Pathophysiological Basis of Endometriosis-Linked Stress Associated with Pain and Infertility: A Conceptual Review
Abstract
:1. Introduction
2. Methods
3. A Brief Overview on How Endometriosis Affects Women’s Health and Beyond
4. Different Facets of Endometriosis-Associated Stress
5. Pathophysiological Impact of Inflammatory Stress in Endometriosis
5.1. Endometriosis-Associated Pain
5.2. Endometriosis-Associated Infertility
6. Physiological Basis of Management of Endometriosis-Associated Medical Problems
7. Potential Impact of Patient Centric Multidisciplinary Healthcare in Endometriosis
8. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Simpson, J.L.; Bischoff, F. Heritability and candidate genes for endometriosis. Reprod. Biomed. Online 2003, 7, 162–169. [Google Scholar] [CrossRef]
- Guo, S.W. Epigenetics of endometriosis. Mol. Hum. Reprod. 2009, 15, 587–607. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Imanaka, S.; Nakamura, H.; Tsuji, A. Understanding the role of epigenomic, genomic and genetic alterations in the development of endometriosis. Mol. Med. Rep. 2014, 9, 1483–1505. [Google Scholar] [CrossRef] [Green Version]
- Borghese, B.; Zondervan, K.T.; Abrao, M.S.; Chapron, C.; Vaiman, D. Recent insights on the genetics and epigenetics of endometriosis. Clin. Genet. 2017, 91, 254–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sengupta, J.; Anupa, G.; Bhat, M.; Ghosh, D. Molecular biology of endometriosis. In Human Reproduction: Updates and New Horizon; Schatten, H., Ed.; John Wiley & Sons: New York, NY, USA, 2017; pp. 71–141. [Google Scholar]
- Kajiyama, H.; Suzuki, S.; Yoshihara, M.; Tamauchi, S.; Yoshikawa, N.; Niimi, K.; Shibata, K.; Kikkawa, F. Endometriosis and cancer. Free Radic. Biol. Med. 2019, 133, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Koninckx, P.R.; Ussia, A.; Adamyan, L.; Wattiez, A.; Gomel, V.; Martin, D.C. Pathogenesis of endometriosis: The genetic/epigenetic theory. Fertil. Steril. 2019, 111, 327–340. [Google Scholar] [CrossRef] [Green Version]
- Giudice, L.C.; Kao, L.C. Endometriosis. Lancet 2004, 364, 1789–1799. [Google Scholar] [CrossRef]
- Parasar, P.; Ozcan, P.; Terry, K.L. Endometriosis: Epidemiology, diagnosis and clinical management. Curr. Obstet. Gynecol. Rep. 2017, 6, 34–41. [Google Scholar] [CrossRef] [Green Version]
- Zondervan, K.T.; Becker, C.M.; Koga, K.; Missmer, S.A.; Taylor, R.N.; Viganò, P. Endometriosis. Nat. Rev. Dis. Primer. 2018, 4, 9. [Google Scholar] [CrossRef]
- Ghosh, D.; Anupa, G.; Bhat, M.A.; Bharti, J.; Mridha, A.R.; Sharma, J.B.; Roy, K.K.; Sengupta, J. How benign is endometriosis: Multi-scale interrogation of documented evidence. Cur. Opin. Gyn. Obs. 2019, 2, 318–345. [Google Scholar] [CrossRef]
- Culley, L.; Law, C.; Hudson, N.; Denny, E.; Mitchell, H.; Baumgarten, M.; Raine-Fenning, N. The social and psychological impact of endometriosis on women’s lives: A critical narrative review. Hum. Reprod. Update 2013, 19, 625–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pope, C.J.; Sharma, V.; Sharma, S.; Mazmanian, D. A systematic review of the association between psychiatric disturbances and endometriosis. J. Obstet. Gynaecol. Can. 2015, 37, 1006–1015. [Google Scholar] [CrossRef] [Green Version]
- Macer, M.L.; Taylor, H.S. Endometriosis and infertility: A review of the pathogenesis and treatment of endometriosis-associated infertility. Obstet. Gynecol. Clin. N. Am. 2012, 39, 535–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerlinger, C.; Faustmann, T.; Hassall, J.J.; Seitz, C. Treatment of endometriosis in different ethnic populations: A meta-analysis of two clinical trials. BMC Women Health 2012, 12, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, A.; Johnstone, E.B.; Bloom, M.S.; Huddleston, H.G.; Fujimoto, V.Y. A higher prevalence of endometriosis among Asian women does not contribute to poorer IVF outcomes. J. Assist. Reprod. Genet. 2017, 34, 765–774. [Google Scholar] [CrossRef]
- Luisi, S.; Pizzo, A.; Pinzauti, S.; Zupi, E.; Centini, G.; Lazzeri, L.; Di Carlo, C.; Petraglia, F. Neuroendocrine and stress-related aspects of endometriosis. Neuro. Endocrinol. Lett. 2015, 36, 15–23. [Google Scholar] [PubMed]
- Kvaskoff, M.; Mu, F.; Terry, K.L.; Harris, H.R.; Poole, E.M.; Farland, L.; Missmer, S.A. Endometriosis: A high-risk population for major chronic diseases? Hum. Reprod. Update 2015, 21, 500–516. [Google Scholar] [CrossRef] [Green Version]
- Mu, F.; Rich-Edwards, J.; Rimm, E.B.; Spiegelman, D.; Forman, J.P.; Missmer, S.A. Association between endometriosis and hypercholesterolemia or hypertension. Hypertension 2017, 70, 59–65. [Google Scholar] [CrossRef]
- Surrey, E.S.; Soliman, A.M.; Johnson, S.J.; Davis, M.; Castelli-Haley, J.; Snabes, M.C. Risk of developing comorbidities among women with endometriosis: A retrospective matched cohort study. J. Women Health 2018, 27, 1114–1123. [Google Scholar] [CrossRef]
- Shigesi, N.; Kvaskoff, M.; Kirtley, S.; Feng, Q.; Fang, H.; Knight, J.C.; Missmer, S.A.; Rahmioglu, N.; Zondervan, K.T.; Becker, C.M. The association between endometriosis and autoimmune diseases: A systematic review and meta-analysis. Hum. Reprod. Update 2019, 25, 486–503. [Google Scholar] [CrossRef]
- Wood, R.; Guidone, H.; Hummelshoj, L. Myths and Misconceptions in Endometriosis. Available online: http://www.endometriosis.org/resources/articles/myths/ (accessed on 3 June 2020).
- Ballard, K.; Lowton, K.; Wright, J. What’s the delay? A qualitative study of women’s experiences of reaching a diagnosis of endometriosis. Fertil. Steril. 2006, 86, 1296–1301. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, A.Y.; Soriano, D.; Seidman, D.S.; Goldenberg, M.; Eisenberg, V.H. Think endometriosis: Delay in diagnosis or delay in referral to adequate treatment? JFIV Reprod. Med. Genet. 2014, 2, 127–134. [Google Scholar] [CrossRef]
- Van Der Zanden, M.; Arens, M.; Braat, D.; Nelen, W.; Nap, A. Gynaecologists’ view on diagnostic delay and care performance in endometriosis in the Netherlands. Reprod. Biomed. Online 2018, 37, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.; Katikireddi, S.V.; Sowden, A.; McKenzie, J.E.; Thomson, H. Improving Conduct and Reporting of Narrative Synthesis of Quantitative Data (ICONS-Quant): Protocol for a mixed methods study to develop a reporting guideline. BMJ Open 2018, 8, e020064. [Google Scholar] [CrossRef]
- Dixon-Woods, M.; Agarwal, S.; Jones, D.; Young, B.; Sutton, A. Synthesising qualitative and quantitative evidence: A review of possible methods. J. Health Serv. Res. Policy 2005, 10, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, M.; Sowden, A.; Petticrew, M.; Arai, L.; Roberts, H.M.; Britten, N.; Popay, J. Testing methodological guidance on the conduct of narrative synthesis in systematic reviews. Evaluation 2009, 15, 49–73. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.G.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Pannucci, C.J.; Wilkins, E.G. Identifying and avoiding bias in research. Plast. Reconstr. Surg. 2010, 126, 619–625. [Google Scholar] [CrossRef]
- Eskenazi, B.; Warner, M.L. Epidemiology of endometriosis. Obstet. Gynecol. Clin. N. Am. 1997, 24, 235–258. [Google Scholar] [CrossRef]
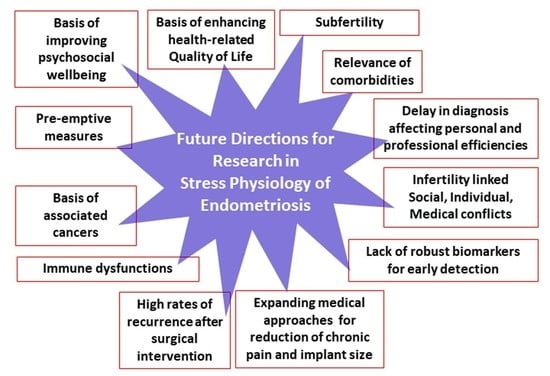
- As-Sanie, S.; Black, R.; Giudice, L.C.; Valbrun, G.T.; Gupta, J.; Jones, B.; Laufer, M.R.; Milspaw, A.T.; Missmer, S.A.; Norman, A.; et al. Assessing research gaps and unmet needs in endometriosis. Am. J. Obstet. Gynecol. 2019, 221, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Gylfason, J.T.; Kristjansson, K.A.; Sverrisdottir, G.; Jonsdottir, K.; Rafnsson, V.; Geirsson, R.T. Pelvic endometriosis diagnosed in an entire nation over 20 years. Am. J. Epidemiol. 2010, 172, 237–243. [Google Scholar] [CrossRef]
- Seear, K. The etiquette of endometriosis: Stigmatisation, menstrual concealment and the diagnostic delay. Soc. Sci. Med. 2009, 69, 1220–1227. [Google Scholar] [CrossRef]
- Agarwal, S.K.; Chapron, C.; Giudice, L.C.; Laufer, M.R.; Leyland, N.; Missmer, S.A.; Singh, S.S.; Taylor, H.S. Clinical diagnosis of endometriosis: A call to action. Am. J. Obstet. Gynecol. 2019, 220, E1–E354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grundström, H.; Gerdle, B.; Alehagen, S.; Berterö, C.; Arendt-Nielsen, L.; Kjølhede, P. Reduced pain thresholds and signs of sensitization in women with persistent pelvic pain and suspected endometriosis. Acta Obstet. Gynecol. Scand. 2018, 98, 327–336. [Google Scholar] [CrossRef] [Green Version]
- Young, K.; Fisher, J.; Kirkman, M. Women’s experiences of endometriosis: A systematic review and synthesis of qualitative research. J. Fam. Plan. Reprod. Health Care 2015, 41, 225–234. [Google Scholar] [CrossRef]
- Arruda, M.; Petta, C.A.; Abrão, M.; Benetti-Pinto, C. Time elapsed from onset of symptoms to diagnosis of endometriosis in a cohort study of Brazilian women. Hum. Reprod. 2003, 18, 756–759. [Google Scholar] [CrossRef] [PubMed]
- Dmowski, W.P.; Lesniewicz, R.; Rana, N.; Pepping, P.; Noursalehi, M. Changing trends in the diagnosis of endometriosis: A comparative study of women with pelvic endometriosis presenting with chronic pelvic pain or infertility. Fertil. Steril. 1997, 67, 238–243. [Google Scholar] [CrossRef]
- Young, K.; Fisher, J.; Kirkman, M. Clinicians’ perceptions of women’s experiences of endometriosis and of psychosocial care for endometriosis. Aust. N. Z. J. Obstet. Gynaecol. 2017, 57, 87–92. [Google Scholar] [CrossRef] [Green Version]
- Aerts, L.; Grangier, L.; Streuli, I.; Dällenbach, P.; Marci, R.; Wenger, J.M.; Pluchino, N. Psychosocial impact of endometriosis: From co-morbidity to intervention. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 50, 2–10. [Google Scholar] [CrossRef]
- Becker, C.M.; Gattrell, W.T.; Gude, K.; Singh, S.S. Reevaluating response and failure of medical treatment of endometriosis: A systematic review. Fertil. Steril. 2017, 108, 125–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epstein, A.J.; Soliman, A.M.; Davis, M.; Johnson, S.J.; Snabes, M.C.; Surrey, E.S. Changes in healthcare spending after diagnosis of comorbidities among endometriosis patients: A difference-in-differences analysis. Adv. Ther. 2017, 34, 2491–2502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilbur, M.A.; Shih, I.M.; Segars, J.H.; Fader, A.N. Cancer implications for patients with endometriosis. Semin. Reprod. Med. 2017, 35, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Bulun, S.E.; Wan, Y.; Matei, D. Epithelial mutations in endometriosis: Link to ovarian cancer. Endocrinology 2019, 160, 626–638. [Google Scholar] [CrossRef] [Green Version]
- Selye, H. A syndrome produced by diverse nocuous agents. Nature 1936, 138, 32. [Google Scholar] [CrossRef]
- Lazarus, R.S.; Folkman, S. Stress, Appraisal and Coping; Springer: New York, NY, USA, 1984. [Google Scholar]
- Goldstein, D.S.; McEwen, B. Allostasis, homeostats, and the nature of stress. Stress 2002, 5, 55–58. [Google Scholar] [CrossRef]
- Epel, E.S.; Crosswell, A.D.; Mayer, S.E.; Prather, A.A.; Slavich, G.M.; Puterman, E.; Mendes, W.B. More than a feeling: A unified view of stress measurement for population science. Front. Neuroendocrinol. 2018, 49, 146–169. [Google Scholar] [CrossRef]
- Peters, A.; McEwen, B.S.; Friston, K. Uncertainty and stress: Why it causes diseases and how it is mastered by the brain. Prog. Neurobiol. 2017, 156, 164–188. [Google Scholar] [CrossRef]
- Rush, G.; Misajon, R. Examining subjective wellbeing and health-related quality of life in women with endometriosis. Health Care Women Int. 2018, 39, 303–321. [Google Scholar] [CrossRef]
- Donatti, L.; Ramos, D.G.; Andres, M.P.; Passman, L.J.; Podgaec, S. Patients with endometriosis using positive coping strategies have less depression, stress and pelvic pain. Einstein 2017, 15, 65–70. [Google Scholar] [CrossRef] [Green Version]
- Barnack, J.L.; Chrisler, J.C. The experience of chronic illness in women: A comparison between women with endometriosis and women with chronic migraine headaches. Women Health 2007, 46, 115–133. [Google Scholar] [CrossRef]
- Facchin, F.; Barbara, G.; Saita, E.; Mosconi, P.; Roberto, A.; Fedele, L.; Vercellini, P. Impact of endometriosis on quality of life and mental health: Pelvic pain makes the difference. J. Psychosom. Obstet. Gynaecol. 2015, 36, 135–141. [Google Scholar] [CrossRef]
- Low, W.Y.; Edelmann, R.J.; Sutton, C. A psychological profile of endometriosis patients in comparison to patients with pelvic pain of other origins. J. Psychosom. Res. 1993, 37, 111–116. [Google Scholar] [CrossRef]
- Harrison, V.; Rowan, K.; Mathias, J. Stress reactivity and family relationships in the development and treatment of endometriosis. Fertil. Steril. 2005, 83, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.P.; Moura, M.D.; Rosa e Silva, A.A. Prolactin and cortisol levels in women with endometriosis. Braz. J. Med. Biol. Res. 2006, 39, 1121–1127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casu, G.; Gremigni, P. Screening for infertility-related stress at the time of initial infertility consultation: Psychometric properties of a brief measure. J. Adv. Nurs. 2016, 72, 693–706. [Google Scholar] [CrossRef]
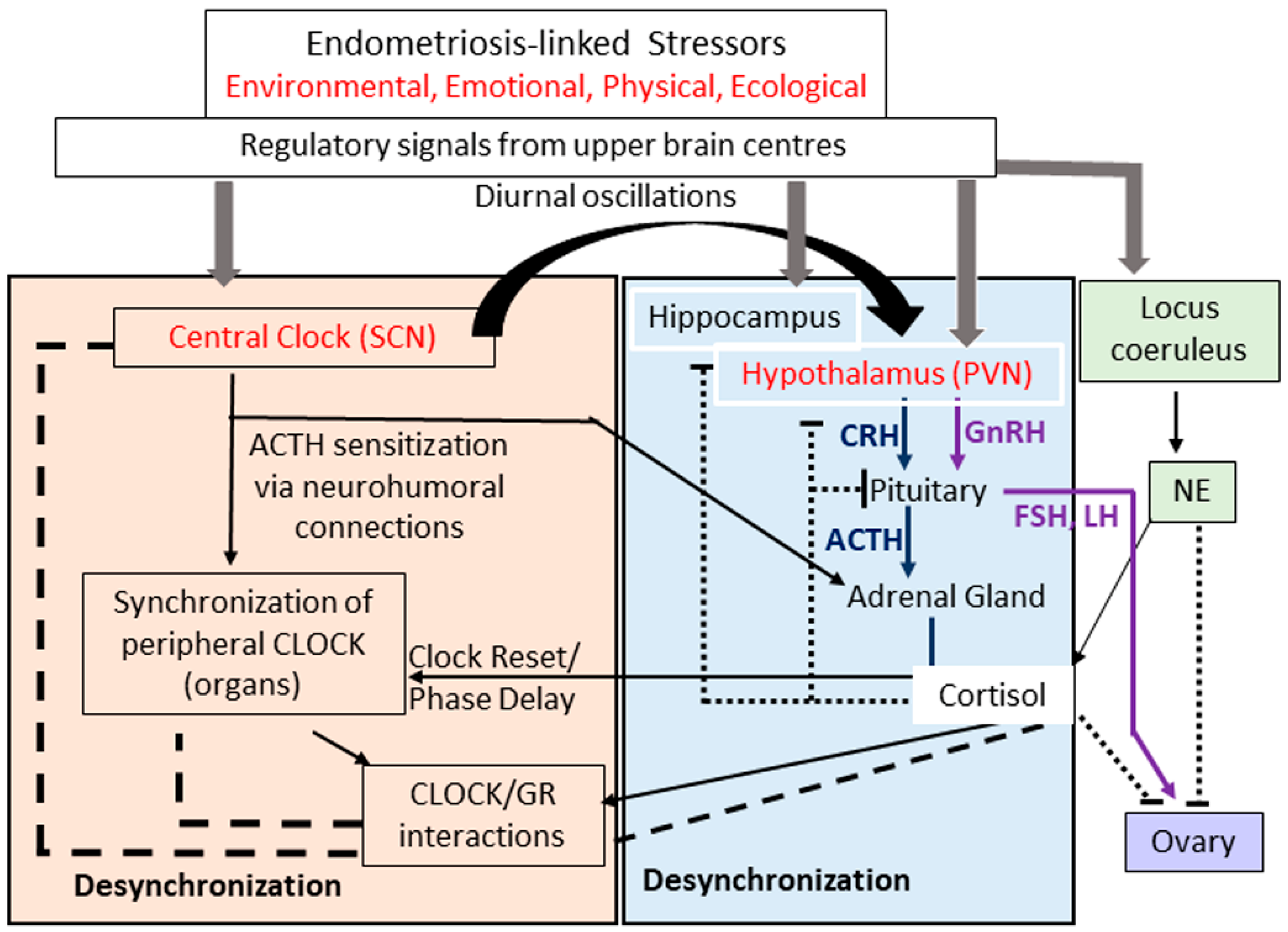
- Herman, J.P.; Figueiredo, H.; Mueller, N.K.; Ulrich-Lai, Y.; Ostrander, M.M.; Choi, D.C.; Cullinan, W.E. Central mechanisms of stress integration: Hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front. Neuroendocrinol. 2003, 24, 151–180. [Google Scholar] [CrossRef] [PubMed]
- Revest, S. Interactions between the immune and neuroendocrine systems. Prog. Brain Res. 2010, 181, 43–53. [Google Scholar] [CrossRef]
- Bellavance, M.A.; Rivest, S. The HPA–Immune axis and the immunomodulatory actions of glucocorticoids in the brain. Front. Immunol. 2014, 5, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yarushkina, N.I.; Filaretova, L.P. The peripheral corticotropin-releasing factor (CRF)-induced analgesic effect on somatic pain sensitivity in conscious rats: Involving CRF, opioid and glucocorticoid receptors. Inflammopharmacology 2018, 26, 305–318. [Google Scholar] [CrossRef]
- Wilsterman, K.; Gotlieb, N.; Kriegsfeld, L.J.; Bentley, G.E. Pregnancy stage determines the effect of chronic stress on ovarian progesterone synthesis. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E987–E994. [Google Scholar] [CrossRef]
- Kassi, E.N.; Chrousos, G.P. The central CLOCK system and the stress axis in health and disease. Hormones 2013, 12, 172–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barut, M.U.; Agacayak, E.; Bozkurt, M.; Aksu, T.; Gul, T. There is a positive correlation between socioeconomic status and ovarian reserve in women of reproductive age. Med. Sci. Monit. 2016, 22, 4386–4392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joseph, D.N.; Whirledge, S. Stress and the HPA Axis: Balancing homeostasis and fertility. Int. J. Mol. Sci. 2017, 18, 2224. [Google Scholar] [CrossRef]
- Nicolaides, N.C.; Charmandari, E.; Kino, T.; Chrousos, G.P. Stress-related and circadian secretion and target tissue actions of glucocorticoids: Impact on health. Front. Endocrinol. 2017, 8, 70. [Google Scholar] [CrossRef] [PubMed]
- Astiz, M.; Oster, H. Perinatal programming of circadian clock-stress crosstalk. Neural Plast. 2018, 2018, 5689165. [Google Scholar] [CrossRef] [Green Version]
- Moenter, S.M. GnRH neurons on LSD: A year of rejecting hypotheses that may have made Karl Popper proud. Endocrinology 2018, 159, 199–205. [Google Scholar] [CrossRef] [Green Version]
- Hu, K.L.; Chang, H.M.; Li, R.; Yu, Y.; Qiao, J. Regulation of LH secretion by RFRP-3—From the hypothalamus to the pituitary. Front. Neuroendocrinol. 2019, 52, 12–21. [Google Scholar] [CrossRef]
- Neumann, A.M.; Schmidt, C.X.; Brockmann, R.M.; Oster, H. Circadian regulation of endocrine systems. Auton. Neurosci. 2019, 216, 1–8. [Google Scholar] [CrossRef]
- Jacobson, L. Hypothalamic-pituitary-adrenocortical axis regulation. Endocrinol. Metab. Clin. N. Am. 2005, 34, 271–292. [Google Scholar] [CrossRef]
- Filaretova, L.P.; Podvigina, T.T.; Bobryshev, P.Y.; Bagaeva, T.R.; Tanaka, A.; Takeuchi, K. Hypothalamic-pituitary-adrenocortical axis: The hidden gold in gastric mucosal homeostasis. Inflammopharmacology 2006, 14, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr. Physiol. 2016, 6, 603–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godoy, L.D.; Rossignoli, M.T.; Delfino-Pereira, P.; Garcia-Cairasco, N.; de Lima Umeoka, E.H. A comprehensive overview on stress neurobiology: Basic concepts and clinical implications. Front. Behav. Neurosci. 2018, 12, 127. [Google Scholar] [CrossRef] [Green Version]
- Tian, R.; Hou, G.; Li, D.; Yuan, T.F. A possible change process of inflammatory cytokines in the prolonged chronic stress and its ultimate implications for health. Sci. World J. 2014, 2014, 780616. [Google Scholar] [CrossRef] [PubMed]
- Tariverdian, N.; Theoharides, T.C.; Siedentopf, F.; Gutiérrez, G.; Jeschke, U.; Rabinovich, G.A.; Blois, S.M.; Arck, P.C. Neuroendocrine-immune disequilibrium and endometriosis: An interdisciplinary approach. Semin. Immunopathol. 2007, 29, 193–210. [Google Scholar] [CrossRef] [Green Version]
- Tariverdian, N.; Siedentopf, F.; Rücke, M.; Blois, S.M.; Klapp, B.F.; Kentenich, H.; Arck, P.C. Intraperitoneal immune cell status in infertile women with and without endometriosis. J. Reprod. Immunol. 2009, 80, 80–90. [Google Scholar] [CrossRef]
- Jeung, I.; Cheon, K.; Kim, M.R. Decreased cytotoxicity of peripheral and peritoneal natural killer cell in endometriosis. Biomed. Res. Int. 2016, 2016, 2916070. [Google Scholar] [CrossRef] [Green Version]
- Basta, P.; Majka, M.; Jozwicki, W.; Lukaszewska, E.; Knafel, A.; Grabiec, M.; Stasienko, E.; Wicherek, L. The frequency of CD25+CD4+ and FOXP3+ regulatory T cells in ectopic endometrium and ectopic decidua. Reprod. Biol. Endocrinol. 2010, 8, 116. [Google Scholar] [CrossRef] [Green Version]
- Berbic, M.; Schulke, L.; Markham, R.; Tokushige, N.; Russell, P.; Fraser, I.S. Macrophage expression in endometrium of women with and without endometriosis. Hum. Reprod. 2009, 24, 325–332. [Google Scholar] [CrossRef] [Green Version]
- Riccio, L.D.G.C.; Santulli, P.; Marcellin, L.; Abrão, M.S.; Batteux, F.; Chapron, C. Immunology of endometriosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 50, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.L.; Zhou, W.J.; Chang, K.K.; Mei, J.; Huang, L.Q.; Wang, M.Y.; Meng, Y.; Ha, S.Y.; Li, D.J.; Li, M.Q. The crosstalk between endometrial stromal cells and macrophages impairs cytotoxicity of NK cells in endometriosis by secreting IL-10 and TGF-β. Reproduction 2017, 154, 815–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malvezzi, H.; Hernandes, C.; Piccinato, C.; Podgaec, S. Interleukin in endometriosis-associated infertility-pelvic pain: Systematic review and meta-analysis. Reproduction 2019, 158, 1–12. [Google Scholar] [CrossRef]
- Anupa, G.; Pooraswamy, J.; Bhat, M.A.; Sharma, J.B.; Sengupta, J.; Ghosh, D. Endometrial stromal cell inflammatory phenotype during severe ovarian endometriosis as a cause of endometriosis-associated infertility. Reprod. Biol. Online 2020. [Google Scholar] [CrossRef]
- Neziri, A.Y.; Bersinger, N.A.; Andersen, O.K.; Arendt-Nielsen, L.; Mueller, M.D.; Curatolo, M. Correlation between altered central pain processing and concentration of peritoneal fluid inflammatory cytokines in endometriosis patients with chronic pelvic pain. Reg. Anesth. Pain Med. 2014, 39, 181–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Podgaec, S.; Abrao, M.S.; Dias, J.A., Jr.; Rizzo, L.V.; de Oliveira, R.M.; Baracat, E.C. Endometriosis: An inflammatory disease with a Th2 immune response component. Hum. Reprod. 2007, 22, 1373–1379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beste, M.T.; Pfäffle-Doyle, N.; Prentice, E.A.; Morris, S.N.; Lauffenburger, D.A.; Isaacson, K.B.; Griffith, L.G. Molecular network analysis of endometriosis reveals a role for c-Jun-regulated macrophage activation. Sci. Transl. Med. 2014, 6, 222ra16. [Google Scholar] [CrossRef] [Green Version]
- Ahn, S.H.; Monsanto, S.P.; Miller, C.; Singh, S.S.; Thomas, R.; Tayade, C. Pathophysiology and immune dysfunction in endometriosis. Biomed. Res. Int. 2015, 2015, 795976. [Google Scholar] [CrossRef] [Green Version]
- Esmaeilzadeh, S.; Mirabi, P.; Basirat, Z.; Zeinalzadeh, M.; Khafri, S. Association between endometriosis and hyperprolactinemia in infertile women. Iran. J. Reprod. Med. 2015, 13, 155–160. [Google Scholar]
- Greaves, E.; Temp, J.; Esnal-Zufiurre, A.; Mechsner, S.; Horne, A.W.; Saunders, P.T. Estradiol is a critical mediator of macrophage-nerve cross talk in peritoneal endometriosis. Am. J. Pathol. 2015, 185, 2286–2297. [Google Scholar] [CrossRef]
- Bungum, H.F.; Nygaard, U.; Vestergaard, C.; Martensen, P.M.; Knudsen, U.B. Increased IL-25 levels in the peritoneal fluid of patients with endometriosis. J. Reprod. Immunol. 2016, 114, 6–9. [Google Scholar] [CrossRef]
- Chen, X.; Gianferante, D.; Hanlin, L.; Fiksdal, A.; Breines, J.G.; Thoma, M.V.; Rohleder, N. HPA-axis and inflammatory reactivity to acute stress is related with basal HPA-axis activity. Psychoneuroendocrinology 2017, 78, 168–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Barros, I.B.L.; Malvezzi, H.; Gueuvoghlanian-Silva, B.Y.; Piccinato, C.A.; Rizzo, L.V.; Podgaec, S. What do we know about regulatory T cells and endometriosis? A systematic review. J. Reprod. Immunol. 2017, 120, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, H.; Hill, A.S.; Beste, M.T.; Kumar, M.P.; Chiswick, E.; Fedorcsak, P.; Isaacson, K.B.; Lauffenburger, D.A.; Griffith, L.G.; Qvigstad, E. Peritoneal fluid cytokines related to endometriosis in patients evaluated for infertility. Fertil. Steril. 2017, 107, 1191–1199.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izumi, G.; Koga, K.; Takamura, M.; Makabe, T.; Nagai, M.; Urata, Y.; Harada, M.; Hirata, T.; Hirota, Y.; Fujii, T.; et al. Mannose receptor is highly expressed by peritoneal dendritic cells in endometriosis. Fertil. Steril. 2017, 107, 167–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izumi, G.; Koga, K.; Takamura, M.; Makabe, T.; Satake, E.; Takeuchi, A.; Taguchi, A.; Urata, Y.; Fujii, T.; Osuga, Y. Involvement of immune cells in the pathogenesis of endometriosis. J. Obstet. Gynaecol. Res. 2018, 44, 191–198. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.M.; Ma, Z.Y.; Song, N. Inflammatory cytokines IL-6, IL-10, IL-13, TNF-α and peritoneal fluid flora were associated with infertility in patients with endometriosis. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 2513–2518. [Google Scholar] [CrossRef]
- Khan, K.N.; Yamamoto, K.; Fujishit, A.; Koshiba, A.; Kuroboshi, H.; Sakabayashi, S.; Teramukai, S.; Nakashima, M.; Kitawaki, J. Association between FOXP3+ regulatory T-cells and occurrence of peritoneal lesions in women with ovarian endometrioma and dermoid cysts. Reprod. Biomed. Online 2019, 38, 857–869. [Google Scholar] [CrossRef]
- Liang, Y.; Wu, J.; Wang, W.; Xie, H.; Yao, S. Pro-endometriotic niche in endometriosis. Reprod. Biomed. Online 2019, 38, 549–559. [Google Scholar] [CrossRef] [Green Version]
- Ozawa, Y.; Murakami, T.; Terada, Y.; Yaegashi, N.; Okamura, K.; Kuriyama, S.; Tsuji, I. Management of the pain associated with endometriosis: An update of the painful problems. Tohoku J. Exp. Med. 2006, 210, 175–188. [Google Scholar] [CrossRef] [Green Version]
- Soliman, A.M.; Coyne, K.S.; Zaiser, E.; Castelli-Haley, J.; Fuldeore, M. The burden of endometriosis symptoms on health-related quality of life in women in the United States: A cross-sectional study. J. Psychosom. Obstet. Gynecol. 2017, 38, 238–248. [Google Scholar] [CrossRef]
- Vitale, S.G.; La Rosa, V.L.; Rapisarda, A.M.C.; Laganà, A.S. Impact of endometriosis on quality of life and psychological well-being. J. Psychosom. Obstet. Gynecol. 2016, 38, 317–319. [Google Scholar] [CrossRef]
- Kobayashi, H.; Yamada, Y.; Morioka, S.; Niiro, E.; Shigemitsu, A.; Ito, F. Mechanism of pain generation for endometriosis-associated pelvic pain. Arch. Gynecol. Obstet. 2013, 289, 13–21. [Google Scholar] [CrossRef]
- Zheng, P.; Zhang, W.; Leng, J.; Lang, J. Research on central sensitization of endometriosis-associated pain: A systematic review of the literature. J. Pain Res. 2019, 12, 1447–1456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morotti, M.; Vincent, K.; Brawn, J.; Zondervan, K.T.; Becker, C.M. Peripheral changes in endometriosis-associated pain. Hum. Reprod. Updat. 2014, 20, 717–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wesselman, U. Neurogenic inflammation and chronic pelvic pain. World J. Urol. 2001, 19, 180–185. [Google Scholar] [CrossRef]
- Butrick, C.W. Patients with chronic pelvic pain: Endometriosis or interstitial cystitis/painful bladder syndrome? JSLS 2007, 11, 182–189. [Google Scholar] [PubMed]
- Harte, S.E.; Harris, R.E.; Clauw, D. The neurobiology of central sensitization. J. Appl. Biobehav. Res. 2018, 23, e12137. [Google Scholar] [CrossRef] [Green Version]
- Latremoliere, A.; Woolf, C.J. Central sensitization: A generator of pain hypersensitivity by central neural plasticity. J. Pain 2009, 10, 895–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woolf, C.J. Evidence for a central component of post-injury pain hypersensitivity. Nature 1983, 306, 686–688. [Google Scholar] [CrossRef]
- Woolf, C.J. Central sensitization: Implications for the diagnosis and treatment of pain. Pain 2010, 152, S2–S15. [Google Scholar] [CrossRef]
- Arendt-Nielsen, L.; Morlion, B.; Perrot, S.; Dahan, A.; Dickenson, A.; Kress, H.; Wells, C.; Bouhassira, D.; Drewes, A.M. Assessment and manifestation of central sensitisation across different chronic pain conditions. Eur. J. Pain 2017, 22, 216–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aredo, J.V.; Heyrana, K.J.; Karp, B.I.; Shah, J.P.; Stratton, P. Relating Chronic Pelvic Pain and Endometriosis to Signs of Sensitization and Myofascial Pain and Dysfunction. Semin. Reprod. Med. 2017, 35, 88–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- As-Sanie, S.; Harris, R.E.; Harte, S.E.; Tu, F.F.; Neshewat, G.; Clauw, D.J. Increased pressure pain sensitivity in women with chronic pelvic pain. Obstet. Gynecol. 2013, 122, 1047–1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bajaj, P.; Bajaj, P.; Madsen, H.; Arendt-Nielsen, L. Endometriosis is associated with central sensitization: A psychophysical controlled study. J. Pain 2003, 4, 372–380. [Google Scholar] [CrossRef]
- He, W.; Liu, X.; Zhang, Y.; Guo, S.-W. Generalized hyperalgesia in women with endometriosis and its resolution following a successful surgery. Reprod. Sci. 2010, 17, 1099–1111. [Google Scholar] [CrossRef]
- Stratton, P.; Khachikyan, I.; Sinaii, N.; Ortiz, R.; Shah, J. Association of chronic pelvic pain and endometriosis with signs of sensitization and myofascial pain. Obstet. Gynecol. 2015, 125, 719–728. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.; Yaksh, T.L. A brief comparison of the pathophysiology of inflammatory versus neuropathic pain. Curr. Opin. Anaesthesiol. 2011, 24, 400–407. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves Dos Santos, G.; Delay, L.; Yaksh, T.L.; Corr, M. Neuraxial cytokines in pain states. Front. Immunol. 2020, 10, 3061. [Google Scholar] [CrossRef] [Green Version]
- Wieseler-Frank, J.; Maie, S.F.; Watkins, L.R. Central proinflammatory cytokines and pain enhancement. Neurosignals 2005, 14, 166–174. [Google Scholar] [CrossRef]
- Cho, Y.J.; Lee, S.H.; Park, J.W.; Ha, M.; Park, M.J.; Han, S.J. Dysfunctional signaling underlying endometriosis: Current state of knowledge. J. Mol. Endocrinol. 2018, 60, R97–R113. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.H.; Ya-Hsin Chen, Y.H.; Chang, H.Y.; Au, H.K.; Tzeng, C.R.; Huang, Y.H. Chronic niche inflammation in endometriosis-associated infertility: Current understanding and future therapeutic strategies. Int. J. Mol. Sci. 2018, 19, 2385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minici, F.; Tiberi, F.; Tropea, A.; Miceli, F.; Orlando, M.; Gangale, M.F.; Romani, F.; Catino, S.; Campo, S.; Lanzone, A.; et al. Paracrine regulation of endometriotic tissue. Gynecol. Endocrinol. 2007, 23, 574–580. [Google Scholar] [CrossRef]
- Ren, K.; Torres, R. Role of interleukin-1beta during pain and inflammation. Brain Res. Rev. 2009, 60, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Drosdzol-Cop, A.; Skrzypulec-Plinta, V. Selected cytokines and glycodelin A levels in serum and peritoneal fluid in girls with endometriosis. J. Obstet. Gynaecol. Res. 2012, 38, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Coelho, A.M.; Fioramonti, J.; Bueno, L. Brain interleukin-1β and tumor necrosis factor-α are involved in lipopolysaccharide-induced delayed rectal allodynia in awake rats. Brain Res. Bullet. 2000, 52, 223–228. [Google Scholar] [CrossRef]
- Safieh-Garabedian, B.; Dardenne, M.; Kanaan, S.A.; Atweh, S.F.; Jabbur, S.J.; Saadé, N.E. The role of cytokines and prostaglandin-E (2) in thymulin induced hyperalgesia. Neuropharmacology 2000, 39, 1653–1661. [Google Scholar] [CrossRef]
- Maier, S.F.; Watkins, L.R. Immune-to-central nervous system communication and its role in modulating pain and cognition: Implications for cancer and cancer treatment. Brain Behav. Immun. 2003, 17 (Suppl. S1), 125–131. [Google Scholar] [CrossRef]
- Fukaya, T.; Hoshiai, H.; Yajima, A. Is pelvic endometriosis always associated with chronic pain? A retrospective study of 618 cases diagnosed by laparoscopy. Am. J. Obstet. Gynecol. 1993, 169, 719–722. [Google Scholar] [CrossRef]
- Vercellini, P.; Trespidi, L.; De Giorgi, O.; Cortesi, I.; Parazzini, F.; Crosignani, P.G. Endometriosis and pelvic pain: Relation to disease stage and localization. Fertil. Steril. 1996, 65, 299–304. [Google Scholar] [CrossRef]
- Vercellini, P.; Fedele, L.; Aimi, G.; Pietropaolo, G.; Consonni, D.; Crosignani, P. Association between endometriosis stage, lesion type, patient characteristics and severity of pelvic pain symptoms: A multivariate analysis of over 1000 patients. Hum. Reprod. 2006, 22, 266–271. [Google Scholar] [CrossRef]
- Berterö, C.; Alehagen, S.; Grundström, H. Striving for a biopsychosocial approach: A secondary analysis of mutual components during healthcare encounters between women with endometriosis and physicians. J. Endometr. Pelvic Pain Disord. 2019, 11, 146–151. [Google Scholar] [CrossRef]
- Stratton, P.; Berkley, K.J. Chronic pelvic pain and endometriosis: Translational evidence of the relationship and implications. Hum. Reprod. Update 2010, 17, 327–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, R.-R.; Nackley, A.; Huh, Y.; Terrando, N.; Maixner, W. Neuroinflammation and central sensitization in chronic and widespread pain. Anesthesiology 2018, 129, 343–366. [Google Scholar] [CrossRef] [PubMed]
- Kissler, S.; Hamscho, N.; Zangos, S.; Gätje, R.; Müller, A.; Rody, A.; Döbert, N.; Menzel, C.; Grünwald, F.; Siebzehnrübl, E.; et al. Diminished pregnancy rates in endometriosis due to impaired uterotubal transport assessed by hysterosalpingoscintigraphy. Br. J. Obstet. Gynaecol. 2005, 112, 1391–1396. [Google Scholar] [CrossRef]
- Reeve, L.; Lashen, H.; Pacey, A.A. Endometriosis affects sperm-endosalpingeal interactions. Hum. Reprod. 2005, 20, 448–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Venecia, C.; Ascher, S.M. Pelvic endometriosis: Spectrum of magnetic resonance imaging findings. Semin. Ultrasound CT MR. 2015, 36, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Meuleman, C.; Vandenabeele, B.; Fieuws, S.; Spiessens, C.; Timmerman, D.; D’Hooghe, T. High prevalence of endometriosis in infertile women with normal ovulation and normospermic partners. Fertil. Steril. 2009, 92, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Harb, H.M.; Gallos, I.D.; Chu, J.; Harb, M.; Coomarasamy, A. The effect of endometriosis on in vitro fertilisation outcome: A systematic review and meta-analysis. Br. J. Obstet. Gynaecol. 2013, 120, 1308–1320. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, K.; Saito, H.; Saito, T.; Ito, M.; Ohta, N.; Takahashi, T.; Hiroi, M. Ovarian fecundity in patients with endometriosis can be estimated by the incidence of apoptotic bodies. Fertil. Steril. 1998, 69, 931–935. [Google Scholar] [CrossRef]
- Kaya, H.; Oral, B. Effect of ovarian involvement on the frequency of luteinized unruptured follicle in endometriosis. Gynecol. Obstet. Invest. 1999, 48, 123–126. [Google Scholar] [CrossRef]
- Sanchez, A.M.; Somigliana, E.; Vercellini, P.; Pagliardini, L.; Candiani, M.; Viganò, P. Endometriosis as a detrimental condition for granulosa cell steroidogenesis and development: From molecular alterations to clinical impact. J. Steroid Biochem. Mol. Biol. 2016, 155, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Anupa, G.; Sharma, J.B.; Roy, K.K.; Sengupta, J.; Ghosh, D. An assessment of the multifactorial profile of steroid-metabolizing enzymes and steroid receptors in the eutopic endometrium during moderate to severe ovarian endometriosis. Reprod. Biol. Endocrinol. 2019, 17, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bulun, S.E.; Yilmaz, B.D.; Sison, C.; Miyazaki, K.; Bernardi, L.; Liu, S.; Kohlmeier, A.; Yin, P.; Milad, M.; Wei, J. Endometriosis. Endocr. Rev. 2019, 40, 1048–1079. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, J.; Ghosh, D. Multi-level and multi-scale integrative approach to the understanding of human blastocyst implantation. Prog. Biophys. Mol. Biol. 2014, 114, 49–60. [Google Scholar] [CrossRef]
- Lessey, B.A.; Kim, J.J. Endometrial receptivity in the eutopic endometrium of women with endometriosis: It is affected, and let me show you why. Fertil. Steril. 2017, 108, 19–27. [Google Scholar] [CrossRef] [Green Version]
- Miravet-Valenciano, J.; Ruiz-Alonso, M.; Gómez, E.; Garcia-Velasco, J.A. Endometrial receptivity in eutopic endometrium in patients with endometriosis: It is not affected, and let me show you why. Fertil. Steril. 2017, 108, 28–31. [Google Scholar] [CrossRef] [Green Version]
- Lessey, B.A.; Young, S.L. What exactly is endometrial receptivity? Fertil. Steril. 2019, 111, 611–617. [Google Scholar] [CrossRef]
- Schatz, F.; Guzeloglu-Kayisli, O.; Arlier, S.; Kayisli, U.A.; Lockwood, C.J. The role of decidual cells in uterine hemostasis, menstruation, inflammation, adverse pregnancy outcomes and abnormal uterine bleeding. Hum. Reprod. Update 2016, 22, 497–515. [Google Scholar] [CrossRef] [Green Version]
- Kitajima, M.; Defrère, S.; Dolmans, M.M.; Colette, S.; Squifflet, J.; Van Langendonckt, A.; Donnez, J. Endometriomas as a possible cause of reduced ovarian reserve in women with endometriosis. Fertil. Steril. 2011, 96, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Regiani, T.; Cordeiro, F.B.; da Costa Ldo, V.; Salgueiro, J.; Cardozo, K.; Carvalho, V.M.; Perkel, K.J.; Zylbersztejn, D.S.; Cedenho, A.P.; Lo Turco, E.G. Follicular fluid alterations in endometriosis: Label-free proteomics by MS (E) as a functional tool for endometriosis. Syst. Biol. Reprod. Med. 2015, 61, 263–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, A.C.; Prefumo, F. The effects of surgery for endometriosis on pregnancy outcomes following in vitro fertilization and embryo transfer: A systematic review and meta-analysis. Arch. Gynecol. Obstet. 2016, 294, 647–655. [Google Scholar] [CrossRef]
- Giacomini, E.; Sanchez, A.M.; Sarais, V.; Beitawi, S.A.; Candiani, M.; Viganò, P. Characteristics of follicular fluid in ovaries with endometriomas. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 209, 34–38. [Google Scholar] [CrossRef]
- Donnez, J.; Donnez, O.; Orellana, R.; Binda, M.M.; Dolmans, M.M. Endometriosis and infertility. Panminerva Med. 2016, 58, 143–150. [Google Scholar] [PubMed]
- Miller, J.E.; Ahn, S.H.; Monsanto, S.P.; Khalaj, K.; Koti, M.; Tayade, C. Implications of immune dysfunction on endometriosis associated infertility. Oncotarget 2017, 8, 7138–7147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Surrey, E.S. Endometriosis-related infertility: The role of the Assisted Reproductive Technologies. Biomed. Res. Int. 2015, 2015, 482959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuivasaari, P.; Hippeläinen, M.; Anttila, M.; Heinonen, S. Effect of endometriosis on IVF/ICSI outcome: Stage III/IV endometriosis worsens cumulative pregnancy and live-born rates. Hum. Reprod. 2005, 20, 3130–3135. [Google Scholar] [CrossRef] [Green Version]
- Freis, A.; Dietrich, J.E.; Binder, M.; Holschbach, V.; Strowitzki, T.; Germeyer, A. Relative morphokinetics assessed by time-lapse imaging are altered in embryos from patients with endometriosis. Reprod. Sci. 2018, 25, 1279–1285. [Google Scholar] [CrossRef]
- Franasiak, J.M.; Forman, E.J.; Patounakis, G.; Hong, K.H.; Werner, M.D.; Upham, K.M.; Treff, N.R.; Scott, R.T., Jr. Investigating the impact of the timing of blastulation on implantation: Management of embryo-endometrial synchrony improves outcomes. Hum. Reprod. Open. 2018, 2018, hoy022. [Google Scholar] [CrossRef] [Green Version]
- Ahn, S.H.; Khalaj, K.; Young, S.L.; Lessey, B.A.; Koti, M.; Tayade, C. Immune-inflammation gene signatures in endometriosis patients. Fertil. Steril. 2016, 106, 1420–1431. [Google Scholar] [CrossRef] [Green Version]
- Barragan, F.; Irwin, J.C.; Balayan, S.; Erikson, D.W.; Chen, J.C.; Houshdaran, S.; Piltonen, T.T.; Spitzer, T.L.; George, A.; Rabban, J.T.; et al. Human endometrial fibroblasts derived from mesenchymal progenitors inherit progesterone resistance and acquire an inflammatory phenotype in the endometrial niche in endometriosis. Biol. Reprod. 2016, 94, 118. [Google Scholar] [CrossRef]
- Nakanishi, K.; Yoshimoto, T.; Tsutsui, H.; Okamura, H. Interleukin-18 is a unique cytokine that stimulates both Th1 and Th2 responses depending on its cytokine milieu. Cytokine Growth Factor Rev. 2001, 12, 53–72. [Google Scholar] [CrossRef]
- Lédée, N.; Petitbarat, M.; Chevrier, L.; Vitoux, D.; Vezmar, K.; Rahmati, M.; Dubanchet, S.; Gahéry, H.; Bensussan, A.; Chaouat, G. The uterine immune profile may help women with repeated unexplained embryo implantation failure after in vitro fertilization. Am. J. Reprod. Immunol. 2016, 75, 388–401. [Google Scholar] [CrossRef] [Green Version]
- McKinnon, B.; Mueller, M.; Montgomery, G. Progesterone resistance in endometriosis: An acquired property? Trends Endocrinol. Metab. 2018, 29, 535–548. [Google Scholar] [CrossRef]
- Schweppe, K.W.; Ring, D. Peritoneal defects and the development of endometriosis in relation to the timing of endoscopic surgery during the menstrual cycle. Fertil. Steril. 2002, 78, 763–766. [Google Scholar] [CrossRef]
- Jacobson, T.Z.; Duffy, J.M.; Barlow, D.; Koninckx, P.R.; Garry, R. Laparoscopic surgery for pelvic pain associated with endometriosis. Cochrane Database Syst. Rev. 2009, 4, CD001300. [Google Scholar] [CrossRef]
- Yeung, P.P., Jr.; Shwayder, J.; Pasic, R.P. Laparoscopic management of endometriosis: Comprehensive review of best evidence. J. Minim. Invasive Gynecol. 2009, 16, 269–281. [Google Scholar] [CrossRef]
- Black, K.I.; Fraser, I.S. Medical management of endometriosis. Aust. Prescr. 2012, 35, 114–117. [Google Scholar] [CrossRef] [Green Version]
- Vercellini, P.; Crosignani, P.; Somigliana, E.; Viganò, P.; Frattaruolo, M.P.; Fedele, L. ‘Waiting for Godot’: A common sense approach to the medical treatment of endometriosis. Hum. Reprod. 2011, 26, 3–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, S.W. Endometriosis and ovarian cancer: Potential benefits and harms of screening and risk-reducing surgery. Fertil. Steril. 2015, 104, 813–830. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Rock, E.J. Modern Approaches to Endometriosis; Springer Netherlands: Amsterdam, The Netherlands, 1991; pp. 228–234. ISBN 978-94-011-3864-2. [Google Scholar]
- Barbieri, R.L.; Evans, S.; Kistner, R.W. Danazol in the treatment of endometriosis: Analysis of 100 cases with a 4-year follow-up. Fertil. Steril. 1982, 37, 737–746. [Google Scholar] [CrossRef]
- Döberl, A.; Bergqvist, A.; Jeppsson, S.; Koskimies, A.I.; Rönnberg, L.; Segerbrand, E.; Starup, J. Regression of endometriosis following shorter treatment with, or lower dose of danazol. Comparison of pre- and post-treatment laparoscopic findings in the Scandinavian multi-center study. Acta Obstet. Gynecol. Scand. 1984, 63 (Suppl. S123), 51–58. [Google Scholar] [CrossRef]
- Miller, J.D.; Shaw, R.W.; Casper, R.F.; Rock, J.A.; Thomas, E.J.; Dmowski, W.P.; Surrey, E.; Malinak, L.R.; Moghissi, K. Historical prospective cohort study of the recurrence of pain after discontinuation of treatment with danazol or a gonadotropin-releasing hormone agonist. Fertil. Steril. 1998, 70, 293–296. [Google Scholar] [CrossRef]
- Selak, V.; Farquhar, C.; Prentice, A.; Singla, A. Danazol for pelvic pain associated with endometriosis. Cochrane Database Syst. Rev. 2007, 4, CD000068. [Google Scholar] [CrossRef]
- Bromham, D.R.; Booker, M.W.; Rose, G.L.; Wardle, P.G.; Newton, J.R. Updating the clinical experience in endometriosis—The European perspective. Br. J. Obstet. Gynaecol. 1995, 102 (Suppl. S12), 12–16. [Google Scholar] [CrossRef]
- Halbe, H.W.; Nakamura, M.S.; Da Silveira, G.P.; Carvalh, W.P. Updating the clinical experience in endometriosis—The Brazilian perspective. Br. J. Obstet. Gynaecol. 1995, 102 (Suppl. S12), 17–21. [Google Scholar] [CrossRef]
- Blackwell, R.E.; Olive, D.L. Chronic Pelvic Pain: Evaluation and Management; Springer: New York, NY, USA, 2012; pp. 106–107. ISBN 978-1-4612-1752-7. [Google Scholar]
- Gnoth, C.H.; Gödtke, K.; Freundl, G.; Godehardt, E.; Kienle, E. Effects of add-back therapy on bone mineral density and pyridinium crosslinks in patients with endometriosis treated with gonadotropin-releasing hormone agonists. Gynecol. Obstet. Invest. 1999, 47, 37–41. [Google Scholar] [CrossRef]
- Agarwal, S.K.; Daniels, A.; Drosman, S.R.; Udoff, L.; Foster, W.G.; Pike, M.C.; Spicer, D.V.; Daniels, J.R. Treatment of endometriosis with the GnRHa Deslorelin and add-back estradiol and supplementary testosterone. Biomed. Res. Int. 2015, 2015, 934164. [Google Scholar] [CrossRef] [Green Version]
- Carr, B.; Dmowski, W.P.; O’Brien, C.; Jiang, P.; Burke, J.; Jimenez, R.; Garner, E.; Chwalisz, K. Elagolix, an oral GnRH antagonist, versus subcutaneous depot medroxyprogesterone acetate for the treatment of endometriosis: Effects on bone mineral density. Reprod. Sci. 2014, 21, 1341–1351. [Google Scholar] [CrossRef] [Green Version]
- Diamond, M.P.; Carr, B.; Dmowski, W.P.; Koltun, W.; O’Brien, C.; Jiang, P.; Burke, J.; Jimenez, R.; Garner, E.; Chwalisz, K. Elagolix treatment for endometriosis-associated pain: Results from a phase 2, randomized, double-blind, placebo-controlled study. Reprod. Sci. 2014, 21, 363–371. [Google Scholar] [CrossRef]
- Taylor, H.S.; Giudice, L.C.; Lessey, B.A.; Abrao, M.S.; Kotarski, J.; Archer, D.F.; Diamond, M.P.; Surrey, E.; Johnson, N.P.; Watts, N.B.; et al. Treatment of endometriosis-associated pain with Elagolix, an oral GnRH antagonist. N. Engl. J. Med. 2017, 377, 28–40. [Google Scholar] [CrossRef]
- Surrey, E.S.; Soliman, A.M.; Agarwal, S.K.; Snabes, M.C.; Diamond, M.P. Impact of Elagolix treatment on fatigue experienced by women with moderate to severe pain associated with endometriosis. Fertil. Steril. 2019, 112, 298–304. [Google Scholar] [CrossRef]
- Gallagher, J.S.; Missmer, S.A.; Hornstein, M.D.; Laufer, M.R.; Gordon, C.M.; DiVasta, A.D. Long-term effects of gonadotropin-releasing hormone agonists and add-back in adolescent endometriosis. J. Pediatr. Adolesc. Gynecol. 2018, 31, 376–381. [Google Scholar] [CrossRef]
- Strowitzki, T.; Faustmann, T.; Gerlinger, C.; Schumacher, U.; Ahlers, C.; Seitz, C. Safety and tolerability of dienogest in endometriosis: Pooled analysis from the European clinical study program. Int. J. Women Health 2015, 7, 393–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, J.; Yu, Q.; Zhang, S.; Li, H.; Gude, K.; von Ludwig, C.; Ren, X.; Dong, L. Dienogest for treatment of endometriosis in Chinese women: A placebo-controlled, randomized, double-blind phase 3 study. J. Women Health 2018, 27, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P.; Bracco, B.; Mosconi, P.; Roberto, A.; Alberico, D.; Dhouha, D.; Somigliana, E. Norethindrone acetate or dienogest for the treatment of symptomatic endometriosis: A before and after study. Fertil. Steril. 2016, 105, 734–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grandi, G.; Barra, F.; Ferrero, S.; Sileo, F.G.; Bertucci, E.; Napolitano, A.; Facchinetti, F. Hormonal contraception in women with endometriosis: A systematic review. Eur. J. Contracept. Reprod. Health Care 2019, 24, 61–70. [Google Scholar] [CrossRef]
- Santen, R.J.; Brodie, H.; Simpson, E.R.; Siiteri, P.K.; Brodie, A. History of aromatase: Saga of an important biological mediator and therapeutic target. Endocr. Rev. 2009, 30, 343–375. [Google Scholar] [CrossRef]
- Patwardhan, S.; Nawathe, A.; Yates, D.; Harrison, G.R.; Khan, K.S. Systematic review of the effects of aromatase inhibitors on pain associated with endometriosis. Br. J. Obstet. Gynaecol. 2008, 115, 818–822. [Google Scholar] [CrossRef]
- Ferrero, S.; Gillott, D.J.; Venturini, P.L.; Remorgida, V. Use of aromatase inhibitors to treat endometriosis-related pain symptoms: A systematic review. Reprod. Biol. Endocrinol. 2011, 9, 89. [Google Scholar] [CrossRef] [Green Version]
- Nezhat, C.; Vang, N.; Tanaka, P.P.; Nezhat, C. Optimal management of endometriosis and pain. Obstet. Gynecol. 2019, 134, 834–839. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.; Crawford, T.J.; Allen, C.; Hopewell, S.; Prentice, A. Nonsteroidal anti-inflammatory drugs for pain in women with endometriosis. Cochrane Database Syst. Rev. 2017, 1, CD004753. [Google Scholar] [CrossRef] [PubMed]
- Somigliana, E.; Viganò, P.; Benaglia, L.; Busnelli, A.; Berlanda, N.; Vercellini, P. Management of endometriosis in the infertile patient. Semin. Reprod. Med. 2017, 35, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Alborzi, S.; Sorouri, Z.; Askari, E.; Poordast, T.; Chamanara, K. The success of various endometrioma treatments in infertility: A systematic review and meta-analysis of prospective studies. Reprod. Med. Biol. 2019, 18, 312–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil. Steril. 1997, 67, 817–821. [CrossRef]
- Haas, D.; Chvatal, R.; Habelsberger, A.; Wurm, P.; Schimetta, W.; Oppelt, P. Comparison of revised American Fertility Society and ENZIAN staging: A critical evaluation of classifications of endometriosis on the basis of our patient population. Fertil. Steril. 2011, 95, 1574–1578. [Google Scholar] [CrossRef]
- Adamson, G.D. Endometriosis Fertility Index: Is it better than the present staging systems? Curr. Opin. Obstet. Gynecol. 2013, 25, 186–192. [Google Scholar] [CrossRef]
- Cook, A.S.; Adamson, G.D. The role of the Endometriosis Fertility Index (EFI) and endometriosis scoring systems in predicting infertility outcomes. Curr. Obstet. Gynecol. Rep. 2013, 2, 186–194. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Liu, D.; Huang, W.; Wang, Q.; Feng, X.; Tan, J. Prediction of Endometriosis Fertility Index in patients with endometriosis-associated infertility after laparoscopic treatment. Reprod. Biomed. Online 2018, 37, 53–59. [Google Scholar] [CrossRef]
- Negi, N.; Roy, K.K.; Kumar, S.; Nair, V.G.; Vanamail, P. Clinical outcome analysis and correlation of reproductive outcome with Endometriosis Fertility Index in laparoscopically managed endometriosis patients: A retrospective cohort study. J. Hum. Reprod. Sci. 2019, 12, 98–103. [Google Scholar] [CrossRef]
- Johnson, N.P.; Hummelshoj, L.; Adamson, G.D.; Keckstein, J.; Taylor, H.S.; Abrao, M.S.; Bush, D.; Kiesel, L.; Tamimi, R.; Sharpe-Timms, K.L.; et al. World Endometriosis Society Sao Paulo Consortium. World Endometriosis Society consensus on the classification of endometriosis. Hum. Reprod. 2017, 32, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Tomassetti, C.; Bafort, C.; Meuleman, C.; Welkenhuysen, M.; Fieuws, S.; D’Hooghe, T. Reproducibility of the Endometriosis Fertility Index: A prospective inter-/intra-rater agreement study. Br. J. Obstet. Gynaecol. 2020, 127, 107–114. [Google Scholar] [CrossRef]
- Parker, V.J.; Douglas, A.J. Stress in early pregnancy: Maternal neuro-endocrine-immune responses and effects. J. Reprod. Immunol. 2010, 85, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Whirledge, S.; Cidlowski, J.A. A role for glucocorticoids in stress-impaired reproduction: Beyond the hypothalamus and pituitary. Endocrinology 2013, 154, 4450–4468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Aken, M.; Oosterman, J.; van Rijn, T.; Ferdek, M.; Ruigt, G.; Kozicz, T.; Braat, D.; Peeters, A.; Nap, A. Hair cortisol and the relationship with chronic pain and quality of life in endometriosis patients. Psychoneuroendocrinology 2018, 89, 216–222. [Google Scholar] [CrossRef]
- Nepomnaschy, P.A.; Welch, K.B.; McConnell, D.S.; Low, B.S.; Strassmann, B.I.; England, B.G. Cortisol levels and very early pregnancy loss in humans. Proc. Natl. Acad. Sci. USA 2006, 103, 3938–3942. [Google Scholar] [CrossRef] [Green Version]
- Nepomnaschy, P.A.; Welch, K.; McConnell, D.; Strassmann, B.I.; England, B.G. Stress and female reproductive function: A study of daily variations in cortisol, gonadotrophins, and gonadal steroids in a rural Mayan population. Am. J. Hum. Biol. 2004, 16, 523–532. [Google Scholar] [CrossRef]
- Wilcox, A.J.; Baird, D.D.; Weinberg, C.R. Time of implantation of the conceptus and loss of pregnancy. N. Engl. J. Med. 1999, 340, 1796–1799. [Google Scholar] [CrossRef]
- Laufer, N.; Navot, D.; Schenker, J.G. The pattern of luteal phase plasma progesterone and estradiol in fertile cycles. Am. J. Obstet. Gynecol. 1982, 143, 808–813. [Google Scholar] [CrossRef]
- Ghosh, D.; Sengupta, J. Patterns of estrogen and progesterone receptors in rhesus monkey endometrium during secretory phase of normal menstrual cycle and preimplantation stages of gestation. J. Steroid Biochem. 1988, 31, 223–239. [Google Scholar] [CrossRef]
- Ghosh, D.; Stewart, D.R.; Nayak, N.R.; Lasley, B.L.; Overstreet, J.W.; Hendrickx, A.G.; Sengupta, J. Serum concentrations of oestradiol-17beta, progesterone, relaxin and chorionic gonadotrophin during blastocyst implantation in natural pregnancy cycle and in embryo transfer cycle in the rhesus monkey. Hum. Reprod. 1997, 12, 914–920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baird, D.D.; Weinberg, C.R.; Zhou, H.; Kamel, F.; McConnaughey, D.R.; Kesner, J.S.; Wilcox, A.J. Preimplantation urinary hormone profiles and the probability of conception in healthy women. Fertil. Steril. 1999, 71, 40–49. [Google Scholar] [CrossRef]
- Gemzell-Danielsson, K.; Swahn, M.L.; Svalander, P.; Bygdeman, M. Early luteal phase treatment with mifepristone (RU 486) for fertility regulation. Hum. Reprod. 1993, 8, 870–873. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Sengupta, J. Anti-nidatory effect of a single, early post-ovulatory administration of mifepristone (RU 486) in the rhesus monkey. Hum. Reprod. 1993, 8, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Lalitkumar, P.G.; Wong, V.J.; Hendrickx, A.G.; Sengupta, J. Preimplantation embryo morphology following early luteal phase anti-nidatory treatment with mifepristone (RU486) in the rhesus monkey. Hum. Reprod. 2000, 15, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Pizarro, A.; Figueroa, P.; Brito, J.; Marín, J.C.; Munroe, D.J.; Croxatto, H.B. Endometrial gene expression reveals compromised progesterone signaling in women refractory to embryo implantation. Reprod. Biol. Endocrinol. 2014, 12, 92. [Google Scholar] [CrossRef] [Green Version]
- Cuevas, C.A.; Tapia-Pizarro, A.; Salvatierra, A.M.; Munroe, D.J.; Velasquez, L.; Croxatto, H.B. Effect of single post-ovulatory administration of mifepristone (RU486) on transcript profile during the receptive period in human endometrium. Reproduction 2016, 151, 331–349. [Google Scholar] [CrossRef] [Green Version]
- Jacobsen, B.M.; Horwitz, K.B. Progesterone receptors, their isoforms and progesterone regulated transcription. Mol. Cell. Endocrinol. 2012, 357, 18–29. [Google Scholar] [CrossRef] [Green Version]
- Arnett-Mansfield, R.L.; DeFazio, A.; Mote, P.A.; Clarke, C.L. Subnuclear distribution of progesterone receptors A and B in normal and malignant endometrium. J. Clin. Endocrinol. Metab. 2004, 89, 1429–1442. [Google Scholar] [CrossRef] [Green Version]
- Wetendorf, M.; Wu, S.P.; Wang, X.; Creighton, C.J.; Wang, T.; Lanz, R.B.; Blok, L.; Tsai, S.Y.; Tsai, M.J.; Lydon, J.P.; et al. Decreased epithelial progesterone receptor A at the window of receptivity is required for preparation of the endometrium for embryo attachment. Biol. Reprod. 2017, 96, 313–326. [Google Scholar] [CrossRef]
- Arnett-Mansfield, R.L.; DeFazio, A.; Wain, G.V.; Jaworski, R.C.; Byth, K.; Mote, P.A.; Clarke, C.L. Relative expression of progesterone receptors A and B in endometrioid cancers of the endometrium. Cancer Res. 2001, 61, 4576–4582. [Google Scholar] [PubMed]
- Stettner, M.; Kaulfuss, S.; Burfeind, P.; Schweyer, S.; Strauss, A.; Ringert, R.H.; Thelen, P. The relevance of estrogen receptor-beta expression to the antiproliferative effects observed with histone deacetylase inhibitors and phytoestrogens in prostate cancer treatment. Mol. Cancer Ther. 2007, 6, 2626–2633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hapangama, D.K.; Turner, M.A.; Drury, J.A.; Quenby, S.; Saretzki, G.; Martin-Ruiz, C.; Von Zglinicki, T. Endometriosis is associated with aberrant endometrial expression of telomerase and increased telomere length. Hum. Reprod. 2008, 23, 1511–1519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flores, V.A.; Vanhie, A.; Dang, T.; Taylor, H.S. Progesterone receptor status predicts response to progestin therapy in endometriosis. J. Clin. Endocrinol. Metab. 2018, 103, 4561–4568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fassbender, A.; Rahmioglu, N.; Vitonis, A.F.; Viganò, P.; Giudice, L.C.; D’Hooghe, T.M.; Hummelshoj, L.; Adamson, G.D.; Becker, C.M.; Missmer, S.A.; et al. World Endometriosis Research Foundation Endometriosis Phenome and Biobanking Harmonisation Project: IV. Tissue collection, processing, and storage in endometriosis research. Fertil. Steril. 2014, 102, 1244–1253. [Google Scholar] [CrossRef]
- Geukens, E.I.; Apers, S.; Meuleman, C.; D’Hooghe, T.M.; Dancet, E.A.F. Patient-centeredness and endometriosis: Definition, measurement, and current status. Best Practice Res. Clin. Obstet. Gynaecol. 2018, 50, 11–17. [Google Scholar] [CrossRef]
- McPeak, A.E.; Allaire, C.; Williams, C.; Albert, A.; Lisonkova, S.; Yong, P.J. Pain catastrophizing and pain health-related quality-of-life in endometriosis. Clin. J. Pain. 2018, 34, 349–356. [Google Scholar] [CrossRef]
- Dancet, E.A.; Apers, S.; Kremer, J.A.; Nelen, W.L.; Sermeus, W.; D’Hooghe, T.M. The patient-centeredness of endometriosis care and targets for improvement: A systematic review. Gynecol. Obstet. Invest. 2014, 78, 69–80. [Google Scholar] [CrossRef]
- Grundström, H.; Alehagen, S.; Kjølhede, P.; Berterö, C. The double-edged experience of healthcare encounters among women with endometriosis: A qualitative study. J. Clin. Nurs. 2017, 27, 205–211. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, S.; Foster, L.; Groessl, E.J. Rethinking endometriosis care: Applying the chronic care model via a multidisciplinary program for the care of women with endometriosis. Int. J. Women Health 2019, 11, 405–410. [Google Scholar] [CrossRef] [Green Version]
- Hållstam, A.; Stålnacke, B.-M.; Svensén, C.; Löfgren, M. Living with painful endometriosis—A struggle for coherence. A qualitative study. Sex. Reprod. Healthc. 2018, 17, 97–102. [Google Scholar] [CrossRef]
- De Graaff, A.A.; D’Hooghe, T.M.; Dunselman, G.A.; Dirksen, C.D.; Hummelshoj, L.; WERF EndoCost Consortium; Simoens, S. The significant effect of endometriosis on physical, mental and social wellbeing: Results from an international cross-sectional survey. Hum. Reprod. 2013, 28, 2677–2685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowlands, I.J.; Teede, H.; Lucke, J.; Dobson, A.J.; Mishra, G.D. Young women’s psychological distress after a diagnosis of polycystic ovary syndrome or endometriosis. Hum. Reprod. 2016, 31, 2072–2081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apers, S.; Dancet, E.A.F.; Aarts, J.W.M.; Kluivers, K.B.; D’Hooghe, T.M.; Nelen, W.L.D.M. The association between experiences with patient-centred care and health-related quality of life in women with endometriosis. Reprod. Biomed. Online 2018, 36, 197–205. [Google Scholar] [CrossRef] [Green Version]
- Ugwumadu, L.; Chakrabarti, R.; Williams-Brown, E.; Rendle, J.; Swift, I.; John, B.; Allen-Coward, H.; Ofuasia, E. The role of the multidisciplinary team in the management of deep infiltrating endometriosis. Gynecol. Surg. 2017, 14, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]






 , increased;
, increased;  , decreased;
, decreased;  , no change. E1, estrone; E2, estradiol-17β, ERα, estrogen receptor alpha; ERβ, estrogen receptor beta; 17βHSD, 17beta hydroxysteroid dehydrogenase subtype; P4, progesterone; PRA, progesterone receptor isoform A; PRB, progesterone receptor isoform B; PREs, progesterone response elements. For details, see Anupa et al. [146].
, no change. E1, estrone; E2, estradiol-17β, ERα, estrogen receptor alpha; ERβ, estrogen receptor beta; 17βHSD, 17beta hydroxysteroid dehydrogenase subtype; P4, progesterone; PRA, progesterone receptor isoform A; PRB, progesterone receptor isoform B; PREs, progesterone response elements. For details, see Anupa et al. [146].
 , increased;
, increased;  , decreased;
, decreased;  , no change. E1, estrone; E2, estradiol-17β, ERα, estrogen receptor alpha; ERβ, estrogen receptor beta; 17βHSD, 17beta hydroxysteroid dehydrogenase subtype; P4, progesterone; PRA, progesterone receptor isoform A; PRB, progesterone receptor isoform B; PREs, progesterone response elements. For details, see Anupa et al. [146].
, no change. E1, estrone; E2, estradiol-17β, ERα, estrogen receptor alpha; ERβ, estrogen receptor beta; 17βHSD, 17beta hydroxysteroid dehydrogenase subtype; P4, progesterone; PRA, progesterone receptor isoform A; PRB, progesterone receptor isoform B; PREs, progesterone response elements. For details, see Anupa et al. [146].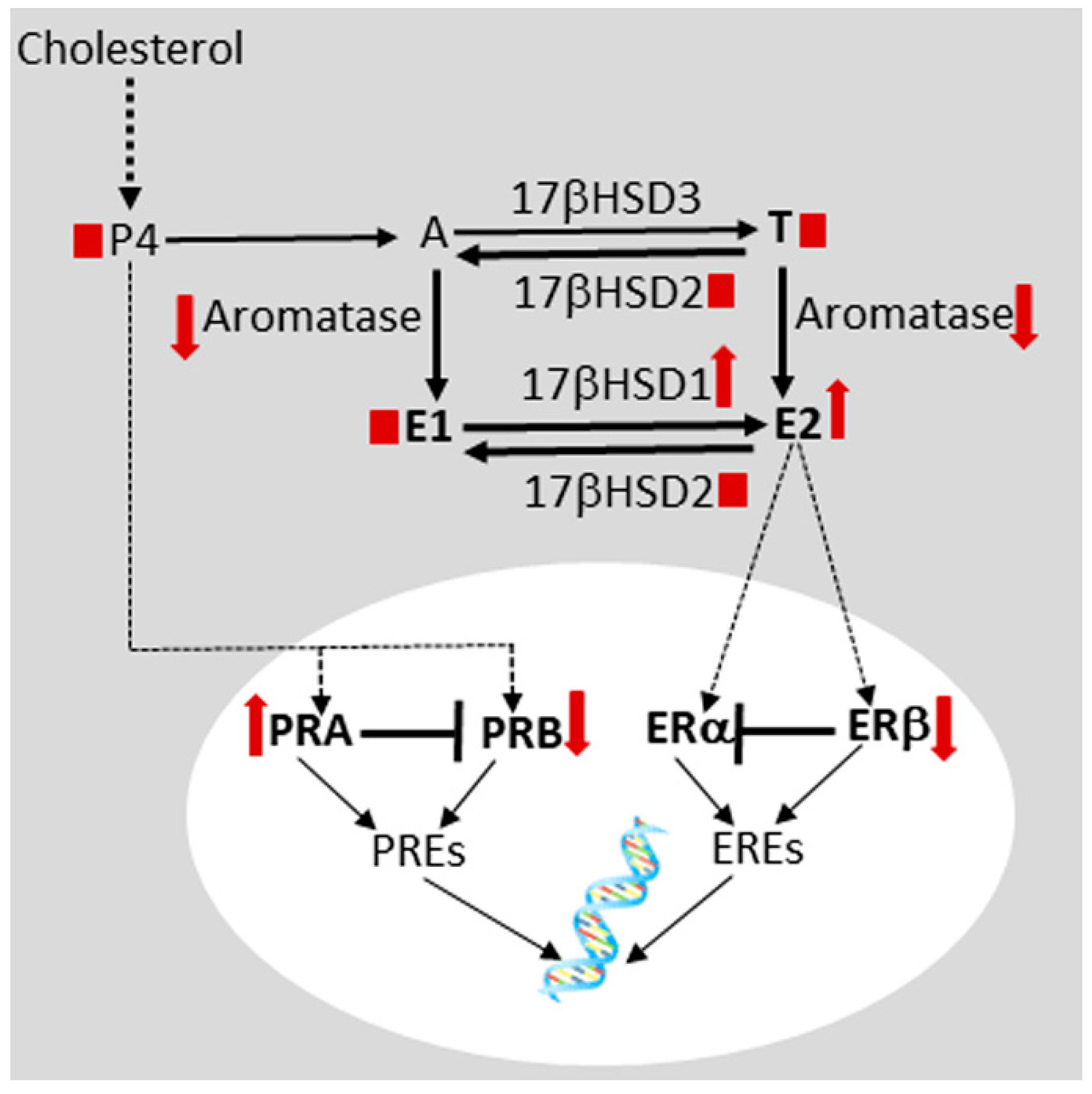

| Factor | Reference [Reference No.] |
|---|---|
| • Disturbed folliculogenesis | Nakahara et al., 1998 [143] |
| • Luteinized unruptured follicle | Kaya and Oral, 1999 [144] |
| • Oocyte quality | Sanchez et al., 2016 [145] |
| • Adhesions | De Venecia, Ascher, 2015 [140] |
| • Dysfunctional uterotubal motility | Kissler et al., 2005 [138] |
| • Detrimental effects on spermatozoa | Reeve et al., 2005 [139] |
| • Peritoneal inflammation | Malvezzi et al., 2019 [85]; Anupa et al. 2020 [86] |
| • Progesterone resistance plus estrogen dominance | Sengupta et al., 2017 [5]; Anupa et al., 2019 [146]; Bulun, 2019 [147] |
| • Endometrial hostility | Sengupta and Ghosh, 2014 [148]; Lessey and Kim, 2017 [149]; Miravet-Valenciano et al., 2017 [150]; Lessey, Young, 2019 [151] |
| • Immune dysfunctions | Schatz et al., 2016 [152]; Jørgensen et al., 2017 [96]; Lin et al., 2018 [124] |
| Therapy | Major Observations [Reference No.] |
|---|---|
| Danazol | Danazol is a weak androgen and anabolic steroid, a weak progestogen, a weak antigonadotropin, a weak steroidogenesis inhibitor and a functional antiestrogen [174]. A large number of patients do not report any significant relief of pain. Moreover, 1 out of 3 patients reported of recurrence of pain at the end of treatment [175,176,177]. According to some reports the treatment may reduce endometriosis related pain but has androgenic effects and causes cysts and infertility [178]. |
| Gestrinone | Gestrinone is a mixed progestogen and antiprogestogen, a weak androgen and anabolic steroid, a weak antigonadotropin, a weak steroidogenesis inhibitor, and a functional antiestrogen [174]. 1 out of 4 patients did not report any significant relieve of pain. Also, 1 out of 4 patients reported of recurrence of pain at the end of treatment or pain remaining at the end of treatment [179,180]. The main side effects are similar but less intense than those caused by danazol [181]. |
| GnRH agonist | 14% of patients did not experience any change in pain, while 40% patients experienced non-pain status remaining at end of treatment and 34% experienced recurrence at the end of treatment [43]. GnRHa alone has adverse side effects of estrogen deficiency and reduction in bone mineral density and sexual functioning [182,183]. |
| GnRH antagonist | Reported studies conducted in industry setup. With Elagolix, 19% of patients did not experience any change in pain [184,185]. Effective in improving dysmenorrhea and non-menstrual pelvic pain with significant lessening of fatigue, however, with associated hypoestrogenic adverse effects [186,187]. |
| GnRH agonist (Leuprolide) + progestin (NETA) | 61% pain reduction during treatment and 52% continued pain reduction after stopping treatment. 80% had at least one long term side effect more than 6 months after the completion of treatment [188]. |
| Synthetic progestogen | With dienogest, pooled analyses from clinical studies showed its tolerability with good safety profile and progressive improvement in pain scores in Caucasian [189] and Chinese population [190]. Comparable results were reported with norethindrone acetate [191]. Reductions in serum high density lipoproteins with use of norethindrone acetate and minor loss of bone density with dienogest are issues of concern [191]. |
| CHCs, COCs, POCs | CHCs and POCs are effective for the relief of endometriosis-related dysmenorrhoea, pelvic pain and dyspareunia and for improving the quality of life. A few COCs (ethinylestradiol/norethisterone acetate, ethinylestradiol/desogestrel and ethinylestradiol/gestodene) decreased risk of recurrence. Additional well-designed, blind, comparative trials required for effective management of endometriosis-related pain. For details, see Grandi et al. [192]. |
| Letrozole | Letrozole is an orally active, nonsteroidal, selective aromatase inhibitor used in the treatment of hormonally responsive breast cancer after surgery [193]. It may be administered in combination with oral contraceptive pills, progestogens or GnRH analogues for treating endometriosis associated pain to the patients with pain from rectovaginal endometriosis, and also to the patients who are refractory to other medical or surgical treatments it can be considered prescribing aromatase inhibitors [194,195]. Major side effects are hot flushes, myalgia and arthralgia [196]. It is as yet not globally approved. |
| NSAIDs | NSAIDs are the most commonly used over the counter drugs for the management of endometriosis related pain and dysmenorrhea; the evidence to indicate whether NSAIDs such as naproxen sodium are indeed effective for the management of pain caused by endometriosis is as yet inconclusive. There are insufficient studies to prove either way, as well as to prove the efficacy and safety of NSAIDs in the management of pain in endometriosis [197]. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghosh, D.; Filaretova, L.; Bharti, J.; Roy, K.K.; Sharma, J.B.; Sengupta, J. Pathophysiological Basis of Endometriosis-Linked Stress Associated with Pain and Infertility: A Conceptual Review. Reprod. Med. 2020, 1, 32-61. https://doi.org/10.3390/reprodmed1010004
Ghosh D, Filaretova L, Bharti J, Roy KK, Sharma JB, Sengupta J. Pathophysiological Basis of Endometriosis-Linked Stress Associated with Pain and Infertility: A Conceptual Review. Reproductive Medicine. 2020; 1(1):32-61. https://doi.org/10.3390/reprodmed1010004
Chicago/Turabian StyleGhosh, Debabrata, Ludmila Filaretova, Juhi Bharti, Kallol K. Roy, Jai B. Sharma, and Jayasree Sengupta. 2020. "Pathophysiological Basis of Endometriosis-Linked Stress Associated with Pain and Infertility: A Conceptual Review" Reproductive Medicine 1, no. 1: 32-61. https://doi.org/10.3390/reprodmed1010004
APA StyleGhosh, D., Filaretova, L., Bharti, J., Roy, K. K., Sharma, J. B., & Sengupta, J. (2020). Pathophysiological Basis of Endometriosis-Linked Stress Associated with Pain and Infertility: A Conceptual Review. Reproductive Medicine, 1(1), 32-61. https://doi.org/10.3390/reprodmed1010004