The History of Cardiopulmonary Resuscitation and Where We Are Today
Abstract
1. Overview
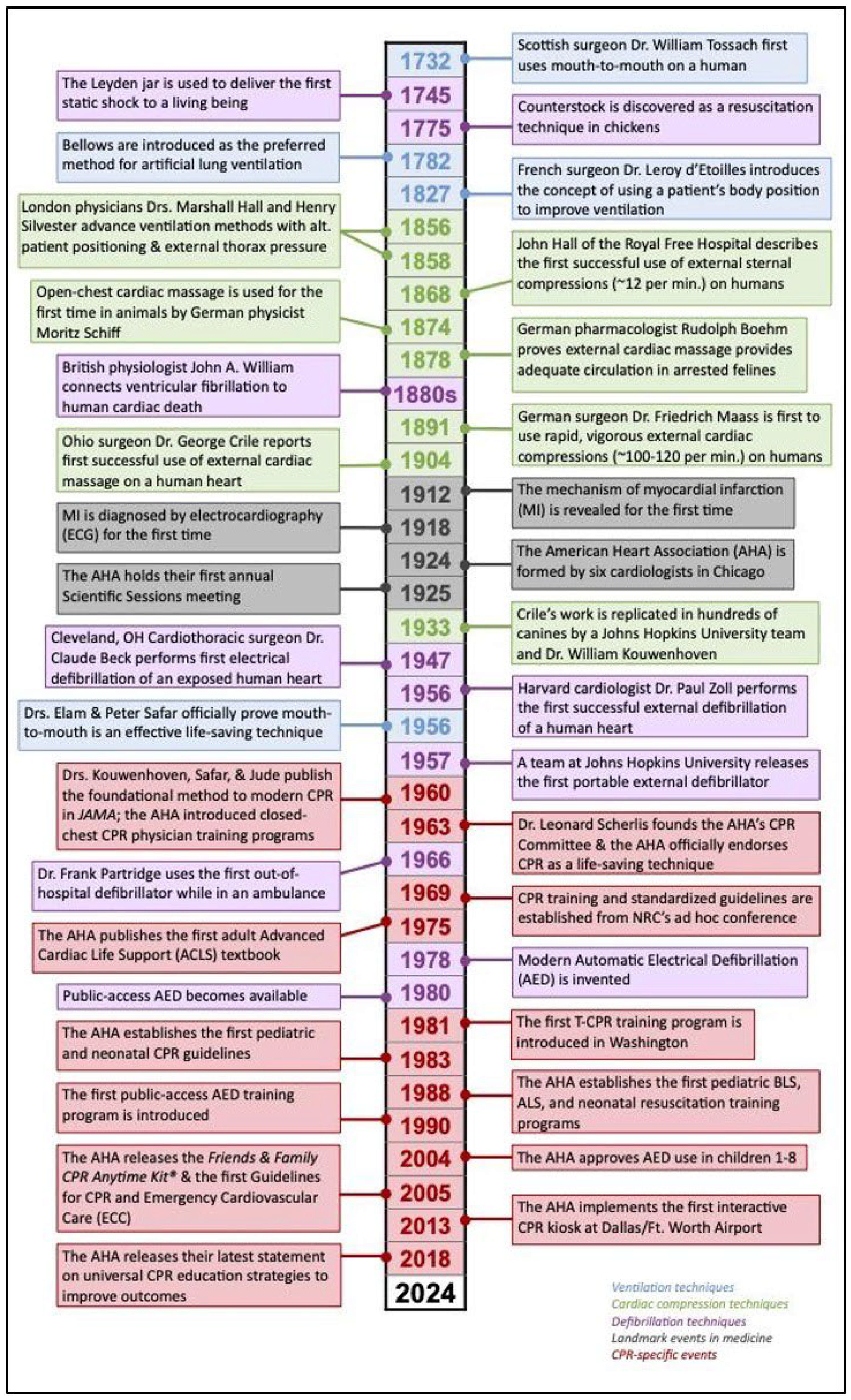
2. CPR Unfolds—From Nostril Bowing to Cardiac Massage
3. CPR Today
3.1. Telecommunicator CPR
3.2. ECMO CPR
3.3. Pediatric Resuscitation
4. CPR Research Today
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CPR | Cardiopulmonary resuscitation |
| CA | Cardiac arrest |
| AED | Automated external defibrillator |
| AHA | American Heart Association |
| CCCPR | Closed-chest CPR |
| OCPR | Open-chest CPR |
| ROSC | Return of spontaneous circulation |
| PAD | Public-access defibrillation |
| OHCA | Out-of-hospital cardiac arrest |
| ECMO | Extracorporeal membrane oxygenation |
| REBOA | Resuscitative endovascular balloon occlusion of the aorta |
References
- Barros, A.J.; Enfield, K.B. In-Hospital Cardiac Arrest. Emerg. Med. Clin. 2023, 41, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Meaney, P.A.; Bobrow, B.J.; Mancini, M.E.; Christenson, J.; De Caen, A.R.; Bhanji, F.; Abella, B.S.; Kleinman, M.E.; Edelson, D.P.; Berg, R.A.; et al. Cardiopulmonary resuscitation quality: Improving cardiac resuscitation outcomes both inside and outside the hospital: A consensus statement from the American Heart Association. Circulation 2013, 128, 417–435. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.C.; Clegg, G.R.; Bray, J.; Deakin, C.D.; Perkins, G.D.; Ringh, M.; Smith, C.M.; Link, M.S.; Merchant, R.M.; Pezo-Morales, J.; et al. Optimizing Outcomes After Out-of-Hospital Cardiac Arrest with Innovative Approaches to Public-Access Defibrillation: A Scientific Statement from the International Liaison Committee on Resuscitation. Circulation 2022, 145, E776–E801. [Google Scholar] [PubMed]
- Maekawa, K.; Tanno, K.; Hase, M.; Mori, K.; Asai, Y. Extracorporeal cardiopulmonary resuscitation for patients with out-of-hospital cardiac arrest of cardiac origin: A propensity-matched study and predictor analysis. Crit. Care Med. 2013, 41, 1186–1196. [Google Scholar] [PubMed]
- Topjian, A.A.; Berg, R.A.; Nadkarni, V.M. Pediatric cardiopulmonary resuscitation: Advances in science, techniques, and outcomes. Pediatrics 2008, 122, 1086–1098. [Google Scholar] [CrossRef]
- Rosen, Z.; Davidson, J.T. Respiratory resuscitation in ancient Hebrew sources. Anesth. Analg. 1972, 51, 502–504. [Google Scholar]
- Hurt, R. Modern cardiopulmonary resuscitation—Not so new after all. J. R. Soc. Med. 2005, 98, 327–331. [Google Scholar]
- American Heart Association. History of CPR: Highlights from the 16th Century to the 21st Century; American Heart Association: Dallas, TX, USA, 2023; Available online: https://cpr.heart.org/en/resources/history-of-cpr (accessed on 4 February 2025).
- Akselrod, H.; Kroll, M.W.; Orlov, M.V. History of defibrillation. In Cardiac Bioelectric Therapy: Mechanisms and Practical Implications; Springer: Berlin/Heidelberg, Germany, 2009; pp. 15–40. [Google Scholar]
- Taw, R.L. Dr. Friedrich Maass: 100th anniversary of “new” CPR. Clin. Cardiol. 1991, 14, 1000–1002. [Google Scholar]
- Nabel, E.G.; Braunwald, E. A tale of coronary artery disease and myocardial infarction. N. Engl. J. Med. 2012, 366, 54–63. [Google Scholar]
- Acosta, P.; Varon, J.; Sternbach, G.L.; Baskett, P. Kouwenhoven, Jude and Knickerbocker: The introduction of defibrillation and external chest compressions into modern resuscitation. Resuscitation 2005, 64, 139–143. [Google Scholar]
- American Heart Association. History of the American Heart Association: Our Lifesaving History; American Heart Association: Dallas, TX, USA, 2023. [Google Scholar]
- Kouwenhoven, W.B.; Jude, J.R.; Knickerbocker, G.G. Closed-chest cardiac massage. JAMA 1960, 173, 1064–1067. [Google Scholar] [CrossRef] [PubMed]
- Safar, P.; Raja, S.N.; Raja, S.N.; Todd, M.M. From control of airway and breathing to cardiopulmonary–cerebral resuscitation. J. Am. Soc. Anesthesiol. 2001, 95, 789–791. [Google Scholar]
- Beaudouin, D. W.B.Kouwenhoven: Reviving the Body Electric. The Johns Hopkins Whiting School of Engineering Magazine: JHUEngineering. 2002. Available online: https://engineering.jhu.edu/magazine/2002/09/w-b-kouwenhoven-reviving-body-electric/ (accessed on 4 February 2025).
- American Heart Association. What Is CPR? American Heart Association: Dallas, TX, USA, 2023. [Google Scholar]
- Endo, A.; Kojima, M.; Hong, Z.J.; Otomo, Y.; Coimbra, R. Open-chest versus closed-chest cardiopulmonary resuscitation in trauma patients with signs of life upon hospital arrival: A retrospective multicenter study. Crit. Care 2020, 24, 541. [Google Scholar] [PubMed]
- Jouffroy, R.; Vivien, B. Open-chest versus closed-chest cardiopulmonary resuscitation in trauma patients: Effect size is probably higher for penetrating injury. Crit. Care 2020, 24, 655. [Google Scholar] [CrossRef]
- Wang, M.; Lu, X.; Gong, P.; Zhong, Y.; Gong, D.; Song, Y. Open-chest cardiopulmonary resuscitation versus closed-chest cardiopulmonary resuscitation in patients with cardiac arrest: A systematic review and meta-analysis. Scand. J. Trauma Resusc. Emerg. Med. 2019, 27, 116. [Google Scholar]
- Public Access Defibrillation Trial Investigators. Public-access defibrillation and survival after out-of-hospital cardiac arrest. N. Engl. J. Med. 2004, 351, 637–646. [Google Scholar]
- Andelius, L.; Malta Hansen, C.; Lippert, F.K.; Karlsson, L.; Torp-Pedersen, C.; Kjær Ersbøll, A.; Køber, L.; Christensen, H.C.; Blomberg, S.N.; Gislason, N. Smartphone activation of citizen responders to facilitate defibrillation in out-of-hospital cardiac arrest. J. Am. Coll. Cardiol. 2020, 76, 43–53. [Google Scholar]
- Yamaguchi, Y.; Woodin, J.A.; Gibo, K.; Zive, D.M.; Daya, M.R. Improvements in out-of-hospital cardiac arrest survival from 1998 to 2013. Prehosp. Emerg. Care 2017, 21, 616–627. [Google Scholar]
- Rybasack-Smith, H.; Joseph Lauro, M.D. A history and overview of telecommunicator cardiopulmonary resuscitation (T-CPR). Rhode Isl. Med. J. 2019, 102, 20–22. [Google Scholar]
- King County, W.A. Emergency Medical Services Division 2023 Annual Report to the King County Council. Public Health-Seattle & King County-Emergency Medical Services-Reports & Publications: Seattle, WA, USA, 2023. [Google Scholar]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart disease and stroke statistics—2022 update: A report from the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar]
- Blewer, A.L.; Ibrahim, S.A.; Leary, M.; Dutwin, D.; McNally, B.; Anderson, M.L.; Morrison, L.J.; Aufderheide, T.P.; Dava, M.; Idris, A.H.; et al. Cardiopulmonary resuscitation training disparities in the United States. J. Am. Heart Assoc. 2017, 6, e006124. [Google Scholar] [PubMed]
- Yannopoulos, D.; Bartos, J.; Raveendran, G.; Walser, E.; Connett, J.; Murray, T.A.; Collins, G.; Zhang, L.; Kalra, R.; Kosmopoulos, M.; et al. Advanced reperfusion strategies for patients with out-of-hospital cardiac arrest and refractory ventricular fibrillation (ARREST): A phase 2, single centre, open-label, randomised controlled trial. Lancet 2020, 396, 1807–1816. [Google Scholar] [PubMed]
- Belohlavek, J.; Smalcova, J.; Rob, D.; Franek, O.; Smid, O.; Pokorna, M.; Horak, J.; Mrazek, V.; Kovarnik, T.; Zemanek, D.; et al. Effect of intra-arrest transport, extracorporeal cardiopulmonary resuscitation, and immediate invasive assessment and treatment on functional neurologic outcome in refractory out-of-hospital cardiac arrest: A randomized clinical trial. JAMA 2022, 327, 737–747. [Google Scholar]
- Richardson AS, C.; Schmidt, M.; Bailey, M.; Pellegrino, V.A.; Rycus, P.T.; Pilcher, D.V. ECMO Cardio-Pulmonary Resuscitation (ECPR), trends in survival from an international multicentre cohort study over 12-years. Resuscitation 2017, 112, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Suverein, M.M.; Delnoij, T.S.; Lorusso, R.; Brandon Bravo Bruinsma, G.J.; Otterspoor, L.; Elzo Kraemer, C.V.; Vlaar, A.P.J.; van der Heijden, J.J.; Scholten, E.; den Uil, C.; et al. Early extracorporeal CPR for refractory out-of-hospital cardiac arrest. N. Engl. J. Med. 2023, 388, 299–309. [Google Scholar]
- Abrams, D.; MacLaren, G.; Lorusso, R.; Price, S.; Yannopoulos, D.; Vercaemst, L.; Bělohlávek, J.; Taccone, F.B.; Aissaoui, N.; Shekar, K.; et al. Extracorporeal cardiopulmonary resuscitation in adults: Evidence and implications. Intensive Care Med. 2022, 48, 1–15. [Google Scholar]
- Pappalardo, F.; Montisci, A. What is extracorporeal cardiopulmonary resuscitation? J. Thorac. Dis. 2017, 9, 1415. [Google Scholar]
- Holmberg, M.J.; Granfeldt, A.; Guerguerian, A.M.; Sandroni, C.; Hsu, C.H.; Gardner, R.M.; Lind, P.C.; Eggertsen, M.A.; Johannsen, C.M.; Andersen, L.W. Extracorporeal cardiopulmonary resuscitation for cardiac arrest: An updated systematic review. Resuscitation 2023, 182, 109665. [Google Scholar] [CrossRef]
- Mir, T.; Shafi, O.M.; Uddin, M.; Nadiger, M.; Llah FS, T.; Qureshi, W.T. Pediatric Cardiac Arrest Outcomes in the United States: A Nationwide Database Cohort Study. Cureus 2022, 14, e26505. [Google Scholar] [CrossRef]
- American Heart Association. Part 4: Pediatric Basic and Advanced Life Support, 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. 2020. Available online: https://cpr.heart.org/en/resuscitation-science/cpr-and-ecc-guidelines/pediatric-basic-and-advanced-life-support#:~:text=For%20infants%20and%20children%2C%20it,(5%20cm)%20in%20children (accessed on 4 February 2025).
- Sutton, R.M.; Reeder, R.W.; Landis, W.P.; Meert, K.L.; Yates, A.R.; Morgan, R.W.; Berger, J.T.; Newth, C.; Carcillo, J.A.; McQuillen, P.A.; et al. Ventilation rates and pediatric in-hospital cardiac arrest survival outcomes. Crit. Care Med. 2019, 47, 1627–1636. [Google Scholar] [CrossRef]
- Nowadly, C.D.; Johnson, M.A.; Hoareau, G.L.; Manning, J.E.; Daley, J.I. The use of resuscitative endovascular balloon occlusion of the aorta (REBOA) for non-traumatic cardiac arrest: A review. J. Am. Coll. Emerg. Physicians Open 2020, 1, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.; Aiello, S.; Govender, K.; Shaw, B.; Tseng, B.; Dawad, Z.; McAulay, M.; Wilkinson, N. The impact of a ventilation timing light on CPR Quality: A randomized crossover study. Resusc. Plus 2023, 14, 100404. [Google Scholar] [PubMed]
- Lee, E.D.; Jang, Y.D.; Kang, J.H.; Seo, Y.S.; Yoon, Y.S.; Kim, Y.W.; Jeong, W.B.; Ji, J.G. Effect of a Real-Time Audio Ventilation Feedback Device on the Survival Rate and Outcomes of Patients with Out-of-Hospital Cardiac Arrest: A Prospective Randomized Controlled Study. J. Clin. Med. 2023, 12, 6023. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, O.J.; Shi, X.; Abella, B.S.; Girotra, S. Mechanical Cardiopulmonary Resuscitation During In-Hospital Cardiac Arrest. J. Am. Heart Assoc. 2023, 12, e027726. [Google Scholar]
- Poole, K.; Couper, K.; Smyth, M.A.; Yeung, J.; Perkins, G.D. Mechanical CPR: Who? When? How? Crit. Care 2018, 22, 140. [Google Scholar]
- Criley, J.M.; Blaufuss, A.H.; Kissel, G.L. Cough-induced cardiac compression: Self-administered form of cardiopulmonary resuscitation. JAMA 1976, 236, 1246–1250. [Google Scholar]
- Dalton, H.J.; Berg, R.A.; Nadkarni, V.M.; Kochanek, P.M.; Tisherman, S.A.; Thiagarajan, R.; Alexander, P.; Bartlett, R.H. Cardiopulmonary resuscitation and rescue therapies. Crit. Care Med. 2021, 49, 1375–1388. [Google Scholar]
- Dankiewicz, J.; Cronberg, T.; Lilja, G.; Jakobsen, J.C.; Levin, H.; Ullén, S.; Rylander, C.; Wise, M.P.; Cariou, A.; Bělohlávek, J.; et al. Hypothermia versus normothermia after out-of-hospital cardiac arrest. N. Engl. J. Med. 2021, 384, 2283–2294. [Google Scholar]
- Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N. Engl. J. Med. 2002, 346, 549–556. [Google Scholar] [CrossRef]
- Bernard, S.A.; Gray, T.W.; Buist, M.D.; Jones, B.M.; Silvester, W.; Gutteridge, G.; Smith, K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N. Engl. J. Med. 2002, 346, 557–563. [Google Scholar]
- Obermaier, M.; Katzenschlager, S.; Kofler, O.; Weilbacher, F.; Popp, E. Advanced and Invasive Cardiopulmonary Resuscitation (CPR) Techniques as an Adjunct to Advanced Cardiac Life Support. J. Clin. Med. 2022, 11, 7315. [Google Scholar] [CrossRef] [PubMed]
- Seigler, S.; Holman, H.; Downing, M.; Kim, J.; Rajab, T.K.; Quinn, K.M. Extremity tourniquets raise blood pressure and maintain heart rate. Am. J. Emerg. Med. 2023, 65, 12–15. [Google Scholar] [PubMed]
- Madurska, M.J.; Abdou, H.; Elansary, N.N.; Edwards, J.; Patel, N.; Stonko, D.P.; Richmond, M.J.; Scalea, T.M.; Rasmussen, T.E.; Morrison, J.J. Whole blood selective aortic arch perfusion for exsanguination cardiac arrest: Assessing myocardial tolerance to the duration of cardiac arrest. Shock Inj. Inflamm. Sepsis Lab. Clin. Approaches 2022, 57, 243–250. [Google Scholar]
- Daudre-Vignier, C.; Bates, D.G.; Scott, T.E.; Hardman, J.G.; Laviola, M. Evaluating current guidelines for cardiopulmonary resuscitation using an integrated computational model of the cardiopulmonary system. Resuscitation 2023, 186, 109758. [Google Scholar]
- Paratz, E.D.; Rowsell, L.; Zentner, D.; Parsons, S.; Morgan, N.; Thompson, T.; James, P.; Pflaumer, A.; Semsarian, C.; Smith, K. Cardiac arrest and sudden cardiac death registries: A systematic review of global coverage. Open Heart 2020, 7, e001195. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Downing, M.; Sakarcan, E.; Quinn, K. The History of Cardiopulmonary Resuscitation and Where We Are Today. Hearts 2025, 6, 8. https://doi.org/10.3390/hearts6010008
Downing M, Sakarcan E, Quinn K. The History of Cardiopulmonary Resuscitation and Where We Are Today. Hearts. 2025; 6(1):8. https://doi.org/10.3390/hearts6010008
Chicago/Turabian StyleDowning, Maren, Eren Sakarcan, and Kristen Quinn. 2025. "The History of Cardiopulmonary Resuscitation and Where We Are Today" Hearts 6, no. 1: 8. https://doi.org/10.3390/hearts6010008
APA StyleDowning, M., Sakarcan, E., & Quinn, K. (2025). The History of Cardiopulmonary Resuscitation and Where We Are Today. Hearts, 6(1), 8. https://doi.org/10.3390/hearts6010008





