Use of Right Ventricular Assist Device Post-Left Ventricular Assist Device Placement
Abstract
1. Introduction
2. Right Ventricular Assist Device Placement
2.1. History
2.2. Availability
3. Current Use
3.1. Percutaneously Implanted Devices
3.2. Surgically Implanted Devices
3.3. Alternative Strategies
4. Indications for Right Ventricular Assist Device Placement
4.1. Right Ventricular Failure
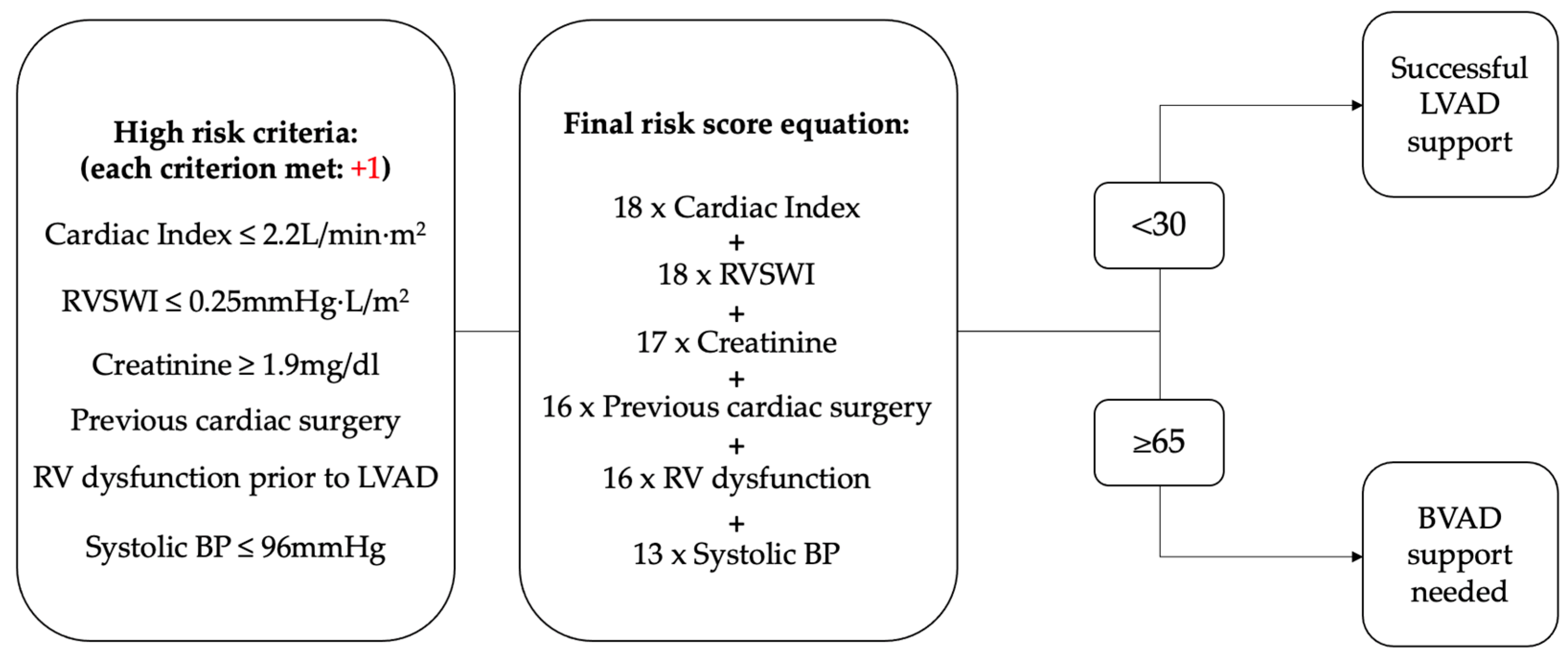
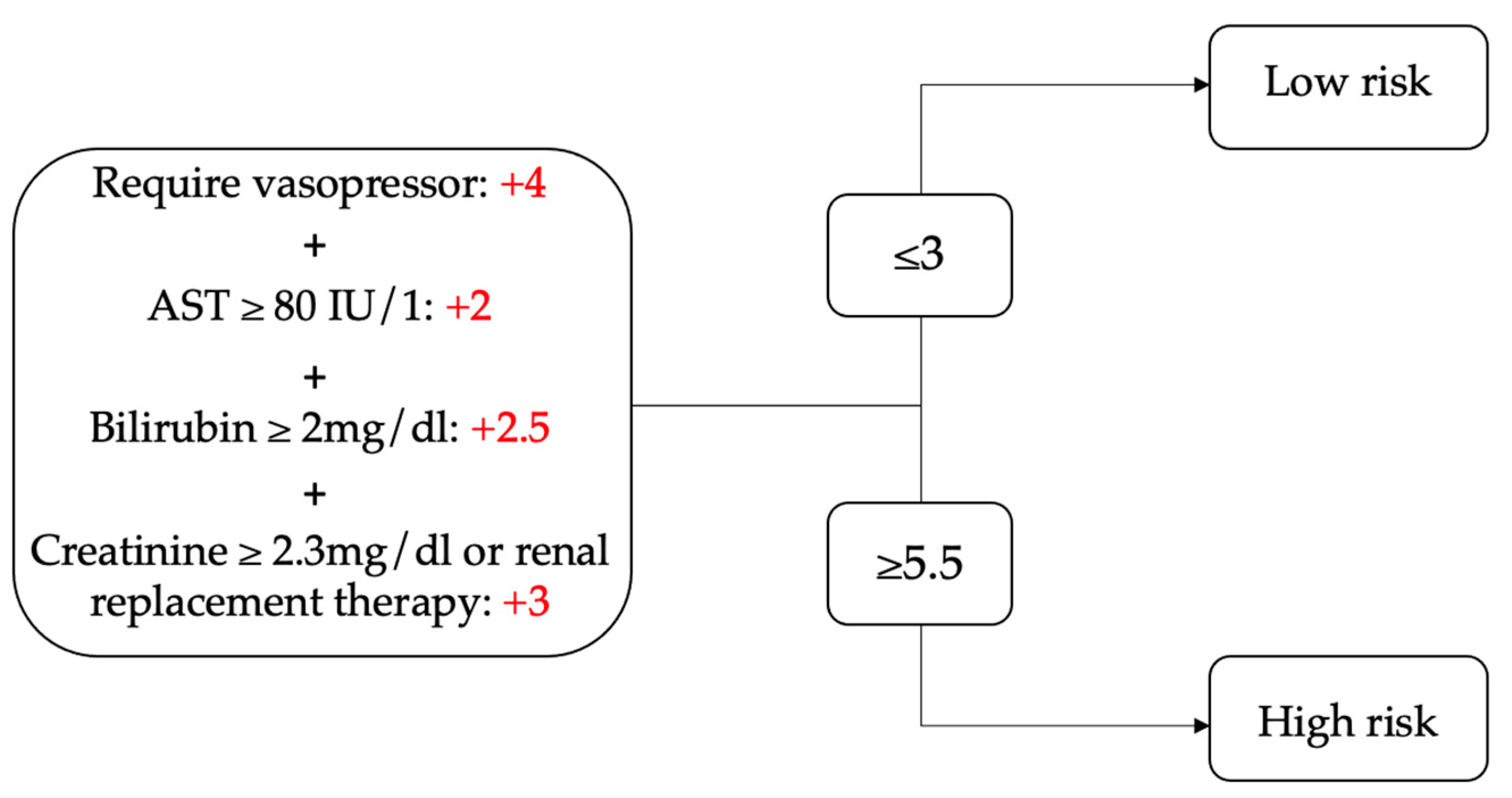
4.2. Scoring Assessments
5. Outcomes After Right Ventricular Assist Device Placement
5.1. Overview
5.2. Timing
5.3. Survival
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hamdan, R.; Charif, F.; Kadri, Z. Right ventricle failure in patients treated with left ventricular assist device. Ann. Cardiol. D’angéiologie 2020, 69, 51–54. [Google Scholar]
- Grant, A.D.; Smedira, N.G.; Starling, R.C.; Marwick, T.H. Independent and incremental role of quantitative right ventricular evaluation for the prediction of right ventricular failure after left ventricular assist device implantation. J. Am. Coll. Cardiol. 2012, 60, 521–528. [Google Scholar] [CrossRef]
- Fitzpatrick, J.R.; Frederick, J.R., 3rd; Hiesinger, W.; Hsu, V.M.; McCormick, R.C.; Kozin, E.D.; Laporte, C.M.; O’Hara, M.; Howell, E.; Doughtery, D.; et al. Early planned institution of biventricular mechanical circulatory support results in improved outcomes compared with delayed conversion of a left ventricular assist device to a biventricular assist device. J. Thorac. Cardiovasc. Surg. 2009, 137, 971–977. [Google Scholar] [PubMed]
- Cogswell, R.; John, R.; Shaffer, A. Right Ventricular Failure After Left Ventricular Assist Device. Cardiol. Clin. 2020, 38, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Pagani, F.D. Right Heart Failure After Left Ventricular Assist Device Placement: Medical and Surgical Management Considerations. Cardiol. Clin. 2020, 38, 227–238. [Google Scholar]
- Turner, K.R. Right Ventricular Failure After Left Ventricular Assist Device Placement-The Beginning of the End or Just Another Challenge? J. Cardiothorac. Vasc. Anesth. 2019, 33, 1105–1121. [Google Scholar]
- James, L.; Smith, D.E. Supporting the “forgotten” ventricle: The evolution of percutaneous RVADs. Front. Cardiovasc. Med. 2022, 9, 1008499. [Google Scholar]
- Logstrup, B.B.; Nemec, P.; Schoenrath, F.; Gummert, J.; Pya, Y.; Potapov, E.; Netuka, I.; Ramjankhan, F.; Parner, E.T.; De By, T.; et al. Heart failure etiology and risk of right heart failure in adult left ventricular assist device support: The European Registry for Patients with Mechanical Circulatory Support (EUROMACS). Scand. Cardiovasc. J. 2020, 54, 306–314. [Google Scholar]
- Salna, M.; Garan, A.R.; Kirtane, A.J.; Karmpaliotis, D.; Green, P.; Takayama, H.; Sanchez, J.; Kurlansky, P.; Yuzefpolskaya, M.; Colombo, P.C.; et al. Novel percutaneous dual-lumen cannula-based right ventricular assist device provides effective support for refractory right ventricular failure after left ventricular assist device implantation. Interact. Cardiovasc. Thorac. Surg. 2020, 30, 499–506. [Google Scholar]
- Coromilas, E.J.; Takeda, K.; Ando, M.; Cevasco, M.; Green, P.; Karmpaliotis, D.; Kirtane, A.; Topkara, V.K.; Yuzefpolskaya, M.; Takayama, H.; et al. Comparison of Percutaneous and Surgical Right Ventricular Assist Device Support After Durable Left Ventricular Assist Device Insertion. J. Card. Fail. 2019, 25, 105–113. [Google Scholar] [CrossRef]
- Schmack, B.; Farag, M.; Kremer, J.; Grossekettler, L.; Brcic, A.; Raake, P.W.; Kreusser, M.M.; Goldwasser, R.; Popov, A.F.; Mansur, A.; et al. Results of concomitant groin-free percutaneous temporary RVAD support using a centrifugal pump with a double-lumen jugular venous cannula in LVAD patients. J. Thorac. Dis. 2019, 11 (Suppl. 6), S913–S920. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, J.R.; Frederick, J.R., 3rd; Hsu, V.M.; Kozin, E.D.; O’Hara, M.L.; Howell, E.; Dougherty, D.; McCormick, R.C.; Laporte, C.A.; Cohen, J.E.; et al. Risk score derived from pre-operative data analysis predicts the need for biventricular mechanical circulatory support. J. Heart Lung Transpl. 2008, 27, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.C.; Koelling, T.M.; Pagani, F.D.; Aaronson, K.D. The right ventricular failure risk score a pre-operative tool for assessing the risk of right ventricular failure in left ventricular assist device candidates. J. Am. Coll. Cardiol. 2008, 51, 2163–2172. [Google Scholar] [CrossRef]
- Shah, H.; Murray, T.; Schultz, J.; John, R.; Martin, C.M.; Thenappan, T.; Cogswell, R. External assessment of the EUROMACS right-sided heart failure risk score. Sci. Rep. 2021, 11, 16064. [Google Scholar] [CrossRef]
- Soliman, O.I.I.; Akin, S.; Muslem, R.; Boersma, E.; Manintveld, O.C.; Krabatsch, T.; Gummert, J.F.; de By, T.M.M.H.; Bogers, A.J.J.C.; Zijlstra, F.; et al. Derivation and Validation of a Novel Right-Sided Heart Failure Model After Implantation of Continuous Flow Left Ventricular Assist Devices: The EUROMACS (European Registry for Patients with Mechanical Circulatory Support) Right-Sided Heart Failure Risk Score. Circulation 2018, 137, 891–906. [Google Scholar]
- Higgins, R.S.; Elefteriades, J.A. Right ventricular assist devices and the surgical treatment of right ventricular failure. Cardiol. Clin. 1992, 10, 185–192. [Google Scholar] [CrossRef]
- DeBakey, M.E. A Simple Continuous-Flow Blood Transfusion Instrument. New Orleans Med. Surg. J. 1934, 87, 386–389. [Google Scholar]
- Gibbon, J.H., Jr. Application of a mechanical heart and lung apparatus to cardiac surgery. Minn. Med. 1954, 37, 171–185. [Google Scholar]
- Dennis, C.; Hall, D.P.; Moreno, J.R.; Senning, A. Left atrial cannulation without thoracotomy for total left heart bypass. Acta Chir. Scand. 1962, 123, 267–279. [Google Scholar]
- Kantrowitz, A.; Tjønneland, S.; Freed, P.S.; Phillips, S.J.; Butner, A.N.; Sherman, J.L. Initial Clinical Experience With Intraaortic Balloon Pumping in Cardiogenic Shock. J. Am. Med. Assoc. 1968, 203, 113–118. [Google Scholar] [CrossRef]
- Miller, D.C.; Moreno-Cabral, R.J.; Stinson, E.B.; Shinn, J.A.; Shumway, N.E. Pulmonary artery balloon counterpulsation for acute right ventricular failure. J. Thorac. Cardiovasc. Surg. 1980, 80, 760–763. [Google Scholar] [CrossRef] [PubMed]
- Kapur, N.K.; Esposito, M.L.; Bader, Y.; Morine, K.J.; Kiernan, M.S.; Pham, D.T.; Burkhoff, D. Mechanical Circulatory Support Devices for Acute Right Ventricular Failure. Circulation 2017, 136, 314–326. [Google Scholar] [CrossRef]
- Taylor, A.J.; Edwards, F.H.; Macon, M.G.; Worley, B.; Graeber, G.M. A comparative evaluation of pulmonary artery balloon counterpulsation and a centrifugal flow pump in an experimental model of right ventricular infarction. J. Extra Corpor. Technol. 1990, 22, 85–90. [Google Scholar] [CrossRef]
- Stretch, R.; Sauer, C.M.; Yuh, D.D.; Bonde, P. National trends in the utilization of short-term mechanical circulatory support: Incidence, outcomes, and cost analysis. J. Am. Coll. Cardiol. 2014, 64, 1407–1415. [Google Scholar] [CrossRef]
- Tran, H.A.; Pollema, T.L.; Silva Enciso, J.; Greenberg, B.H.; Barnard, D.D.; Adler, E.D.; Pretorius, V.G. Durable Biventricular Support Using Right Atrial Placement of the HeartWare HVAD. ASAIO J. 2018, 64, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Potapov, E.V.; Kukucka, M.; Falk, V.; Krabatsch, T. Biventricular support using 2 HeartMate 3 pumps. J. Heart Lung Transpl. 2016, 35, 1268–1270. [Google Scholar] [CrossRef]
- Essandoh, M.M.; Kumar, N. Total artificial heart system. Int. Anesth. Clin. 2022, 60, 39–45. [Google Scholar] [CrossRef]
- Summers, D. BiVACOR Total Artificial Heart Successfully Implanted in Five Patients as Part of FDA Early Feasibility Study; FDA Greenlights Expansion of the EFS; Business Wire: San Francisco, CA, USA, 2024. [Google Scholar]
- Carmat Receives Fda Approval to Use the New Version of Its Artificial Heart in the US Early Feasibility Study (Efs). Available online: https://www.carmatsa.com/en/press-release/carmat-receives-fda-approval-use-new-version-artificial-heart-us-early-feasibility-study-efs/ (accessed on 21 March 2025).
- Sultan, I.; Kilic, A. Short-Term Circulatory and Right Ventricle Support in Cardiogenic Shock: Extracorporeal Membrane Oxygenation, Tandem Heart, CentriMag, and Impella. Heart Fail. Clin. 2018, 14, 579–583. [Google Scholar] [CrossRef]
- DeFilippis, E.M.; Topkara, V.K.; Kirtane, A.J.; Takeda, K.; Naka, Y.; Garan, A.R. Mechanical Circulatory Support for Right Ventricular Failure. Card. Fail. Rev. 2022, 8, e14. [Google Scholar] [CrossRef]
- Vanden Eynden, F.; Mets, G.; De Somer, F.; Bouchez, S.; Bove, T. Is there a place for intra-aortic balloon counterpulsation support in acute right ventricular failure by pressure-overload? Int. J. Cardiol. 2015, 197, 227–234. [Google Scholar] [CrossRef]
- Explore the Minimally Invasive Impella RP® with SmartAssist®Heart Pump for Right-Sided Heart Recovery. Available online: https://www.abiomed.com/en-us/products-and-services/impella/impella-rp-with-smartassist (accessed on 21 March 2025).
- John, K.J.; Nabzdyk, C.G.S.; Chweich, H.; Mishra, A.K.; Lal, A. ProtekDuo percutaneous ventricular support system-physiology and clinical applications. Ann. Transl. Med. 2024, 12, 14. [Google Scholar]
- CentriMag Acute Mechanical Circulatory Support Information. Available online: https://www.cardiovascular.abbott/us/en/hcp/products/heart-failure/mechanical-circulatory-support/centrimag-acute-circulatory-support-system/about.html (accessed on 21 March 2025).
- Takeda, K.; Garan, A.R.; Ando, M.; Han, J.; Topkara, V.K.; Kurlansky, P.; Yuzefpolskaya, M.; Farr, M.A.; Colombo, P.C.; Naka, Y.; et al. Minimally invasive CentriMag ventricular assist device support integrated with extracorporeal membrane oxygenation in cardiogenic shock patients: A comparison with conventional CentriMag biventricular support configuration. Eur. J. Cardiothorac. Surg. 2017, 52, 1055–1061. [Google Scholar] [PubMed]
- Kazui, T.; Tran, P.L.; Echeverria, A.; Jerman, C.F.; Iwanski, J.; Kim, S.S.; Smith, R.G.; Khalpey, Z.I. Minimally invasive approach for percutaneous CentriMag right ventricular assist device support using a single PROTEKDuo Cannula. J. Cardiothorac. Surg. 2016, 11, 123. [Google Scholar] [PubMed]
- Borisenko, O.; Wylie, G.; Payne, J.; Bjessmo, S.; Smith, J.; Yonan, N.; Firmin, R. Thoratec CentriMag for temporary treatment of refractory cardiogenic shock or severe cardiopulmonary insufficiency: A systematic literature review and meta-analysis of observational studies. ASAIO J. 2014, 60, 487–497. [Google Scholar]
- The Novalung System. Available online: https://freseniusmedicalcare.com/en-us/products/novalung-ecmo-therapy/#:~:text=Fresenius%20Medical%20Care’s%20Product%20offering,for%20more%20than%206%20hours (accessed on 28 March 2025).
- Bavaria, J.E.; Ratcliffe, M.B.; Gupta, K.B.; Wenger, R.K.; Bogen, D.K.; Edmunds, L.H. Changes in left ventricular systolic wall stress during biventricular circulatory assistance. Ann. Thorac. Surg. 1988, 45, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Hachamovitch, R.; Kittleson, M.; Patel, J.; Arabia, F.; Moriguchi, J.; Esmailian, F.; Azarbal, B. Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: A meta-analysis of 1,866 adult patients. Ann. Thorac. Surg. 2014, 97, 610–616. [Google Scholar]
- Shudo, Y.; Wang, H.; Ha, R.V.; Hayes, A.D.; Woo, Y.J. Heart transplant after profoundly extended ambulatory central venoarterial extracorporeal membrane oxygenation. J. Thorac. Cardiovasc. Surg. 2018, 156, e7–e9. [Google Scholar] [CrossRef]
- Hess, N.R.; Hickey, G.W.; Murray, H.N.; Fowler, J.A.; Kaczorowski, D.J. Ambulatory simultaneous venoarterial extracorporeal membrane oxygenation and temporary percutaneous left ventricular assist device bridge to heart transplantation. JTCVS Tech. 2022, 13, 131–134. [Google Scholar]
- Ricklefs, M.; Hanke, J.S.; Dogan, G.; Chatterjee, A.; Feldmann, C.; Deniz, E.; Korte, W.; Kirchhoff, F.; Haverich, A.; Schmitto, J.D. Successful HeartMate 3 implantation in isolated right heart failure-first in man experience of right heart configuration. J. Thorac. Dis. 2018, 10 (Suppl. 15), S1834–S1837. [Google Scholar] [CrossRef]
- Mehra, M.R.; Goldstein, D.J.; Uriel, N.; Cleveland, J.C.; Yuzefpolskaya, M.; Salerno, C.; Walsh, M.N.; Milano, C.A.; Patel, C.B.; Ewald, G.A.; et al. Two-Year Outcomes with a Magnetically Levitated Cardiac Pump in Heart Failure. N. Engl. J. Med. 2018, 378, 1386–1395. [Google Scholar]
- Balasubramanian, V.; Bhama, J.K. Technique for “open sternal” chest closure in patients with assist devices and transplant recipients. JTCVS Tech. 2020, 2, 77–79. [Google Scholar] [CrossRef]
- Yanagida, R.; Rajagopalan, N.; Davenport, D.L.; Tribble, T.A.; Bradley, M.A.; Hoopes, C.W. Delayed sternal closure does not reduce complications associated with coagulopathy and right ventricular failure after left ventricular assist device implantation. J. Artif. Organs 2018, 21, 46–51. [Google Scholar]
- Giraldo-Grueso, M.; Desai, S.V.; Webre, K.; Bansal, A.G.; Parrino, P.; Bansal, A. Does Open Chest After LVAD Surgery Affect Blood Transfusion Rates and PRA. J. Heart Lung Transplant. 2022, 41, S487. [Google Scholar] [CrossRef]
- Li, M.; Mazzeffi, M.A.; Gammie, J.S.; Banoub, M.; Pazhani, Y.; Herr, D.; Madathil, R.; Pousatis, S.; Bathula, A. Characterization of Postoperative Infection Risk in Cardiac Surgery Patients With Delayed Sternal Closure. J. Cardiothorac. Vasc. Anesth. 2020, 34, 1238–1243. [Google Scholar] [PubMed]
- Wong, J.K.; Joshi, D.J.; Melvin, A.L.; Aquina, C.T.; Archibald, W.J.; Lidder, A.K.; Probst, C.P.; Massey, H.T.; Hicks, G.L.; Knight, P.A. Early and late outcomes with prolonged open chest management after cardiac surgery. J. Thorac. Cardiovasc. Surg. 2017, 154, 915–924. [Google Scholar] [PubMed]
- Arrigo, M.; Huber, L.C.; Winnik, S.; Mikulicic, F.; Guidetti, F.; Frank, M.; Flammer, A.J.; Ruschitzka, F. Right Ventricular Failure: Pathophysiology, Diagnosis and Treatment. Card. Fail. Rev. 2019, 5, 140–146. [Google Scholar] [PubMed]
- Chatterjee, K. The Swan-Ganz catheters: Past, present, and future. A viewpoint. Circulation 2009, 119, 147–152. [Google Scholar] [CrossRef]
- Rodenas-Alesina, E.; Brahmbhatt, D.H.; Rao, V.; Salvatori, M.; Billia, F. Prediction, prevention, and management of right ventricular failure after left ventricular assist device implantation: A comprehensive review. Front. Cardiovasc. Med. 2022, 9, 1040251. [Google Scholar]
- Takeda, K.; Naka, Y.; Yang, J.A.; Uriel, N.; Colombo, P.C.; Jorde, U.P.; Takayama, H. Outcome of unplanned right ventricular assist device support for severe right heart failure after implantable left ventricular assist device insertion. J. Heart Lung Transpl. 2014, 33, 141–148. [Google Scholar]
- Ende, J.; Wilbring, M.; Ende, G.; Koch, T. The Diagnosis and Treatment of Postoperative Right Heart Failure. Dtsch. Arztebl. Int. 2022, 119, 514–524. [Google Scholar]
- Soliman, O. EUROMACS-RHF Score. MD Calc. Available online: https://www.mdcalc.com/calc/10477/euromacs-rhf-score (accessed on 21 March 2025).
- Abdelshafy, M.; Caliskan, K.; Guven, G.; Elkoumy, A.; Elsherbini, H.; Elzomor, H.; Tenekecioglu, E.; Akin, S.; Soliman, O. Temporary Right-Ventricular Assist Devices: A Systematic Review. J. Clin. Med. 2022, 11, 613. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Naka, Y.; Yang, J.A.; Uriel, N.; Colombo, P.C.; Jorde, U.P.; Takayama, H. Timing of temporary right ventricular assist device insertion for severe right heart failure after left ventricular assist device implantation. ASAIO J. 2013, 59, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Shehab, S.; Macdonald, P.S.; Keogh, A.M.; Kotlyar, E.; Jabbour, A.; Robson, D.; Newton, P.J.; Rao, S.; Wang, L.; Allida, S.; et al. Long-term biventricular HeartWare ventricular assist device support—Case series of right atrial and right ventricular implantation outcomes. J. Heart Lung Transpl. 2016, 35, 466–473. [Google Scholar] [CrossRef]
- Ravichandran, A.K.; Baran, D.A.; Stelling, K.; Cowger, J.A.; Salerno, C.T. Outcomes with the Tandem Protek Duo Dual-Lumen Percutaneous Right Ventricular Assist Device. ASAIO J. 2018, 64, 570–572. [Google Scholar] [CrossRef] [PubMed]
- McGiffin, D.; Kure, C.; McLean, J.; Marasco, S.; Bergin, P.; Hare, J.L.; Leet, A.; Patel, H.; Zimmet, A.; Rix, J.; et al. The results of a single-center experience with HeartMate 3 in a biventricular configuration. J. Heart Lung Transpl. 2021, 40, 193–200. [Google Scholar] [CrossRef]
- Anderson, M.B.; Goldstein, J.; Milano, C.; Morris, L.D.; Kormos, R.L.; Bhama, J.; Kapur, N.K.; Bansal, A.; Garcia, J.; Baker, J.N.; et al. Benefits of a novel percutaneous ventricular assist device for right heart failure: The prospective RECOVER RIGHT study of the Impella RP device. J. Heart Lung Transpl. 2015, 34, 1549–1560. [Google Scholar]
- Potapov, E.; Starck, C.; Falk, V.; Eulert-Grehn, J.-J. Mechanical circulatory support: Technical tips for the implantation of a right ventricular assist device. JTCVS Open 2021, 8, 37–40. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parness, S.; Hester, T.E.; Pandyaram, H.; Tasoudis, P.; Merlo, A.E. Use of Right Ventricular Assist Device Post-Left Ventricular Assist Device Placement. Hearts 2025, 6, 9. https://doi.org/10.3390/hearts6020009
Parness S, Hester TE, Pandyaram H, Tasoudis P, Merlo AE. Use of Right Ventricular Assist Device Post-Left Ventricular Assist Device Placement. Hearts. 2025; 6(2):9. https://doi.org/10.3390/hearts6020009
Chicago/Turabian StyleParness, Shannon, Tori E. Hester, Harish Pandyaram, Panagiotis Tasoudis, and Aurelie E. Merlo. 2025. "Use of Right Ventricular Assist Device Post-Left Ventricular Assist Device Placement" Hearts 6, no. 2: 9. https://doi.org/10.3390/hearts6020009
APA StyleParness, S., Hester, T. E., Pandyaram, H., Tasoudis, P., & Merlo, A. E. (2025). Use of Right Ventricular Assist Device Post-Left Ventricular Assist Device Placement. Hearts, 6(2), 9. https://doi.org/10.3390/hearts6020009





