson, M.W.; Alder, S.; Kumar, S. Invasion and Killing of Human Endothelial Cells by Viridans Group Streptococci. Infect. Immun. 2003, 71, 2365–2372. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.R.; Herrera, D.R.; Francisco, P.A.; Pereira, A.C.; Lemos, J.; Abranches, J.; Gomes, B.P. Detection of Streptococcus mutans in symptomatic and asymptomatic infected root canals. Clin. Oral Investig. 2021, 25, 3535–3542. [Google Scholar] [CrossRef]
- Nomura, R.; Naka, S.; Nemoto, H.; Otsugu, M.; Nakamura, S.; Ooshima, T.; Nakano, K. Potential high virulence for infective endocarditis in Streptococcus mutans strains with collagen-binding proteins but lacking PA expression. Arch. Oral Biol. 2013, 58, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Otsugu, M.; Nomura, R.; Matayoshi, S.; Teramoto, N.; Nakano, K. Contribution of Streptococcus mutans Strains with Collagen-Binding Proteins in the Presence of Serum to the Pathogenesis of Infective Endocarditis. Infect. Immun. 2017, 85, 10–1128. [Google Scholar] [CrossRef]
- Abranches, J.; Miller, J.H.; Martinez, A.R.; Simpson-Haidaris, P.J.; Burne, R.A.; Lemos, J.A. The Collagen-Binding Protein Cnm Is Required for Streptococcus mutans Adherence to and Intracellular Invasion of Human Coronary Artery Endothelial Cells. Infect. Immun. 2011, 79, 2277–2284. [Google Scholar] [CrossRef] [PubMed]
- Larsen, B.; Monif, G.R.G. Understanding the Bacterial Flora of the Female Genital Tract. Clin. Infect. Dis. 2001, 32, e69–e77. [Google Scholar] [CrossRef] [PubMed]
- Fisher, K.; Phillips, C. The ecology, epidemiology and virulence of Enterococcus. Microbiology 2009, 155, 1749–1757. [Google Scholar] [CrossRef] [PubMed]
- Chirouze, C.; Athan, E.; Alla, F.; Chu, V.H.; Corey, G.R.; Selton-Suty, C.; Erpelding, M.L.; Miro, J.M.; Olaison, L.; Hoen, B. Enterococcal endocarditis in the beginning of the 21st century: Analysis from the International Collaboration on Endocarditis-Prospective Cohort Study. Clin. Microbiol. Infect. 2013, 19, 1140–1147. [Google Scholar] [CrossRef] [PubMed]
- Pericas, J.M.; Corredoira, J.; Moreno, A.; García-País, M.J.; Falces, C.; Rabunal, R.; Mestres, C.A.; Alonso, M.P.; Marco, F.; Quintana, E.; et al. Relationship between Enterococcus faecalis Infective Endocarditis and Colorectal Neoplasm: Preliminary Results From a Cohort of 154 Patients. Rev. Española Cardiol. Engl. Ed. 2017, 70, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Escolà-Vergé, L.; Peghin, M.; Givone, F.; Pérez-Rodríguez, M.T.; Suárez-Varela, M.; Meije, Y.; Abelenda, G.; Almirante, B.; Fernández-Hidalgo, N. Prevalence of colorectal disease in Enterococcus faecalis infective endocarditis: Results of an observational multicenter study. Rev. Española Cardiol. (Engl. Ed.) 2020, 73, 711–717. [Google Scholar] [CrossRef]
- Danneels, P.; Hamel, J.F.; Picard, L.; Rezig, S.; Martinet, P.; Lorleac’h, A.; Talarmin, J.P.; Buzelé, R.; Guimard, T.; Le Moal, G.; et al. Impact of Enterococcus faecalis Endocarditis Treatment on Risk of Relapse. Clin. Infect. Dis. 2023, 76, 281–290. [Google Scholar] [CrossRef]
- Ioannou, P.; Tsagkaraki, E.; Vamvoukaki, R.; Kouvidou, C.; Krasagakis, K.; Chamilos, G.; Gikas, A. Infective endocarditis due to Enterococcus faecalis manifesting as pemphigus foliaceus. Hell. J. Cardiol. 2019, 60, 202–204. [Google Scholar] [CrossRef]
- Tamura, M.; Shoji, M.; Fujita, K.; Nakamura, S.; Takahashi, Y.; Suzuki, Y.; Asakura, M.; Kimizuka, S.; Sasaki, M.; Sugawara, K. Postpartum infective endocarditis with Enterococcus faecalis in Japan: A case report. J. Med. Case Rep. 2017, 11, 324. [Google Scholar] [CrossRef] [PubMed]
- Khatri, S.; Teferi, A.; Kashfi, S.; Chamay, S.; Sharma, S. Vancomycin-Resistant Enterococcus Endocarditis Complicated by Splenic Infarction and Embolic Stroke. Cureus 2023, 15, e40633. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, J.C.; Plack, W.F., 3rd. Dental treatment and management of a patient with a prosthetic heart valve. J. Am. Dent. Assoc. 1982, 104, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, P.B.; Brennan, M.T.; Sasser, H.C.; Fox, P.C.; Paster, B.J.; Bahrani-Mougeot, F.K. Bacteremia Associated With Toothbrushing and Dental Extraction. Circulation 2008, 117, 3118–3125. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.C.; Lockhart, P.B.; Firmino, R.T.; Kilmartin, C.; Cahill, T.J.; Dayer, M.; Occhi-Alexandre, I.G.; Lai, H.; Ge, L.; Thornhill, M.H. Bacteremia following different oral procedures: Systematic review and meta-analysis. Oral Dis. 2023, 30, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Bender, I.; Seltzer, S.; Yermish, M. The Incidence of Bacteremia in Endodontic Manipulation: Preliminary Report. J. Endod. 2003, 29, 697–700. [Google Scholar] [CrossRef]
- Debelian, G.J.; Olsen, I.; Tronstad, L. Bacteremia in conjunction with endodontic therapy. Dent. Traumatol. 1995, 11, 142–149. [Google Scholar] [CrossRef]
- Nair, P.N.R. Patogênese da Periodontite Apical e as Causas das Falhas Endodônticas. Crit. Rev. Oral Biol. Med. 2004, 15, 348–381. [Google Scholar] [CrossRef] [PubMed]
- Cahill, T.J.; Dayer, M.; Prendergast, B.; Thornhill, M. Do patients at risk of infective endocarditis need antibiotics before dental procedures? BMJ 2017, 7, 358. [Google Scholar] [CrossRef]
- McGowan, D.A. A dental view of controversies in the prophylaxis of infective endocarditis. J. Antimicrob. Chemother. 1987, 20, 105–109. [Google Scholar] [CrossRef]
- Zadik, Y.; Findler, M.; Livne, S.; Levin, L.; Elad, S. Dentists’ knowledge and implementation of the 2007 American Heart Association guidelines for prevention of infective endocarditis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2008, 106, e16–e19. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.V.; Longman, L.P.; Forde, M.P.; Butterworth, M.L. Infective endocarditis and dentistry: The legal basis for an association. Br. Dent. J. 2007, 203, E1; discussion 38–39. [Google Scholar] [CrossRef] [PubMed]
- Duval, X. Simplification of the prophylaxis of endocarditis: We were right! Arch. Cardiovasc. Dis. 2013, 106, 69–71. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Roberts, G.J. Dentists Are Innocent! “Everyday” Bacteremia Is the Real Culprit: A Review and Assessment of the Evidence That Dental Surgical Procedures Are a Principal Cause of Bacterial Endocarditis in Children. Pediatr. Cardiol. 1999, 20, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, P.B.; Brennan, M.T.; Thornhill, M.; Michalowicz, B.S.; Noll, J.; Bahrani-Mougeot, F.K.; Sasser, H.C. Poor oral hygiene as a risk factor for infective endocarditis–related bacteremia. J. Am. Dent. Assoc. 2009, 140, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Thoresen, T.; Jordal, S.; Lie, S.A.; Wünsche, F.; Jacobsen, M.R.; Lund, B. Infective endocarditis: Association between origin of causing bacteria and findings during oral infection screening. BMC Oral Health 2022, 22, 491. [Google Scholar] [CrossRef] [PubMed]
- Cotti, E.; Cairo, F.; Bassareo, P.P.; Fonzar, F.; Venturi, M.; Landi, L.; Parolari, A.; Franco, V.; Fabiani, C.; Barili, F.; et al. Perioperative dental screening and treatment in patients undergoing cardio-thoracic surgery and interventional cardiovascular procedures. A consensus report based on RAND/UCLA methodology. Int. J. Cardiol. 2019, 292, 78–86. [Google Scholar] [CrossRef]
- Chatterjee, T.; Roy, M.; Reddy, Y.P.S.; Ahmad, S. Finding of Anaerococcus hydrogenalis in blood using cell-free DNA technique in a patient with infective endocarditis. Germs 2023, 13, 282–287. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).